Fe3O4 Coated SiO2 Magnetic Nanoparticles for Enhanced Antibacterial Activity and Electrochemical Sensing
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.1.1. Synthesis of Fe3O4 MNPs
2.1.2. Synthesis of Fe3O4@SiO2 MNPs
3. Characterization
3.1. Phase Evolution Studies
3.2. Surface Morphology Studies
3.3. UV-vis Spectroscopy
3.4. FTIR Studies
3.5. Animicrobial Tests
3.6. Cyclic Voltammetery Studies
4. Results and Discussion
4.1. UV-vis Spectroscopy
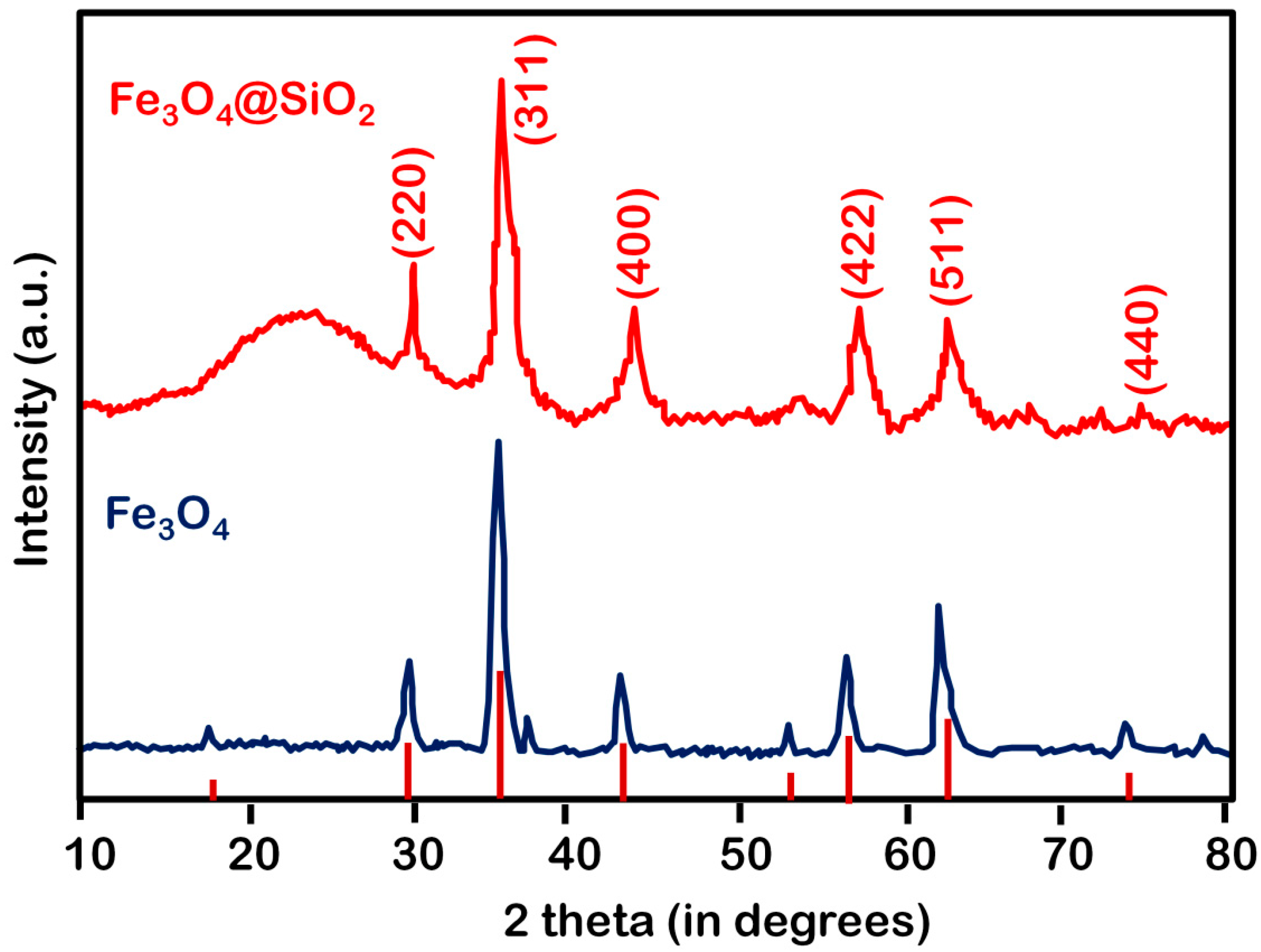
4.2. XRD Analysis
4.3. FTIR Analysis
4.4. Microstructural Analysis using TEM
4.5. Antimicrobial Test
4.6. Cyclic Voltammetry
5. Conclusions
- The solvothermal approach has been used to successfully create Fe3O4 NPs coated with SiO2 NPs. The XRD, UV-vis, and FTIR results demonstrate the creation of spinal Fe3O4@SiO2 structure. A thorough examination of the TEM reveals the formation of Fe3O4 cell structure. Additionally, the size distribution of Fe3O4@SiO2 MNP size distribution ranges from 10 to 30 nm.
