Evolution of Inclusions and Cleanliness in Ti-Bearing IF Steel Produced via the BOF–LF–RH–CC Process
Abstract
1. Introduction
2. Materials and Methods
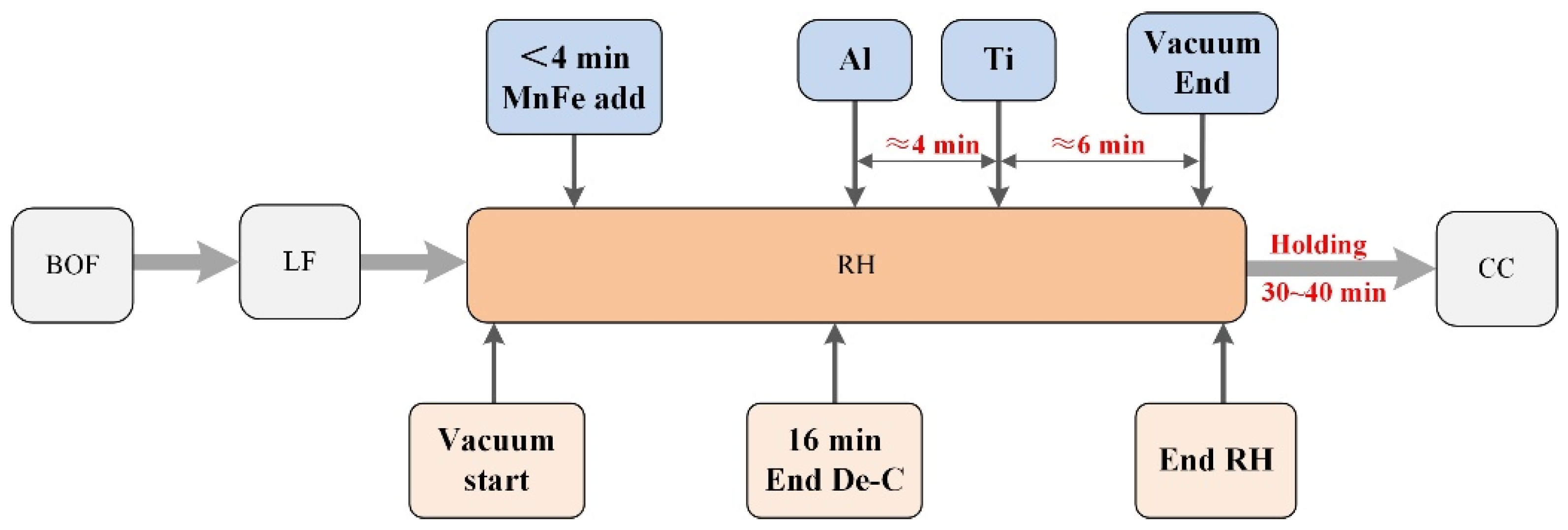
2.1. Industrial Investigations
2.2. Experimental Methods
