Review of Recent Research on AlCoCrFeNi High-Entropy Alloy
Abstract
:1. The History of High-Entropy Alloys
2. The Definition of High-Entropy Alloys
3. The Four “Core Effects”
3.1. High-Entropy Effect
3.2. Sluggish Diffusion Effect
3.3. Severe Lattice Distortion Effect
3.4. Coctail Effect
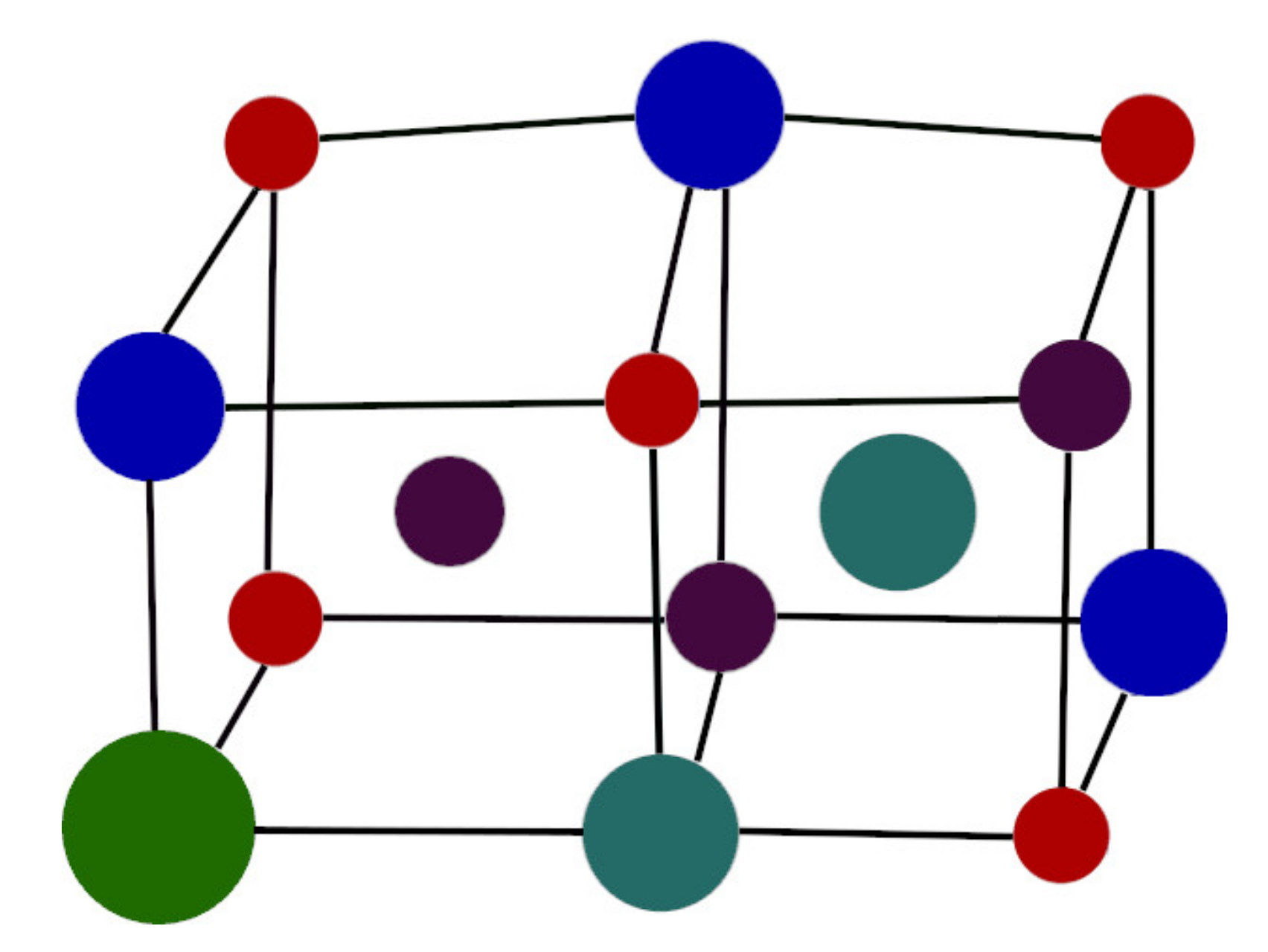
4. The AlCoCrFeNi High-Entropy Alloy
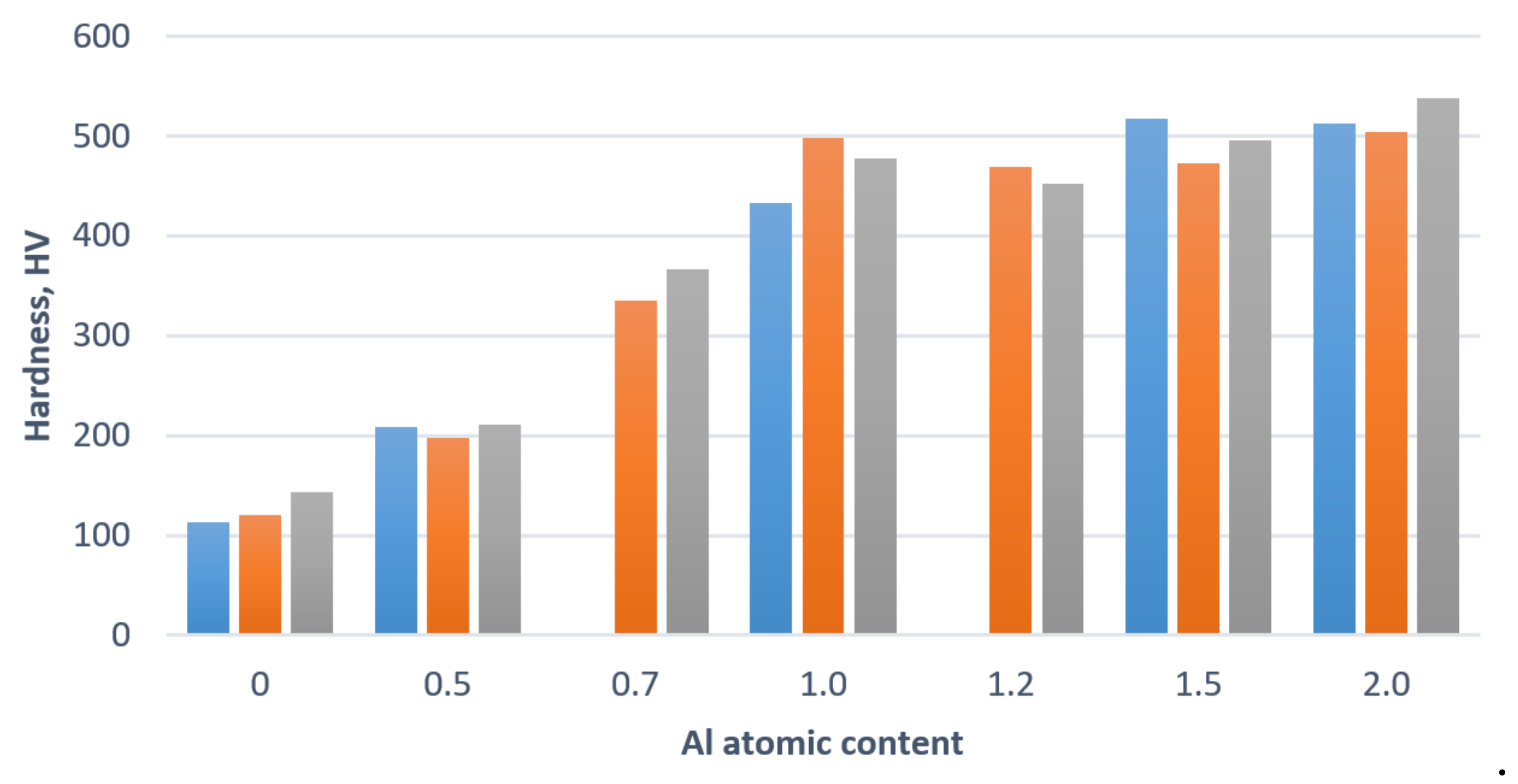
5. The AlxCoCrFeNi High-Entropy Alloys
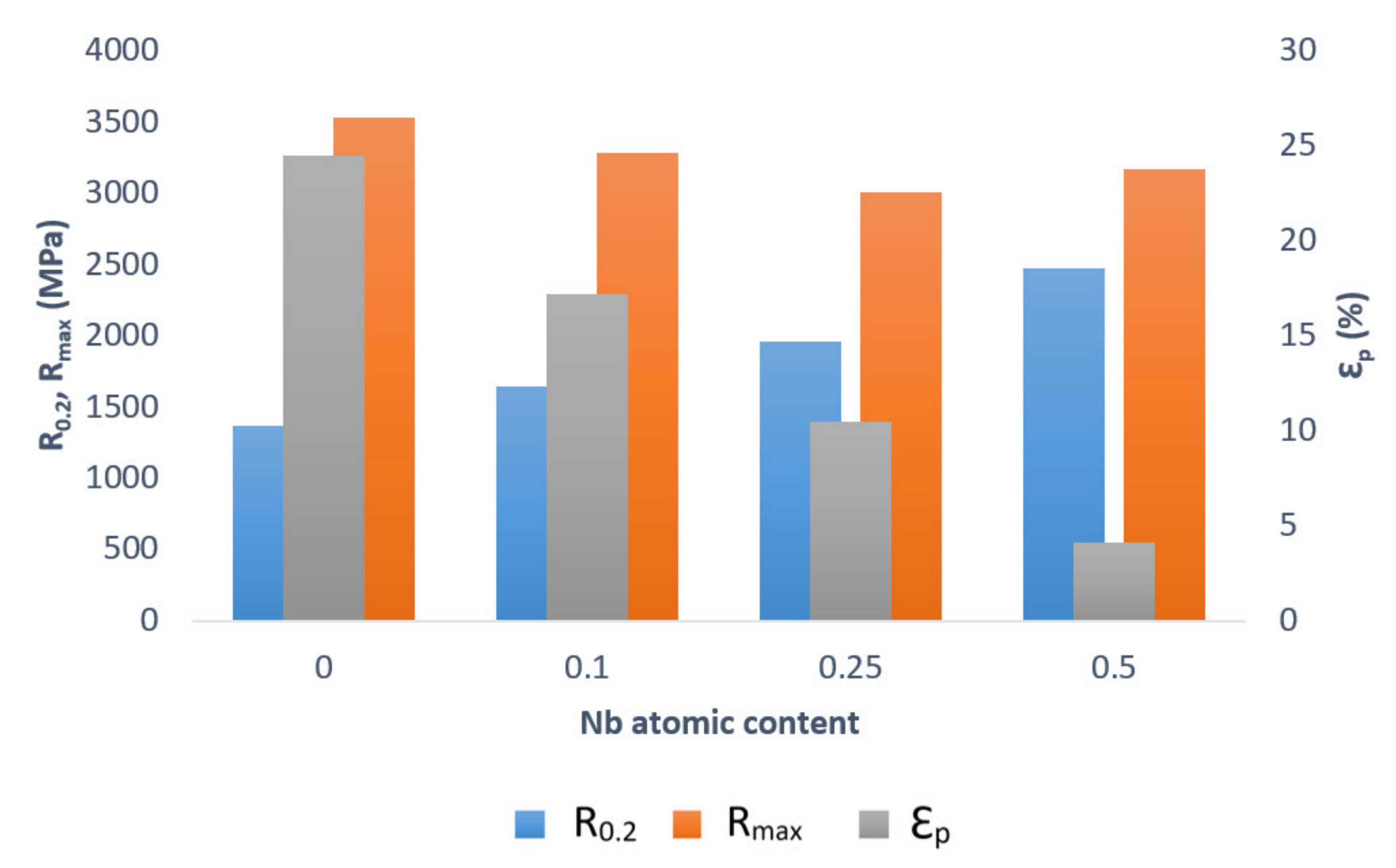
6. Alloying Additives for the AlCoCrFeNi Alloy
7. Manufacturing Methods
8. Effect of Annealing on the Properties of the AlCoCrFeNi Alloy
9. AlCoCrFeNi Coatings
10. Applications of the High-Entropy AlCoCrFeNi Alloy
11. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, B.; Lee, J.; Ryu, H.J.; Hong, S.H. Ultra-High Strength WNbMoTaV High-Entropy Alloys with Fine Grain Structure Fabricated by Powder Metallurgical Process. Mater. Sci. Eng. A 2018, 712, 616–624. [Google Scholar] [CrossRef]
- Raza, A.; Kang, B.; Lee, J.; Ryu, H.J.; Hong, S.H. Transition in Microstructural and Mechanical Behavior by Reduction of Sigma-Forming Element Content in a Novel High Entropy Alloy. Mater. Des. 2018, 145, 11–19. [Google Scholar] [CrossRef]
- Murty, B.S.; Yeh, J.-W.; Ranganathan, S. A Brief History of Alloys and the Birth of High-Entropy Alloys. In High-Entropy Alloys; Butterworth-Heinemann: Oxford, UK, 2014; pp. 1–12. [Google Scholar]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural Development in Equiatomic Multicomponent Alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Zhang, Y. High-Entropy Materials: A Brief Introduction; Springer: Singapore, 2019; ISBN 9789811385254. [Google Scholar]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-Entropy Alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Tsai, M.-H. Physical Properties of High Entropy Alloys. Entropy 2013, 15, 5338–5345. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.M.; Fu, H.M.; Zhang, H.F.; Wang, A.M.; Li, H.; Hu, Z.Q. Synthesis and Properties of Multiprincipal Component AlCoCrFeNiSix Alloys. Mater. Sci. Eng. A 2010, 527, 7210–7214. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Zhang, Y.; Wang, Y.L.; Chen, G.L. Solid Solution Alloys of AlCoCrFeNiTix with Excellent Room-Temperature Mechanical Properties. Appl. Phys. Lett. 2007, 90, 181904. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Juan, C.C.; Wang, W.R.; Sheu, T.S.; Yeh, J.W.; Chen, S.K. On the Superior Hot Hardness and Softening Resistance of AlCoCrxFeMo0.5Ni High-Entropy Alloys. Mater. Sci. Eng. A 2011, 528, 3581–3588. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Zhang, S.; Tang, H.; Zhang, A. Microstructure and oxidation behavior of new refractory high entropy alloys. J. Alloys Compd. 2014, 583, 162–169. [Google Scholar] [CrossRef]
