1. Introduction
The current high demand for neodymium (Nd) and praseodymium (Pr) and their alloys is attributed to their superior magnetic flux density needed for developing very strong permanent magnets [
1,
2]. These rare earth (RE) metals are mostly extracted from their oxides via molten salt electrolysis. The electrolytic production of Nd and Pr has advantages, such as high purity of end product, low energy consumption, and high efficiency [
2,
3,
4]. The efficiency and environmental footprint of the electrolysis process, however, largely depend on the effective dissolution of the rare earth metal oxide (REO) in the electrolyte. The most suitable electrolyte composition for dissolving REO consists of rare earth fluoride (REF), alkali metal fluoride (AF), and in some cases small amounts of alkali earth metal fluorides (AEF) [
2,
3,
5]. In general, the solubility of the REO increases with increasing content of REF in the fluoride electrolyte [
5]. Alkali metal fluoride, usually LiF, is added to reduce the liquidus temperature and improve the ionic conductivity [
5] of the electrolyte.
Suitable levels of REO dissolved in the fluoride electrolyte are important for smooth operations during production [
2]. This is because oxide depletion in molten fluorides generates perfluorocarbon (PFC) gases, notably CF
4 and C
2F
6 [
1,
2,
6], with very high global warming potentials compared with CO
2.
Table 1 presents the main cell reactions during the Nd/Pr electrolysis process with their reversible potentials at 1040 °C. The formation of PFC gases occurs at significantly higher potentials than the CO/CO
2 formation.
Thus, for efficient and environmentally friendly production, the levels of REO electroactive species dissolved in the fluoride electrolyte must be well controlled. Proper control of oxide levels in the fluoride electrolyte can, however, be challenging for the rare earth metal industry due to the current lack of state-of-the-art monitoring technology as compared with, for example, electrowinning of aluminium metal. The use of modified linear sweep voltammetry to determine the concentrations of dissolved aluminium oxide species in cryolite (Na
3AlF
6) melts has been reported for primary aluminium production [
7,
8,
9,
10,
11]. However, no such studies have been reported for RE metal production.
Two principles, namely, voltage and current ramping, may be used to determine the oxide concentration in molten fluoride-based electrolytes with carbon anodes. This phenomenon is triggered by the depletion of dissolved oxide species on the surface of the carbon anode, resulting in the decomposition of the fluoride melt with the generation of PFC gases [
10,
12]. To explain the principles, the common U = R·I equation, where U is voltage, R is resistance, and I is current, can be used. By ramping the voltage U, the current I will also increase as long as the resistance is constant. If the resistance at the electrode increases (e.g., due to PFC gas generation, which also tends to create gas blockage or electrode passivation), the current will decrease. On the other hand, if the current is ramped, increased resistance, as described above, will cause the voltage U to increase until a point where the power supply reaches its voltage limitation. Hence, the oxide concentration can be related to a rapid increase in the voltage or a rapid decrease in the current, depending on what is controlled, the current or the voltage.
Investigations involving electrolytes for aluminium production have shown that the peak current prior to the passive layer formation increases with increasing oxide concentration [
8,
10,
11,
13]. Thus, it is possible to correlate the concentration of the dissolved oxide species with the observed peak current. This makes the voltammetric technique suitable for determining oxide concentrations in fluoride melts using appropriate calibrations gathered from analysed oxide concentration in similar fluoride melts [
8].
The present paper reports on a study of the dissolution of REOs in their corresponding LiF–REF electrolyte. The study aims to provide an online means of monitoring oxide concentrations and, as such, controlling the concentration of dissolved REO species during RE metal electrowinning. This involves incorporating fast voltage sweep voltammetry using a graphite probe as a working electrode. The solubility values of Pr and Nd oxides in their corresponding fluoride electrolytes are also determined.
