Abstract
The global use of lithium-ion batteries of all types has been increasing at a rapid pace for many years. In order to achieve the goal of an economical and sustainable battery industry, the recycling and recirculation of materials is a central element on this path. As the achievement of high 95% recovery rates demanded by the European Union for some metals from today’s lithium ion batteries is already very challenging, the question arises of how the process chains and safety of battery recycling as well as the achievement of closed material cycles are affected by the new lithium battery generations, which are supposed to enter the market in the next 5 to 10 years. Based on a survey of the potential development of battery technology in the next years, where a diversification between high-performance and cost-efficient batteries is expected, and today’s knowledge on recycling, the challenges and chances of the new battery generations regarding the development of recycling processes, hazards in battery dismantling and recycling, as well as establishing a circular economy are discussed. It becomes clear that the diversification and new developments demand a proper separation of battery types before recycling, for example by a transnational network of dismantling and sorting locations, and flexible and high sophisticated recycling processes with case-wise higher safety standards than today. Moreover, for the low-cost batteries, recycling of the batteries becomes economically unattractive, so legal stipulations become important. However, in general, it must be still secured that closing the material cycle for all battery types with suitable processes is achieved to secure the supply of raw materials and also to further advance new developments.
1. Introduction
The goal of economical and sustainable battery cell production remains a key element on the way to establishing electromobility as a green technology of the future [1]. Sustainable process management and development also includes the economic recycling and recirculation of materials used in cell production with a simultaneously low energy input, which leads to a reduction of the ecological CO2 footprint in battery cell production [2,3,4,5]. Therefore, the establishment and sustainable further development of an internationally leading, competitive battery cell production must go hand in hand with the development of appropriate recycling technologies [6,7,8,9]. The recycling technologies must be flexible and adaptable to future production technologies and especially materials that are processed in the future with regard to new battery generations [10].
Moreover, closing the material cycles for batteries on the basis of scalable production and recycling technologies is a central component for a CO2-reduced or CO2-neutral battery cell production and thus for electromobility (e.g., achieving the “Green Deal” goal of the European Union (EU)). Only closed material cycles in batteries can enable a conversion from carbon-based energy sources to sustainably produced electrical energy in ecological, economic, and social terms [7,11,12]. To achieve closed loop material cycles, appropriate recycling technologies must be developed. For example, according to the new battery directive proposal of the EU Nr. 2019/1020, 95% of cobalt (Co), copper (Cu), and nickel (Ni) as well as 70% of lithium (Li) have to be recovered from spent lithium-ion traction batteries by 1 January 2030 [13]. Moreover, according to the report on the circular economy of traction batteries published by the Circular Economy Initiative Deutschland of acatech (National Academy of Science and Engineering in Germany) 90% of Co, Ni, and Cu as well as 85% of Li should be recycled from spent lithium battery systems by 2030. In addition, a recycling rate of 70% of the entire battery should be aimed for [14]. In a worldwide comparison, the EU sets very high requirements with the Battery Directive. In the USA, there are no generally applicable requirements for the return of lithium ion batteries (LIBs). However, voluntary consortia (e.g., the End of Live Vehicle (ELV) Solutions consortium) work together here to close material cycles. China follows a similar approach to the EU. Producers are encouraged to take back batteries that have been put into the market and to return them to the materials cycle [15]. Other countries in Asia (South Korea, Japan) are pursuing similar goals as the EU (South Korean RoHS/ELV/WEEE Act, 2007 and Japan’s End-of-Life Vehicle Recycling Law).
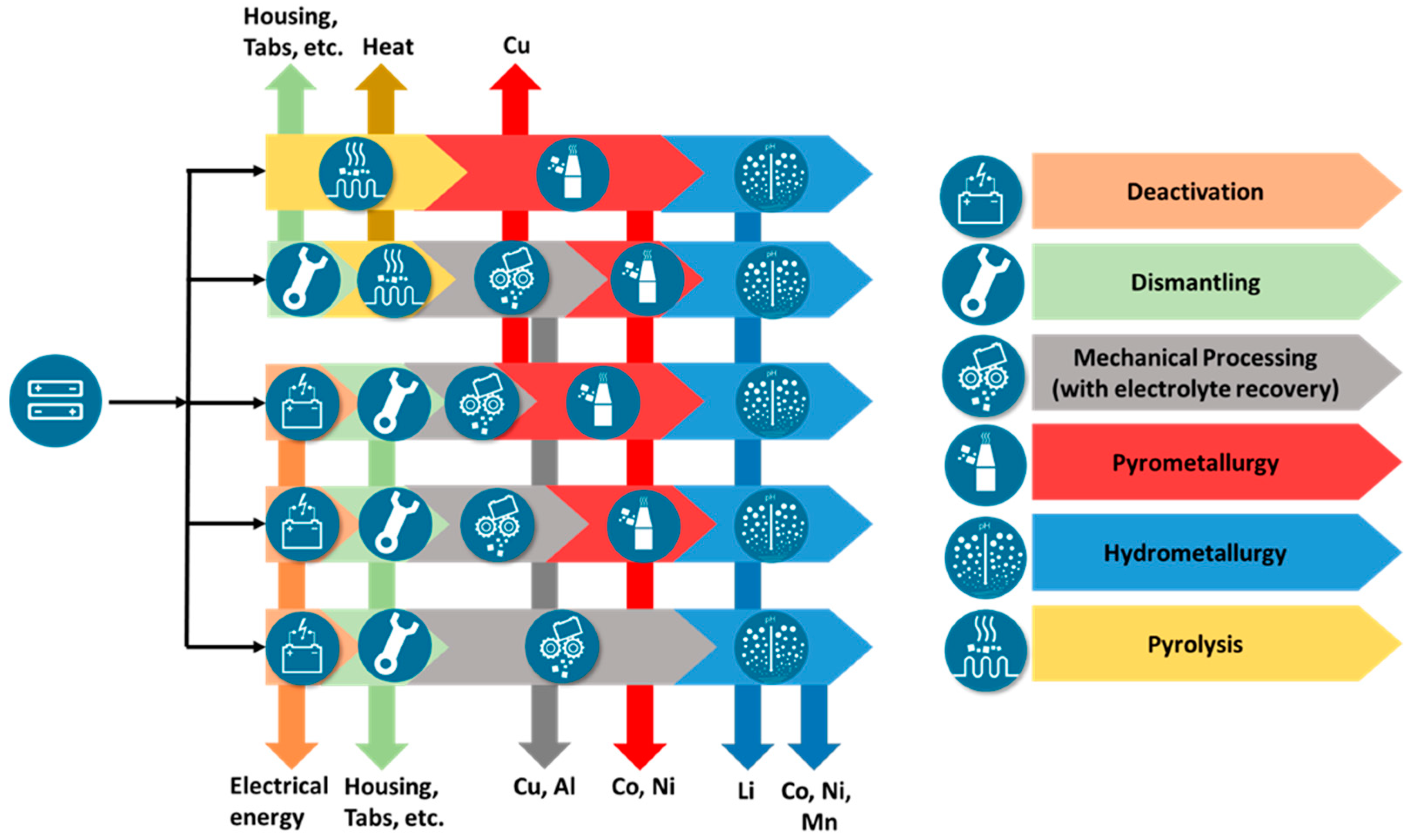
For the recycling of lithium-ion batteries today, usually at first, a deactivation of the battery system is realized, which is followed often (but not compulsory) by a dismantling of the battery system down to the cell modules (or more seldom individual cells). The deactivation can be achieved by full electrical discharge and subsequent short circuiting, by treatment in a saline solution, or by pyrolysis (high heat treatment) of the battery systems at temperatures of more than 200 °C [16,17]. Afterwards, in general, three types of process technologies are used, which are combined in a different manner: mechanical, hydrometallurgical, and pyrometallurgical processes [7,17,18,19,20,21,22]. Figure 1 gives an overview of different possibilities to combine the different process types. The different process steps can be applied in different sequences and above all in different processing depths [19,23]. One lean way is deactivation by pyrolysis, pyrometallurgical processing, and slagging. Pyrometallurgical processes are well established for processing primary materials and can achieve high recovery yields concerning the metals cobalt, nickel, and copper, but they show challenges regarding the recovery of lithium. Therefore, to recover lithium and manganese, pyrometallurgical processes have to be combined with hydrometallurgical processes for processing the slag. Overall, a relatively small overall recovery of the batteries material can be expected in this case due to graphite, polymers, and electrolyte being burned, although a very high recovery of Ni, Co, and Cu is possible [24]. A more elaborate way puts together another combination of processes. Here, the battery cells or modules are discharged in the first step, for example, before they are mechanically crushed [25]. Subsequently, the black mass or the shredded battery material is pyrometallurgically processed before it goes into a final hydrometallurgical step. Here, for example, the degree of mechanical processing can be varied [18,24,26]. The amount of recovered materials increases, as polymer components as well as aluminum can also be recovered. Furthermore, after deactivation and mechanical processing, there is also the possibility of proceeding directly to hydrometallurgical processing (as it was proposed for the LithoRec process [20,27,28]). This route enables early recovery of the polymer battery components as well as Cu and Al. In addition, hydrometallurgical processing can also recover Mn that cannot be recovered by pyrometallurgical processes. In addition to the recovery of the individual substances such as Ni and Co, also, the direct reconditioning of the cathode material by hydrometallurgical treatment is carried out on an industrial scale. Avoiding pyrometallurgical processing during battery preparation theoretically reduces the energy requirement and thus improves the ecological footprint [29]. Furthermore, other process routes are theoretically possible, but they shall not further be discussed here; they are discussed in more detail in relevant literature.
