Hot Compression Behavior of New Al-6Mg and Al-8Mg Alloy with Improved Hot Workability Fabricated by Direct Chill Casting Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Manufacturing Al Alloys for Specimens
2.2. Compressive Test
2.3. Observing Microstructure
3. Results
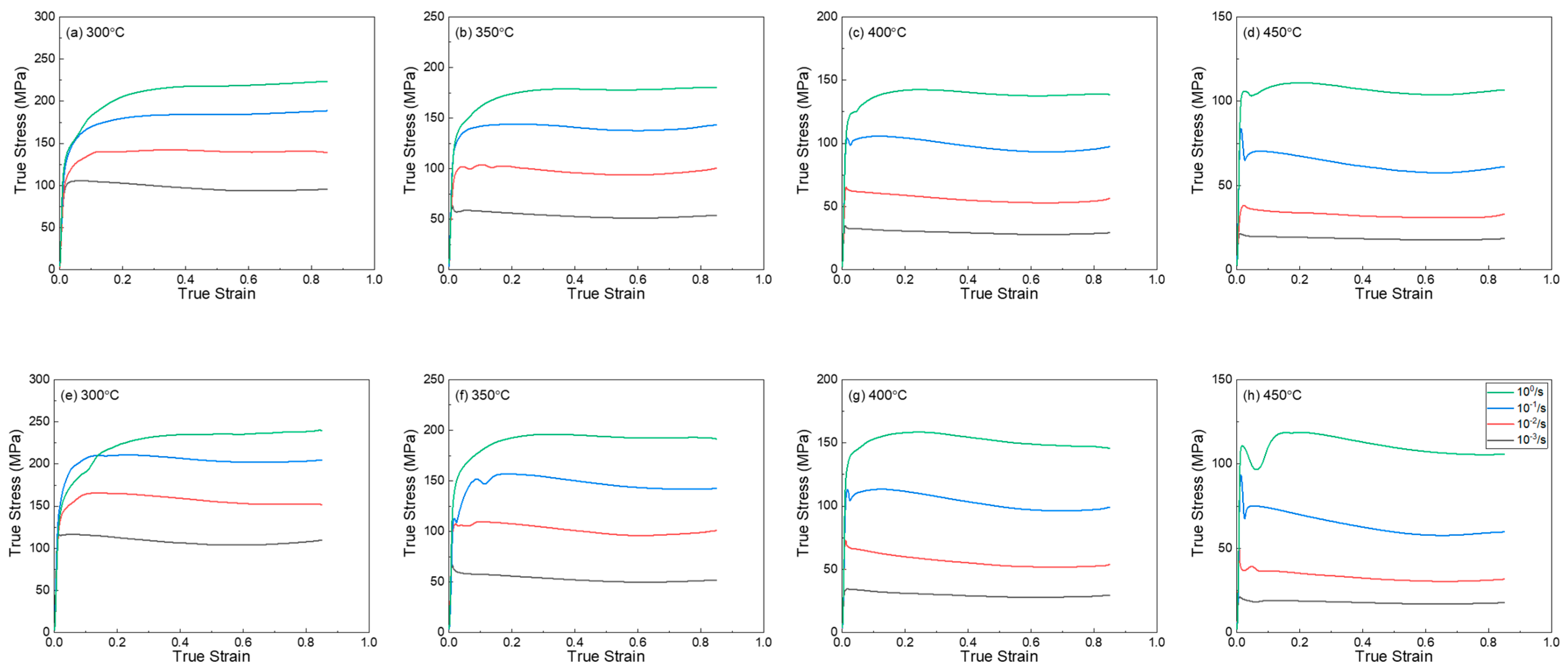
3.1. Flow Stress-Strain Curves Behavior