- The antimicrobial tests showed a larger inhibition zone of Fe3O4 coated by SiO2 as compared to the pure Fe3O4 NPs. Fe3O4@SiO2 MNPs showed strong antibacterial characteristics by killing bacteria on the exterior, as well as inside, of their cell membranes.
- The electrochemical sensor based on Fe3O4 coated with SiO2 NPs is capable of reducing and oxidizing arsenic with outstanding electrocatalytic activity. Fe3O4 covered with SiO2 naturally enhances the sensitivity of the determination of arsenic (III) with a low detection limit because of its distinctive qualities, which include subtle electrical characteristics, good interaction, and strong adsorptive capacity.
- Varying the arsenic content resulted in a shift in potential and an increase in the oxidation peak current, which illustrates the improved electrode’s electrocatalytic capability.
- It was concluded that the combination of SiO2 with the Fe3O4 NPs improves the antibacterial property of Fe3O4 and reduces the adverse effects. Additionally, the composite Fe3O4@SiO2 can be used against bacteria as well as for the detection of arsenic pollutants as an electrochemical sensor.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Ding, X.; Chen, Y.; Guo, M.; Zhang, Y.; Guo, X.; Gu, H. Antibiotic-loaded, silver core-embedded mesoporous silica nano-vehicles as a synergistic antibacterial agent for the treatment of drug-resistant infections. Biomaterials 2016, 101, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Neu, H.C. The crisis in antibiotic resistance. Science 1992, 257, 1064–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ackermann-Liebrich, U.A.; Nocera, S.; Merten, S. A solution for creating competent health-care specialists: The Swiss School of Public Health. Bull. World Health Organ. 2007, 85, 974–976. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Zhang, X.; Wu, F.G.; Chen, Y. Colorimetric and test stripe-based assay of bacteria by using vancomycin-modified gold nanoparticles. Sens. Actuators B Chem. 2019, 281, 408–414. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, K.; Zhou, Z.; Li, Q.; Shao, L.; Hao, R.Z.; Xiao, R.; Wang, S. Vancomycin-modified Fe3O4@SiO2@Ag microflowers as effective antimicrobial agents. Int. J. Nanomed. 2017, 12, 3077–3094. [Google Scholar] [CrossRef] [Green Version]
- Morens, D.M.; Folkers, G.K.; Fauci, A.S. The challenge of emerging and re-emerging infectious diseases. Nature 2004, 430, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Zhao, Q.; Hu, T.; Chen, X.; Cao, Y. Rapid preparation of size-tunable Fe3O4@SiO2 nanoparticles to construct magnetically responsive photonic crystals. J. Nanopart. Res. 2021, 23, 232. [Google Scholar] [CrossRef]
- Tzounis, L.; Logothetidis, S. Fe3O4@SiO2 core shell particles as platforms for the decoration of Ag nanoparticles. Mater. Today Proc. 2017, 4, 7076–7082. [Google Scholar] [CrossRef]
- Quirós, J.; Boltes, K.; Aguado, S.; de Villoria, R.G.; Vilatela, J.J.; Rosal, R. Antimicrobial metal–organic frameworks incorporated into electrospun fibers. Chem. Eng. J. 2015, 262, 189–197. [Google Scholar] [CrossRef]
