Oxidation Behavior of Maraging 300 Alloy Exposed to Nitrogen/Water Vapor Atmosphere at 500 °C
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
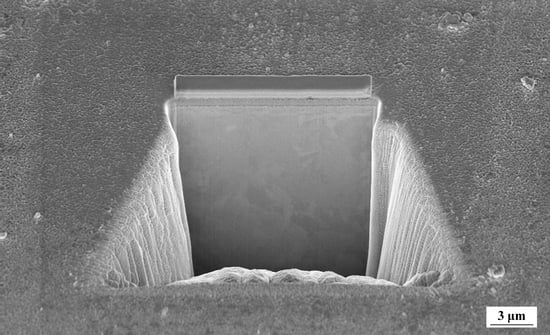
2.2. Microstructural Characterization
2.3. Sliding Properties
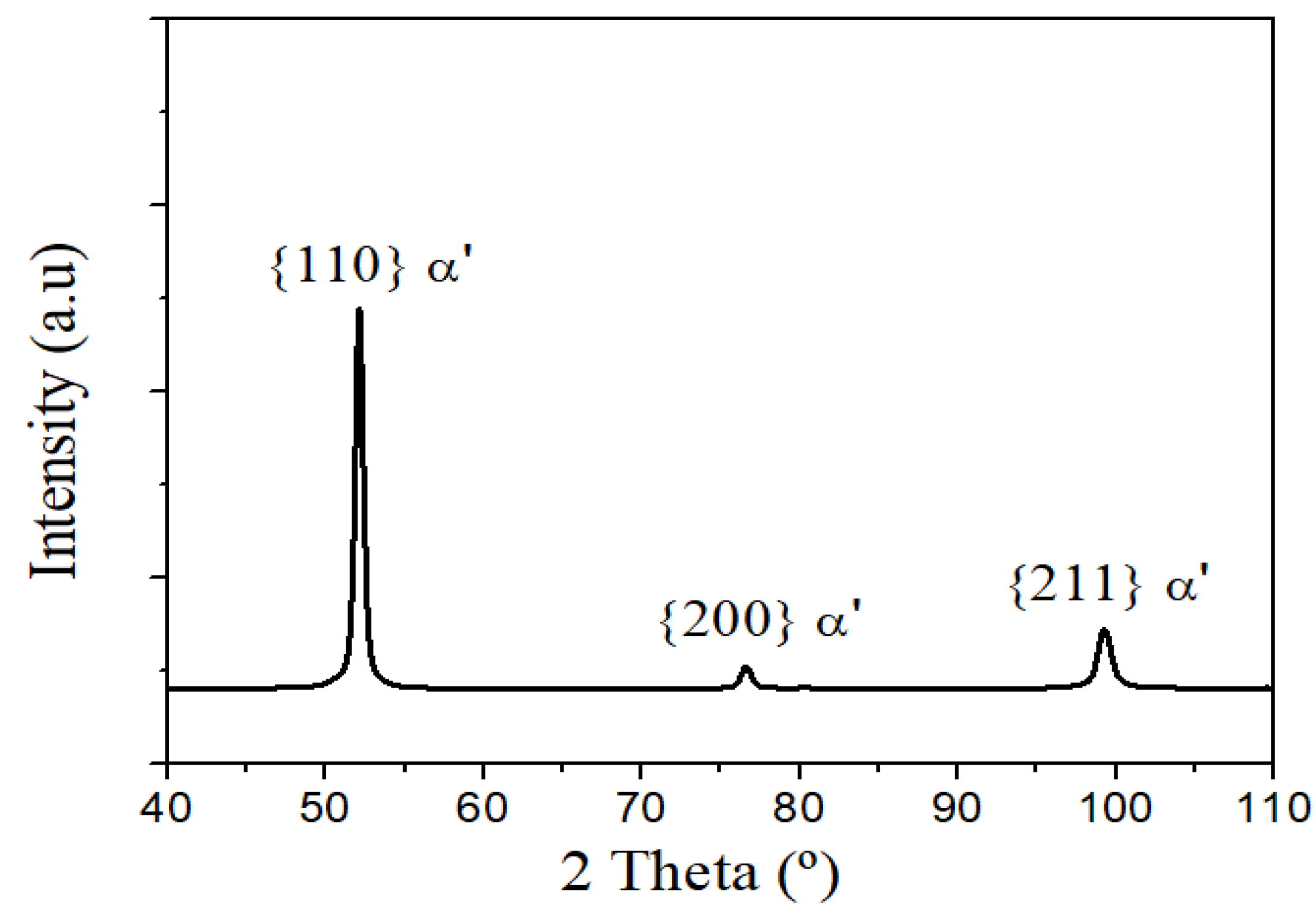
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magnée, A.; Drapier, J.M.; Coutsouradis, D.; Habrakan, L.; Dumont, J. Cobalt-Containing High-Strength Steels; Centre d’Information du Cobalt: Brussels, Belgium, 1974; p. 128. Available online: http://refhub.elsevier.com/S0360-3199(19)31930-5/sref1 (accessed on 10 March 2020).
