Magnetron Sputtering High-Entropy Alloy Coatings: A Mini-Review
Abstract
:1. Introduction
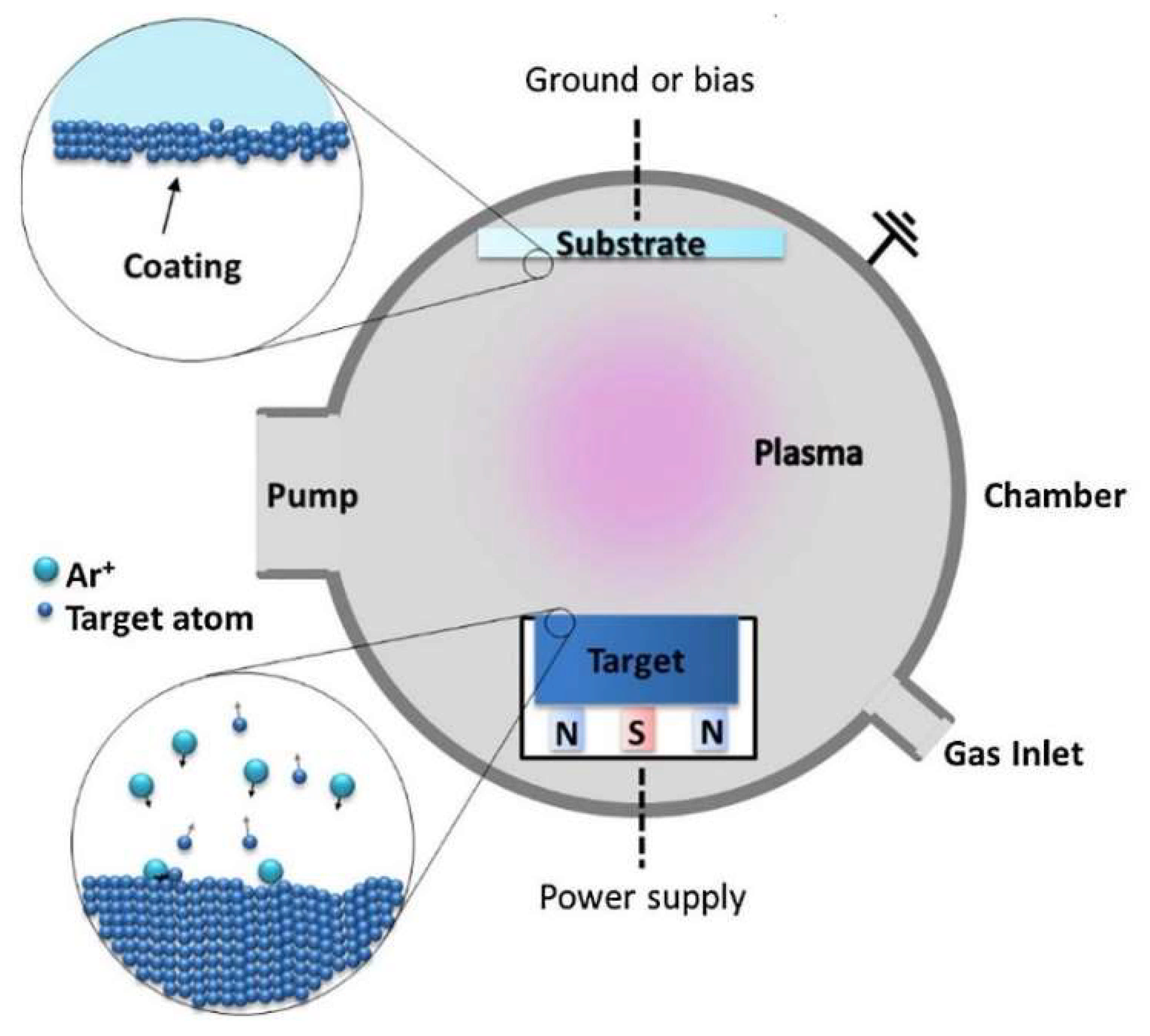
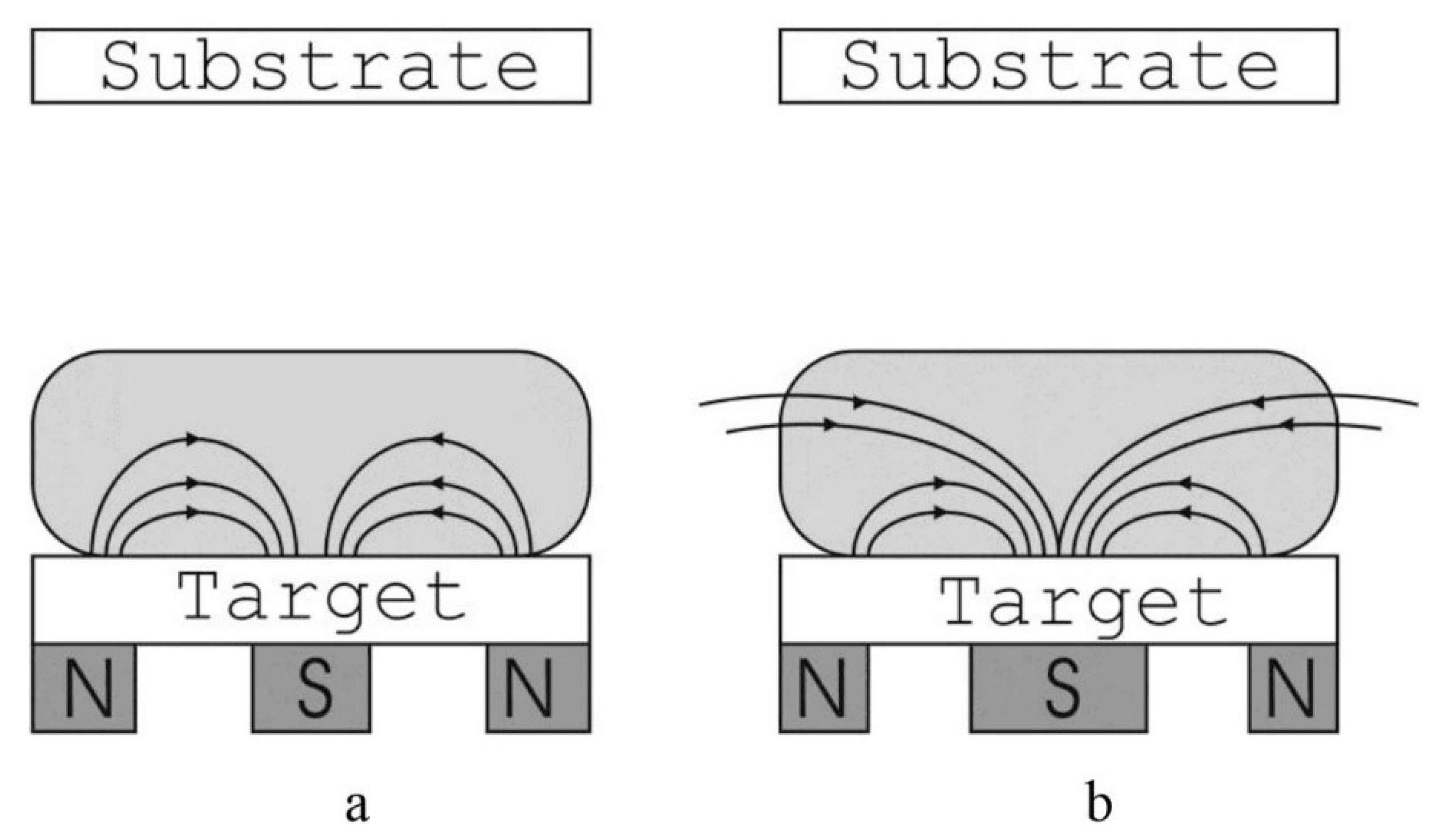
2. Magnetron Sputtering Coating
3. Properties
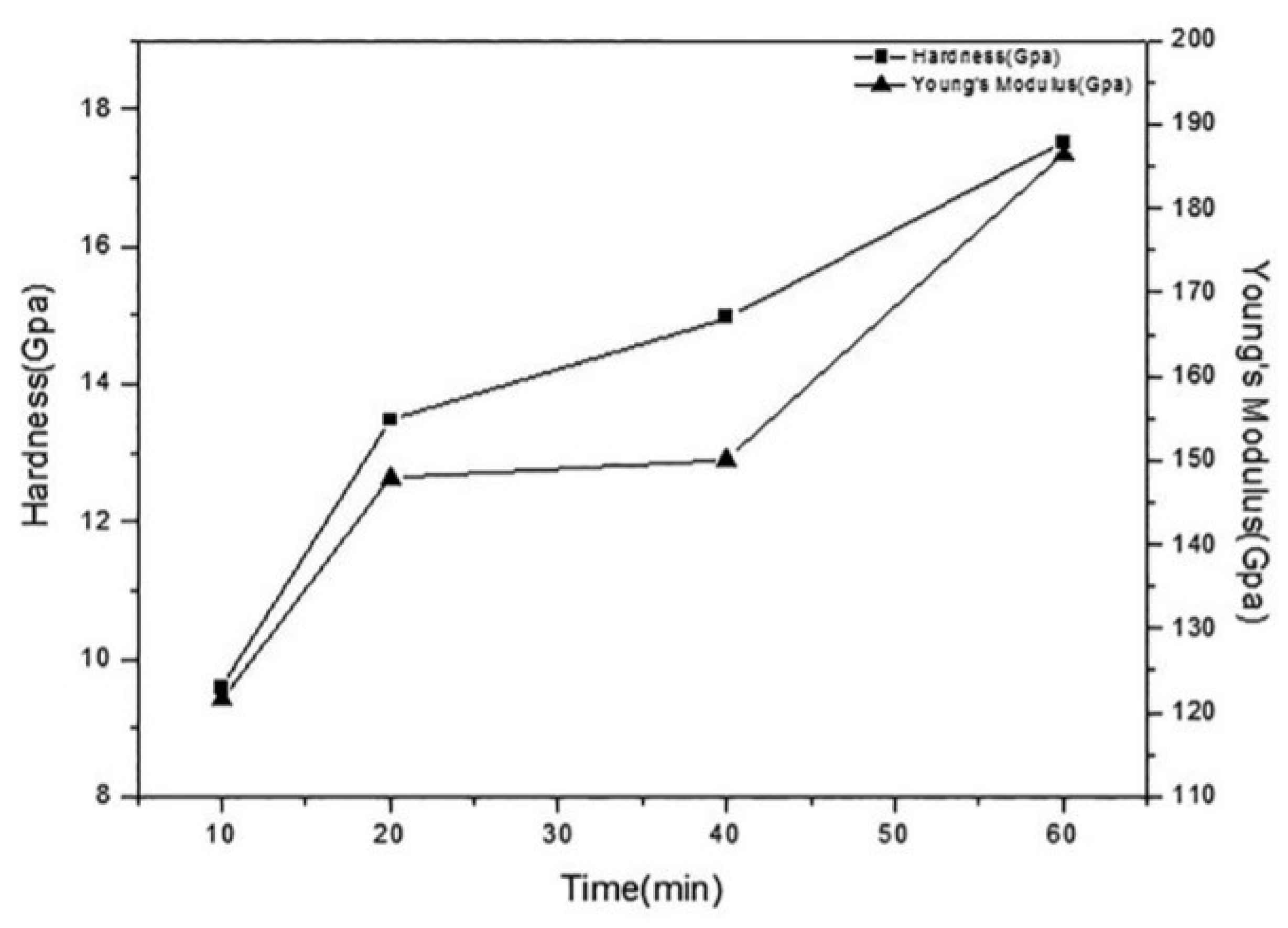
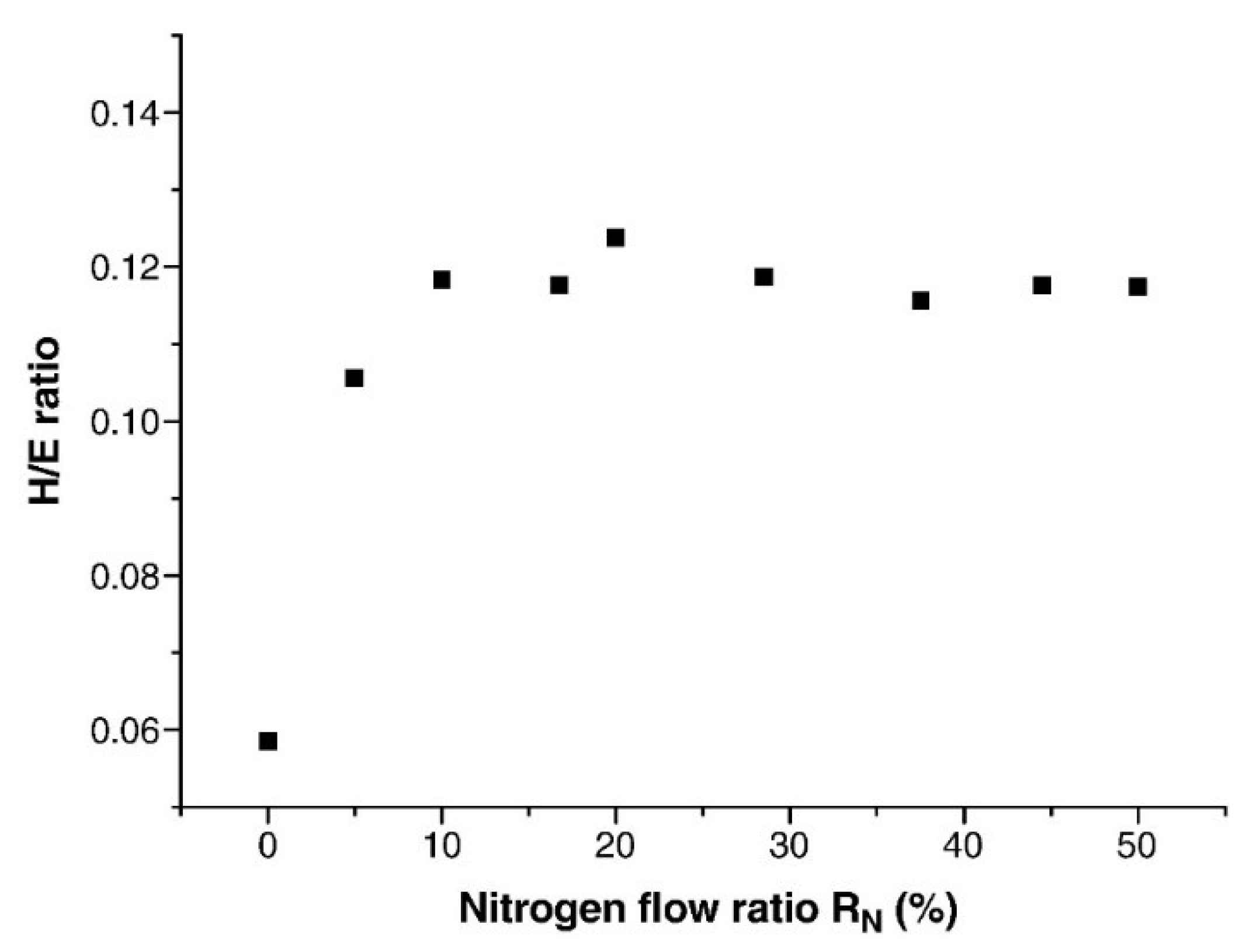
3.1. Mechanical Behavior
3.2. Corrosion Resistance
3.3. Thermal Stability and Oxidation Resistance
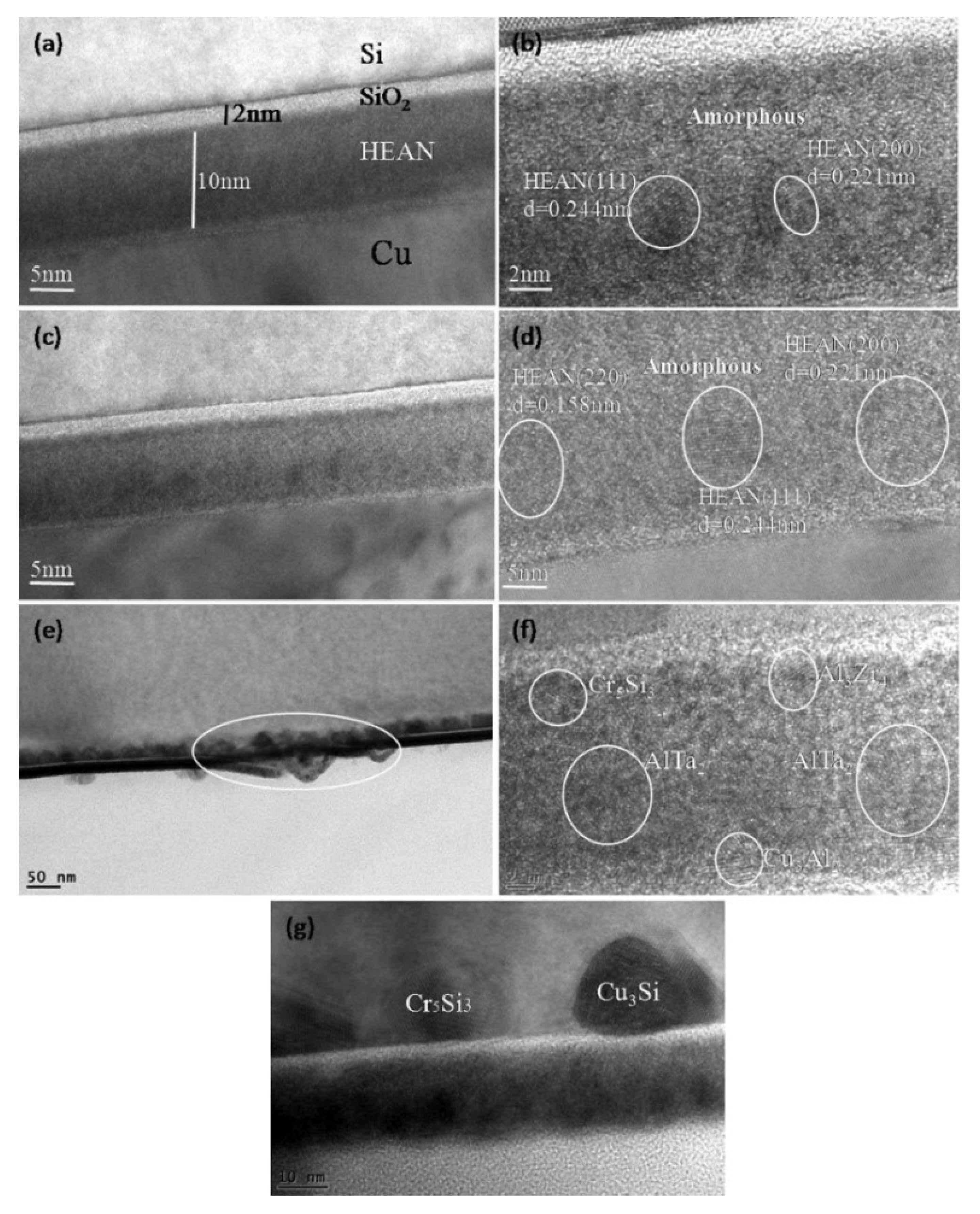
3.4. Diffusion Retardation
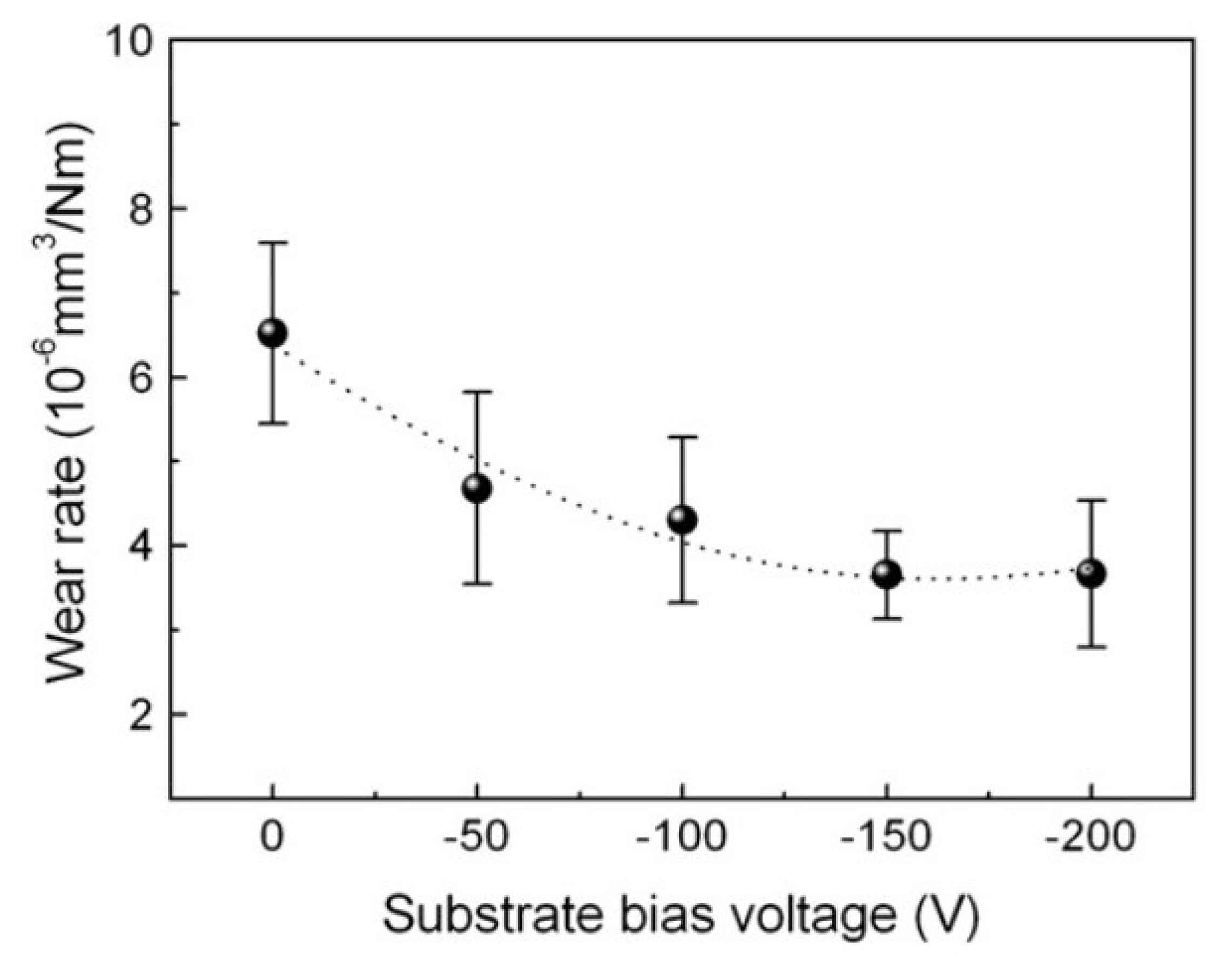
3.5. Wear Resistance
4. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Xu, X.D.; Guo, S.; Nieh, T.G.; Liu, C.T.; Hirata, A.; Chen, M.W. Effects of mixing enthalpy and cooling rate on phase formation of AlxCoCrCuFeNi high-entropy alloys. Materialia 2019, 6, 100292. [Google Scholar] [CrossRef]
- Wang, W.R.; Wang, W.L.; Wang, S.C.; Tsai, Y.C.; Lai, C.H.; Yeh, J.W. Effects of Al addition on the microstructure and mechanical property of AlxCoCrFeNi high-entropy alloys. Intermetallics 2012, 26, 44–51. [Google Scholar] [CrossRef]
- Tsai, M.H.; Yeh, J.W. High-entropy alloys: A critical review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Otto, F.; Dlouhý, A.; Somsen, C.; Bei, H.; Eggeler, G.; George, E.P. The influences of temperature and microstructure on the tensile properties of a CoCrFeMnNi high-entropy alloy. Acta Mater. 2013, 61, 5743–5755. [Google Scholar] [CrossRef] [Green Version]
- Munitz, A.; Kaufman, M.; Nahmany, M.; Derimow, N.; Abbaschian, R. Microstructure and mechanical properties of heat-treated Al1. 25CoCrCuFeNi high entropy alloys. Mater. Sci. Eng. A 2018, 714, 146–159. [Google Scholar] [CrossRef]
- Chuang, M.H.; Tsai, M.H.; Wang, W.R.; Lin, S.J.; Yeh, J.W. Microstructure and wear behavior of AlxCo1.5CrFeNi1.5Tiy high-entropy alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- Chen, Y.; Duval, T.; Hung, U.; Yeh, J.; Shih, H. Microstructure and electrochemical properties of high entropy alloys—A comparison with type-304 stainless steel. Corros. Sci. 2005, 47, 2257–2279. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, M.C.; Yeh, J.W.; Liaw, P.K.; Zhang, Y. (Eds.) High-Entropy Alloys: Fundamentals and Applications; Springer: Cham, Switzerland, 2016; 524p. [Google Scholar]
