Corrosion Mechanisms of High-Mn Twinning-Induced Plasticity (TWIP) Steels: A Critical Review
Abstract
1. Introduction
2. Effect of Alloying Elements on Aqueous Corrosion Behavior of High-Mn TWIP Steels
2.1. Effect of Al Alloying on High-Mn TWIP Steel Corrosion
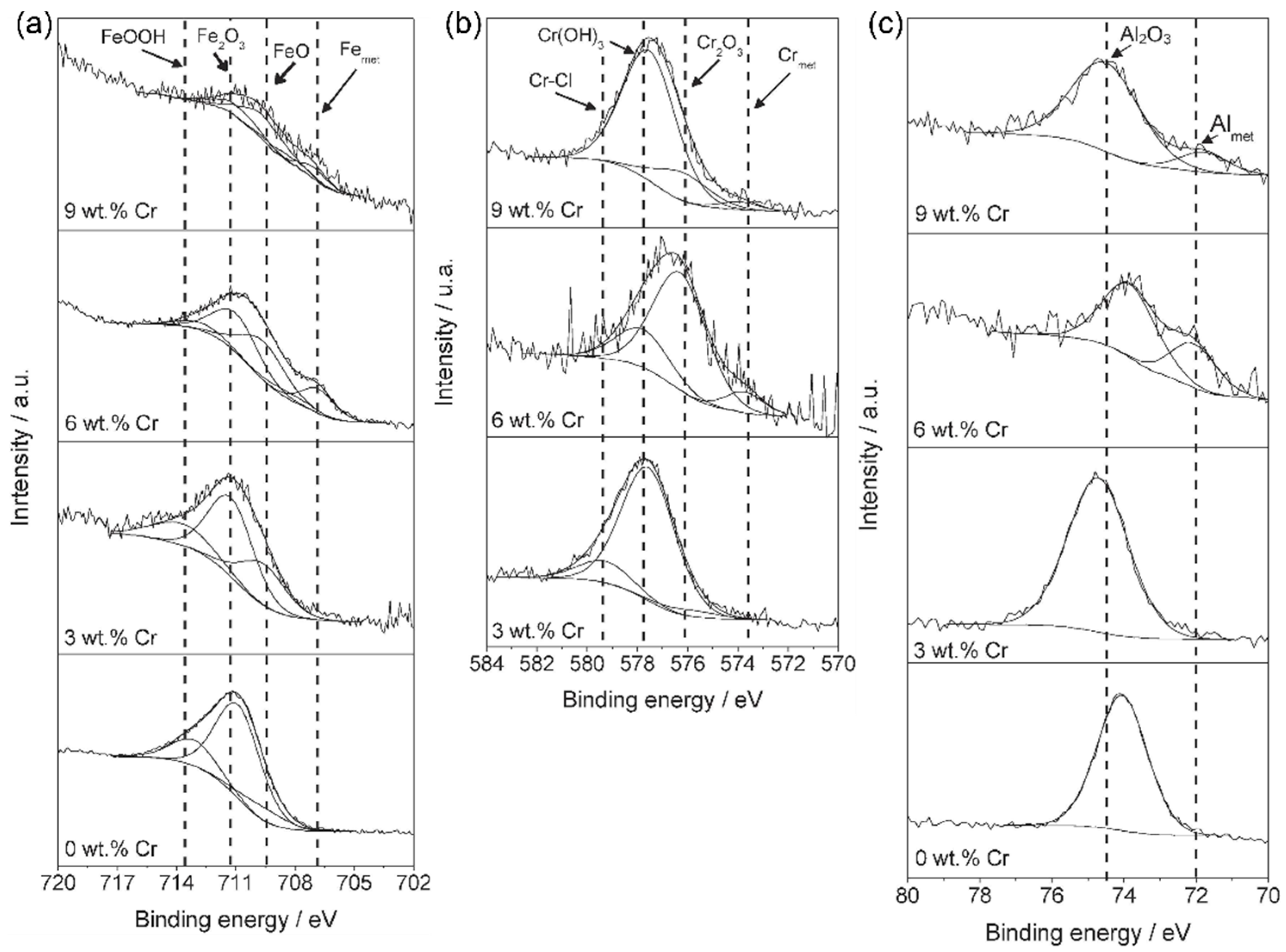
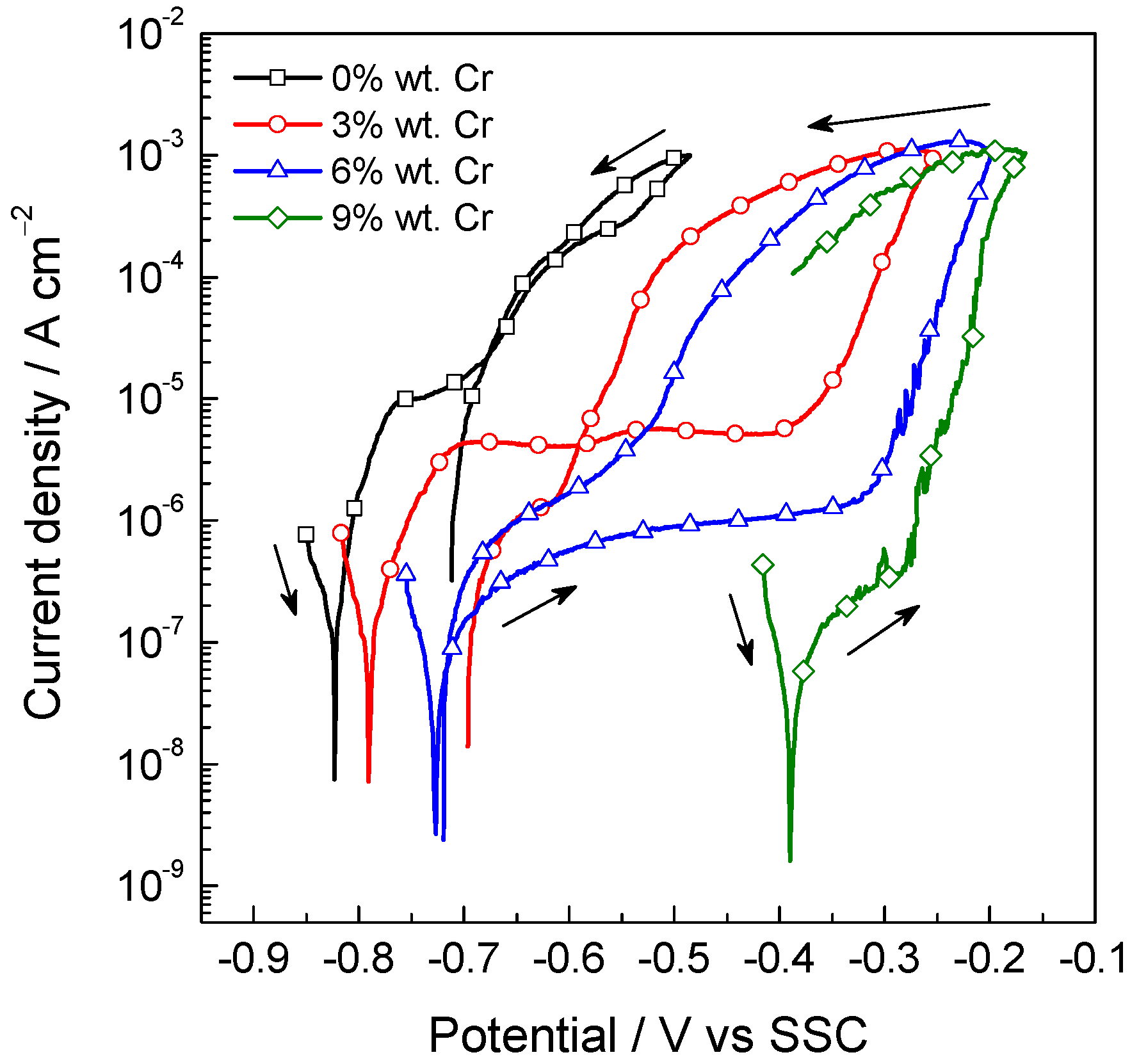
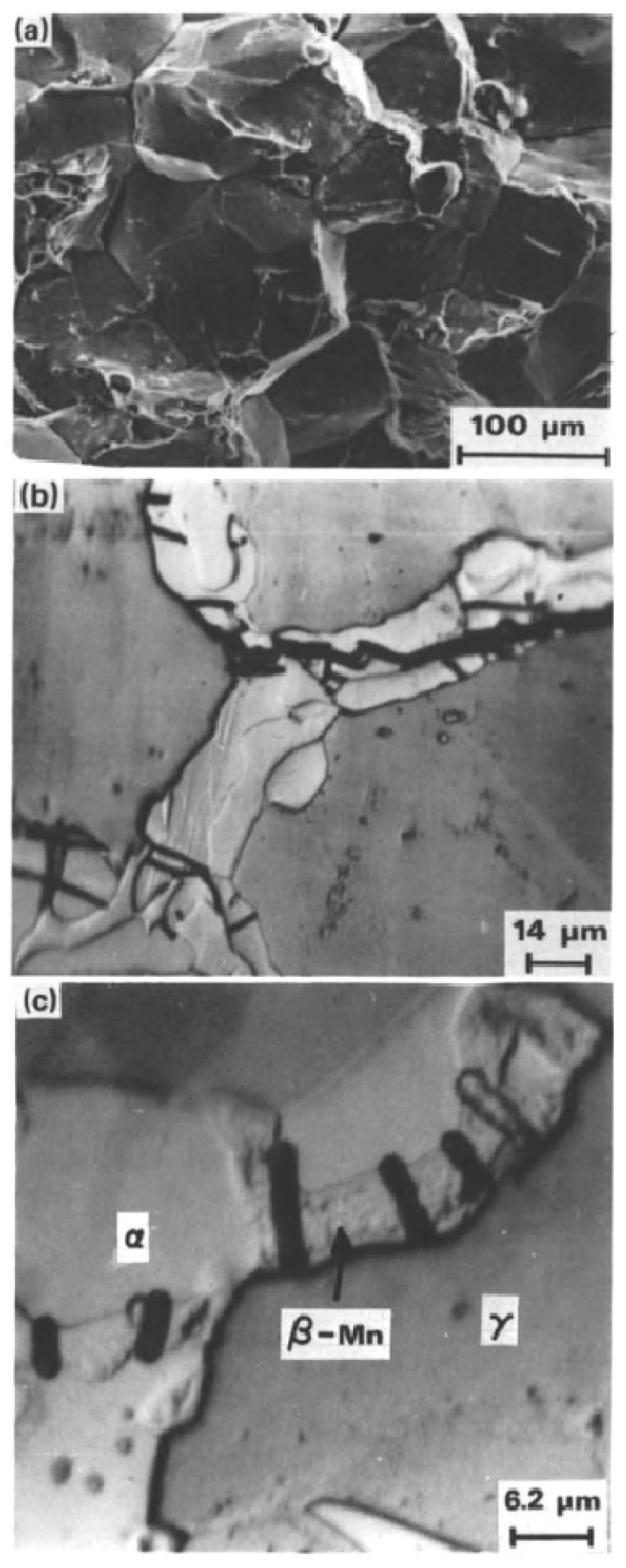
2.2. Effect of Al and Cr Alloying on High-Mn TWIP Steel Corrosion
2.3. Effect of Al and Si Alloying on High-Mn TWIP Steel Corrosion
2.4. Effect of Al, Cu and P Alloying on high-Mn TWIP Steel Corrosion
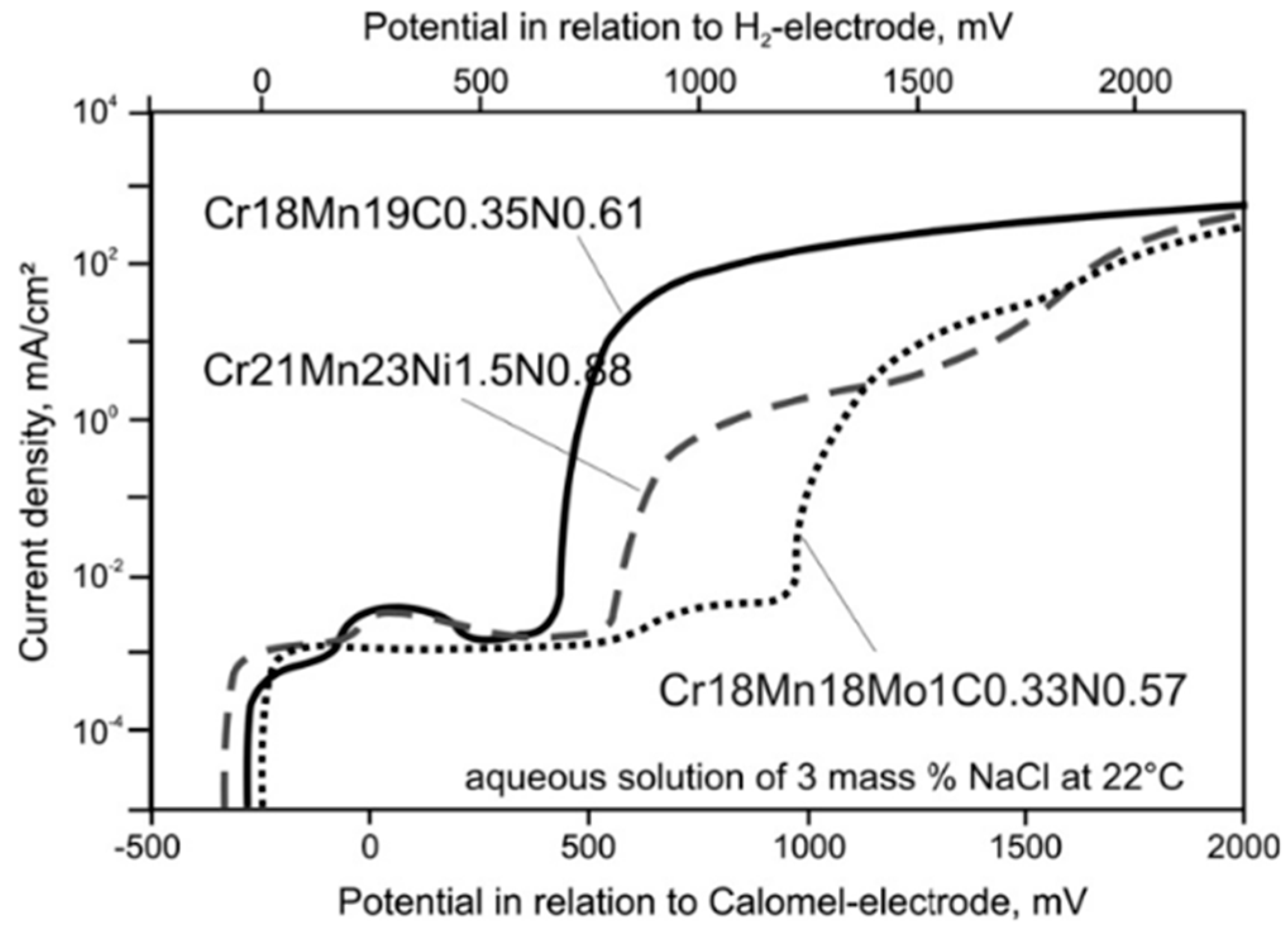
2.5. Effect of Cr, Cu, Si and N Alloying on High-Mn TWIP Steel Corrosion
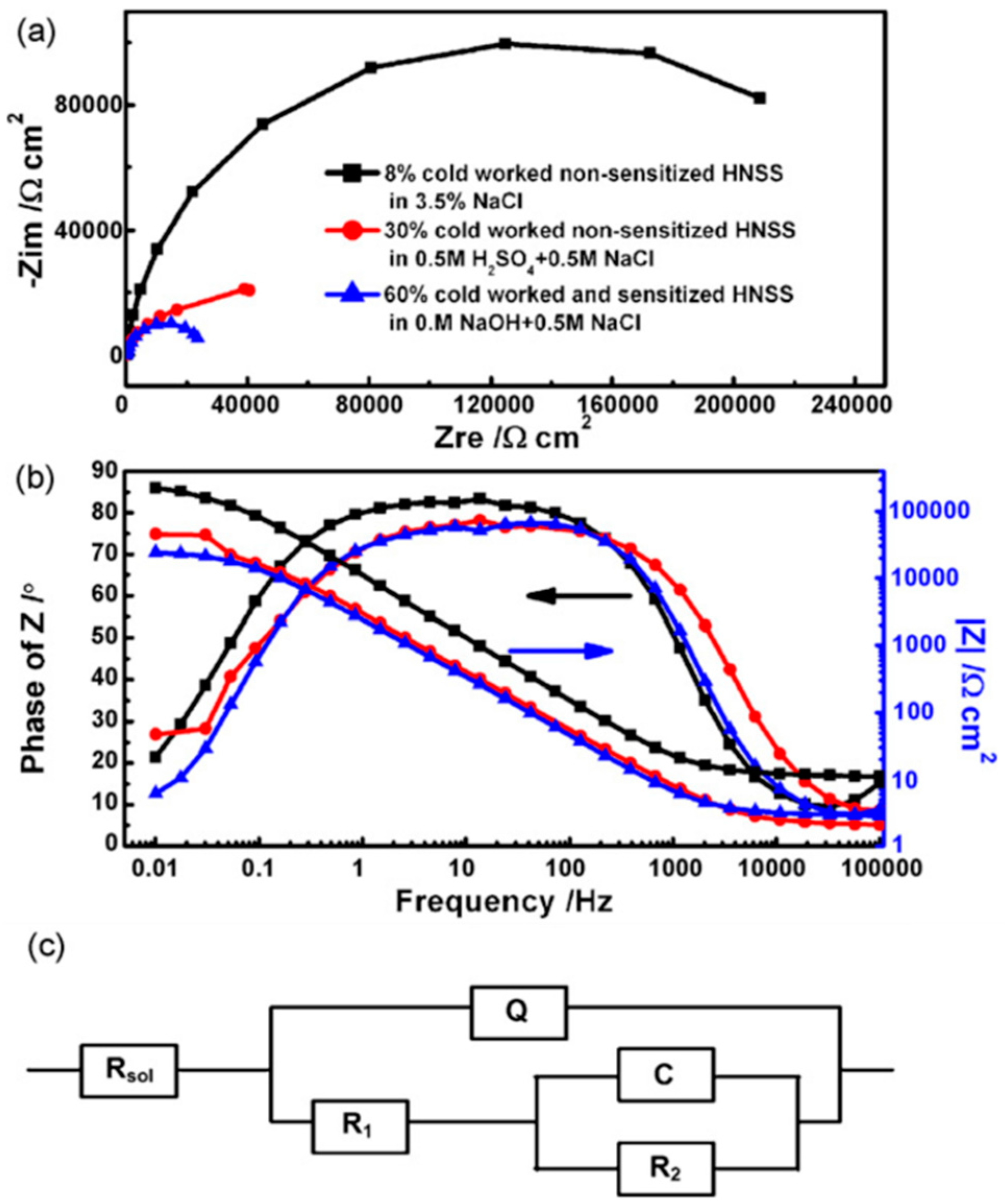
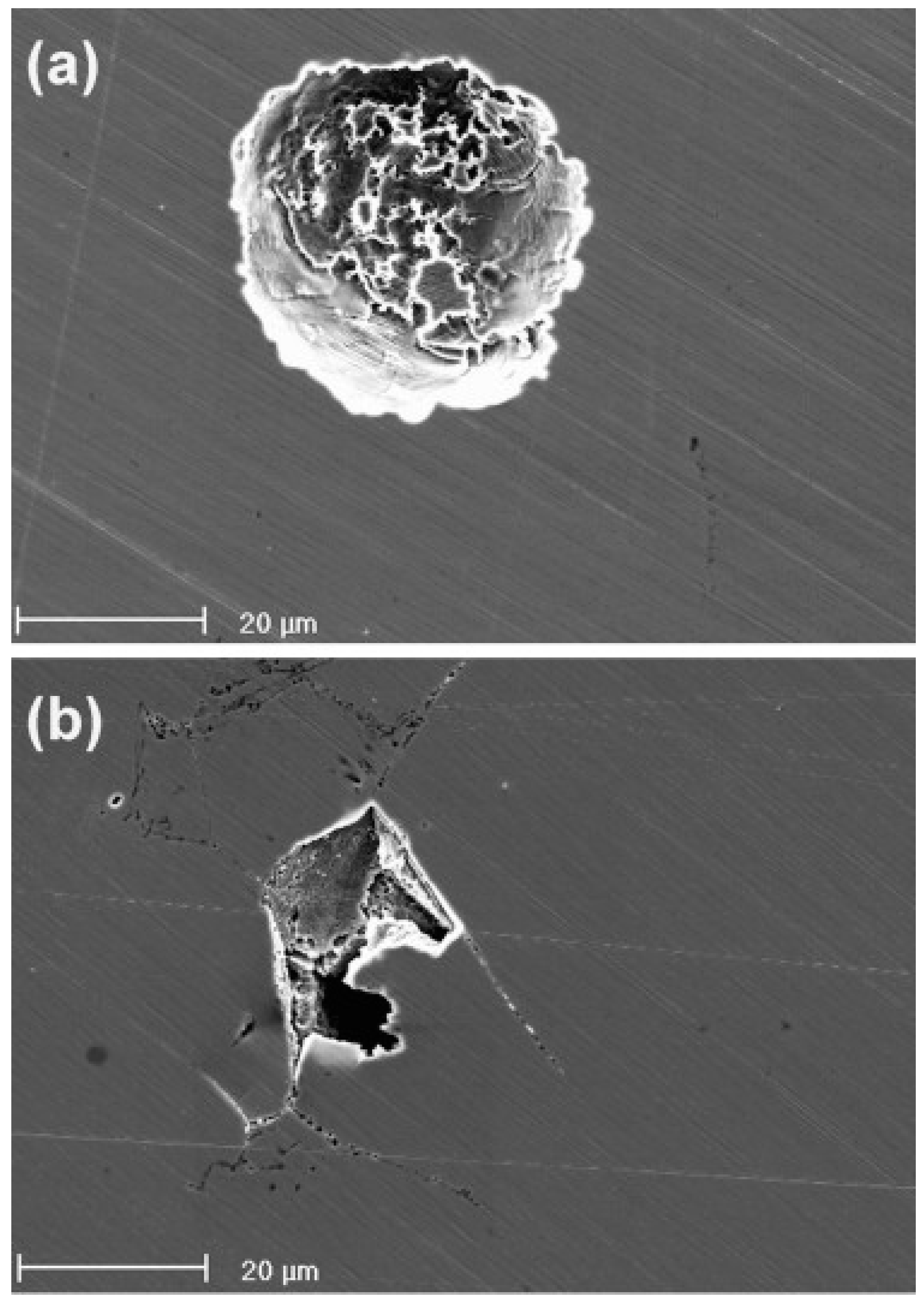
3. Effect of Mechanical Deformation on Corrosion of High-Mn TWIP Steels
4. Stress Corrosion Cracking of High-Mn TWIP Steels
5. Hydrogen Embrittlement of High-Mn TWIP Steels
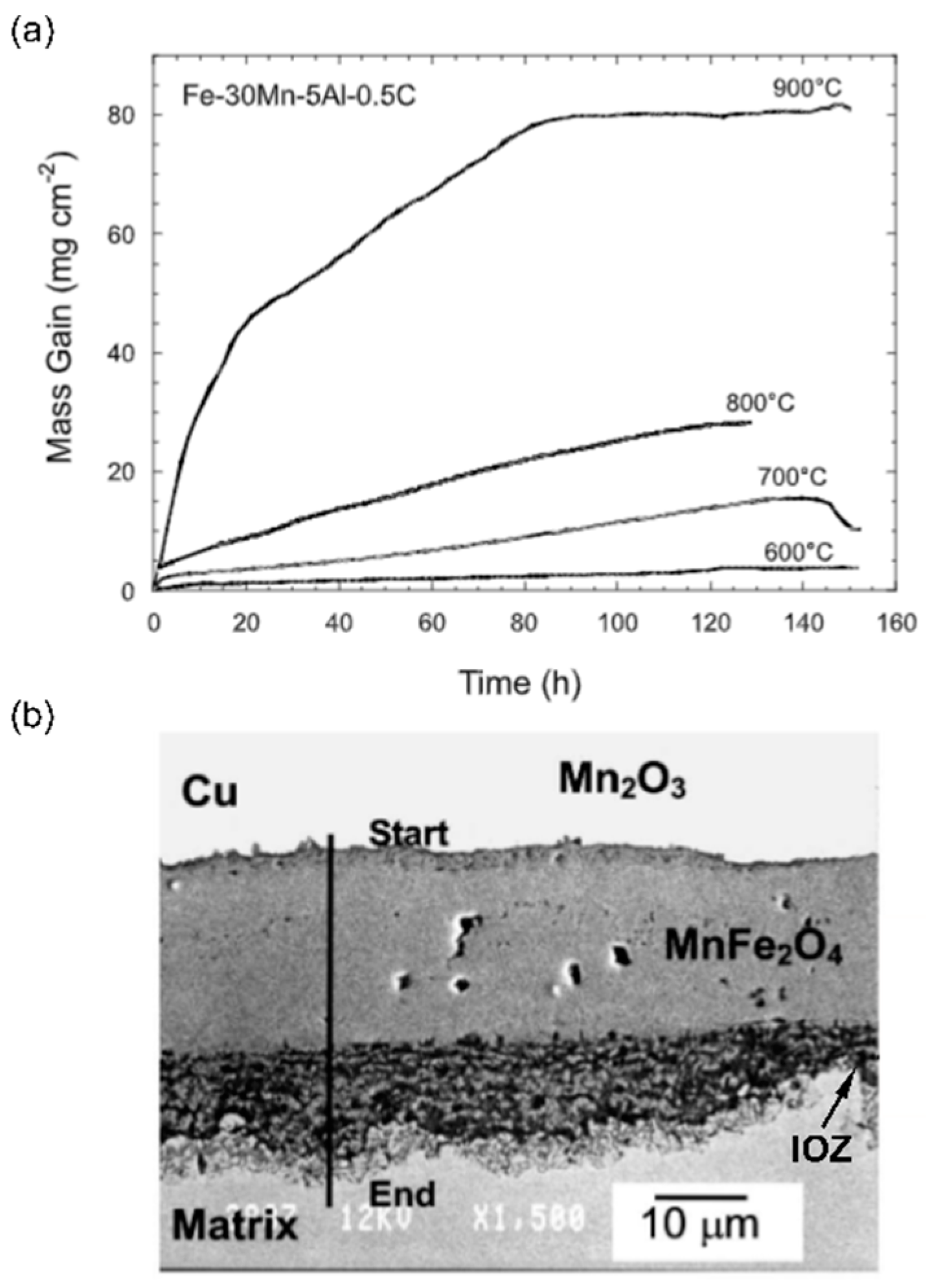
6. Elevated Temperature Oxidation of High-Mn TWIP Steels
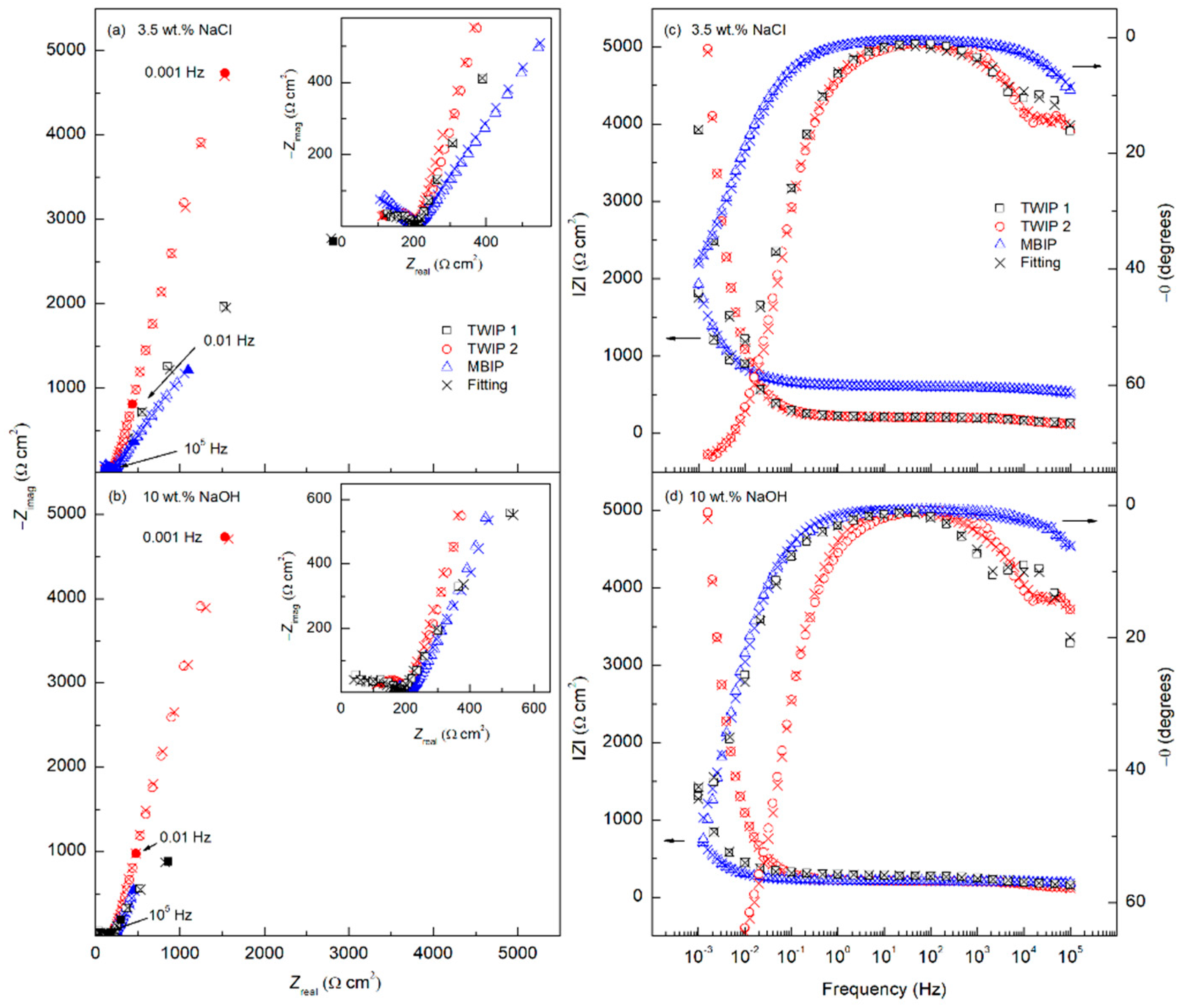
7. Corrosion Behavior of Welded High-Mn TWIP Steels
8. Tribocorrosion of High-Mn TWIP Steels
9. Corrosion of High-Mn TWIP Steels in Harsh Environments
10. Corrosion of Additively Manufactured High-Mn TWIP Steels
11. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Glossary
| AES | Auger electron spectroscopic |
| AHSS | Advanced high-strength steels |
| AM | Additive manufacturing |
| bcc | Body-centered cubic |
| BM | Base material |
| CG | Coarse-grained |
| CP | Complex phase |
| CPP | Cyclic potentiodynamic polarization |
| DP | Dual-phase |
| EBSD | Electron backscattered diffraction |
| Ecorr | Corrosion potential |
| Ef | Flade potential |
| EIS | Electrochemical impedance spectroscopy |
| Epit | Pitting potential |
| FB | Ferritic-bainitic |
| fcc | Face-centered cubic |
| FG | Fine-grained |
| FZ | Fusion zone |
| HAZ | Heat-affected-zone |
| HE | Hydrogen embrittlement |
| HF | Hot formed |
| HDE | Hydrogen-delayed fracture |
| HNSS | Nickel-free high-N SS |
| HSLA | High-strength low alloy |
| HSS | High strength steels |
| icorr | Corrosion current density |
| IF | Interstitial-free |
| ipass | Passive current density |
| PFHT | Post-forming heat-treatable |
| Rp | Polarization resistance |
| RSW | Resistance-spot-welded |
| SCC | Stress corrosion cracking |
| SEM | Scanning electron microscopy |
| SFE | Stacking fault energy |
| SHE | Standard hydrogen electrode |
| SIMS | Secondary ion mass spectroscopy |
| SKPFM | Scanning Kelvin probe force microscopy |
| SLM | Selected laser melting |
| SS | Stainless steel |
| TEM | Transmission electron microscopy |
| TMP | Thermo-mechanically processed |
| TRIP | Transformation-induced plasticity |
| TWIP | Twinning-induced plasticity |
| UHSS | Ultra-high strength steels |
| UTS | Ultimate tensile strength |
| v | Potential scan rate |
| vcorr | Corrosion rate |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
References
- Matlock, D.K.; Speer, J.G. Third Generation of AHSS: Microstructure Design Concepts. In Microstructure and Texture in Steels; Haldar, A., Suwas, S., Bhattacharjee, D., Eds.; Springer: New York, NY, USA, 2009; pp. 185–205. [Google Scholar]
- Cornette, D. SAE Technical Paper of the SAE World Congress; SAE International: Detroit, MI, USA, 2002. [Google Scholar]
- Thorpe, M.D.; Adam, H. SAE Technical Paper of the SAE World Congress; SAE International: Detroit, MI, USA, 2002. [Google Scholar]
- Allain, S.; Chateau, J.-P.; Bouaziz, O. A physical model of the twinning-induced plasticity effect in a high manganese austenitic steel. Mater. Sci. Eng. A 2004, 387–389, 143–147. [Google Scholar] [CrossRef]
- Bouaziz, O.; Allain, S.; Scott, C. Effect of grain and twin boundaries on the hardening mechanisms of twinning-induced plasticity steels. Scr. Mater. 2008, 58, 484–487. [Google Scholar] [CrossRef]