- Chuang, M.H.; Tsai, M.H.; Wang, W.R.; Lin, S.J.; Yeh, J.W. Microstructure and Wear Behavior of AlxCo 1.5CrFeNi1.5Tiy High-Entropy Alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- Lee, C.P.; Chen, Y.Y.; Hsu, C.Y.; Yeh, J.W.; Shih, H.C. The Effect of Boron on the Corrosion Resistance of the High Entropy Alloys Al0.5CoCrCuFeNiBx. J. Electrochem. Soc. 2007, 154, C424. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Prediction of High-Entropy Stabilized Solid-Solution in Multi-Component Alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Borkar, T.; Gwalani, B.; Choudhuri, D.; Mikler, C.V.; Yannetta, C.J.; Chen, X.; Ramanujan, R.V.; Styles, M.J.; Gibson, M.A.; Banerjee, R. A Combinatorial Assessment of AlxCrCuFeNi2 (0 <x <1.5) Complex Concentrated Alloys: Microstructure, Microhardness, and Magnetic Properties. Acta Mater. 2016, 116, 63–76. [Google Scholar] [CrossRef]
- Ng, C.; Guo, S.; Luan, J.; Shi, S.; Liu, C.T. Entropy-Driven Phase Stability and Slow Diffusion Kinetics in an Al0.5CoCrCuFeNi High Entropy Alloy. Intermetallics 2012, 31, 165–172. [Google Scholar] [CrossRef]
- Gao, M.C.; Yeh, J.-W.; Liaw, P.K.; Zhang, Y. High-Entropy Alloys: Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Zhang, W.; Liaw, P.K.; Zhang, Y. Science and Technology in High-Entropy Alloys. Sci. China Mater. 2018, 61, 2–22. [Google Scholar] [CrossRef] [Green Version]
- Yeh, J.W.; Chen, Y.L.; Lin, S.J.; Chen, S.K. High-Entropy Alloys—A New Era of Exploitation. Mater. Sci. Forum 2007, 560, 1–9. [Google Scholar] [CrossRef]
- Yin, X.; Xu, S. Properties and Preparation of High Entropy Alloys. In International Conference on Materials Applications and Engineering 2017 (ICMAE2017); EDP Sciences: Les Ulis, France, 2018; Volume 142. [Google Scholar]
- Zhou, Y.; Zhang, Y.; Wang, Y.; Chen, G. Microstructure and Compressive Properties of Multicomponent Alx(TiVCrMnFeCoNiCu)100-x High-Entropy Alloys. Mater. Sci. Eng. A 2007, 454–455, 260–265. [Google Scholar] [CrossRef]
- Çengel, Y.A.; Boles, M.A. Thermodynamics: An Engineering Approach (SI Units). Available online: https://public.ebookcentral.proquest.com/choice/publicfullrecord.aspx?p=5115249 (accessed on 13 August 2021).
- Tien, C.L.; Lienhard, J.H. Statistical Thermodynamics; HRW Series in Mechanical Engineering; Taylor & Francis: Melbourne, Australia, 1979; ISBN 978-0-89116-828-7. [Google Scholar]
- Zhang, Y.; Xing, Q. High Entropy Alloys: Manufacturing Routes. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 978-0-12-803581-8. [Google Scholar]
- Miracle, D.; Miller, J.; Senkov, O.; Woodward, C.; Uchic, M.; Tiley, J. Exploration and Development of High Entropy Alloys for Structural Applications. Entropy 2014, 16, 494–525. [Google Scholar] [CrossRef]