- Zhou, Y.; Zhang, Y.; Wang, Y.; Chen, G. Microstructure and Compressive Properties of Multicomponent Alx(TiVCrMnFeCoNiCu)100-x High-Entropy Alloys. Mater. Sci. Eng. A 2007, 454–455, 260–265. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Z.P.; Ma, S.G.; Liaw, P.K.; Tang, Z.; Cheng, Y.Q.; Gao, M.C. Guidelines in predicting phase formation of high-entropy alloys. MRS Commun. 2014, 4, 57–62. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Meng, X.; Xie, Y. A Review on High Entropy Alloys Coatings: Fabrication Processes and Property Assessment. Adv. Eng. Mater. 2019, 21, 1900343. [Google Scholar] [CrossRef]
- Ni, C.; Shi, Y.; Liu, J.; Huang, G. Characterization of Al0.5FeCu0.7NiCoCr high-entropy alloy coating on aluminum alloy by laser cladding. Opt. Laser Technol. 2018, 105, 257–263. [Google Scholar] [CrossRef]
- Shon, Y.; Joshi, S.S.; Katakam, S.; Rajamure, R.S.; Dahotr, N.B. Laser additive synthesis of high entropy alloy coating on aluminum: Corrosion behavior. Mater. Lett. 2015, 142, 122–125. [Google Scholar] [CrossRef]
- Li, W.; Liu, P.; Liaw, P.W. Microstructures and properties of high-entropy alloy films and coatings: A review. Mater. Res. Lett. 2018, 6, 199–229. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.; Li, C.; Chiang, S.; Huang, Y. 4-nm thick multilayer structure of multi-component (AlCrRuTaTiZr)Nx as robust diffusion barrier for Cu interconnects. J. Alloys Compd. 2012, 515, 4–7. [Google Scholar] [CrossRef]
- Lin, S.; Chang, S.; Huang, Y.; Shieu, F.; Yeh, J. Mechanical performance and nanoindenting deformation of (AlCrTaTiZr)NCy multi-component coatings co-sputtered with bias. Surf. Coat. Technol. 2012, 206, 5096–5102. [Google Scholar] [CrossRef]
- Meghwal, A.; Anupam, A.; Murty, B.S.; Berndt, C.C.; Kottada, R.S.; Ang, A.S.M. Thermal Spray High-Entropy Alloy Coatings: A Review. J. Therm. Spray Tech. 2020, 29, 857–893. [Google Scholar] [CrossRef]
- Calderon Velasco, S.; Cavaleiro, A.; Carvalho, S. Functional properties of ceramic-Ag nanocomposite coatings produced by magnetron sputtering. Prog. Mater. Sci. 2016, 84, 158–191. [Google Scholar] [CrossRef] [Green Version]