- Nickel Development Institute. 18 per Cent Nickel Maraging Steels: Engineering Properties; No. 4419; Nickel Development Institute: Toronto, ON, Canada, 1976; Available online: https://nickelinstitute.org/media/1598/18_nickelmaragingsteel_engineeringproperties_4419_pdf (accessed on 15 March 2020).
- Schmidt, M.; Rohrbach, K. Heat Treating of Maraging Steel. In ASM International ASM Handbook; ASM International: Materials Park, OH, USA, 1991; Volume 4, pp. 219–228. [Google Scholar] [CrossRef]
- Petty, E.R. Martensite; Fundamentals and Technology: Edited by ER Petty. J. Appl. Cryst. 1970, 4, 402–403. [Google Scholar] [CrossRef]
- Rack, H.J.; Kalish, D. The strength and fracture toughness of 18 Ni (350) maraging steel. Metall. Mater. Trans. B 1971, 2, 3011–3020. [Google Scholar] [CrossRef]
- Cerra Florez, M.A.; Pereira, U.C.; Cardoso, J.L.; Oliveira, F.J.S.; Araújo, W.S.; Ribas, G.F.; De Abreu, H.F.G.; Da Silva, M.J.G. Microstructural characterization of grade 300 and grade 350 maraging steels and electrochemical study in hydrofluoric solution. J. Fluor. Chem. 2021, 243, 109738. [Google Scholar] [CrossRef]
- Viswanathan, U.K.; Dey, G.K.; Asundi, M.K. Precipitation hardening in 350 grade maraging steel. Metall. Trans. A 1993, 24, 2429–2442. [Google Scholar] [CrossRef]
- Klein, I.E.; Yaniv, A.E.; Sharon, J. The oxidation mechanism of Fe-Ni-Co alloys. Oxid. Met. 1981, 16, 99–106. [Google Scholar] [CrossRef]
- Rezek, J.; Klein, I.E.; Yahalom, J. Structure and corrosion resistance of oxides grown on maraging steel in steam at elevated temperatures. Appl. Surf. Sci. 1997, 108, 159–165. [Google Scholar] [CrossRef]
- Greyling, C.J.; Kotzé, I.A.; Viljoen, P.E. The kinetics of oxide film growth on Maraging steel as described by space-charge effects. Surf. Interface Anal. 1990, 16, 293–298. [Google Scholar] [CrossRef]