- Carroll, R.; Lee, C.; Tsai, C.W.; Yeh, J.W.; Antonaglia, J.; Brinkman, B.A.W.; Leblanc, M.; Xie, X.; Chen, S.; Liaw, P.K.; et al. Experiments and Model for Serration Statistics in Low-Entropy, Medium-Entropy, and High-Entropy Alloys. Sci. Rep. 2015, 5, 16997. [Google Scholar] [CrossRef] [Green Version]
- Pickering, E.J.; Jones, N.G. High-Entropy Alloys: A Critical Assessment of Their Founding Principles and Future Prospects. Int. Mater. Rev. 2016, 61, 183–202. [Google Scholar] [CrossRef] [Green Version]
- Yeh, J. Recent Progress in High-Entropy Alloys. Ann. Chim. Sci. Mat. 2006, 31, 633–648. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A Critical Review of High Entropy Alloys and Related Concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Yeh, J.-W. Physical Metallurgy of High-Entropy Alloys. JOM 2015, 67, 2254–2261. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Yeh, J.-W. High-Entropy Alloys: A Critical Review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, X.; Wang, W.; Liu, B.; Lv, Y.; Yang, W.; Xu, D.; Liu, Y. A Review on Fundamental of High Entropy Alloys with Promising High–Temperature Properties. J. Alloys Compd. 2018, 760, 15–30. [Google Scholar] [CrossRef]
- Guo, S.; Hu, Q.; Ng, C.; Liu, C.T. More than Entropy in High-Entropy Alloys: Forming Solid Solutions or Amorphous Phase. Intermetallics 2013, 41, 96–103. [Google Scholar] [CrossRef]
- Chenzhong, Y.A.O.; Huixuan, M.A.; Yexiang, T. Electrochemical Preparation and Magnetic Study of Amorphous Nanostructural Nd-Fe-Co-Ni-Mn High Entropy Alloy Film. Chin. J. Appl. Chem. 2011, 28, 1189. [Google Scholar] [CrossRef]
- Senkov, O.; Wilks, G.; Scott, J.; Miracle, D. Mechanical Properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 Refractory High Entropy Alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Wang, C.-W.; Tsai, C.-W.; Shen, W.-J.; Yeh, J.-W.; Gan, J.-Y.; Wu, W.-W. Thermal Stability and Performance of NbSiTaTiZr High-Entropy Alloy Barrier for Copper Metallization. J. Electrochem. Soc. 2011, 158, H1161. [Google Scholar] [CrossRef]
- Shun, T.-T.; Hung, C.-H.; Lee, C.-F. Formation of Ordered/Disordered Nanoparticles in FCC High Entropy Alloys. J. Alloys Compd. 2010, 493, 105–109. [Google Scholar] [CrossRef]
- Tsai, K.-Y.; Tsai, M.-H.; Yeh, J.-W. Sluggish Diffusion in Co–Cr–Fe–Mn–Ni High-Entropy Alloys. Acta Mater 2013, 61, 4887–4897. [Google Scholar] [CrossRef]
- Yeh, J.-W. Alloy Design Strategies and Future Trends in High-Entropy Alloys. JOM 2013, 65, 1759–1771. [Google Scholar] [CrossRef]
- Vaidya, M.; Trubel, S.; Murty, B.; Wilde, G.; Divinski, S. Ni Tracer Diffusion in CoCrFeNi and CoCrFeMnNi High Entropy Alloys. J. Alloys Compd. 2016, 688, 994–1001. [Google Scholar] [CrossRef]