- Tan, P.; Li, Y.H.; Liu, X.Q.; Jiang, Y.; Sun, L.B. Core–shell AgCl@SiO2 nanoparticles: Ag(I)-based antibacterial materials with enhanced stability. ACS Sustain. Chem. Eng. 2016, 4, 3268–3275. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, H.; Yan, J.; Cai, Y. Immobilizing silver nanoparticles onto the surface of magnetic silica composite to prepare magnetic disinfectant with enhanced stability and antibacterial activity. Colloid. Surf. A Physicochem. Eng. Asp. 2011, 375, 186–192. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Lim, T.P.; Leong, D.T.; Xie, J. Antimicrobial cluster bombs: Silver nanoclusters packed with daptomycin. ACS Nano 2016, 10, 7934–7942. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, N.; Hakamada, M.; Mabuchi, M. Antimicrobial mechanisms due to hyperpolarisation induced by nanoporous Au. Sci. Rep. 2018, 8, 3870. [Google Scholar] [CrossRef] [PubMed]
- Sadighian, S.; Sharifan, K.; Khanmohammadi, A.; Rohani, M.K. A facile synthesis of Fe3O4@SiO2@ZnO for Curcumin delivery. Biointerface Res. Appl. Chem. 2021, 12, 7994–8002. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, D.K.; Cha, H.G.; Kim, C.W.; Kang, Y.C.; Kang, Y.S. Preparation and characterization of the antibacterial Cu nanoparticle formed on the surface of SiO2 nanoparticles. J. Phys. Chem. B 2006, 110, 24923–24928. [Google Scholar] [CrossRef]
- Lei, S.; Zhao, H.; Pang, B.; Qu, R.; Lian, Z.; Jiang, C.; Shao, D.; Huang, Q.; Jin, M.; Shi, J. Capability of iturin from Bacillus subtilis to inhibit Candida albicans in vitro and in vivo. Appl. Microbiol. Biotechnol. 2019, 103, 4377–4392. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.Y.; Jiang, X.P.; Ye, J.J.; Zhang, Y.W.; Zhang, X.Y. Fabrication of graphene oxide decorated with Fe3O4@SiO2 for immobilization of cellulose. J. Nanopart. Res. 2015, 17, 8. [Google Scholar] [CrossRef]
- Babay, S.; Mhiri, T.; Toumi, M. Synthesis, structural and spectroscopic characterizations of maghemite γ-Fe2O3 prepared by one-step coprecipitation route. J. Mol. Struct. 2015, 1085, 286–293. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic nanoparticles: Preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 2012, 7, 144. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; He, Q.; Jiang, C. Magnetic iron oxide nanoparticles: Synthesis and surface functionalization strategies. Nanoscale Res. Lett. 2008, 3, 397. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metals Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology. Experientia Supplementum; Luch, A., Ed.; Springer: Basel, Switzerland, 2012; Volume 101. [Google Scholar]
- Hajipour, P.; Eslami, A.; Bahrami, A.; Hosseini-Abari, A.; Saber, F.Y.; Mohammadi, R.; Mehr, M.Y. Surface modification of TiO2 nanoparticles with CuO for visible-light antibacterial applications and photocatalytic degradation of antibiotics. Ceram. Int. 2021, 47, 33875–33885. [Google Scholar] [CrossRef]
- Beitollahi, H.; Nejad, F.G.; Shakeric, S. GO/Fe3O4@SiO2 core–shell nanocomposite modified graphite screen-printed electrode for sensitive and selective electrochemical sensing of dopamine and uric acid. Anal. Methods 2017, 9, 5541. [Google Scholar] [CrossRef]
