Development of Materials Based on the NiAlCrMoCo System Reinforced with ZrO2 Nanoparticles
Abstract
:1. Introduction
- –
- reducing the grain size, preventing the boundaries from growing and moving, increasing the yield strength and tensile strength, maintaining the distance between them and stabilizing the acquired structure at the stage of cold pressing. This is an obstacle to the movement of the dislocation front, since they retain incoherence at the grain boundary [18,19,20,21];
- –
- –
- –
- limiting the formation, and promoting the annihilation, of vacancies, increasing the creep resistance along the grain boundaries [24];
- –
- preventing the diffusion of oxidant molecules by adsorbing them on its surface [18].
2. Materials and Methods
3. Results
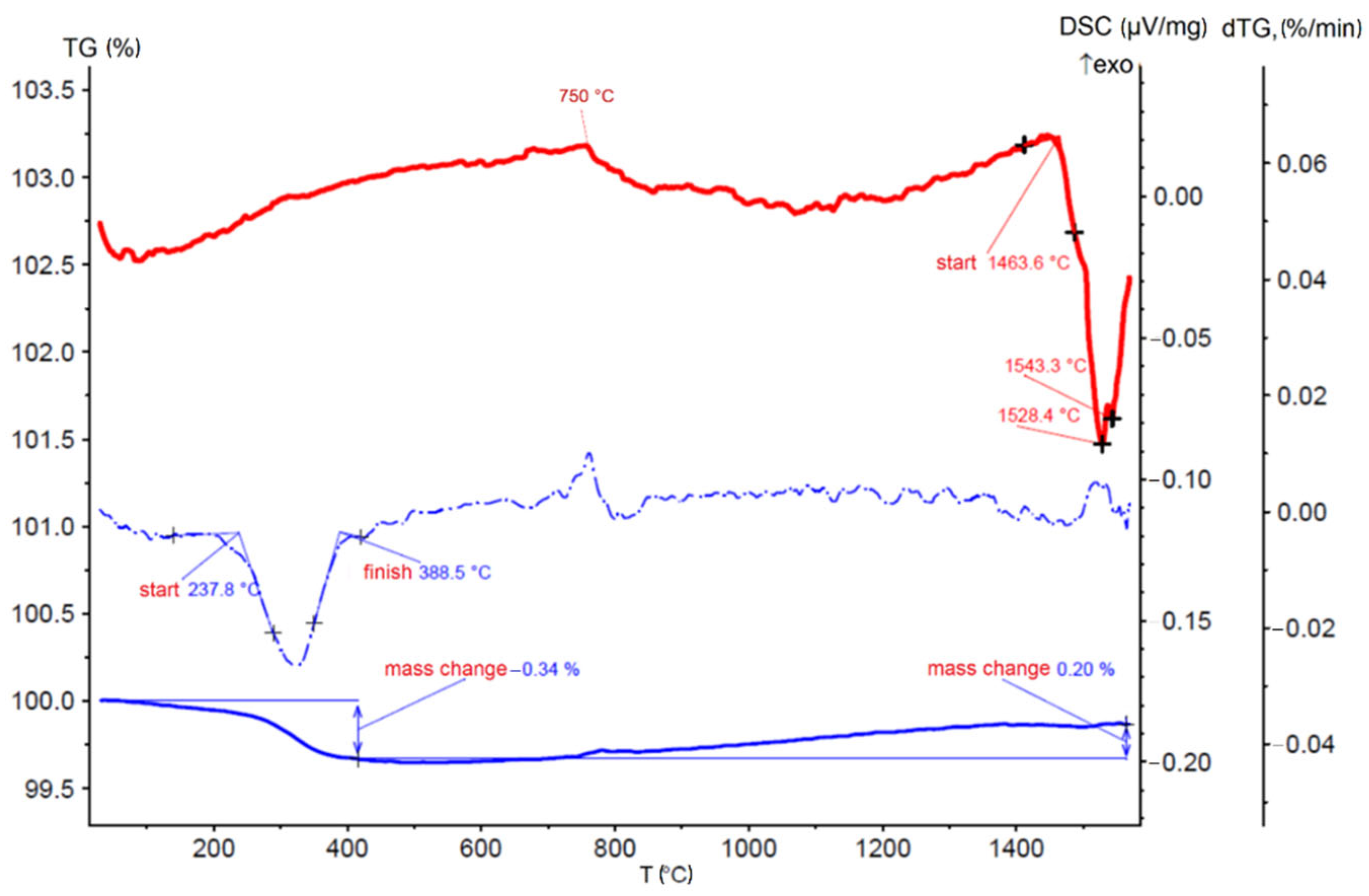
3.1. Thermal Analysis
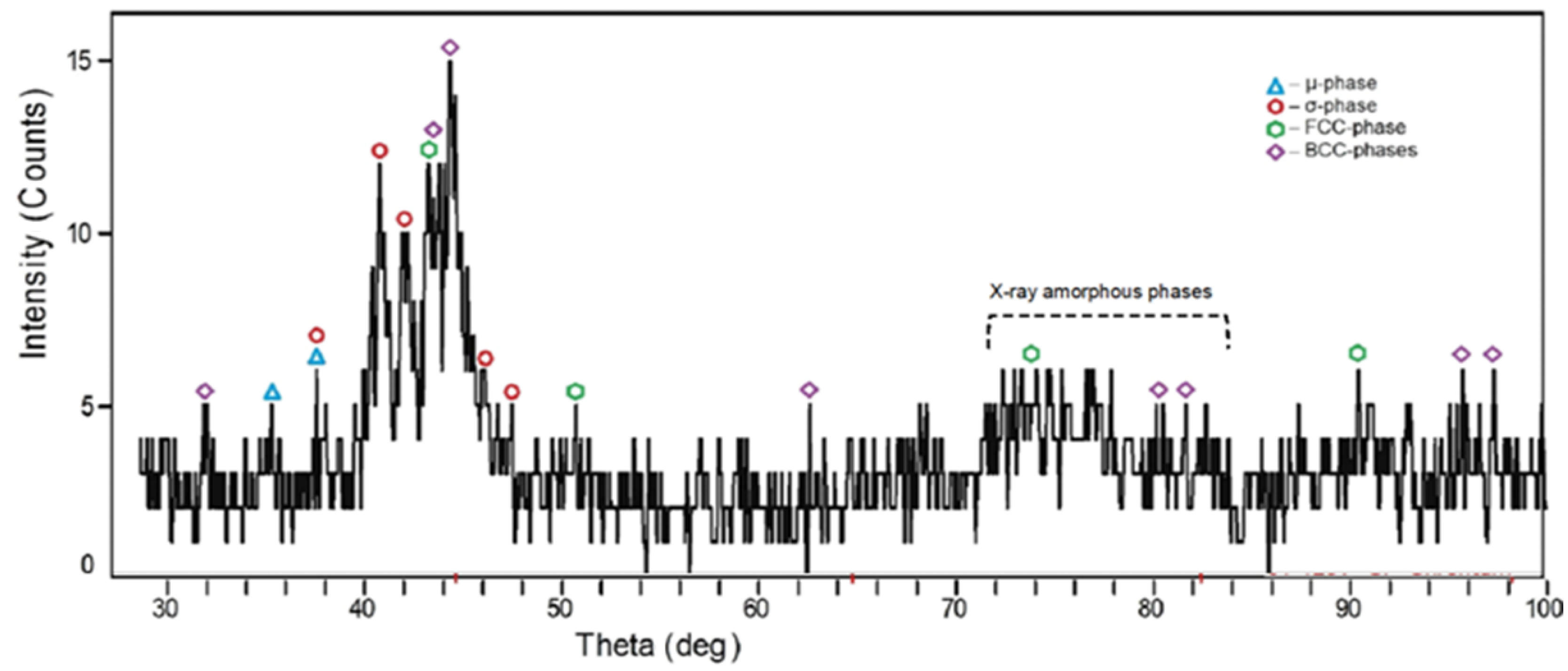
3.2. Phase Composition
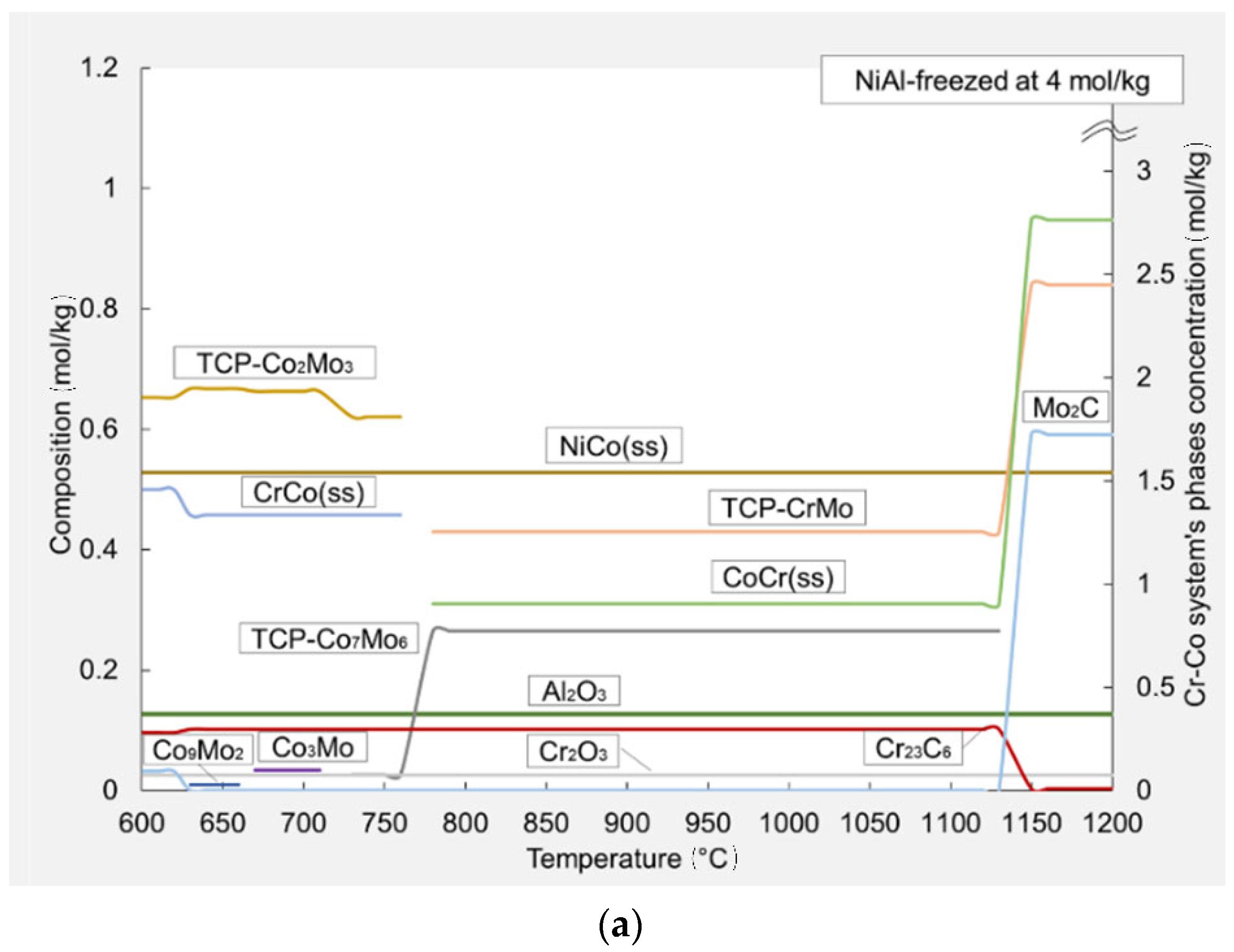
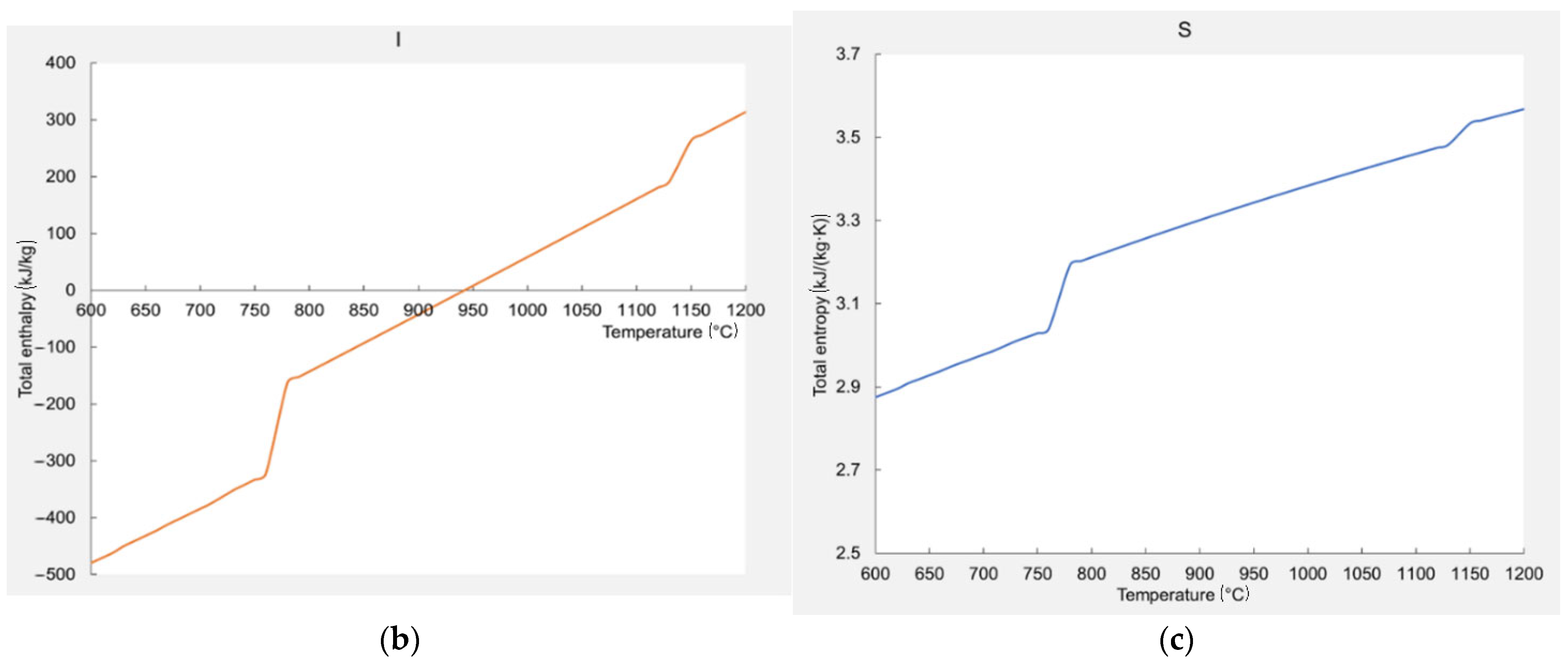
3.3. Thermodynamic Modeling
- –
- closed and isolated thermodynamic systems are considered, where the boundaries are impenetrable for the exchange of matter, heat and energy with the environment;
- –
- systems are analyzed in a state of external and internal thermodynamic equilibrium (full or local);
- –
- it is considered that the system is heterogeneous, consisting of several homogeneous parts (phases) separated by visible boundaries;
- –
- the presence of the gas phase in the system is mandatory;
- –
- all gaseous individual substances (atoms, molecules, atomic and molecular ions, electron gas) are part of one gas phase;
- –
- the gas phase is described by the equation of state of an ideal gas;
- –
- surface effects at the phase boundary are not considered, the solubility of gases in condensed (liquid and solid) phases is absent; condensed matter may be absent;
- –
- condensed substances forming one-component immiscible phases are included in ideal condensed solutions;
- –
- individual substances that have the same chemical formula, but are included in different phases, are considered to be different components;
- –
- substances with the same chemical formula, which are in various polymorphic modifications, crystalline or liquid states, are considered as one component, in which the change in properties occurs abruptly at transformation temperatures;
- –
- the volume of condensed components is negligible.
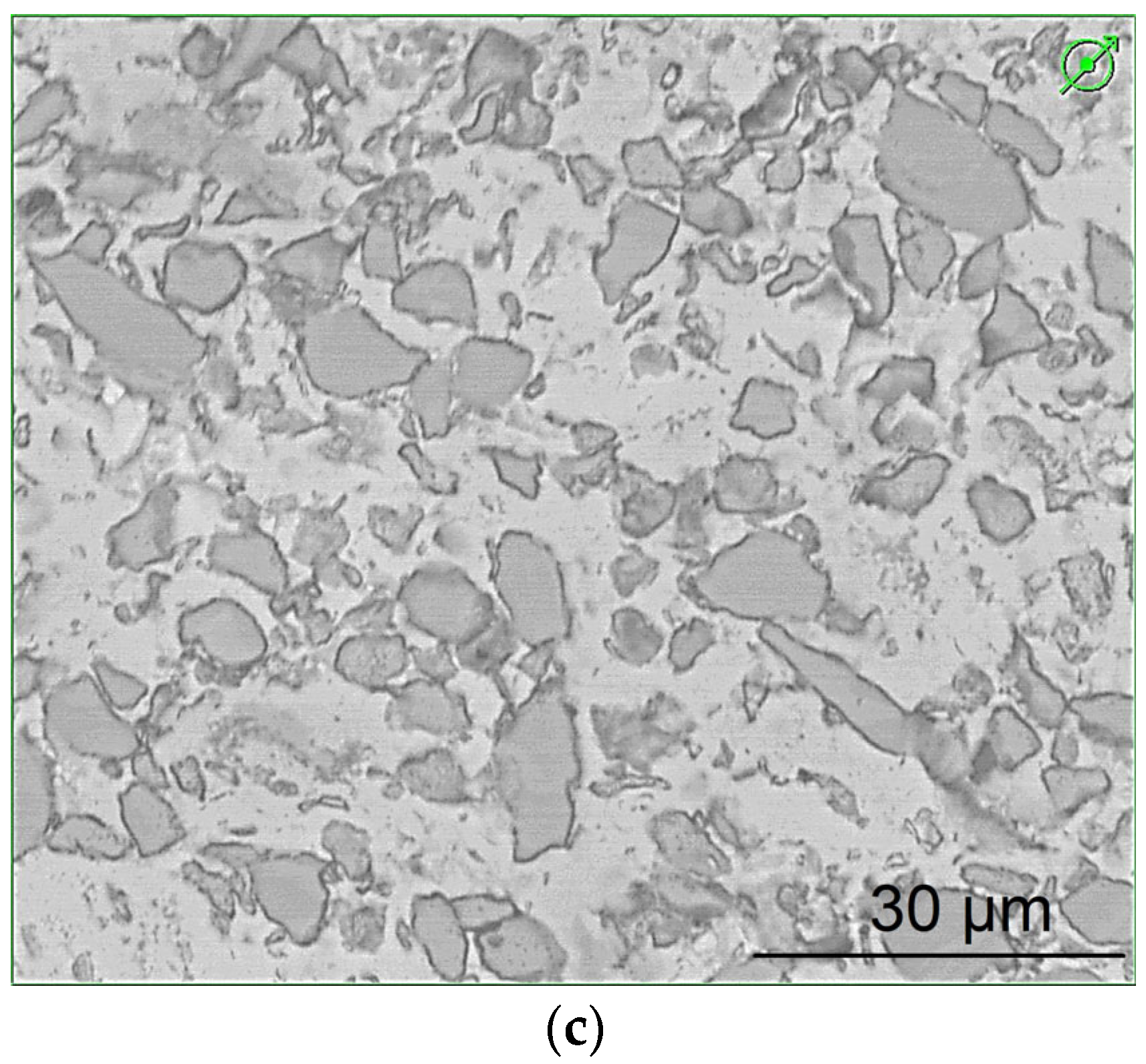
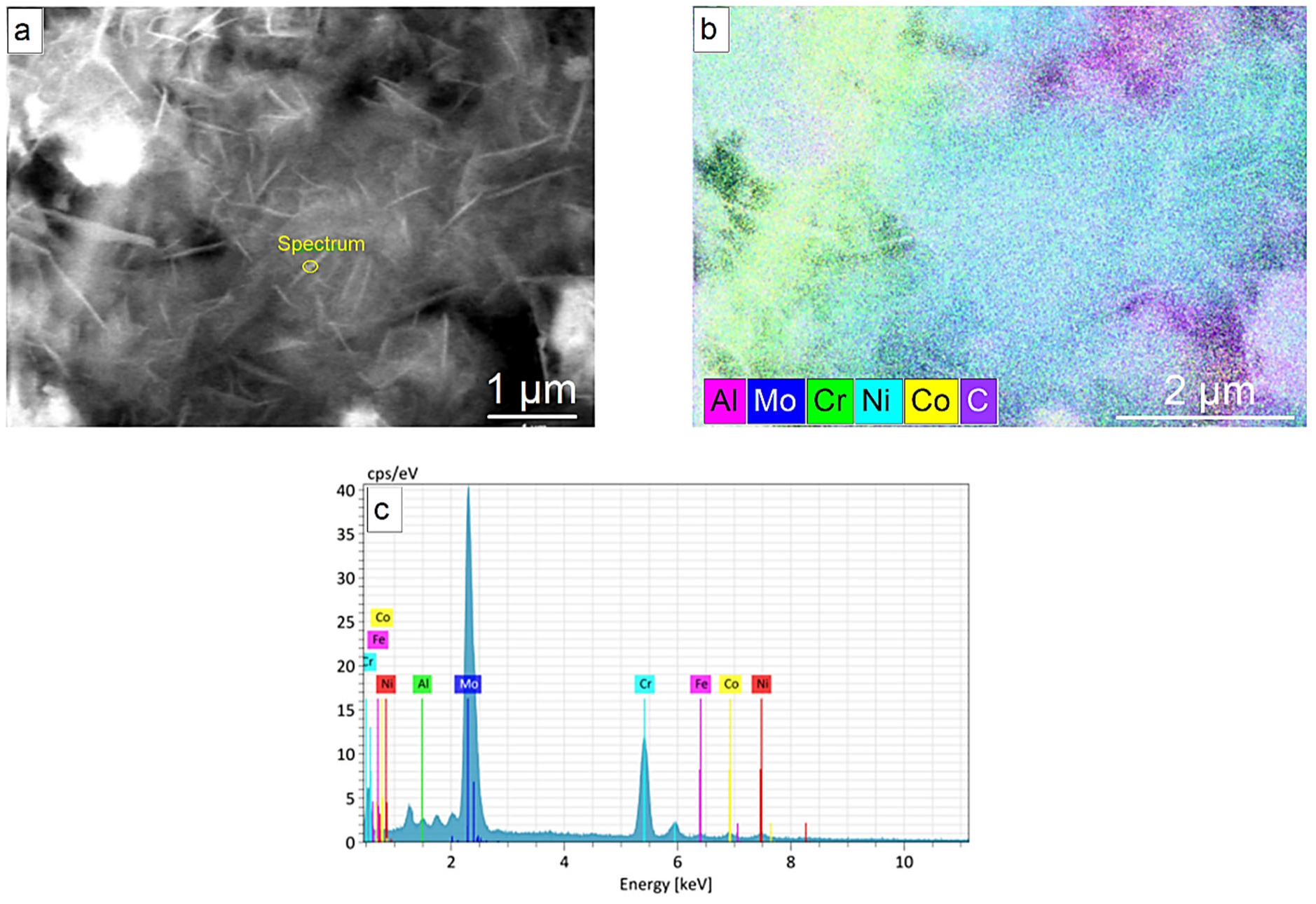
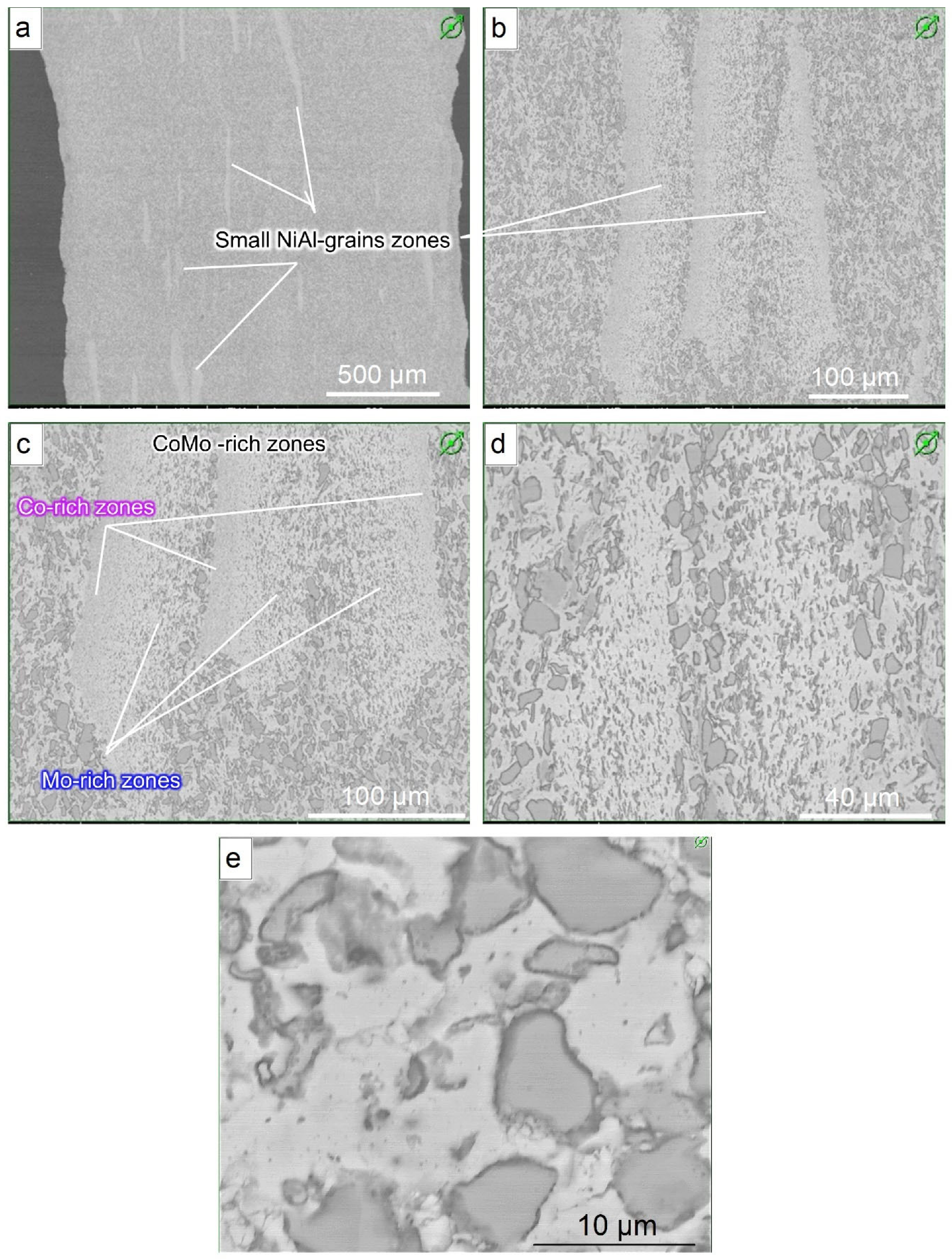
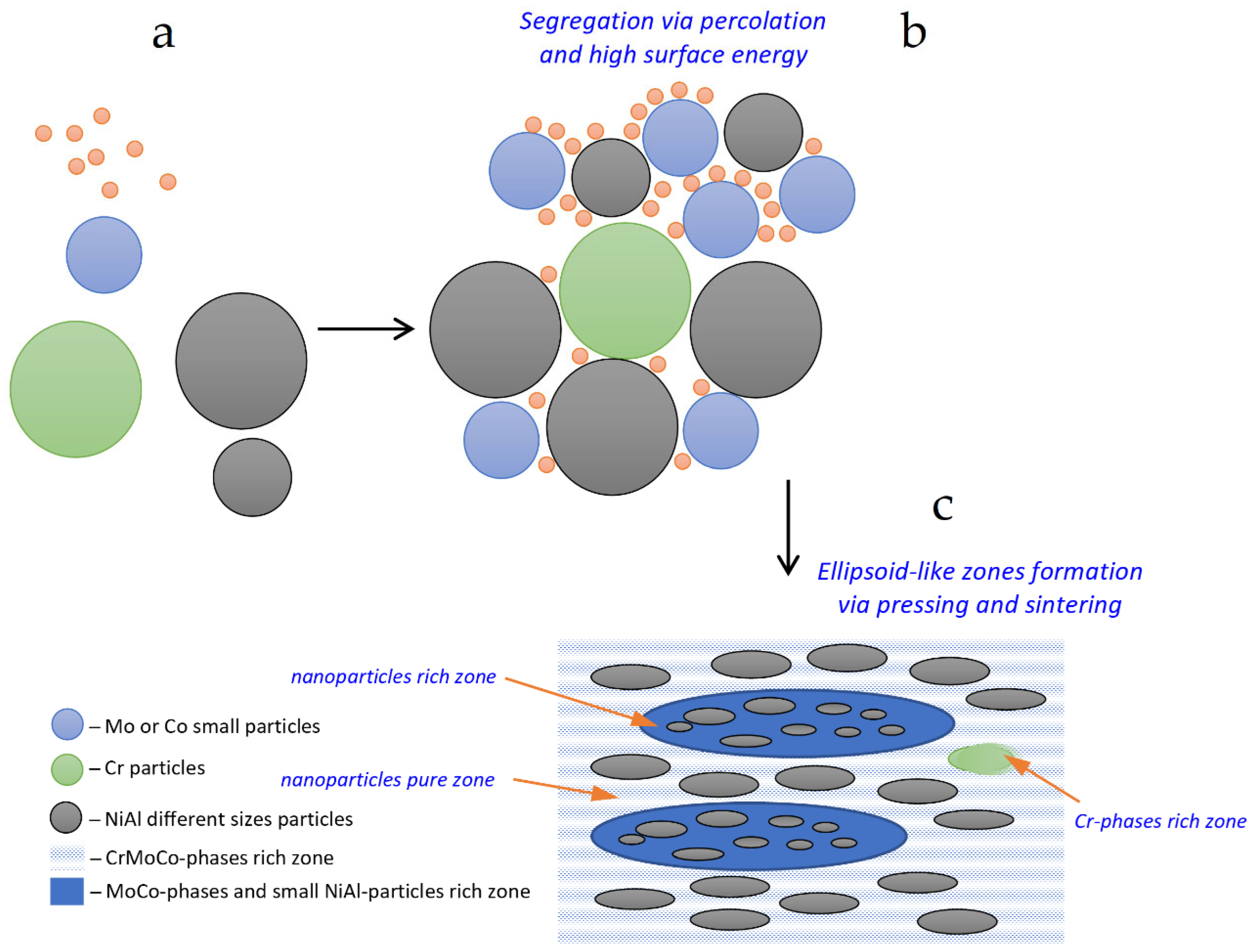
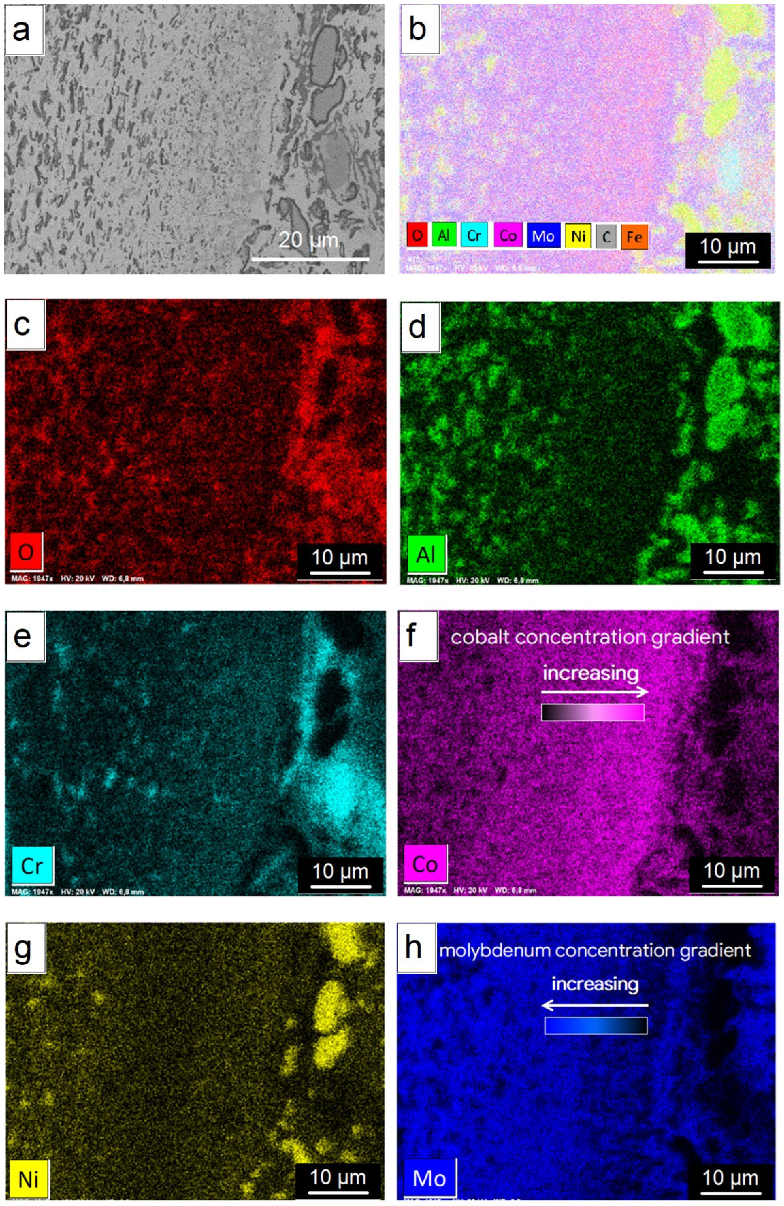
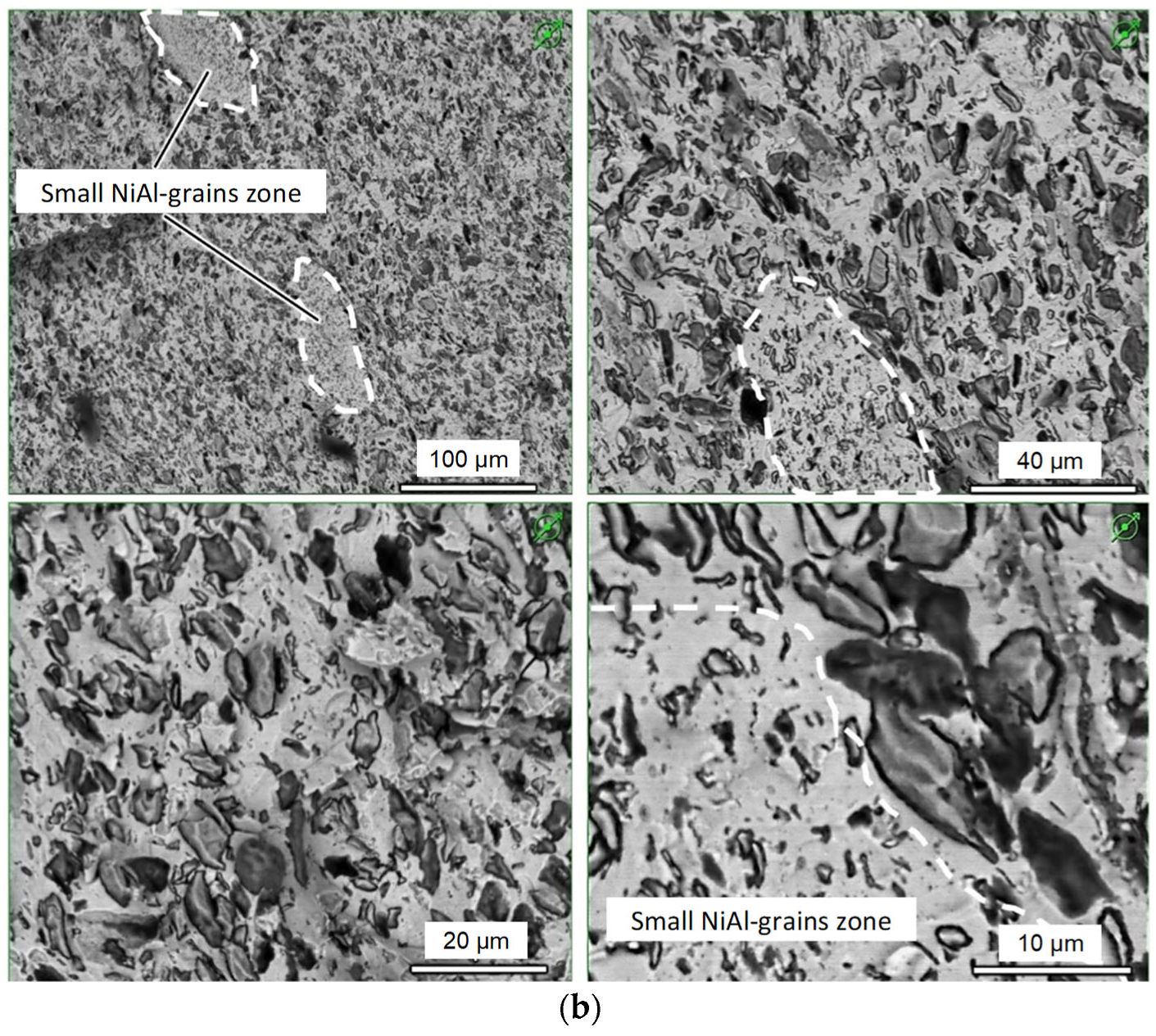
3.4. Microstructure and Composition
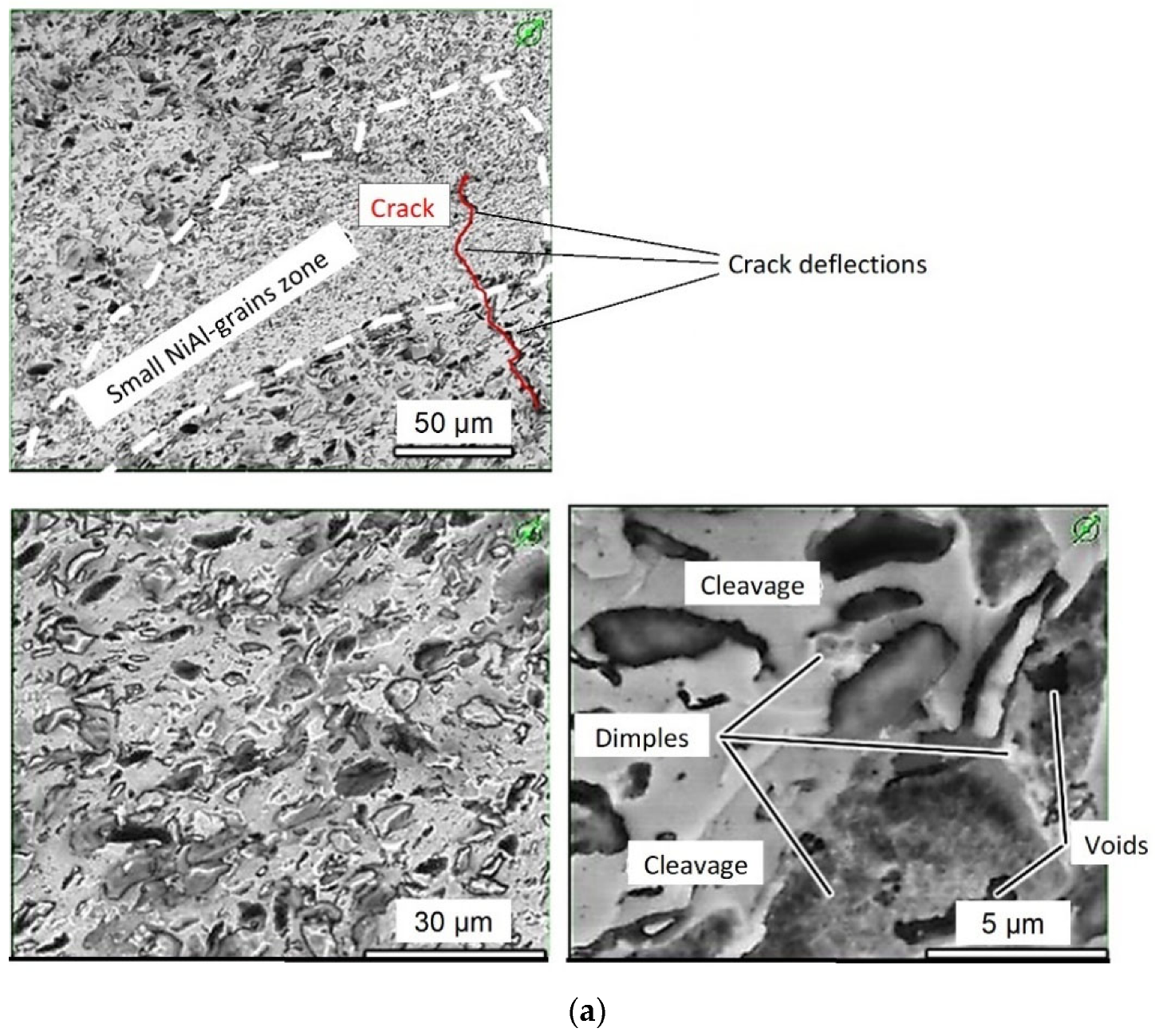
3.5. Fractographic Analysis
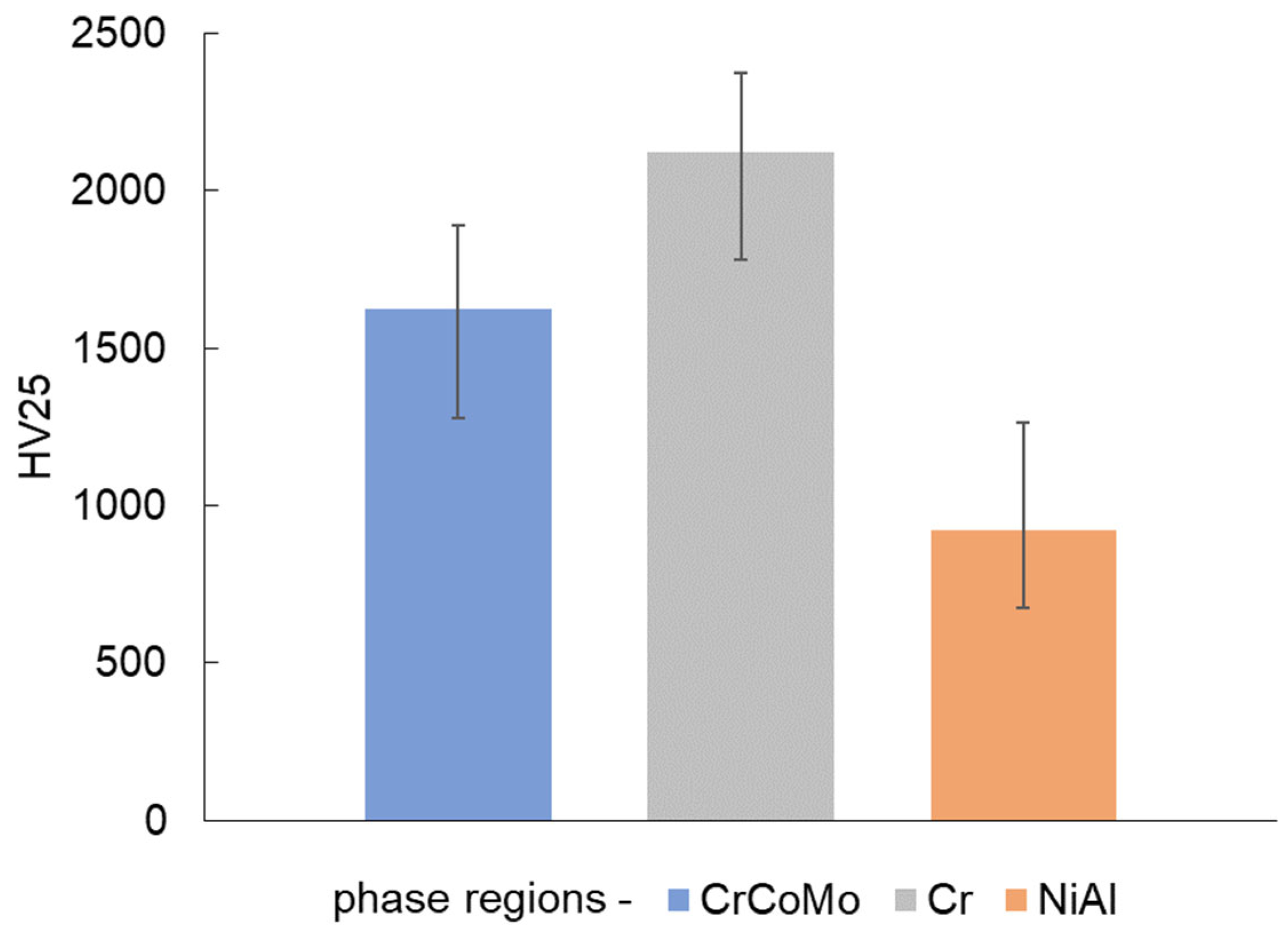
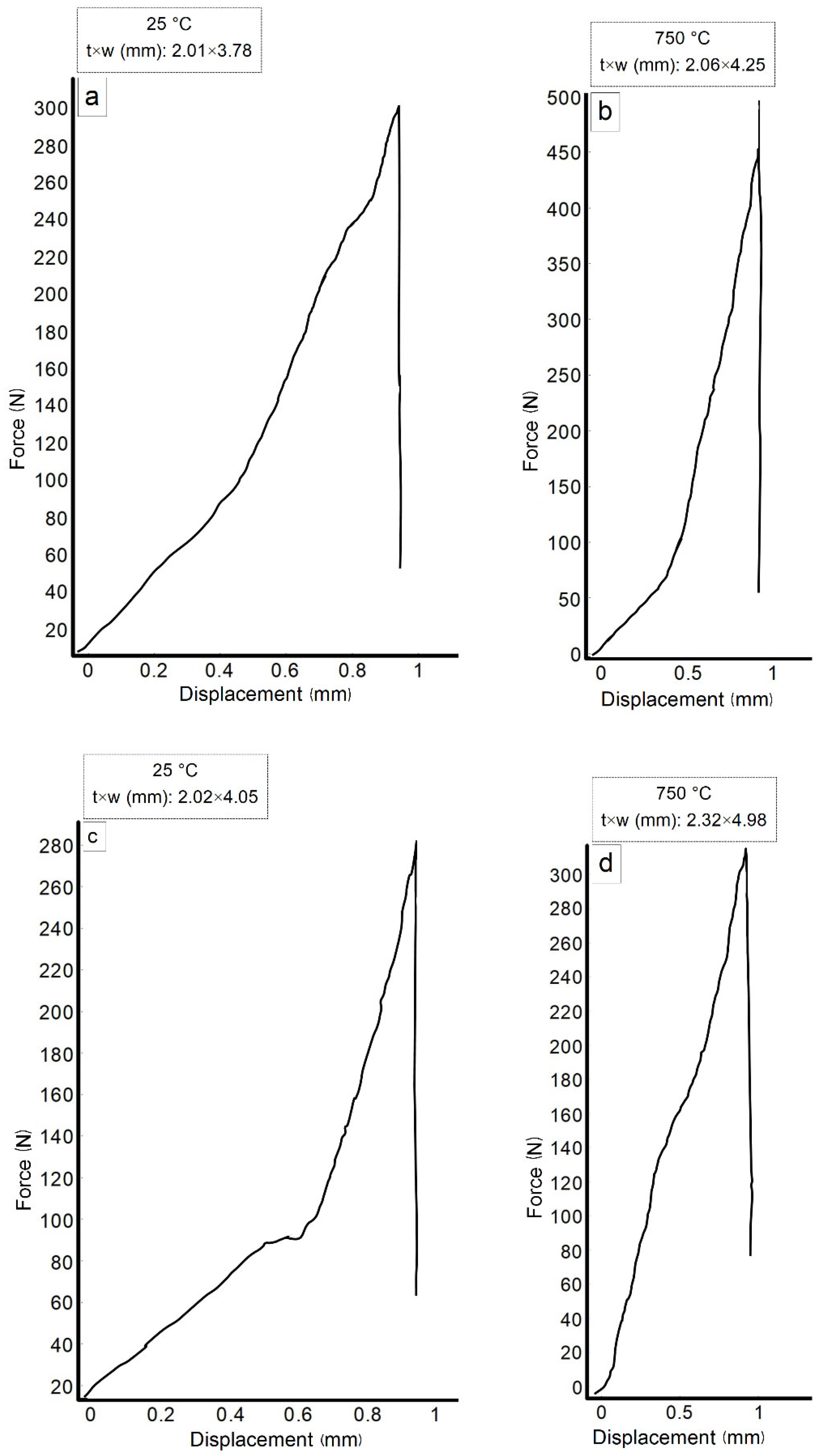
3.6. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miracle, D.B. The physical and mechanical properties of NiAl. Acta Metall. 1993, 41, 649–684. [Google Scholar] [CrossRef]
- Noebe, R.D.; Bowman, R.R.; Nathal, M.V. Physical and mechanical properties of the B2 compound NiAl. Int. Mater. Rev. 1993, 38, 193–232. [Google Scholar] [CrossRef]
- George, E.P.; Liu, C.T. Brittle fracture and grain boundary chemistry of microalloyed NiAl. J. Mater. Res. 1990, 5, 754–762. [Google Scholar] [CrossRef]
- Barrett, C.A. Effect of 0.1 at.% Zirconium on the cyclic oxidation resistance of β-NiAl. Oxid. Met. 1988, 30, 361–390. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Shrivastava, S. Brittle-to-ductile transition in NiAl single crystal. Acta Mater. 1998, 46, 1493–1502. [Google Scholar] [CrossRef]