- De Cooman, B.C.; Chin, K.; Kim, J. High Mn TWIP Steels for Automotive Applications. In New Trends and Developments in Automotive System Engineering; Chiaberge, M., Ed.; InTech Europe: Rijeka, Croatia, 2011; pp. 101–128. [Google Scholar]
- Pierce, D.T.; Jiménez, J.A.; Bentley, J.; Raabe, D.; Wittig, J.E. The influence of stacking fault energy on the microstructural and strain-hardening evolution of Fe–Mn–Al–Si steels during tensile deformation. Acta Mater. 2015, 100, 178–190. [Google Scholar] [CrossRef]
- Bouaziz, O.; Guelton, N. Modelling of TWIP effect on work-hardening. Mater. Sci. Eng. A 2001, 319–321, 246–249. [Google Scholar] [CrossRef]
- Allain, S.; Chateau, J.-P.; Bouaziz, O.; Migot, S.; Guelton, N. Correlations between the calculated stacking fault energy and the plasticity mechanisms in Fe–Mn–C alloys. Mater. Sci. Eng. A 2004, 387–389, 158–162. [Google Scholar] [CrossRef]
- Cohen, J.B.; Weertman, J. A dislocation model for twinning in f.c.c. metals. Acta Metall. 1963, 11, 996–998. [Google Scholar] [CrossRef]
- Venables, J.A. On dislocation pole models for twinning. Philos. Mag. 1974, 30, 1165–1169. [Google Scholar] [CrossRef]
- Chen, S.; Rana, R.; Haldar, A.; Ray, R.K. Current state of Fe-Mn-Al-C low density steels. Prog. Mater. Sci. 2017, 89, 345–391. [Google Scholar] [CrossRef]
- Jacob, R.J.; Sankaranarayanan, S.R.; Babu, S.P.K. Recent advancements in manganese steels—A review. Mater. Today Proc. 2020, 27, 2852–2858. [Google Scholar] [CrossRef]
- De Cooman, B.C.; Kwon, O.; Chin, K.-G. State-of-the-knowledge on TWIP steel. Mater. Sci. Technol. 2012, 28, 513–527. [Google Scholar] [CrossRef]
- Neu, R.W. Performance and characterization of TWIP steels for automotive applications. Mater. Perform. Charact. 2013, 2, 20130009. [Google Scholar] [CrossRef]
- Remy, L. Temperature variation of the intrinsic stacking fault energy of a high manganese austenitic steel. Acta Metall. 1977, 25, 173–179. [Google Scholar] [CrossRef]
- Remy, L. The interaction between slip and twinning systems and the influence of twinning on the mechanical behavior of fcc metals and alloys. Metall. Trans. A 1981, 12, 387–408. [Google Scholar] [CrossRef]
- Remy, L. Kinetics of f.c.c. deformation twinning and its relationship to stress-strain behaviour. Acta Metall. 1978, 26, 443–451. [Google Scholar] [CrossRef]
- Remy, L. Twin-slip interaction in f.c.c. crystals. Acta Metall. 1977, 25, 711–714. [Google Scholar] [CrossRef]
- Remy, L.; Pineau, A.; Thomas, B. Temperature dependence of stacking fault energy in close-packed metals and alloys. Mater. Sci. Eng. 1978, 36, 47–63. [Google Scholar] [CrossRef]
- Remy, L.; Pineau, A. Twinning and strain-induced F.C.C. → H.C.P. transformation in the Fe-Mn-Cr-C system. Mater. Sci. Eng. 1977, 28, 99–107. [Google Scholar] [CrossRef]
- Chalant, G.; Remy, L. The slip character and low cycle fatigue behaviour: The influence of F.C.C. twinning and strain-induced F.C.C. → H.C.P. martensitic transformation. Acta Metall. 1980, 28, 75–88. [Google Scholar] [CrossRef]
- Bouaziz, O.; Allain, S.; Scott, C.P.P.; Cugy, P.; Barbier, D. High manganese austenitic twinning induced plasticity steels: A review of the microstructure properties relationships. Curr. Opin. Solid State Mater. Sci. 2011, 15, 141–168. [Google Scholar] [CrossRef]
- Keeler, S.; Kimchi, M.; Mooney, P.J. Advance High-Strength Steel Applications Guidelines, Version 6.0; World Auto Steel: Middletown, OH, USA, 2017. [Google Scholar]
- Barbier, D.; Gey, N.; Allain, S.; Bozzolo, N.; Humbert, M. Analysis of the tensile behavior of a TWIP steel based on the texture and microstructure evolutions. Mater. Sci. Eng. A 2009, 500, 196–206. [Google Scholar] [CrossRef]
- Idrissi, H.; Renard, K.; Ryelandt, L.; Schryvers, D.; Jacques, P.J. On the mechanism of twin formation in Fe–Mn–C TWIP steels. Acta Mater. 2010, 58, 2464–2476. [Google Scholar] [CrossRef]