- Shi, G.; Sun, B.; Jin, Z.; Liu, Z.; Li, M. Synthesis of SiO2/Fe3O4 nanomaterial and its application as cataluminescence gas sensor material for ether. Sens. Actuators B 2012, 171–172, 699–704. [Google Scholar] [CrossRef]
- Ansari, J.R.; Naseh, M.F.; Singh, N.; Sarkar, T.; Datta, A. Unique photoluminescence response of MoS2 quantum dots over a wide range of As (III) in aqueous media. Nanotechnology 2021, 32, 345708. [Google Scholar] [CrossRef] [PubMed]
- Prucek, R.; Tuček, J.; Kilianová, M.; Panáček, A.; Kvítek, L.; Filip, J.; Kolář, M.; Tománková, K.; Zbořil, R. The targeted antibacterial and antifungal properties of magnetic nanocomposite of iron oxide and silver nanoparticles. Biomaterials 2011, 32, 4704–4713. [Google Scholar] [CrossRef]
- Qu, M.; Chen, Z.; Sun, Z.; Zhou, D.; Xu, W.; Tang, H.; Gu, H.; Liang, T.; Hu, P.; Li, G.; et al. Rational design of asymmetric atomic Ni-P1N3 active sites for promoting electrochemical CO reduction. Nano Res. 2022, in press. [CrossRef]
- Huo, J.; Wei, H.; Fu, L.; Zhao, C.; He, C. Highly active Fe36Co44 bimetallic nanoclusters catalysts for hydrolysis of ammonia borane: The first-principles study. Chin. Chem. Lett. 2022, in press. [CrossRef]
- Ghasemzadeh, M.A.; Abdollahi-Basir, M.H.; Babaei, M. Fe3O4@SiO2–NH2 core-shell nanocomposite as an efficient and green catalyst for the multi-component synthesis of highly substituted chromeno[2,3-b]pyridines in aqueous ethanol media. Green Chem. Lett. Rev. 2015, 8, 40–49. [Google Scholar] [CrossRef] [Green Version]
- Prasad, K.; Lekshmi, G.S.; Ostrikov, K.; Lussini, V.; Blinco, J.; Mohandas, M.; Vasilev, K.; Bottle, S.; Bazaka, K.; Ostrikov, K. Synergic bactericidal effects of reduced graphene oxide and silver nanoparticles against Gram-positive and Gram-negative bacteria. Sci. Rep. 2017, 7, 1591. [Google Scholar] [CrossRef] [Green Version]
- Cong, Y.; Xia, T.; Zou, M.; Li, Z.; Peng, B.; Guo, D.; Deng, Z. Mussel-inspired polydopamine coating as a versatile platform for synthesizing polystyrene/Ag nanocomposite particles with enhanced antibacterial activities. J. Mater. Chem. B 2014, 2, 3450–3461. [Google Scholar] [CrossRef]
- Liu, L.; Yang, J.; Xie, J.; Luo, Z.; Jiang, J.; Yang, Y.Y.; Liu, S. The potent antimicrobial properties of cell penetrating peptide-conjugated silver nanoparticles with excellent selectivity for Gram-positive bacteria over erythrocytes. Nanoscale 2013, 5, 3834. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yin, Y.; Mayers, B.T.; Xia, Y. Modifying the surface properties of superparamagnetic iron oxide nanoparticles through a sol−gel approach. Nano Lett. 2002, 2, 183–186. [Google Scholar] [CrossRef]
- Habila, M.A.; Alothman, Z.A.; El-Toni, A.M.; Labis, J.P.; Soylak, M. Synthesis and application of Fe3O4@SiO2@TiO2 for photocatalytic decomposition of organic matrix simultaneously with magnetic solid phase extraction of heavy metals prior to ICP-MS analysis. Talanta 2016, 154, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Wegmann, M.; Scharr, M. Synthesis of magnetic iron oxide nanoparticles. In Precision Medicine; Elsevier: Amsterdam, The Netherlands, 2018; pp. 145–181. [Google Scholar] [CrossRef]
