Effect of Rare Earth Cerium Content on Manganese Sulfide in U75V Heavy Rail Steel
Abstract
:1. Introduction
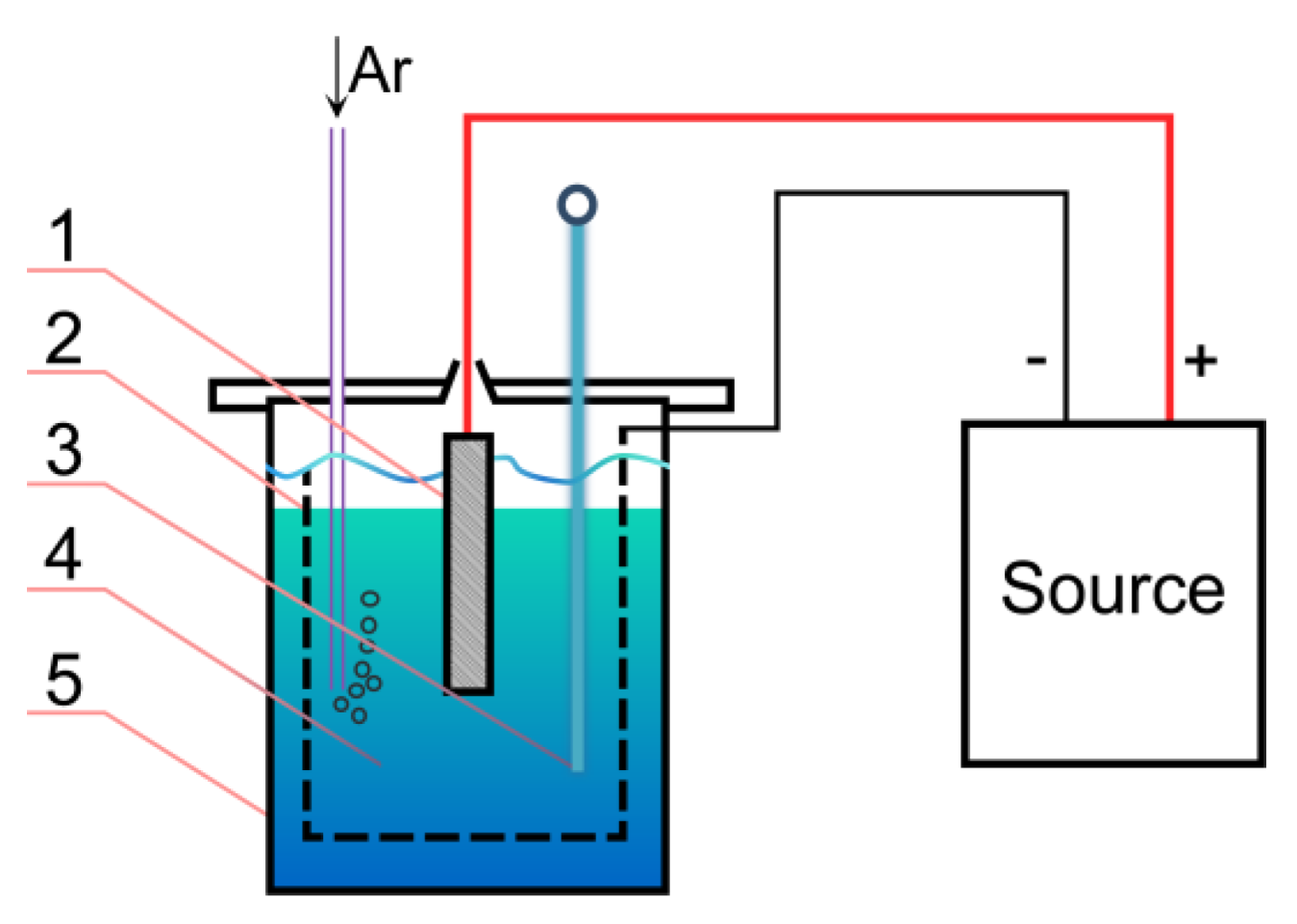
2. Materials and Methods
3. Results and Discussion
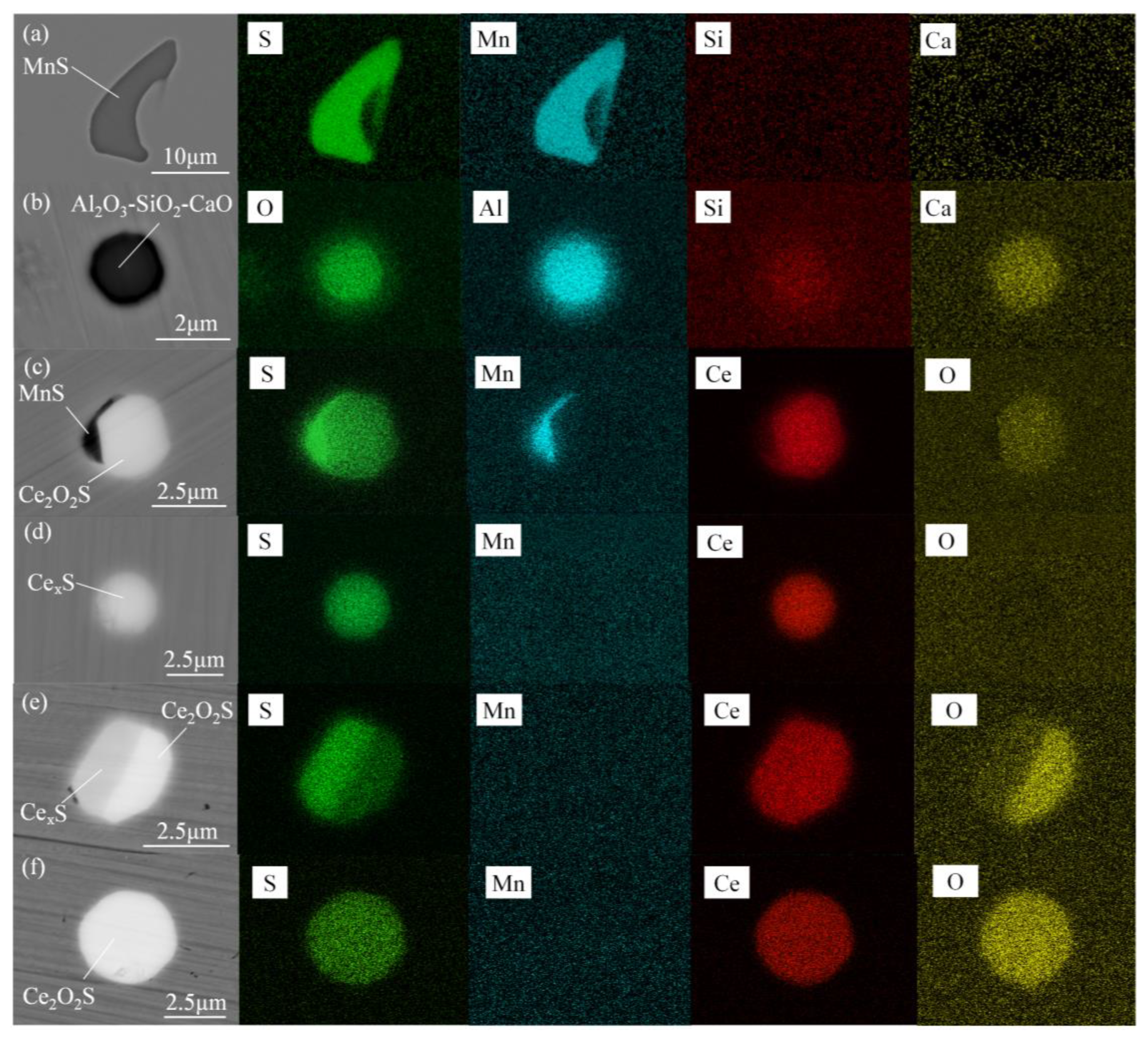