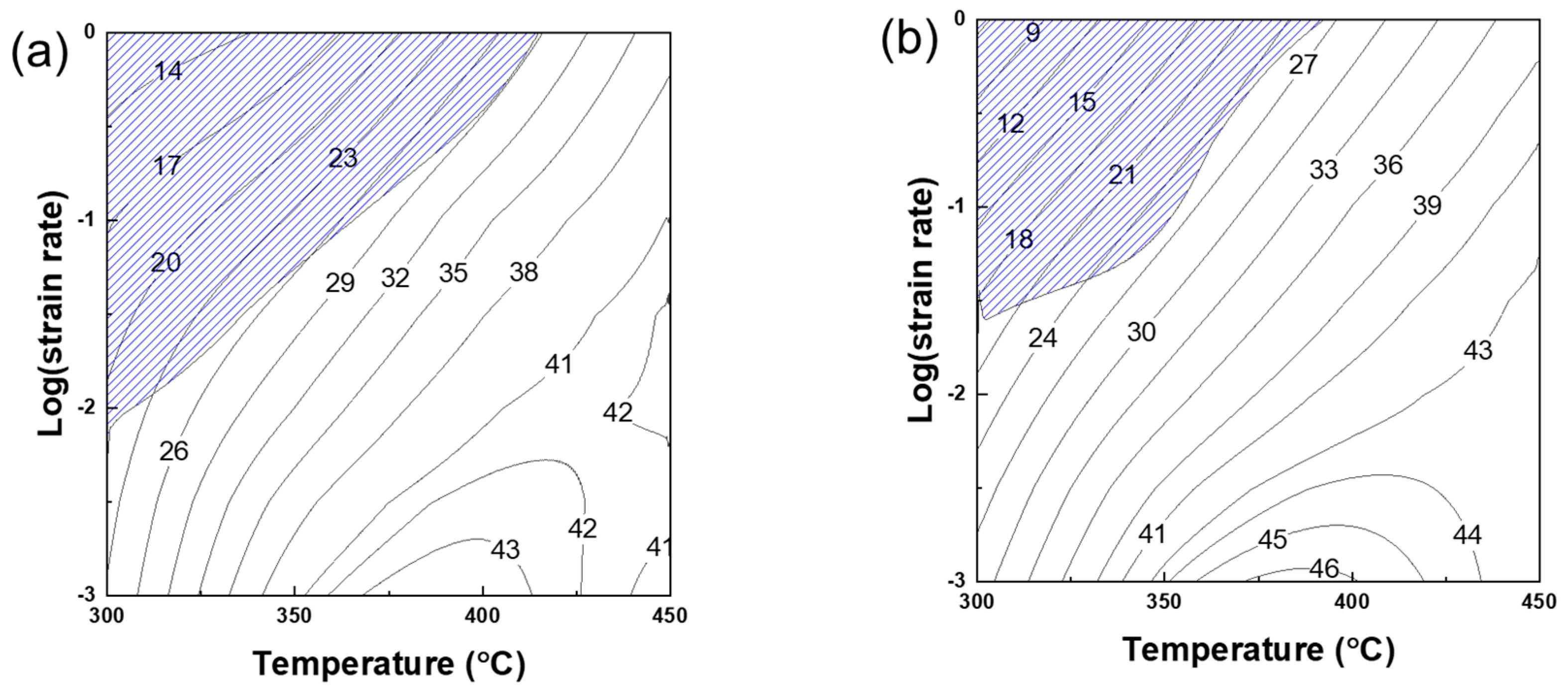
3.2. Processing Maps
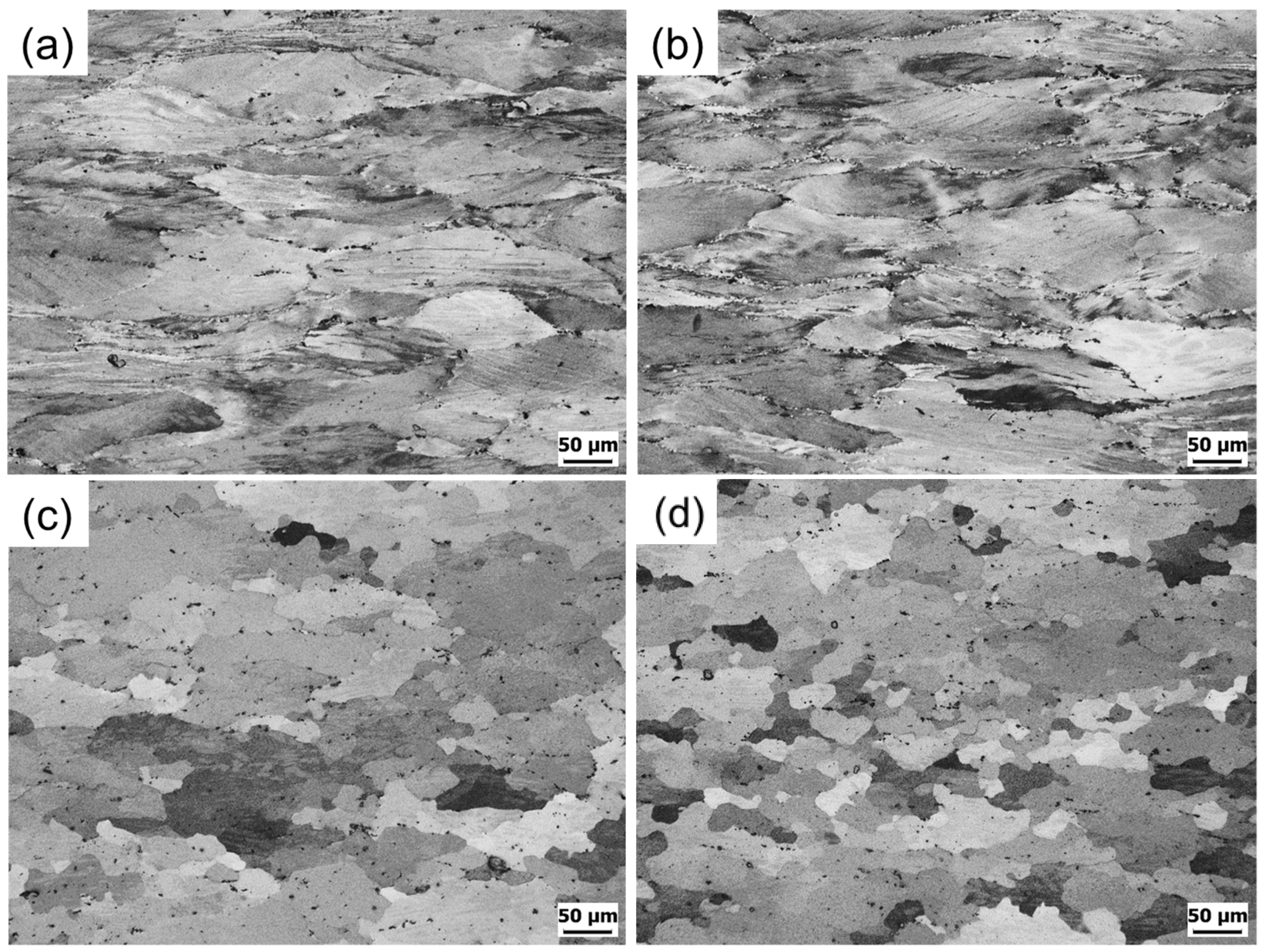
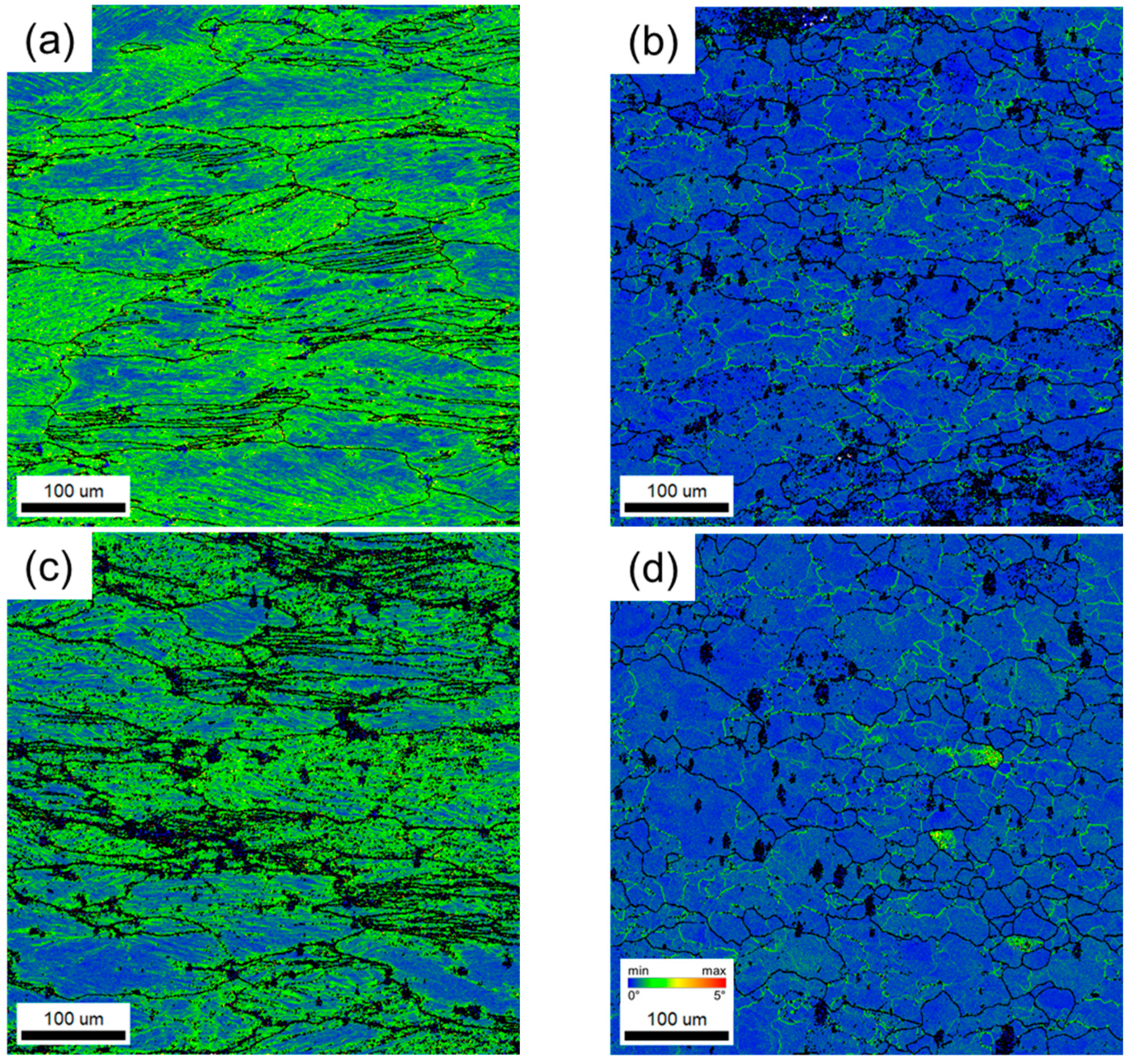
3.3. Microstructure
4. Discussion
5. Conclusions
- The flow curves show that the maximum strength increased as the Mg content increased. In addition, a rapid softening behavior of flow stress was observed after the yield point at a temperature of 350 °C or higher. Dynamic recrystallization may explain this phenomenon.
- According to the results of the processing maps, it is observed that, as the Mg content increases, the maximum power dissipation efficiency increases, whereas the plastic instability region area decreases. The level of power dissipation efficiency increases with the activation of dynamic restoration, especially dynamic recrystallization (DRX). It appears that the inhomogeneous deformation is suppressed by the acceleration of DRX in the Mg content under the dynamic restoration-activated conditions. Deformation band formation is evident in the region where the plastic instability occurs with a low power dissipation efficiency.