- Parkinson, G. Iron oxide surfaces. Surf. Sci. Rep. 2016, 71, 272–365. [Google Scholar] [CrossRef]
- Rodrigues, A.P.G.; Gomes, D.K.S.; Araújo, J.H.; Melo, D.M.A.; Oliveira, N.A.S.; Braga, R.M. Nanoferrites of nickel doped with cobalt: Influence of Co2+ on the structural and magnetic properties. J. Magn. Magn. Mater. 2015, 374, 748–754. [Google Scholar] [CrossRef]
- Diniz, V.C.S.; Dantas, B.B.; Figueiredo, A.R.; Cornejo, D.R.; Costa, A. Microstructural and magnetic evaluation of Fe3O4 synthesized by the combustion reaction method. Cerâmica 2015, 61, 298–302. [Google Scholar] [CrossRef][Green Version]
- Horcas, I.; Fernández, R. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, K.; Gao, Q.; Hussain, S.; Lv, Y. Investigation of morphology and texture properties of WSi2 coatings on W substrate based on contact-mode AFM and EBSD. Surf. Coat. Technol. 2020, 396, 125966. [Google Scholar] [CrossRef]
- Nwaogu, U.C.; Tiedje, N.S.; Hansen, H.N. A non-contact 3D method to characterize the surface roughness of castings. J. Mater. Process. Technol. 2013, 213, 59–68. [Google Scholar] [CrossRef]
- Gadelmawla, E.S.; Koura, M.M.; Maksoud, T.M.A.; Elewa, I.M.; Soliman, H.H. Roughness parameters. J. Mater. Process. Technol. 2002, 123, 133–145. [Google Scholar] [CrossRef]
- Chung, F. Quantitative Interpretation of X-ray Diffraction Patterns of Mixtures. I. Matrix-Flushing Method for Quantitative Multicomponent Analysis. J. Appl. Cryst. 1974, 7, 519. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, D.; Bu, H.; Deng, L.; Liu, H.; Yuan, P.; Du, P.; Song, H. XRD-based quantitative analysis of clay minerals using reference intensity ratios, mineral intensity factors, Rietveld, and full pattern summation methods: A critical review. Solid Earth Sci. 2018, 3, 16–29. [Google Scholar] [CrossRef]
