Influence of Ceramic Particles Character on Resulted Properties of Zinc-Hydroxyapatite/Monetite Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Powder Characterization and Preparation of Powder Mixtures
2.2. Thermomechanical Processing
2.3. Microstructure
2.4. Mechanical Properties
2.5. Corrosion Tests
3. Results
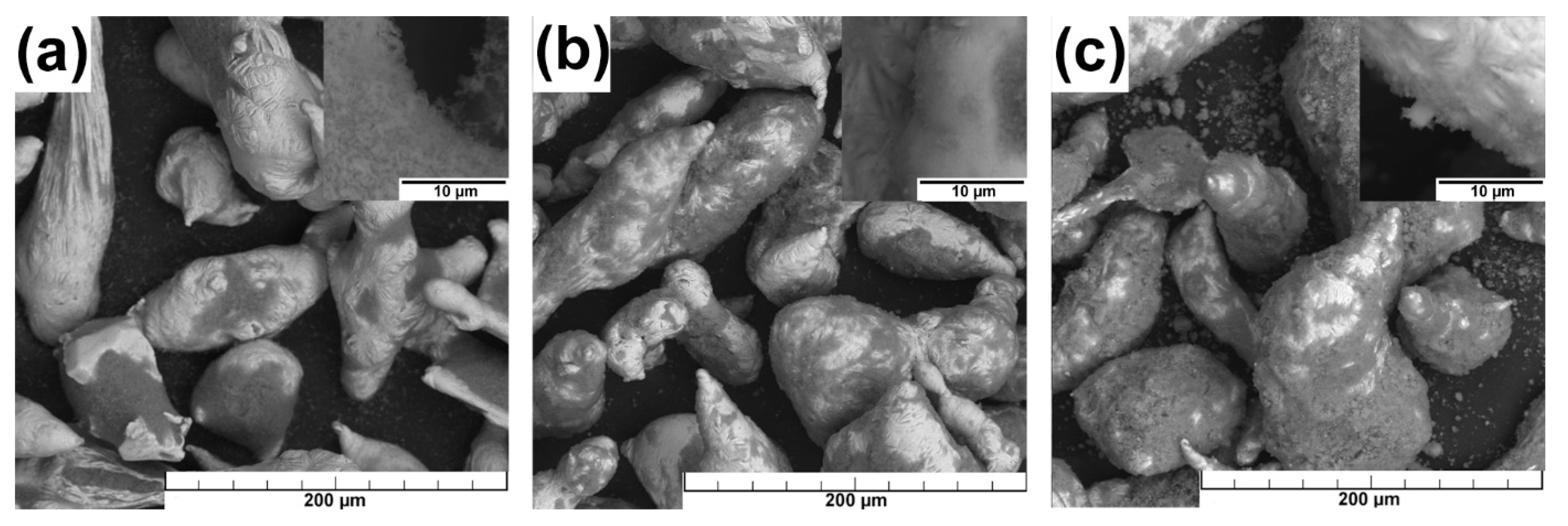
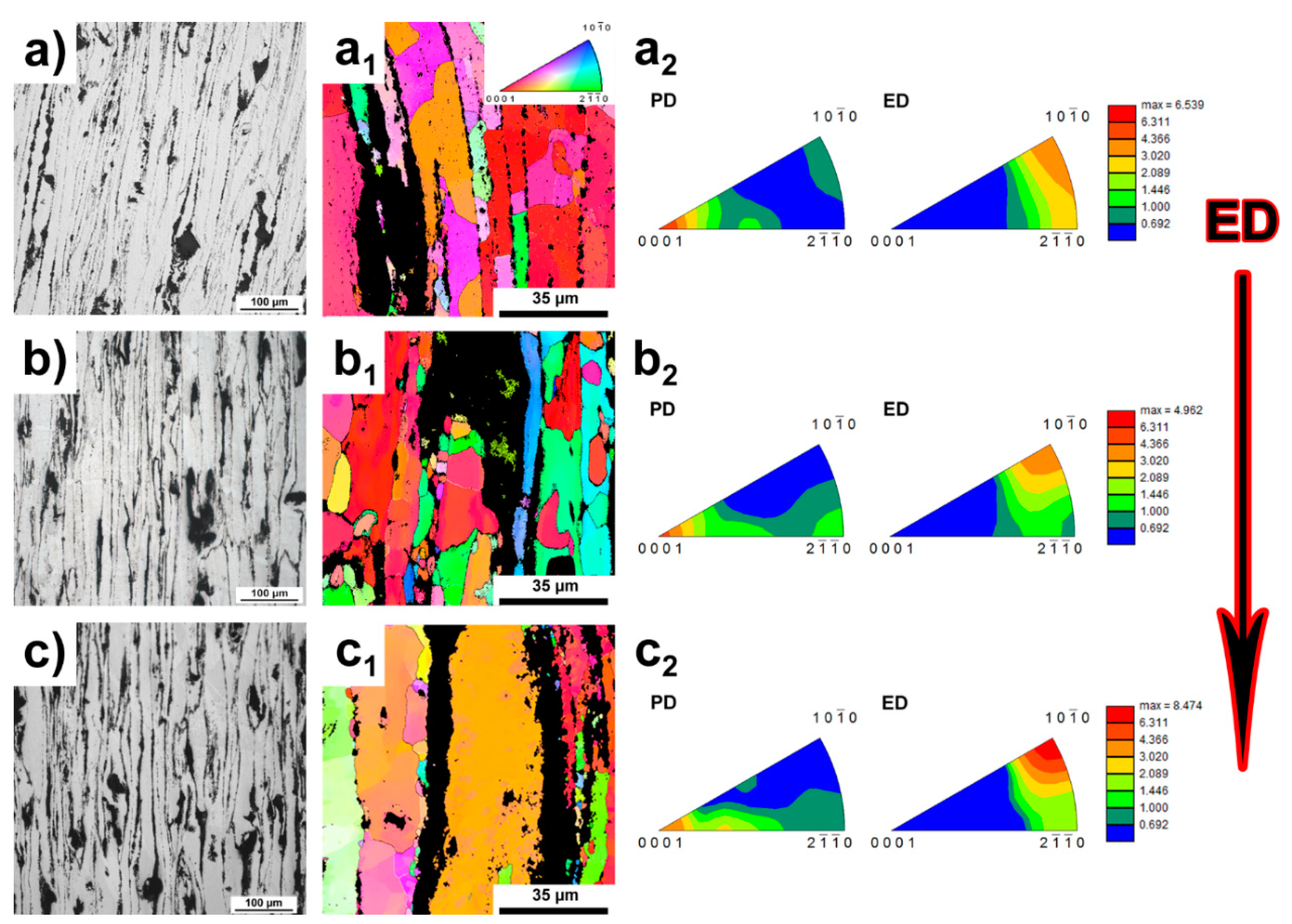
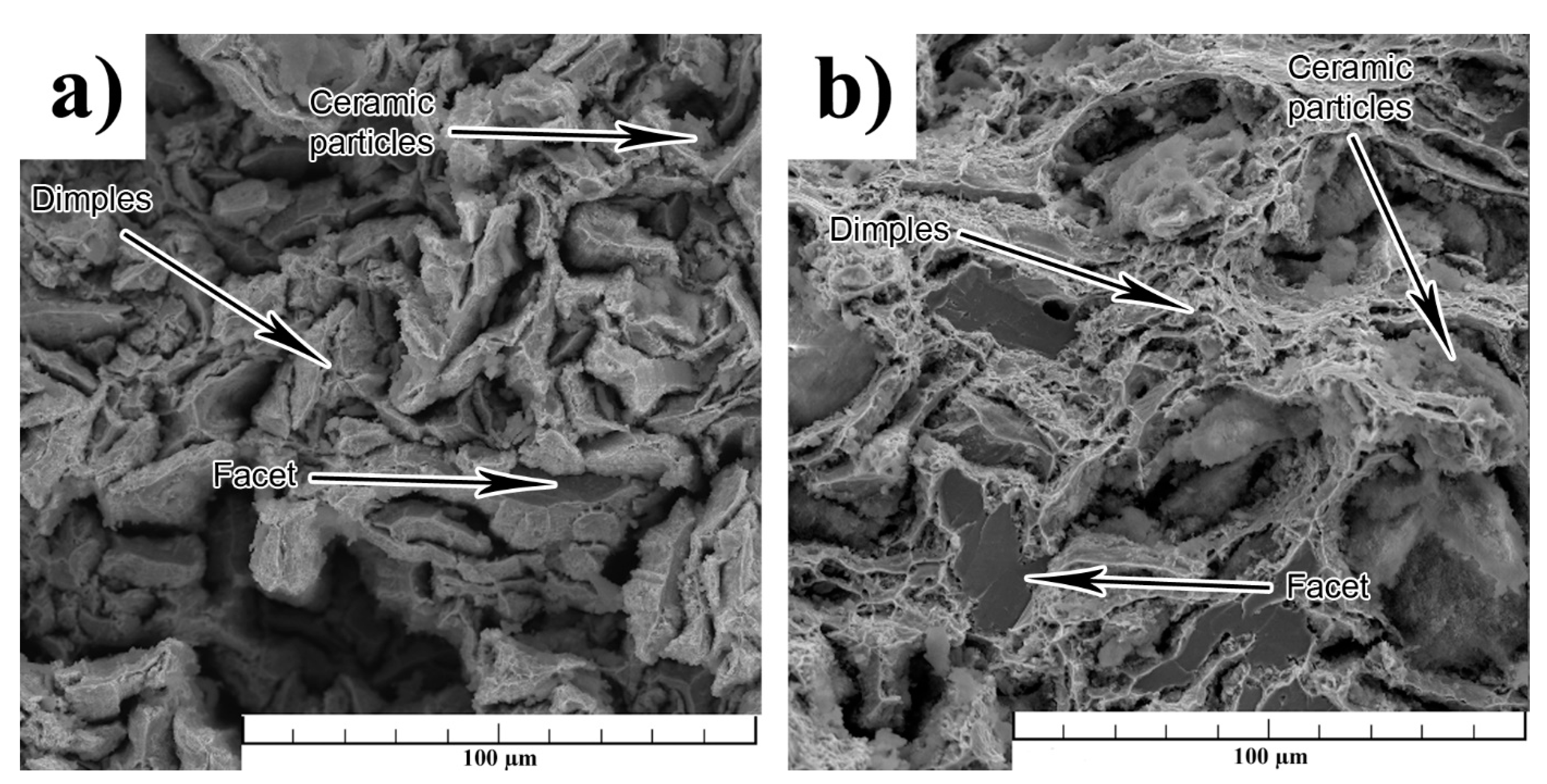
3.1. Microstructure
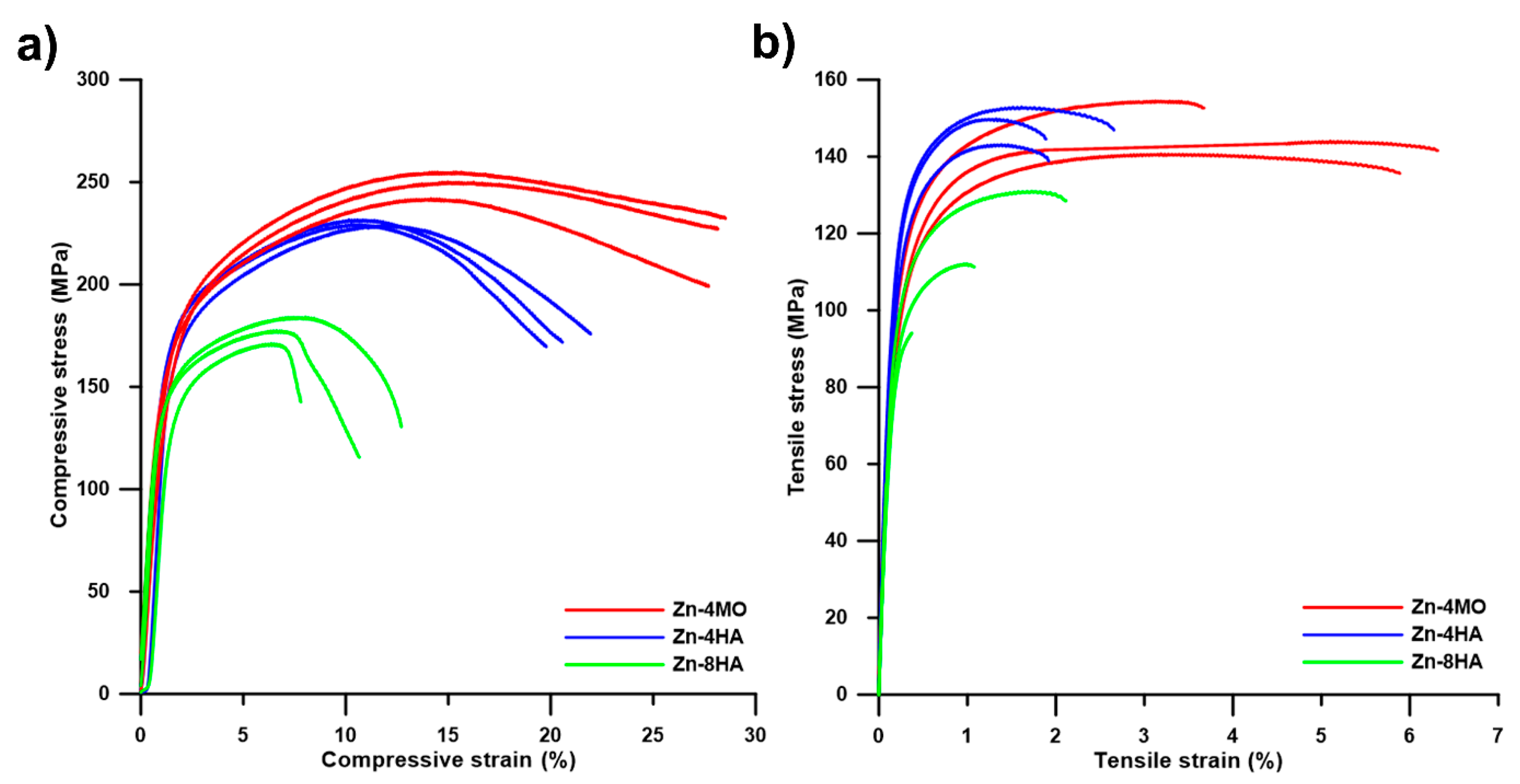
3.2. Mechanical Properties
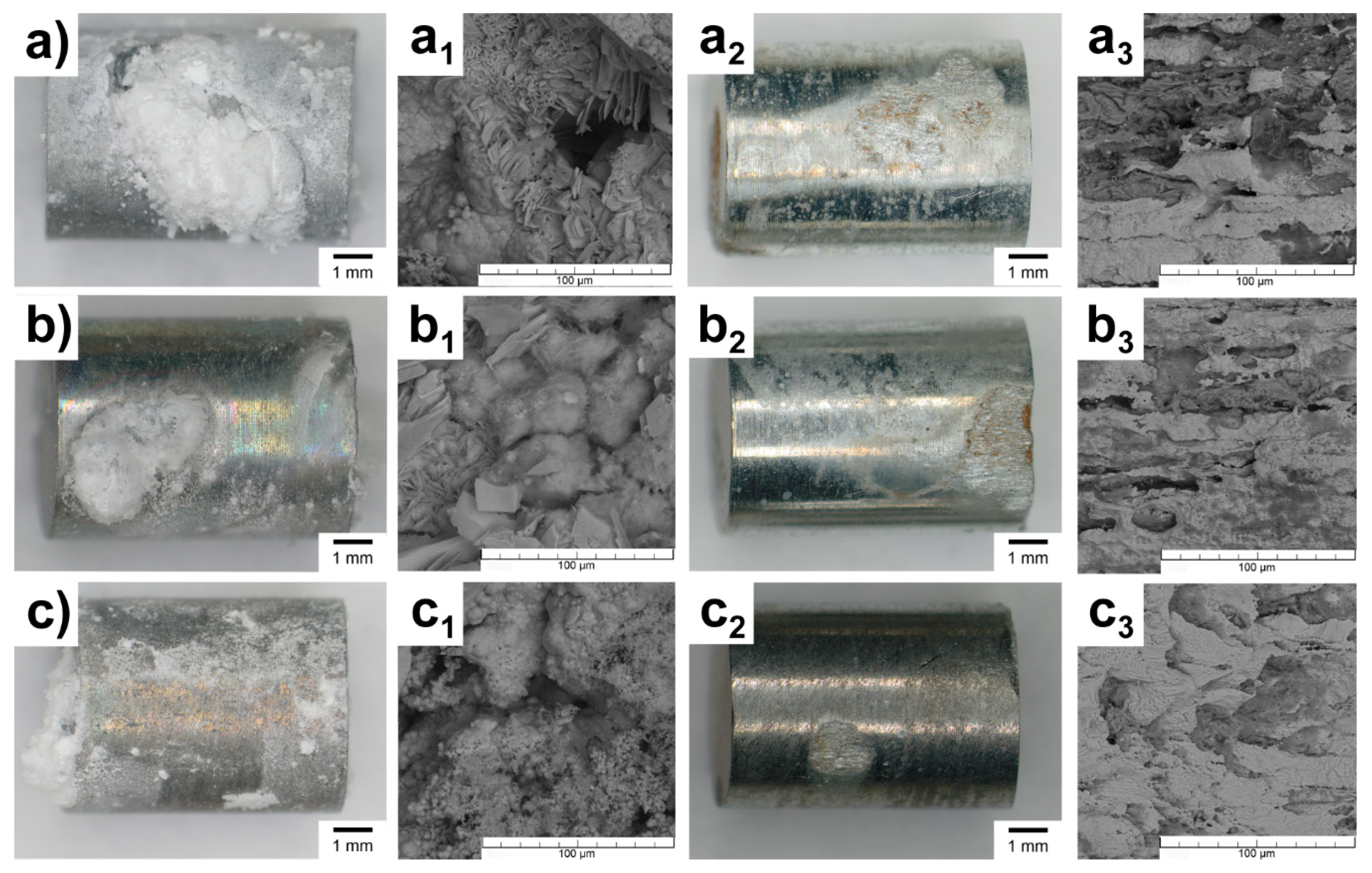
3.3. Immersion Tests
4. Discussion
5. Conclusions
- HA and MO formed spherical conglomerates in the extruded materials due to their physical characteristics. The conglomerates created, with the Zn matrix, only the mechanical connection, which had an influence on the resulting properties of the materials.
- The influence of the amount and morphology of the particles on the mechanical properties was found. The higher content of the ceramic particles resulted in the lower amount of interconnections between individual zinc particles, decreasing the material strength approximately by 40–60 MPa. In addition, the morphology of ceramic particles affected predominantly the ductility of the materials. More precisely, the presence of rod-like particles increased the ductility of the material by 100% (from 2.1 to 5.1%) compared with the globular one.
- The type of reinforcement affected the corrosion behavior. The measurements pointed to a higher degradation rate of the Zn-4MO sample due to the gradual dissolving of the MO. The process characterizes a minor part of material degradation and explains the differences between Zn-4HA and Zn-4MO corrosion rates (0.1 mm/a). Exposure to a corrosive environment results in the formation of corrosion products, primarily hydrozincite, simonkolleite and zinc hydrogen phosphate.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levy, G.K.; Goldman, J.; Aghion, E. The Prospects of Zinc as a Structural Material for Biodegradable Implants—A Review Paper. Metals 2017, 7, 402. [Google Scholar] [CrossRef]