- Miracle, D. High-Entropy Alloys: A Current Evaluation of Founding Ideas and Core Effects and Exploring “Nonlinear Alloys”. JOM 2017, 69, 2130–2136. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhou, Y.; Hui, X.; Wang, M.; Chen, G. Minor Alloying Behavior in Bulk Metallic Glasses and High-Entropy Alloys. Sci. China Ser. G-Phys. Mech. Astron. 2008, 51, 427–437. [Google Scholar] [CrossRef]
- Ranganathan, S. Alloyed Pleasures: Multimetallic Cocktails. Curr. Sci. 2003, 85, 1404–1406. [Google Scholar]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and Properties of High-Entropy Alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Wang, F.J.; Zhang, Y.; Chen, G.L.; Davies, H.A. Cooling Rate and Size Effect on the Microstructure and Mechanical Properties of AlCoCrFeNi High Entropy Alloy. J. Eng. Mater. Technol. 2009, 131. [Google Scholar] [CrossRef]
- Chou, H.-P.; Chang, Y.-S.; Chen, S.-K.; Yeh, J.-W. Microstructure, Thermophysical and Electrical Properties in AlxCoCrFeNi (0≤x≤2) High-Entropy Alloys. Mater. Sci. Eng. B 2009, 163, 184–189. [Google Scholar] [CrossRef]
- Wang, X.F.; Zhang, Y.; Qiao, Y.; Chen, G.L. Novel Microstructure and Properties of Multicomponent CoCrCuFeNiTix Alloys. Intermetallics 2007, 15, 357–362. [Google Scholar] [CrossRef]
- Wang, Y.P.; Li, B.S.; Ren, M.X.; Yang, C.; Fu, H.Z. Microstructure and Compressive Properties of AlCrFeCoNi High Entropy Alloy. Mater. Sci. Eng. A 2008, 491, 154–158. [Google Scholar] [CrossRef]
- Wang, X.-R.; Wang, Z.-Q.; Lin, T.-S.; He, P. Microstructure, Thermodynamics and Compressive Properties of AlCrCuNiZr x (x = 0.1) High-Entropy Alloys. Mater. Sci. Technol. 2016, 32, 1289–1295. [Google Scholar] [CrossRef]
- Qiao, D.X.; Jiang, H.; Chang, X.X.; Lu, Y.P.; Li, T.J. Microstructure and Mechanical Properties of VTaTiMoAlx Refractory High Entropy Alloys. Mater. Sci. Forum 2017, 898, 638–642. [Google Scholar] [CrossRef]
- López Ríos, M.; Socorro Perdomo, P.P.; Voiculescu, I.; Geanta, V.; Crăciun, V.; Boerasu, I.; Mirza Rosca, J.C. Effects of Nickel Content on the Microstructure, Microhardness and Corrosion Behavior of High-Entropy AlCoCrFeNix Alloys. Sci. Rep. 2020, 10, 21119. [Google Scholar] [CrossRef] [PubMed]
- Shun, T.-T.; Hung, W.-J. Effects of Cr Content on Microstructure and Mechanical Properties of AlCoCrxFeNi High-Entropy Alloy. Adv. Mater. Sci. Eng. 2018, 2018, 5826467. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Xiao, D.; Wu, W.; Ni, S.; Song, M. Effect of Fe on Microstructure, Phase Evolution and Mechanical Properties of (AlCoCrFeNi)100-XFex High Entropy Alloys Processed by Spark Plasma Sintering. Intermetallics 2018, 103, 1–11. [Google Scholar] [CrossRef]
- Tong, C.-J.; Chen, Y.-L.; Yeh, J.-W.; Lin, S.-J.; Chen, S.-K.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Microstructure Characterization of AlxCoCrCuFeNi High-Entropy Alloy System with Multiprincipal Elements. Metall. Mater. Trans. A 2005, 36, 881–893. [Google Scholar] [CrossRef]