2. Materials and Methodology
The present study was based on the principle of fast voltage ramping to an electrode assembly consisting of a graphite anode and a graphite cathode in fluoride electrolytes. By monitoring the current passing through the electrode assembly during each sweep, the rapid drop in current when the anode surface is depleted in oxide is recorded.
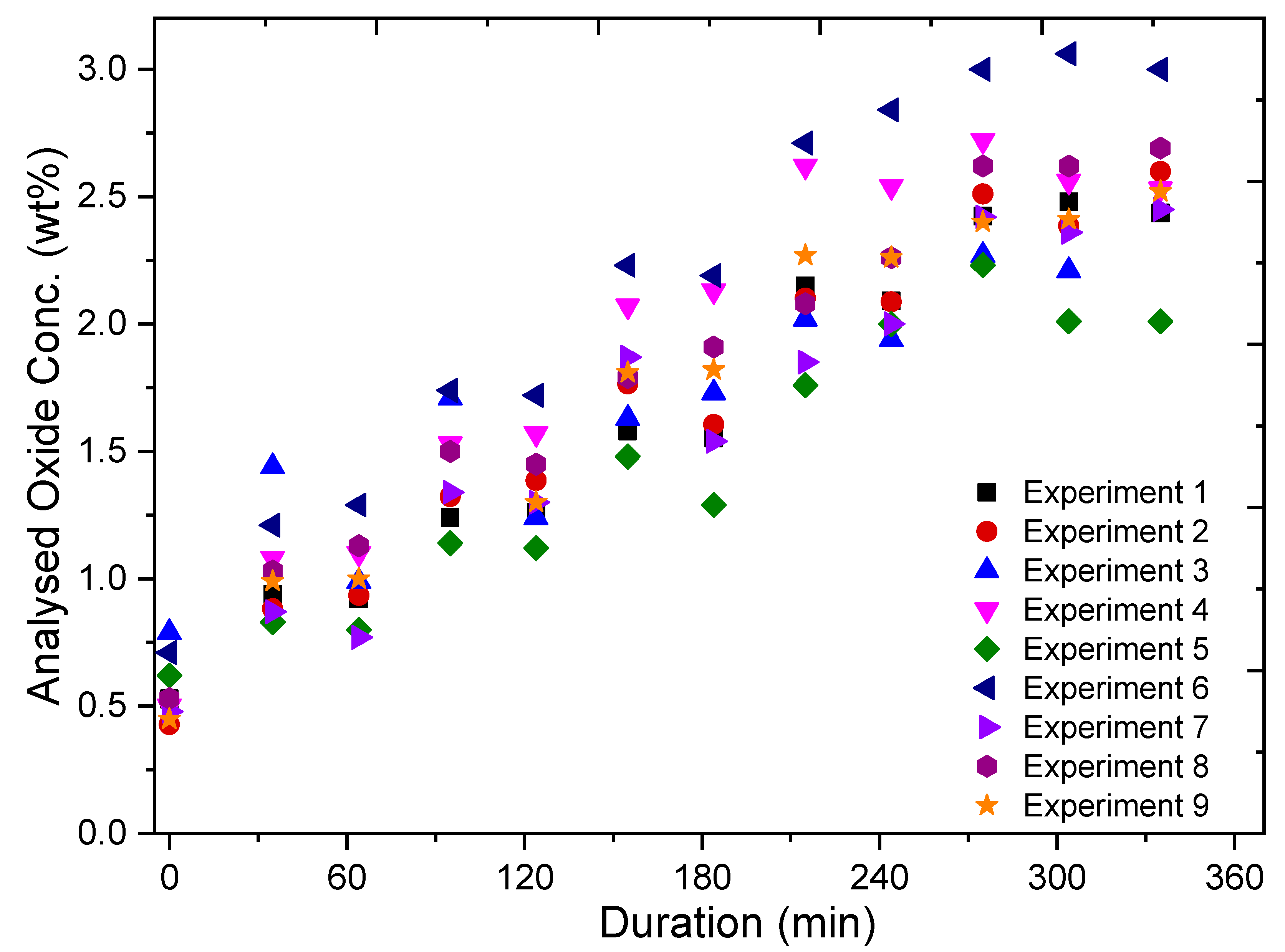
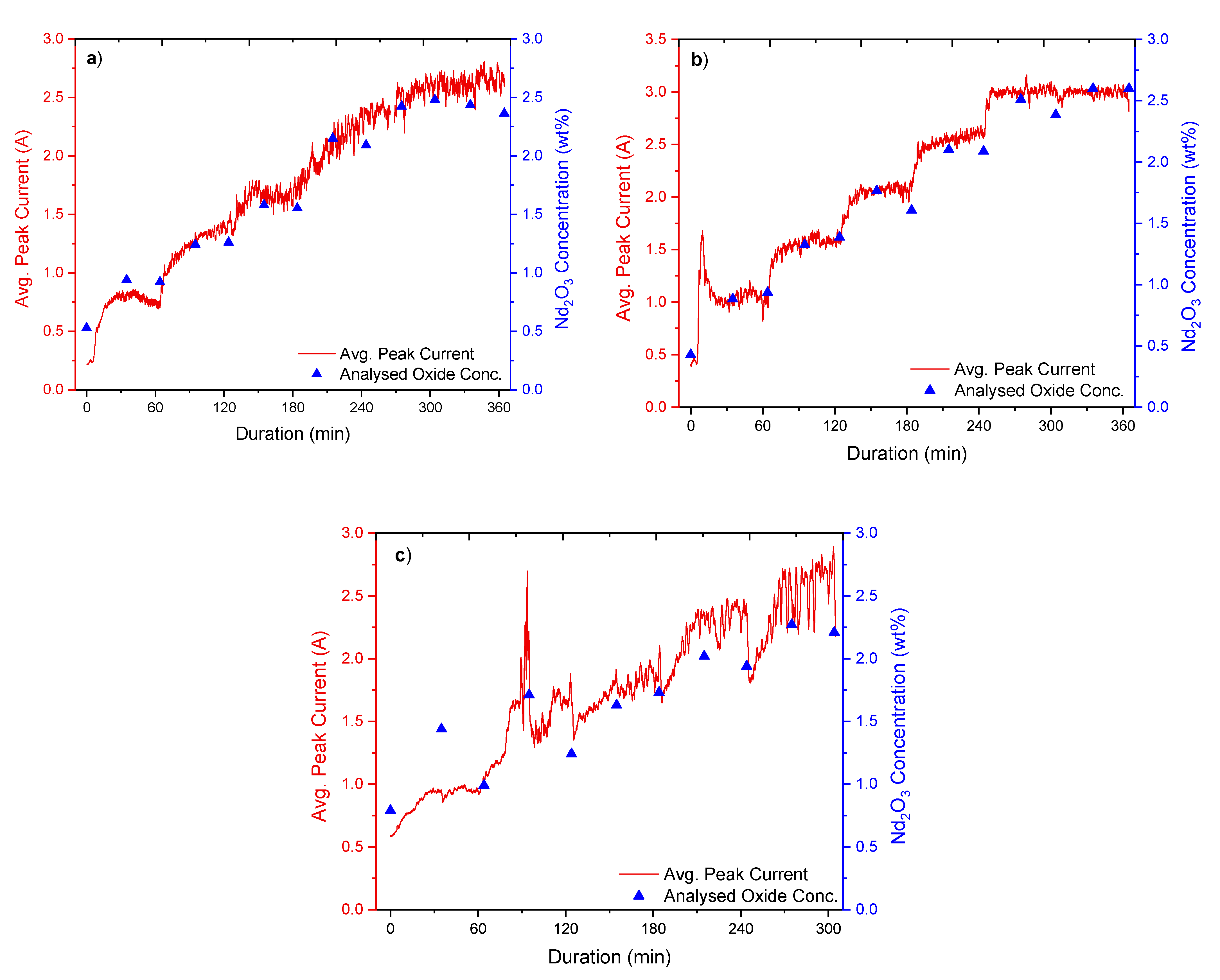
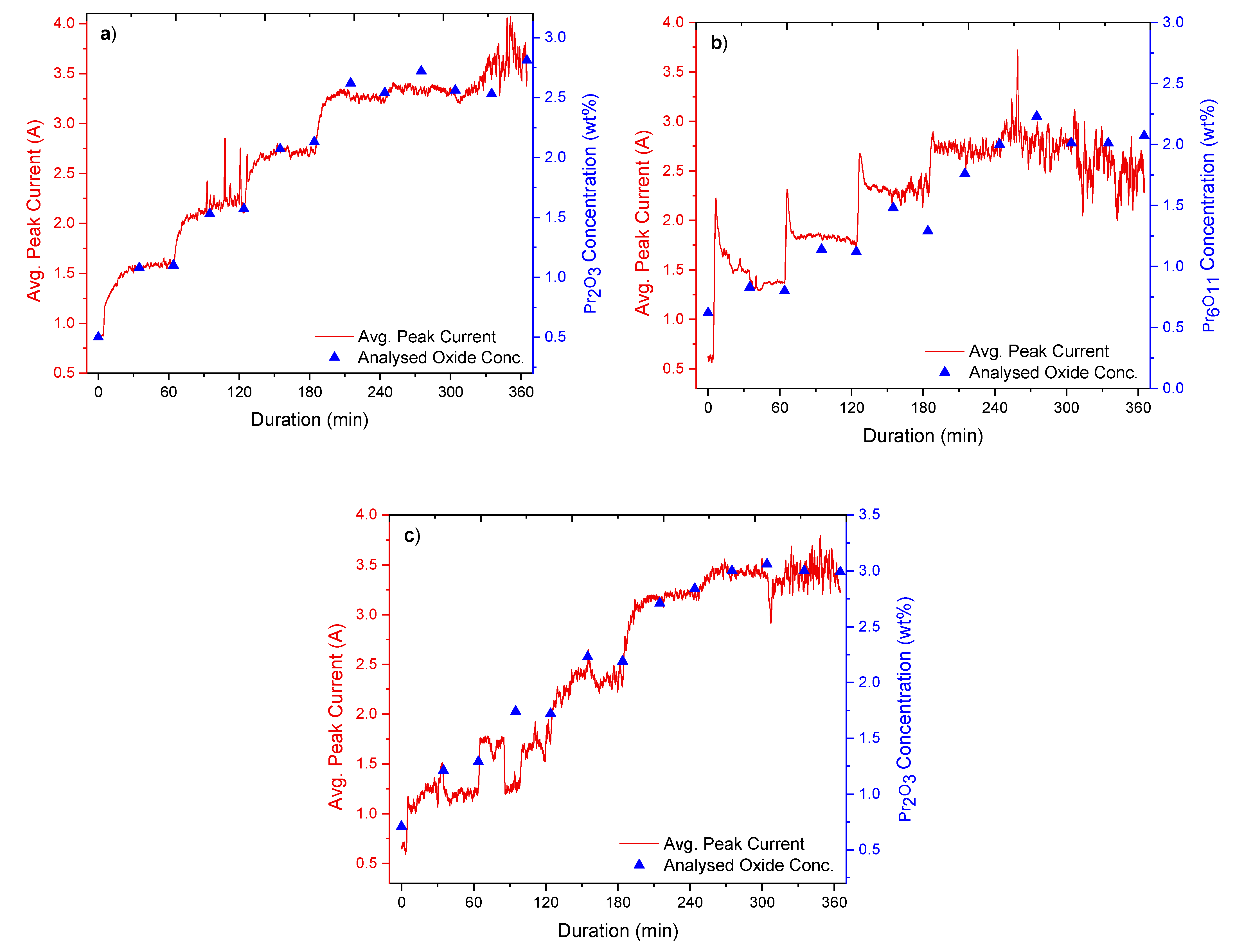
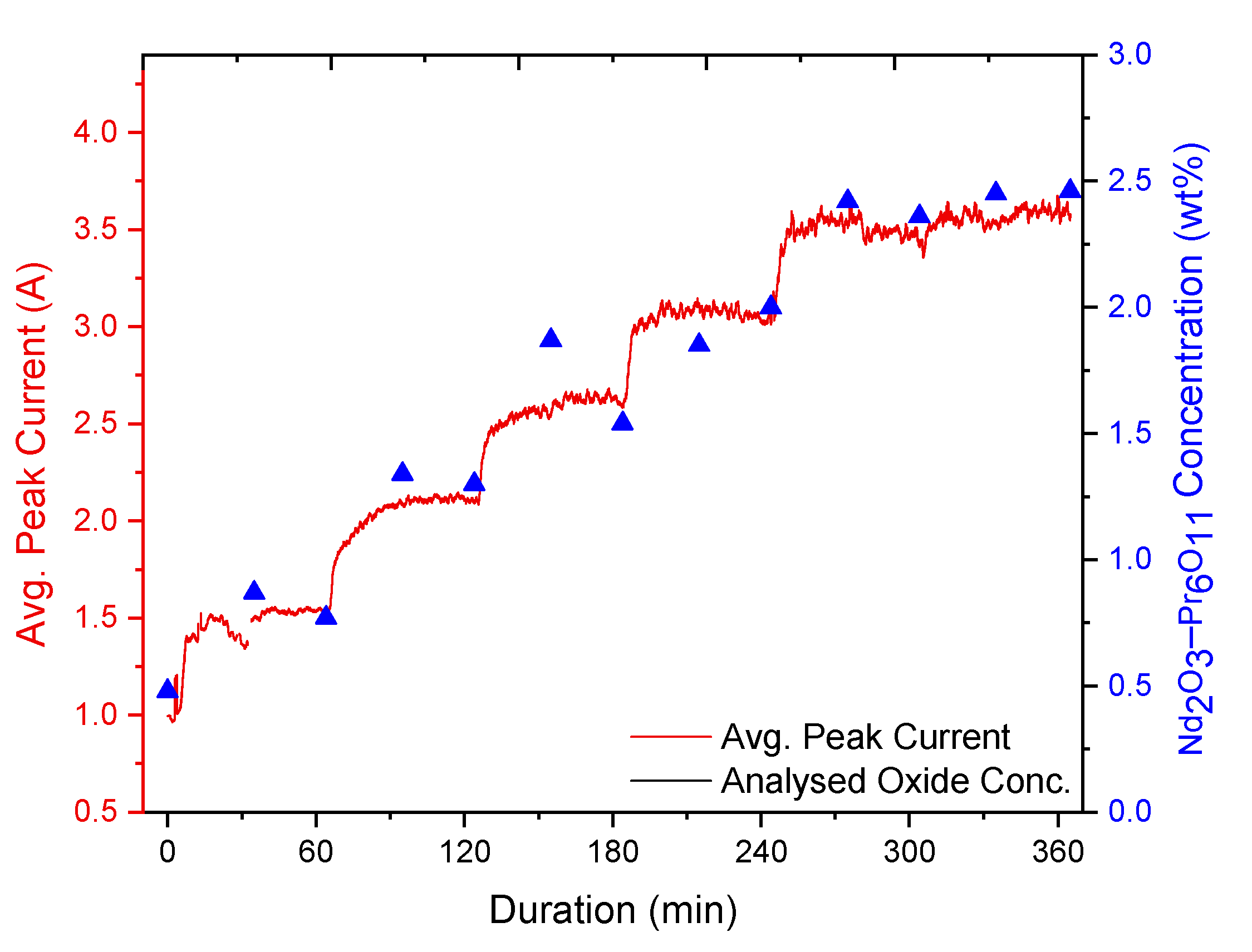
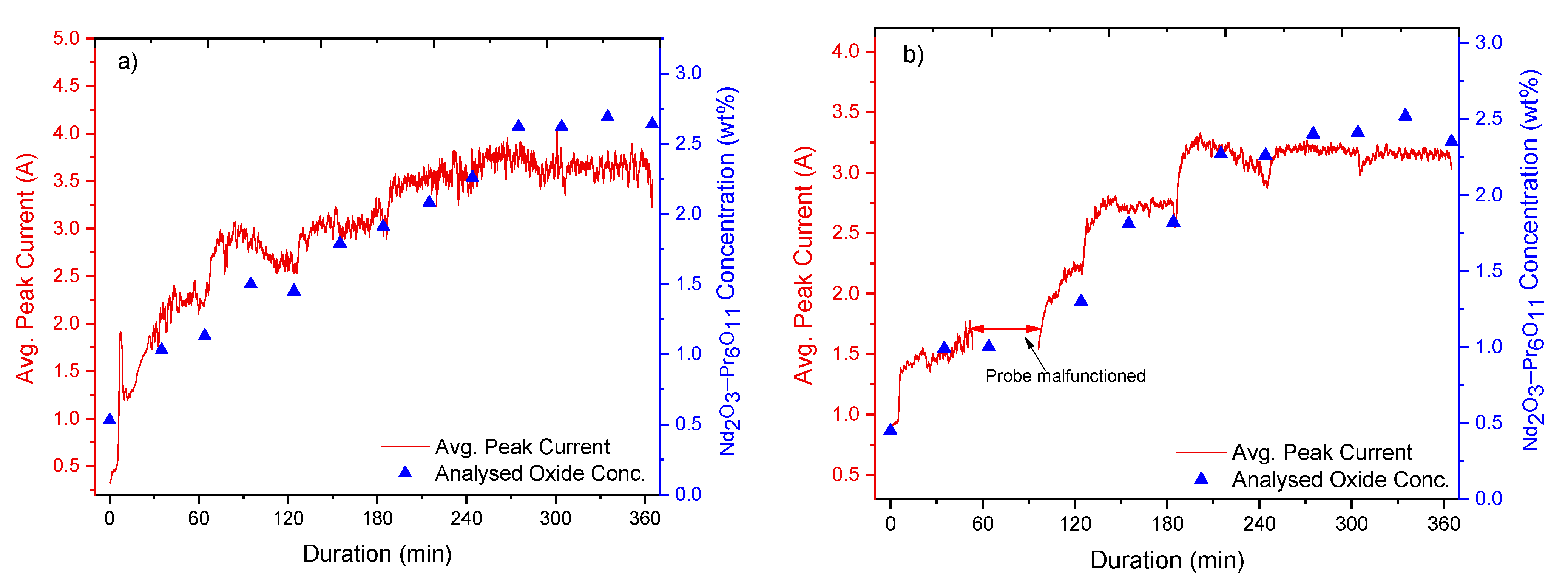
The experiments involved the addition of REOs into their respective fluoride electrolytes, which had been heated up to ca. 1040 °C. Additions were done every 60 min throughout the whole 6 h duration of the experiments, while measurements of the current response from fast voltage ramping were taken continuously. Sampling of the electrolyte was done before every new addition and in the middle between each addition. These samples were later analysed by the inert gas fusion technique using a LECO (ON836) apparatus for determining the oxygen content. The principle of the inert gas fusion technique involves temperature ramping of an electrolyte sample in a high-purity graphite crucible up to about 3000 °C in an inert gas atmosphere. During temperature ramping, the oxygen in the sample reacts with the graphite crucible to form CO and CO2, which are then quantified to obtain the oxygen content. From the oxygen content, the REO concentrations are then determined.