- Mukherjee, S.; Chowdhury, D.; Kotcherlakota, R.; Patra, S.; Vinothkumar, B.; Bhadra, M.P.; Sreedhar, B.; Patra, C.R. Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 System). Theranostics 2014, 4, 316–335. [Google Scholar] [CrossRef] [Green Version]
- Hui, C.; Shen, C.; Tian, J.; Bao, L.; Ding, H.; Li, C.; Tian, Y.; Shi, X.; Gao, H.-J. Core-shell Fe3O4@SiO2 nanoparticles synthesized with well-dispersed hydrophilic Fe3O4 seeds. Nanoscale 2011, 3, 701–705. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.C.; Liu, B.; Sun, X.Y.; Lin, H.C.; Lu, J.Z.; Jin, S.F.; Yan, S.Q.; Li, Y.Y.; Zhao, P. Core/shell structured Fe3O4@TiO2 -DNM nanospheres as multifunctional anticancer platform: Chemotherapy and photodynamic therapy research. J. Nanosci. Nanotechnol. 2018, 18, 4445–4456. [Google Scholar] [CrossRef]
- Bharadishettar, N.; Udaya, B.K.; Panemangalore, D.B. Coating Technologies for Copper Based Antimicrobial Active Surfaces: A Perspective Review. Metals 2021, 11, 711. [Google Scholar] [CrossRef]
- Gaviria, J.; Alcudia, A.; Begines, B.; Beltrán, A.M.; Rodríguez-Ortiz, J.A.; Trueba, P.; Villarraga, J.; Torres, Y. Biofunctionalization of Porous Ti Substrates Coated with Ag Nanoparticles for Potential Antibacterial Behavior. Metals 2021, 11, 692. [Google Scholar] [CrossRef]
- Frida, E.; Bukit, N.; Bukit, F.R.A.; Bukit, B.F. Preparation and characterization of Bentonite-OPBA nanocomposite as filler. J. Phys. Conf. Ser. 2022, 2165, 012023. [Google Scholar] [CrossRef]
- Wang, S.; Tang, J.; Zhao, H.; Wan, J.; Chen, K. Synthesis of magnetite–silica core–shell nanoparticles via direct silicon oxidation. J. Colloid Interf. Sci. 2014, 432, 43–46. [Google Scholar] [CrossRef]
- Qu, H.; Tong, S.; Song, K.; Ma, H.; Bao, G.; Pincus, S.; Zhou, W.; O’Connor, C. Controllable in situ synthesis of magnetite coated silica-core water-dispersible hybrid nanomaterials. Langmuir 2013, 29, 10573–10578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandler, S.E.; Fellows, B.; Mefford, O.T. Best practices for characterization of magnetic nanoparticles for biomedical applications. Anal. Chem. 2019, 91, 14159–14169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, L.; Liu, Z.; Zhang, S. Magnetic solid acid Fe3O4@SiO2-SO3H for cellulose hydrolysis. Biomass Conv. Bioref. 2021, 1–8. [Google Scholar] [CrossRef]
- Shao, D.; Lu, M.; Zhao, Y.; Zhang, F.; Tan, Y.; Zheng, X.; Pan, Y.; Xiao, X.; Wang, Z.; Dong, W.; et al. The shape effect of magnetic mesoporous silica nanoparticles on endocytosis, biocompatibility and biodistribution. Acta Biomater. 2017, 49, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Asab, G.; Zereffa, E.A.; Abdo Seghne, T. Synthesis of silica-coated Fe3O4 nanoparticles by microemulsion method: Characterization and evaluation of antimicrobial activity. Int. J. Biomater. 2020, 2020, 4783612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Inter. Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Prentice-Hall, Inc.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Cao, Y.; Li, C.; Li, J.; Li, Q.; Yang, J. Magnetically separable Fe3O4/AgBr hybrid materials: Highly efficient photocatalytic activity and good stability. Nanoscale Res. Lett. 2015, 10, 251. [Google Scholar] [CrossRef] [Green Version]