- Walter, J.L.; Cline, H.E. The effect of solidification rate on structure and high-temperature strength of the eutectic NiAl-Cr. Metall. Mater. Trans. 1970, 1, 1221–1229. [Google Scholar] [CrossRef]
- Ferrandini, P.; Batista, W.W.; Caram, R. Influence of growth rate on the microstructure and mechanical behavior of a NiAl-Mo eutectic alloy. J. Alloys Compd. 2004, 381, 91–98. [Google Scholar] [CrossRef]
- Bei, H.; George, E.P. Microstructures and mechanical properties of a directionally solidified NiAl-Mo eutectic alloy. Acta Mater. 2005, 53, 69–77. [Google Scholar] [CrossRef]
- Zhang, J.F.; Shen, J.; Shang, Z.; Feng, Z.R.; Wang, L.S.; Fu, H.Z. Microstructure and room temperature fracture toughness of directionally solidified NiAl-Mo eutectic in situ composites. Intermetallics 2012, 21, 18–25. [Google Scholar] [CrossRef]
- Bogner, S.; Hu, L.; Hollad, S.; Hu, W.; Gottstein, G.; Bührig-Polaczek, A. Microstructure of a eutectic NiAl-Mo alloy directionally solidified using an industrial scale and a laboratory scale bridgman furnace. Int. J. Mater. Res. 2012, 103, 17–23. [Google Scholar] [CrossRef]
- Hu, L.; Hu, W.; Gottstein, G.; Bogner, S.; Hollad, S.; Bührig-Polaczek, A. Investigation into microstructure and mechanical properties of NiAl-Mo composites produced by directional solidification. Mater. Sci. Eng. A 2012, 539, 211–222. [Google Scholar] [CrossRef]
- Chen, X.F.; Johnson, D.R.; Noebe, R.D.; Oliver, B.F. Deformation and fracture of a directionally solidified NiAl-28Cr-6Mo eutectic alloy. J. Mater. Res. 1995, 10, 1159–1170. [Google Scholar] [CrossRef] [Green Version]
- Dudová, M.; Kuchařová, K.; Barták, T.; Bei, H.; George, E.P.; Somsen, C.; Dlouhý, A. Creep in directionally solidified NiAl-Mo eutectics. Scr. Mater. 2011, 65, 699–702. [Google Scholar] [CrossRef]
- Sanin, V.V.; Kaplansky, Y.Y.; Aheiev, M.I.; Levashov, E.A.; Petrzhik, M.I.; Bychkova, M.Y.; Samokhin, A.V.; Fadeev, A.A.; Sanin, V.N. Structure and Properties of Heat-Resistant Alloys NiAl–Cr–Co–X (X = La, Mo, Zr, Ta, Re) and Fabrication of Powders for Additive Manufacturing. Materials 2021, 14, 3144. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Sergeev, D.; Dodge, M.F.; Fanicchia, F.; Müller, M.; Paul, S.; Dong, H. Microstructure and Thermal Analysis of Metastable Intermetallic Phases in High-Entropy Alloy CoCrFeMo0.85Ni. Materials 2021, 14, 1073. [Google Scholar] [CrossRef] [PubMed]
- Shun, T.-T.; Chang, L.-Y.; Shiu, M.-H. Microstructure and mechanical properties of multiprincipal component CoCrFeNiMox alloys. Mater. Charact. 2012, 70, 63–67. [Google Scholar] [CrossRef]
- Mironov, V.V.; Agureev, L.E.; Eremeeva, Z.V.; Kostikov, V.I. Dependence of the strength properties of aluminum materials on the concentration of ZrO2 nanoparticles. Dokl. Phys. Chem. 2019, 485, 63–65. [Google Scholar] [CrossRef]
- Chuvil’deev, V.N.; Kopylov, V.I.; Zeiger, W. Non-equilibrium grain boundaries. Theory and its applications for describing nano- and microcrystalline materials processed by ECAP. Ann. Chim. Sci. Mater. 2002, 27, 55–64. [Google Scholar]
- Ohji, T.; Hirano, T.; Nakahira, A.; Niihara, K. Particle/Matrix interface and its role in creep inhibition in aluminasilicon carbide nanocomposites. J. Am. Ceram. Soc. 1996, 79, 33–45. [Google Scholar] [CrossRef]
- Grigorovich, V.K.; Sheftel, E.N. Dispersion Hardening of Refractory Metals; Nauka: Moscow, Russia, 1980. (In Russian) [Google Scholar]
- Gottstein, G. Physical Foundations of Materials Science; Springer: Berlin/Heidelberg, Germany, 2004; 502p. [Google Scholar]
