Influence of Relative Humidity and Oxygen Concentration on Corrosion Behaviour of Copper in H2S-Containing Liquid Petroleum Gas
Abstract
1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Copper Corrosion Tests
2.3. Analysis of Corrosion Products
3. Results and Discussion
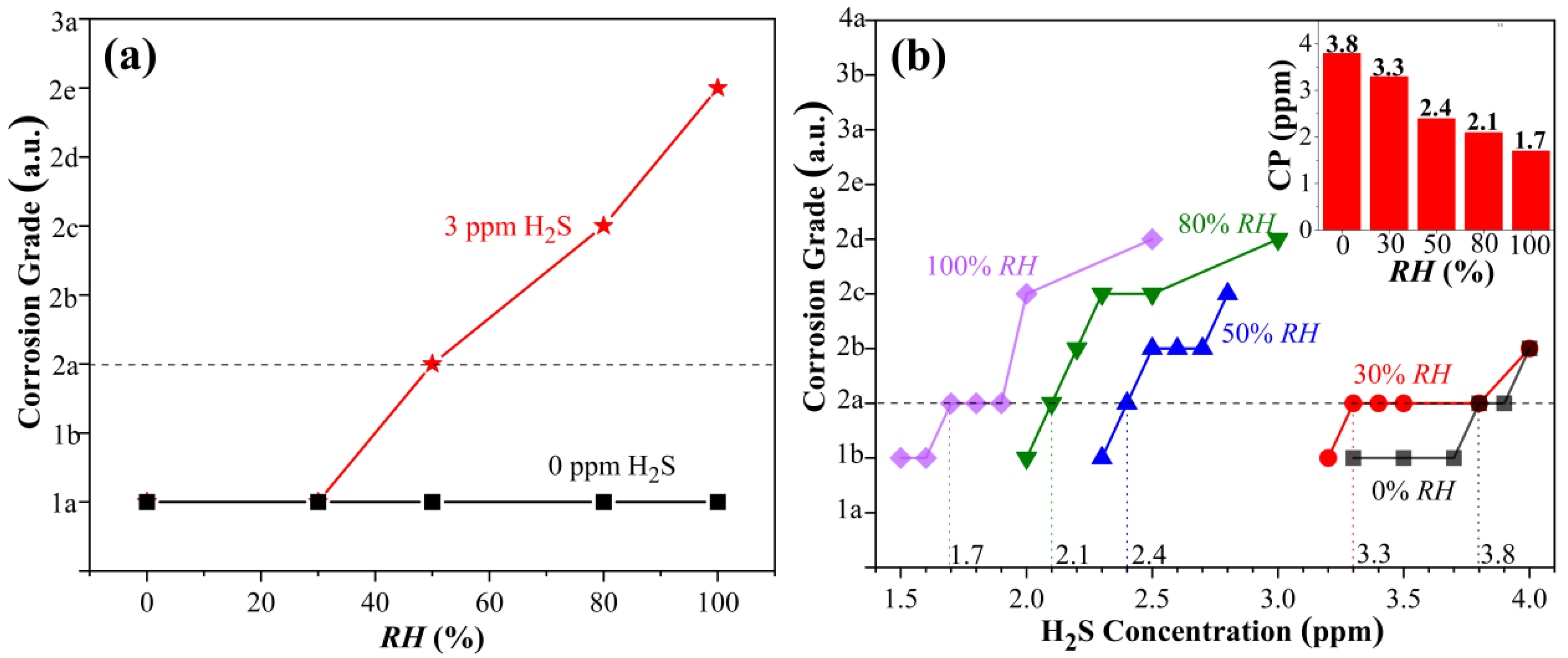
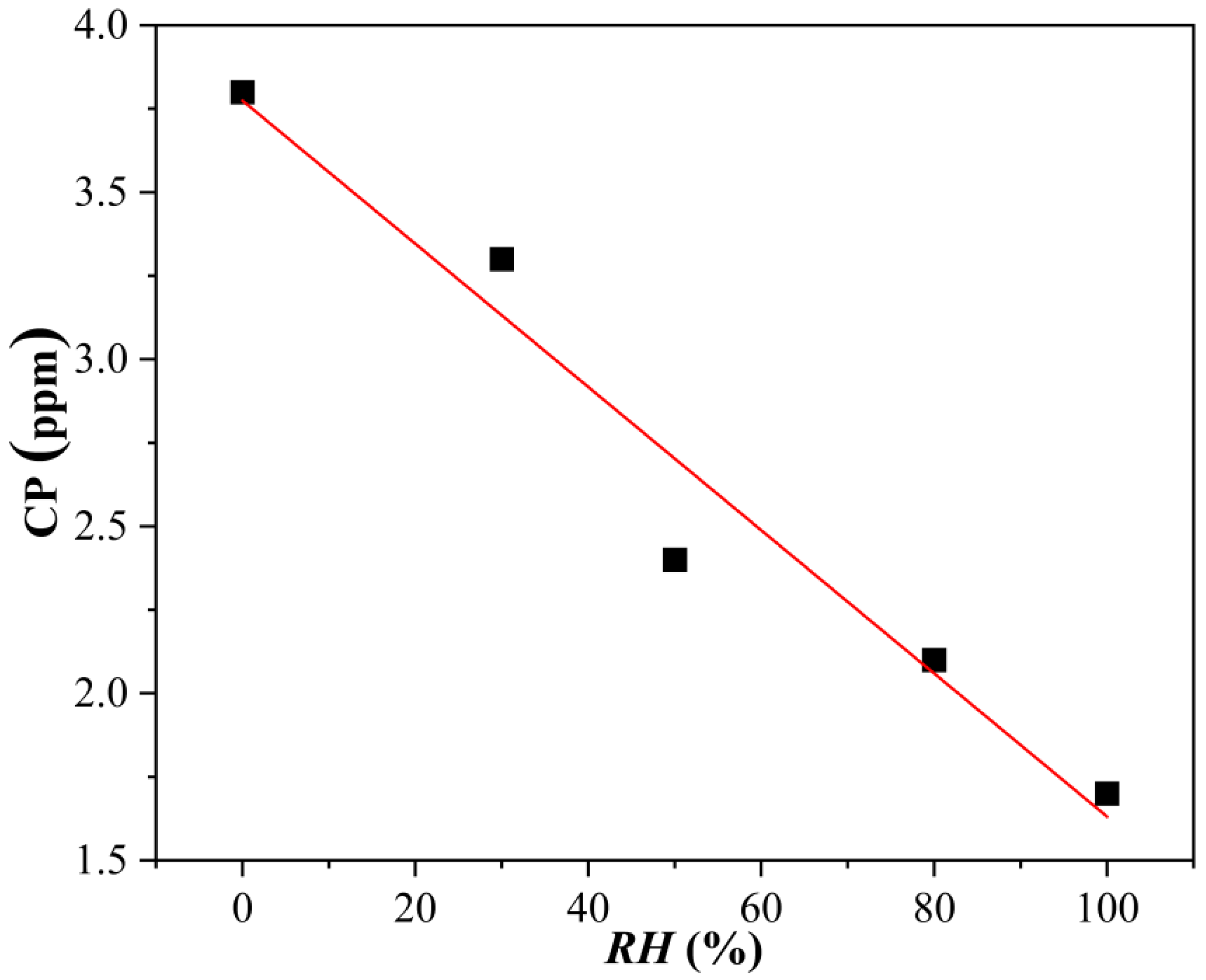
3.1. Influence of Humidity on Copper Corrosion in H2S-Containing LPG
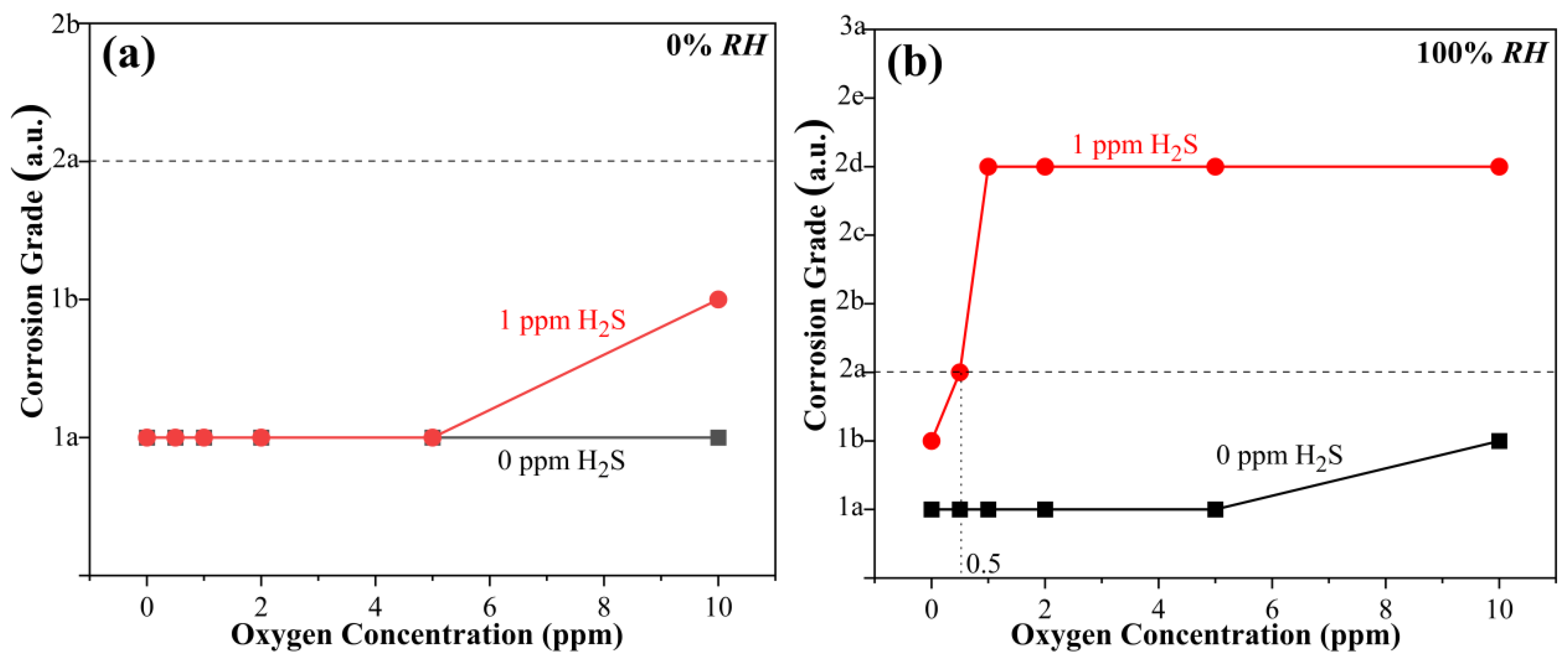
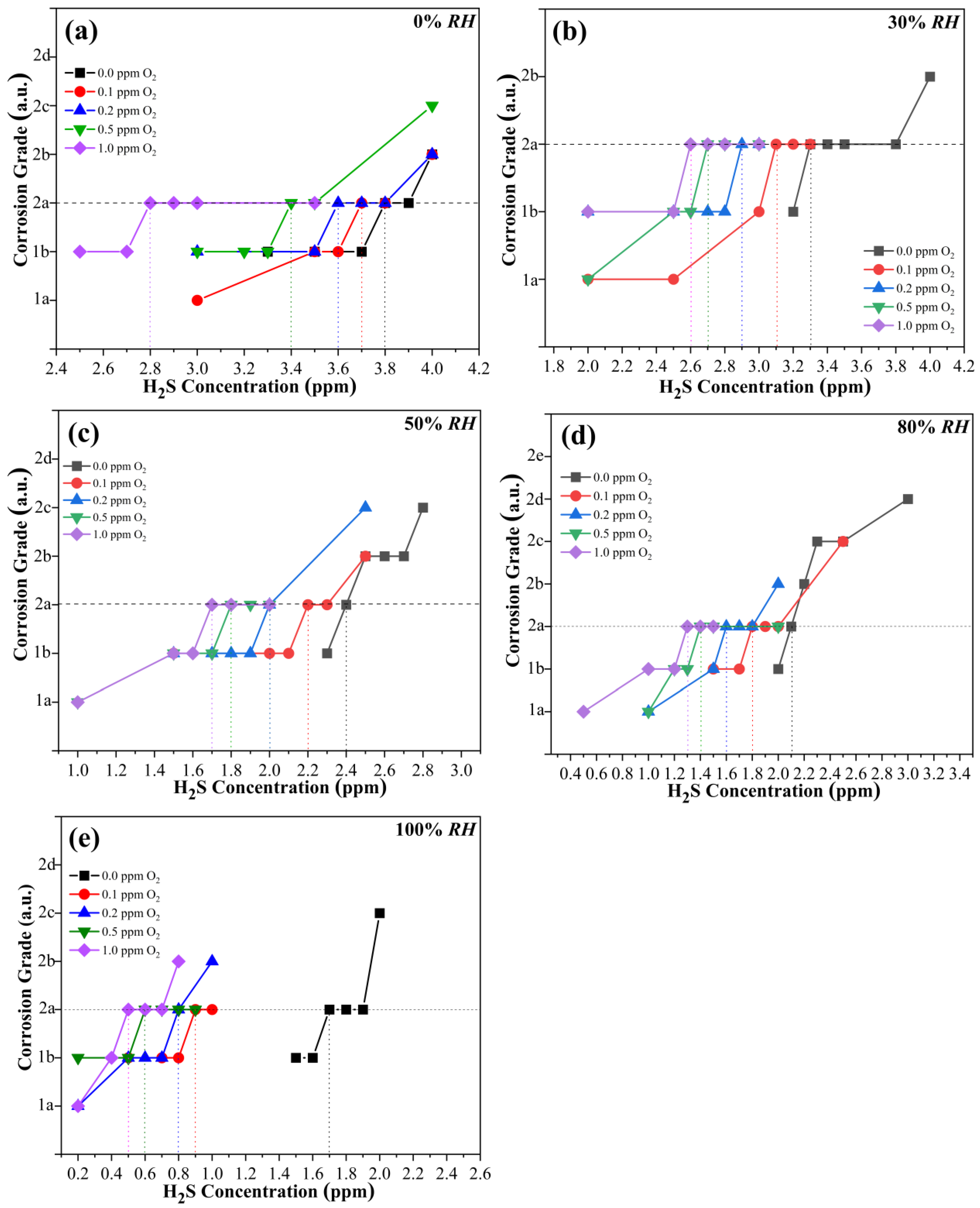
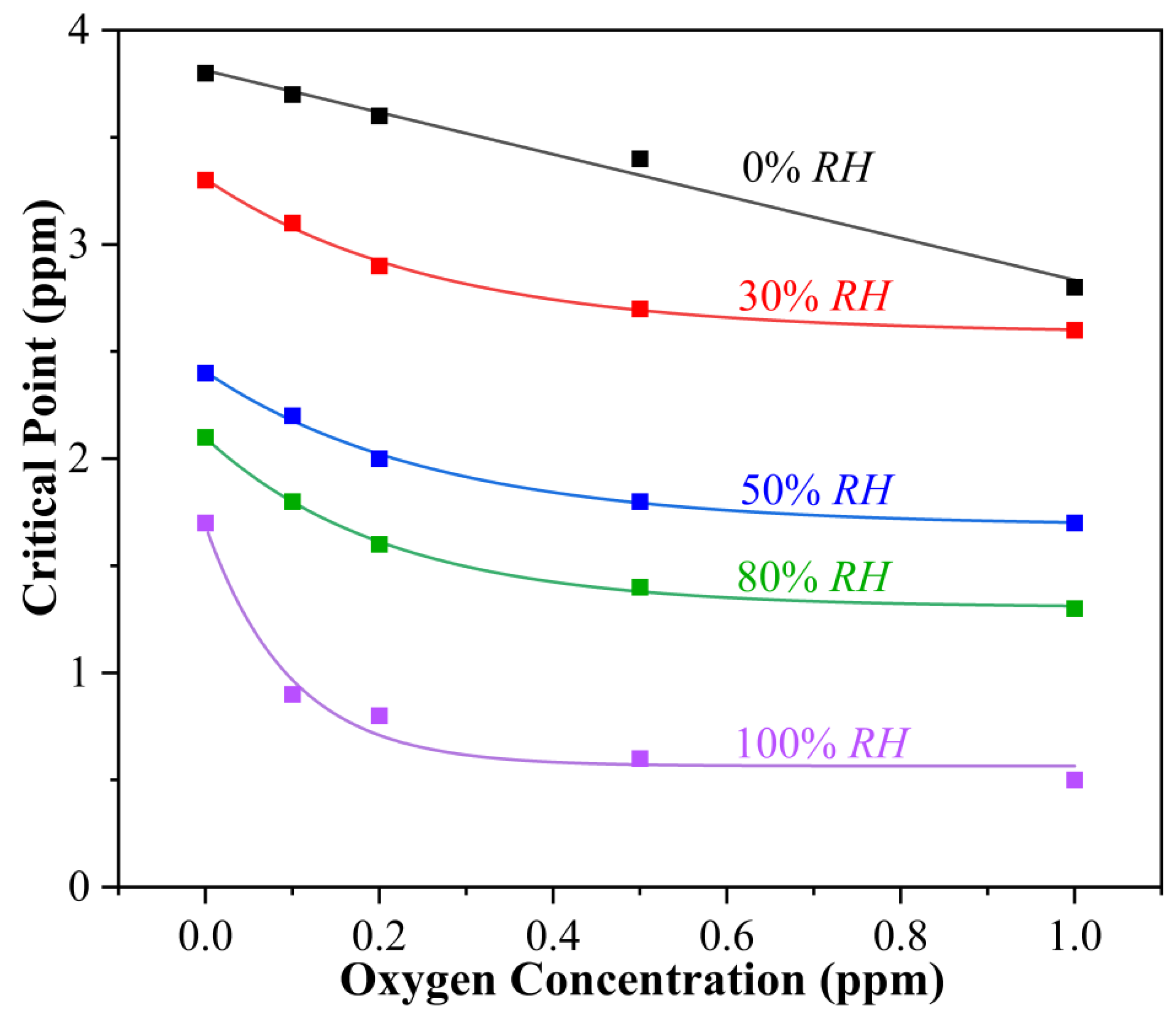
3.2. Synergistic Effect of Oxygen and Humidity on Copper Corrosion in H2S-Containing LPG
