Study of Microbial Cultures for the Bioleaching of Scandium from Alumina Industry By-Products †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bauxite Residue
2.2. Sources of Microorganisms and Cultivation Mineral Media
2.3. Bioleaching Experiments and Sampling
2.4. Analytical Methods
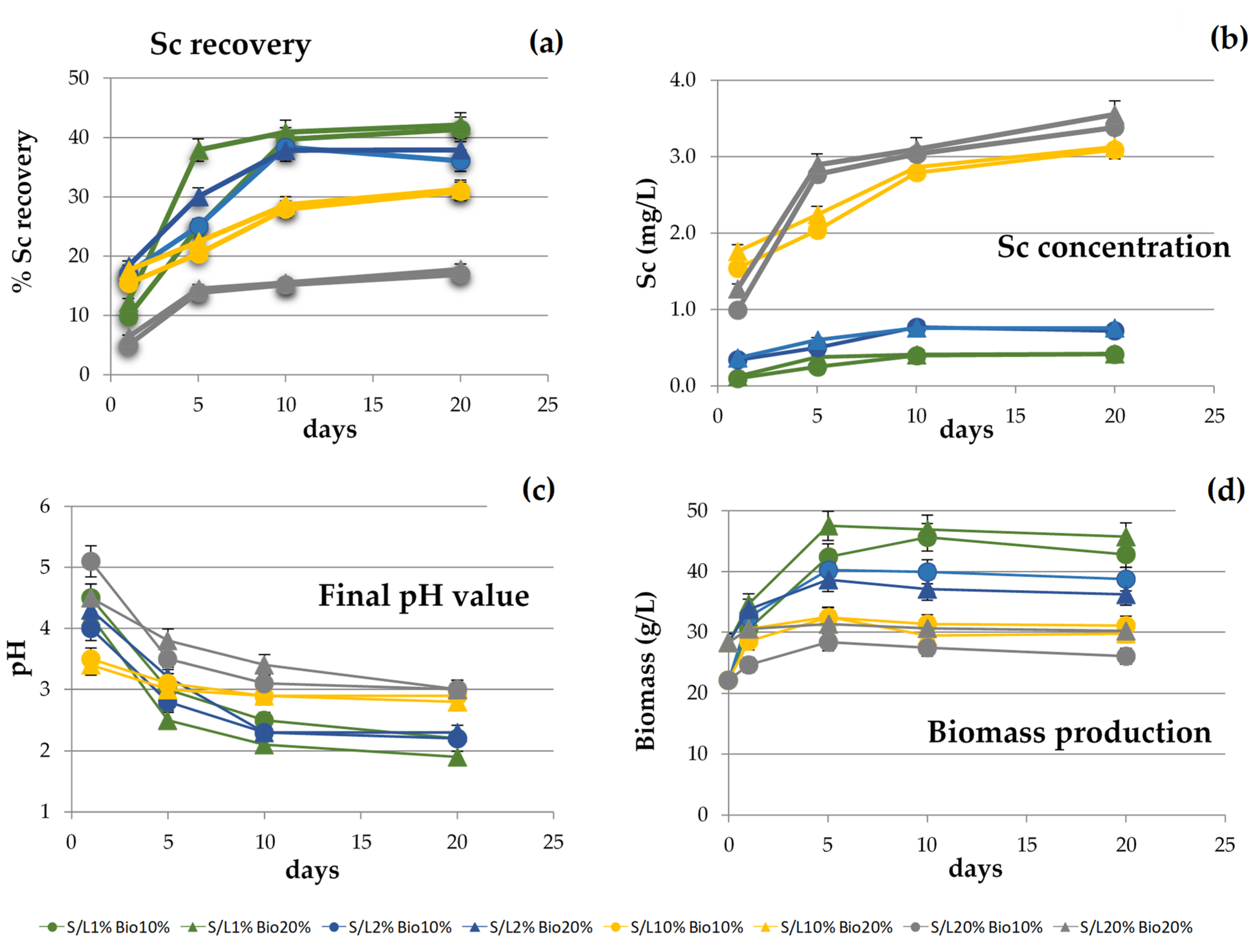
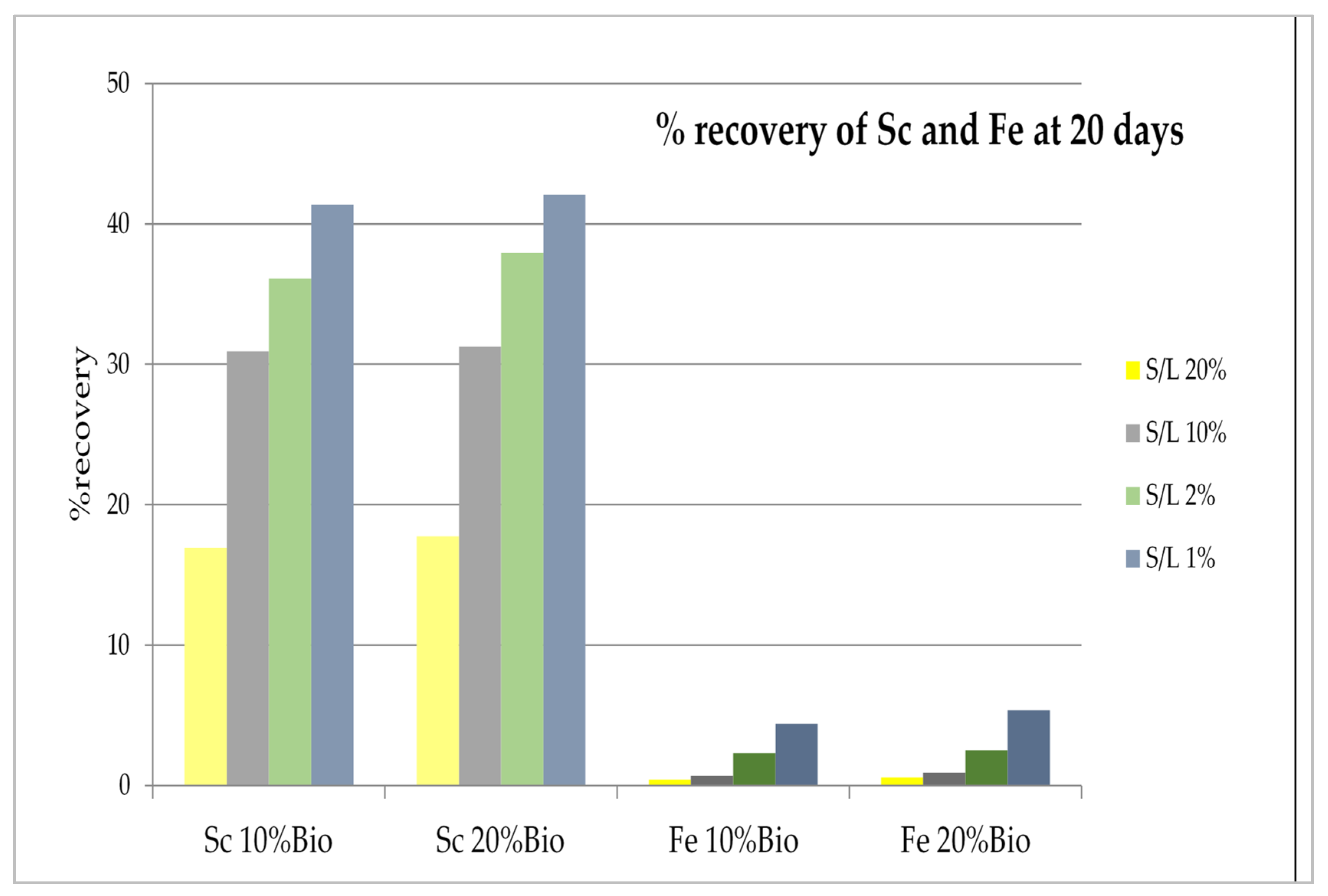
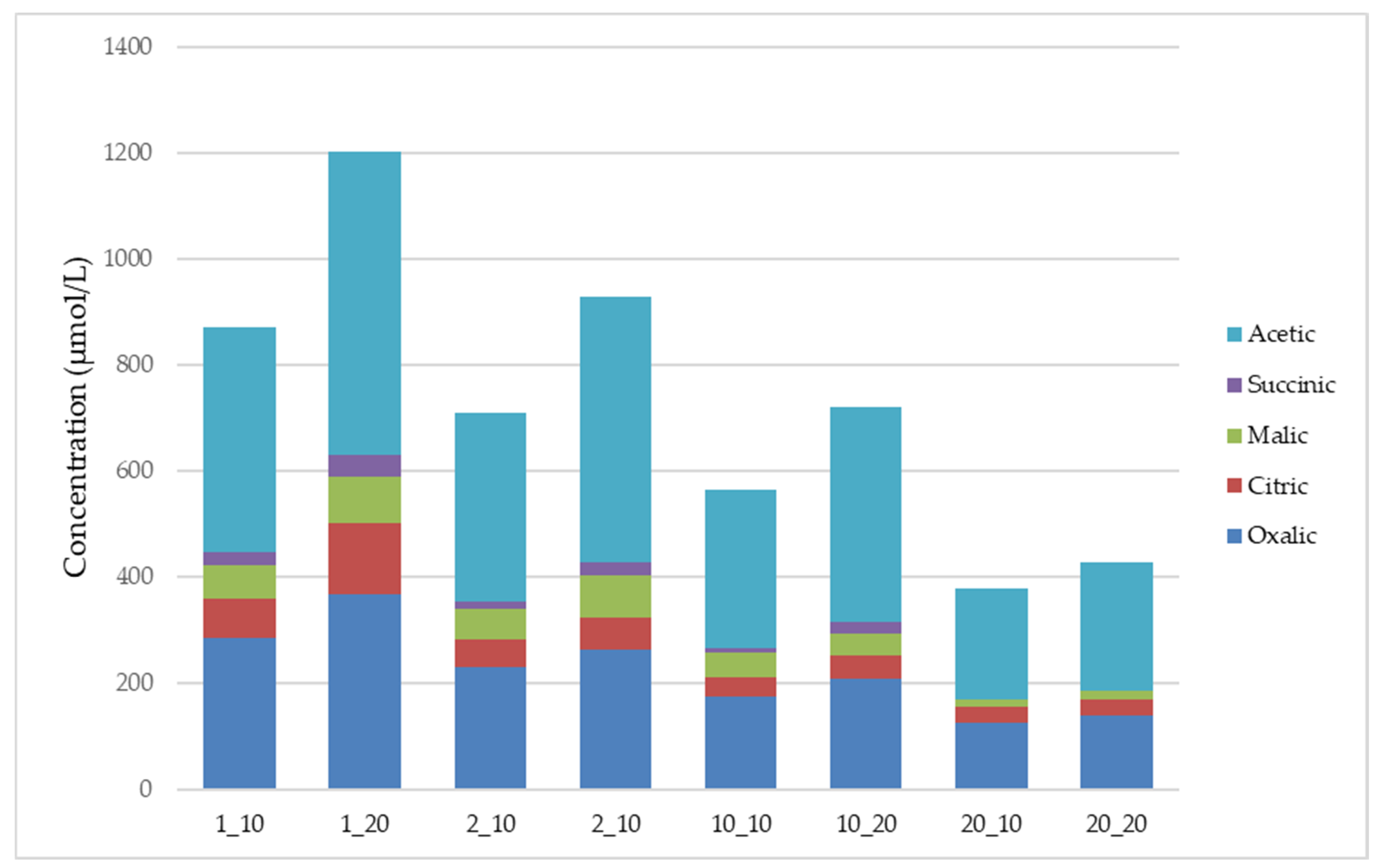
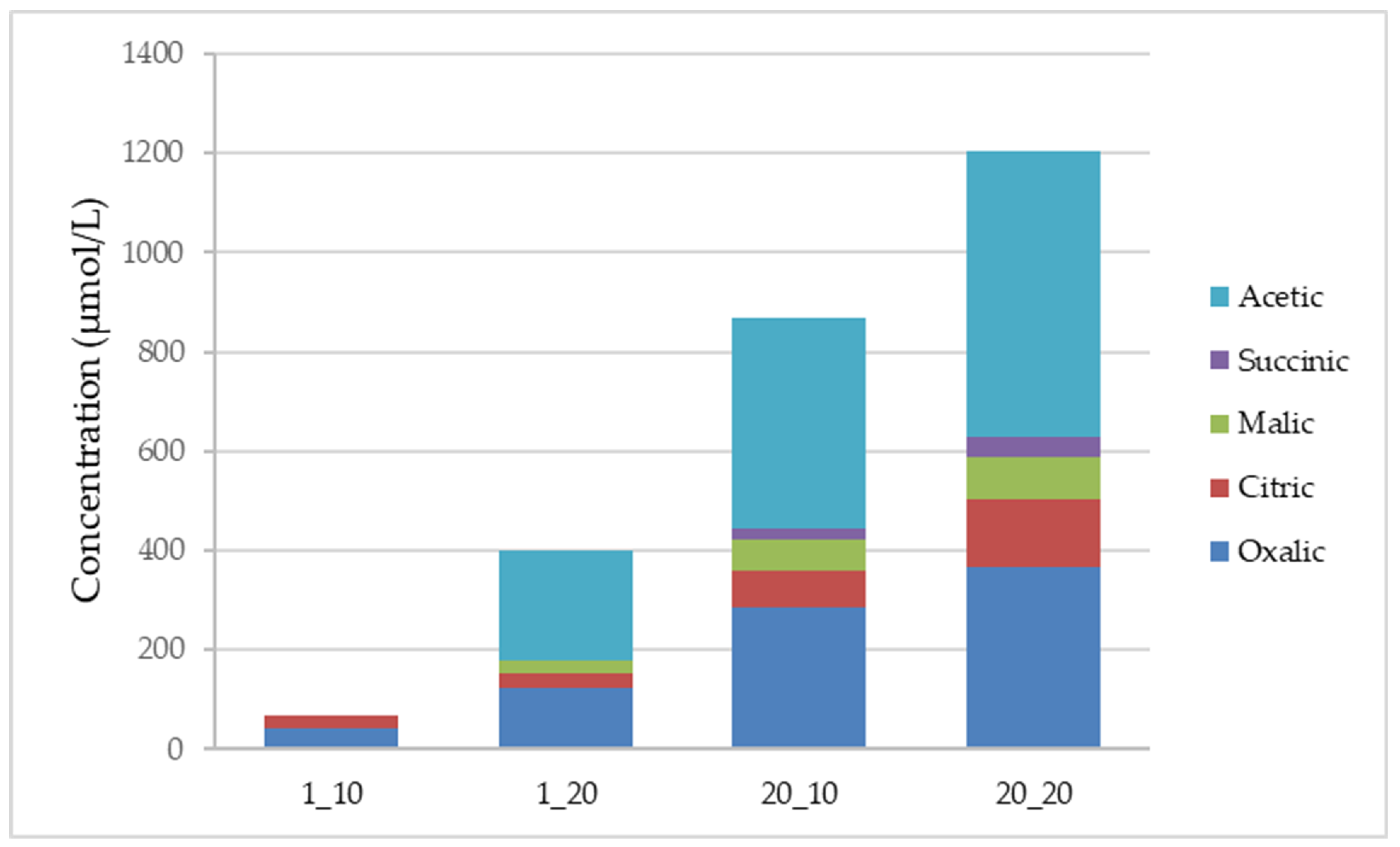
3. Results and Discussion
3.1. Preliminary Tests
3.2. Parameters Evaluation
3.3. Organic Acids Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Sun, N.; Tang, H.; Sun, W. A review on comprehensive utilization of red mud and prospect analysis. Minerals 2019, 9, 362. [Google Scholar] [CrossRef] [Green Version]
- Ochsenkuehn-Petropoulou, M.; Tsakanika, L.A.; Lymperopoulou, T.; Ochsenkuehn, K.M.; Hatzilyberis, K.; Georgiou, P.; Stergiopoulos, C.; Serifi, O.; Tsopelas, F. Efficiency of sulfuric acid on selective scandium leachability from bauxite residue. Metals 2018, 8, 915. [Google Scholar] [CrossRef] [Green Version]
- Ochsenkühn-Petropoulou, M.; Lyberopulu, T.; Parissakis, G. Recovery of lanthanides and yttrium from red mud by selective leaching. Anal. Chim. Acta 1996, 319, 249–254. [Google Scholar] [CrossRef]
- European Commission. Critical Raw Materials. Fourth List of CRMs; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Liu, Y.; Naidu, R. Hidden values in bauxite residue (red mud): Recovery of metals. Waste Manag. 2014, 34, 2662–2673. [Google Scholar] [CrossRef] [PubMed]
