Composition Stability and Cr-Rich Phase Formation in W-Cr-Y and W-Cr-Ti Smart Alloys
Abstract
1. Introduction
2. Computational Methodology
2.1. DFT Computational Details
2.2. Cluster Expansion Formalism
2.3. Chemical Short-Range Order Parameters
3. Phase Stability of Derivative Cr-W-Y Alloys
3.1. Phase Stability at 0 K
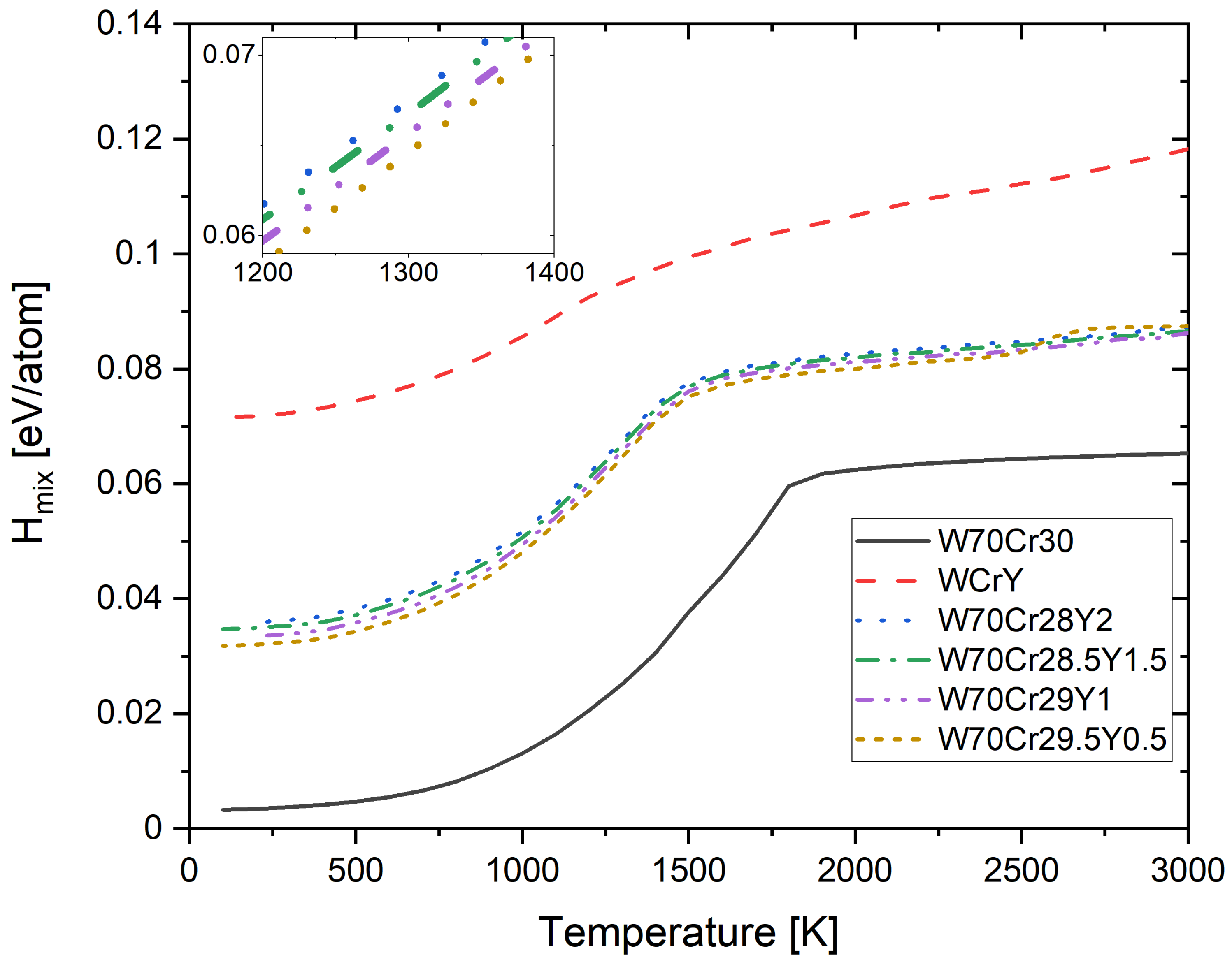
3.2. Finite Temperature Phase Stability and Order-Disorder Transition Temperatures of Cr-W-Y Smart Alloys
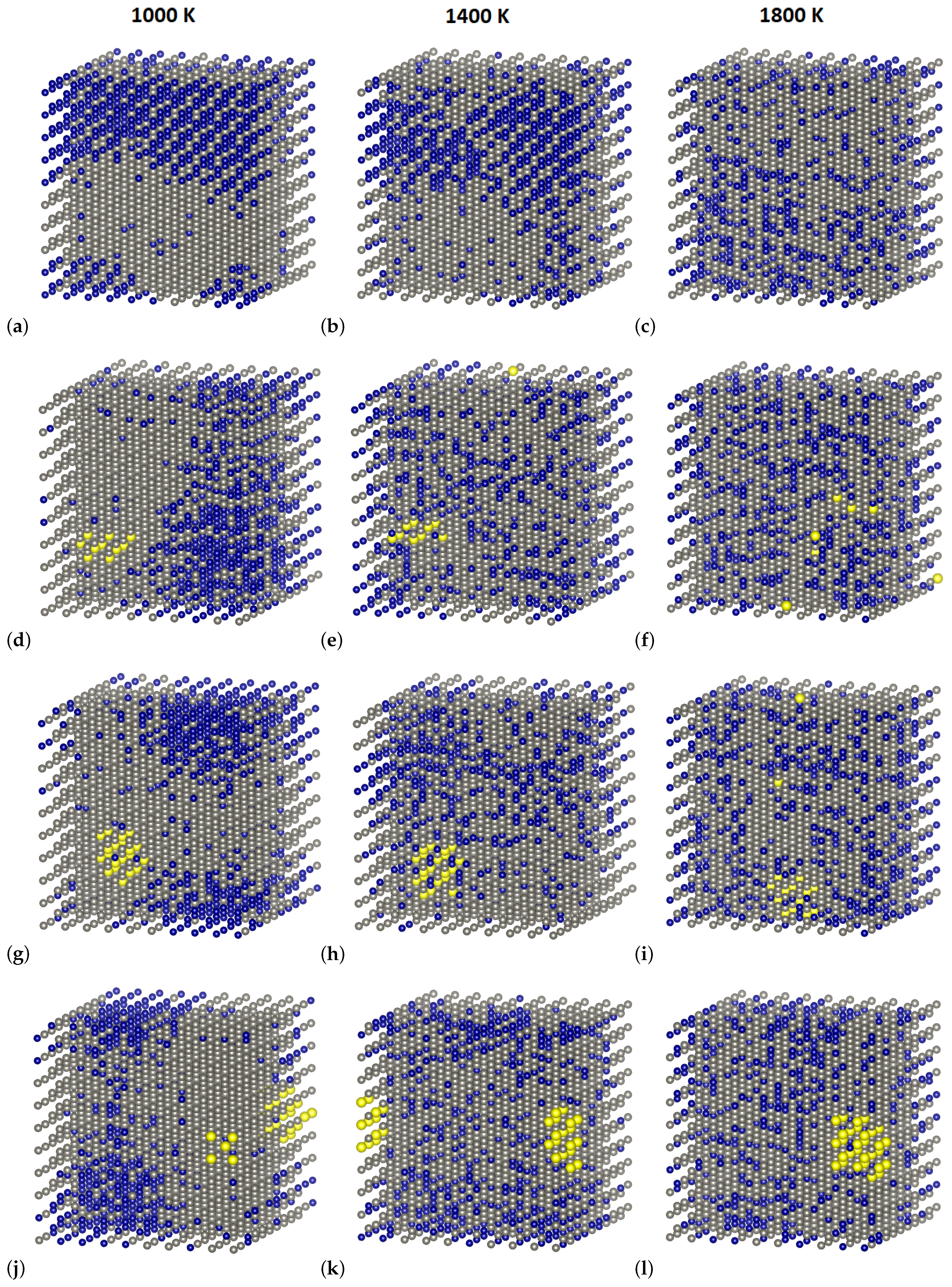
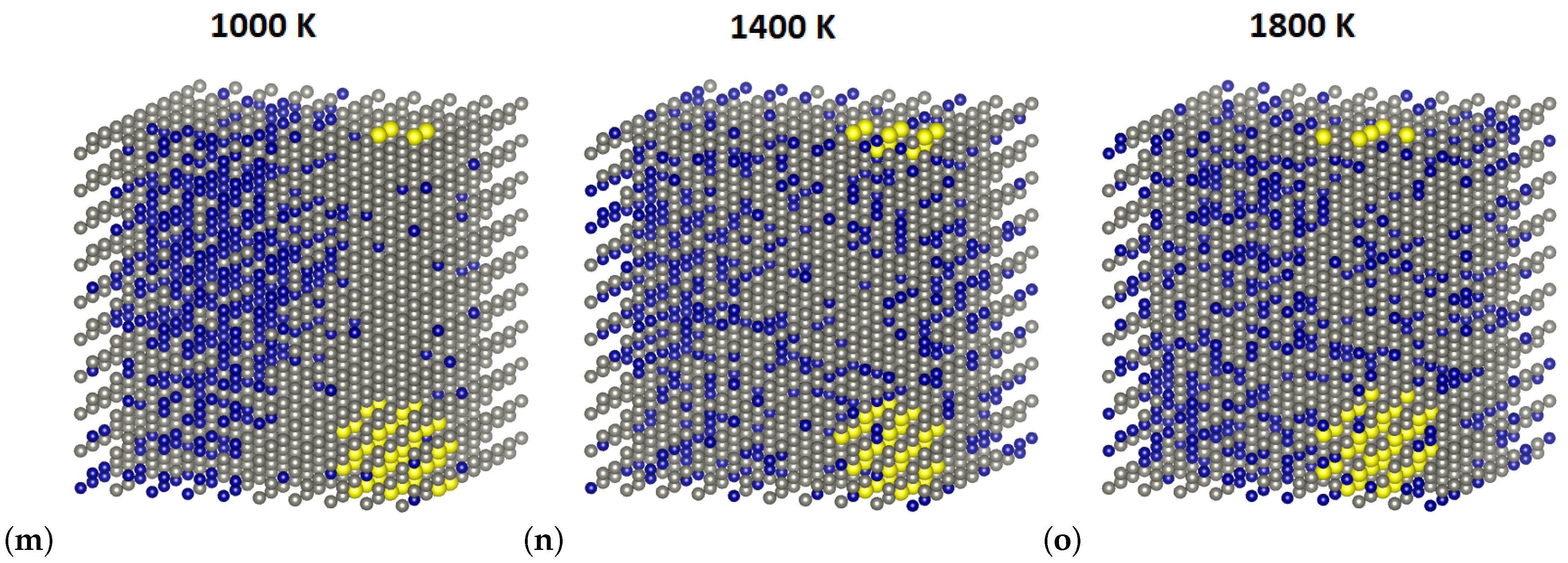
3.3. Short-Range Ordering in Derivative Cr-W-Y Alloys
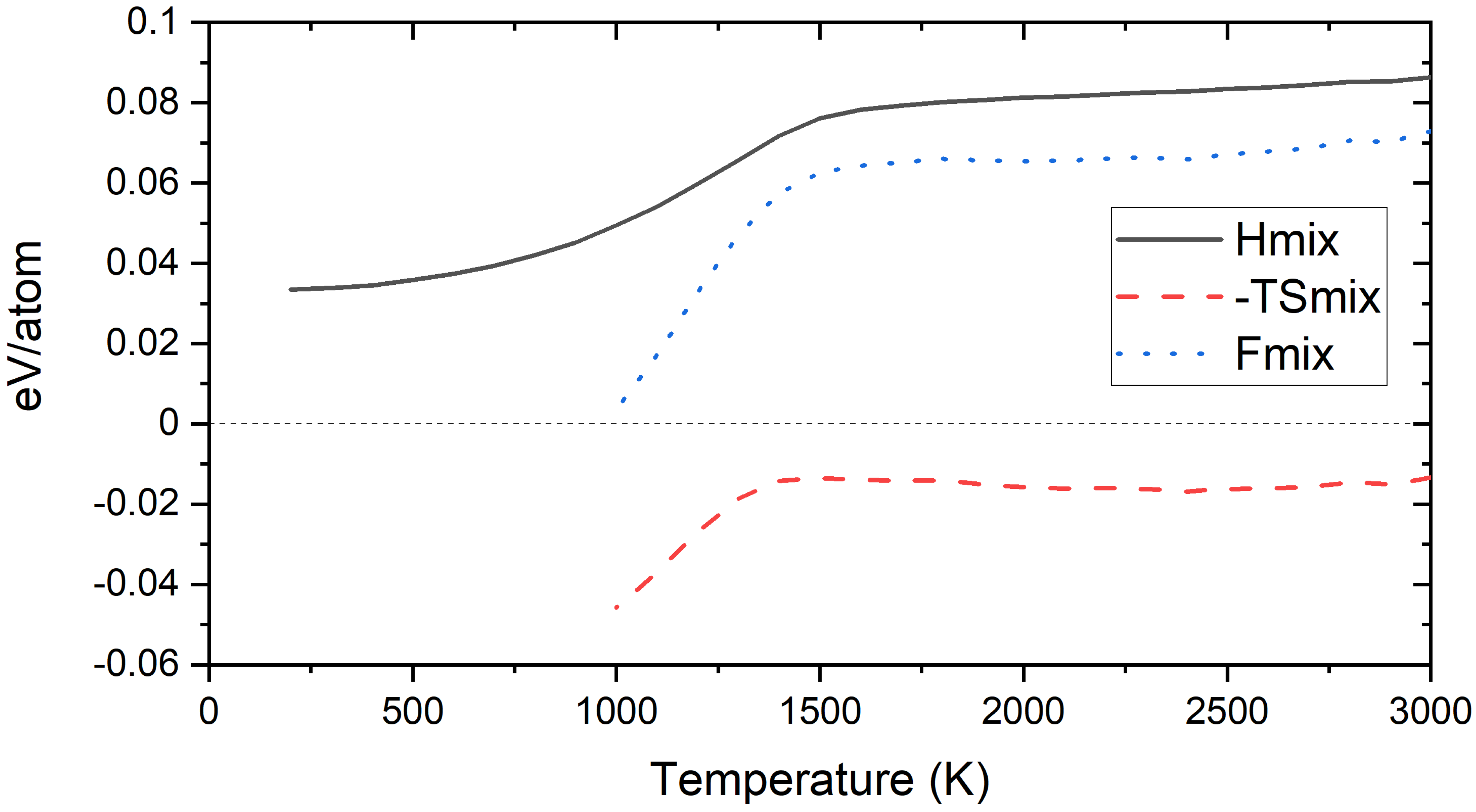
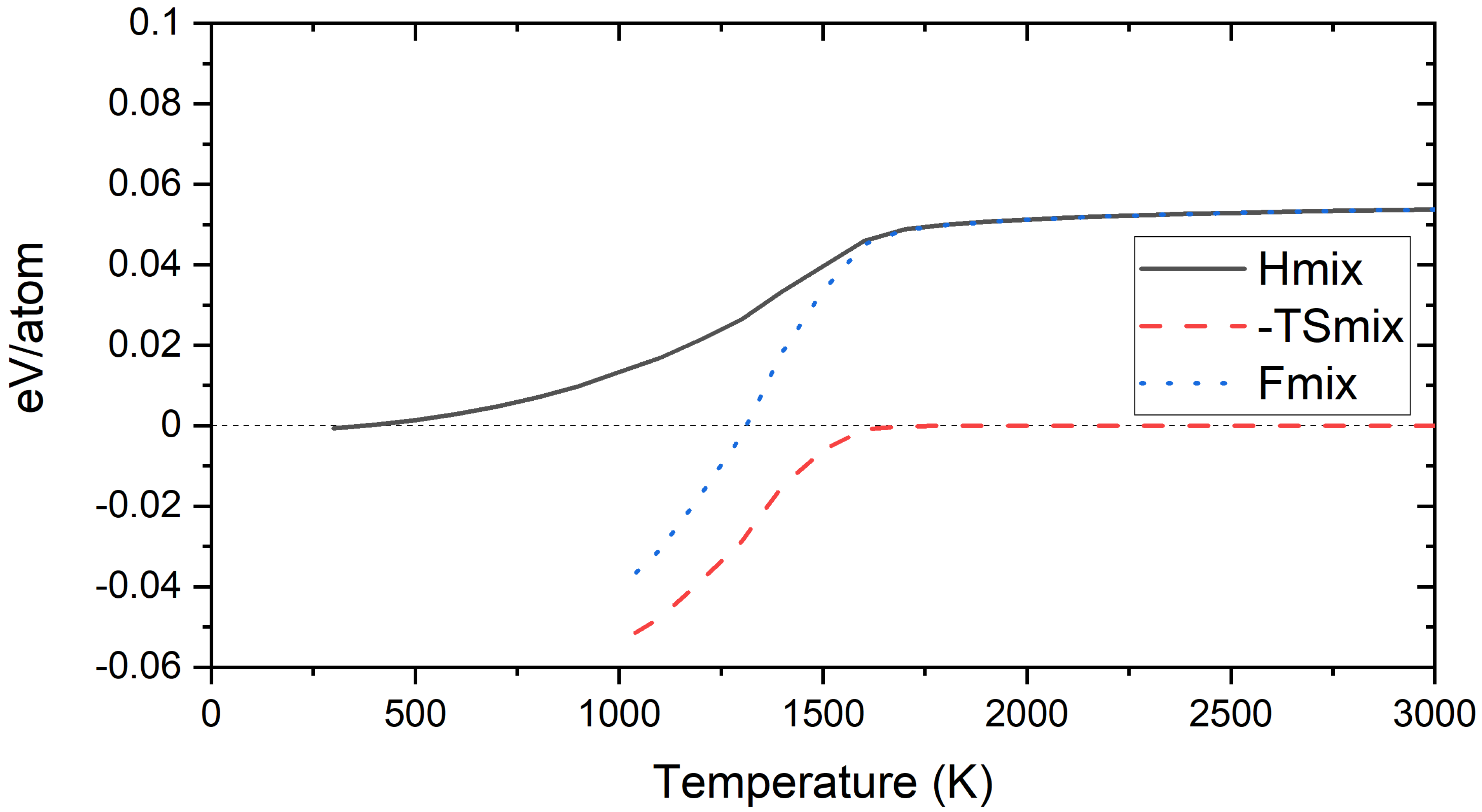
3.4. Free Energy of Mixing of Derivative Cr-W-Y Alloys
4. Phase Stability of Derivative Cr-Ti-W Alloys
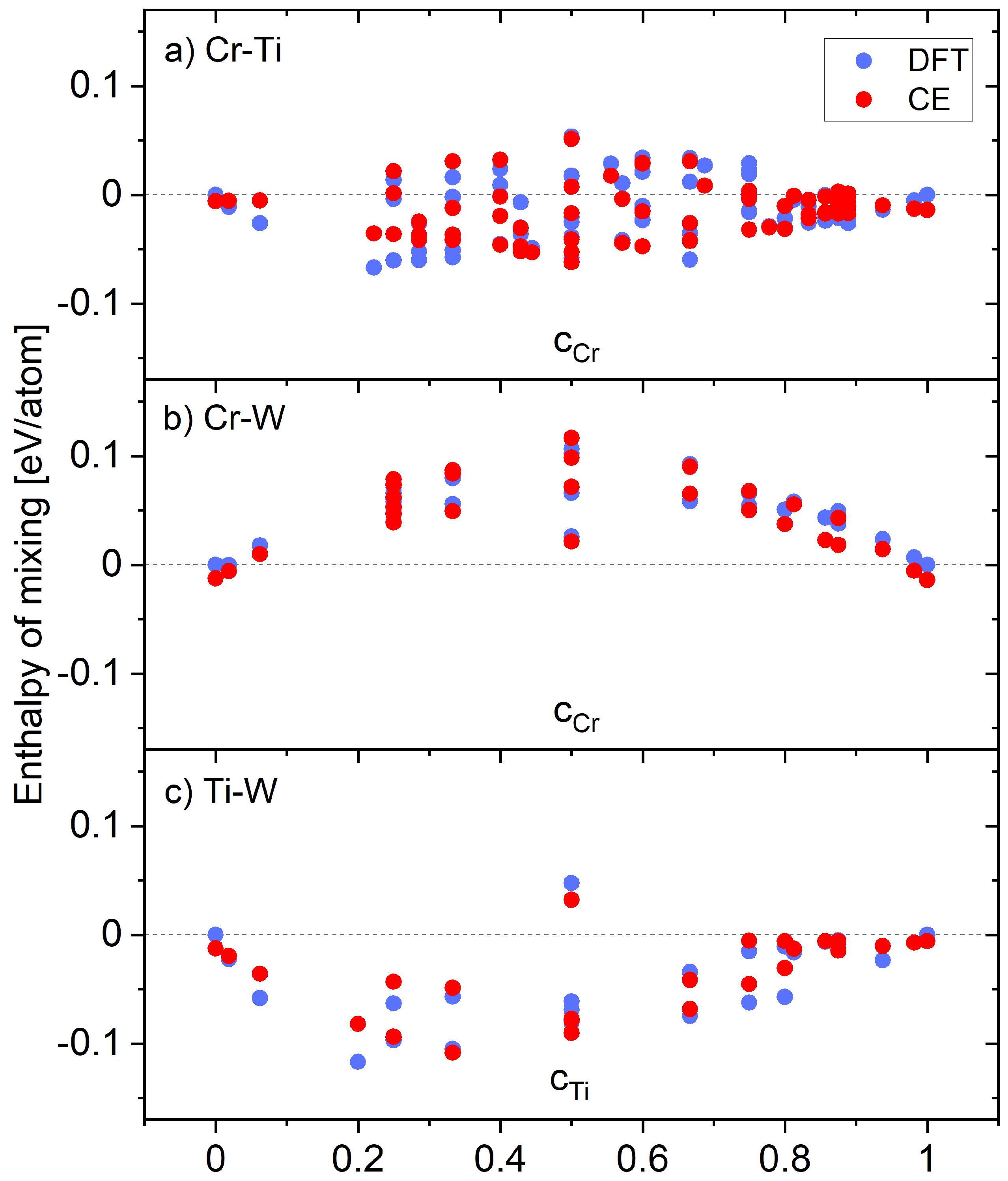
4.1. Phase Stability at 0 K
4.2. Finite Temperature Phase Stability and Order–Disorder Transition Temperatures of Cr-Ti-W Alloys
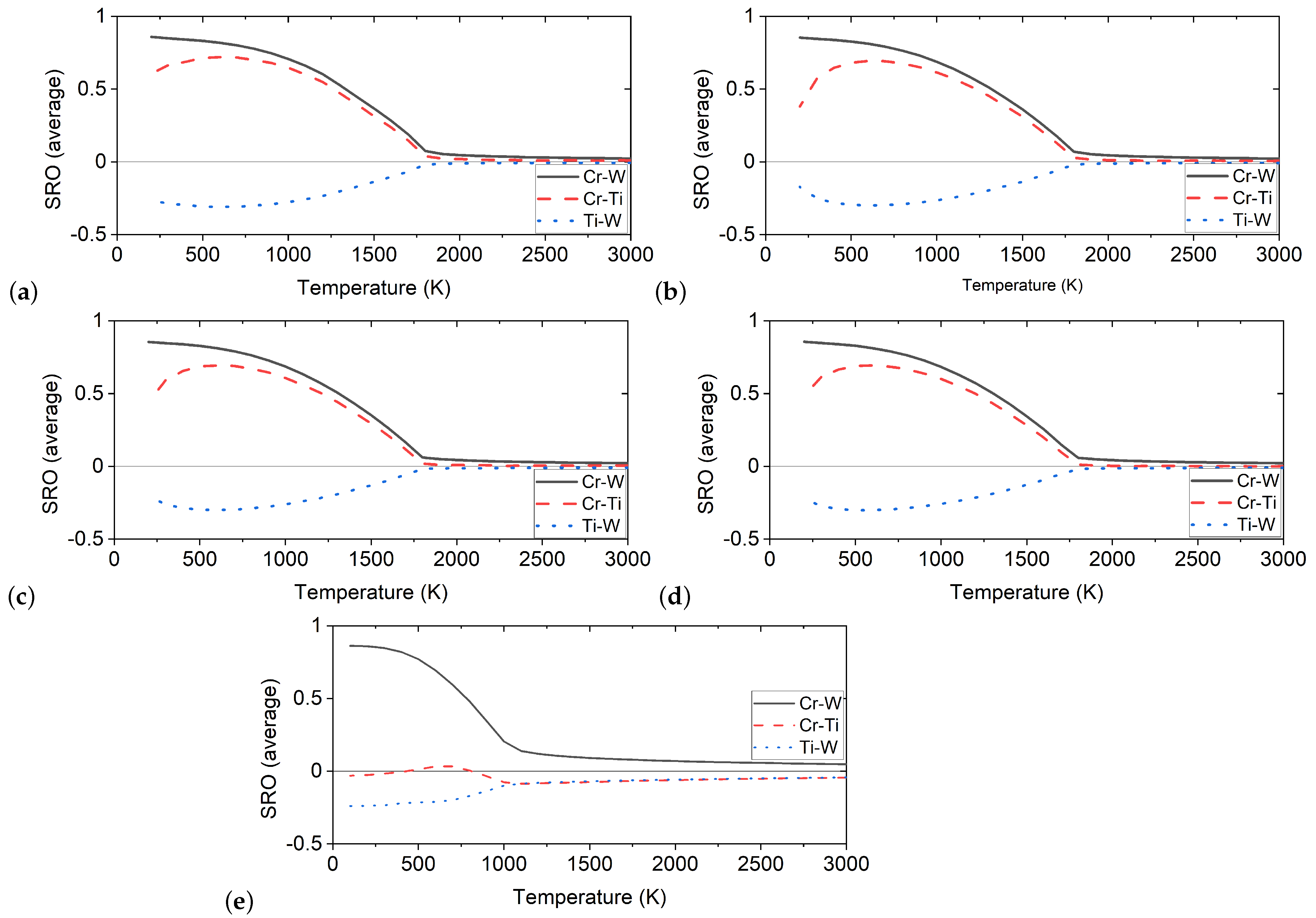
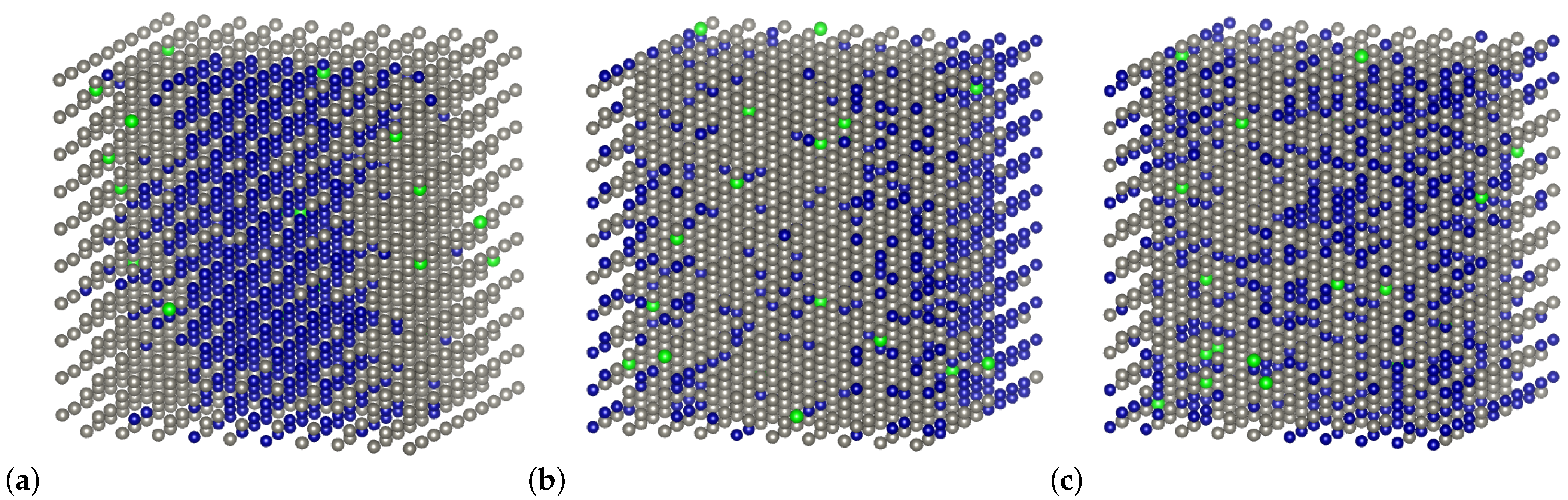
4.3. Short-Range Ordering in Derivative Cr-Ti-W Alloys
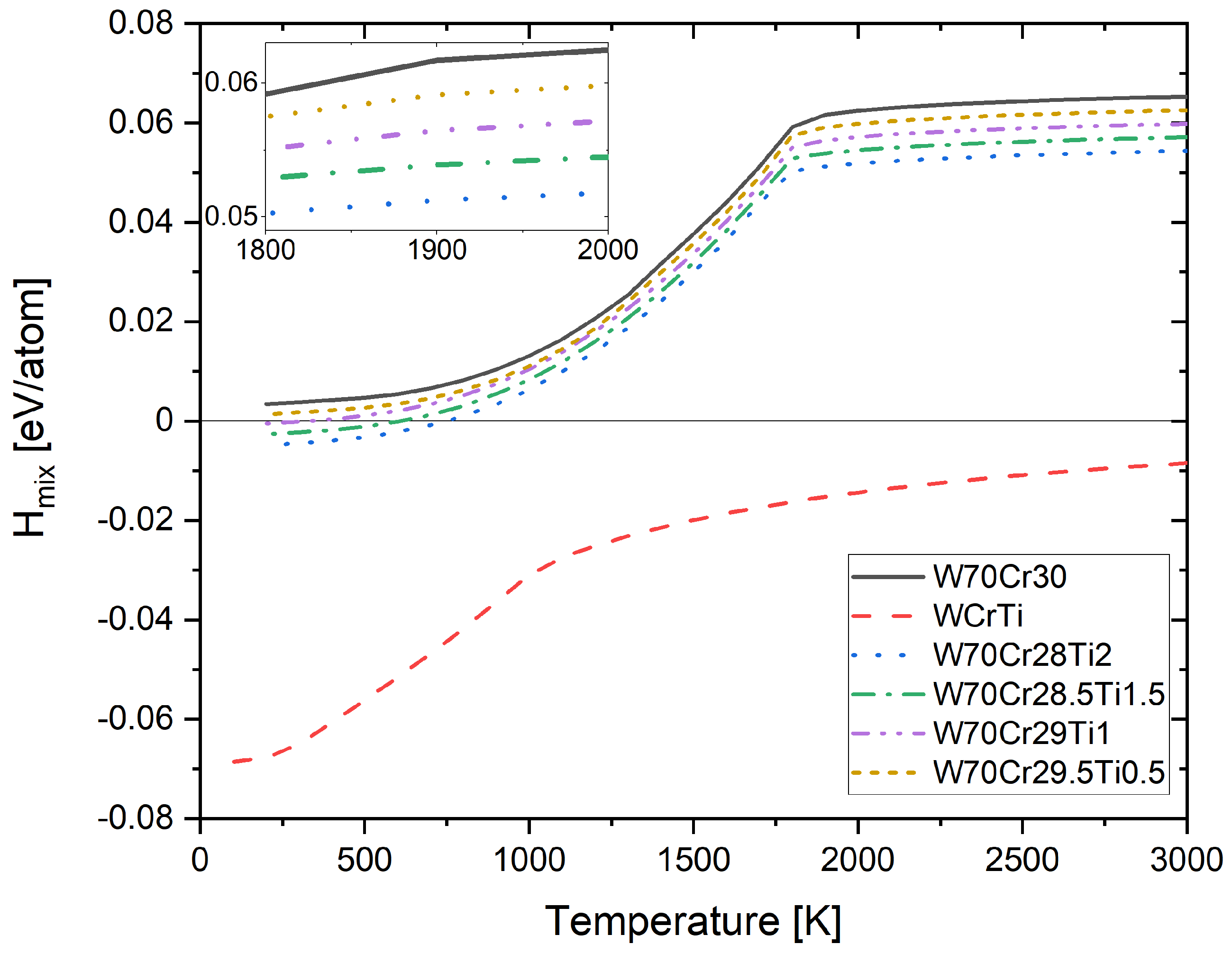
4.4. Free Energy of Mixing of Derivative Cr-Ti-W Alloys
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1NN | First Nearest Neighbours |
| BCC | Body-Centered Cubic |
| CE | Cluster Expansion |
| DEMO | European demonstration fusion power plant |
| DFT | Density Functional Theory |
| LLW | Low Level Waste |
| LOCA | Loss-Of-Coolant Accident |