3. Results and Discussion
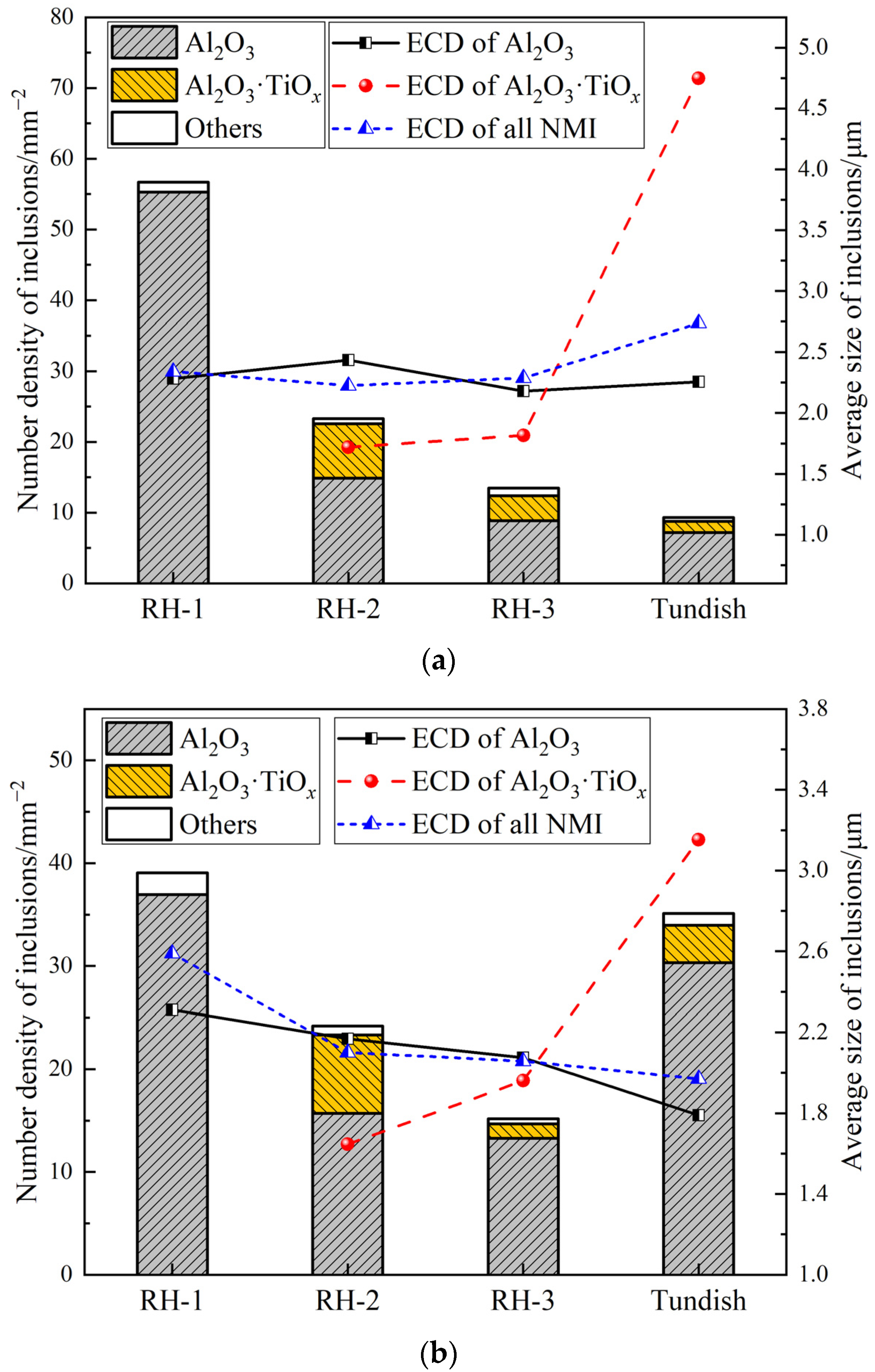
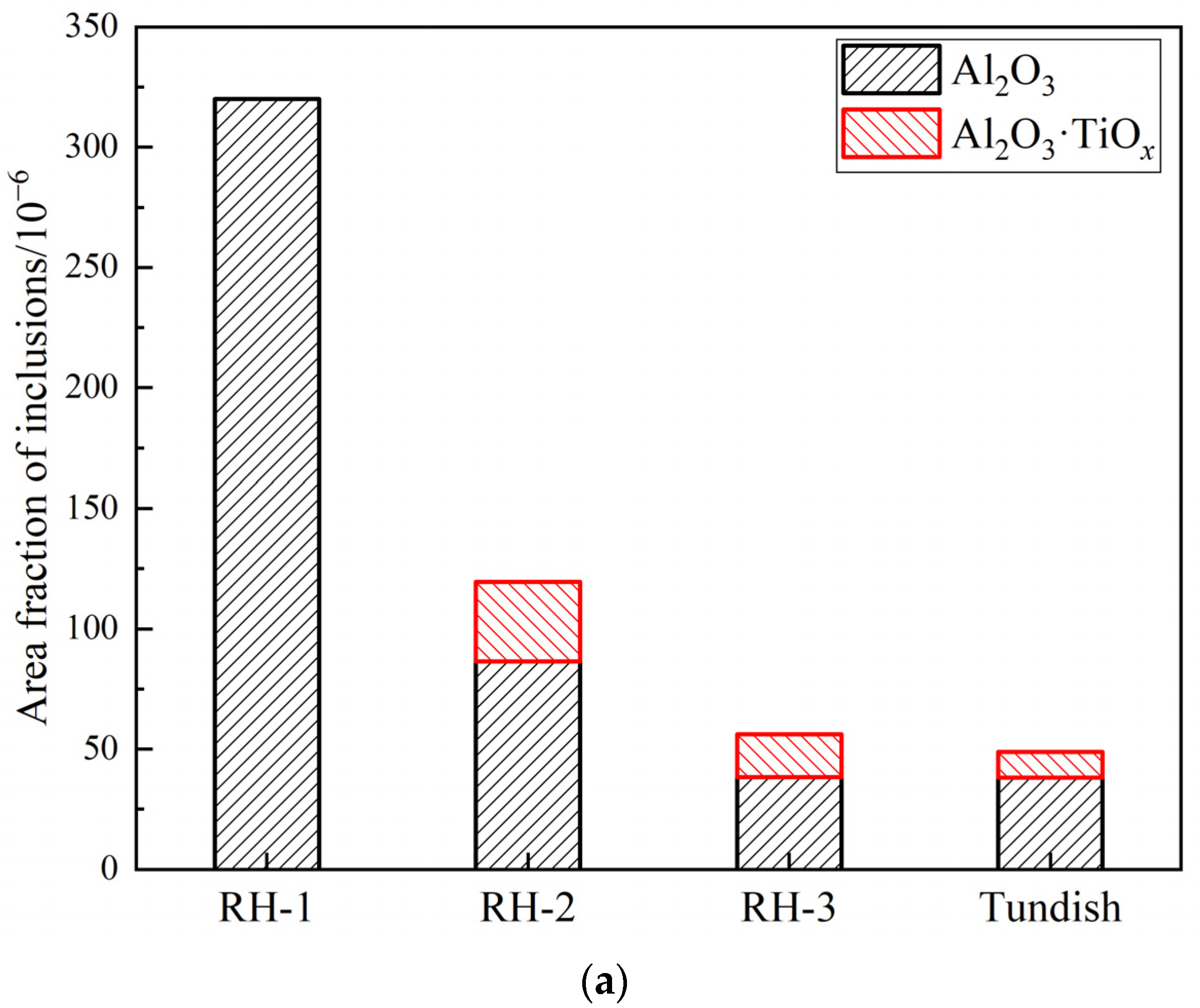
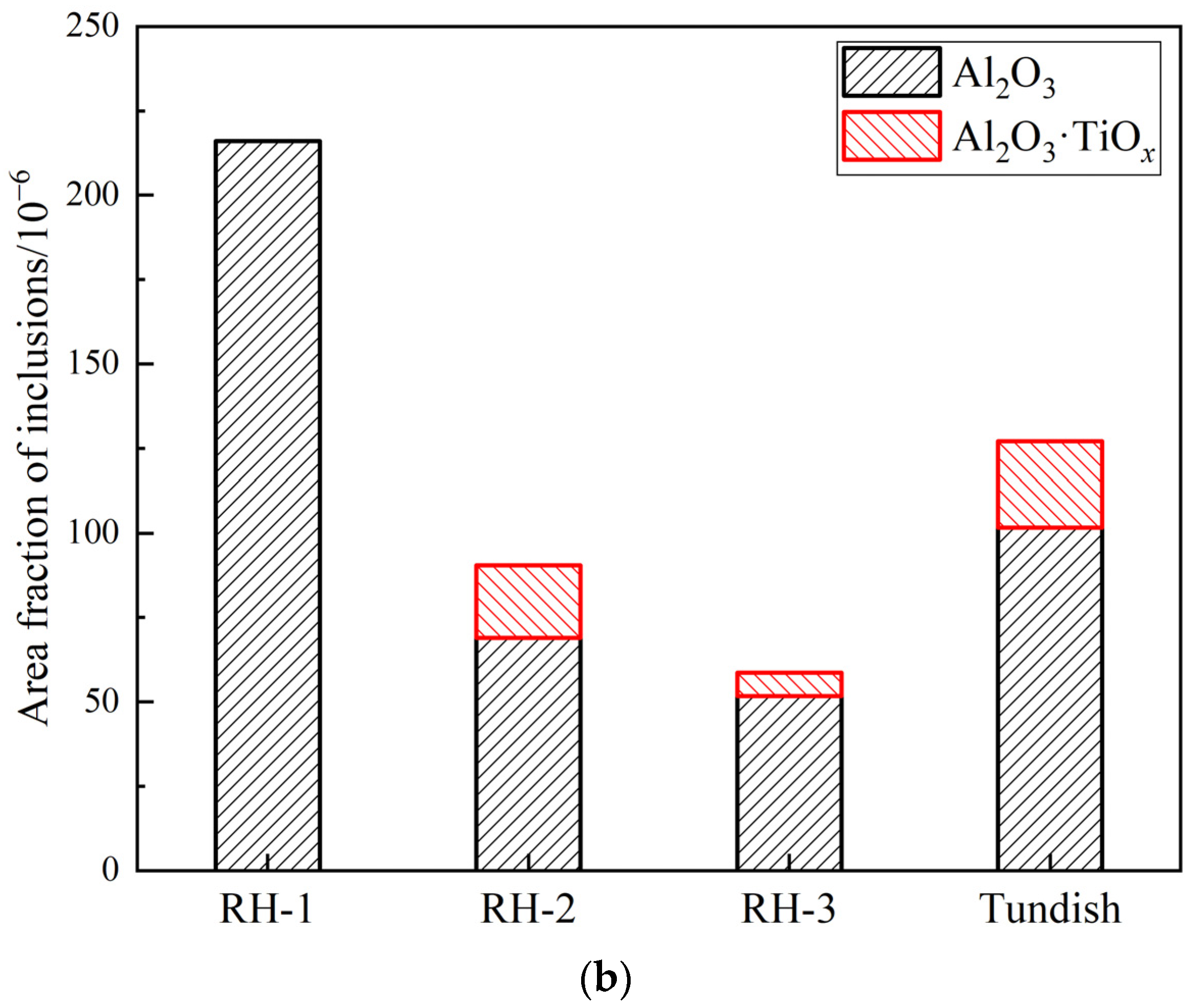
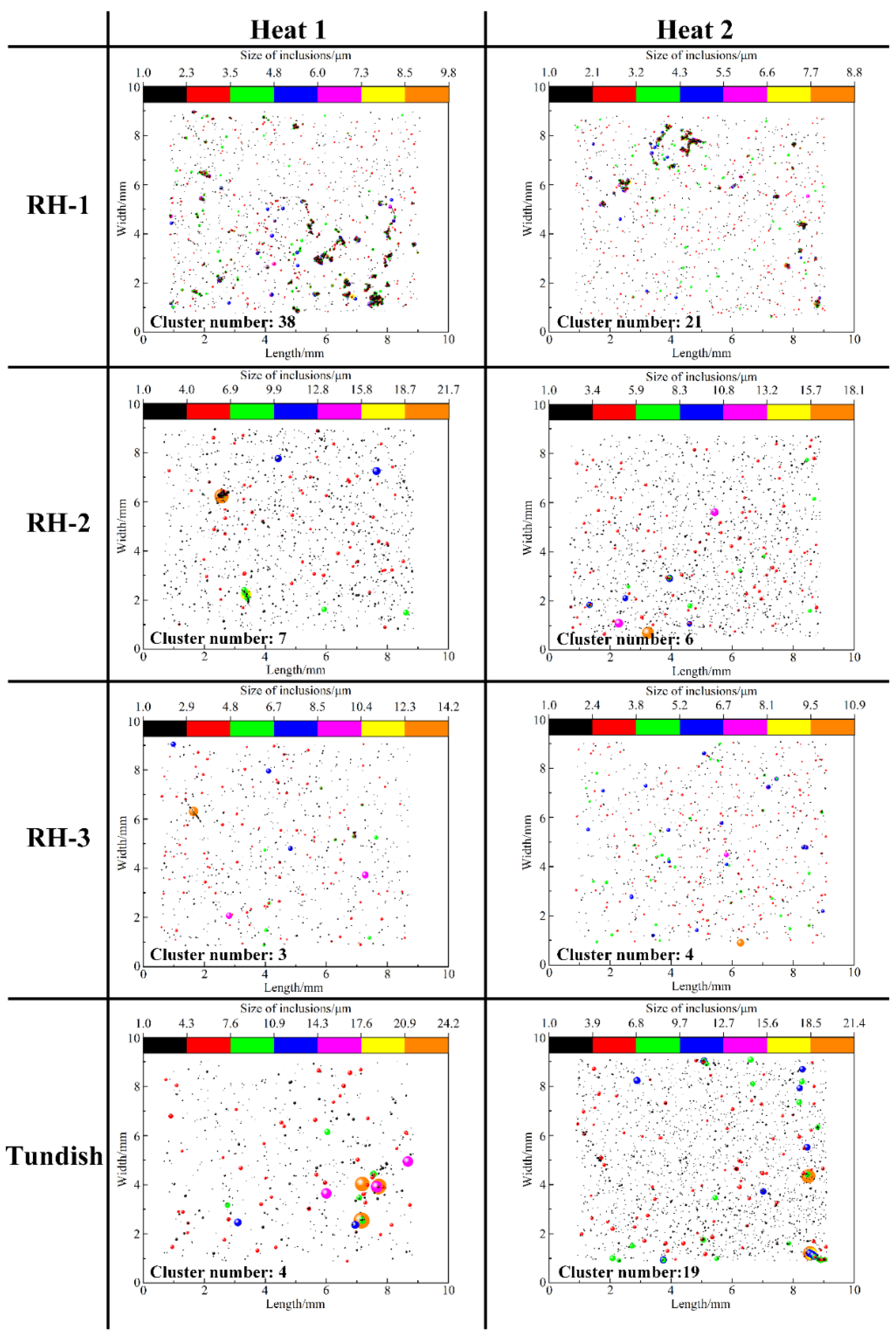
3.1. Number and Size Distribution of Inclusions