- Kao, Y.-F.; Chen, T.-J.; Chen, S.-K.; Yeh, J.-W. Microstructure and Mechanical Property of As-Cast, -Homogenized, and -Deformed AlxCoCrFeNi (0≤x≤2) High-Entropy Alloys. J. Alloys Compd. 2009, 488, 57–64. [Google Scholar] [CrossRef]
- Wang, W.-R.; Wang, W.-L.; Wang, S.-C.; Tsai, Y.-C.; Lai, C.-H.; Yeh, J.-W. Effects of Al Addition on the Microstructure and Mechanical Property of AlxCoCrFeNi High-Entropy Alloys. Intermetallics 2012, 26, 44–51. [Google Scholar] [CrossRef]
- Praveen, S.; Kim, H.S. High-Entropy Alloys: Potential Candidates for High-Temperature Applications—An Overview. Adv. Eng. Mater. 2017, 20, 1700645. [Google Scholar] [CrossRef]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of Valence Electron Concentration on Stability of Fcc or Bcc Phase in High Entropy Alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Zhang, Y.; Chen, G. Atomic Packing Efficiency and Phase Transition in a High Entropy Alloy. J. Alloys Compd. 2009, 478, 321–324. [Google Scholar] [CrossRef]
- Ogura, M.; Fukushima, T.; Zeller, R.; Dederichs, P.H. Structure of the High-Entropy Alloy AlxCrFeCoNi: Fcc versus Bcc. J. Alloys Compd. 2017, 715, 454–459. [Google Scholar] [CrossRef]
- Manzoni, A.; Daoud, H.; Völkl, R.; Glatzel, U.; Wanderka, N. Phase Separation in Equiatomic AlCoCrFeNi High-Entropy Alloy. Ultramicroscopy 2013, 132, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Geanta, V.; Voiculescu, I.; Milosan, I.; Istrate, B.; Mates, I.M. Chemical Composition Influence on Microhardness, Microstructure and Phase Morphology of AlxCrFeCoNi High Entropy Alloys. Rev. Chim. 2018, 69, 798–801. [Google Scholar] [CrossRef]
- Joseph, J.; Jarvis, T.; Wu, X.; Stanford, N.; Hodgson, P.; Fabijanic, D.M. Comparative Study of the Microstructures and Mechanical Properties of Direct Laser Fabricated and Arc-Melted AlxCoCrFeNi High Entropy Alloys. Mater. Sci. Eng. A 2015, 633, 184–193. [Google Scholar] [CrossRef]
- Shafeie, S.; Guo, S.; Hu, Q.; Fahlquist, H.; Erhart, P.; Palmqvist, A. High-Entropy Alloys as High-Temperature Thermoelectric Materials. J. Appl. Phys. 2015, 118, 184905. [Google Scholar] [CrossRef]
- Joseph, J.; Haghdadi, N.; Shamlaye, K.; Hodgson, P.; Barnett, M.; Fabijanic, D. The Sliding Wear Behaviour of CoCrFeMnNi and AlxCoCrFeNi High Entropy Alloys at Elevated Temperatures. Wear 2019, 428–429, 32–44. [Google Scholar] [CrossRef]
- Kao, Y.-F.; Chen, S.-K.; Chen, T.-J.; Chu, P.-C.; Yeh, J.-W.; Lin, S.-J. Electrical, Magnetic, and Hall Properties of AlxCoCrFeNi High-Entropy Alloys. J. Alloys Compd. 2011, 509, 1607–1614. [Google Scholar] [CrossRef]