- Thompson, A.W. Substructure strengthening mechanisms. Met. Trans. 1977, 8, 833–842. [Google Scholar] [CrossRef]
- Lugovskoi, Y.F. Effect of structure on the fatigue strength of dispersion-hardened condensated based on copper II. Analysis of the first coefficient of the Mott—Stroh relation. Powder Metall. Met. Ceram. 1998, 37, 432–437. [Google Scholar] [CrossRef]
- Taira, S.; Otani, R. Theory of High Temperature Strength of Materials; Metallurgiya: Moscow, Russia, 1986. (In Russian) [Google Scholar]
- Agureev, L.; Kostikov, V.; Savushkina, S.; Eremeeva, Z.; Lyakhovetsky, M. Preparation and Study of Composite Materials of the NiAl-Cr-Mo-Nanoparticles (ZrO2, MgAl2O4) System. Materials 2022, 15, 5822. [Google Scholar] [CrossRef]
- Rogal, Ł.; Szklarz, Z.; Bobrowski, P.; Kalita, D.; Garzeł, G.; Tarasek, A.; Szlezynger, M. Microstructure and Mechanical Properties of Al–Co–Cr–Fe–Ni Base High Entropy Alloys Obtained Using Powder Metallurgy. Met. Mater. Int. 2019, 25, 930–945. [Google Scholar] [CrossRef] [Green Version]
- Stryzhyboroda, O.; Witusiewicz, V.T.; Gein, S.; Röhrens, D.; Hecht, U. Phase Equilibria in the Al–Co–Cr–Fe–Ni High Entropy Alloy System: Thermodynamic Description and Experimental Study. Front. Mater. 2020, 7, 270. [Google Scholar] [CrossRef]
- Hecht, U.; Gein, S.; Stryzhyboroda, O.; Eshed, E.; Osovski, S. The BCC-FCC Phase Transformation Pathways and Crystal Orientation Relationships in Dual Phase Materials from Al-(Co)-Cr-Fe-Ni Alloys. Front. Mater. 2020, 7, 287. [Google Scholar] [CrossRef]
- Dong, Y.; Lu, Y.; Kong, J.; Zhang, J.; Li, T. Microstructure and mechanical properties of multi-component AlCrFeNiMox high-entropy alloys. J. Alloys Compd. 2013, 573, 96–101. [Google Scholar] [CrossRef]
- Mathiou, C.; Giorspyros, K.; Georgatis, E.; Poulia, A.; Karantzalis, A.E. NiAl-Cr-Mo Medium Entropy Alloys: Microstructural Verification, Solidification Considerations, and Sliding Wear Response. Materials 2020, 13, 3445. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.diva-portal.org/smash/get/diva2:447253/FULLTEXT01.pdf (accessed on 28 October 2022).
- Shun, T.-T.; Du, Y.-C. Age hardening of the Al0.3CoCrFeNiC0.1 high entropy alloy. J. Alloys Compd. 2009, 478, 269–272. [Google Scholar] [CrossRef]
- Fang, S.; Chen, W.; Fu, Z. Microstructure and mechanical properties of twinned Al0.5CrFeNiCo0.3C0.2 high entropy alloy processed by mechanical alloying and spark plasma sintering. Mater. Des. 2014, 54, 973–979. [Google Scholar] [CrossRef]
- Chen, M.-R.; Lin, S.-J.; Yeh, J.-W.; Chen, S.-K.; Huang, Y.-S.; Tu, C.-P. Microstructure and Properties of Al0.5CoCrCuFeNiTix (x = 0-2.0) High-Entropy Alloys. Jpn. Inst. Met. 2006, 47, 1395–1401. [Google Scholar]
- Stepanov, N.; Yurchenko, N.Y.; Tikhonovsky, M.; Salishchev, G. Effect of carbon content and annealing on structure and hardness of the CoCrFeNiMn-based high entropy alloys. J. Alloys Compd. 2016, 687, 59–71. [Google Scholar] [CrossRef]
- Jiang, L.; Cao, Z.; Jie, J.; Zhang, J.; Lu, Y.; Wang, T.; Li, T. Effect of Mo and Ni elements on microstructure evolution and mechanical properties of the CoFeNixVMoy high entropy alloys. J. Alloys Compd. 2015, 649, 585–590. [Google Scholar] [CrossRef]
- Zaitsev, A.A. Structure and properties of NiAl-Cr(Co,Hf) alloys prepared by centrifugal SHS casting followed by vacuum induction remelting. Part 2–Evolution of the structure and mechanical behavior at high temperature. Mater. Sci. Eng. A 2017, 690, 473–481. [Google Scholar] [CrossRef]
- Cao, Y. First-principles study of NiAl alloyed with Co. Comput. Mater. Sci. 2016, 111, 34–40. [Google Scholar] [CrossRef]