- Viana, N.F.; Dos Santos, N.C.; De Abreu, H.F.G. The variant selection in the transformation from austenite to martensite in samples of maraging-350 steel. J. Mater. Res. Technol. 2013, 2, 298–302. [Google Scholar] [CrossRef][Green Version]
- JCPDS. X-Ray Diffraction Data Cards of the Joint Committee on Powder Diffraction Standards; International Center for Diffraction Data: Swarthmore, PA, USA, 1975. [Google Scholar]
- Dąbrowa, J.; Stygar, M.; Mikuła, A.; Knapik, A.; Mroczka, K.; Tejchman, W.; Martin, M. Synthesis and microstructure of the (Co,Cr,Fe,Mn,Ni)3O4 high entropy oxide characterized by spinel structure. Mater. Lett. 2018, 216, 32–36. [Google Scholar] [CrossRef]
- Conde, F.F.; Escobar, J.D.; Oliveira, J.P.; Béreš, M.; Jardini, A.L.; Bose, W.W.; Avila, J.A. Effect of thermal cycling and aging stages on the microstructure and bending strength of a selective laser melted 300-grade maraging steel. Mater. Sci. Eng. A 2019, 758, 192–201. [Google Scholar] [CrossRef]
- Florez, M.A.C.; Fargas Ribas, G.; Rovira, J.J.R.; Vilarrasa-García, E.; Rodríguez-Castellón, E.; Sousa, A.B.F.; Cardoso, J.L.; Gomes da Silva, M.J. Characterization Study of an Oxide Film Layer Produced under CO2/Steam Atmospheres on Two Different Maraging Steel Grades. Metals 2021, 11, 746. [Google Scholar] [CrossRef]
- Klein, I.E.; Sharon, J.; Yaniv, A.E. A mechanism of oxidation of ferrous aloys by super-heated steam. Scr. Metall. 1981, 15, 141–144. [Google Scholar] [CrossRef]
- Klein, I.E.; Yaniv, A.E.; Sharon, J. The Mechanism of Oxidation of Fe-Ni-Co Alloys; The Role of Ti And Mo. Appl. Surf. Sci. 1983, 14, 351–358. [Google Scholar] [CrossRef]
- Descotes, V.; Migot, S.; Robaut, F.; Bellot, J.P.; Perrin-Guérin, V.; Witzke, S.; Jardy, A. TEM Characterization of a Titanium Nitride (TiN) Inclusion in a Fe-Ni-Co Maraging Steel. Metall. Mater. Trans. A 2015, 46A, 2793. [Google Scholar] [CrossRef]