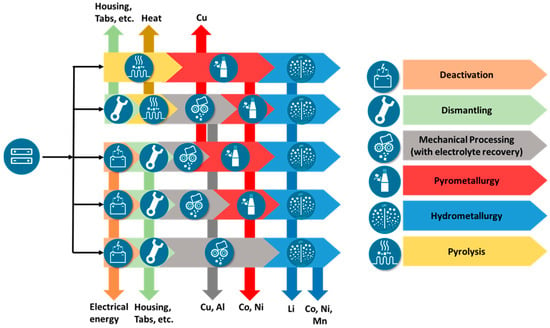
Figure 1.
General overview of some potential recycling process chains in different combinations.
Overall, the overview of the recycling processes shows that many different recycling process routes are possible and in industrial use or under development at pilot scale at least [17,28,29,30]. As a requirement for a future process chain, besides achieving high recovery rates of more than 90% or even 95% [13,14] and in parallel sufficient material purities for further usage as battery material, it is therefore to be set that it should be highly flexible in order to achieve the most energy-efficient multi-material recovery possible. To reach this goal, mechanical, thermal, and chemical process steps are to be used and combined in different ways. Future recycling processes for Li-ion batteries must not only be able to process new materials but should also replace energy-intensive processes currently used for classic Li-ion batteries with low-energy, environmentally friendly process steps.
2. Lithium Battery Development
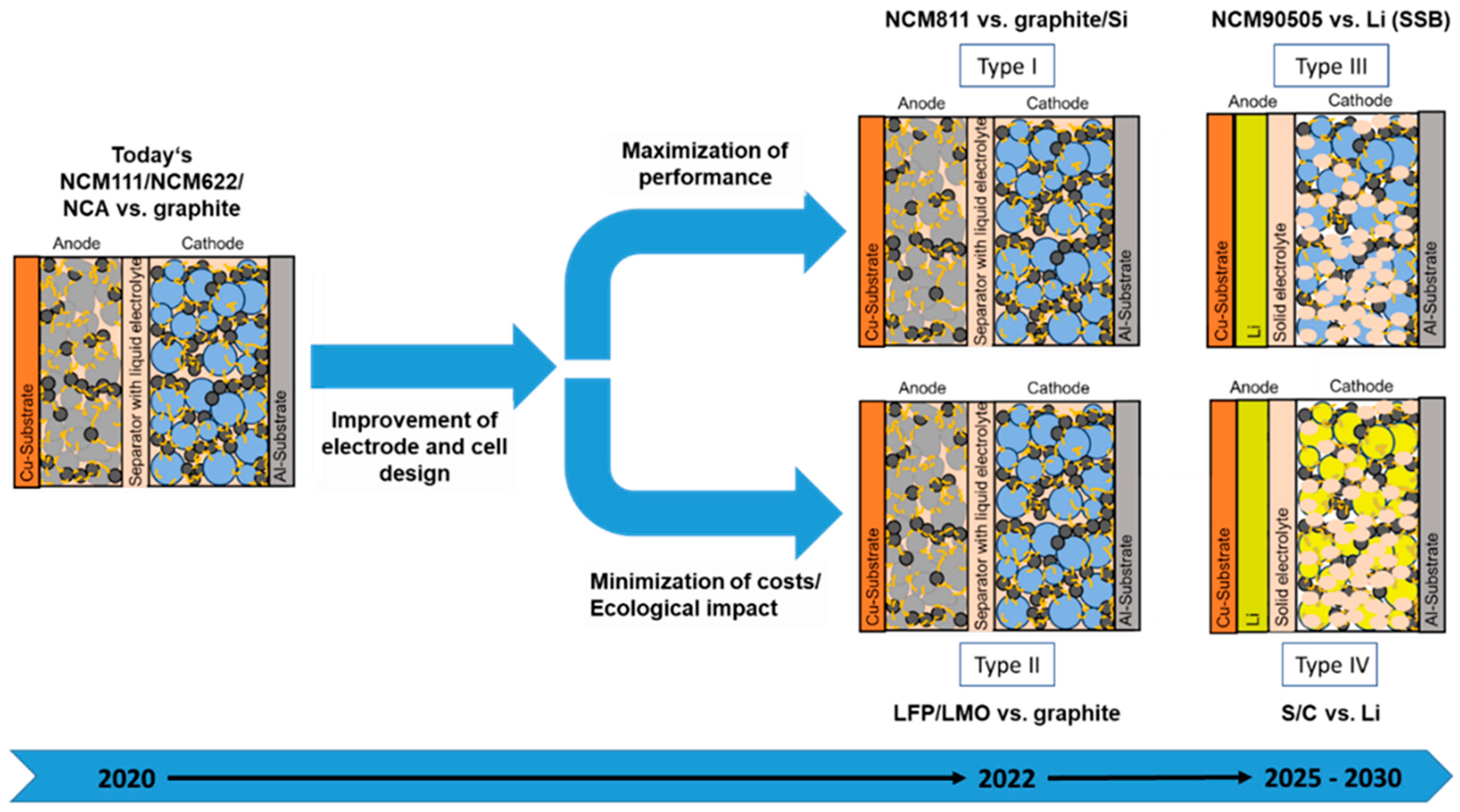
Today, lithium-ion batteries with liquid electrolyte dominate the market for electromobility. They are also used in portable devices as well as in the field of stationary energy storage. On the cathode’s side, particles of lithium transition metal oxides such as lithium nickel manganese cobalt oxide (NMC, LiNixMnyCozO2) and lithium nickel cobalt aluminum oxide (NCA, LiNixCoyAlzO2) or also lithium metal phosphates, especially lithium iron phosphate (LFP), are mainly used. In the case of the NMC and NCA materials, the nickel content increases steadily, and the cobalt content decreases continuously. Polyvinylidene fluoride (PVDF) is currently used as a standard binder on the cathode side. On the anode’s side, graphite is usually used as the anode material; in rare cases, lithium titanium oxide (LTO) is used. Moreover, the first cells with the addition of very small amounts of silicon to the graphite are offered by the cell manufactures. A mixture of carboxymethyl cellulose (CMC) and styrene butadiene rubber (SBR) usually acts as a binder within the anodes. As shown in Figure 2 within the near and medium-term future, the following developments in next-generation lithium battery technology are expected:
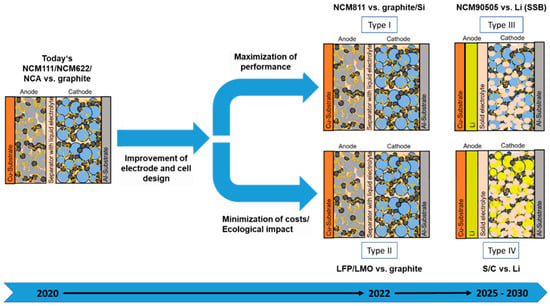
Figure 2.
Diversification of lithium battery technologies.
- High-performance lithium-ion batteries with liquid electrolyte, but anodes with higher content of nanosized silicon and cathodes with minimal or no cobalt content [31,32].
- Cost-efficient lithium-ion batteries with liquid electrolyte, graphite anode, and cathode material based mainly on iron and/or manganese and only small amounts of nickel and eventually cobalt. In addition to lithium-ion based batteries, also sodium-ion based batteries are under development, which could replace at least partly the named cost-efficient lithium-ion batteries [33,34].
- Solid-state lithium batteries with lithium or lithium-free anode structure (eventually graphite anode as intermediate stage) and solid-state electrolytes on the cathode side and as separator [35].
- Lithium sulfur batteries with lithium anode and a cathode made out of sulfur–carbon composites [36].
An overview of the materials and their potential contents in the different battery types is given in Table 1. According to Table 1, it can be concluded that with the use of the upcoming battery generations, the composition in terms of recyclables will also vary. While the Ni content will increase significantly in type I, the presence of Fe or Mn is expected in type II. In both types, the liquid electrolyte including Li conducting salt is also expected to have high potential for recovery. However, for type II, also sodium instead of Li has a significant potential. In contrast, for type III and eventually also type IV, solid electrolytes are employed for the separator and as electrolyte within the composite cathode. The solid electrolytes can have an oxide, sulfide, or polymer nature, whereby the compositions and properties can be highly variable (Table 2).

Table 1.
Material contents of different battery generations (with a focus on lithium-based batteries).

Table 2.
Typical components and properties of separator technologies.
From these points, it is clear that next-generation technologies will include much fewer critical components, such as cobalt, but also new materials such as silicon, or even germanium. In the medium to long term, metallic lithium is expected on the anode side in case of type III and especially type IV. The use of lithium anodes will require the use of polymeric, sulfidic, and/or oxidic solid electrolytes as separator and as electrolyte on the cathode’s side. However, more and more also lithium-free anode structures are shown in the literature [42,43]. In this case, the lithium from the cathode active material moves to the anode side and is deposited on the lithium-free anode layer. In order to minimize the formation of dangerous lithium dendrites, also lanthanum, titanium, zirconium or phosphorus in the solid electrolyte area are presented. Lithium is still indispensable for the time being, but the commercialization of sodium ion or other batteries in the near future is also conceivable. Overall, a further increase in the complexity of the battery cells developed and produced and the use of other economically strategic raw materials can be expected. The recovery of these should be tackled urgently in order to establish sustainable battery production with respect to the increasing sales figures.
In view of the rapidly evolving battery technologies, the best possible recycling routes for the newly developed battery cells should be determined and their recyclability assessed at an early stage. In addition to battery cells with a higher proportion of silicon, pure lithium, and/or solid-state electrolytes, the use of novel binders and fibrous additives or the increase in the adhesive strength of the electrodes can also significantly influence the recycling processes. In addition, active material mixtures are increasingly being used on the cathode’s side, so that iron, among other substances, enters the metallurgical material preparation process. Moreover, a direct reconditioning of the active material gets very difficult to impossible. Sustainable recycling concepts must be evaluated in advance by process-based economic and ecological models for the entire life cycle.
Of great interest is the assessment of the coming battery generations with regard to the change of a future circular production of batteries, the recycling process itself, and the hazard potential within the recycling process. Accordingly, in the first step, the three criteria for today’s LIB are briefly presented, and based on this, the potential challenges posed by the introduction of the different cell generations mentioned above are assessed.