- Li, H. Site preferences and effects of X (X = Mn, Fe, Co, Cu) on the properties of NiAl: A firstprinciples study. Mod. Phys. Lett. B. 2016, 30, 1650133–29. [Google Scholar] [CrossRef]
- Ponomareva, V.; Vekilova, Y.; Abrikosov, I. Effect of Re content on elastic properties of B2 NiAl from ab initio calculations. J. Alloys Compd. 2014, 586, 274–278. [Google Scholar] [CrossRef] [Green Version]
- Jha, S.C.; Ray, R. Carbide-dispersion-strengthened B2 NiAl. Mater. Sci. Eng. A 1989, 119, 103–111. [Google Scholar] [CrossRef]
- Logunov, A.V. Heat Resistant Nickel Alloys for Gas Turbine Blades and Discs; Mosuch: Moscow, Russia, 2018; 590p. (In Russian) [Google Scholar]
- Chen, L.; Han, Y. The microstructure and compressive properties in NiAl(Co) alloys by HPXD technique. Mater. Sci. Eng. A 2002, 329–331, 725–728. [Google Scholar] [CrossRef]
- Chung, D.H.; Liu, X.D.; Yang, Y. Fracture of sigma phase containing CoeCreNieMo medium entropy alloys. J. Alloys Comp. 2020, 846, 156–189. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Yi, H.; Qi, W.; Peng, Q. Effect of Molybdenum Additives on Corrosion Behavior of (CoCrFeNi)100−xMox High-Entropy Alloys. Entropy 2018, 20, 908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasan, H.; Ozcan, A. New Eutectic High-Entropy Alloys Based on Co–Cr–Fe–Mo–Ni–Al: Design, Characterization and Mechanical Properties. Met. Mater. Int. 2020, 26, 1152–1167. [Google Scholar] [CrossRef]
- Liu, W.H.; Lu, Z.P.; He, J.Y.; Luan, J.H.; Wang, Z.J.; Liu, B.; Liu, Y.; Chen, M.W.; Liu, C.T. Ductile CoCrFeNiMox high entropy alloys strengthened by hard intermetallic phases. Acta Mater. 2016, 116, 332–342. [Google Scholar] [CrossRef]
- Shun, T.-T.; Chang, L.-Y.; Shiu, M.-H. Age-hardening of the CoCrFeNiMo0.85 high-entropy alloy. Mater. Charact. 2013, 81, 92–96. [Google Scholar] [CrossRef]
- Cai, B.; Liu, B.; Kabra, S.; Wang, Y.; Yan, K.; Lee, P.D.; Liu, Y. Deformation mechanisms of Mo alloyed FeCoCrNi high entropy alloy: In situ neutron diffraction. Acta Mater. 2017, 127, 2471–2480. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.Y.; Sheu, T.S.; Yeh, J.W.; Chen, S.K. Effect of iron content on wear behavior of AlCoCrFexMo0.5Ni high-entropy alloys. Wear 2010, 268, 653. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Wang, W.R.; Tang, W.Y.; Chen, S.K.; Yeh, J.W. Microstructure and Mechanical Properties of New AlCoxCrFeMo0.5Ni High-Entropy Alloys. Adv. Eng. Mater. 2010, 12, 44–49. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Juan, C.C.; Wang, W.R.; Sheu, T.S.; Yeh, J.W.; Chen, S.K. On the superior hot hardness and softening resistance of AlCoCrxFeMo0.5Ni high-entropy alloys. Mater. Sci. Eng. A 2011, 528, 3581–3588. [Google Scholar] [CrossRef]
- Juan, C.C.; Hsu, C.Y.; Tsai, C.W.; Wang, W.R.; Sheu, T.S.; Yeh, J.W.; Chen, S.K. On microstructure and mechanical performance of AlCoCrFeMo0.5Nix high-entropy alloys. Intermetallics 2013, 32, 401–407. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.; Shen, J.; Zhang, Y.; Xu, H.; Ge, Y.; Fu, H. A true change of NiAl-Cr(Mo) eutectic lamellar structure during high temperature treatment. J. Alloys Compd. 2018, 732, 124–128. [Google Scholar] [CrossRef]
- Liu, Z.; Ning, H.; Lin, Z.; Wang, D. Influence of Spark Plasma Sintering Parameters on the Microstructure and Room-Temperature Mechanical Properties of NiAl-28Cr-5.5Mo-0.5Zr Alloy. Acta Metall. Sin. 2021, 57, 1579–1587. [Google Scholar]
- Röhrens, D.; Navaeilavasani, N.; Stryzhyboroda, O.; Swientek, F.; Pavlov, P.; Meister, D.; Genau, A.; Hecht, U. Microstructure and Mechanical Properties of BCC-FCC Eutectics in Ternary, Quaternary and Quinary Alloys from the Al-Co-Cr-Fe- Ni System. Front. Mater. 2020, 7, 324. [Google Scholar] [CrossRef]