3.2. Morphological Evolution of Inclusions
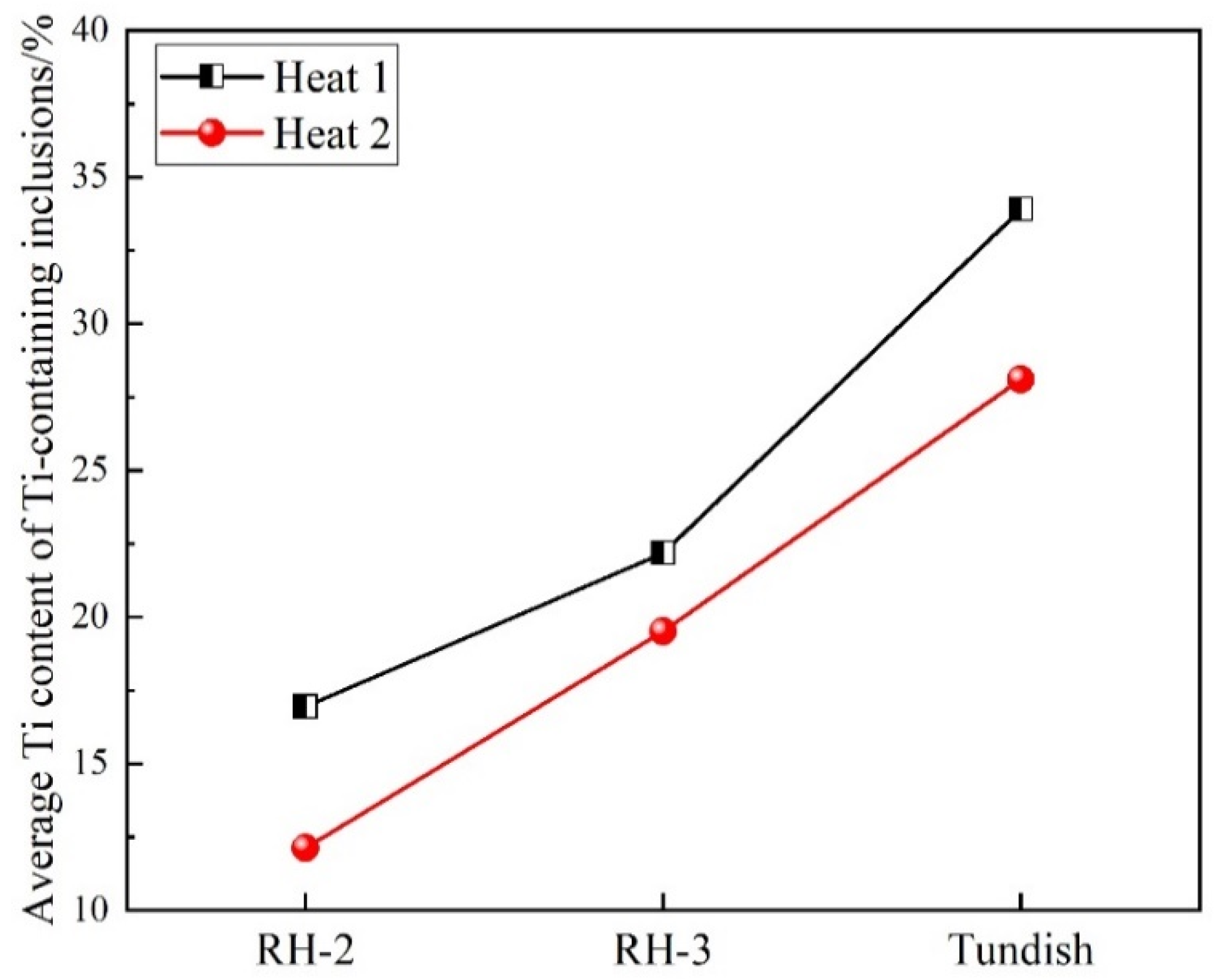
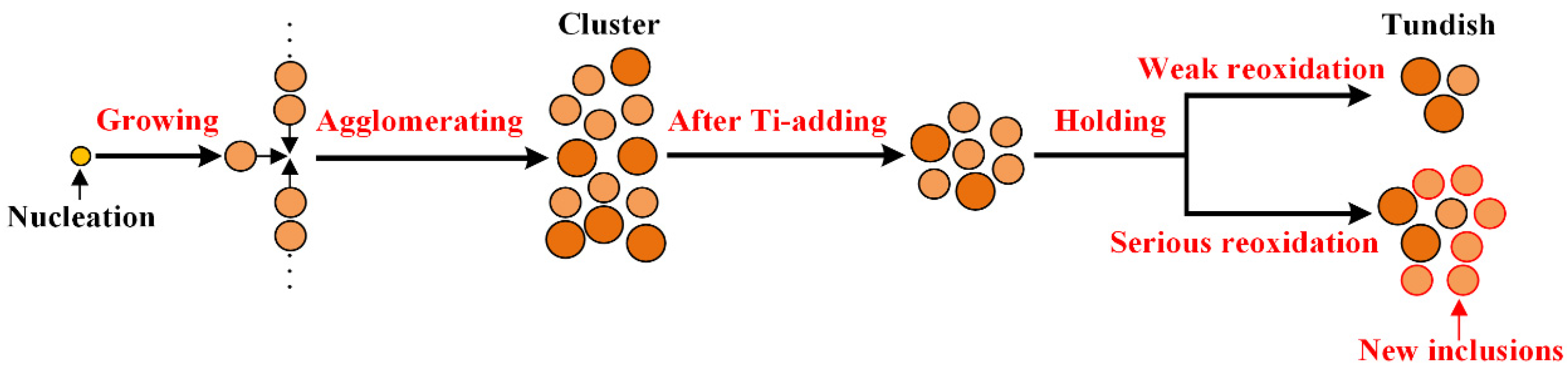
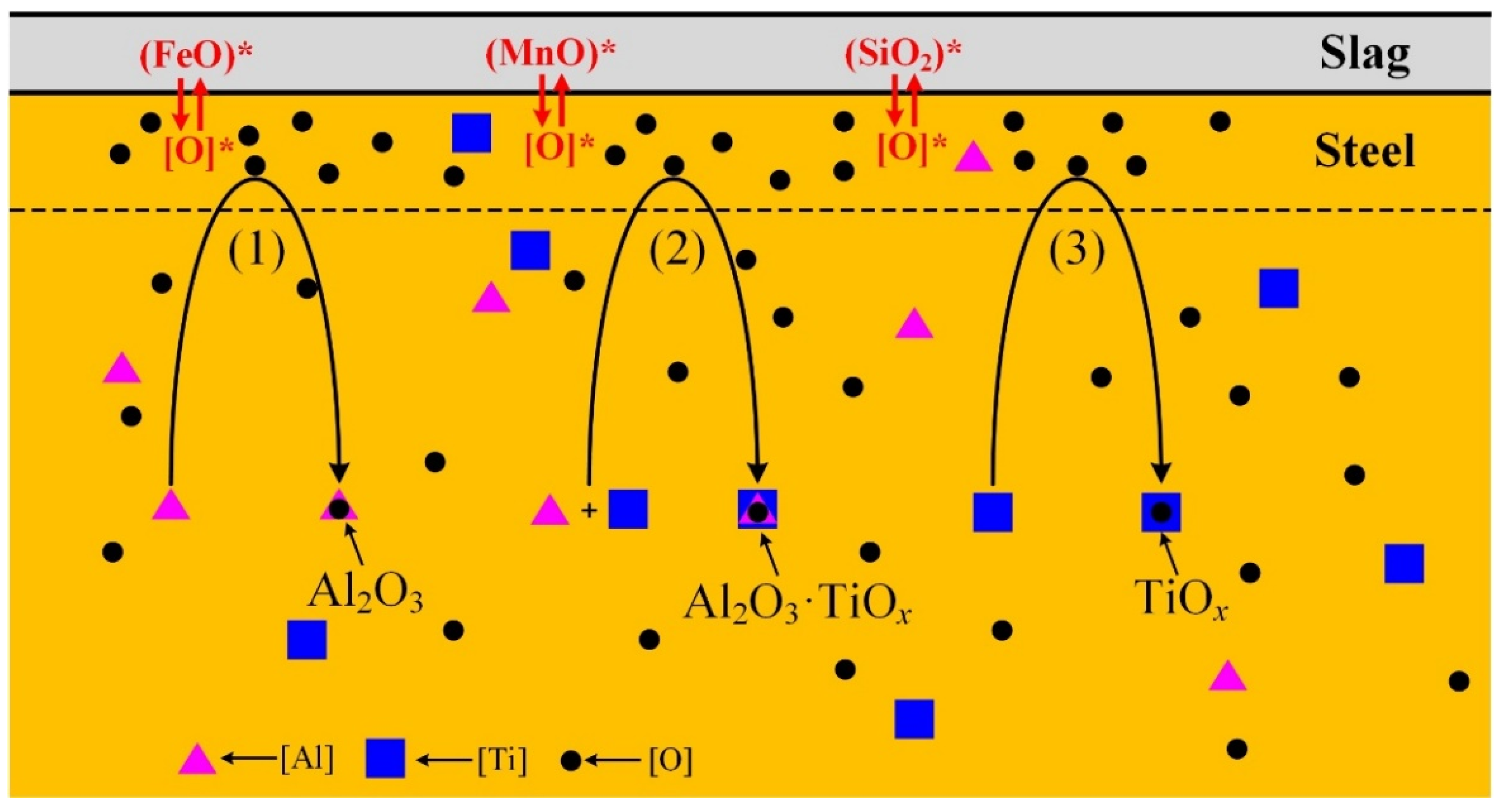
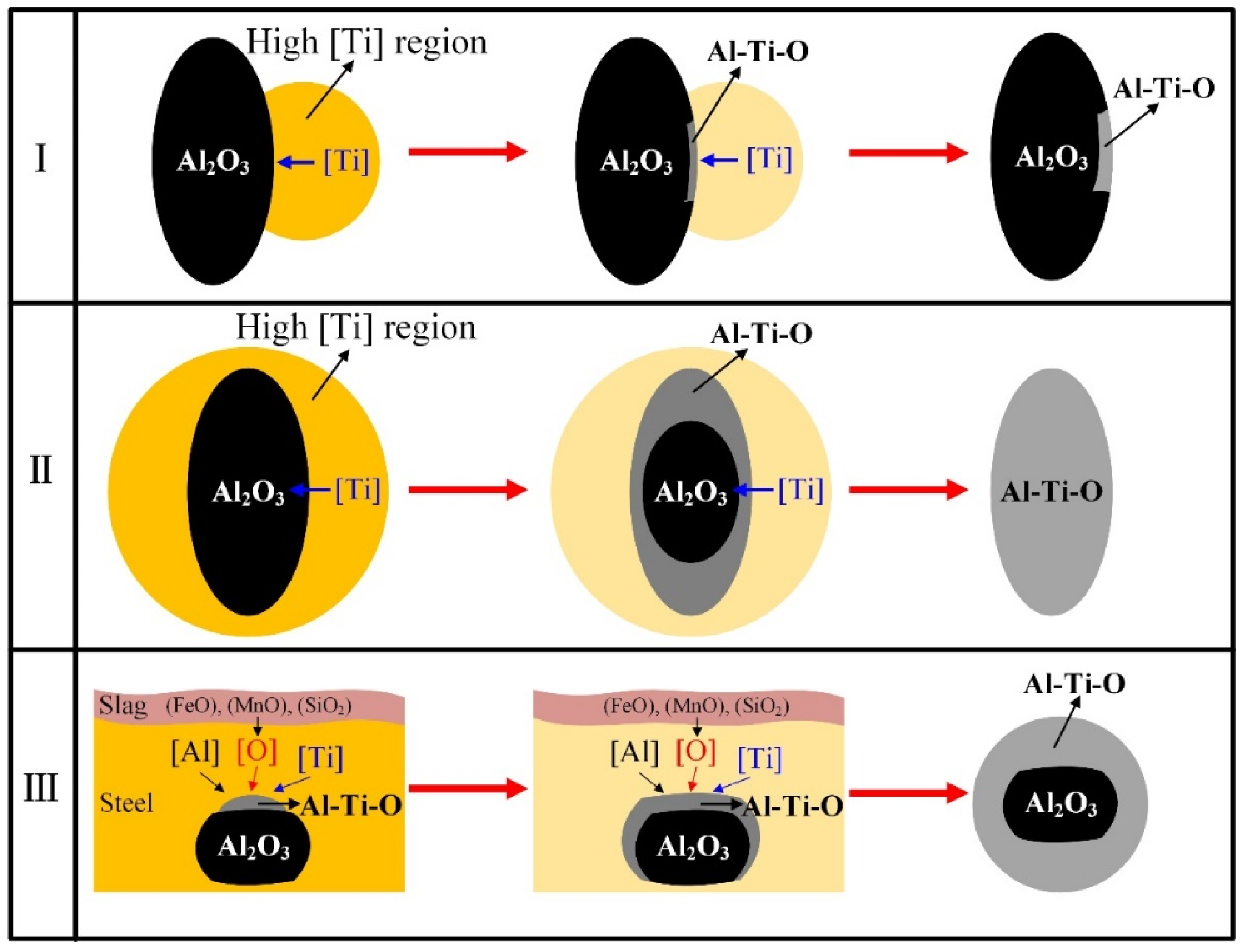
3.3. Formation Evolution of Inclusions