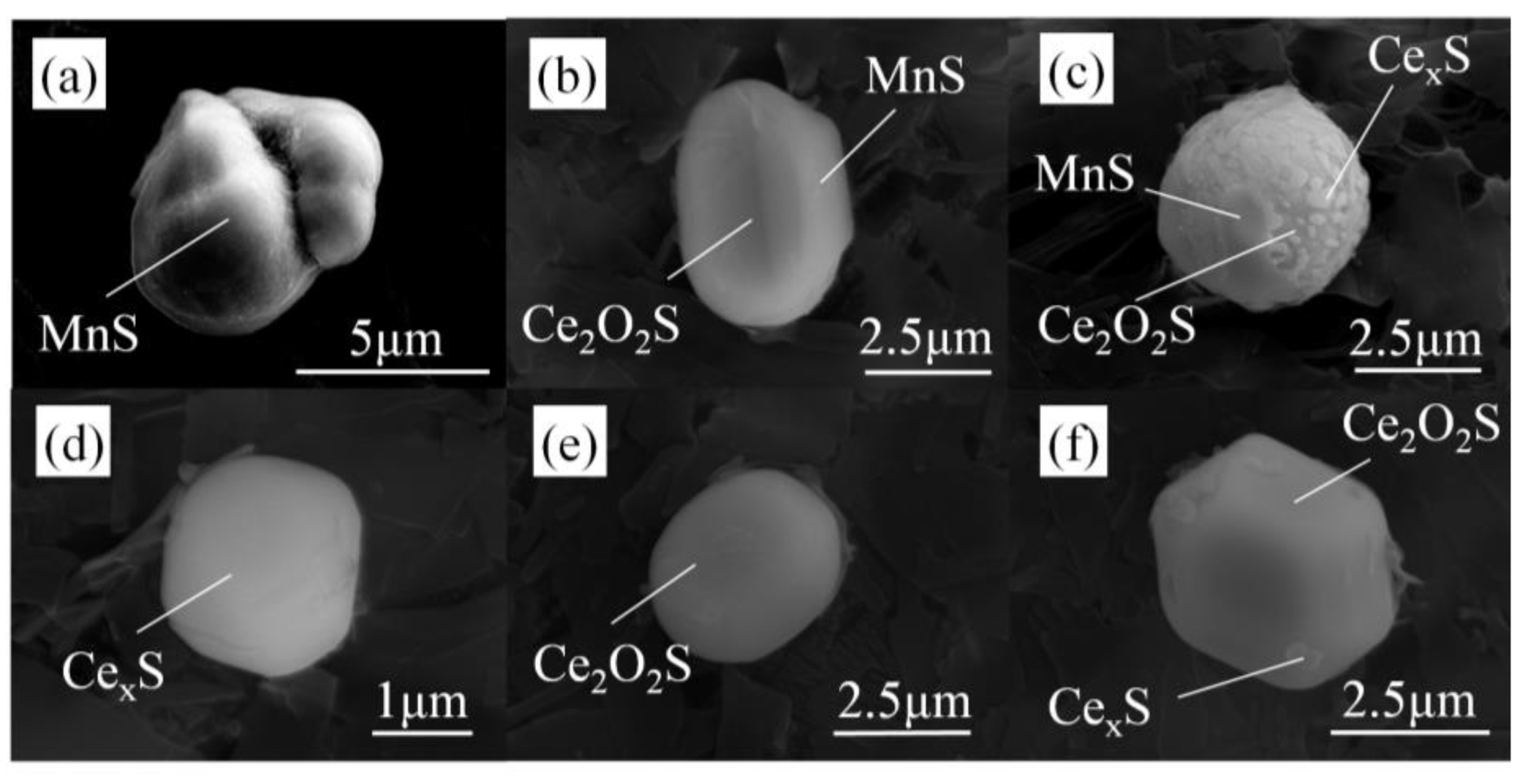
3.1. Composition and Morphology of Inclusions
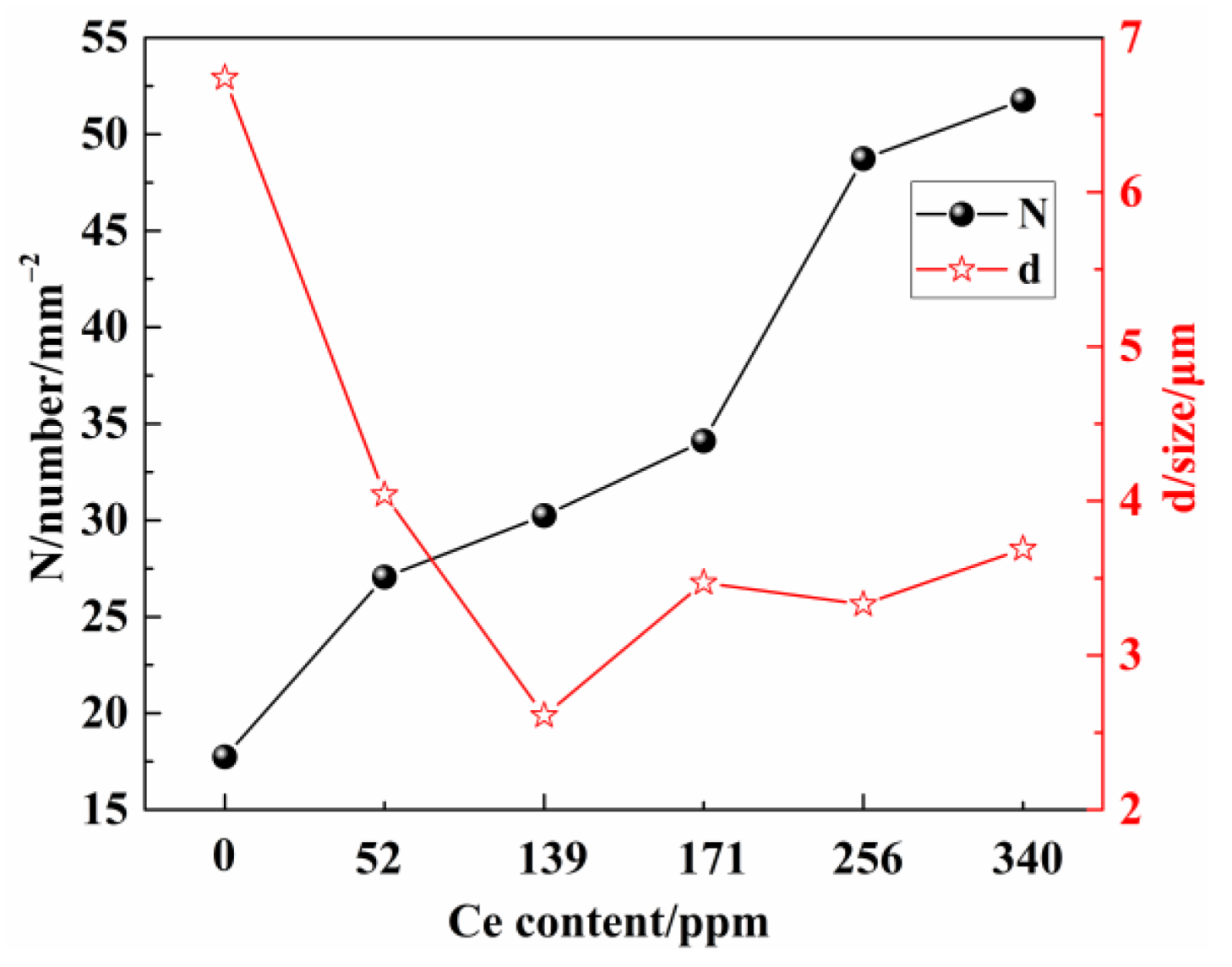
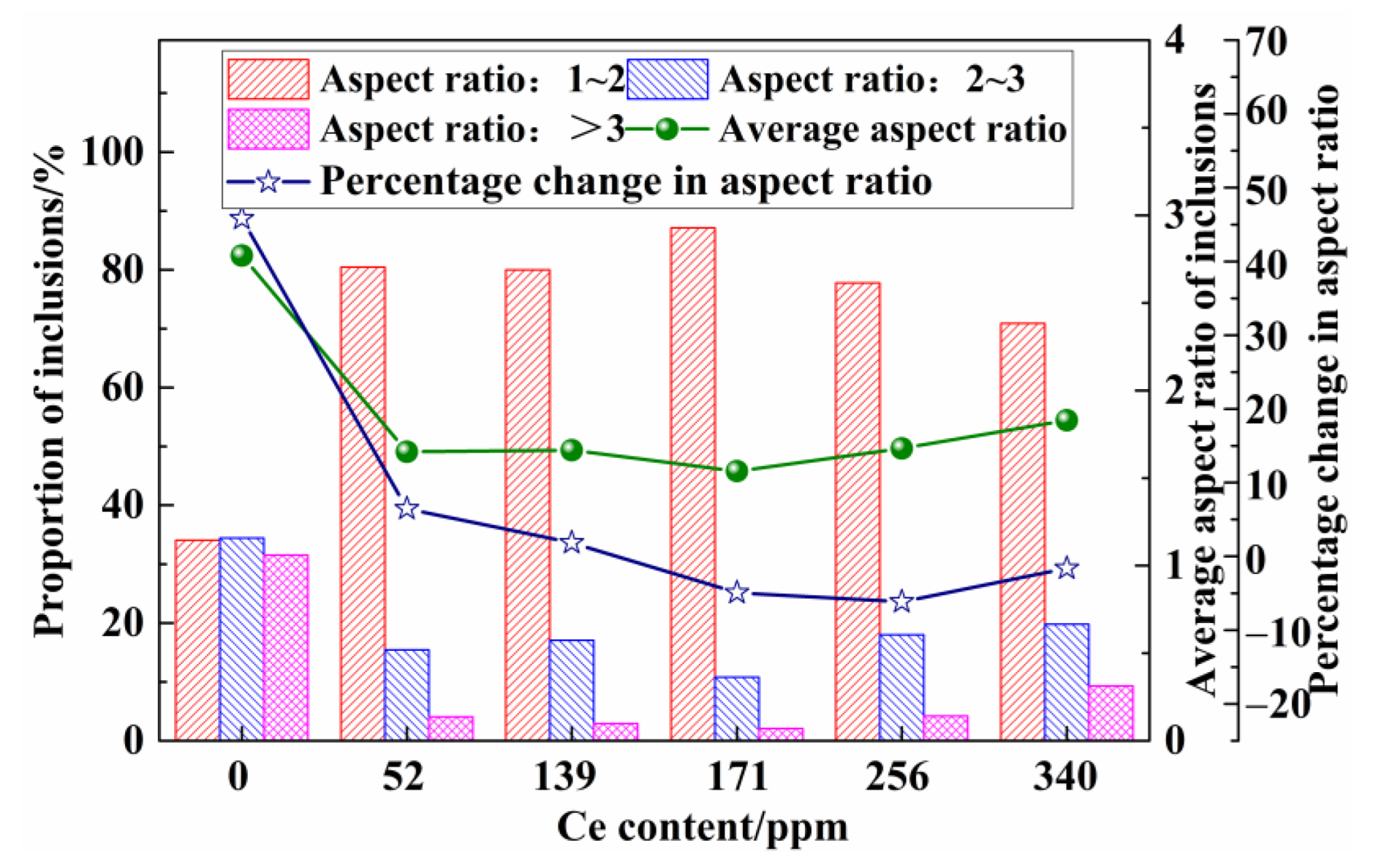
3.2. Number Density, Size, and Aspect Ratio of Inclusions