- Akcil, A.; Akhmadiyeva, N.; Abdulvaliyev, R.; Abhilash, M.P. Overview on extraction and separation of rare earth elements from red mud: Focus on scandium. Miner. Process. Extr. Metall. Rev. 2018, 39, 145–151. [Google Scholar] [CrossRef]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Smelting of bauxite residue (red mud) in view of iron and selective rare earths recovery. J. Sustain. Metall. 2015, 2, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Power, G.; Gräfe, M.; Klauber, C. Bauxite residue issues: I. Current management, disposal and storage practices. Hydrometallurgy 2011, 108, 33–45. [Google Scholar] [CrossRef]
- Ochsenkühn-Petropulu, M.; Hatzilyberis, K.S.; Mendrinos, L.N.; Salmas, C.E. Pilot-plant investigation of the leaching process for the recovery of scandium from red mud. Ind. Eng. Chem. Res. 2002, 41, 5794–5801. [Google Scholar] [CrossRef]
- Tsakanika, L.; Ochsenkühn-Petropoulou, M.; Mendrinos, L. Investigation of the separation of Rare Earth elements from red mud by use of reversed-phase HPLC. Anal. Bioanal. Chem. 2004, 379, 796–802. [Google Scholar] [CrossRef]
- Tsakanika, L.A.; Ochsenkühn-Petropoulou, M. Separation and recovery of rare earths after red mud leaching by cation-exchange chromatography. In Proceedings of the International Bauxite Residue Valorization and Best Practices Conference (BR2015), Leuven, Belgium, 5–7 October 2015. [Google Scholar]
- Project SCALE: Production of Scandium Compounds and Scandium Aluminum Alloys from European Metallurgical By-Products. European Community’s Horizon 2020 Programme. Available online: https://cordis.europa.eu/project/rcn/206331/factsheet/en&http://scale-project.eu/ (accessed on 10 May 2021).