XRD analyses of solidified electrolyte samples were done to investigate the phases present after solidification. A Bruker D8 Advance Davinci 1 X-ray diffractometer was used to obtain the diffractograms. The different phase compositions represented by the different diffractograms were determined by employing the DIFFRAC.EVA Version 5.2.0.3 evaluation software program from Bruker and using the International Centre for Diffraction Data (ICDD) Powder Diffraction File™ (PDF
®) database, PDF-4+, 2019, as given in
Table 2.
The concentration of the electrolyte was kept at 50 mol% REF and 50 mol% LiF for all the tests except for one test where the LiF content was increased to 80 mol% (REF–LiF eutectic composition). The chemicals used for the tests are NdF
3 (in-house, 98%), PrF
3 (Jiayuan Advanced Materials, 99.9%), LiF (Sigma-Aldrich, 99%), Nd
2O
3 (Alfa Aesar, 99.9%), Pr
6O
11 (Alfa Aesar, 99.9%), Nd
2O
3-Pr
6O
11 (in-house, 98%), and Pr
2O
3 (Alfa Aesar, 99.9%). All the chemicals were kept under dry conditions and preheated to ca. 120 °C before use.
Table 3 summarises the chemicals and the amounts used.
The experimental setup consisted of a pure graphite crucible with an internal diameter of ca. 77 mm filled with 1 kg electrolyte placed in a furnace together with a type S thermocouple as well as a graphite probe and a graphite stirrer. The experimental setup as well as a representation of a typical voltammetric measurement data is displayed in
Figure 1.
The setup was purged with argon during the whole experiment. The stirrer and the graphite probe were rotated at 80 and 100 rpm, respectively. Rotation of the probe was done to avoid oxide concentrating around the tip, creating noise in the data. A voltage was applied between the working electrode (graphite probe) and the counter electrode (graphite crucible) such that the probe was polarised anodically. Fast voltage sweeps of 100 V/s were applied to the graphite probe through a computer-controlled voltage sweep generator (inductive proximity sensors, Alumina Sensor Model 003D from New Zealand), and the current response was recorded using a National Instruments acquisition card (NI 9205). A summary of the measurement sequence is given below:
- (1)
The probe is conditioned by carrying out several linear voltage sweeps at 100 V/s before the measurements.
- (2)
Predetermined amounts of the REO are added to the fluoride electrolyte every 60 min.
- (3)
Fast voltage sweeps (100 V/s) are applied every 2 s, and the peak current is recorded. A typical voltammetric measurement plot showing the peak current is given in
Figure 1b.
The analysed REO concentration in the electrolyte is obtained by using the inert gas fusion technique to calibrate and verify the REO concentration derived from the peak current recorded during the measurements.
Figure 1b shows a typical current response during fast voltage sweep applied to the graphite probe. In the first stage of anodic polarisation, the current increases with the probe voltage. At 37 ms, a rapid drop of current occurs, indicating anode passivation. The peak current prior to the passivation, as shown in
Figure 1b, is recorded for further analysis. Due to the large number of voltage sweeps recorded during the experiments, data processing was implemented using LabVIEW.
Author Contributions
Conceptualisation, H.G., A.P.R. and S.S.; methodology, H.G.; software, H.G.; validation, A.P.R., A.M.M. and W.G.; formal analysis, A.P.R. and S.S.; investigation, H.G. and A.S.; resources, A.M.M. and W.G.; data curation, H.G., S.S. and W.G.; writing—original draft preparation, S.S.; writing—review and editing, S.S., A.P.R., A.M.M. and W.G.; visualisation, S.S.; project administration, A.P.R.; funding acquisition, A.P.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from the European Union’s Horizon 2020 and Innovation Programme under Grant Agreement No. 776559, which is very much appreciated.
Institutional Review Board Statement
Not applicable to this article.
Informed Consent Statement
Not applicable to this article.