| MC | Monte Carlo |
| ODTT | Order-Disorder Transition Temperature |
| PFM | Plasma-Facing-Materials |
| PAW | Projector Augmented Wave |
| SRO | Short-Range Order |
| TENDL | Truly general-purpose nuclear data library |
| VASP | Vienna Ab-initio Simulation Package |
References
- Klein, F.; Gilbert, M.R.; Litnovsky, A.; Gonzalez-Julian, J.; Weckauf, S.; Wegener, T.; Schmitz, J.; Linsmeier, C.; Bram, M.; Coenen, J.W. Tungsten–chromium–yttrium alloys as first wall armor material: Yttrium concentration, oxygen content and transmutation elements. Fusion Eng. Des. 2020, 158, 111667. [Google Scholar] [CrossRef]
- Litnovsky, A.; Schmitz, J.; Klein, F.; De Lannoye, K.; Kreter, A.; Rasinski, M.; Coenen, J.W.; Linsmeier, C.; Gonzalez-Julian, J.; Bram, M.; et al. Smart Tungsten-based Alloys for a First Wall of DEMO. Fusion Eng. Des. 2020, 159, 111742. [Google Scholar] [CrossRef]
- Riccardi, B.; Montanari, R.; Casadei, M.; Costanza, G.; Filacchioni, G.; Moriani, A. Optimisation and characterisation of tungsten thick coatings on copper based alloy substrates. J. Nucl. Mater. 2006, 352, 29–35. [Google Scholar] [CrossRef]
- Lloyd, M.J.; Abernethy, R.G.; Gilbert, M.R.; Griffiths, I.; Bagot, P.A.J.; Nguyen-Manh, D.; Moody, M.P.; Armstrong, D.E.J. Decoration of voids with rhenium and osmium transmutation products in neutron irradiated single crystal tungsten. Scr. Mater. 2019, 173, 96–100. [Google Scholar] [CrossRef]