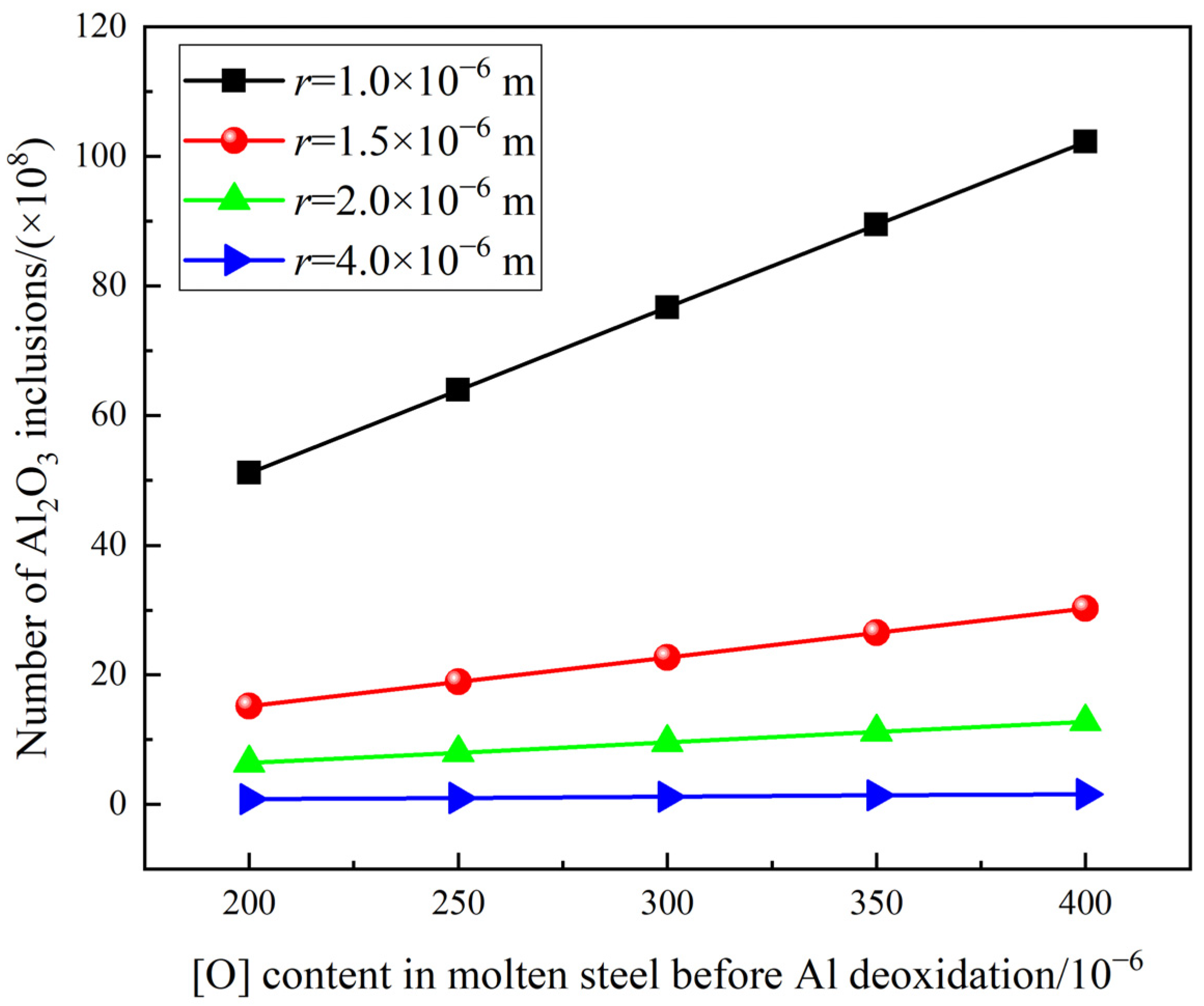
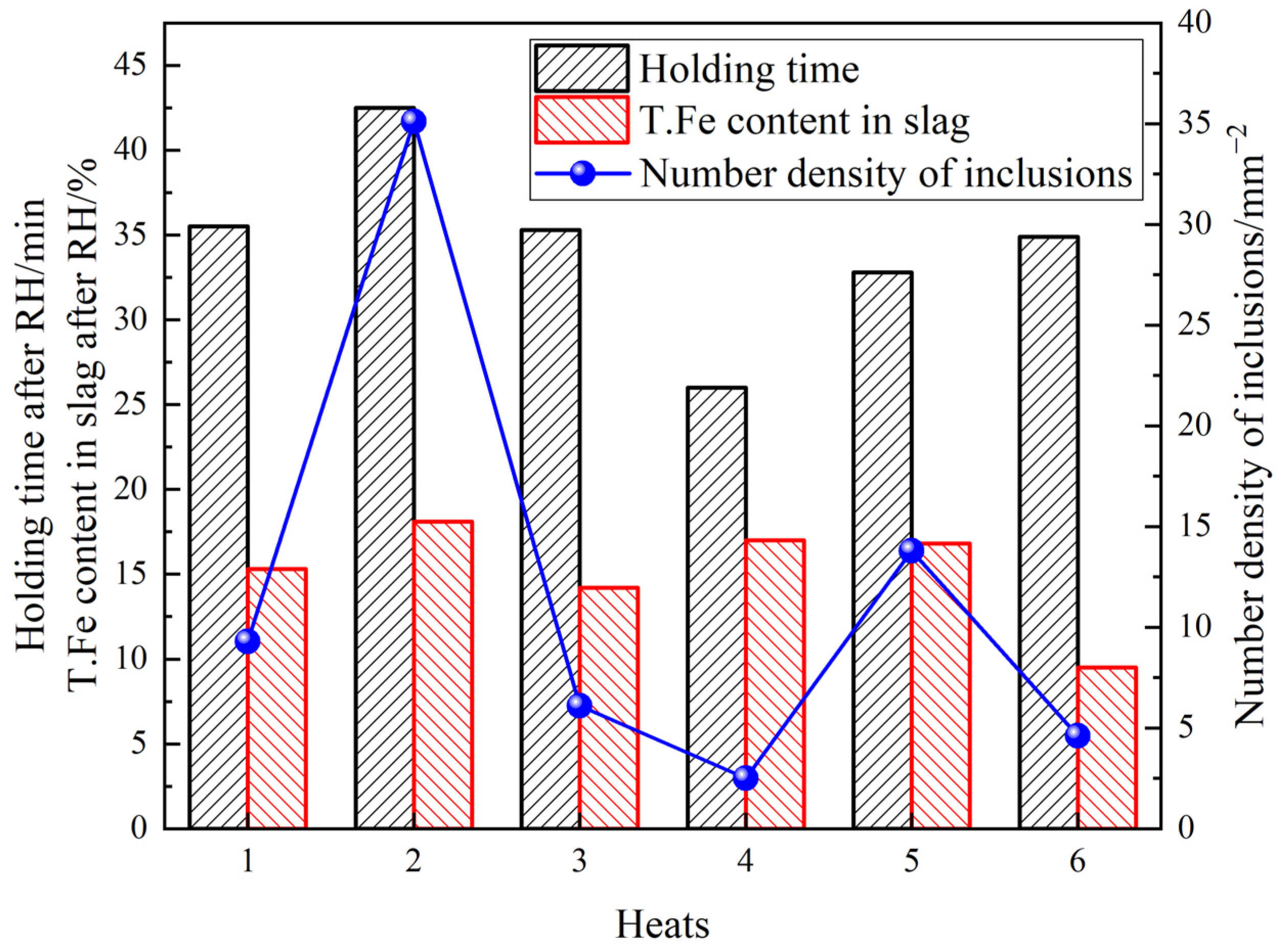
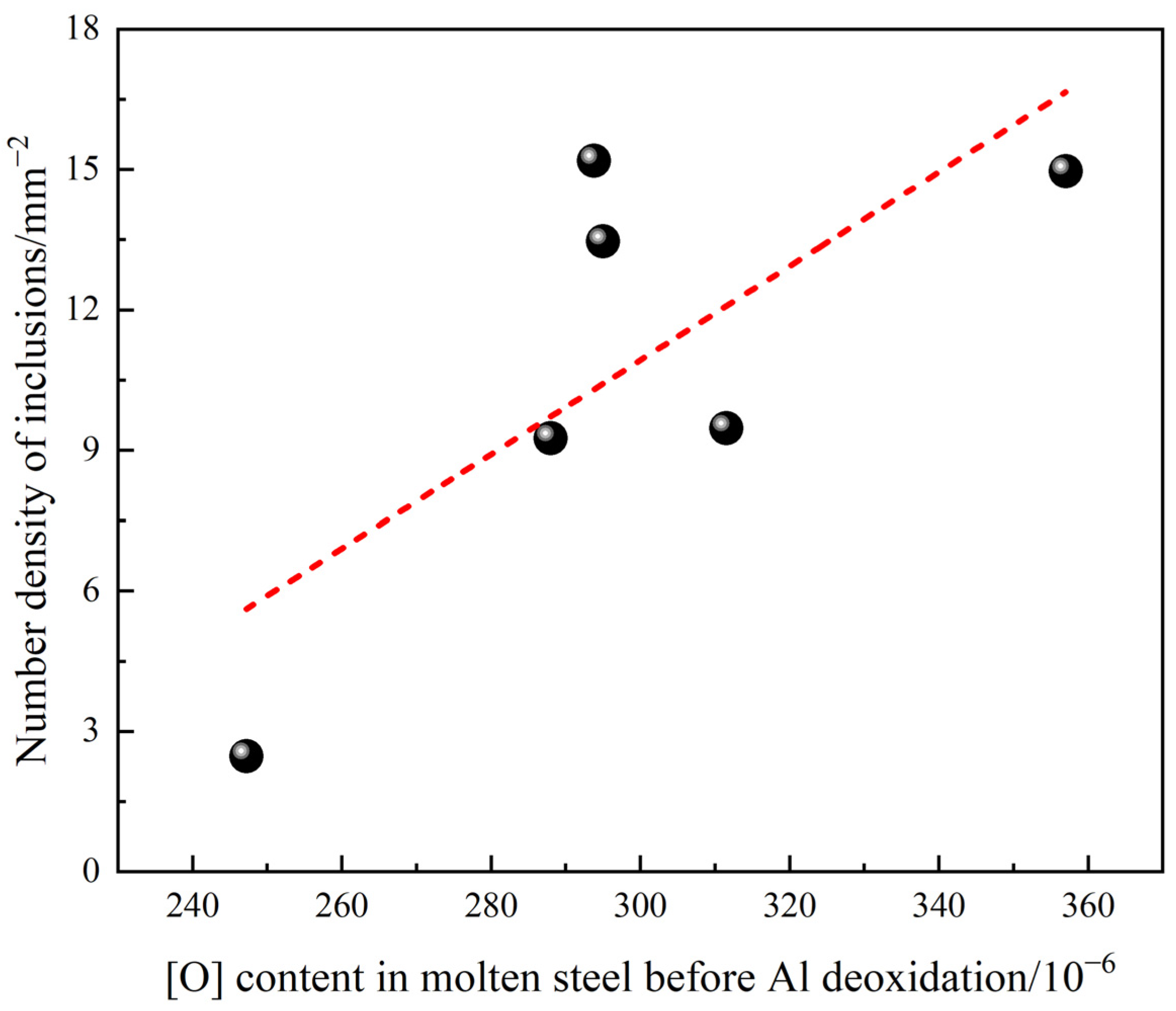
3.4. Influence of Process Parameters on the Cleanliness of Molten Steel
4. Conclusions
- (1)
- During RH treatment, the ND and AF of Al2O3 and Al2O3·TiOx inclusions decrease gradually. In the tundish samples, when the ND and AF of Al2O3 and Al2O3·TiOx inclusions especially from 1 to 2 μm increase significantly, the mean size of all inclusions shows a decreasing trend.