- Misra, R.D.K.; Kumar, B.R.; Somani, M.; Karjalainen, P. Deformation processes during tensile straining of ultrafine/nanograined structures formed by reversion in metastable austenitic steels. Scr. Mater. 2008, 59, 79–82. [Google Scholar] [CrossRef]
- Grässel, O.; Krüger, L.; Frommeyer, G.; Meyer, L.W. High strength Fe-Mn-(Al, Si) TRIP/TWIP steels development—Properties—Application. Int. J. Plast. 2000, 16, 1391–1409. [Google Scholar] [CrossRef]
- Gutierrez-Urrutia, I.; Zaefferer, S.; Raabe, D. The effect of grain size and grain orientation on deformation twinning in a Fe–22wt.% Mn–0.6wt.% C TWIP steel. Mater. Sci. Eng. A 2010, 527, 3552–3560. [Google Scholar] [CrossRef]
- Vercammen, S.; Blanpain, B.; de Cooman, B.C.; Wollants, P. Cold rolling behaviour of an austenitic Fe–30Mn–3Al–3Si TWIP-steel: The importance of deformation twinning. Acta Mater. 2004, 52, 2005–2012. [Google Scholar] [CrossRef]
- Soulami, A.; Choi, K.S.; Shen, Y.F.; Liu, W.N.; Sun, X.; Khaleel, M.A. On deformation twinning in a 17.5% Mn–TWIP steel: A physically based phenomenological model. Mater. Sci. Eng. A 2011, 528, 1402–1408. [Google Scholar] [CrossRef]
- Yu, Q.; Shan, Z.-W.; Li, J.; Huang, X.; Xiao, L.; Sun, J.; Ma, E. Strong crystal size effect on deformation twinning. Nature 2010, 463, 335–338. [Google Scholar] [CrossRef]
- Wei, Y.; Li, Y.; Zhu, L.; Liu, Y.; Lei, X.; Wang, G.; Wu, Y.; Mi, Z.; Liu, J.; Wang, H.; et al. Evading the strength–ductility trade-off dilemma in steel through gradient hierarchical nanotwins. Nat. Commun. 2014, 5, 3580. [Google Scholar] [CrossRef]
- Li, Z.; Pradeep, K.G.; Deng, Y.; Raabe, D.; Tasan, C.C. Metastable high-entropy dual-phase alloys overcome the strength–ductility trade-off. Nature 2016, 534, 227–230. [Google Scholar] [CrossRef]
- Lu, K.; Lu, L.; Suresh, S. Strengthening materials by engineering coherent internal boundaries at the nanoscale. Science 2009, 324, 349–352. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Choi, C. Driving force for γ → ε martensitic transformation and stacking fault energy of γ in Fe-Mn binary system. Metall. Mater. Trans. A 2000, 31, 355–360. [Google Scholar] [CrossRef]
- Mahajan, S.; Chin, G.Y. Formation of deformation twins in f.c.c. crystals. Acta Metall. 1973, 21, 1353–1363. [Google Scholar] [CrossRef]
- Sato, K.; Tanaka, K.; Inoue, Y. Determination of the α/γ equilibrium in the iron rich portion of the Fe-Mn-Al system. ISIJ Int. 1989, 29, 788–792. [Google Scholar] [CrossRef]
- Sugimoto, K.; Kikuchi, R.; Hashimoto, S. Development of high strength low alloy TRIP-aided steels with annealed martensite matrix. Steel Res. 2002, 73, 253–258. [Google Scholar] [CrossRef]
- Dumay, A.; Chateau, J.-P.P.; Allain, S.; Migot, S.; Bouaziz, O. Influence of addition elements on the stacking-fault energy and mechanical properties of an austenitic Fe–Mn–C steel. Mater. Sci. Eng. A 2008, 483–484, 184–187. [Google Scholar] [CrossRef]
- Yoo, J.D.; Park, K.-T.T. Microband-induced plasticity in a high Mn–Al–C light steel. Mater. Sci. Eng. A 2008, 496, 417–424. [Google Scholar] [CrossRef]
- Cheng, W.-C.; Lin, Y.-S.; Chen, K.-F. The formation of ferrite quenching twins in a body-centered cubic Fe–Mn–Al alloy during high-temperature quenching. Scr. Mater. 2014, 81, 36–39. [Google Scholar] [CrossRef]
- Cheng, W.-C.; Lai, C.-K. Observing massive phase transformation in a Fe–Mn–Al alloy. Scr. Mater. 2006, 55, 783–786. [Google Scholar] [CrossRef]
- Charles, J.; Berghézan, A.; Lutts, A. Structure and mechanical properties of high-alloy manganese-aluminum steels. J. Phys. Colloq. 1982, 43, C4–C435. [Google Scholar] [CrossRef]
- Kim, Y.G.; Park, Y.S.; Han, J.K. Low temperature mechanical behavior of microalloyed and controlled-rolled Fe-Mn-Al-C-X alloys. Metall. Trans. A 1985, 16, 1689–1693. [Google Scholar] [CrossRef]
- Kim, Y.G.; Han, J.M.; Lee, J.S. Composition and temperature dependence of tensile properties of austenitic Fe–Mn–Al–C alloys. Mater. Sci. Eng. A 1989, 114, 51–59. [Google Scholar] [CrossRef]