- Lymperopoulou, T.; Georgiou, P.; Tsakanika, L.A.; Hatzilymperis, K.; Ochsenkühn-Petropoulou, M. Optimizing Conditions for Scandium Extraction from Bauxite Residue Using Taguchi Methodology. Minerals 2019, 9, 236–249. [Google Scholar] [CrossRef] [Green Version]
- Hatzilyberis, K.; Tsakanika, L.A.; Lymperopoulou, T.; Georgiou, P.; Kiskira, K.; Tsopelas, F.; Ochsenkühn, K.M.; Ochsenkühn-Petropoulou, M. Design of an advanced hydrometallurgy process for the intensified and optimized industrial recovery of scandium from bauxite residue. Chem. Eng. Process. Process Intensif. 2020, 155, 108015–108033. [Google Scholar] [CrossRef]
- Davris, P.; Balomenos, E.; Panias, D.; Paspaliaris, I. Selective leaching of rare earth elements from bauxite residue (red mud), using a functionalized hydrophobic ionic liquid. Hydrometallurgy 2016, 164, 125–135. [Google Scholar] [CrossRef]
- Davris, P.; Marinos, D.; Balomenos, E.; Alexandri, A.; Gregou, M.; Panias, D.; Paspaliaris, I. Leaching of rare earth elements from ‘Rödberg’ore of Fen carbonatite complex deposit, using the ionic liquid HbetTf2N. Hydrometallurgy 2018, 175, 20–27. [Google Scholar] [CrossRef]
- Borra, C.R.; Mermans, J.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Selective recovery of rare earths from bauxite residue by combination of sulfation, roasting and leaching. Miner. Eng. 2016, 92, 151–159. [Google Scholar] [CrossRef]
- Qu, Y.; Lian, B. Bioleaching of rare earth and radioactive elements from red mud using Penicillium tricolor RM-10. Bioresour. Technol. 2013, 136, 16–23. [Google Scholar] [CrossRef]