3. State of the Art Recycling Processes for Lithium-Ion Batteries
As shown in Figure 1, different process chains and routes are developed for the recycling of today’s lithium-ion batteries. Today, the most common ones are a combination of mechanical and hydrometallurgical processes as well as a combination of dismantling and pyrometallurgical processing. Casing and connection materials of the battery pack and module are removed in advance or even after comminution in the processes presented and fed, in the normal case, to the conventional recycling methods for aluminum, iron, polymers, and others.
3.1. Mechanical–Hydrometallurgical Recycling Technology and Challenges
A common recycling route to be found combines mechanical processing with direct hydrometallurgical processing of the batteries. The mechanical processing can be fulfilled in a dry or a wet mode. Moreover, before mechanical treatment, the battery system has to be deactivated by complete discharge and usually short circuiting and dismantled down to at least the module level. However, it is also reported that the dismantling goes down to the electrode level in order to separate anode and cathode materials already before the mechanical treatment.
The economically and ecologically attractive mechanical processing of the battery cells is carried out down to the level of active and inactive materials, and it can be combined with an evaporation of electrolyte components [20,27,28,44]. Discharged battery cells and modules are comminuted under inert atmosphere in a shredder process or under water [45]. Then, the components are separated by classification and sieving processes. To achieve a high separation quality of the materials, several steps can be run consecutively with different process settings [20,28]. Furthermore, some alternative methods also include electrolyte recovery steps. The pre-dried shredded material is divided in the separation process into metallic components (casing material), current collector foils (Al and Cu), black mass (Li, Co, Ni, and Mn oxides, graphite, PVDF, carbon black, and impurities) and organic components (separator) [19,25]. However, today, the mechanical processes are not able to achieve practical recycling rates of more than 95% regarding cathode active materials and the necessary purities. Subsequently, the black mass is processed without any intermediate steps in the hydrometallurgy.
A particular challenge without pyrolysis or pyrometallurgical processes is the handling of the remaining fluorine components, which can be deviated on the routes including pyrolysis or pyrometallurgical processes due to high temperatures. Hydrometallurgy is a process at low temperatures in aqueous phases and can be performed in three major steps. Leaching is the first step and describes the dissolution of metals via the usage of acids or bases. Typically, at first, the black mass is dissolved in NaOH and afterwards leached using H2SO4, with initial impurities such as iron, aluminum, and copper precipitated by small amounts of NaOH and sieving [46,47]. The purification is the second step in the process chain where the metals are separated and purified via e.g., selective chemical reactions. The last step is the final recovery of the metals or the salts. This can be done by means of e.g., crystallization, ionic precipitation, electrolytic deposition, or further methods. For example, the individual metals can be further precipitated as sulfate salts by adding NaOH or other basic agents and increasing the pH. Selectively, this can be controlled by considering the different solubilities of the metal salts [46,47]. Hydrometallurgical process steps are capable of producing high product purities. However, plants for counter-flow are larger than those used in pyrometallurgy and require a larger financial investment volume for their construction [21,48,49]. In general, important challenges are to achieve the demanded high recovery rates and, at the same time, high material purities.
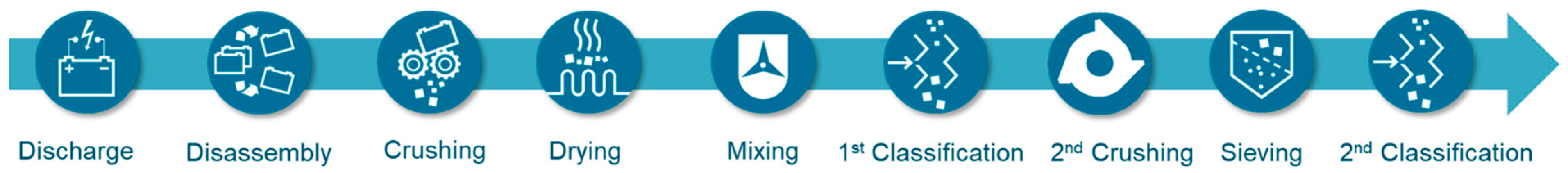
An example of the mechanical–hydrometallurgical process chain is the LithoRec process [19,20,28], which is commercialized in similar form by Duesenfeld and Redux (Figure 3). The developed process has a high potential to close the loop of the circular economy in the battery production as well to reduce the CO2 footprint in the battery production and utilization phase due to the high recovery rates of the recyclables and the low energy consumption. Maximizing the recycling rate was a core objective of the projects. Among other things, a process was developed in which, depending on the active material, 85 to potentially over 95% of the lithium can be recovered by mechanical (includes drying step) and hydrometallurgical means (leaching and subsequent precipitation of the lithium).

Figure 3.
Developed process chain of mechanical/thermal processing of the LithoRec process prior to hydrometallurgical steps [20,28].
Another example is the process used by Accurec GmbH® (Krefeld, Germany). Here, the batteries are discharged prior to mechanical processing and subjected to vacuum thermal pretreatment (pyrolysis) [44,50]. In this process step, volatile organic (electrolyte) as well as polymeric components (separator) and halogenic compounds can be pyrolyzed at 200–400 °C and low pressure. In the further course, the pyrolyzed mass is subjected to mechanical processing, pyrometallurgical processing, and subsequently hydrometallurgical processing to recover also the lithium. The developed process also has a noticeable potential to close the loop of the circular economy. In addition, the company Erlos GmbH represents another interesting approach in the recycling of lithium-ion batteries, in which the battery cells are separated by type (anode, separator, cathode, casing). Subsequently, in the example, the entire cathode coating is separated from the aluminum substrate foil. Then, the cathode coating is separated into its components—active material, binder, and carbon—by wet chemical processes (leaching), and the active material is reconditioned. This step allows the active materials on the cathode and also anode side to be reused after the treatment without having to perform a resynthesis based on the purified substances, i.e., metals. However, the electrolyte, binder, and conductive additives are lost for reuse. Nevertheless, the active material chemistry is retained and cannot be adapted to new requirements [47,51].
3.2. Pyrometallurgical Recycling Technology and Challenges
Another approach for the recycling of lithium ion batteries is the pyrometallurgical (mechanical–pyrometallurgical) way. In this process, the battery cells or modules can be placed in a direct pyrometallurgical step or they can be mechanically processed in a first step (analogous to the mechanical–hydrometallurgical), and the obtained valuable black mass is fed into the pyrometallurgy. Pyrometallurgical processing involves high-temperature processes such as melting and roasting to produce battery slag [52]. A pyrometallurgical recycling process of LIBs starts with an initial heating in the temperature range of 150–500 °C, during which electrolyte components and organic solvents are removed. Subsequently, a high-temperature process with temperatures up to 1450 °C using reducing agents (graphite, coke, NaHSO4, CaCl2, or NH4Cl) is carried out in the furnace to obtain battery alloy and slag (Li2O as well as Li2CO3) as products [17,53,54]. Due to the different meting points, the metals Ni, Co, and Cu can be individually recovered. However, lithium, manganese, and aluminum get into the slag or the kiln dust. The battery alloy produced in this way contains the valuable materials (such as Co, Cu, and Ni) and can then be processed hydrometallurgically. Lithium and other battery components can only be recovered at great expense or not at all by this process. The advantage of this process technology is the comparatively high robustness against changing feed material and a comparatively small plant size with given throughput. The disadvantage is the comparatively lower overall quantity of recyclable materials. However, the critical metals are recycled at a high recovery rate. The processes generate only intermediates that have to be purified by further steps to enable reuse (e.g., further processing by hydrometallurgical steps). In addition, they show low economic efficiency when low concentrations of recyclable materials (e.g., Co, Cu, and Ni) are present [24,52,55].
The process from Umicore Valéas™ (Bruxelles, Belgium) can be cited as an example of the pyrometallurgical processing route. As a great advantage in advance can be mentioned the great robustness of this way to various types of batteries [17]. Prior to thermal processing, the batteries are dismantled, whereby plastic and metal housing parts in particular are removed, and the cells are exposed [56]. Then, these are pyrolyzed in the shaft furnace at three different process temperatures (400–1450 °C). The alloy obtained contains valuable materials such as Cu, Co, and Ni. In turn, the slag contains Li. Both are subsequently hydrometallurgically processed in a leaching process to recover the valuable materials contained for reuse [17].
3.3. Potential Hazards of Lithium-Ion Batteries in Recycling Processes
Battery systems of electrically powered vehicles (e.g., EV, PHEV, HEV) contain chemically stored energy. The systems contain energy quantities of up to 20–100 kWh (TESLA Model S) and reach system voltages of 300–800 V [2,57]. The associated hazards are particularly relevant when the battery is handled separately from its application, such as in the dismantling and the recycling process [28]. In recycling, due to mechanical process steps in particular, this should be of major importance. Today’s and future battery generations combine built-in active materials with high energy densities and partly highly inflammable electrolytes. In normal use, particularly external factors such as short circuits (internal and external), high temperatures or mechanical deformation can trigger critical events and lead to the thermal runaway [58]. Thus, the hazards that can be caused by these faults from the battery in recycling can be divided into three main areas: (1) electrical, (2) thermal, and (3) chemical hazards. However, in the real case, they never occur alone but are usually a combination of hazards. Therefore, in general, a recycling process should be designed and engineered in such a way that the risks can be avoided as far as possible.
- Direct electrical shock is one of the main types of hazard when handling batteries. A direct electric shock can cause severe skin burns at the point of entry and exit, depending on the current, voltage and type of current (AC/DC). In addition, the paralysis of muscles and, in the worst case, electrolysis of the blood may occur.