- Schramm, R.E.; Reed, R.P. Stacking fault energies of seven commercial austenitic stainless steels. Metall. Trans. A 1975, 6, 1345–1351. [Google Scholar] [CrossRef]
- Rosalbino, F.; Carlini, R.; Parodi, R.; Zanicchi, G.; Scavino, G. Investigation of passivity and its breakdown on Fe3Al–Si and Fe3Al–Ge intermetallics in chloride-containing solution. Corros. Sci. 2014, 85, 394–400. [Google Scholar] [CrossRef]
- Park, J.H.; Seo, H.S.; Kim, K.Y.; Kim, S.J. The effect of Cr on the electrochemical corrosion of high Mn steel in a sweet environment. J. Electrochem. Soc. 2016, 163, C791–C797. [Google Scholar] [CrossRef]
- Roncery, L.M.; Weber, S.; Theisen, W. Development of Mn-Cr-(C-N) corrosion resistant twinning induced plasticity steels: Thermodynamic and diffusion calculations, production, and characterization. Metall. Mater. Trans. A 2010, 41, 2471–2479. [Google Scholar] [CrossRef]
- Jiang, B.; Qi, X.; Yang, S.; Zhou, W.; Hsu, T.Y. Effect of stacking fault probability on γ–ε martensitic transformation and shape memory effect in Fe–Mn–Si based alloys. Acta Mater. 1998, 46, 501–510. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Jun, J.-H.; Choi, C.-S. Damping capacity in Fe-Mn binary alloys. ISIJ Int. 1997, 37, 1023–1030. [Google Scholar] [CrossRef]
- Bastidas, D.M.; Medina, S.F.; Ress, J.; Jiménez, J.A.; Bastidas, J.M. Revealing austenite stability in Fe–30Mn–5Al–0.5C twinning-induced plasticity steel using differential thermal analysis. Steel Res. Int. 2020, 91, 2000076. [Google Scholar] [CrossRef]
- Frommeyer, G.; Brüx, U.; Neumann, P. Supra-ductile and high-strength manganese-TRIP/TWIP steels for high energy absorption purposes. ISIJ Int. 2003, 43, 438–446. [Google Scholar] [CrossRef]
- Frommeyer, G.; Grässel, O. Light Constructional Steel and the Use Thereof. Patent PCT/EP98/04044, WO 99/01585 A1, 1998. [Google Scholar]
- Dai, Y.; Tang, D.; Mi, Z.; Lü, J. Microstructure characteristics of an Fe-Mn-C TWIP steel after deformation. J. Iron Steel Res. Int. 2010, 17, 53–59. [Google Scholar] [CrossRef]
- Karaman, I.; Sehitoglu, H.; Beaudoin, A.; Chumlyakov, Y.; Maier, H.; Tomé, C. Modeling the deformation behavior of Hadfield steel single and polycrystals due to twinning and slip. Acta Mater. 2000, 48, 2031–2047. [Google Scholar] [CrossRef]
- ASTM. A128/A128M-19, Standard Specification for Steel Castings, Austenitic Manganese; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar] [CrossRef]
- Banerji, S.K. An austenitic stainless steel without nickel and chromium. Trans. Indian Inst. Met. 1977, 30, 186–189. [Google Scholar]
- World Auto Steel. Future Steel Vehicle Steel Technology Assessment and Design Methodology Interim Report; World Auto Steel: Middletown, OH, USA, 2010. [Google Scholar]
- Zhang, Y.; Zhu, X. Electrochemical polarization and passive film analysis of austenitic Fe–Mn–Al steels in aqueous solutions. Corros. Sci. 1999, 41, 1817–1833. [Google Scholar] [CrossRef]
- Cavallini, M.; Felli, F.; Fratesi, R.; Veniaii, F. Aqueous solution corrosion behaviour of “poor man” high manganese-aluminum steels. Mater. Corros. 1982, 33, 281–284. [Google Scholar] [CrossRef]
- Pourbaix, M.; Deltombe, E.C.; Vanleugenhagle, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; Elsevier: Oxford, UK, 1966. [Google Scholar]
- Delpech, S.; Cannes, C.; Barré, N.; Tran, Q.T.; Sanchez, C.; Lahalle, H.; Lambertin, D.; Gauffinet, S.; Coumes, C.C.D. Kinetic model of aluminum behavior in cement-based matrices analyzed by impedance spectroscopy. J. Electrochem. Soc. 2017, 164, C717–C727. [Google Scholar] [CrossRef]
- Tjong, S.C. Aqueous corrosion properties of austenitic Fe-8.7Al-29.7Mn-1.04C alloy. Surf. Coat. Technol. 1986, 28, 181–186. [Google Scholar] [CrossRef]
- Altstetter, C.J.; Bentley, A.P.; Fourie, J.W.; Kirkbride, A.N. Processing and properties of Fe-Mn-Al alloys. Mater. Sci. Eng. 1986, 82, 13–25. [Google Scholar] [CrossRef]