- Kiskira, K.; Papirio, S.; Van Hullebusch, E.D.; Esposito, G. Fe (II)-mediated autotrophic denitrification: A new bioprocess for iron bioprecipitation/biorecovery and simultaneous treatment of nitrate-containing wastewaters. Int. Biodeterior. Biodegrad. 2017, 119, 631–648. [Google Scholar] [CrossRef]
- Kiskira, K.; Papirio, S.; Pechaud, Y.; Matassa, S.; van Hullebusch, E.D.; Esposito, G. Evaluation of Fe (II)-driven autotrophic denitrification in packed-bed reactors at different nitrate loading rates. Process Saf. Environ. Prot. 2020, 142, 317–324. [Google Scholar] [CrossRef]
- Santini, T.C.; Kerr, J.L.; Warren, L.A. Microbially-driven strategies for bioremediation of bauxite residue. J. Hazard. Mater. 2015, 293, 131–157. [Google Scholar] [CrossRef] [Green Version]
- Qu, Y.; Li, H.; Wang, X.; Tian, W.; Shi, B.; Yao, M.; Zhang, Y. Bioleaching of Major, Rare Earth, and Radioactive Elements from Red Mud by using Indigenous Chemoheterotrophic Bacterium Acetobacter sp. Minerals 2019, 9, 67. [Google Scholar] [CrossRef] [Green Version]
- Valix, M.; Loon, L.O. Adaptive tolerance behaviour of fungi in heavy metals. Miner. Eng. 2003, 16, 193–198. [Google Scholar] [CrossRef]
- Brisson, V.L.; Zhuang, W.Q.; Alvarez-Cohen, L. Bioleaching of rare earth elements from monazite sand. Biotechnol. Bioeng. 2016, 113, 339–348. [Google Scholar] [CrossRef] [Green Version]
- Faramarzi, M.A.; Mogharabi-Manzari, M.; Brandl, H. Bioleaching of metals from wastes and low-grade sources by HCN-forming microorganisms. Hydrometallurgy 2020, 191, 105228–105239. [Google Scholar] [CrossRef]
- Dev, S.; Sachan, A.; Dehghani, F.; Ghosh, T.; Briggs, B.R.; Aggarwal, S. Mechanisms of biological recovery of rare-earth elements from industrial and electronic wastes: A review. Chem. Eng. J. 2020, 397, 124596–124613. [Google Scholar] [CrossRef]