- Kao, Y.-F.; Lee, T.-D.; Chen, S.-K.; Chang, Y.-S. Electrochemical Passive Properties of AlxCoCrFeNi (X=0, 0.25, 0.50, 1.00) Alloys in Sulfuric Acids. Corro. Sci. 2010, 52, 1026–1034. [Google Scholar] [CrossRef]
- Ma, S.G.; Zhang, Y. Effect of Nb Addition on the Microstructure and Properties of AlCoCrFeNi High-Entropy Alloy. Mater. Sci. Eng. A 2012, 532, 480–486. [Google Scholar] [CrossRef]
- Dong, Y.; Zhou, K.; Lu, Y.; Gao, X.; Wang, T.; Li, T. Effect of Vanadium Addition on the Microstructure and Properties of AlCoCrFeNi High Entropy Alloy. Mater. Des. 2014, 57, 67–72. [Google Scholar] [CrossRef]
- Chen, J.; Niu, P.; Liu, Y.; Lu, Y.; Wang, X.; Peng, Y.; Liu, J. Effect of Zr Content on Microstructure and Mechanical Properties of AlCoCrFeNi High Entropy Alloy. Mater. Des. 2016, 94, 39–44. [Google Scholar] [CrossRef]
- Chen, Q.; Lu, Y.; Dong, Y.; Wang, T.; Li, T. Effect of Minor B Addition on Microstructure and Properties of AlCoCrFeNi Multi-Compenent Alloy. Trans. Nonferrous Met. Soc. China 2015, 25, 2958–2964. [Google Scholar] [CrossRef]
- Cheng, B.; Zhang, F.; Lou, H.; Chen, X.; Liaw, P.K.; Yan, J.; Zeng, Z.; Ding, Y.; Zeng, Q. Pressure-Induced Phase Transition in the AlCoCrFeNi High-Entropy Alloy. Scr. Mater. 2019, 161, 88–92. [Google Scholar] [CrossRef] [Green Version]
- Cao, T.; Shang, J.; Zhao, J.; Cheng, C.; Wang, R.; Wang, H. The Influence of Al Elements on the Structure and the Creep Behavior of AlxCoCrFeNi High Entropy Alloys. Mater. Lett. 2016, 164, 344–347. [Google Scholar] [CrossRef]
- Tian, Q.; Zhang, G.; Yin, K.; Wang, W.; Cheng, W.; Wang, Y. The Strengthening Effects of Relatively Lightweight AlCoCrFeNi High Entropy Alloy. Mater. Charact. 2019, 151, 302–309. [Google Scholar] [CrossRef]
- Zhou, P.F.; Xiao, D.H.; Wu, Z.; Song, M. Microstructure and Mechanical Properties of AlCoCrFeNi High Entropy Alloys Produced by Spark Plasma Sintering. Mater. Res. Express 2019, 6, 0865e7. [Google Scholar] [CrossRef]
- Zhang, A.; Han, J.; Meng, J.; Su, B.; Li, P. Rapid Preparation of AlCoCrFeNi High Entropy Alloy by Spark Plasma Sintering from Elemental Powder Mixture. Mater. Lett. 2016, 181, 82–85. [Google Scholar] [CrossRef]
- Al-shataif, Y.A.; Sivasankaran, S.; Al-Mufadi, F.A.; Alaboodi, A.S.; Ammar, H.R. Manufacturing Methods, Microstructural and Mechanical Properties Evolutions of High-Entropy Alloys: A Review. Met. Mater. Int. 2020, 26, 1099–1133. [Google Scholar] [CrossRef]
- Niu, P.; Li, R.; Yuan, T.; Zhu, S.; Chen, C.; Wang, M.; Huang, L. Microstructures and Properties of an Equimolar AlCoCrFeNi High Entropy Alloy Printed by Selective Laser Melting. Intermetallics 2018, 104, 24–32. [Google Scholar] [CrossRef]
- Liang, J.-T.; Cheng, K.-C.; Chen, S.-H. Effect of Heat Treatment on the Phase Evolution and Mechanical Properties of Atomized AlCoCrFeNi High-Entropy Alloy Powders. J. Alloys Compd. 2019, 803, 484–490. [Google Scholar] [CrossRef]