- Gau, Y.J.; Wu, J.K. Galvanic corrosion behaviour of Fe-Mn-Al alloys in sea water. J. Mater. Sci. Lett. 1992, 11, 119–121. [Google Scholar] [CrossRef]
- Park, I.-J.; Lee, S.-M.; Kang, M.; Lee, S.; Lee, Y.-K. Pitting corrosion behavior in advanced high strength steels. J. Alloys Compd. 2015, 619, 205–210. [Google Scholar] [CrossRef]
- Ruščăk, M.; Perng, T.-P. Effect of ferrite on corrosion of Fe-Mn-Al alloys in sodium chloride solution. Corrosion 1995, 51, 738–743. [Google Scholar] [CrossRef]
- Shih, S.T.; Tai, C.Y.; Perng, T.P. Corrosion behavior of two-phase Fe-Mn-Al alloys in 3.5% NaCl solution. Corrosion 1993, 49, 130–134. [Google Scholar] [CrossRef]
- Chen, P.-C.; Chao, C.-G.; Liu, T.-F. A novel high-strength, high-ductility and high-corrosion-resistance FeAlMnC low-density alloy. Scr. Mater. 2013, 68, 380–383. [Google Scholar] [CrossRef]
- Bastidas, J.M.; Polo, J.L.; Torres, C.L.; Cano, E. A stochastic approach to study localized corrosion of AISI 304L and AISI 316L stainless steels as a function of potential scan rate. Corrosion 2001, 57, 666–669. [Google Scholar] [CrossRef]
- Zhu, X.M.; Zhang, Y.S. An XPS study of passive film formation on Fe 30Mn 9Al alloy in sodium sulphate solution. Appl. Surf. Sci. 1998, 125, 11–16. [Google Scholar] [CrossRef]
- Hamada, A.S.; Karjalainen, L.P. Corrosion behaviour of high-Mn TWIP steels with electroless Ni-P coating. Open Corros. J. 2010, 3, 1–6. [Google Scholar] [CrossRef]
- Yang, M.; Yue, L.; Xie, K.; Zhang, S.; Sun, Y.; Tan, Y. Study on corrosion behavior of TWIP steel and properties of surface electroless Ni-P coatings. Mater. Res. E Express 2020, 7, 036517. [Google Scholar] [CrossRef]
- Hamada, A.S.; Karjalainen, L.P.; El-Zeky, M.A. Effect of anodic passivation on the corrosion behaviour of Fe-Mn-Al steels in 3.5%NaCl. In Passivation of Metals and Semiconductors, and Properties of Thin Oxide Layers; Marcus, P., Maurice, V., Eds.; Elsevier: New York, NY, USA, 2006; pp. 77–82. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Duh, J.-B.; Lee, J.-T. Electrochemical polarization and pitting behaviour of Fe-Al-Mn alloys in chloride solutions. J. Mater. Sci. 1987, 22, 3517–3521. [Google Scholar] [CrossRef]
- Jabłońska, M.; Michalik, R. Studies on the corrosion properties of high-Mn austenitic steels. Solid State Phenom. 2015, 227, 75–78. [Google Scholar] [CrossRef]
- Heger, J.J. Austenitic iron-aluminum-manganese alloys as possible substitutes for austenitic stainless steels. J. Test. Eval. 1985, 13, 446. [Google Scholar] [CrossRef]
- Kolman, D.G.; Ford, D.K.; Butt, D.P.; Nelson, T.O. Corrosion of 304 stainless steel exposed to nitric acid-chloride environments. Corros. Sci. 1997, 39, 2067–2093. [Google Scholar] [CrossRef]
- Wang, W.; Wang, D.; Han, F. Improvement of corrosion resistance of twinning-induced plasticity steel by hot-dipping aluminum with subsequent thermal diffusion treatment. Mater. Lett. 2019, 248, 60–64. [Google Scholar] [CrossRef]
- Hamada, A.S.; Karjalainen, L.P. Nitric acid resistance of new type Fe-Mn-Al stainless steels. Can. Metall. Q. 2006, 45, 41–48. [Google Scholar] [CrossRef]
- Fajardo, S.; Llorente, I.; Jiménez, J.A.; Bastidas, J.M.; Bastidas, D.M. Effect of Mn additions on the corrosion behaviour of TWIP Fe-Mn-Al-Si austenitic steel in chloride solution. Corros. Sci. 2019, 154, 246–253. [Google Scholar] [CrossRef]
- Tuan, Y.H.; Wang, C.S.; Tsai, C.Y.; Chao, C.G.; Liu, T.F. Corrosion behaviors of austenitic Fe–30Mn–7Al–xCr–1C alloys in 3.5% NaCl solution. Mater. Chem. Phys. 2009, 114, 595–598. [Google Scholar] [CrossRef]
- Fajardo, S.; Llorente, I.; Jiménez, J.A.; Calderón, N.; Herrán-Medina, D.; Bastidas, J.M.; Ress, J.; Bastidas, D.M. Influence of chromium on the passivity of thermo-mechanically processed high-Mn TWIP steels. Appl. Surf. Sci. 2020, 513, 145852. [Google Scholar] [CrossRef]