- Qu, Y.; Li, H.; Wang, X.; Tian, W.; Shi, B.; Yao, M.; Cao, L.; Yue, L. Selective parameters and bioleaching kinetics for leaching vanadium from red mud using Aspergillus niger and Penicillium tricolor. Minerals 2019, 9, 697. [Google Scholar] [CrossRef] [Green Version]
- Qu, Y.; Li, H.; Tian, W.; Wang, X.; Wang, X.; Jia, X.; Shi, B.; Song, G.; Tang, Y. Leaching of valuable metals from red mud via batch and continuous processes by using fungi. Miner. Eng. 2015, 81, 1–4. [Google Scholar] [CrossRef]
- Pedram, H.; Hosseini, M.R.; Bahrami, A. Utilization of A. niger strains isolated from pistachio husk and grape skin in the bioleaching of valuable elements from red mud. Hydrometallurgy 2020, 198, 105495–105507. [Google Scholar] [CrossRef]
- Čížková, M.; Mezricky, D.; Rucki, M.; Tóth, T.M.; Náhlík, V.; Lanta, V.; Bišová, K.; Zachleder, V.; Vítová, M. Bio-mining of lanthanides from red mud by green microalgae. Molecules 2019, 24, 1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiskira, K.; Papirio, S.; van Hullebusch, E.D.; Esposito, G. Influence of pH, EDTA/Fe (II) ratio, and microbial culture on Fe (II)-mediated autotrophic denitrification. Environ. Sci. Pollut. Res. 2017, 24, 21323–21333. [Google Scholar] [CrossRef]
- Kiskira, K.; Papirio, S.; Mascolo, M.C.; Fourdrin, C.; Pechaud, Y.; van Hullebusch, E.D.; Esposito, G. Mineral characterization of the biogenic Fe (III)(hydr) oxides produced during Fe (II)-driven denitrification with Cu, Ni and Zn. Sci. Total Environ. 2019, 687, 401–412. [Google Scholar] [CrossRef]