- The thermal hazards of a battery cell are mainly due to the electrolyte components used. The main components of the current electrolytes are a mixture of organic solvents (e.g., ethyl carbonate, EC; ethyl methyl carbonate, EMC; and others) and a conducting salt (lithium hexaflourophosphate, LiPF6). The carbonates used are highly flammable hydrocarbons. The reaction of LiPF6 with water can result in the highly toxic and corrosive hydrogen fluoride (HF) [59,60]. Partially high evaporation rates of electrolyte components in moderate temperature ranges and partially closed process rooms can lead to explosive mixtures in combination with an oxygen-containing atmosphere [61]. In addition, if higher temperatures have occurred, the reaction products of reactions of the different components of a battery cell can also lead to fire and explosions in the processes (hydrogen, methane, carbon monoxide).
- Chemical hazards of battery cells are mainly determined by the ingredients but also by accessible reaction products in case of failure. The materials and products have irritating, human-toxic, carcinogenic, respiratory, environmentally harmful, and water-damaging effects. Particularly noteworthy in this case is the active material of the cathode. The cathode active materials consist mainly of lithium transition metal oxides such as NMC and NCA or also lithium metal phosphates, especially lithium iron phosphate (LFP). Especially the significant amounts of the heavy metals nickel and cobalt are both known to be carcinogenic and toxic for mammals. In addition, the small particle size (10–15 µm) of these can increase the exposure through the human respiratory system.
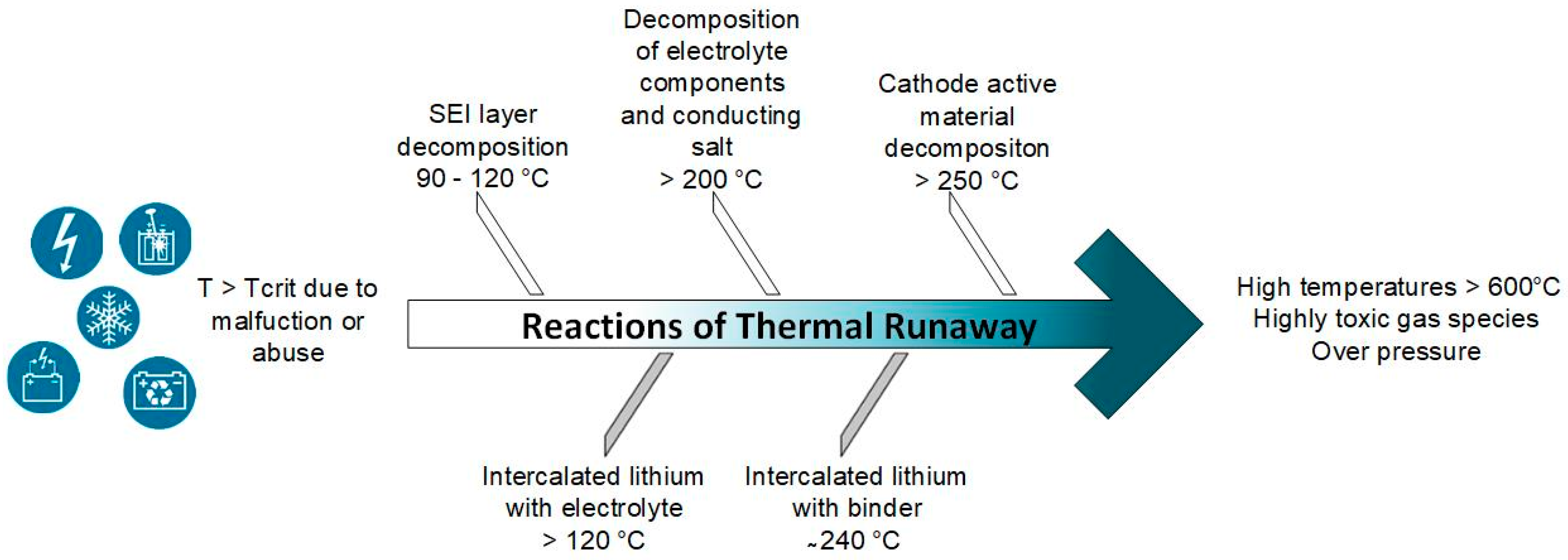
Uncontrolled temperature rises up to the so-called thermal runaway of the battery cells can be caused by external as well as internal short circuits if a critical temperature is exceeded, which can be generated by handling in recycling. These effects can be triggered during the process by mechanical penetration of foreign bodies, internal cell defects, or external arrester/electrode contacts [62,63,64,65]. In addition, overloading or high ambient temperatures can cause the thermal runaway to occur. This type of hazard mainly occurs during the storage and discharge of spent batteries and not during the recycling process. The thermal runaway involves the reaction of cathode, anode, and electrolyte (Figure 4). In the first step, the process runaway begins with the decomposition of the solid electrolyte interface (SEI) on the anode. At a critical temperature of about 90–120 °C in a battery, the chemical decomposition processes start. Under an exothermic reaction process, the formed SEI is decomposed and leads to different gaseous reaction products (e.g., carbon dioxide, ethane, ethane) [66]. The energy released from the first exothermic reactions leads to further heating of the battery cell and can dissolve the subsequent chain reaction processes. Then, the intercalated lithium begins to react with the electrolyte.
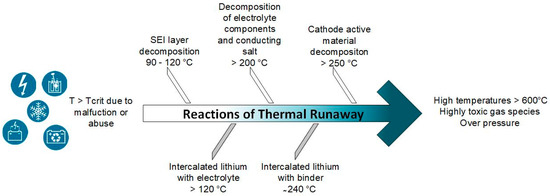
Figure 4.
Schematic sequence of thermal runaway between different battery cell components after a critical cell temperature has been exceeded.
Electrolyte decomposition starts at approximately 200 °C with the formation of CO2, hydrogen fluoride, ethene, and other hydrocarbons containing fluoride [67,68]. The exothermic reactions of the embedded lithium with the binder and the decomposition of the cathode active material start at 240 °C and 250 °C, respectively [69]. In actual recycling processes, the steps that still involve electrolytes are to be regarded as particularly critical. To reduce hazards related to the electrolyte, it is advisable to remove the electrolyte as early as possible in the recycling process chain and, in an ideal case, to purify and return it back into the material cycle. However, how valuable a repeated use of the electrolyte is regarding costs and environmental protection is an open question. If a primarily mechanical process strategy is used, comminution can be carried out under an inert gas atmosphere (e.g., nitrogen), followed by evaporation of the electrolyte at moderate temperature increases and/or at negative pressures [20,28] or even by further extraction methods [70,71]. Then, the evaporated electrolyte components can be condensed out as in the Lithorec process. In the case of upstream pyrolysis, the electrolyte can already be removed in advance at temperatures of approximately 200–400 °C in specialized ovens (Accurec GmbH®). The comminution takes place downstream. The pyrometallurgical process route can handle the electrolyte removal even more easily. Due to the high temperatures during the processing of the battery materials (up to 1450 °C) and the addition of reducing agents, the electrolyte can be safely removed from the cell materials [17]. Moreover, at temperatures above about 650 °C also, the binder PVDF can be decomposed and removed under the challenge of handling hydrofluorocarbons. In summary, an improperly handled battery cell represents some potential hazards. However, with appropriate and orderly process control, these risks can be reduced to a minimum, and safe process control should be ensured.
4. Circular Economy in the Context of Battery Production
Establishing closed material cycles for batteries on the basis of scalable production and recycling technologies is a central component for CO2-reduced or CO2-neutral battery cell production and thus for electromobility, the provision of energy in household and handicraft appliances, as well as for the stationary storage of renewable energies [7,72,73]. As a matter of fact, closed material cycles in batteries are the only way to convert carbon-based energy sources into sustainably generated electrical energy in ecological, economic, and social contexts. Consequently, a circular economy for traction batteries, i.e., lithium-ion battery systems is demanded by the European Union as written in the Battery Directive 2006/66/EC and [13] as well as the Circular Economy Initiative Deutschland (CEID) of the National Academy of Science and Engineering in Germany [14]. In addition, many research studies are being done on this topic while highlighting the challenges that still exist and need to be overcome [17,21,52,72]. Such closed circles improve the sustainability of lithium-ion batteries; they especially decrease the carbon footprint, decrease the material costs, and ensure secondary material resources for Europe [73,74]. However, in general, when closing material cycles, it should be noted that the energy used is in proportion to the products. With regard to recycling, mechanical treatments are energy-wise recommended, as they are less energy-intensive than metallurgical processes. Examples of specific consumption parameters of energy are electrical energy and wastewater. Pyrometallurgical processing requires 4.68 MJ of electrical energy per kg of battery; hydrometallurgical processing requires 0.125 MJ of electrical energy per kg of battery [75,76]. In addition, approximately 3.76 L of wastewater are produced per kg of battery during hydrometallurgy. However, as described above, a combined process strategy is necessary for good product properties and qualities [29].