- Martin, U.; Ress, J.; Bosch, J.; Bastidas, D.M. Effect of thermo-mechanical processing on the corrosion behavior of Fe−30Mn−5Al−0.5C TWIP steel. Appl. Sci. 2020, 10, 9104. [Google Scholar] [CrossRef]
- Orazem, M.E.; Miller, M.G. The distribution of current and formation of a salt film on an iron disk below the passivation potential. J. Electrochem. Soc. 1987, 134, 392–399. [Google Scholar] [CrossRef]
- Bosch, J.; Martin, U.; Aperador, W.; Bastidas, J.M.; Ress, J.; Bastidas, D.M. Corrosion behavior of high-Mn austenitic Fe-Mn-Al-Cr TWIP steel in NaCl and NaOH solutions. Materials 2021, 14, 425. [Google Scholar] [CrossRef]
- Mujica, L.; Weber, S.; Theisen, W. Development of high-strength corrosion-resistant austenitic TWIP steel. Metall. Ital. 2011, 6, 31–35. [Google Scholar]
- Zhu, X.M.; Zhang, Y.S. Investigation of the electrochemical corrosion behavior and passive film of Fe-Mn, Fe-Mn-Al, and Fe-Mn-Al-Cr alloys in aqueous solution. Corrosion 1998, 54, 3–12. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, X.; Liu, M.; Che, R. Effects of anodic passivation on the constitution, stability and resistance to corrosion of passive film formed on an Fe-24Mn-4Al-5Cr alloy. Appl. Surf. Sci. 2004, 222, 89–101. [Google Scholar] [CrossRef]
- Schinhammer, M.; Steiger, P.; Moszner, F.; Löffler, J.F.; Uggowitzer, P.J. Degradation performance of biodegradable FeMnC (Pd) alloys. Mater. Sci. Eng. C 2013, 33, 1882–1893. [Google Scholar] [CrossRef]
- Zhu, S.M.; Tjong, S.C. Serrated flow in Fe-28Mn-9Al-xC alloys in a temperature range of 573–873 K. Scr. Mater. 1997, 36, 317–321. [Google Scholar] [CrossRef]
- Tjong, S.C.; Ku, J.S.; Wu, C.S. Corrosion behavior of laser consolidated chromium and molybdenum plasma spray coatings on Fe-28Mn-7Al-1C alloy. Scr. Metall. Mater. 1994, 31, 835–839. [Google Scholar] [CrossRef]
- Tjong, S.C. Performance of laser-consolidated plasma-spray coatings on Fe-28Mn-7A1-1C alloy. Thin Solid Films 1996, 274, 95–100. [Google Scholar] [CrossRef]
- Lee, J.-W.; Duh, J.-G.; Tsai, S.-Y. Corrosion resistance and microstructural evaluation of the chromized coating process in a dual phase Fe-Mn-Al-Cr alloy. Surf. Coat. Technol. 2002, 153, 59–66. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, X.; Zhong, S. Effect of alloying elements on the electrochemical polarization behavior and passive film of Fe–Mn base alloys in various aqueous solutions. Corros. Sci. 2004, 46, 853–876. [Google Scholar] [CrossRef]
- Dieudonné, T.; Marchetti, L.; Wery, M.; Miserque, F.; Tabarant, M.; Chêne, J.; Allely, C.; Cugy, P.; Scott, C.P.P. Role of copper and aluminum on the corrosion behavior of austenitic Fe–Mn–C TWIP steels in aqueous solutions and the related hydrogen absorption. Corros. Sci. 2014, 83, 234–244. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Lin, C.-L.; Chao, C.-G.; Liu, T.-F. Excellent enhancement of corrosion properties of Fe–9Al–30Mn–1.8C alloy in 3.5% NaCl and 10% HCl aqueous solutions using gas nitriding treatment. J. Alloys Compd. 2015, 633, 137–144. [Google Scholar] [CrossRef]
- Yuan, X.; Zhao, Y.; Li, X.; Chen, L. Effect of Cr on mechanical properties and corrosion behaviors of Fe-Mn-C-Al-Cr-N TWIP steels. J. Mater. Sci. Technol. 2017, 33, 1555–1560. [Google Scholar] [CrossRef]
- Wang, C.S.; Tsai, C.Y.; Chao, C.G.; Liu, T.F. Effect of chromium content on corrosion behaviors of Fe-9Al-30Mn-(3, 5, 6.5, 8) Cr-1C alloys. Mater. Trans. 2007, 48, 2973–2977. [Google Scholar] [CrossRef]
- Tsay, G.; Lin, C.; Chao, C.; Liu, T. A new austenitic FeMnAlCrC alloy with high-strength, high-ductility, and moderate corrosion resistance. Mater. Trans. 2010, 51, 2318–2321. [Google Scholar] [CrossRef]
- Zhu, X.M.; Zhang, Y.S. Electrochemical polarisation and passive film of austenitic Fe-Mn-Cr-Al alloy in aqueous solution. Br. Corros. J. 1997, 32, 127–132. [Google Scholar] [CrossRef]
- Duh, J.-G.; Lee, J.W.; Wang, C.-J. Microstructural development in the oxidation-induced phase transformation of Fe-Al-Cr-Mn-C alloys. J. Mater. Sci. 1988, 23, 2649–2660. [Google Scholar] [CrossRef]