- Munitz, A.; Salhov, S.; Hayun, S.; Frage, N. Heat Treatment Impacts the Micro-Structure and Mechanical Properties of AlCoCrFeNi High Entropy Alloy. J. Alloys Compd. 2016, 683, 221–230. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Chen, J.-H.; Stadler, S.; Chen, S.-H. Properties of Atomized AlCoCrFeNi High-Entropy Alloy Powders and Their Phase-Adjustable Coatings Prepared via Plasma Spray Process. Appl. Surf. Sci. 2019, 478, 478–486. [Google Scholar] [CrossRef]
- An, Z.; Jia, H.; Wu, Y.; Rack, P.D.; Patchen, A.D.; Liu, Y.; Ren, Y.; Li, N.; Liaw, P.K. Solid-Solution CrCoCuFeNi High-Entropy Alloy Thin Films Synthesized by Sputter Deposition. Mater. Res. Lett. 2015, 3, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Park, H.J.; Mun, S.C.; Jumaev, E.; Hong, S.H.; Song, G.; Kim, J.T.; Park, Y.K.; Kim, K.S.; Jeong, S.I.; et al. Investigation of Structure and Mechanical Properties of TiZrHfNiCuCo High Entropy Alloy Thin Films Synthesized by Magnetron Sputtering. J. Alloys Compd. 2019, 797, 834–841. [Google Scholar] [CrossRef]
- Yue, T.M.; Xie, H.; Lin, X.; Yang, H.O.; Meng, G.H. Solidification Behaviour in Laser Cladding of AlCoCrCuFeNi High-Entropy Alloy on Magnesium Substrates. J. Alloys Compd. 2014, 587, 588–593. [Google Scholar] [CrossRef]
- Wang, W.; Qi, W.; Xie, L.; Yang, X.; Li, J.; Zhang, Y. Microstructure and Corrosion Behavior of (CoCrFeNi)95Nb5 High-Entropy Alloy Coating Fabricated by Plasma Spraying. Materials 2019, 12, 694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sistla, H.R.; Newkirk, J.; Liou, F. Effect of Al/Ni Ratio, Heat Treatment on Phase Transformations and Microstructure of AlxFeCoCrNi2x (x = 0.3, 1) High Entropy Alloys. Mater. Des. 2015, 81, 113–121. [Google Scholar] [CrossRef]
- Cui, W.; Karnati, S.; Zhang, X.; Burns, E.; Liou, F. Fabrication of AlCoCrFeNi High-Entropy Alloy Coating on an AISI 304 Substrate via a CoFe2Ni Intermediate Layer. Entropy 2019, 21, 2. [Google Scholar] [CrossRef] [Green Version]
- Kemény, D.M.; Miskolcziné Pálfi, N.; Fazakas, É. Examination of Microstructure and Corrosion Properties of Novel AlCoCrFeNi Multicomponent Alloy. Mater. Today Proc. 2021, 45, 4250–4253. [Google Scholar] [CrossRef]
- Li, Q.H.; Yue, T.M.; Guo, Z.N.; Lin, X. Microstructure and Corrosion Properties of AlCoCrFeNi High Entropy Alloy Coatings Deposited on AISI 1045 Steel by the Electrospark Process. Metall. Mater. Trans. A 2013, 44, 1767–1778. [Google Scholar] [CrossRef]
- Meghwal, A.; Anupam, A.; Luzin, V.; Schulz, C.; Hall, C.; Murty, B.S.; Kottada, R.S.; Berndt, C.C.; Ang, A.S.M. Multiscale Mechanical Performance and Corrosion Behaviour of Plasma Sprayed AlCoCrFeNi High-Entropy Alloy Coatings. J. Alloys Compd. 2021, 854, 157140. [Google Scholar] [CrossRef]