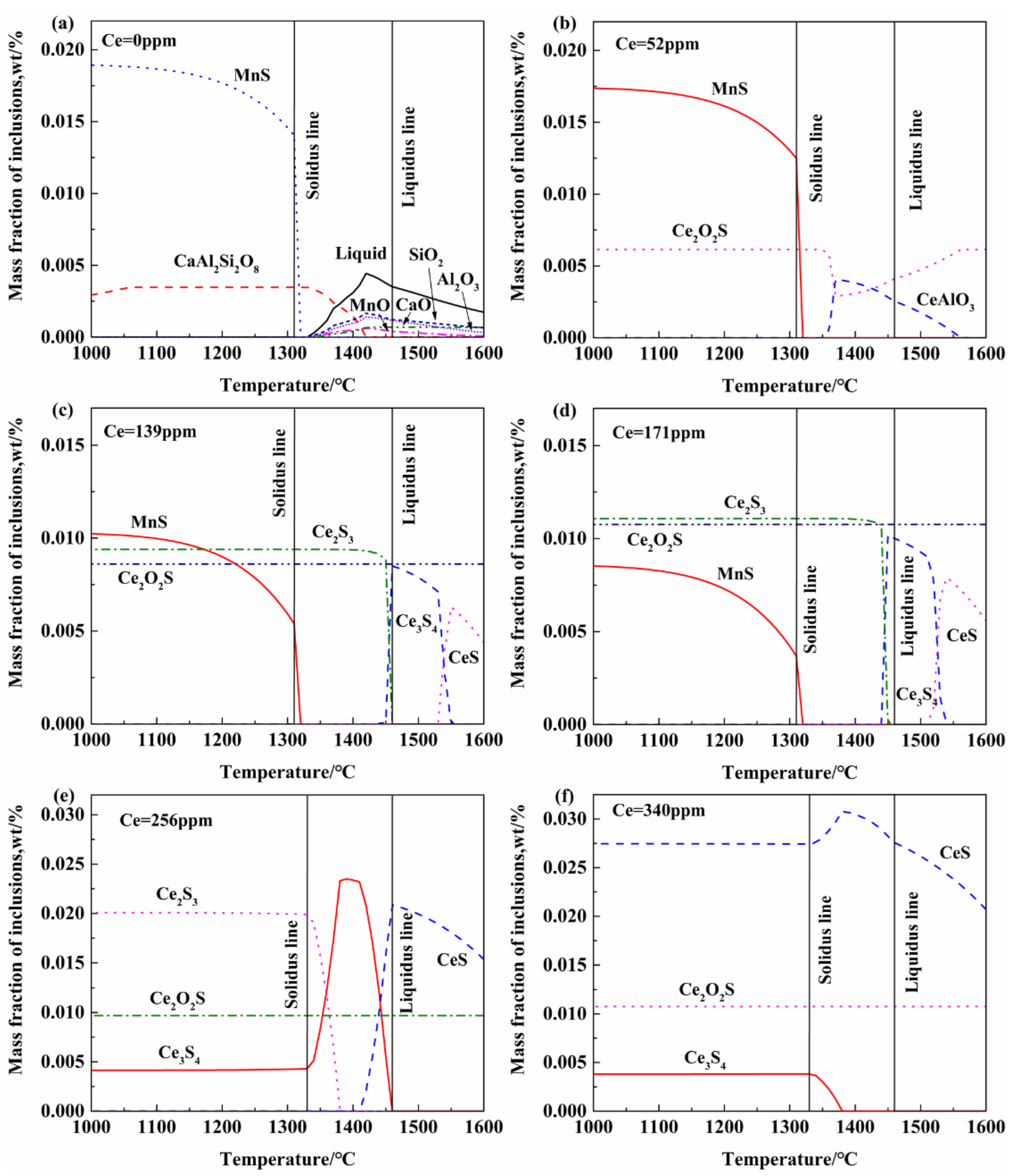
3.3. Thermodynamic Analysis of Inclusion Formation in Steel
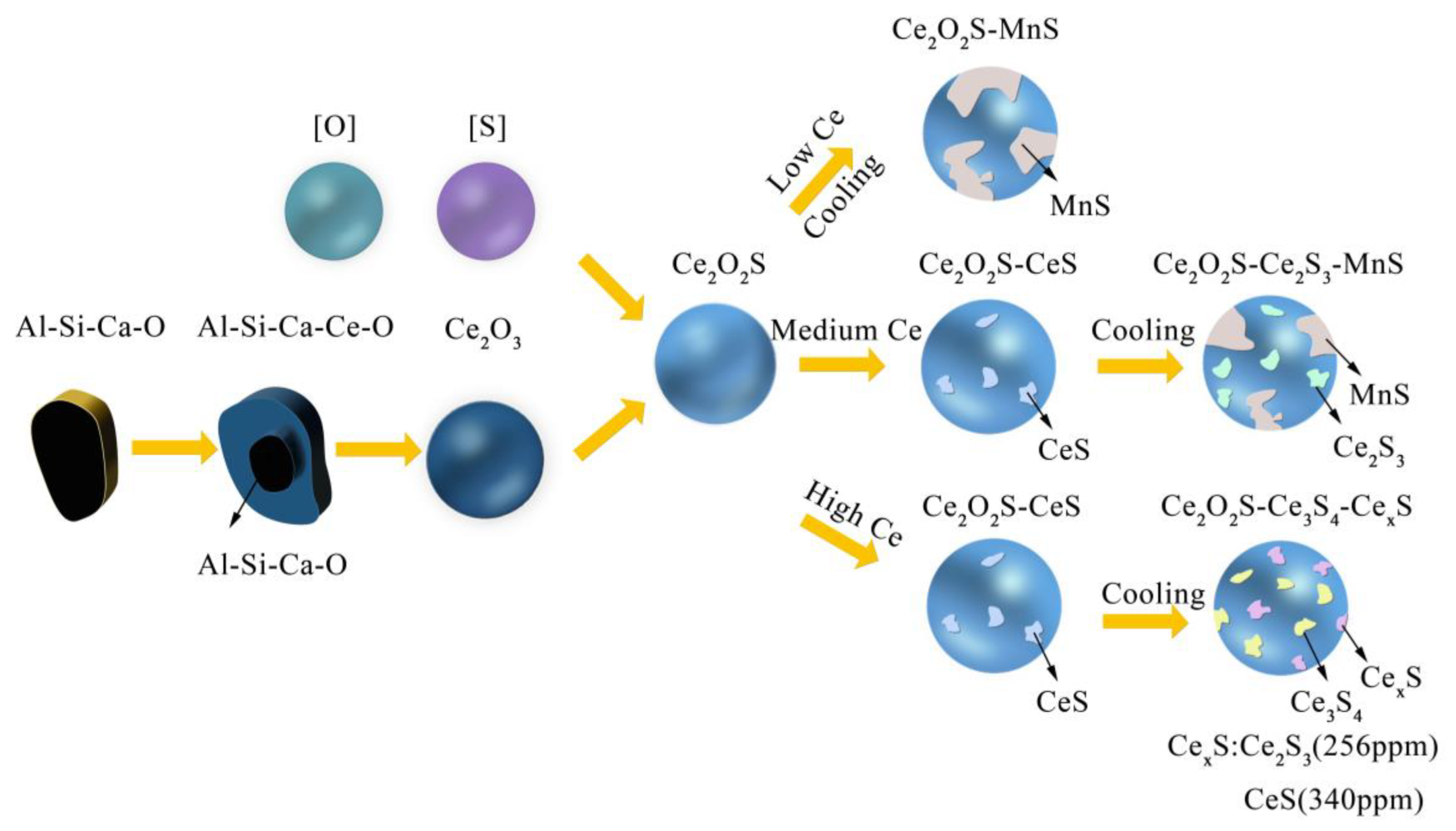
3.4. Evolution Mechanism of Inclusions in Heavy Rail Steel after Adding Ce
3.5. Effect of Ce on Sulfide after Simulated Rolling
4. Conclusions
- (1)
- Without Ce addition to steel, the inclusions in heavy rail steel were elongated MnS and irregular Al-Si-Ca-O inclusions. With the increase in the Ce content from 52 to 340 ppm, the composition of the main inclusions changed in order of Ce2O2S-MnS → Ce2O2S-MnS-Ce2S3 → Ce2O2S-Ce3S4-Ce2S3 → Ce2O2S-Ce3S4-CeS.
- (2)
- The addition of Ce to molten steel causes a significant increase in the number density and a considerable reduction in the size and aspect ratio of the inclusions. The average size of the inclusions without Ce was 6.74 μm. The average size of inclusions upon Ce addition was 2.01–4.04 μm, the size of inclusions was the smallest at 139 ppm Ce.