- (a)
- In the absence of water (0% RH), CP follows a linear relationship with O2 concentration. The fitted data obey Equation (2) as follows:
- (b)
- In the presence of water (30% RH), CP follows a negative exponential function with the O2 concentration. The fitted data obey Equation (3) as follows:
- (c)
- In the presence of water (50% RH), CP follows a negative exponential function with the O2 concentration. The fitted data obey Equation (4) as follows:
- (d)
- In the presence of water (80% RH), CP follows a negative exponential function with the O2 concentration. The fitted data obey Equation (5) as follows:
- (e)
- In the presence of water (100% RH), CP follows a negative exponential function with the O2 concentration. The fitted data obey Equation (6) as follows:
3.3. Corrosion Mechanism of Copper in Different H2S-Containing LPG Environments
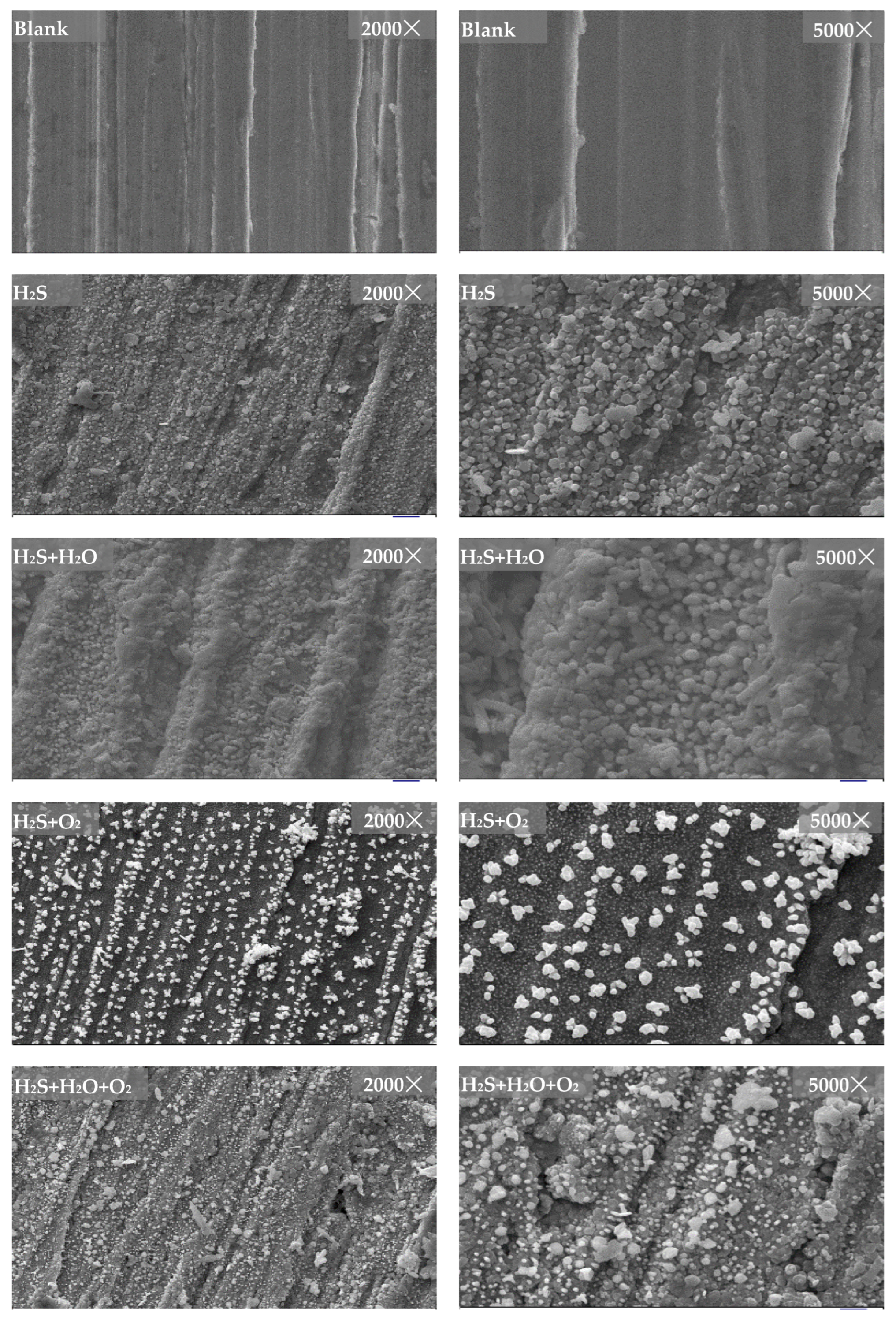
3.3.1. Surface Morphologies after Corrosion
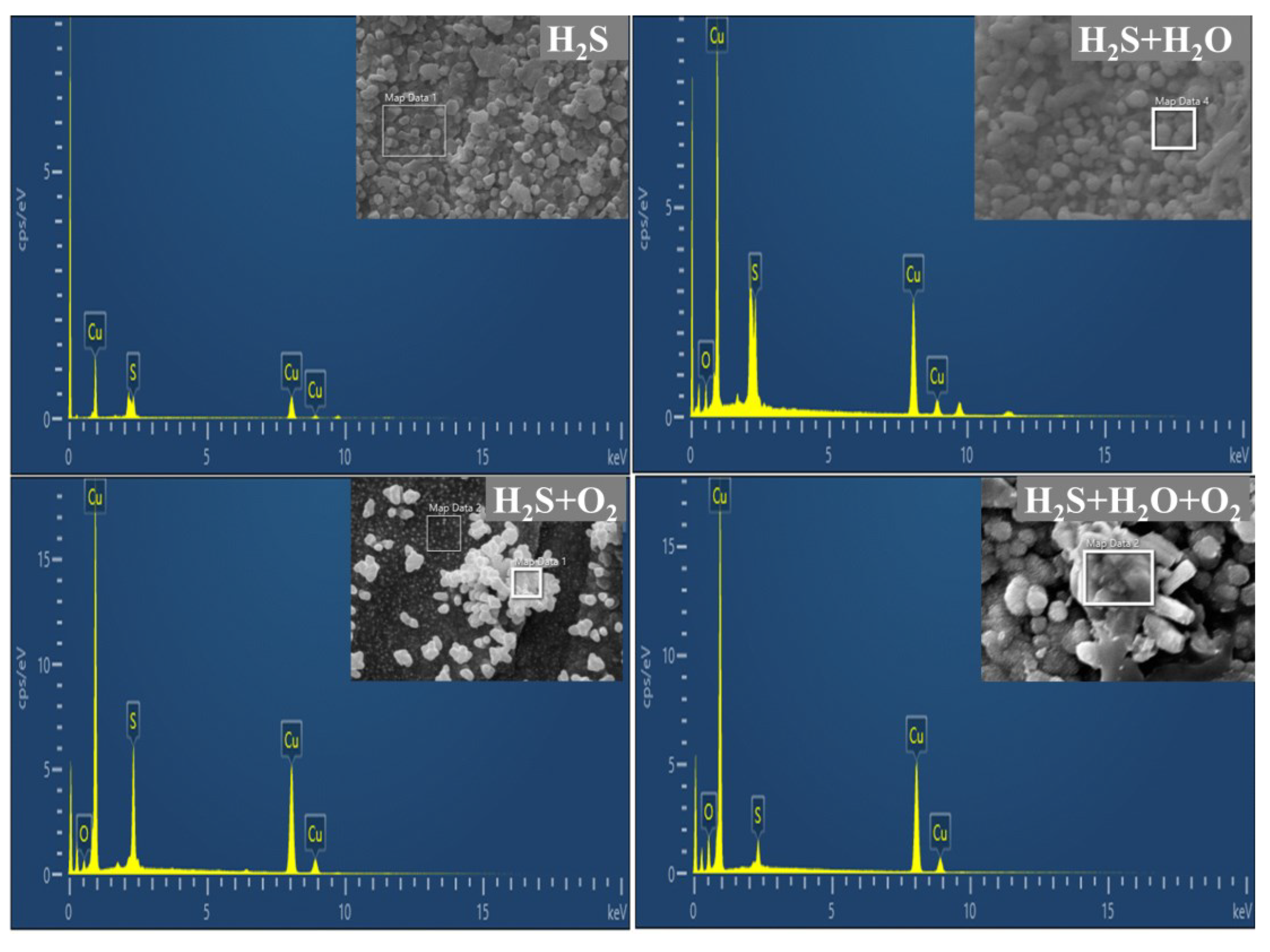
3.3.2. EDS Analysis of Corrosion Products
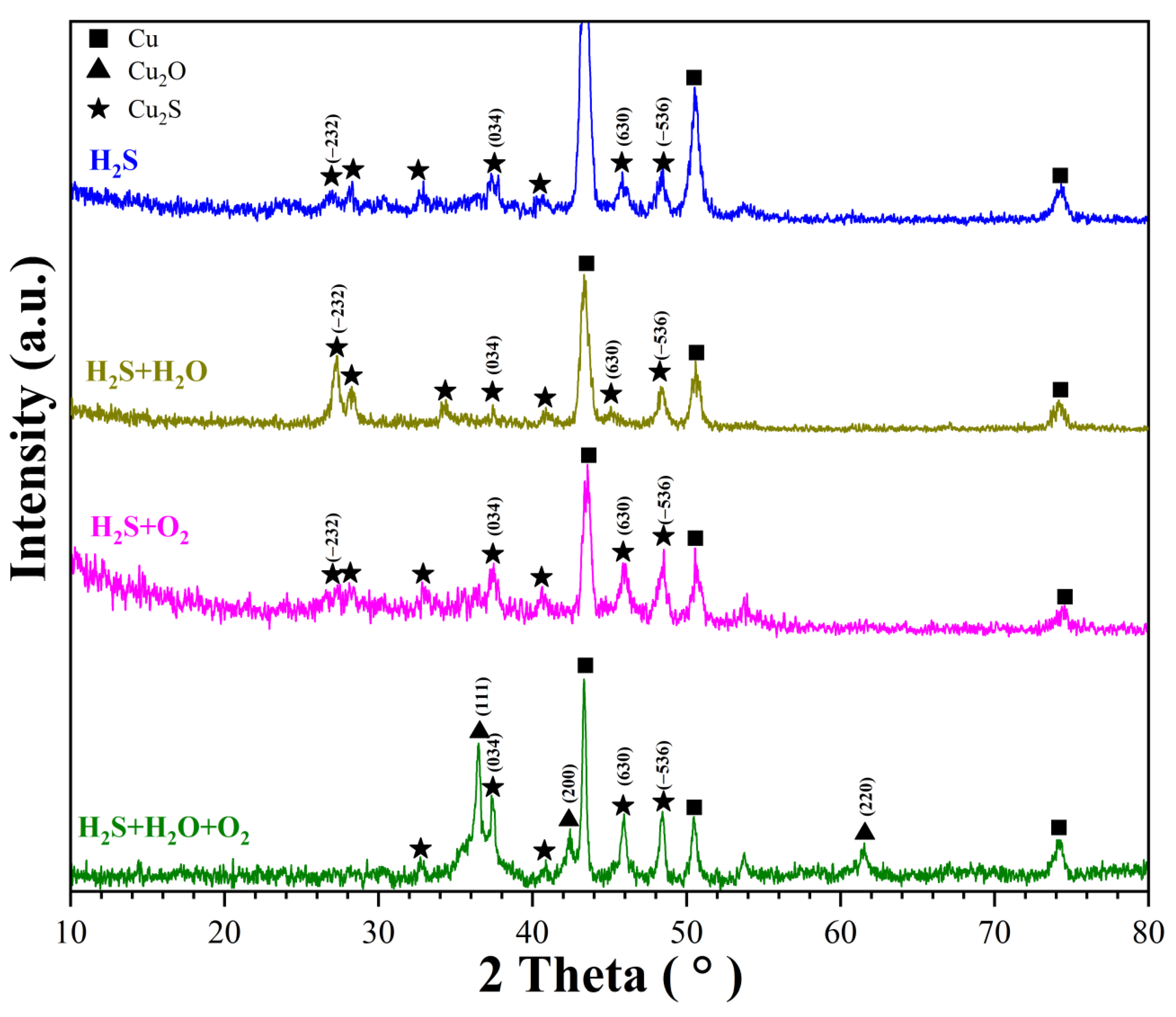
3.3.3. GIXRD Analysis of Corrosion Products
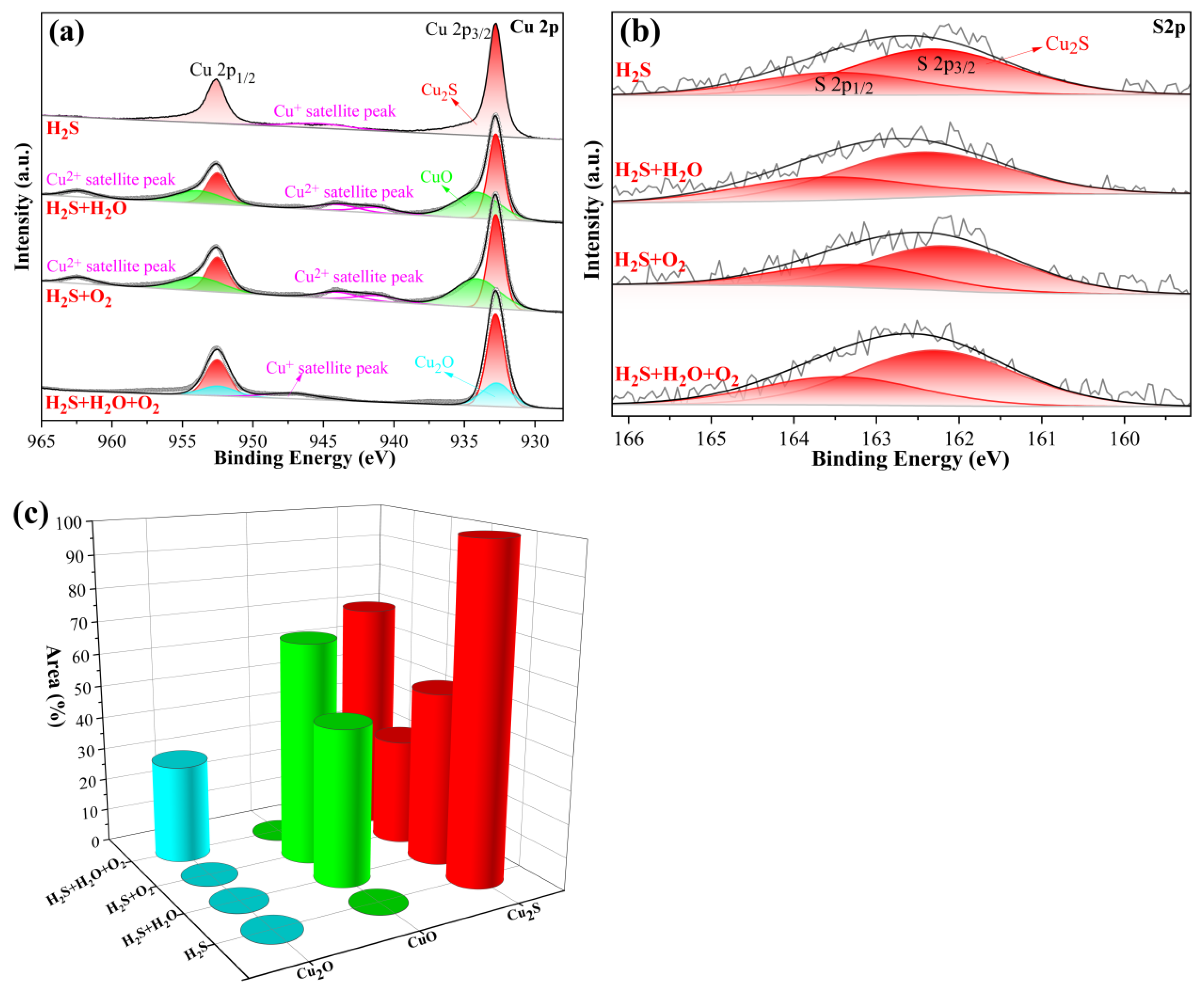
3.3.4. XPS Analysis of Corrosion Products
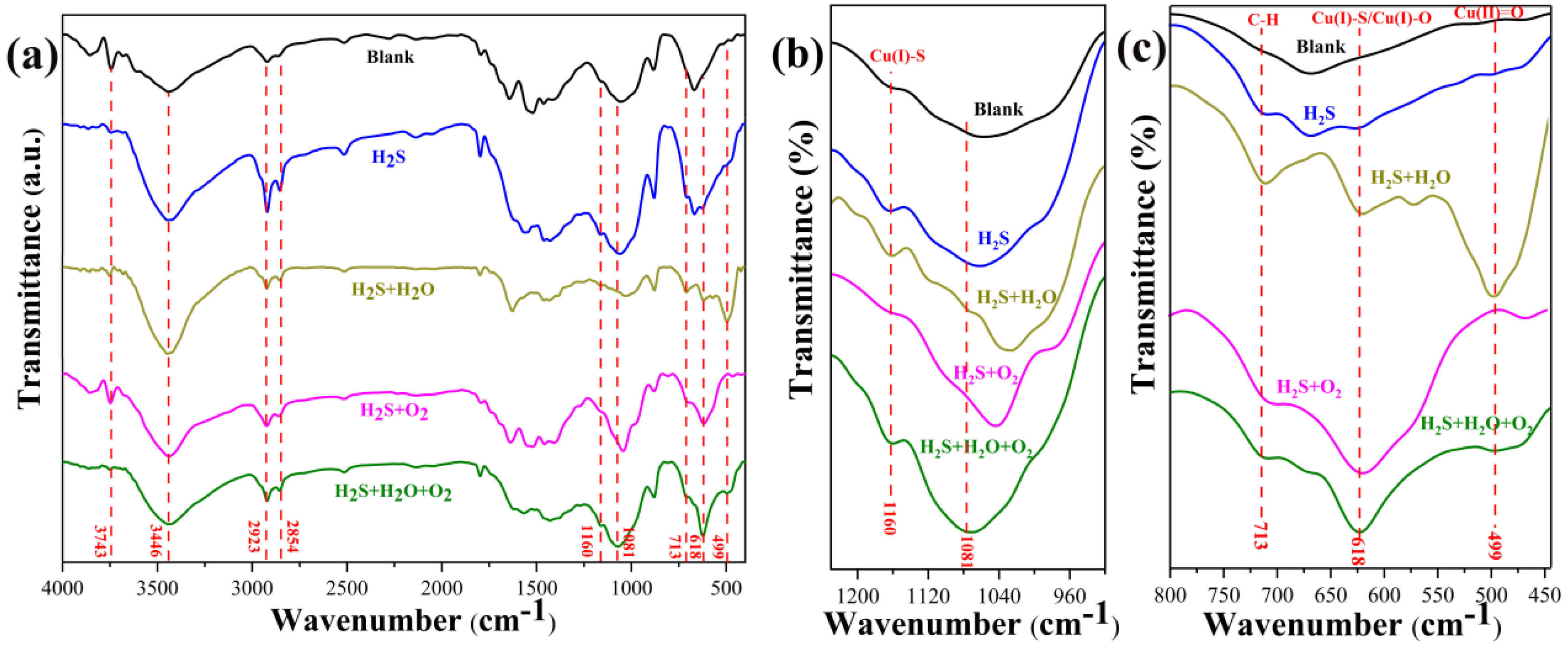