- Frey, H.; Khan, H.R. Handbook of Thin Film Technology; Springer: Cham, Switzerland, 2010; 380p. [Google Scholar]
- Golosov, D.A. Balanced magnetic field in magnetron sputtering systems. Vacuum 2017, 139, 109–116. [Google Scholar] [CrossRef]
- Schwarz, H.; Uhlig, T.; Rösch, N.; Lindner, T.; Ganss, F.; Hellwig, O.; Lampke, T.; Wagner, G.; Seyller, T. CoCrFeNi High-Entropy Alloy Thin Films Synthesised by Magnetron Sputter Deposition from Spark Plasma Sintered Targets. Coatings 2021, 11, 468. [Google Scholar] [CrossRef]
- Cemin, F.; Jimenez, M.J.M.; Leidens, L.M.; Figueroa, C.A.; Alvarez, F. A thermodynamic study on phase formation and thermal stability of AlSiTaTiZr high-entropy alloy thin films. J. Alloys Compd. 2020, 838, 155580. [Google Scholar] [CrossRef]
- Cemin, F.; de Mello, S.R.S.; Figueroa, C.A.; Alvarez, F. Influence of substrate bias and temperature on the crystallization of metallic NbTaTiVZr high-entropy alloy thin films. Surf. Coat. Technol. 2021, 421, 127357. [Google Scholar] [CrossRef]
- Khan, N.A.; Akhavan, B.; Zhou, C.; Zhou, H.; Chang, L.; Wang, Y.; Liu, Y.; Fu, L.; Bilek, M.M.; Liu, Z. RF magnetron sputtered AlCoCrCu0.5FeNi high entropy alloy (HEA) thin films with tuned microstructure and chemical composition. J. Alloys Compd. 2020, 836, 155348. [Google Scholar] [CrossRef]
- Huang, P.K.; Yeh, J.W. Effects of substrate temperature and post-annealing on microstructure and properties of (AlCrNbSiTiV)N coatings. Thin Solid Films 2009, 518, 180–184. [Google Scholar] [CrossRef]
- Shaginyan, L.R.; Gorban, V.F.; Krapivka, N.A.; Firstov, S.A.; Kopylov, I.F. Properties of Coatings of the Al–Cr–Fe–Co–Ni–Cu–V High Entropy Alloy Produced by the Magnetron Sputtering. J. Superhard. Mater. 2016, 38, 25–33. [Google Scholar] [CrossRef]
- Cheng, K.H.; Lai, C.H.; Lin, S.J.; Yeh, J.W. Structural and mechanical properties of multi-element (AlCrMoTaTiZr)Nx coatings by reactive magnetron sputtering. Thin Solid Films 2011, 519, 3185–3190. [Google Scholar] [CrossRef]
- Kao, W.H.; Su, Y.L.; Horng, J.H.; Wu, H.M. Effects of carbon doping on mechanical, tribological, structural, anti-corrosion and anti-glass-sticking properties of CrNbSiTaZr high entropy alloy coatings. Thin Solid Films 2021, 717, 138448. [Google Scholar] [CrossRef]