- (3)
- The change in the aspect ratio of the inclusions before and after thermal deformation was minimal, indicating that Ce can significantly inhibit the deformation of inclusions during the hot compression process. Therefore, when the Ce content of molten steel was 139 ppm, substantial amounts of dispersed, fine, and deformation-resistant inclusions can be obtained.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luu, W.; Wu, J. Effects of sulfide inclusion on hydrogen transport in steels. Mater. Lett. 1995, 24, 175–179. [Google Scholar] [CrossRef]
- Garet, M.; Brass, A.M.; Haut, C.; Guttierez-Solana, F. Hydrogen trapping on non metallic inclusions in Cr-Mo low alloy steels. Corros. Sci. 1998, 40, 1073–1086. [Google Scholar] [CrossRef]
- Domizzi, G.; Anteri, G.; Ovejero-Garcıa, J. Influence of sulphur content and inclusion distribution on the hydrogen induced blister cracking in pressure vessel and pipeline steels. Corros. Sci. 2001, 43, 325–339. [Google Scholar] [CrossRef]
- Wu, X.; Wu, L.; Xie, J.; Shen, P.; Fu, J. Modification of sulfide by Te in Y1Cr13 free-cutting stainless steel. Metall. Res. Technol. 2020, 117, 107. [Google Scholar] [CrossRef] [Green Version]
- Brimacombe, J.K.; Sorimachi, K. Crack formation in the continuous casting of steel. Metall. Trans. B 1977, 8, 489–505. [Google Scholar] [CrossRef]
- Oikawa, K.; Ishida, K.; Nishizawa, T. Effect of titanium addition on the formation and distribution of MnS inclusions in steel during solidification. ISIJ Int. 1997, 37, 332–338. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, D.; Yang, Q.; An, J.; Huang, Z.; Fu, J. Exploration of morphology evolution of the inclusions in Mg-treated 16MnCrS5 steel. Ironmak. Steelmak. 2018, 46, 564–573. [Google Scholar] [CrossRef]
- Lu, J.; Cheng, G.; Chen, L.; Xiong, G.; Wang, L. Distribution and morphology of MnS inclusions in resulfurized non-quenched and tempered steel with Zr addition. ISIJ Int. 2018, 58, 1307–1315. [Google Scholar] [CrossRef] [Green Version]
- Blais, C.; L’Espérance, G.; LeHuy, H.; Forget, C. Development of an integrated method for fully characterizing multiphase inclusions and its application to calcium-treated steels. Mater. Charact. 1997, 38, 25–37. [Google Scholar] [CrossRef]
- Chen, L. Study on the effects of Ti micro-addition on the characteristics of MnS inclusions in rail steel. Ironmak. Steelmak. 2019, 46, 508–512. [Google Scholar] [CrossRef]
- Ji, S.; Zhang, L.; Wang, X. Effect of Magnesium on Inclusions in a High Sulfur Steel. Metall. Mater. Trans. B 2022, 53, 848–863. [Google Scholar] [CrossRef]
- Shen, P.; Fu, J. Morphology study on inclusion modifications using Mg–Ca treatment in resulfurized special steel. Materials 2019, 12, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; He, S.; Chen, G.; Wang, Q. Thermodynamics of complex sulfide inclusion formation in Ca-treated Al-killed structural steel. Metall. Mater. Trans. B 2016, 47, 2549–2557. [Google Scholar] [CrossRef]
- Wang, H.; Xiong, L.; Zhang, L.; Wang, Y.; Shu, Y.; Zhou, Y. Investigation of RE-OS-As inclusions in high carbon steels. Metall. Mater. Trans. B 2017, 48, 2849–2858. [Google Scholar] [CrossRef]
- Adabavazeh, Z.; Hwang, W.; Su, Y. Effect of adding cerium on microstructure and morphology of Ce-based inclusions formed in low-carbon steel. Sci. Rep. 2017, 7, 46503. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bao, Y.; Zhao, M.; Wang, M.; Yuan, X.; Gao, S. Effect of Ce on the cleanliness, microstructure and mechanical properties of high strength low alloy steel Q690E in industrial production process. Int. J. Miner. Metall. Mater. 2019, 26, 1372–1384. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Liu, Y.; Wang, F.; Wang, Q. Cerium Addition Effect on Modification of Inclusions, Primary Carbides and Microstructure Refinement of H13 Die Steel. ISIJ Int. 2021, 61, 1850–1859. [Google Scholar] [CrossRef]
- Torkamani, H.; Raygan, S.; Mateo, C.G.; Rassizadehghani, J.; Vivas, J.; Palizdar, Y.; San-Martin, D. The influence of La and Ce addition on inclusion modification in cast niobium microalloyed steels. Metals 2017, 7, 377. [Google Scholar] [CrossRef] [Green Version]
- Ren, Q.; Zhang, L. Effect of cerium content on inclusions in an ultra-low-carbon aluminum-killed steel. Metall. Mater. Trans. B 2020, 51, 589–600. [Google Scholar] [CrossRef]