3.3.5. FTIR Analysis of Corrosion Products
3.4. Corrosion Mechanism of Copper in Different LPG Environments
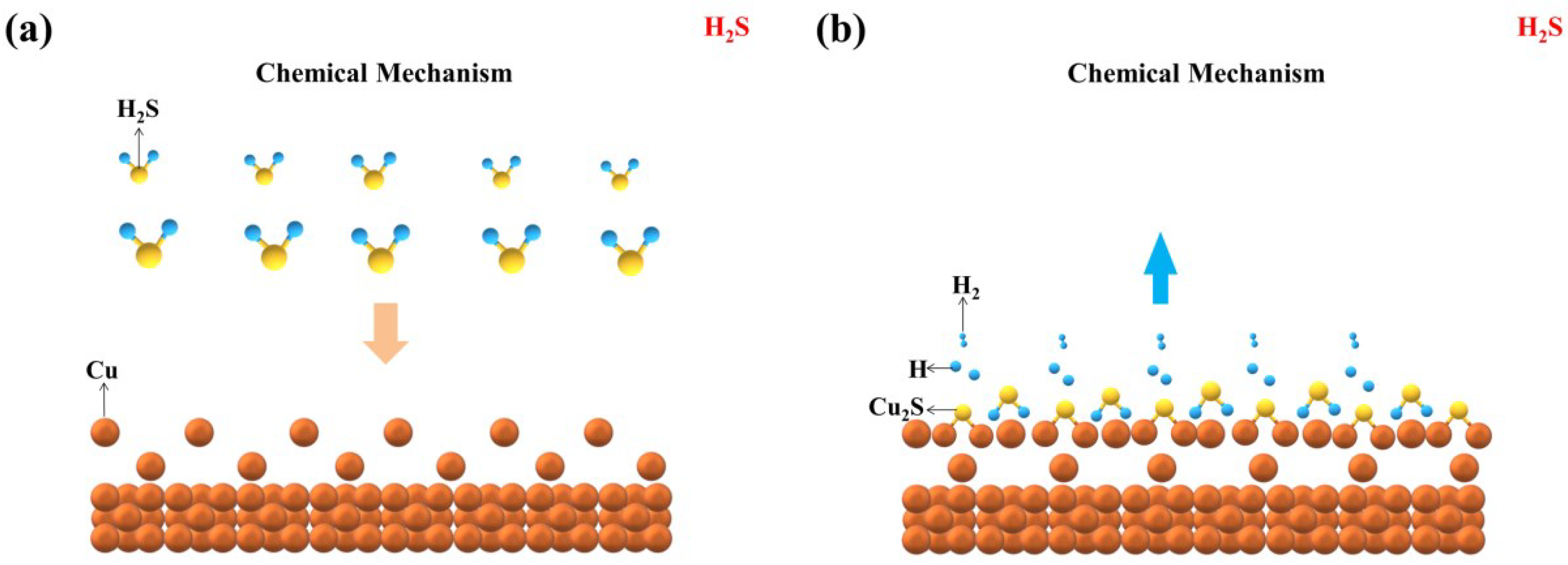
3.4.1. Corrosion Mechanism in H2S
3.4.2. Corrosion Mechanism in H2S+H2O
3.4.3. Corrosion Mechanism in H2S + O2
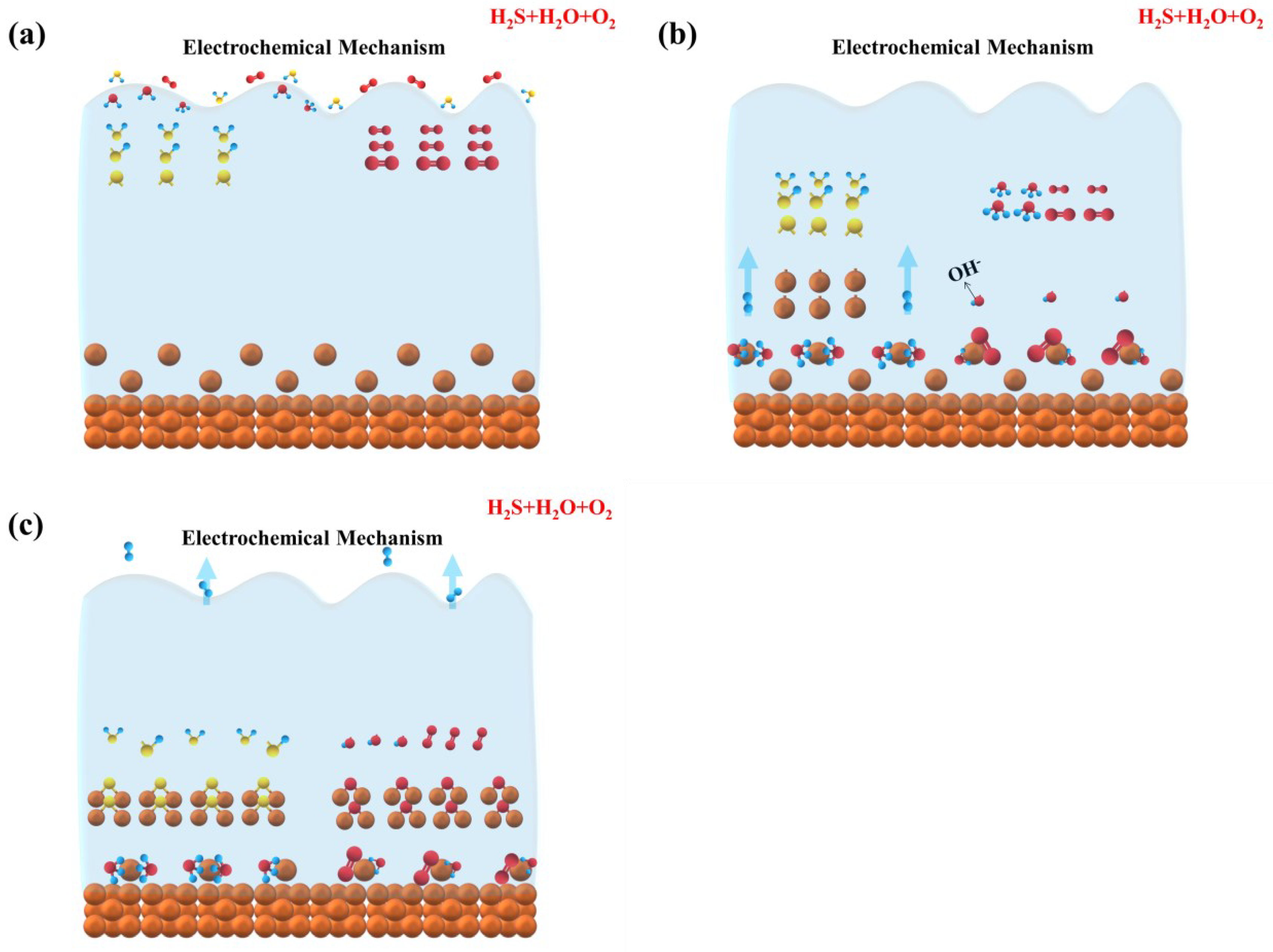
3.4.4. Corrosion Mechanism in H2S + H2O + O2
4. Conclusions
- In H2S-containing LPG, RH has pronounced influence on the corrosion grade of copper. The variation in the CP with RH in LPG is a linear relationship.
- The presence of O2 in dry H2S has limited influence on the corrosion of copper. The CP decreases linearly with the increase in O2 concentration. In the presence of different RHs, the CP always follows a negative exponential function with O2 concentration.
- Surface morphologies of corrosion products obtained in different environments are quite different. Gas humidity and the presence of O2 notably affect the microscopic morphology of corrosion products. In individual H2S, the morphology of copper corrosion products is a regular hexagon block with sharp edges and corners. In H2S + H2O (100% RH), the morphology of copper corrosion products is uniform spherical shape. In H2S + O2, the morphology of copper corrosion products is irregular in shape and size. In H2S + H2O + O2, the morphology of the corrosion products is a regular hexagon block with sharp edges and corners, spherical and irregular in shape and size.
- In H2S-containing LPG, RH and O2 have obvious influence on the composition and distribution of corrosion products. In individual H2S, the corrosion product of copper is only Cu2S. In H2S + H2O, corrosion products of copper are mainly Cu2S. In H2S + O2, corrosion products of copper are composed of a large amount of Cu2S and a small amount of CuO and Cu2O. In H2S + H2O + O2, corrosion products are composed of a large amount of Cu2O, Cu2S and a small amount of CuO.