- Liao, W.B.; Zhang, H.; Liu, Z.Y.; Li, P.F.; Huang, J.J.; Yu, C.Y.; Lu, Y. High Strength and Deformation Mechanisms of Al0.3CoCrFeNi High-Entropy Alloy Thin Films Fabricated by Magnetron Sputtering. Entropy 2019, 21, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tüten, N.; Canadinc, D.; Motallebzadeh, A.; Bal, B. Microstructure and tribological properties of TiTaHfNbZr high entropy alloy coatings deposited on Ti−6Al−4V substrates. Intermetallics 2019, 105, 99–106. [Google Scholar] [CrossRef]
- Yu, X.; Wang, J.; Wang, L.; Huang, W. Fabrication and characterization of CrNbSiTiZr high-entropy alloy films by radio-frequency magnetron sputtering via tuning substrate bias. Surf. Coat. Technol. 2021, 412, 127074. [Google Scholar] [CrossRef]
- Zeng, Q.; Xu, Y. A comparative study on the tribocorrosion behaviors of AlFeCrNiMo high entropy alloy coatings and 304 stainless steel. Mater. Today Commun. 2020, 24, 101261. [Google Scholar] [CrossRef]
- Chang, H.W.; Huang, P.K.; Davison, A.; Yeh, J.W.; Tsau, C.H.; Yang, C.C. Nitride films deposited from an equimolar Al–Cr–Mo–Si–Ti alloy target by reactive direct current magnetron sputtering. Thin Solid Films 2008, 516, 6402–6408. [Google Scholar] [CrossRef]
- Lin, C.H.; Duh, J.G. Corrosion behavior of (Ti–Al–Cr–Si–V)xNy coatings on mild steels derived from RF magnetron sputtering. Surf. Coat. Technol. 2008, 203, 558–561. [Google Scholar] [CrossRef]
- Braic, V.; Vladescu, A.; Balaceanu, M.; Luculescu, C.R.; Braic, M. Nanostructured multi-element (TiZrNbHfTa)N and (TiZrNbHfTa)C hard coatings. Surf. Coat. Technol. 2012, 211, 117–121. [Google Scholar] [CrossRef]
- Huang, P.K.; Yeh, J.W. Effects of nitrogen content on structure and mechanical properties of multi-element (AlCrNbSiTiV)N coating. Surf. Coat. Technol. 2009, 203, 1891–1896. [Google Scholar] [CrossRef]
- Johansson, K.; Riekehr, L.; Fritze, S.; Lewin, E. Multicomponent Hf-Nb-Ti-V-Zr nitride coatings by reactive magnetron sputter deposition. Surf. Coat. Technol. 2018, 349, 529–539. [Google Scholar] [CrossRef]