- Wang, D.; Ning, H.; Wang, B.; Liu, W.; Yuan, S. Fabrication of a NiAl–Cr(Mo) eutectic alloy with network microstructure for high-temperature strengthening. Mater. Sci. Eng. A 2022, 835, 142628. [Google Scholar] [CrossRef]
- Demirtas, H.; Gungor, A. Effect of alloying elements on the microstructure and Mechanical properties of NiAl-Cr(Mo) eutectic alloy. International scientific journal "materials science. Non-Equilib. Phase Transform. 2015, 1, 25–29. [Google Scholar]
- Zhu, J.M.; Fu, H.M.; Zhang, H.F.; Wang, A.M.; Li, H.; Hu, Z.Q. Microstructures and compressive properties of multicomponent AlCoCrFeNiMox alloys. Mater. Sci. Eng. A 2010, 527, 6975–6979. [Google Scholar] [CrossRef]
- Kimura, Y.; Miura, S.; Suzuki, T.; Mishima, Y. Microstructure and mechanical properties of two-phase alloys based on the B2-type intermetallic compound CoAl in the Co-Al-Ni Ternary system. Mat. Trans. 1972, 35, 800–807. [Google Scholar] [CrossRef] [Green Version]
- Begunov, A.I.; Kuzmin, M.P. Thermodynamic Stability of Intermetallic Compounds in Technical Aluminum. J. Sib. Fed. Univ. Eng. Technol. 2014, 2, 132–137. [Google Scholar]
- Onderka, B.; Sypień, A.; Wierzbicka-Miernik, A.; Czeppe, T.; Zabdyr, L.A. Heat capacities of some binary intermetallic compounds in Al-Fe-Ni-Ti system. Arch. Metall. Mater. 2010, 55, 435–439. [Google Scholar]
- Yao, Z.-H.; Ruan, Y.-T.; Dong, J.-X.; Yu, Q.-Y.; Zhang, S.-Q.; Chen, X. Study on Gamma Prime and Carbides of Alloy A286 by Traditional Thermodynamic Calculation. High Temp. Mater. Proc. 2018, 37, 495–507. [Google Scholar] [CrossRef]
- Geramifard, G. Thermodynamic Investigation of Oxidation of Nial-(Cr, Mo) Alloys Used for Manufacturing Metal Matrix Composites by Directional Solidification; Karlsruher Institut für Technologie: Karlsruhe, Germany, 2021. [Google Scholar]
- Zhang, Z.; Li, M.; Flores, K.; Mishra, R. Machine learning formation enthalpies of intermetallics. J. Appl. Phys. 2020, 128, 105103. [Google Scholar] [CrossRef]
- Akande, T.; Matthew-Ojelabi, F.; Agunbiade, G.; Faweya, E. Thermodynamic properties of V, Cr, Mo, and Fe metals and their binary alloys. Turk. J. Phys. 2019, 43, 606–617. [Google Scholar] [CrossRef]
- Turchi, P.E.A.; Kaufman, L.; Liu, Z.-K. Modeling of Ni–Cr–Mo based alloys: Part I—Phase stability. Calphad 2006, 30, 70–87. [Google Scholar] [CrossRef]
- Cutler, R.W. The 1200 °C Isothermal Sections of the Ni-Al-Cr and the Ni-Al-Mo Ternary Phase. Master’s Thesis, The Ohio State University, Columbus, OH, USA, 2011. [Google Scholar]
- Peng, J. Experimental Investigation and Thermodynamic Modeling of the Al-Cr-Mo-Ni System and Its Subsystems. Ph.D. Thesis, Karlsruher Institut für Technologie, Karlsruhe, Germany, 2016. [Google Scholar]
- Sigli, C.; Kosugi, M.; Sanchez, J.M. Calculation of Thermodynamic Properties and Phase Diagrams of Binary Transition-Metal Alloys. Phys. Rev. Lett. 1986, 57, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Huang, Z.; Zhao, Z.; Hu, C. First-principles study on the structural, elastic, and thermodynamics properties of Ni3X (X: Al, Mo, Ti, Pt, Si, Nb, V, and Zr) intermetallic compounds. Appl. Phys. A 2014, 116, 1161–1172. [Google Scholar] [CrossRef]
- Lide, D.R.; Kehiaian, H.V. CRC Handbook of Thermophysical and Thermochemical Data; Standard Reference Data National Institute of Standards and Technology; CRC Press: Boca Raton, FL, USA; New York, NY, USA, 1994. [Google Scholar]
- Oikawa, K.; Kattner, U.R.; Sato, J.; Omori, T.; Jiang, M.; Anzai, K.; Ishida, K. Experimental Determination and Thermodynamic Assessment of Phase Equilibria in the CoMo System. Mater. Trans. 2012, 11, M2012149. [Google Scholar]