- Descotes, V.; Quatravaux, T.; Bellot, J.P.; Witzke, S.; Jardy, A. Titanium Nitride (TiN) Germination and Growth during Vacuum Arc Remelting of a Maraging Steel. Metals 2020, 10, 541. [Google Scholar] [CrossRef]
- Rodrigues, A.C.; Bernardi, H.H.; Otubo, J. Microstructural Analysis of Co-Free Maraging Steel Aged. J. Aerosp. Technol. Manag. 2014, 6, 389–394. [Google Scholar] [CrossRef]
- Moshka, O.; Pinkas, M.; Brosh, E.; Ezersky, V.; Meshi, L. Addressing the issue of precipitates in maraging steels—Unambiguous answer. Mater. Sci. Eng. A 2015, 638, 232–239. [Google Scholar] [CrossRef]
- De Faria, D.L.A.; Lopes, F.N. Heated goethite and natural hematite: Can Raman spectroscopy be used to differentiate them? Vib. Spectrosc. 2007, 45, 117–121. [Google Scholar] [CrossRef]
- Guo, R.; Dang, L.; Liu, Z.; Lei, Z. Incorporation of electroactive NiCo2S4 and Fe2O3 into graphene aerogel for high-energy asymmetric supercapacitor. Colloids Surf. A 2020, 602, 125110. [Google Scholar] [CrossRef]
- Kumar, P.R.; Jung, Y.H.; Bharathi, K.K.; Lim, C.H.; Kim, D.K. High capacity and low cost spinel Fe3O4 for the Na-ion battery negative electrode materials. Electrochim. Acta 2014, 146, 503–510. [Google Scholar] [CrossRef]
- Robinson, M.R.; Abdelmoula, M.; Mallet, M.; Coustel, R. Starch functionalized magnetite nanoparticles: New insight into the structural and magnetic properties. J. Solid State Chem. 2019, 277, 587–593. [Google Scholar] [CrossRef]
- Gao, X.; Bi, J.; Wang, W.; Liu, H.; Chen, Y.; Hao, X.; Sun, X.; Liu, R. Morphology-controllable synthesis of NiFe2O4 growing on graphene nanosheets as advanced electrode material for high performance supercapacitors. J. Alloys Compd. 2020, 826, 154088. [Google Scholar] [CrossRef]