- (2)
- The deteriorated cleanliness of molten steel is closely related to the serious reoxidation of molten steel caused by the slag with high oxidability during the holding process. Meanwhile, the number of clusters counted by the location maps shows basically the same trend with the ND and AF of inclusions.
- (3)
- During the whole steelmaking process, Type 1 and Type 2 inclusions are the main two types of titanium-containing inclusions. In the tundish sample with serious reoxidation, there are still some small-sized Al2O3 clusters, and the number of Type 1 and Type 2 inclusions and Ti content of Type 2 and Type 3 inclusions will increase obviously.
- (4)
- In the case of serious reoxidation, Al2O3·TiOx inclusions can not only form directly at the steel/slag interface, but also form indirectly: Al2O3 particles generated from reoxidation may be transferred into Al2O3·TiOx inclusions under the action of a local high [Ti] region.
- (5)
- It is beneficial to weaken the reoxidation process and improve the cleanliness of molten steel by reducing the oxygen content in molten steel before Al deoxidation, minimizing the holding time and reducing the slag oxidability after RH.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, C.; Nuhfer, N.T.; Sridhar, S. Transient behavior of inclusion chemistry, shape, and structure in Fe-Al-Ti-O melts: Effect of titanium source and laboratory deoxidation simulation. Metall. Mater. Trans. B 2009, 40, 1005–1021. [Google Scholar] [CrossRef]
- Wang, C.; Nuhfer, N.T.; Sridhar, S. Transient behavior of inclusion chemistry, shape, and structure in Fe-Al-Ti-O melts: Effect of titanium/aluminum ration. Metall. Mater. Trans. B 2009, 40, 1022–1034. [Google Scholar] [CrossRef]
- Deng, X.X.; Ji, C.X.; Zhu, G.S.; Liu, Q.M.; Huang, F.X.; Tian, Z.H.; Wang, X.H. Quantitative evaluations of surface cleanliness in IF Steel slabs at unsteady casting. Metall. Mater. Trans. B 2019, 50, 1974–1987. [Google Scholar] [CrossRef]
- Matsuura, H.; Wang, C.; Wen, G.H.; Sridhar, S. The transient stages of inclusion evolution during Al and/or Ti additions to molten iron. ISIJ Int. 2007, 47, 1265–1274. [Google Scholar] [CrossRef]
- Deng, X.X.; Liu, G.L.; Wang, Q.Q.; Liu, B.S.; Ji, C.X.; Li, H.B.; Shao, X.J.; Zhu, G.S. Effect of the weir structure in the tundish on the cleanliness of IF steels and elimination of spot-like defects in deep drawing automobile steel sheets. Metall. Res. Technol. 2020, 117, 609. [Google Scholar] [CrossRef]
- Deng, X.X.; Ji, C.X.; Guan, S.K.; Wang, L.C.; Xu, J.F.; Tian, Z.H.; Cui, Y. Inclusion behaviour in aluminium-killed steel during continuous casting. Ironmak. Steelmak. 2019, 46, 1428420. [Google Scholar] [CrossRef]
- Choucair, H.; Zhou, J.Z.; Ali Jawad, B.; Liu, L.P. Study of the fatigue failure of engine valve springs due to non-metallic inclusions. SAE Int. 2012, 5, 388–394. [Google Scholar] [CrossRef]
- Murakami, Y. Metal Fatigue: Effects of Small Defects and Nonmetallic Inclusions, 2nd ed.; Elsevier: London, UK, 2002; pp. 681–682. [Google Scholar]
- Story, S.R.; Goldsmith, G.E.; Klepzig, G.L. Study of cleanliness and castability in Ti-stabilized ultra low carbon steels using automated SEM inclusion analysis. Metall. Res. Technol. 2008, 105, 272–279. [Google Scholar] [CrossRef]
- Salgado, U.D.; Weiß, C.; Michelic, S.K.; Bernhard, C. Fluid force-induced detachment criteria for nonmetallic inclusions adhered to a refractory/molten steel interface. Metall. Mater. Trans. B 2018, 49, 1632–1643. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, M.H.; Kim, S.K.; Kim, J.H.; Kim, M.S.; Kang, Y.B. Influence of Al/Ti Ratio in Ti-ULC steel and refractory components of submerged entry nozzle on formation of clogging deposits. ISIJ Int. 2019, 59, 749–758. [Google Scholar] [CrossRef]