- Ren, B.; Shen, Z.; Liu, Z. Structure and mechanical properties of multi-element (AlCrMnMoNiZr)Nx coatings by reactive magnetron sputtering. J. Alloys Compd. 2013, 560, 171–176. [Google Scholar] [CrossRef]
- Kao, W.H.; Su, Y.L.; Horng, J.H.; Wu, W.C. Mechanical, tribological, anti-corrosion and anti-glass sticking properties of high-entropy TaNbSiZrCr carbide coatings prepared using radio-frequency magnetron sputtering. Mater. Chem. Phys. 2021, 268, 124741. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, R.; Yang, Z.B.; Liu, C.H.; Chang, H.; Yang, J.J.; Liao, J.L.; Yang, Y.Y.; Liu, N. Preparation, structure, and properties of an AlCrMoNbZr high-entropy alloy coating for accident-tolerant fuel cladding. Surf. Coat. Technol. 2018, 347, 13–19. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, X.; Wang, C.; Sui, X.; Wang, Y.; Zhou, H.; Hao, J. Tailoring the microstructure, mechanical and tribocorrosion performance of (CrNbTiAlV)N x high-entropy nitride films by controlling nitrogen flow. J. Mater. Sci. Technol. 2022, 107, 172–182. [Google Scholar] [CrossRef]
- Chang, Z.C.; Liang, J.Y. Oxidation Behavior and Structural Transformation of (CrTaTiVZr)N Coatings. Coatings 2020, 10, 415. [Google Scholar] [CrossRef]
- Lai, C.H.; Cheng, K.H.; Lin, S.J.; Yeh, J.W. Mechanical and tribological properties of multi-element (AlCrTaTiZr)N coatings. Surf. Coat. Technol. 2008, 202, 3732–3738. [Google Scholar] [CrossRef]
- Behravan, N.; Farhadizadeh, A.; Ghasemi, S.; Khademi, A.; Shojaei, H.; Ghomi, H. The pressure dependence of structure and composition of sputtered AlCrSiTiMoO high entropy thin film. J. Alloys Compd. 2021, 852, 156421. [Google Scholar] [CrossRef]
- Li, X.; Zheng, Z.; Dou, D.; Li, J. Microstructure and Properties of Coating of FeAlCuCrCoMn High Entropy Alloy Deposited by Direct Current Magnetron Sputtering. Mater. Res. 2016, 19, 802–806. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhu, J.B.; Sun, Z.Y.; Li, J.C. Preparation of Amorphous Coatings of AlFeCoNiCuZrV Alloy by Direct Current Magnetron Sputtering Method. Asian J. Chem. 2014, 26, 5627–5630. [Google Scholar] [CrossRef]
- Xu, Y.; Li, G.; Xia, Y. Synthesis and characterization of super-hard AlCrTiVZr high-entropy alloy nitride films deposited by HiPIMS. Appl. Surf. Sci. 2020, 523, 146529. [Google Scholar] [CrossRef]