- Wang, W.; Ding, Z.; Zhao, X. Microstructure and magnetic properties of MFe2O4 (M = Co, Ni, and Mn) ferrite nanocrystals prepared using colloid mill and hydrothermal method. J. Appl. Phys. 2015, 117, 17A328. [Google Scholar] [CrossRef]
- Routray, K.L.; Saha, S.; Behera, D. Nanosized CoFe2O4-graphene nanoplatelets with massive dielectric enhancement for high frequency device application. Mater. Sci. Eng. B 2020, 257, 114548. [Google Scholar] [CrossRef]
- Luo, D.W.; Shen, Z.S. Oxidation behavior of Kovar alloy in controlled atmosphere. Acta Metall. Sin. Engl. Lett. 2008, 21, 409–418. [Google Scholar] [CrossRef]
- Surman, P.L. The oxidation of iron at controlled oxygen partial pressures—I. Hydrogen/water vapour. Corros. Sci. 1973, 13, 113–124. [Google Scholar] [CrossRef]
- Subbaraman, R.; Deshmukh, S.A.; Sankaranarayanan, S.K. Atomistic insights into early stage oxidation and nanoscale oxide growth on Fe (100), Fe (111) and Fe (110) surfaces. J. Phys. Chem. C 2013, 117, 5195–5207. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, S.; Yang, Z.; Xu, X. Kinetics and intermediate phases in epitaxial growth of Fe3O4 films from deposition and thermal reduction. J. Appl. Phys. 2016, 120, 085313. [Google Scholar] [CrossRef]
- Hong, H.; Memon, N.K.; Dong, Z.; Kear, B.H.; Stephen, D.T. Flame synthesis of gamma-iron-oxide (γ-Fe2O3) nanocrystal films and carbon nanotubes on stainless-steel substrates. Proc. Combust. Inst. 2019, 37, 1249–1256. [Google Scholar] [CrossRef]
- Jeon, B.; Van Overmeere, Q.; Van Duin, A.C.; Ramanathan, S. Nanoscale oxidation and complex oxide growth on single crystal iron surfaces and external electric field effects. Phys. Chem. Chem. Phys. 2013, 15, 1821–1830. [Google Scholar] [CrossRef]
- Genuzio, F.; Sala, A.; Schmidt, T.; Menzel, D.; Freund, H.J. Phase transformations in thin iron oxide films: Spectromicroscopic study of velocity and shape of the reaction fronts. Surf. Sci. 2016, 648, 177–187. [Google Scholar] [CrossRef]