- Bernhard, C.; Xia, G.; Karasangabo, A.; Egger, M.; Pissenberger, A. Investigating the influence of Ti and P on the clogging of ULC steels in the continuous casting process. In Proceedings of the 7th European Continuous Casting Conference, Duesseldorf, Germany, 27 June 2011. [Google Scholar]
- Kimura, H. Advances in High-Purity IF Steel Manufacturing Technology. Nippon. Steel Tech. Rep. 1994, 61, 65–69. [Google Scholar]
- Basu, S.; Chouahary, S.K.; Girase, N.U. Nozzle clogging behaviour of Ti-bearing Al-killed ultra low carbon steel. ISIJ Int. 2004, 44, 1653–1660. [Google Scholar] [CrossRef]
- Lyons, C.; Kaushik, P. Inclusion characterization of titanium stabilized ultra low carbon steels: Impact of oxygen activity before deoxidation. Steel Res. Int. 2011, 82, 1394–1403. [Google Scholar] [CrossRef]
- Gao, S.; Wang, M.; Guo, J.L.; Wang, H.; Zhi, J.G.; Bao, Y.P. Characterization transformation of inclusions using rare earth Ce treatment on Al-killed titanium alloyed interstitial free steel. Steel Res. Int. 2019, 10, 1900194. [Google Scholar] [CrossRef]
- Gao, S.; Wang, M.; Guo, J.L.; Wang, H.; Zhi, J.G.; Bao, Y.P. Extraction, distribution, and precipitation mechanism of TiN–MnS complex inclusions in Al-killed titanium alloyed interstitial free steel. Met. Mater. Int. 2021, 27, 1306–1314. [Google Scholar] [CrossRef]
- Yuan, B.H.; Liu, J.H.; Zhou, H.L.; Huang, J.H.; Zhang, S.; Shen, Z.P. Refining effect of IF steel produced by RH forced and natural decarburization process. Chin. J. Eng. 2021, 43, 1107–1115. [Google Scholar]
- Dorrer, P.; Michelic, S.K.; Bernhard, C.; Penz, A.; Rössler, R. Study on the influence of FeTi-addition on the inclusion population in Ti-stabilized ULC steels and its consequences for SEN-clogging. Steel Res. Int. 2019, 90, 1800635. [Google Scholar] [CrossRef]
- Park, D.C.; Jung, I.H.; Rhee, P.C.H.; Lee, H.G. Reoxidation of Al–Ti containing steels by CaO–Al2O3–MgO–SiO2 slag. ISIJ Int. 2004, 44, 1669–1678. [Google Scholar] [CrossRef]
- Jiang, M.F.; Zhang, Z.X.; Wang, D.Y.; Liu, J. Inclusions in characteristics of Ti/Al deoxidation steel and the analysis of nozzle clogging problem. Ind. Heat. 2011, 40, 60–63. [Google Scholar]
- Deng, X.X.; Ji, C.X.; Cui, Y.; Tian, Z.H.; Yin, X.; Shao, X.J.; Yang, Y.D.; Mclean, A. Formation and evolution of macro inclusions in IF steels during continuous casting. Ironmak. Steelmak. 2017, 44, 739–749. [Google Scholar] [CrossRef]
- Yang, W.; Li, S.S.; Li, Y.B.; Wang, X.H.; Zhang, L.F.; Liu, X.F.; Shan, Q.L. Evolution of inclusions in Ti-bearing ultra-low carbon steels during RH refining process. Mater. Pro. Fund. 2013, 3–16. [Google Scholar] [CrossRef]
- Wang, C.; Nuhfer, N.T.; Sridhar, S. Transient behavior of inclusion chemistry, shape, and structure in Fe-Al-Ti-O melts: Effect of gradual increase in Ti. Metall. Mater. Trans. B 2010, 41, 1084–1094. [Google Scholar] [CrossRef]
- Bai, X.F.; Sun, Y.H.; Zhang, Y.M. Transient evolution of inclusions during Al and Ti additions in Fe-20 mass pct Cr alloy. Metals 2019, 9, 702. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, Y.; Zhang, L.F.; Duan, H.J.; Wang, L. Population evolution od oxide inclusions in Ti-stabilized ultra-low carbon steels after deoxidation. J. Iron Steel Res. 2015, 22, 1069–1077. [Google Scholar] [CrossRef]
- Wang, M.; Bao, Y.P.; Cui, H.; Wu, H.J.; Wu, W.S. The composition and morphology evolution of oxide inclusions in Ti-bearing ultra low-carbon steel melt refined in the RH process. ISIJ Int. 2010, 50, 1606–1611. [Google Scholar] [CrossRef]
- Doo, W.C.; Kim, D.Y.; Kang, S.C.; Yi, K.W. The morphology of Al-Ti-O complex oxide inclusions formed in an ultra low-carbon steel melt during the RH process. Met. Mater. Int. 2007, 13, 249–255. [Google Scholar] [CrossRef]