- Charles, J.; Berghezan, A.; Lutts, A.; Dancoisne, P.L. New cryogenic material: Fe-Mn-Al alloys. Met. Prog. 1981, 119, 71–74. [Google Scholar]
- Chou, J.S.; Chao, C.G. Tensile properties of a DO3− containing Fe–Mn–Al–Si–C alloy at elevated temperatures. Scr. Metall. Mater. 1992, 26, 1417–1421. [Google Scholar] [CrossRef]
- Wang, R.; Beck, F.H. New stainless steel without nickel or chromium for marine applications. Met. Prog. 1983, 123, 72–76. [Google Scholar]
- Kannan, M.B.; Raman, R.K.S.; Khoddam, S. Comparative studies on the corrosion properties of a Fe–Mn–Al–Si steel and an interstitial-free steel. Corros. Sci. 2008, 50, 2879–2884. [Google Scholar] [CrossRef]
- Lins, V.F.C.; Freitas, M.A.; Silva, E.M.P. Corrosion resistance study of Fe–Mn–Al–C alloys using immersion and potentiostatic tests. Appl. Surf. Sci. 2005, 250, 124–134. [Google Scholar] [CrossRef]
- Kwon, H.; Park, K. Effects of manganese on the passivity of Fe-18Cr-xMn (x = 0, 6, 12). ECS Trans. 2019, 1, 313–320. [Google Scholar] [CrossRef]
- Roberge, P.R. Handbook of Corrosion Engineering; McGraw-Hill: New York, NY, USA, 1999. [Google Scholar]
- Abuzriba, M.B.; Musa, S.M. Substitution for Chromium and Nickel in Austenitic Stainless Steels. In 2nd International Multidisciplinary Microscopy and Microanalysis Congress; Polychroniadis, E., Oral, A., Ozer, M., Eds.; Springer: Cham, Switzerland, 2015; Volume 164, pp. 205–214. [Google Scholar]
- Moon, K.M.; Kim, D.A.; Kim, Y.H.; Lee, M.H. Effect of Mn content on corrosion characteristics of lean Mn TWIP steel. Int. J. Mod. Phys. B 2018, 32, 1840083. [Google Scholar] [CrossRef]
- Dieudonné, T.; Marchetti, L.; Wery, M.; Chêne, J.; Allely, C.; Cugy, P.; Scott, C.P. Role of copper and aluminum additions on the hydrogen embrittlement susceptibility of austenitic Fe–Mn–C TWIP steels. Corros. Sci. 2014, 82, 218–226. [Google Scholar] [CrossRef]
- Gavriljuk, V.G.; Shanina, B.D.; Berns, H. Ab initio development of a high-strength corrosion-resistant austenitic steel. Acta Mater. 2008, 56, 5071–5082. [Google Scholar] [CrossRef]
- Setiadi, A.; Milestone, N.B.; Hill, J.; Hayes, M. Corrosion of aluminium and magnesium in BFS composite cements. Adv. Appl. Ceram. 2006, 105, 191–196. [Google Scholar] [CrossRef]
- Wan, H.; Cai, Y.; Song, D.; Chen, C. Effect of Cr/Mo carbides on corrosion behaviour of Fe-Mn-C twinning induced plasticity steel. Corros. Sci. 2020, 167, 108518. [Google Scholar] [CrossRef]
- Gómez, J.A.M.; Antonissen, J.; Palacio, C.A.; de Grave, E. Effects of Si as alloying element on corrosion resistance of weathering steel. Corros. Sci. 2012, 59, 198–203. [Google Scholar] [CrossRef]
- Li, D.G.; Wang, J.D.; Chen, D.R.; Liang, P. Molybdenum addition enhancing the corrosion behaviors of 316 L stainless steel in the simulated cathodic environment of proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2015, 40, 5947–5957. [Google Scholar] [CrossRef]
- Kannan, M.B.; Raman, R.K.S.; Khoddam, S.; Liyanaarachchi, S. Corrosion behavior of twinning-induced plasticity (TWIP) steel. Mater. Corros. 2013, 64, 231–235. [Google Scholar] [CrossRef]
- Peguet, L.; Malki, B.; Baroux, B. Influence of cold working on the pitting corrosion resistance of stainless steels. Corros. Sci. 2007, 49, 1933–1948. [Google Scholar] [CrossRef]
- Fu, Y.; Wu, X.; Han, E.-H.; Ke, W.; Yang, K.; Jiang, Z. Effects of cold work and sensitization treatment on the corrosion resistance of high nitrogen stainless steel in chloride solutions. Electrochim. Acta 2009, 54, 1618–1629. [Google Scholar] [CrossRef]
- Mudali, U.K.; Shankar, P.; Ningshen, S.; Dayal, R.K.; Khatak, H.S.; Raj, B. On the pitting corrosion resistance of nitrogen alloyed cold worked austenitic stainless steels. Corros. Sci. 2002, 44, 2183–2198. [Google Scholar] [CrossRef]