- Tsukimura, K.; Sasaki, S.; Kimizuka, N. Cation Distributions in Nickel Ferrites. Jpn. J. Appl. Phys. 1997, 36, 3609. [Google Scholar] [CrossRef]
- Bliem, R.; Pavelec, J.; Gamba, O.; McDermott, E.; Wang, Z.; Gerhold, S.; Blaha, P. Adsorption and incorporation of transition metals at the magnetite Fe3O4 (001). Phys. Rev. B 2015, 92, 75440. [Google Scholar] [CrossRef]
- Pardavi-Horvath, M. Microwave applications of soft ferrites. J. Magn. Magn. Mater. 2000, 215–216, 171–183. [Google Scholar] [CrossRef]
- Ghasemi Parizi, M.J.; Shahverdi, H.; Roa, J.J.; Pipelzadeh, E.; Martinez, M.; Cabot, A.; Guardia, P. Improving Mechanical Properties of Glass Fiber Reinforced Polymers through Silica-Based Surface Nanoengineering. ACS Appl. Polym. Mater. 2020, 2, 2667–2675. [Google Scholar] [CrossRef]
- ASTM C1624. Standard Test Method for Adhesion Strength and Mechanical Failure Modes of Ceramic Coatings by Quantitative Single Point Scratch Testing; Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]



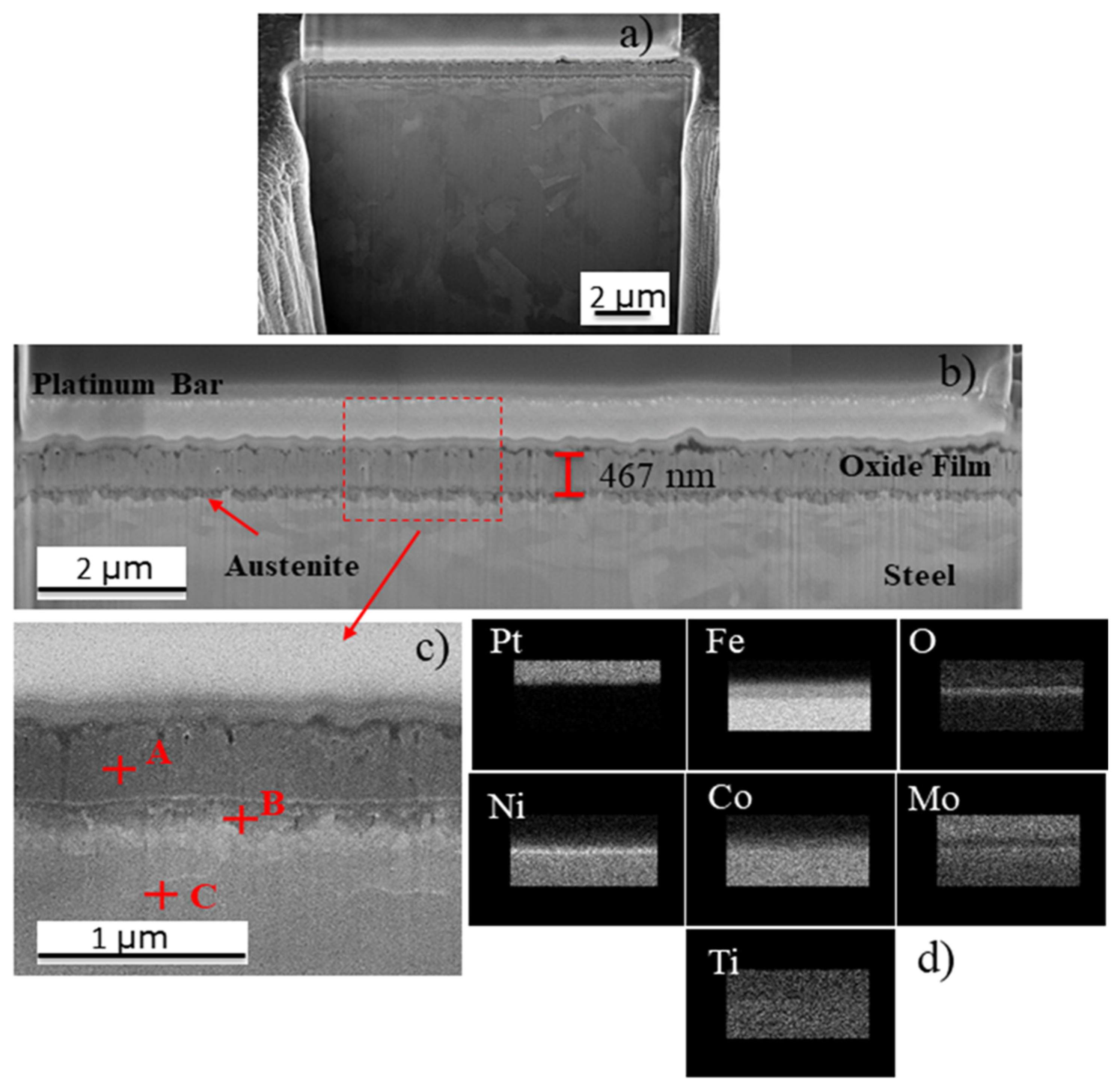

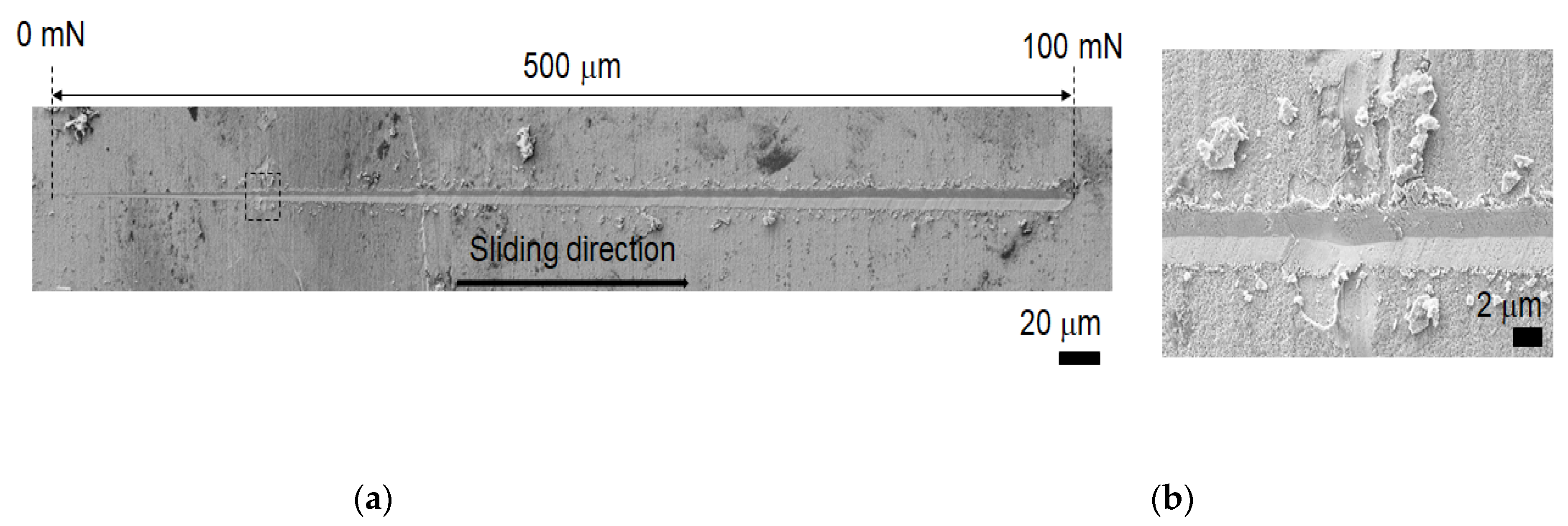
| Ni | Co | Mo | Ti | Cr | V | Si | Al | Cu | Mn | C | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 18.28 | 9.51 | 4.80 | 0.73 | 0.12 | 0.10 | 0.08 | 0.07 | 0.05 | 0.03 | <0.01 | Bal. |
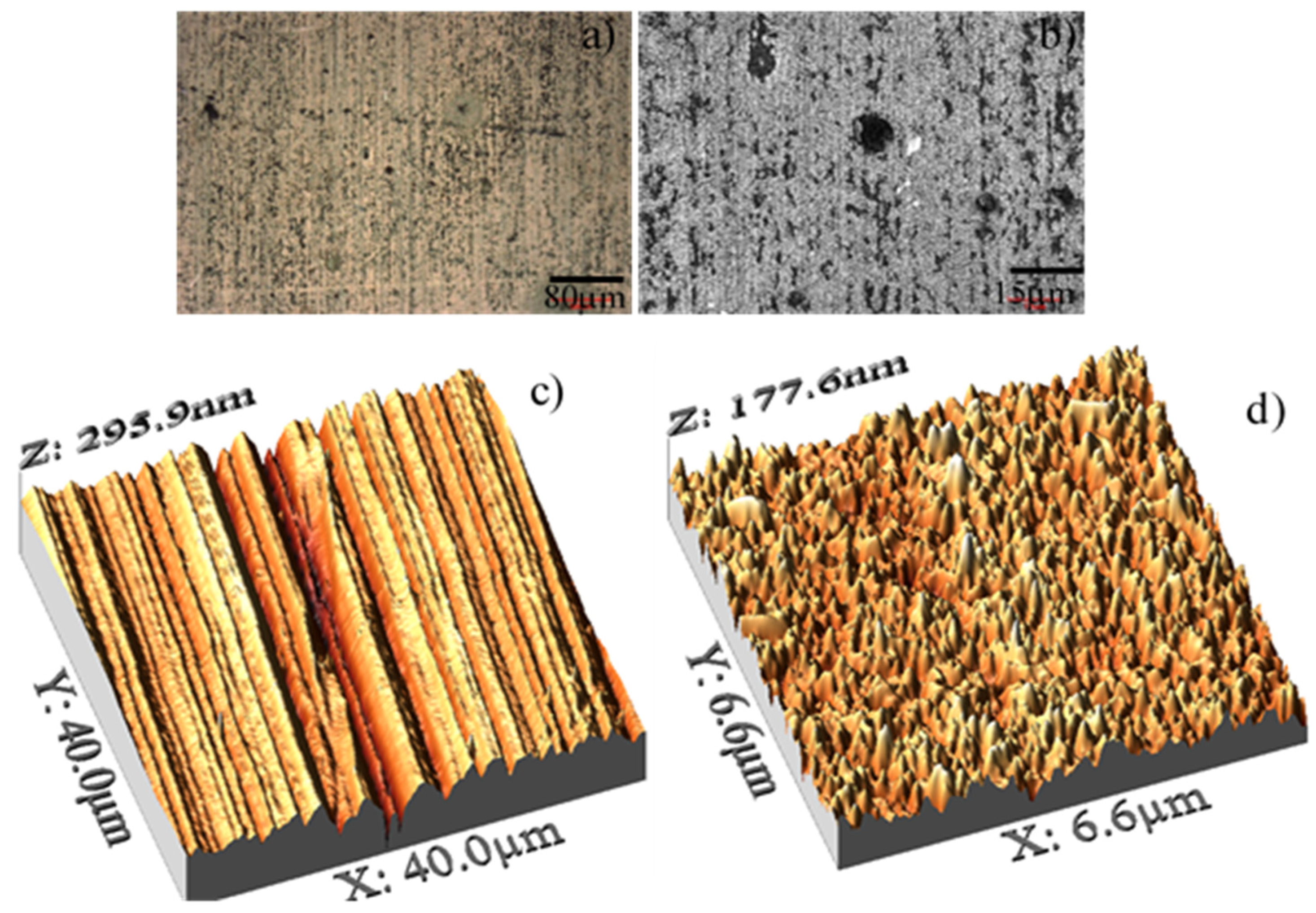
| Roughness (nm) | ||
|---|---|---|
| Parameter | Steel surface | Oxide surface |
| Roughness Average (Ra) | 52 ± 5 | 17 ± 0.5 |
| Roughness Maximum (Rz) | 295 | 177 |
| Max Peak ht (Rp) | 110 | 96 |
| Maximum Depth (Rv) | −185 | −81 |
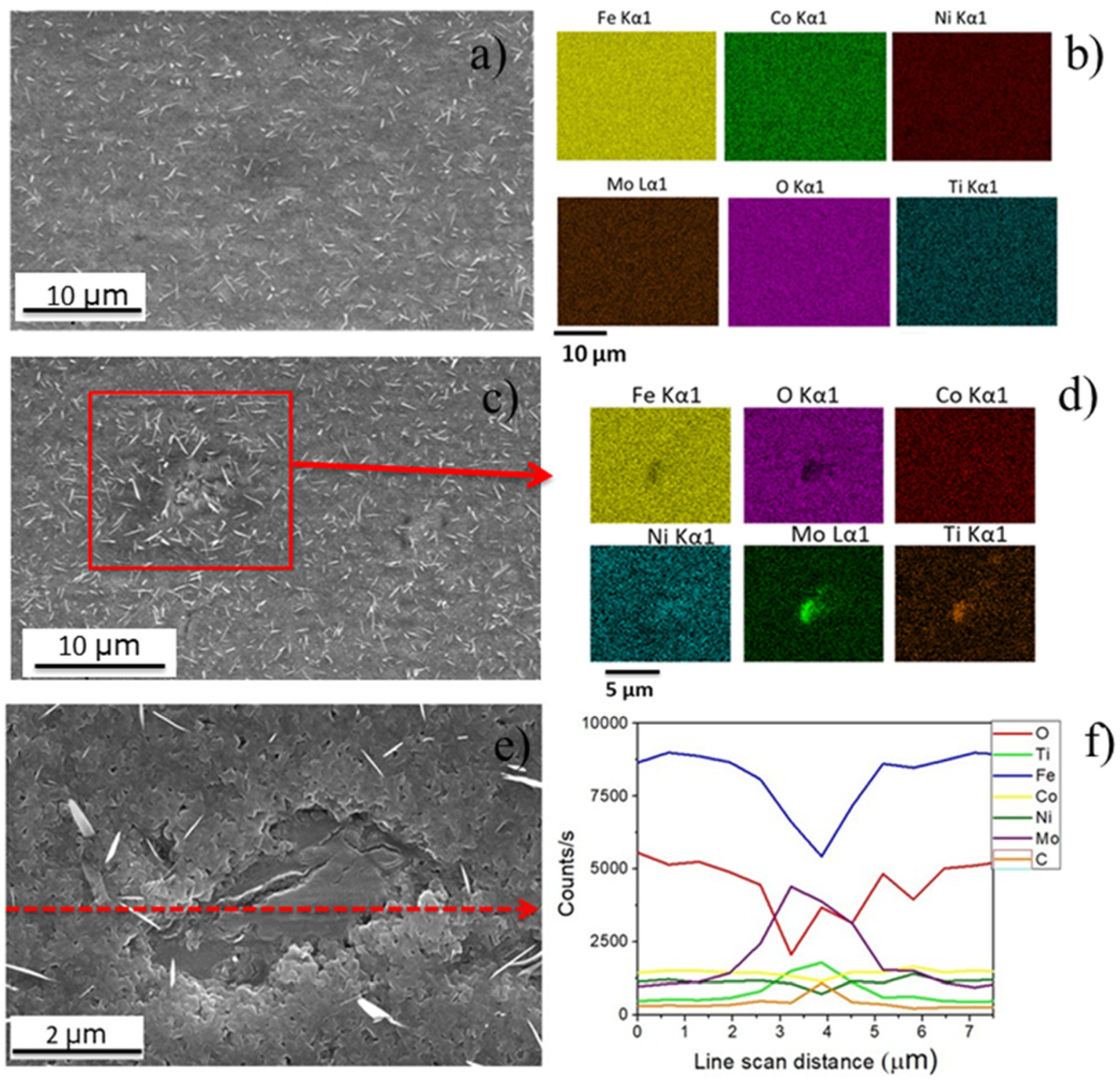
| EDS Quantitative Analysis from A and B Points (wt %) | |||
|---|---|---|---|
| Element Number | Element Symbol | Point A | Point B |