- El-Atwani, O.; Li, N.; Li, M.; Devaraj, A.; Baldwin, J.K.S.; Schneider, M.M.; Sobieraj, D.; Wróbel, J.S.; Nguyen-Manh, D.; Maloy, S.A.; et al. Outstanding radiation resistance of tungsten-based high-entropy alloys. Sci. Adv. 2019, 5, aav2020. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Eade, T.; Bachmann, C.; Fischer, U.; Taylor, N.P. Activation, decay heat, and waste classification studies of the European DEMO concept. Nucl. Fusion 2017, 57, 046015. [Google Scholar] [CrossRef]
- Schmitz, J.; Litnovsky, A.; Klein, F.; De Lannoye, K.; Kreter, A.; Rasinski, M.; Breuer, U.; Gonzalez-Julian, J.; Bram, M.; Coenen, J.W.; et al. On the plasma suitability of WCrY smart alloys—the effect of mixed D+Ar/He plasmas. Phys. Scr. 2020, T171, 014002. [Google Scholar] [CrossRef]
- Schmitz, J.; Litnovsky, A.; Klein, F.; Tan, X.Y.; Breuer, U.; Rasinski, M.; Ertmer, S.; Kreter, A.; Gonzalez-Julian, J.; Bram, M.; et al. Argon-seeded plasma exposure and oxidation performance of tungsten-chromium-yttrium smart alloys. Tungsten 2019, 1, 1–3. [Google Scholar] [CrossRef]
- Klein, F.; Litnovsky, A.; Wegener, T.; Tan, X.Y.; Gonzalez-Julian, J.; Rasinski, M.; Schmitz, J.; Linsmeier, C.; Bram, M.; Coenen, J.W. Sublimation of advanced tungsten alloys under DEMO relevant accidental conditions. Fusion Eng. Des. 2019, 146A, 1198–1202. [Google Scholar] [CrossRef]