- The corrosion mechanism of copper in LPG in the presence of different corrosive gases was proposed. The corrosive gas influences the corrosion mechanism remarkably. In individual H2S and H2S + O2, the corrosion process is chemical in nature. H2S and O2 react with copper directly at the interface. The corrosion mechanism of copper in LPG containing H2S + H2O and H2S + H2O + O2 is an electrochemical corrosion process. In H2S + H2O, the corrosion proceeds with an anodic reaction of copper oxidization and a cathodic reaction of traditional hydrogen depolarization. In H2S + H2O + O2, two different electrochemical reactions happen: one is the same as in H2S + H2O, and the other electrochemical reaction displays as the corrosion of O2 in neutral medium, in which the cathodic process is oxygen depolarization.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, B.W.; Jiang, Z.M.; Hu, X.C.; Guo, B.L. Discussion on corrosion control of LPG copper sheet in a terminal plant of Bohai oil field. Chem. Manag. 2015, 21, 3. [Google Scholar]
- Yi, F.; Lu, Y.; Zhao, J.M. Influence and mechanism of sulfide in liquefied petroleum gas on copper corrosion. J. Beijing Univ. Chem. Technol. (Nat. Sci. Ed.) 2021, 48, 59–65. [Google Scholar]
- Zhong, X.Y. Research on Sulfur Corrosion and Removal of Light Oil Products; China University of Petroleum: Beijing, China, 2011. [Google Scholar]
- Halldin Stenlid, J.; Campos dos Santos, E.; Johansson, A.J.; Pettersson, L.G.M. Properties of interfaces between copper and copper sulphide/oxide films. Corros. Sci. 2021, 183, 109313. [Google Scholar] [CrossRef]
- Long, Y.; Song, W.; Fu, A.; Xie, J.; Feng, Y.; Bai, Z.; Yin, C.; Ma, Q.; Ji, N.; Kuang, X. Combined effect of hydrogen embrittlement and corrosion on the cracking behaviour of C110 low alloy steel in O2-contaminated H2S environment. Corros. Sci. 2022, 194, 109926. [Google Scholar] [CrossRef]
- Johnsen, R.; Lange, T.; Stenerud, G.; Olsen, J.S. Environmentally assisted degradation of spinodal copper alloy C72900. Corros. Sci. 2018, 142, 45–55. [Google Scholar] [CrossRef]
- Dou, W.; Jia, R.; Jin, P.; Liu, J.; Chen, S.; Gu, T. Investigation of the mechanism and characteristics of copper corrosion by sulphate reducing bacteria. Corros. Sci. 2018, 144, 237–248. [Google Scholar] [CrossRef]
- Echeverria, F.; Botero, C.A.; Correa, E.; Meza, D.; Castano, J.G.; Gomez, M.A. High resolution morphological changes of Cu, Ni, Al, and Au surfaces due to atmospheric corrosion. IEEE Trans. Device Mater. Reliab. 2017, 17, 331–339. [Google Scholar] [CrossRef]
- Majtás, D.; Kreislová, K.; Turek, L. Failure of electric products by H2S. Koroze Ochr. Mater. 2018, 62, 71–77. [Google Scholar] [CrossRef][Green Version]
- Zhu, Z.; Zuo, X.; Ying, Z. Corrosion analysis of copper T2 exposed to polluted atmospheres and study on prediction model. Corros. Rev. 2017, 35, 35–46. [Google Scholar] [CrossRef]
- Odnevall, I. Atmospheric corrosion of copper in a rural atmosphere. J. Electrochem. Soc. 1995, 142, 3682–3689. [Google Scholar] [CrossRef]
- Monzó, J.; García-Antón, J.; Guiñón, J.L. Influence of elemental sulphur and mercaptans on corrosion of copper strips in the ASTM D-130 test by means of electronic microscopy (SEM) and energy dispersive X-ray (EDX). Fresenius J. Anal. Chem. 1991, 341, 606–610. [Google Scholar] [CrossRef]
- Monzó, J.; García-Antón, J.; Guiñón, J.L. Study of corrosion on copper strips by mixtures of mercaptans, sulphides and disulphides with elemental sulphur in the ASTM D-130 test by means of electron microscopy (SEM) and energy dispersive X-ray (EDX). Fresenius J. Anal. Chem. 1992, 343, 593–596. [Google Scholar] [CrossRef]
- Toyoda, E.; Watanabe, M.; Higashi, Y.; Tanaka, T.; Miyata, Y.; Ichino, T. Depth profiling analysis of tarnish films on copper formed during short-term outdoor exposure by glow discharge optical emission spectroscopy. Corrosion 2004, 60, 729–735. [Google Scholar] [CrossRef]
- Graedel, T.E.; Franey, J.P.; Gualtieri, G.J.; Kammlott, G.W.; Malm, D.L. On the mechanism of silver and copper sulfidation by atmospheric H2S and OCS. Corros. Sci. 1985, 25, 1163–1180. [Google Scholar] [CrossRef]
- Kong, D.; Dong, C.; Xu, A.; Man, C.; He, C.; Li, X. Effect of sulphide concentration on copper corrosion in anoxic chloride-containing solutions. J. Mater. Eng. Perform. 2017, 26, 1741–1750. [Google Scholar] [CrossRef]
- Su, W.; Lv, W.; Liu, Z.; Zhang, Z. Corrosion of copper exposed to Zhanjiang and Zhuhai atmospheric environments. Anti-Corros. Methods Mater. 2017, 64, 286–292. [Google Scholar] [CrossRef]
- Fujimoto, S.; Umemura, H.; Shibata, T. Atmospheric corrosion of electroplated cu thin film in moist oxygen environment. ECS Trans. 2006, 1, 243–247. [Google Scholar] [CrossRef]
- Araban, V.; Kahram, M.; Rezakhani, D. Evaluation of copper atmospheric corrosion in different environments of Iran. Corros. Eng. Sci. Technol. 2016, 51, 498–506. [Google Scholar] [CrossRef]
- FitzGerald, K.P.; Nairn, J.; Skennerton, G.; Atrens, A. Atmospheric corrosion of copper and the colour, structure and composition of natural patinas on copper. Corros. Sci. 2006, 48, 2480–2509. [Google Scholar] [CrossRef]
- Kong, D.C.; Dong, C.F.; Fang, Y.H.; Xiao, K.; Guo, C.Y.; He, G.; Li, X.G. Copper corrosion in hot and dry atmosphere environment in Turpan, China. Trans. Nonferrous Met. Soc. China 2016, 26, 1721–1728. [Google Scholar] [CrossRef]
- Lopesino, P.; Alcántara, J.; Fuente, D.; Chico, B.; Jiménez, J.; Morcillo, M. Corrosion of copper in unpolluted chloride-rich atmospheres. Metals 2018, 8, 866. [Google Scholar] [CrossRef]
- Yan, F.; Li, X.; Jiang, B.; Lin, D.; Fu, M.; Li, W. Initial corrosion behaviour of pure copper in atmospheric environment. IOP Conf. Ser. Earth Environ. Sci. 2019, 384, 012039. [Google Scholar] [CrossRef]
- Zhang, F.; Örnek, C.; Liu, M.; Müller, T.; Lienert, U.; Ratia-Hanby, V.; Carpén, L.; Isotahdon, E.; Pan, J. Corrosion-induced microstructure degradation of copper in sulphide-containing simulated anoxic groundwater studied by synchrotron high-energy X-ray diffraction and ab-initio density functional theory calculation. Corros. Sci. 2021, 184, 109390. [Google Scholar] [CrossRef]
- Saha, D.; Pandya, A.; Singh, J.K.; Paswan, S.; Singh, D.D.N. Role of environmental particulate matters on corrosion of copper. Atmos. Pollut. Res. 2016, 7, 1037–1042. [Google Scholar] [CrossRef]
- Hedin, A.; Johansson, A.J.; Lilja, C.; Boman, M.; Berastegui, P.; Berger, R.; Ottosson, M. Corrosion of copper in pure O2-free water. Corros. Sci. 2018, 137, 1–12. [Google Scholar] [CrossRef]
- Sharma, S.P. Reaction of copper and copper oxide with H2S. J. Electrochem. Soc. 1980, 127, 21–26. [Google Scholar] [CrossRef]