- Li, Y.; Pavanram, P.; Zhou, J.; Lietaert, K.; Taheri, P.; Li, W.; San, H.; Leeflang, M.; Mol, J.; Jahr, H.; et al. Additively manufactured biodegradable porous zinc. Acta Biomater. 2020, 101, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Drelich, A.J.; Zhao, S.; Guillory, R.J.; Drelich, J.W.; Goldman, J. Long-term surveillance of zinc implant in murine artery: Surprisingly steady biocorrosion rate. Acta Biomater. 2017, 58, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Godavitarne, C.; Robertson, A.; Peters, J.; Rogers, B. Biodegradable materials. Orthop. Trauma 2017, 31, 316–320. [Google Scholar] [CrossRef]
- Li, H.; Zheng, Y.; Qin, L. Progress of biodegradable metals. Prog. Nat. Sci. 2014, 24, 414–422. [Google Scholar] [CrossRef]
- Tan, L.; Yu, X.; Wan, P.; Yang, K. Biodegradable Materials for Bone Repairs: A Review. J. Mater. Sci. Technol. 2013, 29, 503–513. [Google Scholar] [CrossRef]
- Obayi, C.S.; Tolouei, R.; Paternoster, C.; Turgeon, S.; Okorie, B.A.; Obikwelu, D.O.; Cassar, G.; Buhagiar, J.; Mantovani, D. Influence of cross-rolling on the micro-texture and biodegradation of pure iron as biodegradable material for medical implants. Acta Biomater. 2015, 17, 68–77. [Google Scholar] [CrossRef]
- Wegener, B.; Sievers, B.; Utzschneider, S.; Müller, P.; Jansson, V.; Rößler, S.; Nies, B.; Stephani, G.; Kieback, B.; Quadbeck, P. Microstructure, cytotoxicity and corrosion of powder-metallurgical iron alloys for biodegradable bone replacement materials. Mater. Sci. Eng. B 2011, 176, 1789–1796. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef]