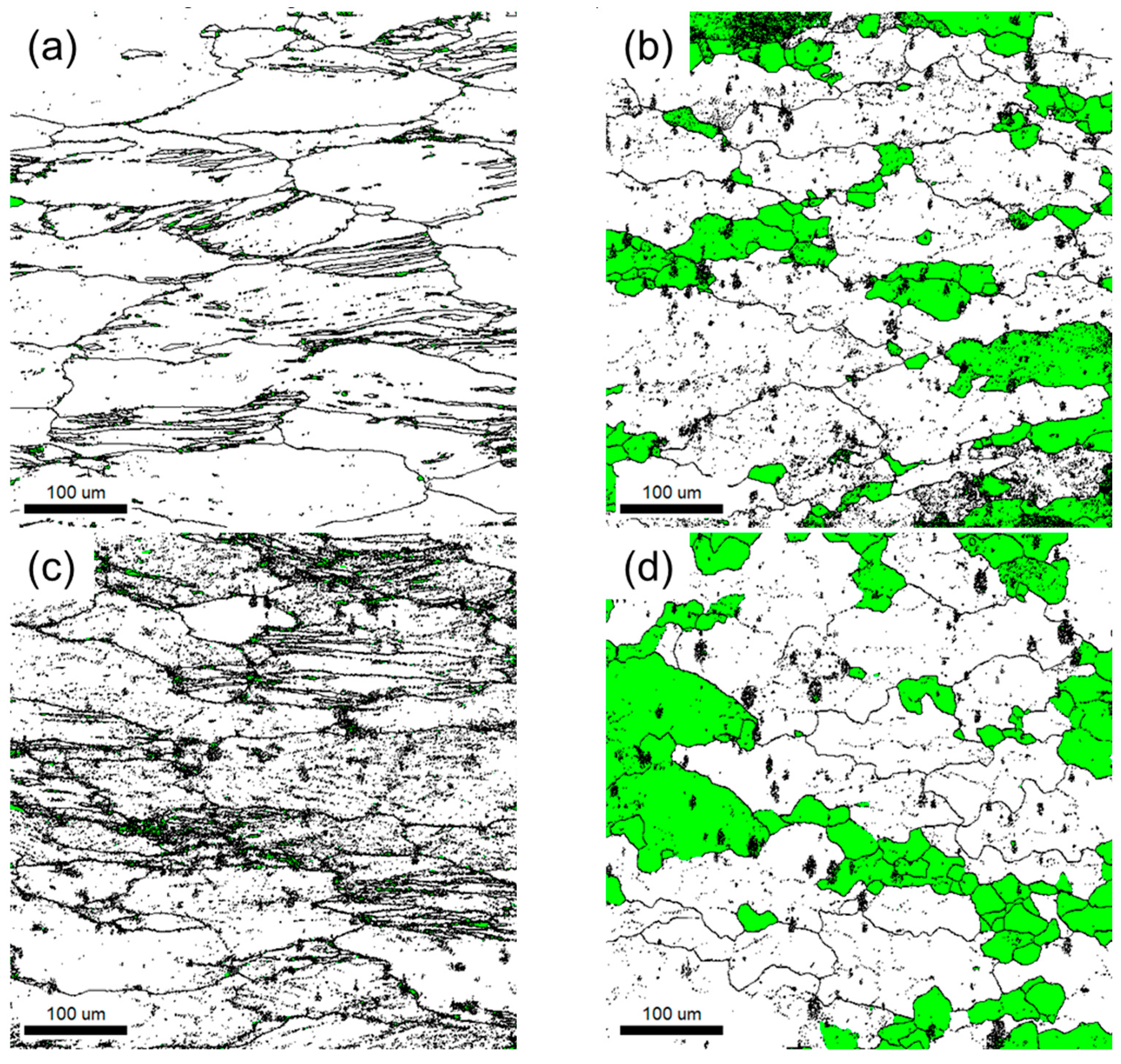
- The kernel average misorientation map visually indicates that the remaining local strain is much smaller in the maximum power dissipation condition than the low condition in both Al-6Mg and Al-8Mg alloys. The accumulated local strains during hot deformation of the maximum efficiency region appears to lead to the DRX formation.