- Michalopoulos, I.; Lytras, G.M.; Mathioudakis, D.; Lytras, C.; Goumenos, A.; Zacharopoulos, I.; Papadopoulou, K.; Lyberatos, G. Hydrogen and Methane Production from Food Residue Biomass Product (FORBI). Waste Biomass Valoris. 2019, 1, 1–9. [Google Scholar] [CrossRef]
- WEF. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1995. [Google Scholar]
- Bosecker, K. Bioleaching: Metal solubilization by microorganisms. FEMS Microbiol. Rev. 1997, 20, 591–604. [Google Scholar] [CrossRef]
- Burgstaller, W.; Schinner, F. Leaching of metals with fungi. J. Biotechnol. 1993, 27, 91–116. [Google Scholar] [CrossRef]
- Amiri, F.; Yaghmaei, S.; Mousavi, S.M.; Sheibani, S. Recovery of metals from spent refinery hydrocracking catalyst using adapted Aspergillus niger. Hydrometallurgy 2011, 109, 65–71. [Google Scholar] [CrossRef]
- Bosshard, P.P.; Bachofen, R.; Brandl, H. Metal leaching of fly ash from municipal waste incineration by Aspergillus niger. Environ. Sci. Technol. 1996, 30, 3066–3070. [Google Scholar] [CrossRef]
| Culture | Acetobacter tropicalis | Digestate Inoculum | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Temp | 30 °C | 20 °C | 30 °C | ||||||
| S/L | 10% | 20% | 30% | 10% | 20% | 30% | 10% | 20% | 30% |
| days | % Sc recovery | ||||||||
| 1 | 20.4 | 5.5 | 2.3 | 8.2 | 1.9 | 0.6 | 9.9 | 3.7 | 2.9 |
| 5 | 24.7 | 10.6 | 2.3 | 9.0 | 2.1 | 0.6 | 18.1 | 8.4 | 4.8 |
| 10 | 34.6 | 11.5 | 2.3 | 24.2 | 1.7 | 0.5 | 29.9 | 11.1 | 5.2 |
| 30 | 38.4 | 14.4 | 2.2 | 25.1 | 1.9 | 0.5 | 30.0 | 12.1 | 5.2 |
| Culture | Acetobacter tropicalis | Digestate Inoculum | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Temp | 30 °C | 20 °C | 30 °C | ||||||