- Liu, Z.; Song, B.; Yang, Z.; Cui, X.; Li, L.; Wang, L.; Song, Z. Effect of Cerium Content on the Evolution of Inclusions and Formation of Acicular Ferrite in Ti-Mg-Killed EH36 Steel. Metals 2020, 10, 863. [Google Scholar] [CrossRef]
- Liu, H.; Fu, P.; Liu, H.; Sun, C.; Sun, M.; Li, D. A novel large cross-section quenching and tempering mold steel matching excellent strength–hardness–toughness properties. Mat. Sci. Eng. A 2018, 737, 274–285. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Chou, K. Effect of cerium on the cleanliness of spring steel used in fastener of high-speed railway. J. Rare Earths 2014, 32, 759–766. [Google Scholar] [CrossRef]
- Gao, S.; Wang, M.; Guo, J.; Wang, H.; Zhi, J.; Bao, Y. Characterization Transformation of Inclusions Using Rare Earth Ce Treatment on Al-Killed Titanium Alloyed Interstitial Free Steel. Steel Res. Int. 2019, 90, 1900194. [Google Scholar] [CrossRef]
- Luo, S.; Shen, Z.; Yu, Z.; Wang, W.; Zhu, M. Effect of Ce Addition on Inclusions and Grain Structure in Gear Steel 20CrNiMo. Steel Res. Int. 2021, 92, 2000394. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Wang, L.; Xiong, X.; Chen, L.; Zhuang, C. Modification of Alumina Inclusions in SWRS82B Steel by Adding Rare Earth Cerium. Metals 2020, 10, 1696. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, Y.; Wang, S.; Hou, Y.; Yin, S. Effects of Rare Earth on Mechanical Properties of LZ50 Axle Steels and Its Formation Mechanism. High Temp. Mater. Process 2018, 37, 509–519. [Google Scholar] [CrossRef]
- Cai, G.; Pang, Y.; Huang, Y.; Misra, R.D.K. Roles of Inclusion, Texture and Grain Boundary in Corrosion Resistance of Low-Nickel Austenite Stainless Steel Containing Ce. ISIJ Int. 2019, 59, 2302–2310. [Google Scholar] [CrossRef] [Green Version]
- Shen, P.; Yang, Q.; Zhang, D.; Yang, S.; Fu, J. The effect of tellurium on the formation of MnTe-MnS composite inclusions in non-quenched and tempered steel. Metals 2018, 8, 639. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Jiang, Z.; Geng, X.; Chen, M.; Peng, L. Evolution mechanism of inclusions in H13 steel with rare earth magnesium alloy addition. ISIJ Int. 2019, 59, 1552–1561. [Google Scholar] [CrossRef] [Green Version]
- Gong, W.; Wang, P.; Zhang, L.; Jiang, Z. Effects of Ce on Microstructure and Mechanical Properties of LDX2101 Duplex Stainless Steel. Metals 2020, 10, 1233. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Z.; Geng, X.; Chen, M.; Cui, S. Effect of Rare Earth–Magnesium Alloy on Inclusion Evolution in Industrial Production of Die Steel. Steel Res. Int. 2019, 90, 1900103. [Google Scholar] [CrossRef]




| Composition | C | Si | Mn | P | S | V | Als |
|---|---|---|---|---|---|---|---|
| Content | 0.77 | 0.64 | 0.89 | 0.02 | 0.007 | 0.04 | 0.0015 |
| No. | C0 | C1 | C2 | C3 | C4 | C5 |
|---|---|---|---|---|---|---|
| Ce | 0 | 0.0052 | 0.0139 | 0.0171 | 0.0256 | 0.0340 |
| Typical Inclusions | Si-Al-Ca-O | MnS | Ce2O2S-MnS | CexS | Ce2O2S | Ce2O2S-CexS | Ce2O2S-CexS-MnS |
|---|---|---|---|---|---|---|---|
| C0 | √√ | √√ | – | – | – | – | – |
| C1 | – | – | √√ | – | – | – | – |
| C2 | – | – | √√ | √ | – | – | √ |
| C3 | – | – | √√ | √ | √ | √ | √ |
| C4 | – | – | √ | √ | √ | √√ | – |
| C5 | – | – | √ | √ | √ | √√ | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuo, C.; Liu, R.; Zhao, Z.; Zhang, Y.; Hao, X.; Wu, H.; Sun, Y. Effect of Rare Earth Cerium Content on Manganese Sulfide in U75V Heavy Rail Steel. Metals 2022, 12, 1012. https://doi.org/10.3390/met12061012
Zhuo C, Liu R, Zhao Z, Zhang Y, Hao X, Wu H, Sun Y. Effect of Rare Earth Cerium Content on Manganese Sulfide in U75V Heavy Rail Steel. Metals. 2022; 12(6):1012. https://doi.org/10.3390/met12061012
Chicago/Turabian StyleZhuo, Chao, Rui Liu, Zirong Zhao, Yulei Zhang, Xiaoshuai Hao, Huajie Wu, and Yanhui Sun. 2022. "Effect of Rare Earth Cerium Content on Manganese Sulfide in U75V Heavy Rail Steel" Metals 12, no. 6: 1012. https://doi.org/10.3390/met12061012
APA StyleZhuo, C., Liu, R., Zhao, Z., Zhang, Y., Hao, X., Wu, H., & Sun, Y. (2022). Effect of Rare Earth Cerium Content on Manganese Sulfide in U75V Heavy Rail Steel. Metals, 12(6), 1012. https://doi.org/10.3390/met12061012