Data Availability Statement
Data sharing is not applicable to this article.
Acknowledgments
Financial support from the European Union’s Horizon 2020 and Innovation Programme under Grant Agreement No. 776559 is very much appreciated.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Milicevic, K.; Feldhaus, D.; Friedrich, B. Conditions and Mechanisms of Gas Emissions from Didymium Electrolysis and Its Process Control. In Light Metals; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1435–1441. [Google Scholar]
- Guo, X.; Sun, Z.; Sietsma, J.; Blanpain, B.; Guo, M.; Yang, Y. Quantitative Study on Dissolution Behavior of Nd2O3 in Fluoride Melts. Ind. Eng. Chem. Res. 2018, 57, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Abbasalizadeh, A.; Malfliet, A.; Seetharaman, S.; Sietsma, J.; Yang, Y. Electrochemical Extraction of Rare Earth Metals in Molten Fluorides: Conversion of Rare Earth Oxides into Rare Earth Fluorides Using Fluoride Additives. J. Sustain. Met. 2017, 3, 627–637. [Google Scholar] [CrossRef]
- Abbasalizadeh, A.; Sridar, S.; Chen, Z.; Sluiter, M.; Yang, Y.; Sietsma, J.; Seetharaman, S.; Kumar, K.H. Experimental investigation and thermodynamic modelling of LiF-NdF3-DyF3 system. J. Alloy. Compd. 2018, 753, 388–394. [Google Scholar] [CrossRef]
- Guo, X.; Sietsma, J.; Yang, Y. Solubility of Rare Earth Oxides in Molten Fluorides. In Proceedings of the European Rare Earth Resources Conference (ERES2014), Milos, Greece, 4–7 September 2014; pp. 149–155. [Google Scholar]
- Aarhaug, T.A.; Ferber, A.; Kjos, O.S.; Gaertner, H. Online Monitoring of Aluminium Primary Production Gas Composition by Use of Fourier-Transform Infrared Spectrometry. In Light Metals; Springer: Berlin/Heidelberg, Germany, 2014; pp. 647–652. [Google Scholar]
- Kibby, R.M.; Marshall, H.C., Jr.; Richards, N.E. Alumina Concentration Meter. U.S. Patent 3471390, 7 October 1969. [Google Scholar]
- Haverkamp, R.G.; Welch, B.J. Measurement of Alumina in Reduction Pots. U.S. Patent 6010611, 4 January 2000. [Google Scholar]
- Crottaz, O.; Haverkamp, R.G.; Yellepeddi, R.; de Nora, V. Development of Techniques for Measuring the Composition of Low Temperature Electrolytes. In Light Metals; Springer: Berlin/Heidelberg, Germany, 2001; pp. 1195–1199. [Google Scholar]
- Haverkamp, R.G.; Rolseth, S.; Thonstad, J.; Gudbrandsen, H. Voltammetry and electrode reactions in AlF3-rich electrolyte. In Light Metals; Springer: Berlin/Heidelberg, Germany, 2001; pp. 481–486. [Google Scholar]
- Haverkamp, R.G.; Welch, B.J.; McMullen, A. Real Time Alumina Measurement in Industrial Cells. In Light Metals; Springer: Berlin/Heidelberg, Germany, 2001; pp. 1193–1194. [Google Scholar]
- Thonstad, J.; Fellner, P.; Haarberg, G.M.; Hives, J.; Kvande, H.; Sterten, A. Aluminium Electrolysis: Fundamentals of the Hall-Héroult Process, 3rd ed.; Aluminium-Verlag Marketing and Kommunikation GmbH: Düsseldorf, Germany, 2001. [Google Scholar]
- Nikolaev, A.; Pavlenko, O.; Zaikov, Y.; Suzdaltsev, A. Electrochemical sensor for monitoring the alumina dissolution and concentration in a cryolite-alumina melt. J. Electrochem. Soc. 2020, 167, 126511. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).