- In a grain misorientation spread (GOS) analysis, both the average GOS value and the low GOS value fraction (<3°) are used. While the Mg content does not affect the microstructure evolution when dynamic restorations are not activated, it seems that the higher Mg concentration in Al-Mg alloy boosts the DRX rate and retards dynamic recovery by inhibiting dislocation movements.
- Further research needs to be done by comparing the plastic deformation behavior and processing map results for each temperature and strain rate domain to reach high reliability in mass-scale products. This research might help design lightweight automotive components, such as aluminum forged parts (such as arm, rod, knuckle, axle, etc.).
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wen, W.; Zhao, Y.; Morris, J.G. The Effect of Mg Precipitation on the Mechanical Properties of 5xxx Aluminum Alloys. Mater. Sci. Eng. A 2005, 392, 136–144. [Google Scholar] [CrossRef]
- Milovanoff, A.; Kim, H.C.; De Kleine, R.; Wallington, T.J.; Posen, I.D.; MacLean, H.L. A Dynamic Fleet Model of U.S Light-Duty Vehicle Lightweighting and Associated Greenhouse Gas Emissions from 2016 to 2050. Environ. Sci. Technol. 2019, 53, 2199–2208. [Google Scholar] [CrossRef]
- Cantor, B.; Grant, P.; Johnston, C. Automotive Engineering: Lightweight, Functional, and Novel Materials; CRC Press: Boca Raton, FL, USA, 2008; ISBN 9781420011906. [Google Scholar]
- Shore, D.; Kestens, L.A.I.; Sidor, J.; Van Houtte, P.; Van Bael, A. Process Parameter Influence on Texture Heterogeneity in Asymmetric Rolling of Aluminium Sheet Alloys. Int. J. Mater. Form. 2018, 11, 297–309. [Google Scholar] [CrossRef]
- Verdier, M.; Janecek, M.; Bréchet, Y.; Guyot, P. Microstructural Evolution during Recovery in Al–2.5% Mg Alloys. Mater. Sci. Eng. A 1998, 248, 187–197. [Google Scholar] [CrossRef]
- Curtin, W.A.; Olmsted, D.L.; Hector, L.G. A Predictive Mechanism for Dynamic Strain Ageing in Aluminium–Magnesium Alloys. Nat. Mater. 2006, 5, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.H.; Vu, H.C. Forming Limit Prediction of Anisotropic Aluminum Magnesium Alloy Sheet AA5052-H32 Using Micromechanical Damage Model. J. Mater. Eng. Perform. 2020, 29, 4677–4691. [Google Scholar] [CrossRef]
- Feng, F.; Li, J.; Chen, R.; Yuan, P.; Su, H.; Zhang, Q.; Huang, P.; Zheng, Z. Effect of Die Geometry on the Formability of 5052 Aluminum Alloy in Electromagnetic Impaction Deformation. Materials 2018, 11, 1379. [Google Scholar] [CrossRef]
- Silva, M.P.; Talbot, D.E.J. Oxidation of Liquid Aluminum—Magnesium Alloys. In Essential Readings in Light Metals: Cast Shop for Aluminum Production; Grandfield, J.F., Eskin, D.G., Eds.; Springer International Publishing: Cham, Switzerland, 2016; Volume 3, pp. 137–142. ISBN 9783319482286. [Google Scholar]