- Li, W.; Liu, P.; Liaw, P.K. Microstructures and Properties of High-Entropy Alloy Films and Coatings: A Review. Mater. Res. Lett. 2018, 6, 199–229. [Google Scholar] [CrossRef] [Green Version]
- Lim, K.R.; Lee, K.S.; Lee, J.S.; Kim, J.Y.; Chang, H.J.; Na, Y.S. Dual-Phase High-Entropy Alloys for High-Temperature Structural Applications. J. Alloys Compd. 2017, 728, 1235–1238. [Google Scholar] [CrossRef]
- Chikumba, S.; Rao, V.V. High Entropy Alloys: Development and Applications. In Proceedings of the 7th Int. Conf. Latest Trends Eng. Technology, Irene, South Africa, 26–27 November 2015; pp. 1–5. [Google Scholar]
- Yan, X.; Zhang, Y. Functional Properties and Promising Applications of High Entropy Alloys. Scr. Mater. 2020, 187, 188–193. [Google Scholar] [CrossRef]
- Li, D.; Li, C.; Feng, T.; Zhang, Y.; Sha, G.; Lewandowski, J.J.; Liaw, P.K.; Zhang, Y. High-Entropy Al0.3CoCrFeNi Alloy Fibers with High Tensile Strength and Ductility at Ambient and Cryogenic Temperatures. Acta Mater. 2017, 123, 285–294. [Google Scholar] [CrossRef] [Green Version]
- Liao, W.-B.; Zhang, H.; Liu, Z.-Y.; Li, P.-F.; Huang, J.-J.; Yu, C.-Y.; Lu, Y. High Strength and Deformation Mechanisms of Al0.3CoCrFeNi High-Entropy Alloy Thin Films Fabricated by Magnetron Sputtering. Entropy 2019, 21, 146. [Google Scholar] [CrossRef] [Green Version]
- Yeh, J.-W.; Lin, S.-J. Breakthrough Applications of High-Entropy Materials. J. Mater. Res. 2018, 33, 3129–3137. [Google Scholar] [CrossRef]
- Socorro-Perdomo, P.P.; Florido-Suárez, N.R.; Voiculescu, I.; Mirza-Rosca, J.C. Comparative EIS Study of AlxCoCrFeNi Alloys in Ringer’s Solution for Medical Instruments. Metals 2021, 11, 928. [Google Scholar] [CrossRef]
| Alloy | Yield Strength [MPa] | Compression Strength [MPa] | Plastic Strain [%] |
|---|---|---|---|
| AlCrFeCoNi | 1250.96 | 2004.23 | 32.7 |
| CrFeCoNiCuTi | 1272 | 1272 | 1.6 |
| CoCrCuFeNi | 230 | 888 | 0.89 |
| AlCrMnFeCoNiCuTiV | 1862 | 2431 | 0.95 |
| CrFeCoNiCuTi0.5 | 700 | 1650 | 21.26 |
| AlCrCuNi | 1140.6 | 1428.7 | 6.6 |
| VTaTiMoAl0.2 | 1021 | 1249 | 6.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokarewicz, M.; Grądzka-Dahlke, M. Review of Recent Research on AlCoCrFeNi High-Entropy Alloy. Metals 2021, 11, 1302. https://doi.org/10.3390/met11081302
Tokarewicz M, Grądzka-Dahlke M. Review of Recent Research on AlCoCrFeNi High-Entropy Alloy. Metals. 2021; 11(8):1302. https://doi.org/10.3390/met11081302
Chicago/Turabian StyleTokarewicz, Marzena, and Małgorzata Grądzka-Dahlke. 2021. "Review of Recent Research on AlCoCrFeNi High-Entropy Alloy" Metals 11, no. 8: 1302. https://doi.org/10.3390/met11081302
APA StyleTokarewicz, M., & Grądzka-Dahlke, M. (2021). Review of Recent Research on AlCoCrFeNi High-Entropy Alloy. Metals, 11(8), 1302. https://doi.org/10.3390/met11081302