- Gu, X.-N.; Zheng, Y.-F. A review on magnesium alloys as biodegradable materials. Front. Mater. Sci. China 2010, 4, 111–115. [Google Scholar] [CrossRef]
- Vojtěch, D.; Kubásek, J.; Šerák, J.; Novák, P. Mechanical and corrosion properties of newly developed biodegradable Zn-based alloys for bone fixation. Acta Biomater. 2011, 7, 3515–3522. [Google Scholar] [CrossRef]
- Kubásek, J.; Vojtěch, D.; Jablonská, E.; Pospíšilová, I.; Lipov, J.; Ruml, T. Structure, mechanical characteristics and in vitro degradation, cytotoxicity, genotoxicity and mutagenicity of novel biodegradable Zn–Mg alloys. Mater. Sci. Eng. C 2016, 58, 24–35. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, Y.; Peng, S.; Xu, L.; He, C.; Qi, F.; Zhao, M.; Shuai, C. Microstructure evolution and texture tailoring of reduced graphene oxide reinforced Zn scaffold. Bioact. Mater. 2021, 6, 1230–1241. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Cockerill, I.; Wang, Y.; Qin, Y.-X.; Chang, L.; Zheng, Y.; Zhu, D. Zinc-Based Biomaterials for Regeneration and Therapy. Trends Biotechnol. 2019, 37, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Kabir, H.; Munir, K.; Wen, C.; Li, Y. Recent research and progress of biodegradable zinc alloys and composites for biomedical applications: Biomechanical and biocorrosion perspectives. Bioact. Mater. 2021, 6, 836–879. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, B.; Cai, Z. Recent Progress on Mg- and Zn-Based Alloys for Biodegradable Vascular Stent Applications. J. Nanomater. 2019, 2019, 1–16. [Google Scholar] [CrossRef]
- Vojtěch, D.; KUBÁSEK, J.; Čapek, J. Comparative mechanical and corrosion studies on magnesium, zinc and iron alloys as biodegradable metals. Mater. Tehnol. 2015, 49, 877–882. [Google Scholar] [CrossRef]
- Liu, L.; Meng, Y.; Dong, C.; Yan, Y.; Volinsky, A.A.; Wang, L.-N. Initial formation of corrosion products on pure zinc in simulated body fluid. J. Mater. Sci. Technol. 2018, 34, 2271–2282. [Google Scholar] [CrossRef]