- Sosiati, H.; Budi, S.; Alaydrus, M.; Handoko, E. Microwave absorbing characteristics of Fe3O4@SiO2 core–shell polyaniline-based composites. Mater. Res. Express 2021, 8, 046101. [Google Scholar]
- Nazarabady, M.M.; Farzi, G. The effect of tunable morphology on the potential application of p(acrylic acid-co-2-ethylhexyl acrylate)/silica nanohybrids. e-Polymers 2017, 17, 471–480. [Google Scholar] [CrossRef]
- Subhan, F.; Aslam, S.; Yan, Z.; Khan, M.; Etim, U.J.; Naeem, M. Effective adsorptive performance of Fe3O4@SiO2 core shell spheres for methylene blue: Kinetics, isotherm and mechanism. J. Porous Mater. 2019, 26, 1465–1474. [Google Scholar] [CrossRef]
- Mirzabe, G.H.; Keshtkar, A.R. Application of response surface methodology for thorium adsorption on PVA/Fe3O4/SiO2/APTES nanohybrid adsorbent. J. Ind. Eng. Chem. 2015, 26, 277–285. [Google Scholar] [CrossRef]
- Ayyappan, S.; Panneerselvam, G.; Antony, M.P.; Rama Rao, N.V.; Thirumurugan, N.; Bharathi, A.; Philip, J. Effect of initial particle size on phase transformation temperature of surfactant capped Fe3O4 nanoparticles. J. Appl. Phys. 2011, 109, 084303. [Google Scholar] [CrossRef]
- Cai, W.; Wan, J. Facile synthesis of superparamagnetic magnetite nanoparticles in liquid polyols. J. Colloid Interf. Sci. 2007, 305, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Hong, R.Y.; Zhang, S.Z.; Di, G.Q.; Li, H.Z.; Zheng, Y.; Ding, J.; Wei, D.G. Preparation, characterization and application of Fe3O4/ZnO core/shell magnetic nanoparticles. Mater. Res. Bull. 2008, 43, 2457–2468. [Google Scholar] [CrossRef]
- Sadeghi, S.; Azhdari, H.; Arabi, H.; Moghaddam, A.Z. Surface modified magnetic Fe3O4 nanoparticles as a selective sorbent for solid phase extraction of uranyl ions from water samples. J. Hazard. Mater. 2012, 215–216, 208–216. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Zhao, L.; Zhang, S.; Huang, Y.; Wu, X.; Wang, X. Synthesis of amidoxime-functionalized Fe3O4@SiO2 core–shell magnetic microspheres for highly efficient sorption of U(VI). Chem. Eng. J. 2014, 235, 275–283. [Google Scholar] [CrossRef]
- Maboudi, S.A.; Shojaosadati, S.A.; Arpanaei, A. Synthesis and characterization of multilayered nanobiohybrid magnetic particles for biomedical applications. Mater. Des. 2017, 115, 317–324. [Google Scholar] [CrossRef]
- Bini, R.A.; Marques, R.F.C.; Santos, F.J.; Chaker, J.A.; Jafelicci, M. Synthesis and functionalization of magnetite nanoparticles with different amino-functional alkoxysilanes. J. Magn. Magn. Mater. 2012, 324, 534–539. [Google Scholar] [CrossRef] [Green Version]
- Prabhu, Y.T.; Rao, K.V.; Kumari, B.S.; Kumar, V.S.S.; Pavani, T. Synthesis of Fe3O4 nanoparticles and its antibacterial application. Int. Nano Lett. 2015, 5, 85–92. [Google Scholar] [CrossRef]
- Kim, B.H.; Yang, J.; Lee, D.; Choi, B.K.; Hyeon, T.; Park, J. Liquid-phase transmission electron microscopy for studying colloidal inorganic nanoparticles. Adv. Mater. 2018, 30, 1703316. [Google Scholar] [CrossRef]