- Lu, P.; Saal, J.E.; Olson, G.B.; Li, T.; Swanson, O.J.; Frankel, G.S.; Gerard, A.Y.; Quiambao, K.F.; Scully, J.R. Computational materials design of a corrosion resistant high entropy alloy for harsh environments. Scr. Mater. 2018, 153, 19–21. [Google Scholar] [CrossRef]
- Dou, D.; Li, X.C.; Zheng, Z.Y.; Li, J.C. Coatings of FeAlCoCuNiV high entropy alloy. Surf. Eng. 2016, 32, 766–770. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, C.; Yang, J.; Zhang, W.; He, L.; Zhang, R.; Yang, H.; Wang, J.; Long, J.; Chang, H. Mechanical and high-temperature corrosion properties of AlTiCrNiTa high entropy alloy coating prepared by magnetron sputtering for accident-tolerant fuel cladding. Surf. Coat. Technol. 2021, 417, 127228. [Google Scholar] [CrossRef]
- Gao, L.; Liao, W.; Zhang, H.; Surjadi, J.U.; Sun, D.; Lu, Y. Microstructure, Mechanical and Corrosion Behaviors of CoCrFeNiAl0.3 High Entropy Alloy (HEA) Films. Coatings 2017, 7, 156. [Google Scholar] [CrossRef]
- Xing, Q.; Wang, H.; Chen, M.; Chen, Z.; Li, R.; Jin, P.; Zhang, Y. Mechanical Properties and Corrosion Resistance of NbTiAlSiZrNx High-Entropy Films Prepared by RF Magnetron Sputtering. Entropy 2019, 21, 396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, W.; Yang, X.; Wang, C.; Zhang, Y. Nano-Crystallization of High-Entropy Amorphous NbTiAlSiWxNy Films Prepared by Magnetron Sputtering. Entropy 2016, 18, 226. [Google Scholar] [CrossRef]
- Shen, W.J.; Tsai, M.H.; Tsai, K.Y.; Juan, C.C.; Tsai, C.W.; Yeh, J.W.; Chang, Y.S. Superior Oxidation Resistance of (Al0.34Cr0.22Nb0.11Si0.11Ti0.22)50N50 High-Entropy Nitride. J. Electrochem. Soc. 2013, 160, C531–C534. [Google Scholar] [CrossRef]
- Kretschmer, A.; Kirnbauer, A.; Moraes, V.; Primetzhofer, D.; Yalamanchili, K.; Rudigier, H.; Mayrhofer, P.H. Improving phase stability, hardness, and oxidation resistance of reactively magnetron sputtered (Al,Cr,Nb,Ta,Ti)N thin films by Si-alloying. Surf. Coat. Technol. 2021, 416, 127162. [Google Scholar] [CrossRef]