- Jia, B.; Yang, H.; Zhang, Z.; Qu, X.; Jia, X.; Wu, Q.; Han, Y.; Zheng, Y.; Dai, K. Biodegradable Zn–Sr alloy for bone regeneration in rat femoral condyle defect model: In vitro and in vivo studies. Bioact. Mater. 2021, 6, 1588–1604. [Google Scholar] [CrossRef]
- Jia, B.; Yang, H.; Han, Y.; Zhang, Z.; Qu, X.; Zhuang, Y.; Wu, Q.; Zheng, Y.; Dai, K. In vitro and in vivo studies of Zn-Mn biodegradable metals designed for orthopedic applications. Acta Biomater. 2020, 108, 358–372. [Google Scholar] [CrossRef]
- Li, Z.; Shi, Z.-Z.; Hao, Y.; Li, H.-F.; Zhang, H.-J.; Liu, X.-F.; Wang, L.-N. Insight into role and mechanism of Li on the key aspects of biodegradable Zn Li alloys: Microstructure evolution, mechanical properties, corrosion behavior and cytotoxicity. Mater. Sci. Eng. C 2020, 114, 111049. [Google Scholar] [CrossRef]
- Gong, H.; Wang, K.; Strich, R.; Zhou, J.G. In vitro biodegradation behavior, mechanical properties, and cytotoxicity of biodegradable Zn-Mg alloy. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1632–1640. [Google Scholar] [CrossRef]
- Shi, Z.-Z.; Gao, X.-X.; Chen, H.-T.; Liu, X.-F.; Li, A.; Zhang, H.-J.; Wang, L.-N. Enhancement in mechanical and corrosion resistance properties of a biodegradable Zn-Fe alloy through second phase refinement. Mater. Sci. Eng. C 2020, 116, 111197. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, L.; Zhang, Z.; Wang, X.; Wang, R.; Cui, C. Fabrication and properties of porous Zn-Ag alloy scaffolds as biodegradable materials. Mater. Chem. Phys. 2018, 219, 433–443. [Google Scholar] [CrossRef]
- Čapek, J.; KUBÁSEK, J.; Pinc, J.; Maňák, J.; Molnárová, O.; Drahokoupil, J.; Čavojský, M. ZnMg0.8Ca0.2 (wt%) biodegradable alloy—The influence of thermal treatment and extrusion on microstructural and mechanical characteristics. Mater. Charact. 2020, 162, 110230. [Google Scholar] [CrossRef]