- Tan, X.Y.; Klein, F.; Litnovsky, A.; Wegener, T.; Schmitz, J.; Linsmeier, C.; Coenen, J.W.; Breuer, U.; Rasinski, M.; Li, P.; et al. Evaluation of the high temperature oxidation of W-Cr-Zr self-passivating alloys. Corros. Sci. 2019, 147, 201–211. [Google Scholar] [CrossRef]
- Klein, F.; Wegener, T.; Litnovsky, A.; Rasinski, M.; Tan, X.Y.; Schmitz, J.; Linsmeier, C.; Coenen, J.W.; Du, H.; Mayer, J.; et al. On Oxidation Resistance Mechanisms at 1273 K of Tungsten-Based Alloys Containing Chromium and Yttria. Metals 2018, 8, 488. [Google Scholar] [CrossRef]
- Klein, F.; Wegener, T.; Litnovsky, A.; Rasinski, M.; Tan, X.Y.; Gonzalez-Julian, J.; Schmitz, J.; Bram, M.; Coenen, J.W.; Linsmeier, C. Oxidation resistance of bulk plasma-facing tungsten alloys. Nucl. Mater. Energy 2018, 15, 226–231. [Google Scholar] [CrossRef]
- Litnovsky, A.; Wegener, T.; Klein, F.; Linsmeier, C.; Rasinski, M.; Kreter, A.; Tan, X.Y.; Schmitz, J.; Mao, Y.; Coenen, J.W.; et al. Oxidation resistance of bulk plasma-facing tungsten alloys. Plasma Phys. Control. Fusion 2017, 59, 064003. [Google Scholar] [CrossRef]
- Litnovsky, A.; Wegener, T.; Klein, F.; Linsmeier, C.; Rasinski, M.; Kreter, A.; Unterberg, B.; Coenen, J.W.; Du, H.; Mayer, J.; et al. Smart tungsten alloys as a material for the first wall of a future fusion power plant. Nucl. Fusion 2017, 57, 066020. [Google Scholar] [CrossRef]
- Fischer, U.; Bachmann, C.; Catalan, J.P.; Eade, T.; Flammini, D.; Gilbert, M.R.; Jaboulay, J.-C.; Konobeev, A.; Leichtle, D.; Lu, L.; et al. Methodological approach for DEMO neutronics in the European PPPT programme: Tools, data and analyses. Fusion Eng. Des. 2017, 123, 26–31. [Google Scholar] [CrossRef]