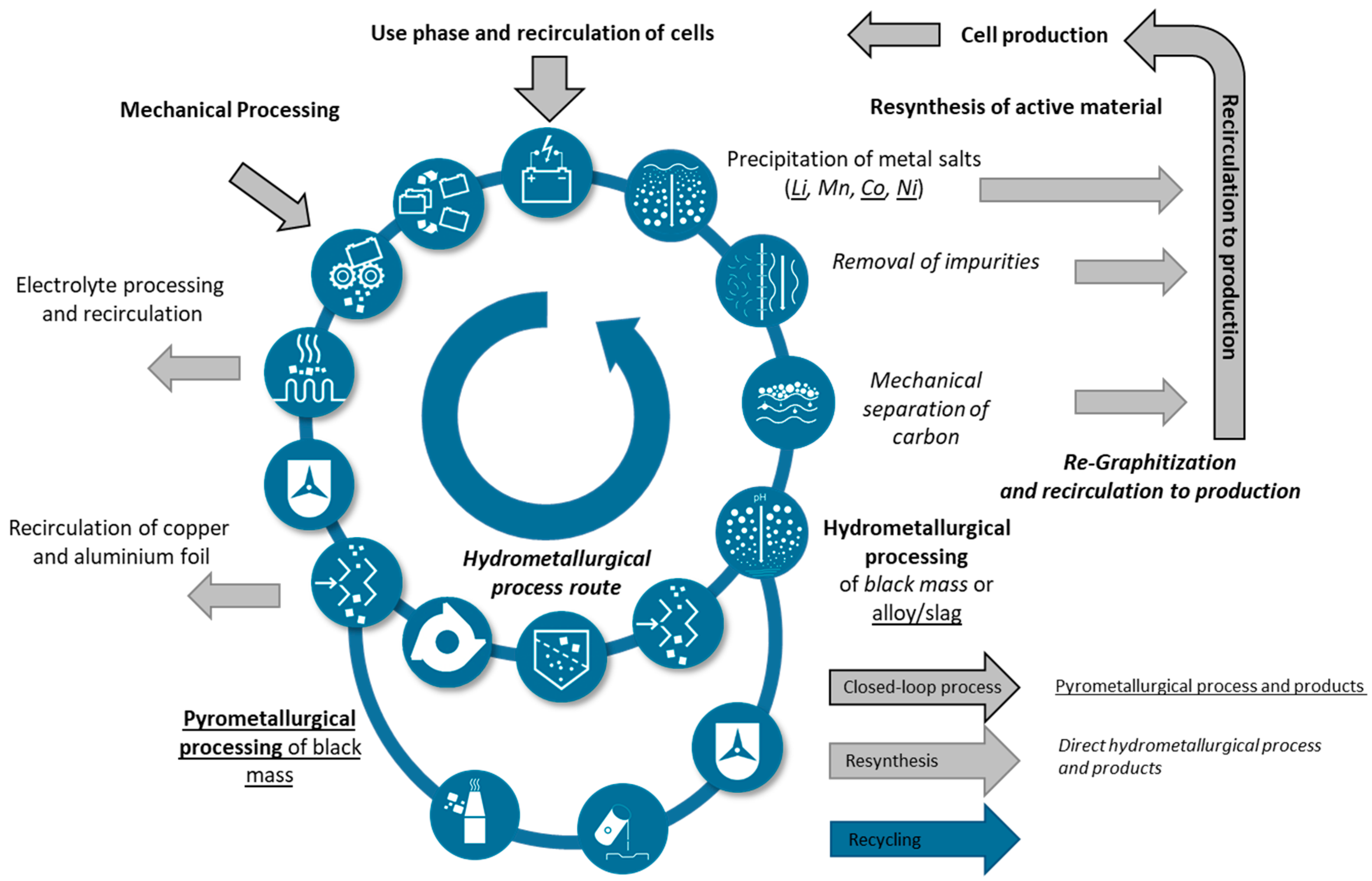
A possible implementation of such a closed-loop production is shown in Figure 5. After the utilization phase, batteries are mechanically disassembled, whereby the safety of the processes is an important aspect of development. Depending on the level of detail of the mechanical process chain, components such as copper and aluminum foil can already be separated. Pyro- or hydrometallurgical processes must be applied for further purification at the latest after the active materials, the so-called black mass, have been exposed. Depending on the process, graphite and binder can also be separated. Finally, metal salts are to be precipitated and re-synthesized to produce new active materials [48,52]. Alternatively, the anode and cathode active materials can be reconditioned by means of purification processes, lithium enhancement, and functionalization [47,51,77,78,79]. Direct reconditioning has the advantage of being a fast and less energy-consuming process, but it cannot directly adapt the cathode chemistry to new developments. For short life cycle phases or rejects from production (e.g., losses of battery cells during formation), direct reconditioning can be very attractive [80].
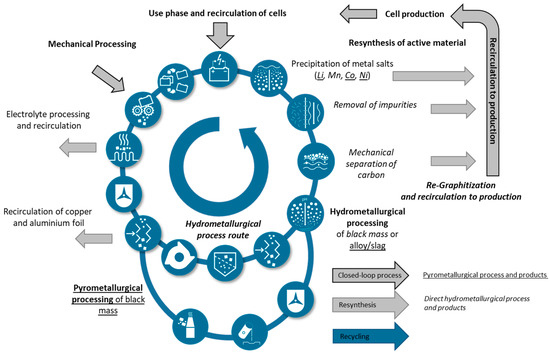
Figure 5.
Exemplary approach for the cycle of circular battery production with the presentation of both established processing routes, direct hydrometallurgical and upstream pyrometallurgical processing after previous mechanical processing.
In order to realize a circular battery cell production, a number of technological and organizational prerequisites must be created:
- Almost 100% of end-of-life batteries must be collected and recycled at the latest after any second-life application [73]. The expected lifetime of batteries in the automotive sector is at least 8 years, and, thus, in the mean, it tends to be more than 10 years. In the future, the lifetime will probably increase further. However, this value is highly dependent on the loads (fast charging, temperatures, etc.) [81]. Before recycling, it is important to check whether a second-life application can be reasonable.
- The condition of the batteries, especially the material composition, must be documented for the subsequent recycling process [82]. Alternatively, a uniform interface for reading out specific battery data could be implemented in the systems.
- With regard to the material composition of batteries, robust recycling processes must be developed and industrially implemented, especially with regard to future battery generations.
- The re-synthesis and eventual reconditioning of the active materials, such as Si-containing anode materials and cathode materials from lithium mixed oxides, has to function on a large scale without any loss of performance of the later battery. The synthesis processes should be as robust as possible against material contamination [83,84].
- The design of the battery cells should not only be based on requirements such as performance, cost, and safety, but also on sustainability and thus recyclability [85].
- The production of the battery cells themselves must be ecologically and economically sustainable [2].
- For objective evaluation of the individual technologies, new software tools should be developed for an “as objective as possible” cost and environmental life cycle assessment of different battery cells and process technologies.
In order to get an overview of the technological difficulties, an example is given below to obtain a recycling rate of 95% from a recyclable material in five process steps. If each of these process steps has a yield of 99%, a total yield of 95.09% can just be obtained with all five steps. Thus, it is very important to use few very good process steps in the reprocessing to generate the highest possible yield.
5. Perspective on Recycling and Circular Economy of Future Battery Generations
The focus in future developments is a highly energy-efficient multi-material recovery with recovery rates of at least more than 90%, most probably more than 95%, which should be adaptable to variations of respective input streams and current output demand. This results in the requirements for future recycling processes, which must either have a high degree of flexibility or which are focused on certain battery types, which in this case have to be sorted efficiently. In addition, the process routes to be developed must be specialized with regard to the individual components to be purified, e.g., electrolyte, electrode components, and active materials. To achieve this, combinations of mechanical, thermal, and chemical process steps have to be used and interconnected. These processes have to be designed also with regard to novel battery materials and chemistry (e.g., solid-state batteries). They should over a long term replace the current energy-intensive processes for classical LIB. This will render future recycling processes significantly more environmentally friendly and less energy-intensive.
Various challenges and requirements for recycling processes, closed material cycles (circular economy), and safety aspects arise from the large-scale use of the four battery types expected to dominate the future as mentioned in Section 1.
Battery type I (Si-containing anodes, Ni-rich cathodes, liquid electrolyte): Closed-loop production is economically attractive because of the high nickel content, and regeneration of the anode materials should be very feasible both ecologically and economically. The recycling processes known today, using hydrometallurgy alone or a combination of pyro- and hydrometallurgy, should be very well able to process these battery cells, which will be available on the market in the near future. However, the recycling process chains must ensure high recovery rates of 95% and higher for critical metals and most possible lithium. For active materials with high nickel and low cobalt content and already high specific capacities such as NMC 811, also a reconditioning of the active material can be attractive because a further increase in specific capacity due to new material developments is probably relatively small. These reconditioned active materials could also be used for the production of battery type II in the near future, especially if the performance is slightly reduced due to the multiple usage. Regarding safety, battery type I cells will be more sensitive to mechanical damage and external short circuits due to the high nickel content and the presence of nanoparticulate silicon. This increases the risk of thermal runaway and resulting fires.
Battery type II (Graphite anodes, Mn- and Fe-rich cathodes, liquid electrolyte): This type of battery, which is mainly used in stationary applications and low-cost mobility concepts, can be well recycled with the existing processes such as the previous one. The preferential use of Mn and Fe leads to a low-cost batteries and thus, economically less attractive recycling processes. Therefore, regulations have to be set up to ensure that these batteries are recycled at the EoL. Due to the high risk of contamination, cells with active materials containing iron must most probably be separated from batteries of other types even before recycling. Otherwise, complex purification processes must be used to separate the transition metals (Ni, Co) from the iron especially in case of hydrometallurgical process routes. For such batteries, the reconditioning of the active materials is probably also a cost-efficient and sustainable option for material recovery.
Battery type III (Lithium or lithium-free anodes, Ni-rich cathodes, solid electrolyte): The solid-state electrolytes used in this type of battery will lead to obstacles in establishing a closed-loop economy [35]. It makes it difficult to recover the individual materials used in the battery in high degrees of purity. On the anode’s side, thin lithium foils or, in the future, metallic lithium deposited on 3D structures (depending on the transfer of current research results to industry) or even lithium-free 3D-structures are used. These anodes require that the mechanical treatment of the battery cells be performed in an inert gas atmosphere. Furthermore, this results in additional equipment and work safety requirements during processing. This is also due to additionally required hydrometallurgical steps of solving and recovering lithium in the form of salt (LiOH), which leads to the formation of gaseous H2. When considering the possible recycling processes for cathodes and separators, a distinction must be made between the solid-state electrolytes used:
- The use of polymer electrolytes both in the cathode and in the separator results in a complex task of separation of the individual materials used. As it stands now, there are two options: On the one hand, the polymer electrolyte can be burned using thermal processes, and the materials exposed can be further processed in a similar way to classical LIB. However, the polymer-type solid electrolyte is lost. On the other hand, complex wet chemical processes can be chosen. Here, the electrolyte is dissolved in a suitable solvent, and the polymer can be recovered in the process, but it is not known today if this can be fulfilled with a sufficient quality or purity, respectively. However, the wet chemical route is not expected to be economically or environmentally viable, despite an associated increase in recycling yield.