- Čapek, J.; Pinc, J.; Kubásek, J.; Molnárová, O.; Maňák, J.; Drahokoupil, J. ZnMg0.8Ca/Sr0.2 ternary alloys—The influence of the third element on material properties. Procedia Struct. Integr. 2019, 23, 3–8. [Google Scholar] [CrossRef]
- Venezuela, J.; Dargusch, M. The influence of alloying and fabrication techniques on the mechanical properties, biodegradability and biocompatibility of zinc: A comprehensive review. Acta Biomater. 2019, 87, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.-Z.; Li, Z.-L.; Bai, W.-S.; Tuoliken, A.; Yu, J.; Liu, X.-F. (Fe, Mn)Zn13 phase and its core-shell structure in novel biodegradable Zn-Mn-Fe alloys. Mater. Des. 2019, 162, 235–245. [Google Scholar] [CrossRef]
- Shi, Z.-Z.; Yu, J.; Liu, X.-F.; Zhang, H.-J.; Zhang, D.-W.; Yin, Y.-X.; Wang, L.-N. Effects of Ag, Cu or Ca addition on microstructure and comprehensive properties of biodegradable Zn-0.8Mn alloy. Mater. Sci. Eng. C 2019, 99, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Cockerill, I.; Su, Y.; Zhang, Z.; Fu, J.; Lee, K.-W.; Ma, J.; Okpokwasili, C.; Tang, L.; Zheng, Y.; et al. Mechanical Strength, Biodegradation, and in Vitro and in Vivo Biocompatibility of Zn Biomaterials. ACS Appl. Mater. Interfaces 2019, 11, 6809–6819. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Luo, Q.; Zhang, X.; Qiu, J.; Qian, S.; Liu, X. Co-implantation of magnesium and zinc ions into titanium regulates the behaviors of human gingival fibroblasts. Bioact. Mater. 2021, 6, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Ratha, I.; Datta, P.; Balla, V.K.; Nandi, S.K.; Kundu, B. Effect of doping in hydroxyapatite as coating material on biomedical implants by plasma spraying method: A review. Ceram. Int. 2021, 47, 4426–4445. [Google Scholar] [CrossRef]
- Foltz, J.V.; Blackmon, C.M. Metal-Matrix Composites. 2013. Available online: http://hdl.handle.net/11115/214 (accessed on 16 March 2021).