- Rajan, K. Thermodynamic assessment of heat treatments for a Co-Cr-Mo alloy. J. Mater. Sci. 1983, 18, 257–264. [Google Scholar] [CrossRef]
- Jindal, V. A thermodynamic assessment of the Cr–Mo system using CE-CVM. Calphad 2013, 43, 80–85. [Google Scholar] [CrossRef]
- Colinet, C.; Tedenac, J.-C. Enthalpies of formation of TM–X compounds(X-Al, Ga,Si,Ge,Sn). Comparison of ab-initio values and experimental data. Calphad Comput. Coupling Phase Diagr. Thermochem. 2016, 54, 16–34. [Google Scholar] [CrossRef]
- Rank, M.; Gotcu, P.; Franke, P.; Seifert, H.J. Thermodynamic investigations in the Al-Fe system: Heat capacity measurements of three intermetallic phases. Intermetallics 2018, 94, 73–82. [Google Scholar] [CrossRef]
- Yao, Q.; Shang, S.-L.; Wang, K.; Liu, F.; Wang, Y.; Wang, Q.; Lu, T.; Liu, Z.K. Phase stability, elastic, and thermodynamic properties of the L12 (Co,Ni)3(Al,Mo,Nb) phase from first-principles calculations. J. Mater. Res. 2017, 32, 2100–2108. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.-K.; Chen, L.-Q. Thermodynamic properties of Al, Ni, NiAl, and Ni3Al from first-principles calculations. Acta Mater. 2004, 52, 2665–2671. [Google Scholar] [CrossRef]
- Asadikiya, M.; Drozd, V.; Yang, S.; Zhong, Y. Enthalpies and Elastic Properties of Ni-Co Binary System by ab initio Calculations and an Energy Comparison with the CALPHAD Approach. Mater. Today Commun. 2020, 23, 100905. [Google Scholar] [CrossRef]
- Liu, X.L.; Gheno, T.; Lindahl, B.B.; Lindwall, G.; Gleeson, B.; Liu, Z.-K. First-Principles Calculations, Experimental Study, and Thermodynamic Modeling of the Al-Co-Cr System. PLoS ONE 2015, 10, e0121386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuzmin, M.P.; Begunov, A.I. Priblizhonnyye raschoty termodinamicheskikh kharakteristik intermetallicheskih soedinenij na osnove alyuminiya. Vestn. IrGTU 2013, 72, 98–101. (In Russian) [Google Scholar]
- Moiseyev, G.K.; Vatolin, N.A.; Marshuk, L.A.; Il’inykh, N.I. Temperaturnyye Zavisimosti Privedennoy Energii Gibbsa Nekotorykh Neorganicheskikh Veshchestv (Al’ternativnyy Bank Dannykh ACTPA.OWN); UrO RAN: Yekaterinburg, Russia, 1997; 231p. (In Russian) [Google Scholar]
- Khina, B.B. Thermodynamic Models of Condensed Phases and Their Application in Materials Science. Adv. Mater. Technol. 2018, 1, 41–47. [Google Scholar] [CrossRef]
- Kubashevskij, O.; Olkokk, S.B. Metallurgicheskaja Termohimija [Metallurgical Thermochemistry]; Metallurgija: Moscow, Russia, 1982; 392p. (In Russian) [Google Scholar]
- Moiseyev, G.K. Otsenka termokhimicheskikh svoystv i termodinamicheskikh funktsiy nekotorykh letuchikh i kondensirovannykh klasterov shchelochnykh metallov (SHCHM). RASPL 2003, 4, 59–84. (In Russian) [Google Scholar]
- Gurvich, L.V.; Bergman, G.A.; Veitz, I.V.; Medvedev, V.A.; Khachkuruzov, G.A.; Yungman, V.S. Thermodynamic Properties of Individual Substances; Glushko, V.P., Ed.; High Temperature Institute: Moscow, Russia, 1982; Volume 1–4. [Google Scholar]
- Berdnikov, V.I.; Gudim, Y.A. Termodinamicheskiye svoystva binarnykh metallichskikh sistem, soderzhashchikh intermetallidnyye soyedineniya. Izv. Vyssh. Uch. Zaved. Chern. Met. 2013, 5, 37–41. (In Russian) [Google Scholar]
- Maier, C.G.; Kelly, K.K. An equation for the representation of high temperature heat content data. J. Am. Chem. Soc. 1932, 54, 3243–3246. [Google Scholar] [CrossRef]
- Morachevskiy, A.G.; Sladkov, I.B. Termodinamicheskie Raschety v Metallurgii: Spravochnik (Thermodynamic Calculations in Metallurgy: Reference Book); Metallurgia: Moscow, Russia, 1993; 304p. [Google Scholar]