- When using sulfidic solid electrolytes, the formation of toxic hydrogen sulfide compounds must be avoided during recycling. A mechanical separation of the solid sulfidic electrolyte from the active material is very difficult and probably not possible with the required purity or separation efficiency, respectively. Therefore, a reconditioning of the solid electrolyte and the active materials seems to be not possible from the today´s experience. In addition, the frequent use of other elements, such as germanium, makes it more difficult to recycle these substances in a pure form by hydrometallurgical processes. Therefore, complex hydrometallurgical processes are probably required to recover the different materials.
- If oxidic solid electrolytes are used in the separator and/or cathode, the electrolyte particles will be firmly sintered together. Thus, mechanical separation is associated with significantly higher costs, so that pyrometallurgical treatment of entire cells or at least larger cell fragments probably becomes more attractive compared to a mechanical/hydrometallurgical process.
Battery type IV (Lithium anodes, S/C-containing cathodes, liquid or solid electrolyte): Apart from lithium, copper, and aluminum, no further valuable materials are used in lithium–sulfur batteries. This results in the task of efficiently separating these three materials from the mechanically treated battery mixture. Presumably, the recovery of sulfur and carbon for direct use in a battery is not practical, since there are other inexpensive and reliable sources of sulfur and carbon that promise less effort and the needed higher purity. Accordingly, a closed-loop production of this cell type does not necessarily make sense economically and probably also ecologically according to the current state of knowledge. If solid instead of liquid electrolytes are used, the challenges described for battery type III apply additionally.
In conclusion, with the exception of battery type II, all other battery types that potentially lead to a higher energy density in Wh/L (battery types I and III) or an increased specific energy in Wh/kg (Li-sulfur battery) cause additional challenges with regard to circular economy and closed material cycles, recycling processes as well as safety in the handling and recycling of batteries. From the current state of research and industrial implementation, already established or partially established processes can be adapted and expanded to meet the challenges of the coming battery generations. Nevertheless, this can be ambitious for some battery types (i.e., battery type III/IV) with regard to the required high recycling rates of the total battery of 70% and higher or 95% of individual metals and make the development of new process steps necessary. Today’s established recycling processes allow a recycling rate for individual materials of up to more than 90% in some cases, depending on the combination of the processes. However, if for example the recovery of the transition metals such as nickel and cobalt is maximized by a mainly pyrometallurgical process, a recovery of graphite as an anode material is not practical; i.e., the graphite serves as an energy source for the heating. Therefore, depending on the regulations, a certain process route can be worthwhile or not useful at all. Processes routes based on only one process type (e.g., only mechanical) enable at least lower overall recovery values. Thus, with the position today, it can be assumed that almost all battery materials can be recycled and reused within new batteries. However, an open question is how far the substances have to be purified so that there is no effect on the electrochemical performance, and if also a reconditioning of the active materials, especially also the cathode materials, is possible on large scale, as it has been shown on a lab scale. This question is the subject of actual research and can hopefully be quantitatively answered in the near future. However, it is expected that the original performance will be achieved in any case when the current active material is reprocessed to the original material purity via complex processes similar to the ones applied for primary materials [65,66,68]. For types I and III, this is also expected for the cathode materials, as the reprocessing processes will be very similar. For type II, a good performance retention result can also be expected for the cathode, since these battery chemistries and materials are already well researched. For type IV, reuse cannot be reasonably implemented from today’s economic perspectives. In addition, direct reconditioning of the cathode material can be used to recycle type II and, in some cases, type I materials. These materials are subject to a longer period of use and application than new and even higher-energy materials in example for type III. With regard to the graphite-containing anode in particular (types I and III), recycling is not yet an option from an economic point of view. However, technically, there are approaches that show a reuse with the same performance [86]. Since types II and IV contain a lithium anode, recycling is an economical option for reuse here.
It becomes clear that the diversification and new developments demand a proper separation of battery types before recycling, for example by a transnational network of dismantling and sorting locations, and flexible and high sophisticated recycling processes with case-wise higher safety standards than today. Moreover, for the low-cost batteries, recycling of the batteries becomes economically unattractive, so that legal stipulations become important. However, in general, it must be still secured that closing the material cycle for all battery types with suitable processes is achieved to secure the supply of raw materials and also to further advance new developments. Last but not least, it can be stated that in absolute percentage values, a relatively small step has to be realized until we meet the targets, but it will be enormously demanding to achieve the last percentage points in the quotas especially if more than only the transition metals shall be recovered.
Author Contributions
Writing, S.D., J.K.M. and P.M., with support from A.K.; project supervision, A.K. with support from P.M. All authors have read and agreed to the published version of the manuscript.
Funding
We have received financial support for the preparation of this manuscript from the German Research Foundation and the Open Access Publication Funds of Technische Universität Braunschweig.
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge support by the German Research Foundation and the Open Access Publication Funds of Technische Universität Braunschweig.
Conflicts of Interest
The authors declare no conflict of interest.
References
- The European Dimension of Germany’s Energy Transition. Opportunities and Conflicts; Gawel, E., Strunz, S., Lehmann, P., Purkus, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-03374-3. [Google Scholar]
- Kwade, A.; Haselrieder, W.; Leithoff, R.; Modlinger, A.; Dietrich, F.; Droeder, K. Current status and challenges for automotive battery production technologies. Nat. Energy 2018, 3, 290–300. [Google Scholar] [CrossRef]
- Peters, J.F.; Baumann, M.; Zimmermann, B.; Braun, J.; Weil, M. The environmental impact of Li-Ion batteries and the role of key parameters—A review. Renew. Sustain. Energy Rev. 2017, 67, 491–506. [Google Scholar] [CrossRef]
- Habib, K.; Hansdóttir, S.T.; Habib, H. Critical metals for electromobility: Global demand scenarios for passenger vehicles, 2015–2050. Resour. Conserv. Recycl. 2020, 154, 104603. [Google Scholar] [CrossRef]
- Behaviour of Lithium-Ion Batteries in Electric Vehicles. Battery Health, Performance, Safety, and Cost; Pistoia, G., Liaw, B., Eds.; Springer: Cham, Switzerland, 2018; ISBN 978-3-319-69950-9. [Google Scholar]
- Mohr, M.; Peters, J.F.; Baumann, M.; Weil, M. Toward a cell-chemistry specific life cycle assessment of lithium-ion battery recycling processes. J. Ind. Ecol. 2020, 24, 1310–1322. [Google Scholar] [CrossRef]
- Velázquez-Martínez, O.; Valio, J.; Santasalo-Aarnio, A.; Reuter, M.; Serna-Guerrero, R. A Critical Review of Lithium-Ion Battery Recycling Processes from a Circular Economy Perspective. Batteries 2019, 5, 68. [Google Scholar] [CrossRef]
- Dai, Q.; Kelly, J.C.; Gaines, L.; Wang, M. Life Cycle Analysis of Lithium-Ion Batteries for Automotive Applications. Batteries 2019, 5, 48. [Google Scholar] [CrossRef]
- Chen, M.; Zheng, Z.; Wang, Q.; Zhang, Y.; Ma, X.; Shen, C.; Xu, D.; Liu, J.; Liu, Y.; Gionet, P.; et al. Closed Loop Recycling of Electric Vehicle Batteries to Enable Ultra-high Quality Cathode Powder. Sci. Rep. 2019, 9, 173. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.; Li, L.; Wang, Z.; Lin, J.; Huang, Y.; Yao, Y.; Chen, R.; Wu, F. Sustainable Recycling Technology for Li-Ion Batteries and Beyond: Challenges and Future Prospects. Chem. Rev. 2020, 120, 7020–7063. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.; Singh, N. Recycling of Spent Lithium-Ion Battery: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1129–1165. [Google Scholar] [CrossRef]
- Xu, C.; Dai, Q.; Gaines, L.; Hu, M.; Tukker, A.; Steubing, B. Future material demand for automotive lithium-based batteries. Commun. Mater. 2020, 1, 437. [Google Scholar] [CrossRef]
- Proposal for a Regulation of the European Parliament and of the Council Concerning Batteries and Waste Batteries, Repealing Directive 2006/66/EC and Amending Regulation (EU) No 2019/1020. 2020. Available online: https://ec.europa.eu/environment/waste/batteries/pdf/Proposal_for_a_Regulation_on_batteries_and_waste_batteries.pdf (accessed on 22 December 2020).
- Kwade, A.; Hagelüken, C.; Kohl, H.; Buchert, M.; Herrmann, C.; Vahle, T.; von Wittken, R.; Carrara, M.; Daelemans, S.; Ehrenberg, H.; et al. Ressourcenschonende Batteriekreisläufe—mit Circular Economy die Elektromobilität Antreiben; Acatech: München, Germany; London, UK, 2020; Available online: https://www.acatech.de/publikation/ressourcenschonende-batteriekreislaeufe/ (accessed on 22 December 2020).