- Witte, F.; Feyerabend, F.; Maier, P.; Fischer, J.; Störmer, M.; Blawert, C.; Dietzel, W.; Hort, N. Biodegradable magnesium–hydroxyapatite metal matrix composites. Biomaterials 2007, 28, 2163–2174. [Google Scholar] [CrossRef]
- Yin, X.; Calderin, L.; Stott, M.J.; Sayer, M. Density functional study of structural, electronic and vibrational properties of mg- and zn-doped tricalcium phosphate biomaterials. Biomaterials 2002, 23, 4155–4163. [Google Scholar] [CrossRef]
- Fujii, E.; Ohkubo, M.; Tsuru, K.; Hayakawa, S.; Osaka, A.; Kawabata, K.; Bonhomme, C.; Babonneau, F. Selective protein adsorption property and characterization of nano-crystalline zinc-containing hydroxyapatite. Acta Biomater. 2006, 2, 69–74. [Google Scholar] [CrossRef]
- Mensah-Darkwa, K.; Gupta, R.; Kumar, D. Mechanical and Corrosion Properties of Magnesium–Hydroxyapatite (Mg–HA) Composite Thin Films. J. Mater. Sci. Technol. 2013, 29, 788–794. [Google Scholar] [CrossRef]
- Gu, X.; Zhou, W.; Zheng, Y.; Dong, L.; Xi, Y.; Chai, D. Microstructure, mechanical property, bio-corrosion and cytotoxicity evaluations of Mg/HA composites. Mater. Sci. Eng. C 2010, 30, 827–832. [Google Scholar] [CrossRef]
- Yang, H.; Qu, X.; Lin, W.; Wang, C.; Zhu, D.; Dai, K.; Zheng, Y. In vitro and in vivo studies on zinc-hydroxyapatite composites as novel biodegradable metal matrix composite for orthopedic applications. Acta Biomater. 2018, 71, 200–214. [Google Scholar] [CrossRef]
- Pinc, J.; Čapek, J.; Hybášek, V.; Průša, F.; Hosová, K.; Maňák, J.; Vojtěch, D. Characterization of Newly Developed Zinc Composite with the Content of 8 wt.% of Hydroxyapatite Particles Processed by Extrusion. Materials 2020, 13, 1716. [Google Scholar] [CrossRef]
- Pathak, D.K.; Pandey, P.M. Evaluation of in vitro corrosion behavior of zinc–hydroxyapatite and zinc–hydroxyapatite–iron as biodegradable composites. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 436–450. [Google Scholar] [CrossRef]
- Pinc, J.; Čapek, J.; KUBÁSEK, J.; Průša, F.; Hybášek, V.; Veřtát, P.; Sedlářová, I.; Vojtěch, D. Characterization of a Zn-Ca5(PO4)3(OH) Composite with a High Content of the Hydroxyapatite Particles Prepared by the Spark Plasma Sintering Process. Metals 2020, 10, 372. [Google Scholar] [CrossRef]
- Nawang, R.; Hussein, M.Z.; Matori, K.A.; Abdullah, C.A.C.; Hashim, M. Physicochemical properties of hydroxyapatite/montmorillonite nanocomposite prepared by powder sintering. Results Phys. 2019, 15, 102540. [Google Scholar] [CrossRef]
- Švecová, M.; Bartůněk, V. Facile synthesis of monetite nanoparticles from basic raw materials. Ceram. Int. 2018, 44, 16079–16082. [Google Scholar] [CrossRef]
- Liu, L.; Gebresellasie, K.; Collins, B.; Zhang, H.; Xu, Z.; Sankar, J.; Lee, Y.-C.; Yun, Y. Degradation Rates of Pure Zinc, Magnesium, and Magnesium Alloys Measured by Volume Loss, Mass Loss, and Hydrogen Evolution. Appl. Sci. 2018, 8, 1459. [Google Scholar] [CrossRef]