- Ha, S.-H.; Yoon, Y.-O.; Kim, B.-H.; Lee, T.-W.; Lim, S.-H.; Kim, S.K. Precipitation of Oxide Particles in Surface Mixed Layer of Al–Mg Alloys with a Trace of Ca During Oxidation. J. Nanosci. Nanotechnol. 2017, 17, 8232–8235. [Google Scholar] [CrossRef]
- Kim, B.-H.; Ha, S.-H.; Yoon, Y.-O.; Lim, H.-K.; Kim, S.K.; Kim, D.-H. Effect of Ca Addition on Selective Oxidation of Al3Mg2 Phase in Al-5 mass% Mg Alloy. Mater. Lett. 2018, 228, 108–111. [Google Scholar] [CrossRef]
- Ha, S.-H.; Yoon, Y.-O.; Kim, B.-H.; Lim, H.-K.; Lee, T.-W.; Lim, S.-H.; Kim, S.K. Oxide Scale Behavior and Surface Protection of Al–Mg Alloys Containing a Trace of Ca. Int. J. Met. 2019, 13, 121–129. [Google Scholar] [CrossRef]
- Jenab, A.; Karimi Taheri, A. Experimental Investigation of the Hot Deformation Behavior of AA7075: Development and Comparison of Flow Localization Parameter and Dynamic Material Model Processing Maps. Int. J. Mech. Sci. 2014, 78, 97–105. [Google Scholar] [CrossRef]
- Jin, N.; Zhang, H.; Han, Y.; Wu, W.; Chen, J. Hot Deformation Behavior of 7150 Aluminum Alloy during Compression at Elevated Temperature. Mater. Charact. 2009, 60, 530–536. [Google Scholar] [CrossRef]
- Ding, S.; Khan, S.A.; Yanagimoto, J. Flow Behavior and Dynamic Recrystallization Mechanism of A5083 Aluminum Alloys with Different Initial Microstructures during Hot Compression. Mater. Sci. Eng. A 2020, 787, 139522. [Google Scholar] [CrossRef]
- Prasad, Y.V.R.K.; Gegel, H.L.; Doraivelu, S.M.; Malas, J.C.; Morgan, J.T.; Lark, K.A.; Barker, D.R. Modeling of Dynamic Material Behavior in Hot Deformation: Forging of Ti-6242. Metall. Trans. A 1984, 15, 1883–1892. [Google Scholar] [CrossRef]
- Malas, J.C.; Seetharaman, V. Using Material Behavior Models to Develop Process Control Strategies. JOM 1992, 44, 8–13. [Google Scholar] [CrossRef]
- Ravichandran, N.; Prasad, Y.V.R.K. Dynamic Recrystallization during Hot Deformation of Aluminum: A Study Using Processing Maps. Metall. Trans. A 1991, 22, 2339–2348. [Google Scholar] [CrossRef]
- Ziegler, H. Progress in Solid Mechanics; Springer: Berlin/Heidelberg, Germany, 1963; pp. 63–193. [Google Scholar]
- Field, D.P.; Bradford, L.T.; Nowell, M.M.; Lillo, T.M. The Role of Annealing Twins during Recrystallization of Cu. Acta Mater. 2007, 55, 4233–4241. [Google Scholar] [CrossRef]
- Shen, X.; Song, W.; Sevsek, S.; Ma, Y.; Hüter, C.; Spatschek, R.; Bleck, W. Influence of Microstructural Morphology on Hydrogen Embrittlement in a Medium-Mn Steel Fe-12Mn-3Al-0.05C. Metals 2019, 9, 929. [Google Scholar] [CrossRef]
- Sakai, T.; Belyakov, A.; Kaibyshev, R.; Miura, H.; Jonas, J.J. Dynamic and Post-Dynamic Recrystallization under Hot, Cold and Severe Plastic Deformation Conditions. Prog. Mater. Sci. 2014, 60, 130–207. [Google Scholar] [CrossRef]
- Valdes-Tabernero, M.A.; Sancho-Cadenas, R.; Sabirov, I.; Murashkin, M.Y.; Ovid’ko, I.A.; Galvez, F. Effect of SPD Processing on Mechanical Behavior and Dynamic Strain Aging of an Al-Mg Alloy in Various Deformation Modes and Wide Strain Rate Range. Mater. Sci. Eng. A 2017, 696, 348–359. [Google Scholar] [CrossRef]