- Tsai, D.C.; Deng, M.J.; Chang, Z.C.; Kuo, B.H.; Chen, E.C.; Chang, S.Y.; Shieu, F.S. Oxidation resistance and characterization of (AlCrMoTaTi)-Six-Ncoating deposited via magnetron sputtering. J. Alloys Compd. 2015, 647, 179–188. [Google Scholar] [CrossRef]
- Tsai, M.H.; Yeh, J.W.; Gan, J.Y. Diffusion barrier properties of AlMoNbSiTaTiVZr high-entropy alloy layer between copper and silicon. Thin Solid Films 2008, 516, 5527–5530. [Google Scholar] [CrossRef]
- Chang, S.Y.; Chen, D.S. 10-nm-thick quinary (AlCrTaTiZr)N film as effective diffusion barrier for Cu interconnects at 900 °C. Appl. Phys. Lett. 2009, 94, 231909. [Google Scholar] [CrossRef]
- Li, R.; Chen, T.; Jiang, C.; Zhang, J.; Zhang, Y.; Liaw, P.K. Applications of High Diffusion Resistance Multicomponent AlCrTaTiZrRu/(AlCrTaTiZrRu)N0.7 Film in Cu Interconnects. Adv. Eng. Mater. 2020, 22, 2000557. [Google Scholar] [CrossRef]
- Li, R.; Qiao, B.; Shang, H.; Zhang, J.; Jiang, C.; Zhang, W. Multi-component AlCrTaTiZrMo-nitride film with high diffusion resistance in copper metallization. J. Alloys Compd. 2018, 748, 258–264. [Google Scholar] [CrossRef]
- Chang, S.Y.; Chen, M.K.; Chen, D.S. Multiprincipal-Element AlCrTaTiZr-Nitride Nanocomposite Film of Extremely High Thermal Stability as Diffusion Barrier for Cu Metallization. J. Electrochem. Soc. 2009, 156, G37–G42. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, P.; Wang, W.; Shi, Q.; Yang, H.; Wang, D.; Hong, Y.; Wang, L.; Guo, C.; Lin, S.; et al. AlCoCrNiMo high-entropy alloy as diffusion barrier between NiAlHf coating and Ni-based single crystal superalloy. Surf. Coat. Technol. 2021, 414, 127101. [Google Scholar] [CrossRef]
- Luo, D.; Zhou, Q.; Ye, W.; Ren, Y.; Greiner, C.; He, Y.; Wang, H. Design and Characterization of self-lubricating refractory high entropy alloy based multilayered films. ACS Appl. Mater. Interfaces 2021, 13, 55712–55725. [Google Scholar] [CrossRef] [PubMed]


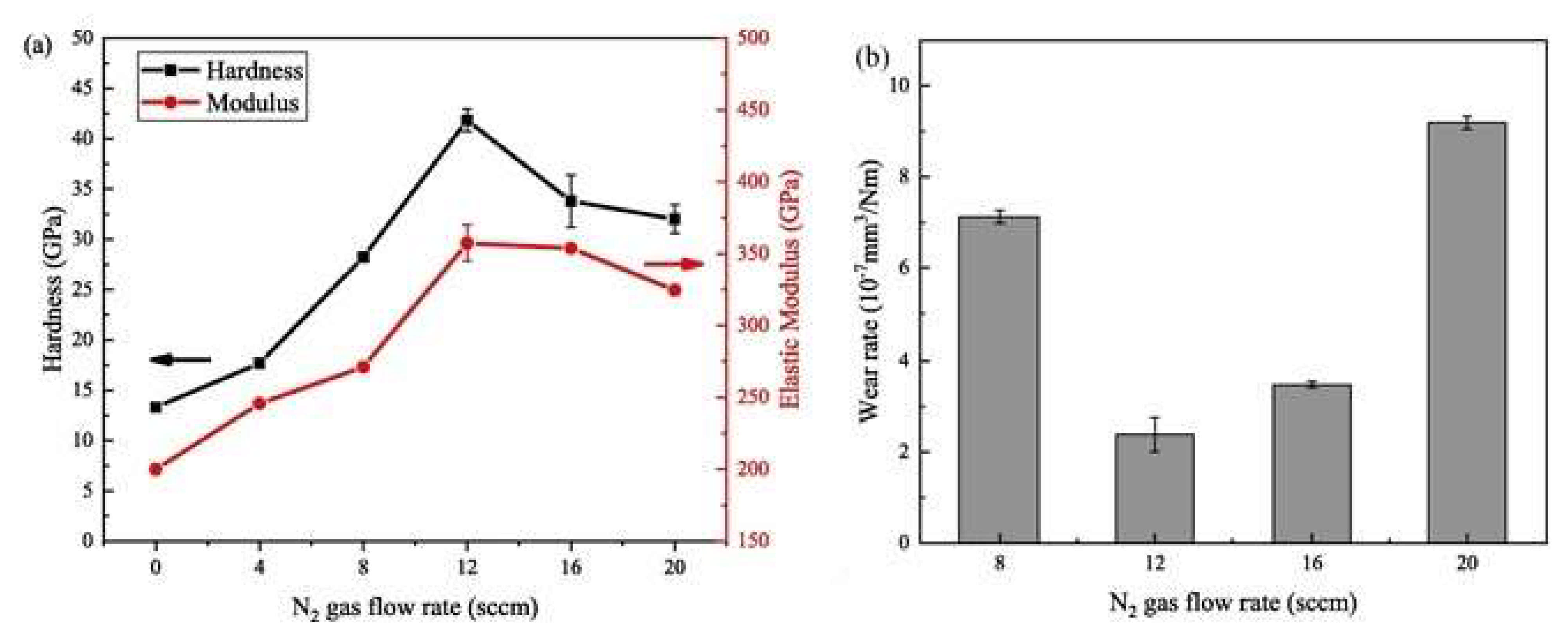
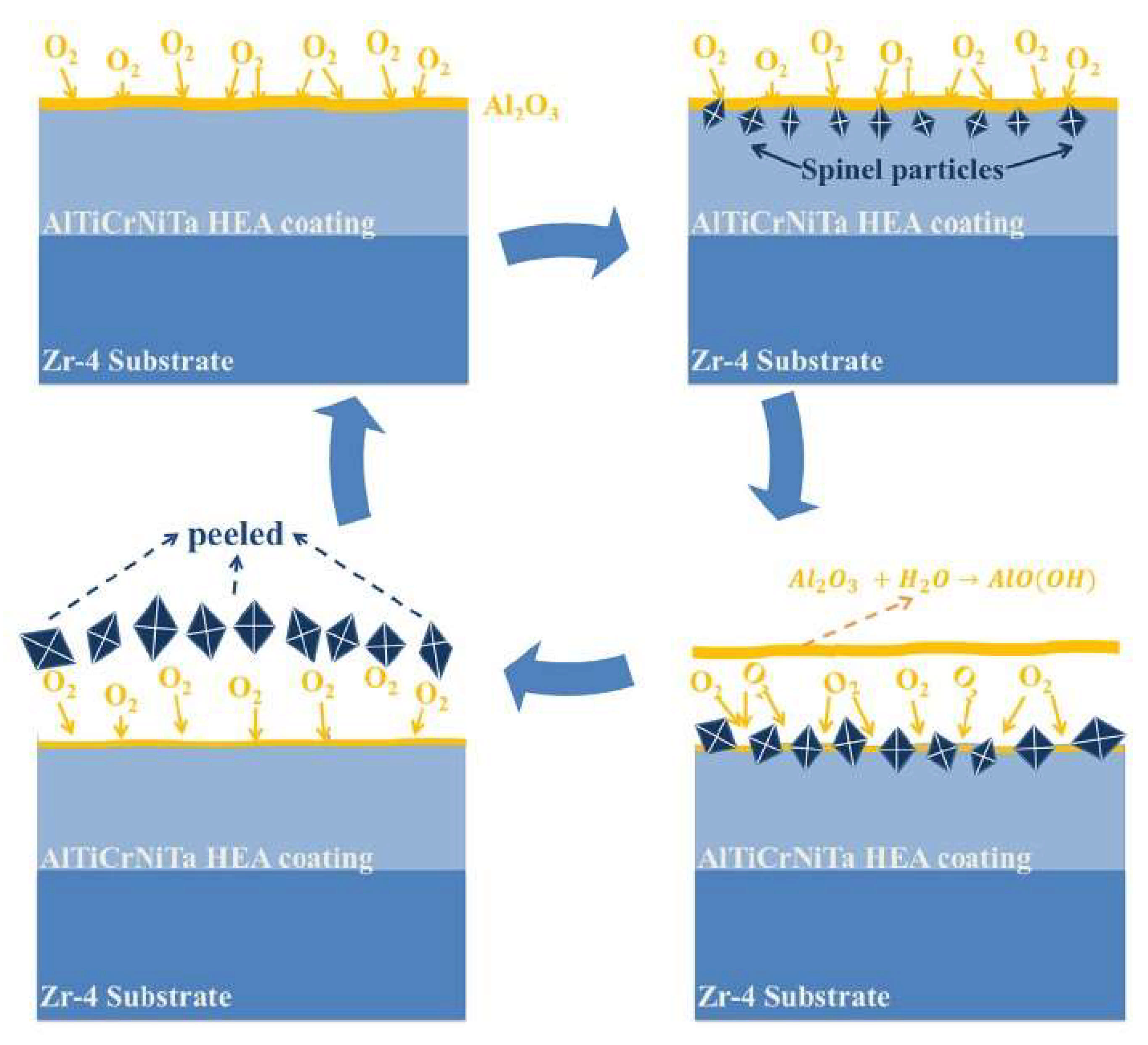
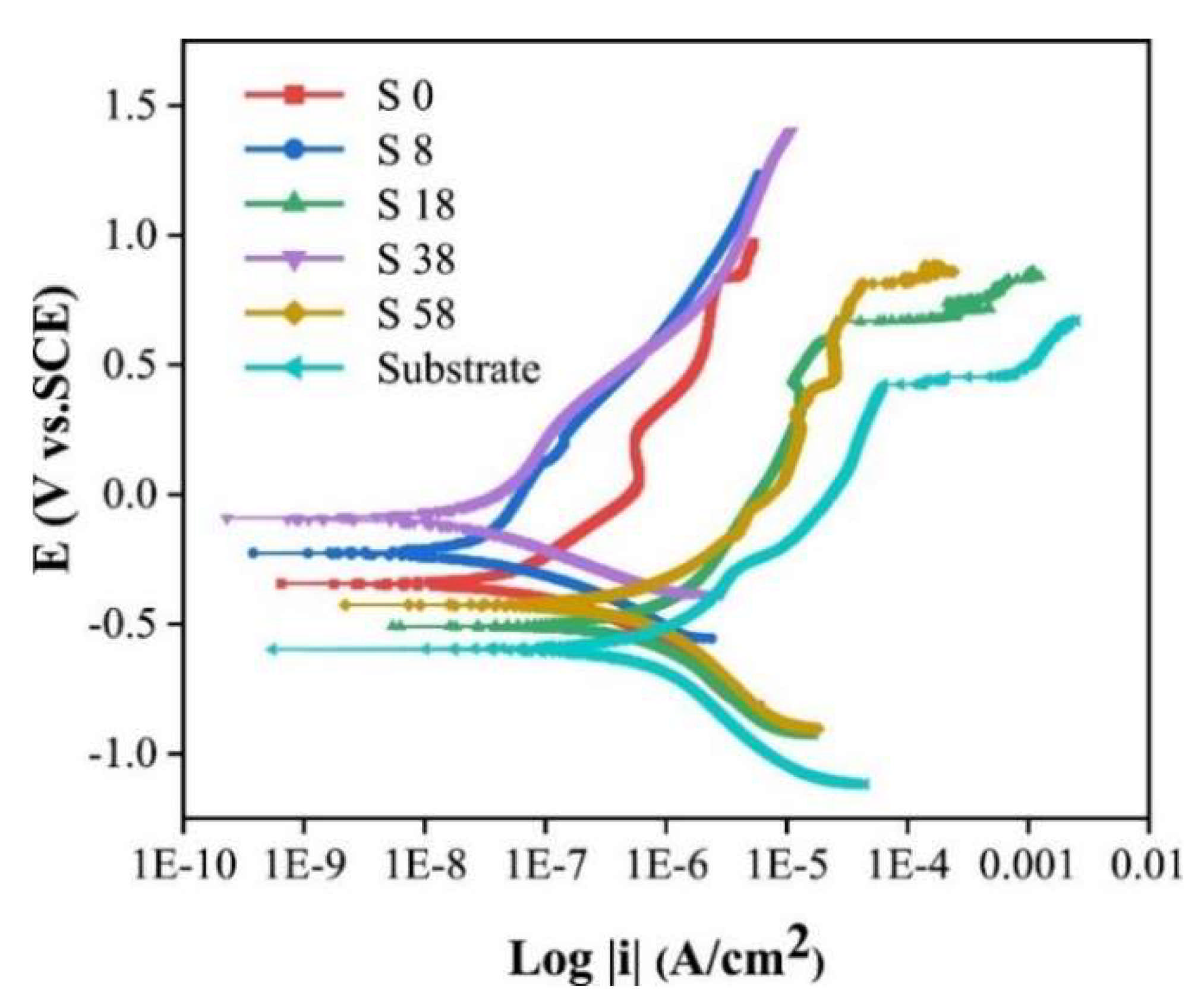
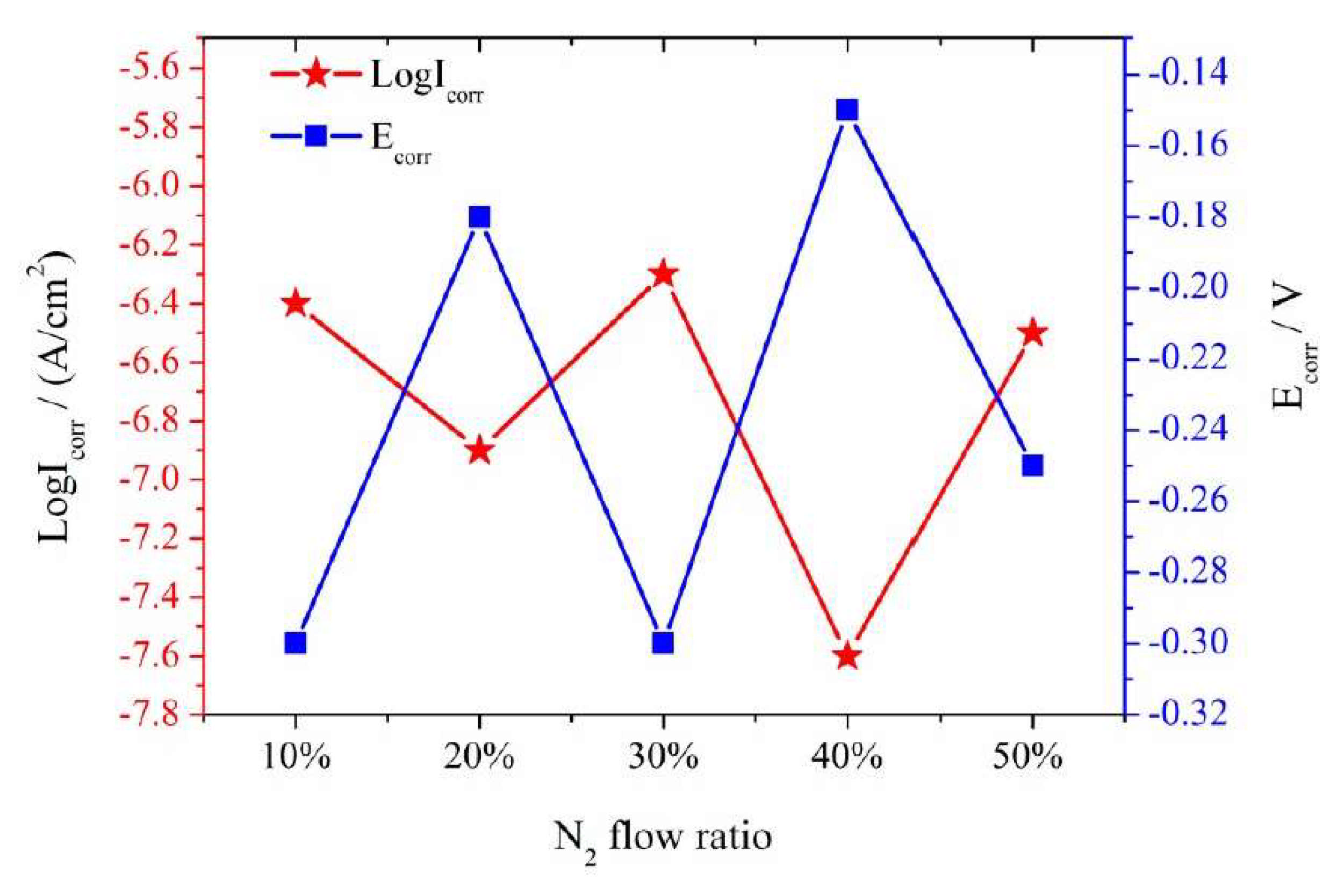
| Technique | Advantages | Disadvantages |
|---|---|---|
| Magnetron sputtering |
|
|
| Arc cladding |
|
|
| Laser cladding |
|
|
| Thermal spraying |
|
|
| Electrodeposition |
|
|