- Müller, L.; Müller, F.A. Preparation of SBF with different HCO3- content and its influence on the composition of biomimetic apatites. Acta Biomater. 2006, 2, 181–189. [Google Scholar] [CrossRef]
- Levoguer, C. Using laser diffraction to measure particle size and distribution. Met. Powder Rep. 2013, 68, 15–18. [Google Scholar] [CrossRef]
- Bystrov, V.S.; Paramonova, E.; Dekhtyar, Y.; Katashev, A.; Karlov, A.; Polyaka, N.; Bystrova, A.V.; Patmalnieks, A.; Kholkin, A.L. Computational and experimental studies of size and shape related physical properties of hydroxyapatite nanoparticles. J. Phys. Condens. Matter 2011, 23, 065302. [Google Scholar] [CrossRef]
- Robson, J.; Henry, D.; Davis, B. Particle effects on recrystallization in magnesium–manganese alloys: Particle-stimulated nucleation. Acta Mater. 2009, 57, 2739–2747. [Google Scholar] [CrossRef]
- Wang, X.; Hu, X.; Nie, K.; Deng, K.; Wu, K.; Zheng, M. Dynamic recrystallization behavior of particle reinforced Mg matrix composites fabricated by stir casting. Mater. Sci. Eng. A 2012, 545, 38–43. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, J. Texture analysis in hexagonal materials. Mater. Chem. Phys. 2003, 81, 11–26. [Google Scholar] [CrossRef]
- Solas, D.E.; Tomé, C.N.; Engler, O.; Wenk, H.R. Deformation and recrystallization of hexagonal metals: Modeling and experimental results for zinc. Acta Mater. 2001, 49, 3791–3801. [Google Scholar] [CrossRef]
- Laasri, S.; Taha, M.; Hajjaji, A.; Laghzizil, A.; Hlil, E. Mechanical properties of calcium phosphate biomaterials. Mol. Cryst. Liq. Cryst. 2016, 628, 198–203. [Google Scholar] [CrossRef]
- Tamimi, F.; Sheikh, Z.; Barralet, J. Dicalcium phosphate cements: Brushite and monetite. Acta Biomater. 2012, 8, 474–487. [Google Scholar] [CrossRef]
- Tromans, D. Elastic Anisotropy of HCP Metal Crystals and Polycrystals. Int. J. Res. Rev. Appl. Sci. 2011, 6, 462–483. [Google Scholar]
- Da Silva, M.P.; Lima, J.; Soares, G.; Elias, C.; De Andrade, M.; Best, S.; Gibson, I. Transformation of monetite to hydroxyapatite in bioactive coatings on titanium. Surf. Coatings Technol. 2001, 137, 270–276. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Qiao, H.; Hao, M.; Zhang, H.; Xu, Z.; Zhang, X.; Pang, X.; Lin, H. Corrosion resistance and cytocompatibility studies of zinc-doped fluorohydroxyapatite nanocomposite coatings on titanium implant. Ceram. Int. 2016, 42, 1903–1915. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, Y.; Xu, X.; Lu, Y.; Chen, L.; Li, D.; Dai, Y.; Kang, Y.; Yu, K. Investigation on the microstructure, mechanical properties, in vitro degradation behavior and biocompatibility of newly developed Zn-0.8%Li-(Mg, Ag) alloys for guided bone regeneration. Mater. Sci. Eng. C 2019, 99, 1021–1034. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, H.; Xiong, P.; Li, W.; Huang, H.-H.; Zheng, Y. Comparative studies of Tris-HCl, HEPES and NaHCO3/CO2 buffer systems on the biodegradation behaviour of pure Zn in NaCl and SBF solutions. Corros. Sci. 2019, 157, 205–219. [Google Scholar] [CrossRef]