- Kaibyshev, R.; Musin, F.; Lesuer, D.R.; Nieh, T.G. Superplastic Behavior of an Al–Mg Alloy at Elevated Temperatures. Mater. Sci. Eng. A 2003, 342, 169–177. [Google Scholar] [CrossRef]
- Rupp, R.E.; Weldon, A.J.; Watt, T.J.; Perez-Bustamante, R.; Takata, K.; Taleff, E.M. Recrystallization in Al-Mg Alloys after Hot Compression. In Light Metals; Williams, E., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 163–167. ISBN 9783319482514. [Google Scholar]
- Miura, H.; Sakai, T.; Hamaji, H.; Jonas, J.J. Preferential Nucleation of Dynamic Recrystallization at Triple Junctions. Scr. Mater. 2004, 50, 65–69. [Google Scholar] [CrossRef]
- Zhou, P.; Deng, L.; Zhang, M.; Gong, P.; Wang, X. Characterization of Hot Workability of 5052 Aluminum Alloy Based on Activation Energy-Processing Map. J. Mater. Eng. Perform. 2019, 28, 6209–6218. [Google Scholar] [CrossRef]
- Du, N.; Qi, Y.; Krajewski, P.E.; Bower, A.F. The Effect of Solute Atoms on Aluminum Grain Boundary Sliding at Elevated Temperature. Metall. Mater. Trans. A 2011, 42, 651–659. [Google Scholar] [CrossRef]

| Alloy | Mg | Ti | Ca | Al |
|---|---|---|---|---|
| Al-6Mg | 6.22 | 0.03 | 0.06 | Bal. |
| Al-8Mg | 7.82 | 0.03 | 0.08 | Bal. |
| Process Conditions | GOS Value | GOS < 3° Fraction |
|---|---|---|
| (a) Al-6Mg, 300 °C, 100/s | 1.724 | 0.031 |
| (b) Al-6Mg, 400 °C, 10−3/s | 0.582 | 0.231 |
| (c) Al-8Mg, 300 °C, 100/s | 1.631 | 0.026 |
| (d) Al-8Mg, 400 °C, 10−3/s | 1.080 | 0.278 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, N.-S.; Choi, K.-H.; Yang, S.-Y.; Ha, S.-H.; Yoon, Y.-O.; Kim, B.-H.; Lim, H.-K.; Kim, S.K.; Hyun, S.-K. Hot Compression Behavior of New Al-6Mg and Al-8Mg Alloy with Improved Hot Workability Fabricated by Direct Chill Casting Method. Metals 2021, 11, 288. https://doi.org/10.3390/met11020288
Kim N-S, Choi K-H, Yang S-Y, Ha S-H, Yoon Y-O, Kim B-H, Lim H-K, Kim SK, Hyun S-K. Hot Compression Behavior of New Al-6Mg and Al-8Mg Alloy with Improved Hot Workability Fabricated by Direct Chill Casting Method. Metals. 2021; 11(2):288. https://doi.org/10.3390/met11020288
Chicago/Turabian StyleKim, Nam-Seok, Kweon-Hoon Choi, Seung-Yoon Yang, Seong-Ho Ha, Young-Ok Yoon, Bong-Hwan Kim, Hyun-Kyu Lim, Shae K. Kim, and Soong-Keun Hyun. 2021. "Hot Compression Behavior of New Al-6Mg and Al-8Mg Alloy with Improved Hot Workability Fabricated by Direct Chill Casting Method" Metals 11, no. 2: 288. https://doi.org/10.3390/met11020288
APA StyleKim, N.-S., Choi, K.-H., Yang, S.-Y., Ha, S.-H., Yoon, Y.-O., Kim, B.-H., Lim, H.-K., Kim, S. K., & Hyun, S.-K. (2021). Hot Compression Behavior of New Al-6Mg and Al-8Mg Alloy with Improved Hot Workability Fabricated by Direct Chill Casting Method. Metals, 11(2), 288. https://doi.org/10.3390/met11020288