- Qin, Y.M.; Wang, X.H.; Huang, F.X.; Ji, C.X. Behavior of non-metallic inclusions of IF steel during production process. J. Northeast Univ. (Nat. Sci.) 2015, 36, 1614–1618. [Google Scholar]
- Sun, M.K.; Jung, I.-H.; Lee, H.-G. Morphology and Chemistry of Oxide inclusions after Al and Ti complex deoxidation. Met. Mater. Int. 2008, 14, 791–798. [Google Scholar] [CrossRef]
- Marie-Aline, V.E.; Guo, M.X.; Zinngrebe, E.; Dekkers, R.; Proost, J.; Blanpain, B.; Wollants, P. Morphology and growth of alumina inclusions in Fe–Al alloys at low oxygen partial pressure. Ironmak. Steelmak. 2009, 36, 201–208. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, Z.Z.; Takata, R. Nucleation and growth of alumina inclusion in early stages of deoxidation: Numerical modeling. ISIJ Int. 2010, 50, 371–379. [Google Scholar] [CrossRef][Green Version]
- Van Ende, M.-A.; Guo, M.X.; Proost, J.; Blanpain, B.; Wollants, P. Formation and Morphology of Al2O3 inclusions at the onset of liquid Fe deoxidation by Al addition. ISIJ Int. 2011, 51, 27–34. [Google Scholar] [CrossRef]
- Yang, G.W.; Wang, X.H.; Huang, F.X.; Wang, W.J.; Yin, Y.Q. Transient inclusion evolution during RH degassing. Steel Res. Int. 2014, 85, 26–34. [Google Scholar] [CrossRef]
- Wakoh, M.; Sano, N. Behavior of alumina inclusions just after deoxidation. ISIJ Int. 2007, 47, 627–632. [Google Scholar] [CrossRef]

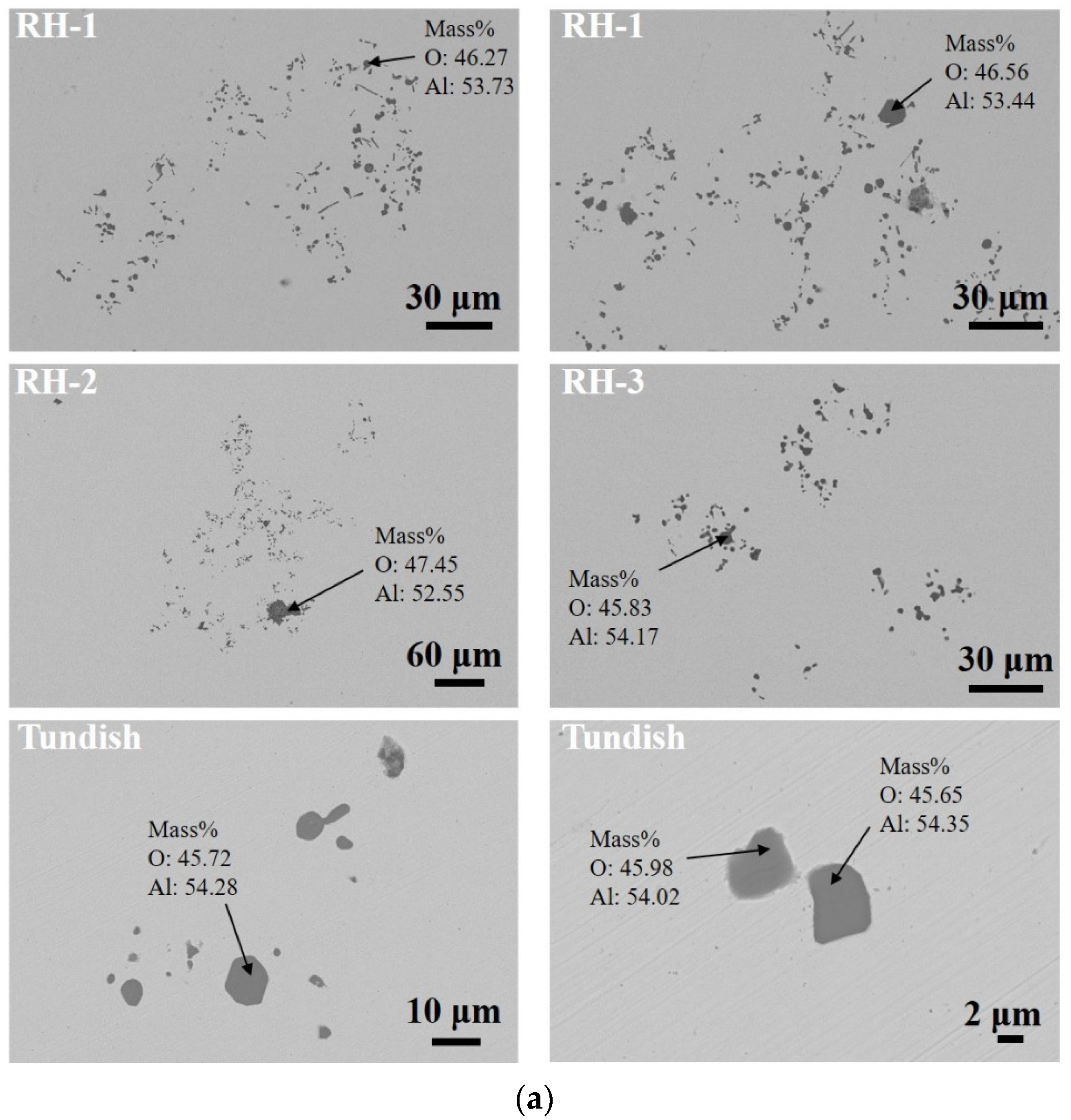
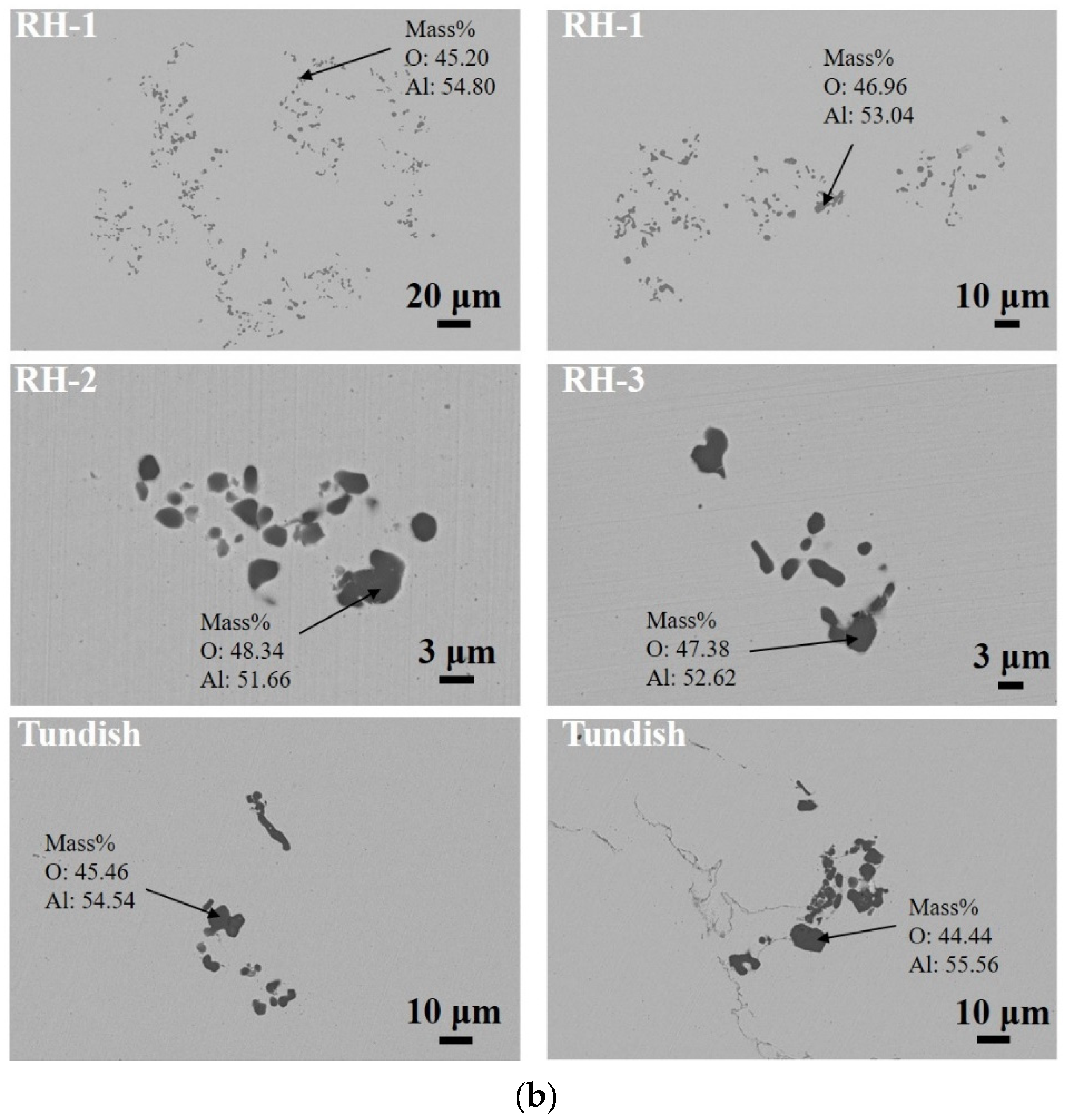

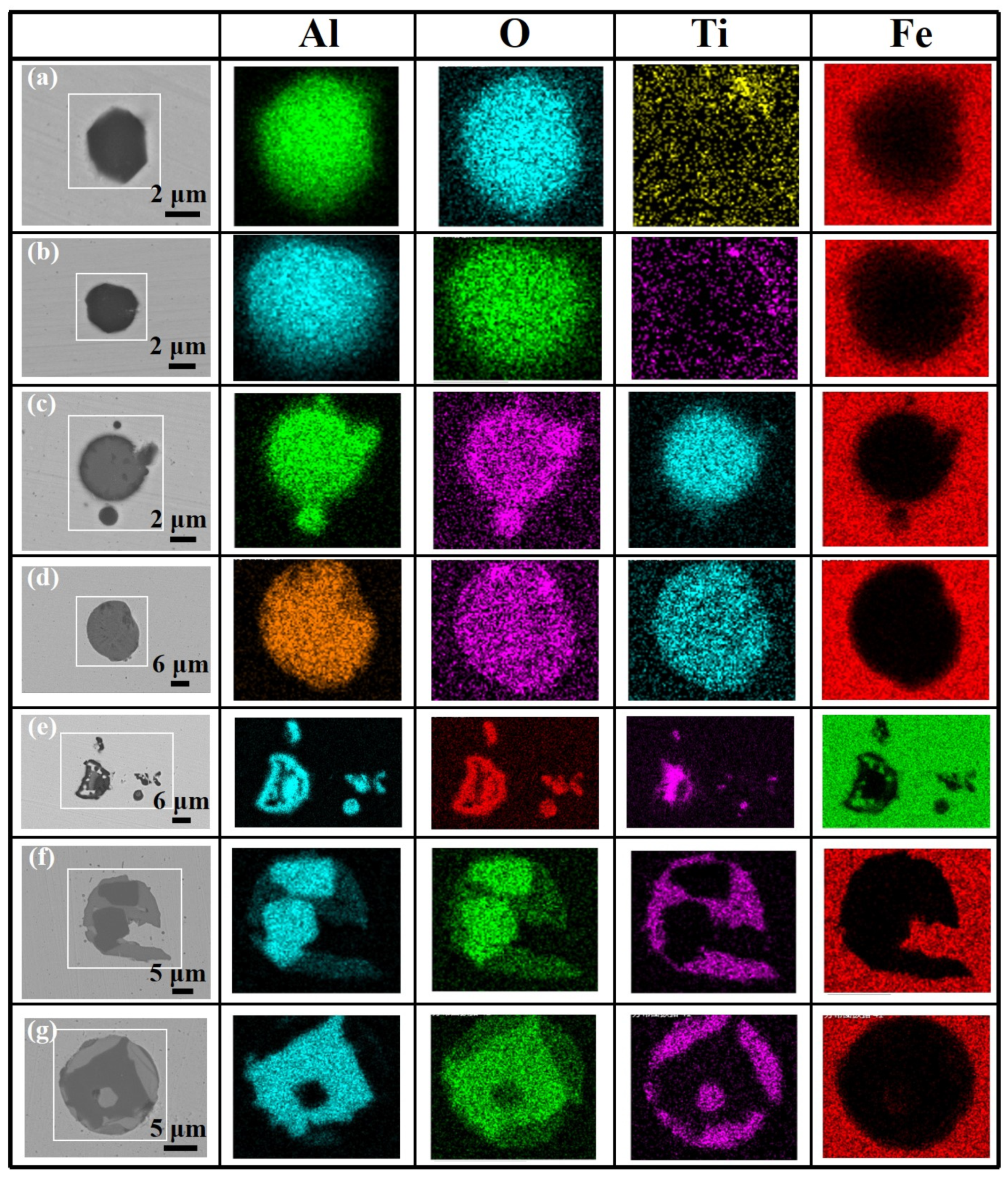





| Sample | Heat 1 | Heat 2 | ||||
|---|---|---|---|---|---|---|
| Description | T.O % | N % | Description | T.O % | N % | |
| RH-1 | 2.0 min after Al addition | 0.0080 | 0.0014 | 2.5 min after Al addition | 0.0120 | 0.0011 |
| RH-2 | 3.0 min after Ti addition | 0.0027 | 0.0018 | 3.3 min after Ti addition | 0.0052 | 0.0010 |
| RH-3 | 6.0 min after Ti addition | 0.0020 | 0.0018 | 5.0 min after Ti addition | 0.0013 | 0.0010 |
| Tundish | - | 0.0017 | 0.0020 | - | 0.0150 | 0.0014 |
| Heats | C | Si | Mn | P | S | Als | Ti | N |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.0018 | 0.0030 | 0.1300 | 0.0080 | 0.0060 | 0.0250 | 0.0550 | 0.0027 |
| 2 | 0.0022 | 0.0030 | 0.1300 | 0.0060 | 0.0060 | 0.0250 | 0.0540 | 0.0018 |
| Heats | [O] before Al Deoxidation/10−6 | Holding Time/Min | T.Fe in Slag after RH/% | Number Density of Inclusions/mm−2 | |
|---|---|---|---|---|---|
| After RH | Tundish | ||||
| 1 | 295 | 35.5 | 15.3 | 13.5 | 9.3 |
| 2 | 294 | 42.5 | 18.1 | 15.2 | 35.1 |
| 3 | 288 | 35.3 | 14.2 | 9.3 | 6.1 |
| 4 | 357 | 26.0 | 17.0 | 15.0 | 2.5 |
| 5 | 247 | 32.8 | 16.8 | 2.5 | 13.8 |
| 6 | 312 | 34.9 | 9.5 | 9.5 | 4.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, B.; Liu, J.; Zeng, J.; Zhang, M.; Huang, J.; Yang, X. Evolution of Inclusions and Cleanliness in Ti-Bearing IF Steel Produced via the BOF–LF–RH–CC Process. Metals 2022, 12, 434. https://doi.org/10.3390/met12030434
Yuan B, Liu J, Zeng J, Zhang M, Huang J, Yang X. Evolution of Inclusions and Cleanliness in Ti-Bearing IF Steel Produced via the BOF–LF–RH–CC Process. Metals. 2022; 12(3):434. https://doi.org/10.3390/met12030434
Chicago/Turabian StyleYuan, Baohui, Jianhua Liu, Jianhua Zeng, Min Zhang, Jihong Huang, and Xiaodong Yang. 2022. "Evolution of Inclusions and Cleanliness in Ti-Bearing IF Steel Produced via the BOF–LF–RH–CC Process" Metals 12, no. 3: 434. https://doi.org/10.3390/met12030434
APA StyleYuan, B., Liu, J., Zeng, J., Zhang, M., Huang, J., & Yang, X. (2022). Evolution of Inclusions and Cleanliness in Ti-Bearing IF Steel Produced via the BOF–LF–RH–CC Process. Metals, 12(3), 434. https://doi.org/10.3390/met12030434