- Wu, T.; Zhou, Z.; Xu, S.; Xie, Y.; Huang, L.; Yin, F. A corrosion failure analysis of copper wires used in outdoor terminal boxes in substation. Eng. Failure Anal. 2019, 98, 83–94. [Google Scholar] [CrossRef]
- Thethwayo, B.M.; Garbers-Craig, A.M. Laboratory scale investigation into the corrosion of copper in a sulphur-containing environment. Corros. Sci. 2011, 53, 3068–3074. [Google Scholar] [CrossRef]
- Chen, J.; Qin, Z.; Martino, T.; Shoesmith, D.W. Non-uniform film growth and micro/macro-galvanic corrosion of copper in aqueous sulphide solutions containing chloride. Corros. Sci. 2017, 114, 72–78. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Y.; Liu, M.; Wang, Z. Corrosion behaviour of copper T2 and brass H62 in simulated Nansha marine atmosphere. J. Mater. Sci. Technol. 2019, 35, 1831–1839. [Google Scholar] [CrossRef]
- Schindelholz, E.J.; Cong, H.; Jove-Colon, C.F.; Li, S.; Ohlhausen, J.A.; Moffat, H.K. Electrochemical aspects of copper atmospheric corrosion in the presence of sodium chloride. Electrochim. Acta. 2018, 276, 194–206. [Google Scholar] [CrossRef]
- Deutscher, R.L.; Woods, R. Characterization of oxide layers on copper by linear potential sweep voltammetry. J. Appl. Electrochem. 1986, 16, 413–421. [Google Scholar] [CrossRef]
- Tran, T.T.M.; Fiaud, C.; Sutter, E.M.M.; Villanova, A. The atmospheric corrosion of copper by hydrogen sulphide in underground conditions. Corros. Sci. 2003, 45, 2787–2802. [Google Scholar] [CrossRef]
- Tran, T.T.M.; Fiaud, C.; Sutter, E.M.M. Oxide and sulphide layers on copper exposed to H2S containing moist air. Corros. Sci. 2005, 47, 1724–1737. [Google Scholar] [CrossRef]
- Nielsen, B.C.; Doğan, Ö.N.; Howard, B.H. Effect of temperature on the corrosion of Cu-Pd hydrogen separation membrane alloys in simulated syngas containing H2S. Corros. Sci. 2015, 96, 74–86. [Google Scholar] [CrossRef]
- Cai, L.; Chen, M.; Wang, Y.; Chen, C.; Zhang, L.; Zhou, H.; Wu, L.; Yan, Y. Electrochemical corrosion behaviour of bronze materials in an acid-containing simulated atmospheric environment. Mater. Corros. 2019, 71, 464–473. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Z.; Lu, Z. In situ investigation of atmospheric corrosion behaviour of copper under thin electrolyte layer and static magnetic field. Microelectron. Reliab. 2020, 108, 113630. [Google Scholar] [CrossRef]
- Bellakhal, N.; Draou, K.; Brisset, J.L. Electrochemical investigation of copper oxide films formed by oxygen plasma treatment. J. Appl. Electrochem. 1997, 27, 414–421. [Google Scholar] [CrossRef]
- ASTM Standard D-130-80, Part 23; American Society for Testing and Materials: West Conshohocken, PA, USA, 1981; p. 104.
- Wang, H.; Xie, J.; Yan, K.P.; Duan, M.; Zuo, Y. The nucleation and growth of metastable pitting on pure iron. Corros. Sci. 2009, 51, 181–185. [Google Scholar] [CrossRef]
- Persson, D.; Leygraf, C. In situ infrared reflection absorption spectroscopy for studies of atmospheric corrosion. J. Electrochem. Soc. 1993, 140, 1256–1260. [Google Scholar] [CrossRef]
- Zhao, W.; Babu, R.P.; Chang, T.; Odnevall, I.; Hedström, P.; Johnson, C.M.; Leygraf, C. Initial atmospheric corrosion studies of copper from macroscale to nanoscale in a simulated indoor atmospheric environment. Corros. Sci. 2022, 195, 109995. [Google Scholar] [CrossRef]
- Paul, A.M.; Sajeev, A.; Nivetha, R.; Gothandapani, K.; Bhardwaj, P.; Raghavan, V.; Jacob, G.; Sellapan, R.; Jeong, S.K.; Grace, A.N. Cuprous oxide/graphitic carbon nitride (g-C3N4) nanocomposites for electrocatalytic hydrogen evolution reaction. Diamond Relat. Mater. 2020, 107, 107899. [Google Scholar] [CrossRef]
- Shanghai Institute of Organic Chemistry of CAS. Chemistry Database. 1978–2022. Available online: http://www.organchem.csdb.cn. (accessed on 1 February 2022).
- Li, K.; Chen, Z.; Li, J.; Sun, X.; Xu, F.; Xu, L. Corrosion mechanism of copper immersed in ammonium sulphate solution. Mater. Corros. 2018, 69, 1597–1608. [Google Scholar] [CrossRef]
- Chialvo, M.R.G.; Marchiano, S.L.; Arvía, A.J. The mechanism of oxidation of copper in alkaline solutions. J. Appl. Electrochem. 1984, 14, 165–175. [Google Scholar] [CrossRef]


| Component | Propane | Isobutane | N-Butane |
|---|---|---|---|
| wt./% | 70 | 14 | 16 |
| Corrosion Level | Copper Colour | Detailed Description |
|---|---|---|
| 1 | mild discolouration | a. pale orange, almost the same as freshly polished copper |
| b. dark orange | ||
| 2 | moderate discolouration | a. fuchsia |
| b. lavender | ||
| c. multicoloured with lavender blue, silver or both, overlaid on fuchsia | ||
| d. silver | ||
| e. brass or golden yellow | ||
| 3 | deep discolouration | a. multicolour magenta overlay brass |
| b. multicolour (malachite green) shown by red and green, no grey | ||
| 4 | corrosion | a. transparent black, dark grey or brown with only malachite green |
| b. graphite or matte black | ||
| c. glossy black or jet-black glossy black |
| Element | H2S | H2S + H2O | H2S + O2 | H2S + O2 + H2O | ||||
|---|---|---|---|---|---|---|---|---|
| wt.% | Atomic% | wt.% | Atomic% | wt.% | Atomic% | wt.% | Atomic% | |
| O | - | - | 5.18 | 16.61 | 2.85 | 9.20 | 8.40 | 25.99 |
| S | 9.94 | 17.95 | 8.66 | 13.85 | 14.98 | 24.10 | 3.50 | 5.40 |
| Cu | 90.06 | 82.05 | 86.15 | 69.53 | 82.17 | 66.70 | 88.09 | 68.60 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Element | Peak Type | Condition | BE (ev) | Area% |
|---|---|---|---|---|
| Cu | Cu2S | H2S | 932.75 | 100.00 |
| H2S + H2O | 932.77 | 52.74 | ||
| H2S + O2 | 932.70 | 32.56 | ||
| H2S + H2O + O2 | 932.80 | 70.26 | ||
| Cu2O | H2S | - | - | |
| H2S + H2O | - | - | ||
| H2S + O2 | - | - | ||
| H2S + H2O + O2 | 932.51 | 29.74 | ||
| CuO | H2S | - | - | |
| H2S + H2O | 934.10 | 47.26 | ||
| H2S + O2 | 934.27 | 67.44 | ||
| H2S + H2O + O2 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Lu, Y.; Wei, Q.; Wang, H.; Xie, J. Influence of Relative Humidity and Oxygen Concentration on Corrosion Behaviour of Copper in H2S-Containing Liquid Petroleum Gas. Metals 2022, 12, 2015. https://doi.org/10.3390/met12122015
Li X, Lu Y, Wei Q, Wang H, Xie J. Influence of Relative Humidity and Oxygen Concentration on Corrosion Behaviour of Copper in H2S-Containing Liquid Petroleum Gas. Metals. 2022; 12(12):2015. https://doi.org/10.3390/met12122015
Chicago/Turabian StyleLi, Xianqiang, Yuan Lu, Qiang Wei, Hu Wang, and Juan Xie. 2022. "Influence of Relative Humidity and Oxygen Concentration on Corrosion Behaviour of Copper in H2S-Containing Liquid Petroleum Gas" Metals 12, no. 12: 2015. https://doi.org/10.3390/met12122015
APA StyleLi, X., Lu, Y., Wei, Q., Wang, H., & Xie, J. (2022). Influence of Relative Humidity and Oxygen Concentration on Corrosion Behaviour of Copper in H2S-Containing Liquid Petroleum Gas. Metals, 12(12), 2015. https://doi.org/10.3390/met12122015