| Coatings | Substrates | Phases | Thickness | Hardness | Ref. |
|---|---|---|---|---|---|
| (CrNbSiTaZr)C | WC | Amorphous | 1.2 − 2.1 µm | 6.53–20.12 GPa | [30] |
| Al0.3CoCrFeNi | Si wafer | FCC | 4.0 µm | 11.09 GPa | [31] |
| TiTaHfNbZr | Ti-6Al-4V | Amorphous | 0.8 µm | 12.51 GPa | [32] |
| CrNbSiTiZr | Stainless steel | Amorphous | 1.0 µm | 12.4 GPa | [33] |
| AlFeCrNiMo | Stainless steel | BCC | 1.5 µm | - | [34] |
| AlCrMoSiTi | Si wafer and SiO2-Si | Amorphous | 1.0 µm | 10–16 GPa | [35] |
| (TiAlCrSiV)xNy | Mild steel | Amorphous + FCC | ≈1.7 µm | - | [36] |
| (TiZrNbHfTa)N | C45 steel | FCC | 2.0 µm | ≈33 GPa | [37] |
| (TiZrNbHfTa)N | M2 steel | FCC | 2.0 µm | ≈28 GPa | [37] |
| (AlCrNbSiTiV)N | Si wafer | Amorphous + FCC | ≈1.0 µm | ≈41 GPa | [38] |
| (HfNbTiVZr)N | Si wafer, α-SiO2, α-Al2O3 | FCC | ≈1.2 µm | ≈7.6–18.8 GPa | [39] |
| (AlCrMnMoNiZr)Nx | Si wafer | FCC | 1.5 µm | ≈11.9 GPa | [40] |
| (TaNbSiZrCr)C | WC and Si | FCC | 0.7–1.9 µm | 34.1 GPa | [41] |
| AlCrMoNbZr | N36 Zr alloy | Amorphous + BCC | 3.0 µm | 11.8 GPa | [42] |
| (CrNbTiAlV)Nx | AISI 440 C steel | FCC | 0.67 µm | 49.95 GPa | [43] |
| (CrTaTiVZr)N | Si wafer | FCC | 1.0 µm | 34.3 GPa | [44] |
| (AlCrTaTiZr)N | Si and cemented carbide substrate | FCC | 1.3 & 1.0 µm | 36.9 GPa | [45] |
| AlCrSiTiMoO | Si | Amorphous | 3.3–3.8 µm | 8.9 GPa | [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padamata, S.K.; Yasinskiy, A.; Yanov, V.; Saevarsdottir, G. Magnetron Sputtering High-Entropy Alloy Coatings: A Mini-Review. Metals 2022, 12, 319. https://doi.org/10.3390/met12020319
Padamata SK, Yasinskiy A, Yanov V, Saevarsdottir G. Magnetron Sputtering High-Entropy Alloy Coatings: A Mini-Review. Metals. 2022; 12(2):319. https://doi.org/10.3390/met12020319
Chicago/Turabian StylePadamata, Sai Krishna, Andrey Yasinskiy, Valentin Yanov, and Gudrun Saevarsdottir. 2022. "Magnetron Sputtering High-Entropy Alloy Coatings: A Mini-Review" Metals 12, no. 2: 319. https://doi.org/10.3390/met12020319
APA StylePadamata, S. K., Yasinskiy, A., Yanov, V., & Saevarsdottir, G. (2022). Magnetron Sputtering High-Entropy Alloy Coatings: A Mini-Review. Metals, 12(2), 319. https://doi.org/10.3390/met12020319