- Siqi, Z.; Guangming, L.; Wenzhi, H.; Juwen, H.; Haochen, Z. Recovery methods and regulation status of waste lithium-ion batteries in China: A mini review. Waste Manag. Res. 2019, 37, 1142–1152. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, Y. An overview of recycling and treatment of spent LiFePO 4 batteries in China. Resour. Conserv. Recycl. 2017, 127, 233–243. [Google Scholar] [CrossRef]
- Georgi-Maschler, T.; Friedrich, B.; Weyhe, R.; Heegn, H.; Rutz, M. Development of a recycling process for Li-ion batteries. J. Power Sources 2012, 207, 173–182. [Google Scholar] [CrossRef]
- Sommerfeld, M.; Vonderstein, C.; Dertmann, C.; Klimko, J.; Oráč, D.; Miškufová, A.; Havlík, T.; Friedrich, B. A Combined Pyro- and Hydrometallurgical Approach to Recycle Pyrolyzed Lithium-Ion Battery Black Mass Part 1: Production of Lithium Concentrates in an Electric Arc Furnace. Metals 2020, 10, 1069. [Google Scholar] [CrossRef]
- Hanisch, C.; Loellhoeffel, T.; Diekmann, J.; Markley, K.J.; Haselrieder, W.; Kwade, A. Recycling of lithium-ion batteries: A novel method to separate coating and foil of electrodes. J. Clean. Prod. 2015, 108, 301–311. [Google Scholar] [CrossRef]
- Diekmann, J.; Hanisch, C.; Froböse, L.; Schälicke, G.; Loellhoeffel, T.; Fölster, A.-S.; Kwade, A. Ecological Recycling of Lithium-Ion Batteries from Electric Vehicles with Focus on Mechanical Processes. J. Electrochem. Soc. 2017, 164, A6184–A6191. [Google Scholar] [CrossRef]
- Chagnes, A.; Pospiech, B. A brief review on hydrometallurgical technologies for recycling spent lithium-ion batteries. J. Chem. Technol. Biotechnol. 2013, 88, 1191–1199. [Google Scholar] [CrossRef]
- Sommerville, R.; Shaw-Stewart, J.; Goodship, V.; Rowson, N.; Kendrick, E. A review of physical processes used in the safe recycling of lithium ion batteries. Sustain. Mater. Technol. 2020, 25, e00197. [Google Scholar] [CrossRef]
- Sojka, R.T. Safe Treatment of Lithium-based Batteries through Thermal Conditioning. In Recycling und Sekundärrohstoffe, Band 13; Thomé-Kozmiensky, E., Holm, O., Friedrich, B., Goldmann, D., Eds.; Thomé-Kozmiensky Verlag GmbH: Nietwerder, Germany, 2020; pp. 506–523. ISBN 3944310519. [Google Scholar]
- Hiskey, B. Metallurgy, Survey. In Encyclopedia of Chemical Technology; Kirk, R.E., Othmer, D.F., Eds.; Wiley: New York, NY, USA, 2003; ISBN 0471238961. [Google Scholar]
- Zhang, G.; Yuan, X.; He, Y.; Wang, H.; Zhang, T.; Xie, W. Recent advances in pretreating technology for recycling valuable metals from spent lithium-ion batteries. J. Hazard. Mater. 2020, 124332. [Google Scholar] [CrossRef]
- Träger, T.; Friedrich, B.; Weyhe, R. Recovery Concept of Value Metals from Automotive Lithium-Ion Batteries. Chem. Ing. Tech. 2015, 87, 1550–1557. [Google Scholar] [CrossRef]
- Diekmann, J.; Hanisch, C.; Loellhoeffel, T.; Schalicke, G.; Kwade, A. (Invited) Ecologically Friendly Recycling of Lithium-Ion Batteries—The LithoRec Process. ECS Trans. 2016, 73, 1–9. [Google Scholar] [CrossRef]
- Kwade, A.; Diekmann, J. Recycling of Lithium-Ion Batteries; Springer International Publishing: Cham, Germany, 2018; ISBN 978-3-319-70571-2. [Google Scholar]
- Dai, Q.; Spangenberger, J.; Ahmed, S.; Gaines, L.; Kelly, J.C.; Wang, M. EverBatt. A Closed-loop Battery Recycling Cost and Environmental Impacts Model; Argonne National Laboratory: Lemont, IL, USA, 2019. Available online: https://publications.anl.gov/anlpubs/2019/0-7/153050.pdf (accessed on 3 February 2021).
- Ciez, R.E.; Whitacre, J.F. Examining different recycling processes for lithium-ion batteries. Nat. Sustain. 2019, 2, 148–156. [Google Scholar] [CrossRef]
- Zhao, X.; Lehto, V.-P. Challenges and prospects of nanosized silicon anodes in lithium-ion batteries. Nanotechnology 2021, 32, 42002. [Google Scholar] [CrossRef]
- Heck, C.A.; Horstig, M.-W.; von Huttner, F.; Mayer, J.K.; Haselrieder, W.; Kwade, A. Review—Knowledge-Based Process Design for High Quality Production of NCM811 Cathodes. J. Electrochem. Soc. 2020, 167, 160521. [Google Scholar] [CrossRef]
- Cheruvally, G. Lithium Iron Phosphate. A Promising Cathode-Active Material for Lithium Secondary Batteries; Trans Tech Publishers: Zurich, Switzerland, 2008; ISBN 978-0-87849-477-4. [Google Scholar]
- Omar, N.; Monem, M.A.; Firouz, Y.; Salminen, J.; Smekens, J.; Hegazy, O.; Gaulous, H.; Mulder, G.; van den Bossche, P.; Coosemans, T.; et al. Lithium iron phosphate based battery—Assessment of the aging parameters and development of cycle life model. Appl. Energy 2014, 113, 1575–1585. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 294. [Google Scholar] [CrossRef]
- Wild, M.; O’Neill, L.; Zhang, T.; Purkayastha, R.; Minton, G.; Marinescu, M.; Offer, G.J. Lithium sulfur batteries, a mechanistic review. Energy Environ. Sci. 2015, 8, 3477–3494. [Google Scholar] [CrossRef]
- Golubkov, A.W.; Fuchs, D.; Wagner, J.; Wiltsche, H.; Stangl, C.; Fauler, G.; Voitic, G.; Thaler, A.; Hacker, V. Thermal-runaway experiments on consumer Li-ion batteries with metal-oxide and olivin-type cathodes. RSC Adv. 2014, 4, 3633–3642. [Google Scholar] [CrossRef]
- Olivetti, E.A.; Ceder, G.; Gaustad, G.G.; Fu, X. Lithium-Ion Battery Supply Chain Considerations: Analysis of Potential Bottlenecks in Critical Metals. Joule 2017, 1, 229–243. [Google Scholar] [CrossRef]
- Lee, Y.-G.; Fujiki, S.; Jung, C.; Suzuki, N.; Yashiro, N.; Omoda, R.; Ko, D.-S.; Shiratsuchi, T.; Sugimoto, T.; Ryu, S.; et al. High-energy long-cycling all-solid-state lithium metal batteries enabled by silver–carbon composite anodes. Nat. Energy 2020, 5, 299–308. [Google Scholar] [CrossRef]
- Kato, Y.; Hori, S.; Saito, T.; Suzuki, K.; Hirayama, M.; Mitsui, A.; Yonemura, M.; Iba, H.; Kanno, R. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 2016, 1, 652. [Google Scholar] [CrossRef]
- Wang, H.; Sheng, L.; Yasin, G.; Wang, L.; Xu, H.; He, X. Reviewing the current status and development of polymer electrolytes for solid-state lithium batteries. Energy Storage Mater. 2020, 33, 188–215. [Google Scholar] [CrossRef]
- Son, I.H.; Hwan Park, J.; Kwon, S.; Park, S.; Rümmeli, M.H.; Bachmatiuk, A.; Song, H.J.; Ku, J.; Choi, J.W.; Choi, J.-M.; et al. Silicon carbide-free graphene growth on silicon for lithium-ion battery with high volumetric energy density. Nat. Commun. 2015, 6, 7393. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Cheng, H.; Choi, H.; Lee, J.-H.; Han, H.; Lee, D.H.; Yoo, D.S.; Kwon, M.-S.; Choi, J.-M.; Doo, S.G.; et al. Si/Ge double-layered nanotube array as a lithium ion battery anode. ACS Nano 2012, 6, 303–309. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Li, M.; Chen, R.; Wu, F.; Amine, K.; Lu, J. The Recycling of Spent Lithium-Ion Batteries: A Review of Current Processes and Technologies. Electrochem. Energ. Rev. 2018, 1, 461–482. [Google Scholar] [CrossRef]
- Zhang, T.; He, Y.; Ge, L.; Fu, R.; Zhang, X.; Huang, Y. Characteristics of wet and dry crushing methods in the recycling process of spent lithium-ion batteries. J. Power Sources 2013, 240, 766–771. [Google Scholar] [CrossRef]
- Zou, H.; Gratz, E.; Apelian, D.; Wang, Y. A novel method to recycle mixed cathode materials for lithium ion batteries. Green Chem. 2013, 15, 1183. [Google Scholar] [CrossRef]
- Larouche, F.; Tedjar, F.; Amouzegar, K.; Houlachi, G.; Bouchard, P.; Demopoulos, G.P.; Zaghib, K. Progress and Status of Hydrometallurgical and Direct Recycling of Li-Ion Batteries and Beyond. Materials 2020, 13, 801. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, M.; Zhao, Z.; Tong, B.; Fan, Y.; Hua, Z. Hydrometallurgical Processes for Recycling Spent Lithium-Ion Batteries: A Critical Review. ACS Sustain. Chem. Eng. 2018, 6, 13611–13627. [Google Scholar] [CrossRef]
- Zheng, Z.; Chen, M.; Wang, Q.; Zhang, Y.; Ma, X.; Shen, C.; Xu, D.; Liu, J.; Liu, Y.; Gionet, P.; et al. High Performance Cathode Recovery from Different Electric Vehicle Recycling Streams. ACS Sustain. Chem. Eng. 2018, 6, 13977–13982. [Google Scholar] [CrossRef]