| S/L | 10% | 20% | 30% | 10% | 20% | 30% | 10% | 20% | 30% |
| days | pH | ||||||||
| 1 | 3.2 | 3.6 | 3.4 | 3.5 | 4.5 | 4.9 | 5.1 | 6.8 | 7.0 |
| 5 | 2.4 | 3.0 | 3.1 | 3.6 | 4.3 | 4.8 | 3.6 | 4.9 | 6.9 |
| 10 | 2.0 | 3.0 | 3.1 | 3.0 | 4.2 | 4.8 | 3.0 | 4.2 | 5.6 |
| 30 | 1.9 | 2.9 | 3.1 | 2.6 | 4.2 | 4.8 | 2.7 | 4.0 | 5.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiskira, K.; Lymperopoulou, T.; Tsakanika, L.-A.; Pavlopoulos, C.; Papadopoulou, K.; Ochsenkühn, K.-M.; Lyberatos, G.; Ochsenkühn-Petropoulou, M. Study of Microbial Cultures for the Bioleaching of Scandium from Alumina Industry By-Products. Metals 2021, 11, 951. https://doi.org/10.3390/met11060951
Kiskira K, Lymperopoulou T, Tsakanika L-A, Pavlopoulos C, Papadopoulou K, Ochsenkühn K-M, Lyberatos G, Ochsenkühn-Petropoulou M. Study of Microbial Cultures for the Bioleaching of Scandium from Alumina Industry By-Products. Metals. 2021; 11(6):951. https://doi.org/10.3390/met11060951
Chicago/Turabian StyleKiskira, Kyriaki, Theopisti Lymperopoulou, Lamprini-Areti Tsakanika, Charalampos Pavlopoulos, Konstantina Papadopoulou, Klaus-Michael Ochsenkühn, Gerasimos Lyberatos, and Maria Ochsenkühn-Petropoulou. 2021. "Study of Microbial Cultures for the Bioleaching of Scandium from Alumina Industry By-Products" Metals 11, no. 6: 951. https://doi.org/10.3390/met11060951
APA StyleKiskira, K., Lymperopoulou, T., Tsakanika, L.-A., Pavlopoulos, C., Papadopoulou, K., Ochsenkühn, K.-M., Lyberatos, G., & Ochsenkühn-Petropoulou, M. (2021). Study of Microbial Cultures for the Bioleaching of Scandium from Alumina Industry By-Products. Metals, 11(6), 951. https://doi.org/10.3390/met11060951