- Federici, G.; Biel, W.; Gilbert, M.R.; Kemp, R.; Taylor, N.; Wenninger, R. European DEMO design strategy and consequences for materials. Nucl. Fusion 2017, 57, 092002. [Google Scholar] [CrossRef]
- Federici, G.; Bachmann, C.; Barucca, L.; Biel, W.; Boccaccini, L.; Brown, R.; Bustreo, C.; Ciattaglia, S.; Cismondi, F.; Coleman, M.; et al. DEMO design activity in Europe: Progress and updates. Fusion Eng. Des. 2018, 136, 729–741. [Google Scholar] [CrossRef]
- Sublet, J.-C.; Eastwood, J.W.; Morgan, J.G.; Gilbert, M.R.; Fleming, M.; Arter, W. FISPACT-II: An Advanced Simulation System for Activation, Transmutation and Material Modelling. Nucl. Data Sheets 2017, 139, 77–137. [Google Scholar] [CrossRef]
- Koning, A.J.; Rochman, D.; Sublet, J. TENDL-2019; Release Date: 31 December 2019. Available online: https://tendl.web.psi.ch/tendl_2019/tendl2019.html (accessed on 29 April 2020).
- Gilbert, M.R.; Eade, T.; Rey, T.; Vale, R.; Bachmann, C.; Fischer, U.; Taylor, N. Waste implications from minor impurities in European DEMO materials. Nucl. Fus. 2019, 59, 076015. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Sublet, J.-C. Handbook of Activation, Transmutation, and Radiation Damage Properties of the Elements Simulated Using FISPACT-II & TENDL-2015; Magnetic Fusion Plants, CCFE-R(16)36, UKAEA 2016. Available online: http://fispact.ukaea.uk (accessed on 29 April 2020).