- Perry, R.H.; Green, D.W. (Eds.) Perry’s Chemical Engineer’s Handbook, 7th ed.; McGraw-Hill: New York, NY, USA, 1997. [Google Scholar]
- Ilinykh, N.I.; Krivorogova, A.S.; Gelchinsky, B.R.; Ilinykh, S.A.; Leontiev, L.I. Thermodynamic modeling of Ni-Cr-B-C-Si system in "air+propane" atmosphere. J. Phys. Conf. Ser. 2021, 1954, 012012. [Google Scholar] [CrossRef]
- Gelchinski, B.R.; Balyakin, I.A.; Ilinykh, N.I.; Rempel, A.A. Analysis of the Probability of Synthesizing High-Entropy Alloys in the Systems Ti-Zr-Hf-V-Nb, Gd-Ti-Zr-Nb-Al, and Zr-Hf-V-Nb-Ni. Phys. Mesomech. 2021, 24, 701–706. [Google Scholar] [CrossRef]
- Khil’ko, A.A.; Simonyan, L.M.; Glinskaya, I.V. Determining the composition of electrosmelting dust. Steel Transl. 2014, 44, 1–5. [Google Scholar] [CrossRef]
- Vatolin, N.L.; Moiseev, G.K.; Trusov, B.G. Thermodynamic Simulation in High-Temperature Inorganic Systems; Metallurgia: Moscow, Russia, 1994; 352p. [Google Scholar]
- Williams, J.C. Mixing and segregation in powders. In Principles of Powder Technology; Martin, R., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 1992; pp. 71–90. [Google Scholar]
- Hersey, J.A.; Thiel, W.J.; Yeung, C.C. Partially ordered randomized powder mixtures. Powder Technol. 1979, 24, 251–256. [Google Scholar] [CrossRef]
- Zimon, A.D.; Pavlov, A.N. Colloidal Chemistry of Nanoparticles; Scientific World: Singapore, 2012; 224p. (In Russian) [Google Scholar]
- Kostikov, V.I.; Agureev, L.E.; Eremeeva, Z.V. Development of nanoparticle-hardened aluminum composites for rocket and space technology. Russ. J. Non-Ferr. Met. 2015, 56, 325–328. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, H.; Yee, J.K.; Mo, X.; Topping, T.; Lavernia, E.J.; Schoenung, J.M. Toughening of aluminum matrix nanocomposites via spatial arrays of boron carbide spherical nanoparticles. Acta Mater. 2016, 103, 128–140. [Google Scholar] [CrossRef] [Green Version]
- Ponomareva, A.V.; Isaev, E.; Vekilov, Y.K.; Abrikosov, I. Site preference and effect of alloying on elastic properties of ternary B2 NiAl-based alloys. Phys. Rev. B. Condens. Matter Mater. Phys. 2012, 85, 144117. [Google Scholar] [CrossRef] [Green Version]
- Lurie, S.; Volkov-Bogorodskiy, D.; Solyaev, Y.; Rizahanov, R.; Agureev, L. Multiscale modelling of aluminium-based metal-matrix composites with oxide nanoinclusions. Comput. Mater. Sci. 2016, 116, 62–73. [Google Scholar] [CrossRef]






| Alloys Composition (HT—Heat Treated) | Material Type | Phase Structure | Preparation Method | Mechanical Properties at 25 °C | References |
|---|---|---|---|---|---|
| AlCrFe(0.6;1)CoNiMo0.5 | HEA | BCC + σ | arc smelting | 750 HV (Fe 0.6) 725 HV(Fe 1) | [50] |
| AlCrFeCo(1;1.5)NiMo0.5 | HEA | 780 HV(Co1) 730 HV(Co1.5) | [51] | ||
| AlCrFeCo2NiMo0.5 | HEA | FCC + BCC + σ | 600 HV | [51] | |
| AlCr(1;1.5;2)FeCoNiMo0.5 | HEA | BCC + σ | 725 HV(Cr1) 810 HV(Cr1) 860 HV(Cr2) | [52,53] | |
| AlCrCoFeMo0.5Ni(1.5;2) | HEA | FCC + BCC + σ | - | [53] | |
| NiAl-28Cr-5.5Mo-0.5Hf at.% | eutectic alloy | Cr(Mo)—plates, NiAl, Ni2AlHf (Heusler) | induction melting | - | [54] |
| NiAl-20Cr-20Mo at.% | eutectic alloy | BCC-B2 NiAl, BCC-Cr, AlMo3, NiMo orth., oxide | arc melting | 680 HV | [30] |
| NiAl-15Cr-25Mo at.% | eutectic alloy | BCC-B2 NiAl, BCC-Cr, NiMo orth. | 650 HV | ||
| NiAl-28Cr-5.5Mo-0.5Zr at.% | eutectic alloy | NiAl, Cr(Mo) | SPS | YS 1321 MPa UCS 2360 MPa plasticity strain 0.313 | [55] |
| NiAl-13Cr-13Mo, at.% (modified with 0.01 wt.% ZrO2) | eutectic alloy | NiAl, (CrMo), Al5Mo | MA + SPS | bending strength 210 MPa (at 20 °C) 475 MPa (at 700 °C). | [25] |