- Juel, C. Regulation of pH in human skeletal muscle: Adaptations to physical activity. Acta Physiol. 2008, 193, 17–24. [Google Scholar] [CrossRef] [PubMed]
| Ions | c (mmol/L) |
|---|---|
| Na+ | 142.0 |
| K+ | 5.0 |
| Ca2+ | 2.5 |
| Mg2+ | 1.0 |
| Cl− | 109.0 |
| HCO3− | 27.0 |
| SO42− | 1.0 |
| HPO42− | 1.0 |
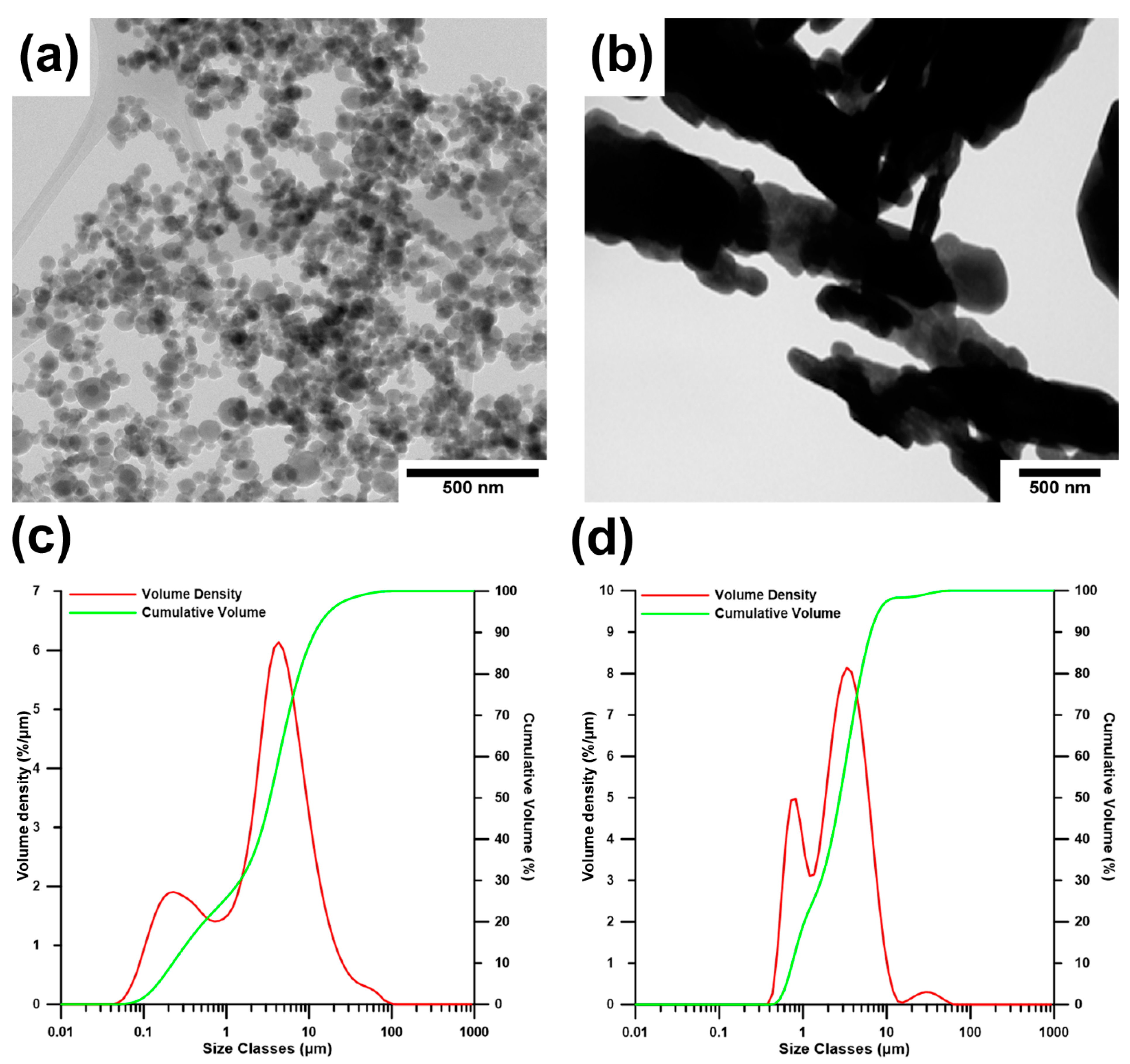
| Nanoparticles | d10 (µm) | d50 (µm) | d90 (µm) |
|---|---|---|---|
| MO | 0.74 | 2.74 | 6.45 |
| HA | 0.22 | 3.36 | 11.70 |
| Powder | Mineral | Chemical Formula | SemiQuant (%) |
|---|---|---|---|
| Zn-4MO | Zinc | Zn | 86 |
| Monetite | Ca(PO3OH) | 14 | |
| Zn-4HA | Zinc | Zn | ≥85 |
| Hydroxyapatite | Ca5(PO4)3(OH) | ≥15 | |
| Zn-8HA | Zinc | Zn | ≥80 |
| Hydroxyapatite | Ca5(PO4)3(OH) | ≥20 |
| Sample | Zn-4MO | Zn-4HA | Zn-8HA |
|---|---|---|---|
| Average | 8.6% | 7.6% | 13.7% |
| Standard deviation | 0.6% | 0.8% | 0.8% |
| Sample | CYS (MPa) | UCS (MPa) | TYS (MPa) | UTS (MPa) |
|---|---|---|---|---|
| Zn-4MO | 146 ± 4 | 249 ± 5 | 117 ± 4 | 146 ± 6 |
| Zn-4HA | 151 ± 1 | 229 ± 1 | 131 ± 4 | 149 ± 4 |
| Zn-8HA | 129 ± 2 | 176 ± 6 | 101 ± 7 | 113 ± 15 |
| Sample | Weight Loss | Corrosion Rate Determined through the Weight Loss | AAS (Zn2+) | Corrosion Rate Determined from the AAS (Zn2+) | |
|---|---|---|---|---|---|
| - | (mg) | (mm/a) | (mg/cm2/Day) | (mg/L) | (mg/cm2/Day) |
| Zn-4MO | 12.2 ± 0.4 | 0.26 ± 0.02 | 0.51 ± 0.02 | 15.0 ± 5.6 | 0.09 ± 0.03 |
| Zn-4HA | 7.6 ± 0.9 | 0.18 ± 0.02 | 0.35 ± 0.04 | 15.0 ± 6.4 | 0.09 ± 0.04 |
| Zn-8HA | 3.9 ± 0.2 | 0.10 ± 0.01 | 0.19 ± 0.01 | 11.5 ± 3.7 | 0.06 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosová, K.; Pinc, J.; Školáková, A.; Bartůněk, V.; Veřtát, P.; Školáková, T.; Průša, F.; Vojtěch, D.; Čapek, J. Influence of Ceramic Particles Character on Resulted Properties of Zinc-Hydroxyapatite/Monetite Composites. Metals 2021, 11, 499. https://doi.org/10.3390/met11030499
Hosová K, Pinc J, Školáková A, Bartůněk V, Veřtát P, Školáková T, Průša F, Vojtěch D, Čapek J. Influence of Ceramic Particles Character on Resulted Properties of Zinc-Hydroxyapatite/Monetite Composites. Metals. 2021; 11(3):499. https://doi.org/10.3390/met11030499
Chicago/Turabian StyleHosová, Klára, Jan Pinc, Andrea Školáková, Vilém Bartůněk, Petr Veřtát, Tereza Školáková, Filip Průša, Dalibor Vojtěch, and Jaroslav Čapek. 2021. "Influence of Ceramic Particles Character on Resulted Properties of Zinc-Hydroxyapatite/Monetite Composites" Metals 11, no. 3: 499. https://doi.org/10.3390/met11030499
APA StyleHosová, K., Pinc, J., Školáková, A., Bartůněk, V., Veřtát, P., Školáková, T., Průša, F., Vojtěch, D., & Čapek, J. (2021). Influence of Ceramic Particles Character on Resulted Properties of Zinc-Hydroxyapatite/Monetite Composites. Metals, 11(3), 499. https://doi.org/10.3390/met11030499