| 26 | Fe | 38.87 | 33.46 |
| 8 | O | 36.37 | 62.30 |
| 27 | Co | 11.19 | 1.91 |
| 28 | Ni | 12.93 | 2.33 |
| 42 | Mo | 0.54 | - |
| 22 | Ti | 0.10 | - |
| EDS Quantitative Analysis from Points A, B and C (wt %) | ||||
|---|---|---|---|---|
| Elem. Number | Elem. Symbol | Point A | Point B | Point C |
| 26 | Fe | 44.08 | 38.11 | 63.69 |
| 8 | O | 26.07 | 2.33 | - |
| 27 | Co | 13.28 | 11.40 | 12.08 |
| 28 | Ni | 15.81 | 44.17 | 19.64 |
| 42 | Mo | 0.63 | 3.27 | 3.99 |
| 22 | Ti | 0.15 | 0.72 | 0.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerra Florez, M.A.; Ribas, G.F.; Cardoso, J.L.; Mateo García, A.M.; Roa Rovira, J.J.; Bastos-Neto, M.; de Abreu, H.F.G.; da Silva, M.J.G. Oxidation Behavior of Maraging 300 Alloy Exposed to Nitrogen/Water Vapor Atmosphere at 500 °C. Metals 2021, 11, 1021. https://doi.org/10.3390/met11071021
Cerra Florez MA, Ribas GF, Cardoso JL, Mateo García AM, Roa Rovira JJ, Bastos-Neto M, de Abreu HFG, da Silva MJG. Oxidation Behavior of Maraging 300 Alloy Exposed to Nitrogen/Water Vapor Atmosphere at 500 °C. Metals. 2021; 11(7):1021. https://doi.org/10.3390/met11071021
Chicago/Turabian StyleCerra Florez, Mauro Andres, Gemma Fargas Ribas, Jorge Luiz Cardoso, Antonio Manuel Mateo García, Joan Josep Roa Rovira, Moises Bastos-Neto, Hamilton Ferreira Gomes de Abreu, and Marcelo José Gomes da Silva. 2021. "Oxidation Behavior of Maraging 300 Alloy Exposed to Nitrogen/Water Vapor Atmosphere at 500 °C" Metals 11, no. 7: 1021. https://doi.org/10.3390/met11071021
APA StyleCerra Florez, M. A., Ribas, G. F., Cardoso, J. L., Mateo García, A. M., Roa Rovira, J. J., Bastos-Neto, M., de Abreu, H. F. G., & da Silva, M. J. G. (2021). Oxidation Behavior of Maraging 300 Alloy Exposed to Nitrogen/Water Vapor Atmosphere at 500 °C. Metals, 11(7), 1021. https://doi.org/10.3390/met11071021