- Pinegar, H.; Smith, Y.R. Recycling of End-of-Life Lithium Ion Batteries, Part I: Commercial Processes. J. Sustain. Metall. 2019, 5, 402–416. [Google Scholar] [CrossRef]
- Sloop, S.; Crandon, L.; Allen, M.; Koetje, K.; Reed, L.; Gaines, L.; Sirisaksoontorn, W.; Lerner, M. A direct recycling case study from a lithium-ion battery recall. Sustain. Mater. Technol. 2020, 25, e00152. [Google Scholar] [CrossRef]
- Brückner, L.; Frank, J.; Elwert, T. Industrial Recycling of Lithium-Ion Batteries—A Critical Review of Metallurgical Process Routes. Metals 2020, 10, 1107. [Google Scholar] [CrossRef]
- Xiao, S.; Ren, G.; Xie, M.; Pan, B.; Fan, Y.; Wang, F.; Xia, X. Recovery of Valuable Metals from Spent Lithium-Ion Batteries by Smelting Reduction Process Based on MnO–SiO2–Al2O3 Slag System. J. Sustain. Metall. 2017, 3, 703–710. [Google Scholar] [CrossRef]
- Assefi, M.; Maroufi, S.; Yamauchi, Y.; Sahajwalla, V. Pyrometallurgical recycling of Li-ion, Ni–Cd and Ni–MH batteries: A minireview. Curr. Opin. Green Sustain. Chem. 2020, 24, 26–31. [Google Scholar] [CrossRef]
- Encyclopedia of Chemical Technology; Kirk, R.E., Othmer, D.F., Eds.; Wiley: New York, NY, USA, 2003; ISBN 0471238961. [Google Scholar]
- Werner, D.; Peuker, U.A.; Mütze, T. Recycling Chain for Spent Lithium-Ion Batteries. Metals 2020, 10, 316. [Google Scholar] [CrossRef]
- Iclodean, C.; Varga, B.; Burnete, N.; Cimerdean, D.; Jurchiş, B. Comparison of Different Battery Types for Electric Vehicles. Iop Conf. Ser. Mater. Sci. Eng. 2017, 252, 12058. [Google Scholar] [CrossRef]
- Ruiz, V.; Pfrang, A.; Kriston, A.; Omar, N.; van den Bossche, P.; Boon-Brett, L. A review of international abuse testing standards and regulations for lithium ion batteries in electric and hybrid electric vehicles. Renew. Sustain. Energy Rev. 2018, 81, 1427–1452. [Google Scholar] [CrossRef]
- Tebbe, J.L.; Fuerst, T.F.; Musgrave, C.B. Mechanism of hydrofluoric acid formation in ethylene carbonate electrolytes with fluorine salt additives. J. Power Sources 2015, 297, 427–435. [Google Scholar] [CrossRef]
- Yang, H.; Zhuang, G.V.; Ross, P.N. Thermal stability of LiPF6 salt and Li-ion battery electrolytes containing LiPF6. J. Power Sources 2006, 161, 573–579. [Google Scholar] [CrossRef]
- Nedjalkov, A.; Meyer, J.; Köhring, M.; Doering, A.; Angelmahr, M.; Dahle, S.; Sander, A.; Fischer, A.; Schade, W. Toxic Gas Emissions from Damaged Lithium Ion Batteries—Analysis and Safety Enhancement Solution. Batteries 2016, 2, 5. [Google Scholar] [CrossRef]
- Lamb, J.; Orendorff, C.J. Evaluation of mechanical abuse techniques in lithium ion batteries. J. Power Sources 2014, 247, 189–196. [Google Scholar] [CrossRef]
- Feng, X.; Sun, J.; Ouyang, M.; Wang, F.; He, X.; Lu, L.; Peng, H. Characterization of penetration induced thermal runaway propagation process within a large format lithium ion battery module. J. Power Sources 2015, 275, 261–273. [Google Scholar] [CrossRef]
- Diekmann, J.; Doose, S.; Weber, S.; Münch, S.; Haselrieder, W.; Kwade, A. Development of a New Procedure for Nail Penetration of Lithium-Ion Cells to Obtain Meaningful and Reproducible Results. J. Electrochem. Soc. 2020, 167, 90504. [Google Scholar] [CrossRef]
- Doose, S.; Haselrieder, W.; Kwade, A. Effects of the Nail Geometry and Humidity on the Nail Penetration of High-Energy Density Lithium Ion Batteries. Batteries 2021, 7, 6. [Google Scholar] [CrossRef]
- Yoon, T.; Milien, M.S.; Parimalam, B.S.; Lucht, B.L. Thermal Decomposition of the Solid Electrolyte Interphase (SEI) on Silicon Electrodes for Lithium Ion Batteries. Chem. Mater. 2017, 29, 3237–3245. [Google Scholar] [CrossRef]
- Campion, C.L.; Li, W.; Lucht, B.L. Thermal Decomposition of LiPF6-Based Electrolytes for Lithium-Ion Batteries. J. Electrochem. Soc. 2005, 152, A2327. [Google Scholar] [CrossRef]
- Diaz, F.; Wang, Y.; Weyhe, R.; Friedrich, B. Gas generation measurement and evaluation during mechanical processing and thermal treatment of spent Li-ion batteries. Waste Manag. 2019, 84, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Grützke, M.; Mönnighoff, X.; Horsthemke, F.; Kraft, V.; Winter, M.; Nowak, S. Extraction of lithium-ion battery electrolytes with liquid and supercritical carbon dioxide and additional solvents. RSC Adv. 2015, 5, 43209–43217. [Google Scholar] [CrossRef]
- Nowak, S.; Winter, M. The Role of Sub- and Supercritical CO2 as “Processing Solvent” for the Recycling and Sample Preparation of Lithium Ion Battery Electrolytes. Molecules 2017, 22, 403. [Google Scholar] [CrossRef] [PubMed]
- Baars, J.; Domenech, T.; Bleischwitz, R.; Melin, H.E.; Heidrich, O. Circular economy strategies for electric vehicle batteries reduce reliance on raw materials. Nat. Sustain. 2020, 69, 37. [Google Scholar] [CrossRef]
- Pagliaro, M.; Meneguzzo, F. Lithium battery reusing and recycling: A circular economy insight. Heliyon 2019, 5, e01866. [Google Scholar] [CrossRef] [PubMed]
- Richa, K.; Babbitt, C.W.; Gaustad, G. Eco-Efficiency Analysis of a Lithium-Ion Battery Waste Hierarchy Inspired by Circular Economy. J. Ind. Ecol. 2017, 21, 715–730. [Google Scholar] [CrossRef]
- Xie, Y.H.; Yu, H.J.; Ou, Y.N.; Li, C.D. Environmental impact assessment of recycling waste traction battery. Inorg. Chem. Ind. 2015, 47, 43–46. [Google Scholar]
- Heulens, J.; Van Horebeek, D.; Quix, M.; Brouwe, S. Process for smelting lithium-ion batteries. EP2015/067809, 8 March 2015. [Google Scholar]
- Xu, P.; Dai, Q.; Gao, H.; Liu, H.; Zhang, M.; Li, M.; Chen, Y.; An, K.; Meng, Y.S.; Liu, P.; et al. Efficient Direct Recycling of Lithium-Ion Battery Cathodes by Targeted Healing. Joule 2020, 4, 2609–2626. [Google Scholar] [CrossRef]
- Gao, H.; Yan, Q.; Xu, P.; Liu, H.; Li, M.; Liu, P.; Luo, J.; Chen, Z. Efficient Direct Recycling of Degraded LiMn2O4 Cathodes by One-Step Hydrothermal Relithiation. ACS Appl. Mater. Interfaces 2020, 12, 51546–51554. [Google Scholar] [CrossRef]
- Wang, T.; Luo, H.; Bai, Y.; Li, J.; Belharouak, I.; Dai, S. Direct Recycling of Spent NCM Cathodes through Ionothermal Lithiation. Adv. Energy Mater. 2020, 10, 2001204. [Google Scholar] [CrossRef]
- Hanisch, C.; Schunemann, J.-H.; Diekmann, J.; Westphal, B.; Loellhoeffel, T.; Prziwara, P.F.; Haselrieder, W.; Kwade, A. In-Production Recycling of Active Materials from Lithium-Ion Battery Scraps. ECS Trans. 2015, 64, 131–145. [Google Scholar] [CrossRef]
- Tomaszewska, A.; Chu, Z.; Feng, X.; O’Kane, S.; Liu, X.; Chen, J.; Ji, C.; Endler, E.; Li, R.; Liu, L.; et al. Lithium-ion battery fast charging: A review. eTransportation 2019, 1, 100011. [Google Scholar] [CrossRef]
- Bai, Y.; Muralidharan, N.; Sun, Y.-K.; Passerini, S.; Stanley Whittingham, M.; Belharouak, I. Energy and environmental aspects in recycling lithium-ion batteries: Concept of Battery Identity Global Passport. Mater. Today 2020, 41, 304–315. [Google Scholar] [CrossRef]
- Krüger, S.; Hanisch, C.; Kwade, A.; Winter, M.; Nowak, S. Effect of impurities caused by a recycling process on the electrochemical performance of Li[Ni0.33Co0.33Mn0.33]O2. J. Electroanal. Chem. 2014, 726, 91–96. [Google Scholar] [CrossRef]
- Zhang, R.; Zheng, Y.; Yao, Z.; Vanaphuti, P.; Ma, X.; Bong, S.; Chen, M.; Liu, Y.; Cheng, F.; Yang, Z.; et al. Systematic Study of Al Impurity for NCM622 Cathode Materials. ACS Sustain. Chem. Eng. 2020, 8, 9875–9884. [Google Scholar] [CrossRef]
- Thompson, D.L.; Hartley, J.M.; Lambert, S.M.; Shiref, M.; Harper, G.D.J.; Kendrick, E.; Anderson, P.; Ryder, K.S.; Gaines, L.; Abbott, A.P. The importance of design in lithium ion battery recycling—A critical review. Green Chem. 2020, 22, 7585–7603. [Google Scholar] [CrossRef]
- Rothermel, S.; Evertz, M.; Kasnatscheew, J.; Qi, X.; Grützke, M.; Winter, M.; Nowak, S. Graphite Recycling from Spent Lithium-Ion Batteries. ChemSusChem 2016, 9, 3473–3484. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).