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17978. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmiiller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmiiller, J. Efficiency of ab initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Hafner, J. Ab-initio simulations of materials using VASP: Density-functional theory and beyond. J. Comput. Chem. 2008, 29, 2044–2078. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1997, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Sobieraj, D.; Wróbel, J.S.; Rygier, T.; Kurzydłowski, K.J.; El-Atwani, O.; Devaraj, A.; Martinez, E.; Nguyen-Manh, D. Chemical short-range order in derivative Cr-Ta-Ti-V-W high entropy alloys from the first-principles thermodynamic study. Phys. Chem. Chem. Phys. 2020, 22, 23929. [Google Scholar] [CrossRef] [PubMed]
- Pankhurst, D.A.; Nguyen-Manh, D.; Pettifor, D.G. Electronic origin of structural trends across early transition-metal disilicides: Anomalous behavior of CrSi2. Phys. Rev. B 2004, 69, 075113. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- De Fontaine, D. The number of independent pair-correlation functions in multicomponent systems. J. Appl. Crystallogr. 1971, 4, 15–19. [Google Scholar] [CrossRef]
- Sanchez, J.M.; Ducastelle, F.; Gratias, D. Generalized cluster description of multicomponent systems. Physica A 1984, 128, 334–350. [Google Scholar] [CrossRef]
- Sanchez, J.M. Foundations and Practical Implementations of the Cluster Expansion. J. Phase Equilibria Diffus. 2017, 38, 238–251. [Google Scholar] [CrossRef]
- Nguyen-Manh, D.; Lavrentiev, M.Y.; Dudarev, S.L. Magnetic origin of nano-clustering and point defect interaction in Fe-Cr alloys: An ab initio study. J. Comput. Aided Mater. Des. 2007, 14, 159–169. [Google Scholar] [CrossRef]
- Lavrentiev, M.Y.; Drautz, D.; Nguyen-Manh, D.; Klaver, T.P.C.; Dudarev, S.L. Monte Carlo study of thermodynamic properties and clustering in the bcc Fe-Cr system. Phys. Rev. B 2007, 75, 014208. [Google Scholar] [CrossRef]
- Nguyen-Manh, D.; Lavrentiev, M.Y.; Dudarev, S.L. The Fe—Cr system: Atomistic modelling of thermodynamics and kinetics of phase transformations. C. R. Phys. 2008, 9, 379–388. [Google Scholar] [CrossRef]
- Muzyk, M.; Nguyen-Manh, D.; Kurzydłowski, K.J.; Baluc, N.L.; Dudarev, S.L. Phase stability, point defects, and elastic properties of W-V and W-Ta alloys. Phys. Rev. B 2011, 84, 104115. [Google Scholar] [CrossRef]
- Wu, Q.; He, B.; Song, T.; Gao, J.; Shi, S. Cluster expansion method and its application in computational materials science. Comput. Mater. Sci. 2016, 125, 243–254. [Google Scholar] [CrossRef]