| Ni34.4Fe16.4Co16.4Cr16.4Al16.4 HT 750 °C (at.%) | HEA | cellular/“dendritic” eutectic, BCC-B2, FCC-A1 | arc melting | flexural yield strength reached 1291 MPa; ultimate flexure strength 1968 MPa | [56] |
| NiAl–28Cr-5.5Mo-0.5Zr (at.%) | eutectic alloy | NiAl, Cr(Mo), Heusler | metallic powders were obtained by atomization technique, then hot pressing | UTS 405 MPa (at 1000 °C) 1300 MPa (25 °C) | [57] |
| Ni-29Al-28Cr-6Mo-4Ti HT at 1300 °C | eutectic alloy | NiAl, Cr(Mo), Ni2AlTi | vacuum arc melting | UCS 3430 MPa; YS 1549 MPa 340 MPa (at 1000 °C) YS 293 MPa 401HB εy 3.6% εu 27.71% | [58] |
| NiAl-5.5Co-11Cr-8 Mo at.% | eutectic alloy | NiAl, (Ni,Cr,Co)3Mo3C, Ni3Al, Cr(Mo) | centrifugal SHS casting | UCS 1728 MPa YS 1566 MPa degree of plastic deformation εpd 0.95% | [21] |
| CoCrFeNiMo0.3 HT 1173K, 5 h or 2 days | HEA | FCC + σ (5 h) FCC + σ+μ (2 days) | arc melting | UTS/YS/El% 709/305 MPa/49.3 (as cast) 1186/815 MPa/18.9 (1123 K, 1 h), 1042/646 MPa/32.5 (1173 K, 5 h) | [47] |
| CoCrFeMo0.85Ni | HEA powder alloy | FCC, μ, σ | gas atomization | - | [22] |
| CoCrFeNiMo0.85 | HEA | FCC-A1, σ, μ-D85 (same as Co7Mo6) | arc melting | as the Mo content increases from 0 to 0.85, the yield stress and compressive strength rise from 136 MPa and 871 MPa to 929 MPa and 1441 MPa. fracture strain of 21 %, | [23] |
| AlCoCrFeNiMo0.5 | HEA | Only simple BCC solid solution structure and α phase are identified. | arc melting | yield strength reached 2757 MPa when Mo content was 0.5; Compressive strength max (MPa) 3036, plastic strain 2.5% | [59] |
| Compound | Cp (T) | T, K | J/(kg·K) | , J/mol | , J/(mol·K) | J/mol | , J/(mol·K) | ||
|---|---|---|---|---|---|---|---|---|---|
| a | b | c | |||||||
| TCP-Co2Mo3 | 114.265 | 0.051 | −6.314 | 1273–1893 | 113.94 | −7000 | 146.2 | 15,586.5 | 325.078 |
| TCP-Co7Mo6 | 292.796 | 0.145 | −12.627 | 673–1783 | 290.106 | −7000 | 382.5 | 41,135.9 | 385.316 |
| Co9Mo2 | 240.41 | 0.141 | −4.209 | 1285–1473 | 240.336 | −3000 | 327.8 | 36,509.6 | 701.215 |
| Co3Mo | 13.3 | 32.4 | −0.3 | 673–1498 | 88.7 | −5000 | 118.8 | 13,220.5 | - |
| Compound | Range of T, K | Φ = φ1 + φ2lnx + φ3x−2 + φ4x−1 + φ5x + φ6x2 + φ7x3, (x = T × 10−4, K) | ||||||
|---|---|---|---|---|---|---|---|---|
| φ1 | φ2 | φ3 | φ4 | φ5 | φ6 | φ7 | ||
| TCP-Co2Mo3 | 1273–1893 | 267.199 | 114.2652 | −0.00316 | 13.0411 | 0.257 | - | - |
| TCP-Co7Mo6 | 673–1783 | 878.350 | 292.7961 | −0.006315 | 15.7826 | 0.727 | - | - |
| Co9Mo2 | 1285–1473 | 580.361 | 240.41021 | −0.00211 | 27.2862 | 0.708 | - | - |
| Co3Mo | 673–1498 | 112.05 | 13.3181 | −0.030902 | 1.26433 | 162.003 | 5.02 | 666.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agureev, L.; Savushkina, S.; Laptev, I.; Vysotina, E.; Lyakhovetsky, M. Development of Materials Based on the NiAlCrMoCo System Reinforced with ZrO2 Nanoparticles. Metals 2022, 12, 2014. https://doi.org/10.3390/met12122014
Agureev L, Savushkina S, Laptev I, Vysotina E, Lyakhovetsky M. Development of Materials Based on the NiAlCrMoCo System Reinforced with ZrO2 Nanoparticles. Metals. 2022; 12(12):2014. https://doi.org/10.3390/met12122014
Chicago/Turabian StyleAgureev, Leonid, Svetlana Savushkina, Ivan Laptev, Elena Vysotina, and Maxim Lyakhovetsky. 2022. "Development of Materials Based on the NiAlCrMoCo System Reinforced with ZrO2 Nanoparticles" Metals 12, no. 12: 2014. https://doi.org/10.3390/met12122014
APA StyleAgureev, L., Savushkina, S., Laptev, I., Vysotina, E., & Lyakhovetsky, M. (2022). Development of Materials Based on the NiAlCrMoCo System Reinforced with ZrO2 Nanoparticles. Metals, 12(12), 2014. https://doi.org/10.3390/met12122014