- Chen, S.S.; Xu, H.; Xu, H.J.; Yu, G.J.; Gong, X.L.; Fang, Q.L.; Leung, K.C.F.; Xuan, S.H.; Xiong, Q.R. A facile ultrasonication assisted method for Fe3O4@SiO2-Ag nanospheres with excellent antibacterial activity. Dalton Trans. 2015, 44, 9140–9148. [Google Scholar] [CrossRef]
- Gong, P.; Li, H.; He, X.; Wang, K.; Hu, J.; Tan, W.; Zhang, S.; Yang, X. Preparation and antibacterial activity of Fe3O4@Ag nanoparticles. Nanotechnology 2007, 18, 285604. [Google Scholar] [CrossRef]
- Reddy, K.M.; Feris, K.; Bell, J.; Wingett, D.G.; Hanley, C.; Punnoose, A. Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Appl. Phys. Lett. 2007, 90, 213902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, N.; Cai, T.; Sun, Y.; Jiang, C.; Xiong, H.; Li, Y.; Peng, H. A novel antibacterial agent based on AgNPs and Fe3O4 loaded chitin microspheres with peroxidase-like activity for synergistic antibacterial activity and wound-healing. Int. J. Pharmaceut. 2018, 552, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.A.; Qasim, Q.A.; Mahdi, A.B.; Izzat, S.E.; Alnassar, Y.S.; Abood, E.S.; Alhakim, Z.J.; Mahmoud, Z.H.; Rheima, A.M.; Al-Salman, H.N.K. Supercapacitor performance of Fe3O4 and Fe3O4@SiO2-bis(aminopyridine)-Cu hybrid nanocomposite. Int. J. Electrochem. Sci. 2022, 17, 221057. [Google Scholar] [CrossRef]
- Suresh, R.; Giriabu, K.; Manigandan, R.; Vijayalakshmi, L.; Stephen, A.; Narayanan, V. Electrochemical sensing behaviour of Ni doped Fe3O4 nanoparticles. AIP Conf. Proc. 2014, 1576, 122. [Google Scholar] [CrossRef]
- Vatandost, E.; Ghorbani-Hasan Saraei, A.; Chekin, F.; Raeisi, S.N.; Shahidi, S.A. Electrochemical sensor based on magnetic Fe3O4–reduced graphene oxide hybrid for sensitive detection of Binaphthol. Russ. J. Electrochem. 2021, 57, 490–498. [Google Scholar] [CrossRef]
- Lee, K.S.; Seo, Y.J.; Jeong, H.T. Capacitive behavior of functionalized activated carbon-based all-solid-state supercapacitor. Carbon Lett. 2021, 31, 1041–1049. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madhavi; Kumar, M.; Ansari, J.R.; Kumar, V.; Nagar, S.; Sharma, A. Fe3O4 Coated SiO2 Magnetic Nanoparticles for Enhanced Antibacterial Activity and Electrochemical Sensing. Metals 2022, 12, 2145. https://doi.org/10.3390/met12122145
Madhavi, Kumar M, Ansari JR, Kumar V, Nagar S, Sharma A. Fe3O4 Coated SiO2 Magnetic Nanoparticles for Enhanced Antibacterial Activity and Electrochemical Sensing. Metals. 2022; 12(12):2145. https://doi.org/10.3390/met12122145
Chicago/Turabian StyleMadhavi, Mukesh Kumar, Jamilur R. Ansari, Vinay Kumar, Sushil Nagar, and Ashutosh Sharma. 2022. "Fe3O4 Coated SiO2 Magnetic Nanoparticles for Enhanced Antibacterial Activity and Electrochemical Sensing" Metals 12, no. 12: 2145. https://doi.org/10.3390/met12122145
APA StyleMadhavi, Kumar, M., Ansari, J. R., Kumar, V., Nagar, S., & Sharma, A. (2022). Fe3O4 Coated SiO2 Magnetic Nanoparticles for Enhanced Antibacterial Activity and Electrochemical Sensing. Metals, 12(12), 2145. https://doi.org/10.3390/met12122145