- Chinnappan, R.; Panigrahi, B.K.; van de Walle, A. First-principles study of phase equilibrium in Ti-V, Ti-Nb, and Ti-Ta alloys. Calphad 2016, 54, 125–133. [Google Scholar] [CrossRef]
- Wróbel, J.S.; Nguyen-Manh, D.; Lavrentiev, M.Y.; Muzyk, M.; Dudarev, S.L. Phase stability of ternary fcc and bcc Fe-Cr-Ni alloys. Phys. Rev. B 2015, 91, 024108. [Google Scholar] [CrossRef]
- Connolly, J.W.D.; Williams, A.R. Density-functional theory applied to phase transformations in transition-metal alloys. Phys. Rev. B 1983, 27, 5169. [Google Scholar] [CrossRef]
- Van de Walle, A.; Asta, M.; Ceder, G. The alloy theoretic automated toolkit: A user guide. Calphad 2002, 26, 539–553. [Google Scholar] [CrossRef]
- Warren, B.E. X-ray Diffraction; Dover: New York, NY, USA, 1990. [Google Scholar]
- Cowley, J.M. An Approximate Theory of Order in Alloys. Phys. Rev. 1950, 77, 669. [Google Scholar] [CrossRef]
- Fernández-Caballero, A.; Fedorov, M.; Wróbel, J.S.; Mummery, P.M.; Nguyen-Manh, D. Configurational Entropy in Multicomponent Alloys: Matrix Formulation from Ab Initio Based Hamiltonian and Application to the FCC Cr-Fe-Mn-Ni System. Entropy 2019, 21, 68. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, M.; Wróbel, J.S.; Fernadez-Caballero, A.; Kurzydłowski, K.J.; Nguyen-Manh, D. Phase stability and magnetic properties in fcc Fe-Cr-Mn-Ni alloys from first-principles modeling. Phys. Rev. B 2020, 101, 174416. [Google Scholar] [CrossRef]
- Fernández-Caballero, A.; Wróbel, J.S.; Mummery, P.M.; Nguyen-Manh, D. Short-Range Order in High Entropy Alloys: Theoretical Formulation and Application to Mo-Nb-Ta-V-W System. J. Phase Equilibria Diffus. 2017, 38, 391–403. [Google Scholar] [CrossRef]
- Mirebeau, I.; Hennion, M.; Parette, G. First Measurement of Short-Range-Order Inversion as a Function of Concentration in a Transition Alloy. Phys. Rev. Lett. 1984, 53, 687–690. [Google Scholar] [CrossRef]
- Naidu, S.V.N.; Sriramamurthy, A.M.; Rao, P.R. The Cr-W (Chromium-Tungsten) system. Bull. Alloy Phase Diagr. 1984, 5, 289. [Google Scholar] [CrossRef]
- Klein, F. Studies of Oxidation Resistant Tungsten Alloys at Temperatures of 1100 K to 1475 K. Ph.D. Thesis, Ruhr-Universität Bochum, Universitätsbibliothek, Bochum, Germany, 2020. [Google Scholar]
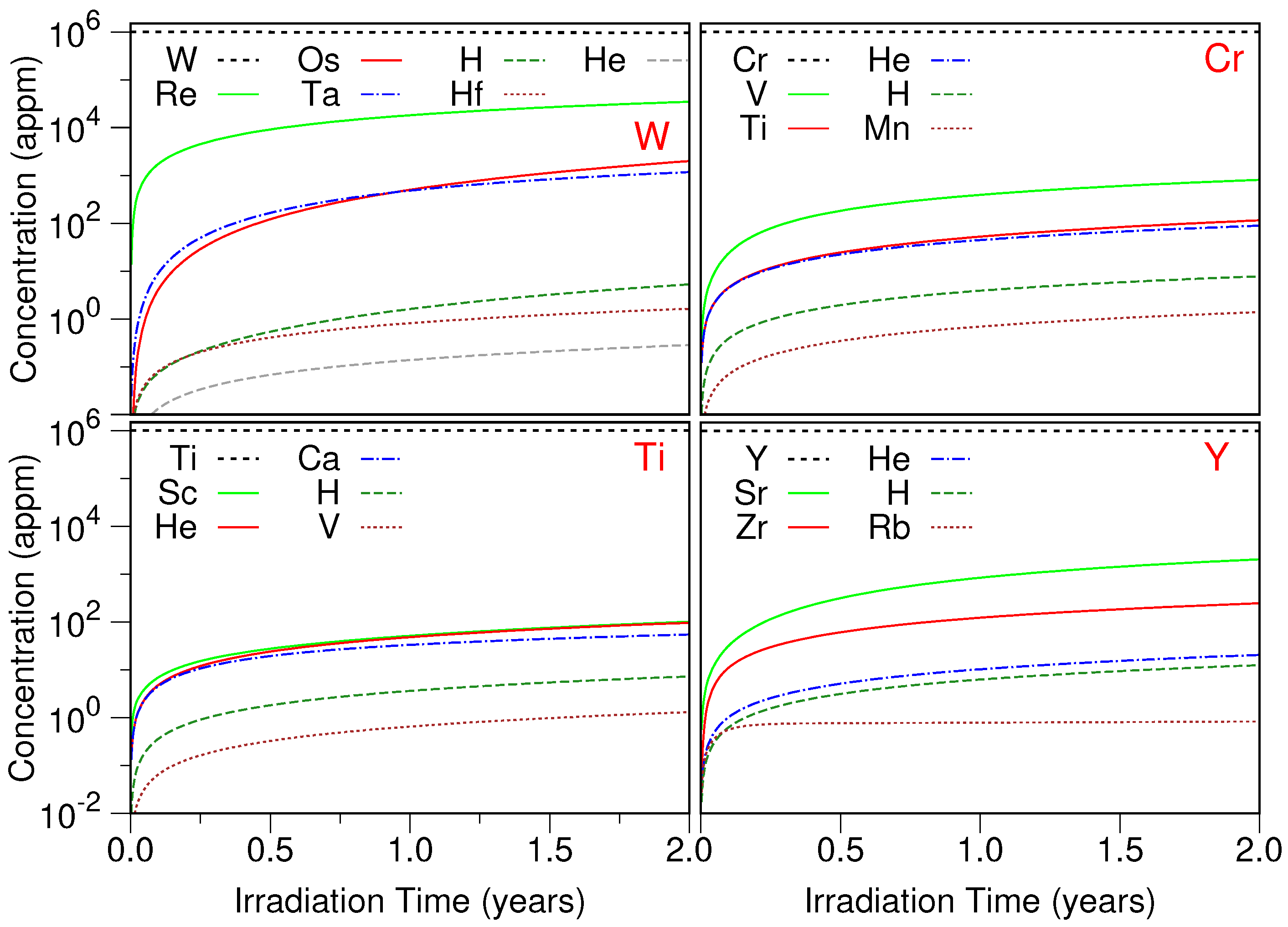
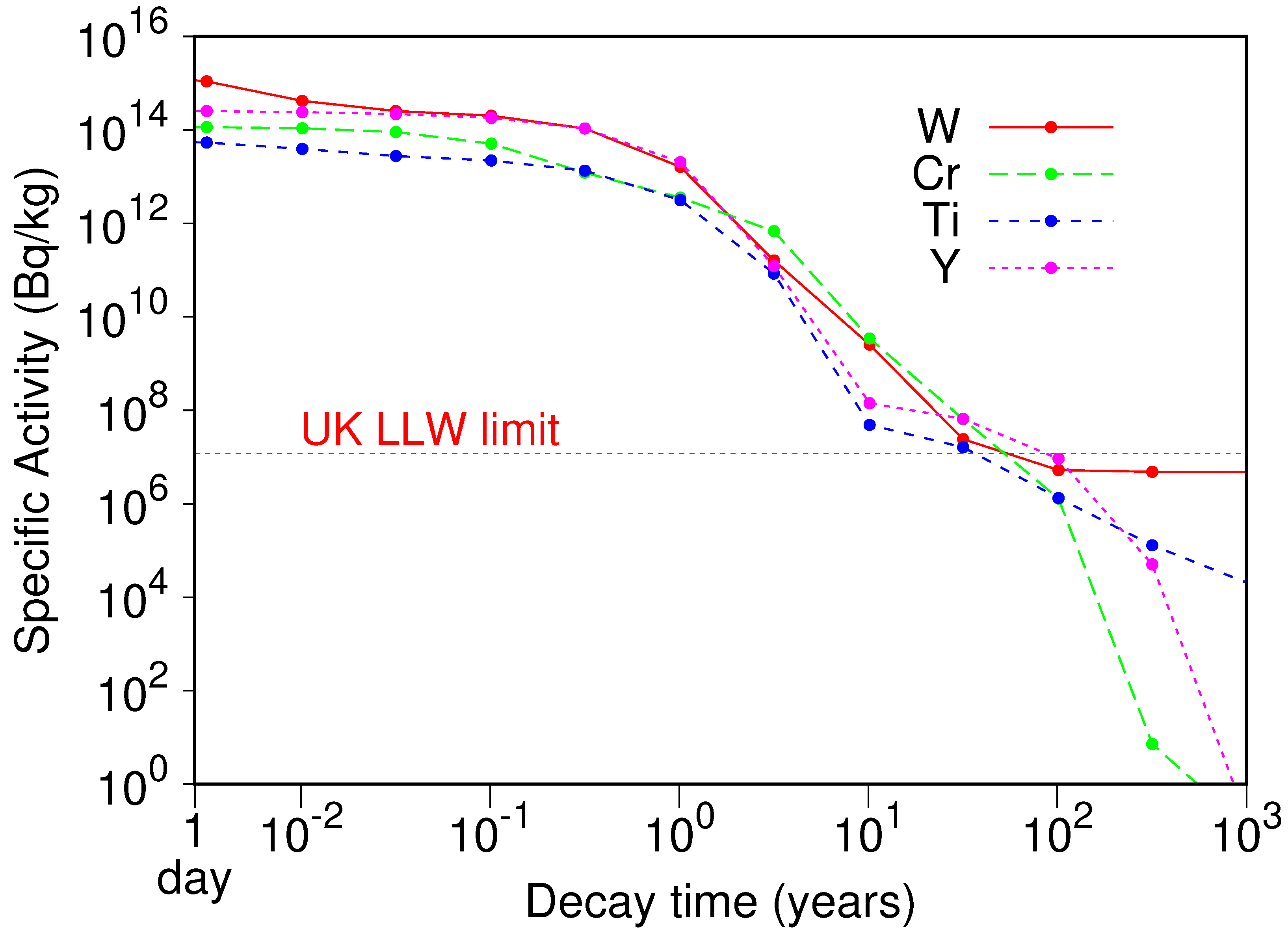
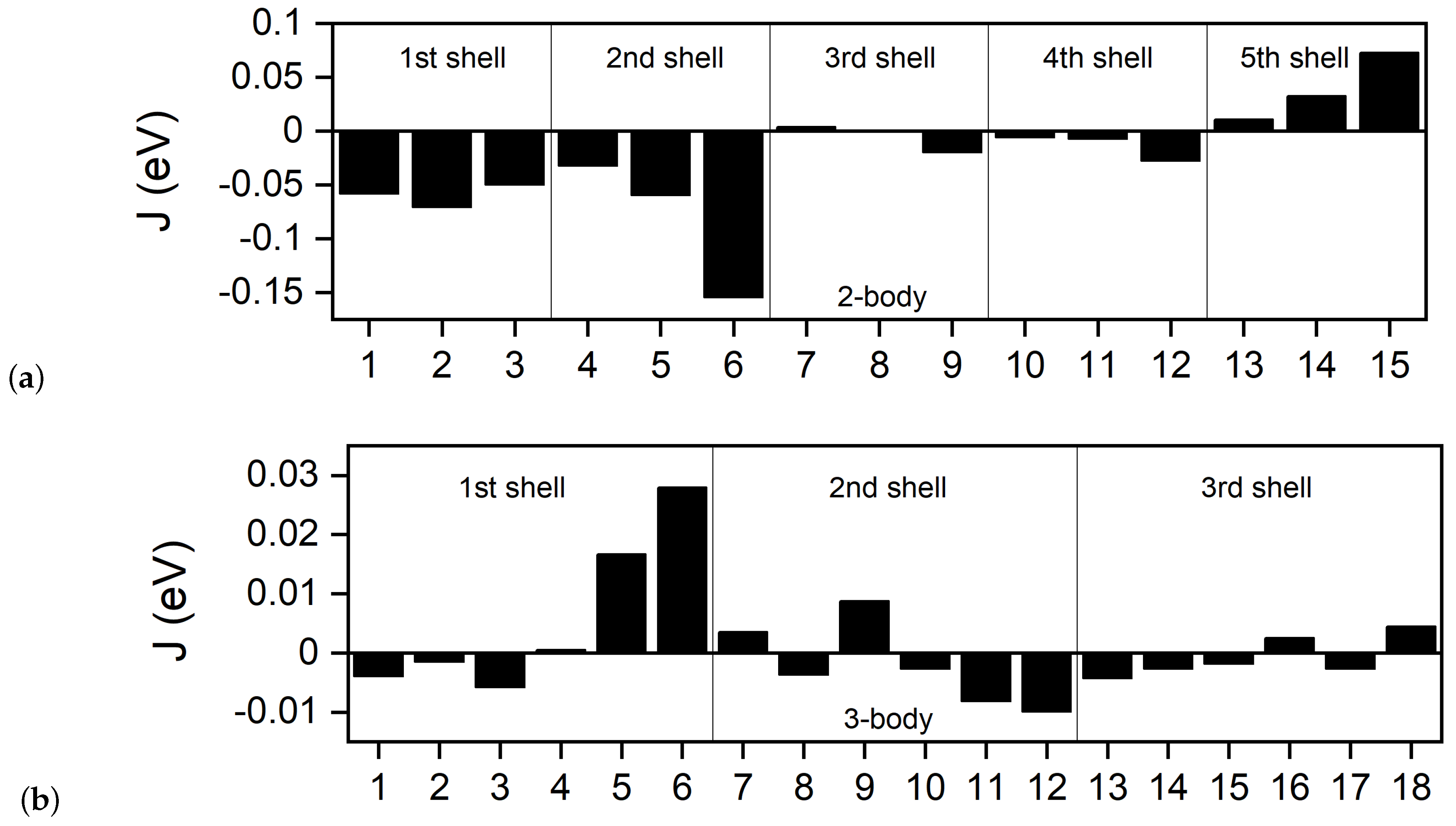
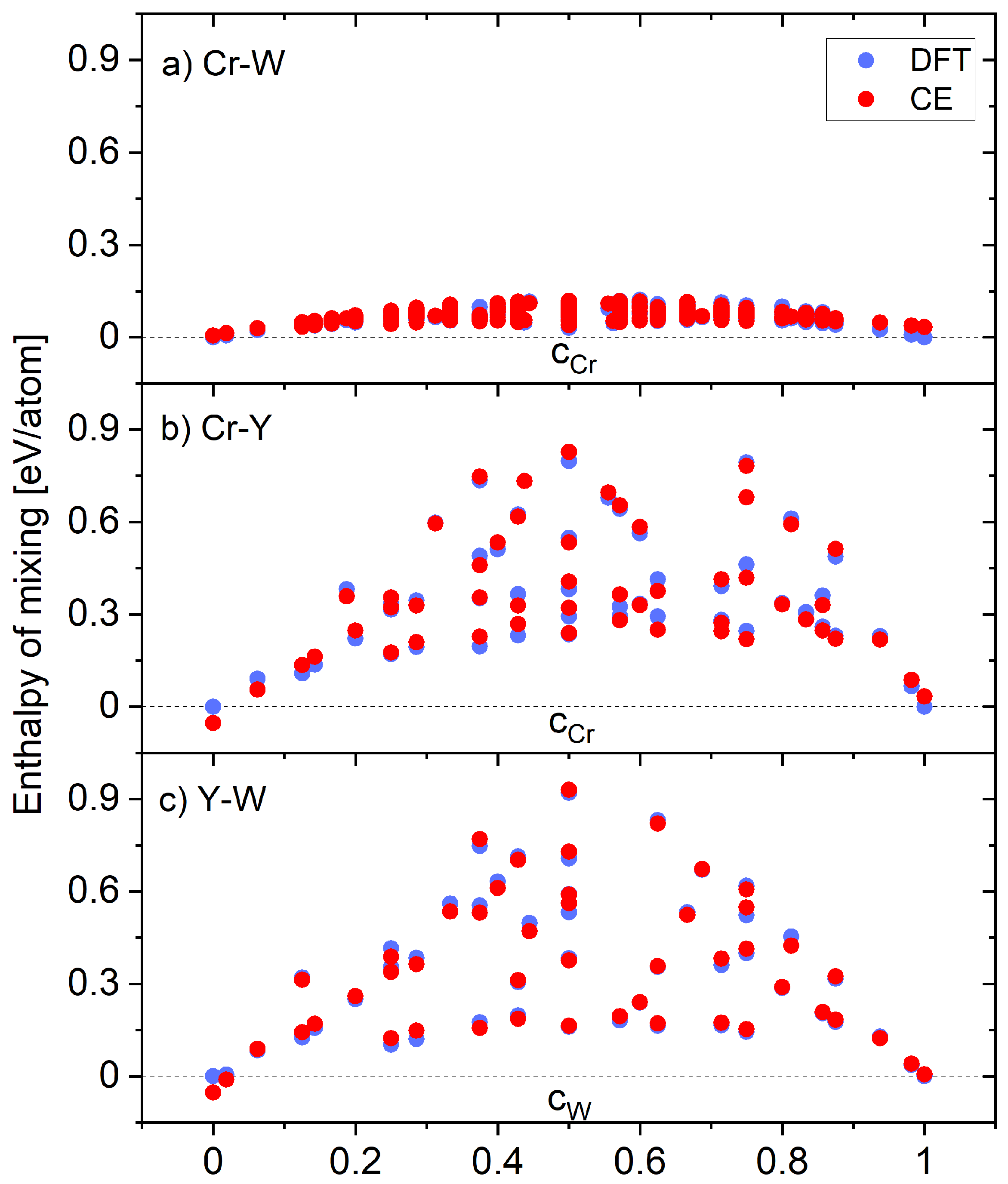



| n | Coordinates | ||||
|---|---|---|---|---|---|
| 1 | 1 | (0) | (0,0,0) | 1 | 538.452 |
| (1) | 1 | 257.288 | |||
| (2) | 1 | 211.843 | |||
| 2 | 1 | (1,1) | (0, 0, 0; , , ) | 4 | −58.172 |
| (1,2) | 8 | −70.914 | |||
| (2,2) | 4 | −50.004 | |||
| 2 | 2 | (1,1) | (0,0,0; 1,0,0) | 3 | −32.089 |
| (1,2) | 6 | −59.610 | |||
| (2,2) | 3 | −154.668 | |||
| 2 | 3 | (1,1) | (0,0,0; 1,0,1) | 6 | 3.656 |
| (1,2) | 12 | 0.321 | |||
| (2,2) | 6 | −19.614 | |||
| 2 | 4 | (1,1) | (0,0,0; 1,,) | 12 | −5.678 |
| (1,2) | 24 | −7.450 | |||
| (2,2) | 12 | −27.653 | |||
| 2 | 5 | (1,1) | (0,0,0; 1,1,1) | 4 | 10.940 |
| (1,2) | 8 | 32.636 | |||
| (2,2) | 4 | 72.744 | |||
| 3 | 1 | (1,1,1) | (1,0,0; ,,; 1,1,1) | 12 | −3.971 |
| (2,1,1) | 24 | −1.483 | |||
| (1,2,1) | 12 | −5.822 | |||
| (2,2,1) | 24 | 0.522 | |||
| (2,1,2) | 12 | 16.694 | |||
| (2,2,2) | 12 | 27.950 | |||
| 3 | 2 | (1,1,1) | (,−,−; 0,0,0; −,−,) | 12 | 3.509 |
| (2,1,1) | 24 | −3.726 | |||
| (1,2,1) | 12 | 8.773 | |||
| (2,2,1) | 24 | −2.666 | |||
| (2,1,2) | 12 | −8.127 | |||
| (2,2,2) | 12 | −9.910 | |||
| 3 | 3 | (1,1,1) | (0,0,1; 0,0,0; 0,1,0) | 12 | −4.250 |
| (2,1,1) | 24 | −2.652 | |||
| (1,2,1) | 12 | −1.870 | |||
| (2,2,1) | 24 | 2.550 | |||
| (2,1,2) | 12 | −2.719 | |||
| (2,2,2) | 12 | 4.426 |
| Alloy | ODTT [K] |
|---|---|
| WCr | 1700 |
| WCrY | 1100 |
| WCrY | 1300 |
| WCrY | 1300 |
| WCrY | 1300 |
| WCrY | 1300 |
| Alloy | ODTT [K] |
|---|---|
| WCr | 1700 |
| WCrTi | 900 |
| WCrTi | 1700 |
| WCrTi | 1700 |
| WCrTi | 1700 |
| WCrTi | 1700 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobieraj, D.; Wróbel, J.S.; Gilbert, M.R.; Litnovsky, A.; Klein, F.; Kurzydłowski, K.J.; Nguyen-Manh, D. Composition Stability and Cr-Rich Phase Formation in W-Cr-Y and W-Cr-Ti Smart Alloys. Metals 2021, 11, 743. https://doi.org/10.3390/met11050743
Sobieraj D, Wróbel JS, Gilbert MR, Litnovsky A, Klein F, Kurzydłowski KJ, Nguyen-Manh D. Composition Stability and Cr-Rich Phase Formation in W-Cr-Y and W-Cr-Ti Smart Alloys. Metals. 2021; 11(5):743. https://doi.org/10.3390/met11050743
Chicago/Turabian StyleSobieraj, Damian, Jan S. Wróbel, Mark R. Gilbert, Andrey Litnovsky, Felix Klein, Krzysztof J. Kurzydłowski, and Duc Nguyen-Manh. 2021. "Composition Stability and Cr-Rich Phase Formation in W-Cr-Y and W-Cr-Ti Smart Alloys" Metals 11, no. 5: 743. https://doi.org/10.3390/met11050743
APA StyleSobieraj, D., Wróbel, J. S., Gilbert, M. R., Litnovsky, A., Klein, F., Kurzydłowski, K. J., & Nguyen-Manh, D. (2021). Composition Stability and Cr-Rich Phase Formation in W-Cr-Y and W-Cr-Ti Smart Alloys. Metals, 11(5), 743. https://doi.org/10.3390/met11050743