- Grajcar, A. Corrosion Resistance of High-Mn Austenitic Steels for the Automotive Industry. In Corrosion Resistance; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Wang, K.; Wei, A.; Tong, X.; Lin, J.; Jin, L.; Zhong, X.; Wang, D. Improvement of the anti-corrosion property of twinning-induced plasticity steel by twin-induced grain boundary engineering. Mater. Lett. 2018, 211, 118–121. [Google Scholar] [CrossRef]
- Grajcar, A.; Kciuk, M.; Topolska, S.; Płachcińska, A. Microstructure and corrosion behavior of hot-deformed and cold-strained high-Mn steels. J. Mater. Eng. Perform. 2016, 25, 2245–2254. [Google Scholar] [CrossRef]
- Ghayad, I.M.; Hamada, A.S.; Girgis, N.N.; Ghanem, W.A. Effect of cold working on the aging and corrosion behaviour of Fe-Mn-Al stainless steel. Steel GRIPS 2006, 4, 133–137. [Google Scholar]
- Bastidas, D.M. Interpretation of impedance data for porous electrodes and diffusion processes. Corrosion 2007, 63, 515–521. [Google Scholar] [CrossRef]
- Bastidas, J.; Polo, J.; Torres, C.; Cano, E. A study on the stability of AISI 316L stainless steel pitting corrosion through its transfer function. Corros. Sci. 2001, 43, 269–281. [Google Scholar] [CrossRef]
- Karaman, I.; Sehitoglu, H.; Gall, K.; Chumlyakov, Y.; Maier, H. Deformation of single crystal Hadfield steel by twinning and slip. Acta Mater. 2000, 48, 1345–1359. [Google Scholar] [CrossRef]
- Speidel, M.O. High Nitrogen Steels. In Proceedings of the International Conference on High Nitrogen Steels HNS 88, Lille, France, 18–20 May 1988; Foct, J., Hendry, A., Eds.; The Institute of Metals: London, UK, 1989; p. 251. [Google Scholar]
- Ralston, K.D.; Birbilis, N. Effect of grain size on corrosion: A review. Corrosion 2010, 66, 075005–075013. [Google Scholar] [CrossRef]
- Dini, G.; Najafizadeh, A.; Ueji, R.; Monir-Vaghefi, S.M. Tensile deformation behavior of high manganese austenitic steel: The role of grain size. Mater. Des. 2010, 31, 3395–3402. [Google Scholar] [CrossRef]
- Yeganeh, M.; Eskandari, M.; Alavi-Zaree, S.R. A comparison between corrosion behaviors of fine-grained and coarse-grained structures of high-Mn steel in NaCl solution. J. Mater. Eng. Perform. 2017, 26, 2484–2490. [Google Scholar] [CrossRef]
- Ueji, R.; Tsuchida, N.; Terada, D.; Tsuji, N.; Tanaka, Y.; Takemura, A.; Kunishige, K. Tensile properties and twinning behavior of high manganese austenitic steel with fine-grained structure. Scr. Mater. 2008, 59, 963–966. [Google Scholar] [CrossRef]
- Lu, C.-W.; Lu, Y.-S.; Lai, Z.-H.; Yen, H.-W.; Lee, Y.-L. Comparative corrosion behavior of Fe50Mn30Co10Cr10 dual-phase high-entropy alloy and CoCrFeMnNi high-entropy alloy in 3.5 wt% NaCl solution. J. Alloys Compd. 2020, 842, 155824. [Google Scholar] [CrossRef]
- So, K.; Kim, J.; Chun, Y.; Park, K.-T.; Lee, Y.-K.; Lee, C.S. Hydrogen delayed fracture properties and internal hydrogen behavior of a Fe–18Mn–1.5Al–0.6C TWIP Steel. ISIJ Int. 2009, 49, 1952–1959. [Google Scholar] [CrossRef]
- Park, I.-J.; Lee, S.; Jeon, H.; Lee, Y.-K. The advantage of grain refinement in the hydrogen embrittlement of Fe–18Mn–0.6C twinning-induced plasticity steel. Corros. Sci. 2015, 93, 63–69. [Google Scholar] [CrossRef]
- Sun, M.; Xiao, K.; Dong, C.; Li, X.; Zhong, P. Stress corrosion cracking of ultrahigh strength martensite steel Cr9Ni5MoCo14 in 3.5% NaCl solution. Aerosp. Sci. Technol. 2014, 36, 125–131. [Google Scholar] [CrossRef]
- Eliaz, N.; Shachar, A.; Tal, B.; Eliezer, D. Characteristics of hydrogen embrittlement, stress corrosion cracking and tempered martensite embrittlement in high-strength steels. Eng. Fail. Anal. 2002, 9, 167–184. [Google Scholar] [CrossRef]
- Jin, L.Z. The chloride stress-corrosion cracking behavior of stainless steels under different test methods. J. Mater. Eng. Perform. 1994, 3, 734–739. [Google Scholar] [CrossRef]
- Tjong, S.C. Stress corrosion cracking of the austenitic Fe-Al-Mn alloy in chloride environment. Mater. Corros. 1986, 37, 444–447. [Google Scholar] [CrossRef]
- Huang, Y.-J.; Yamaguchi, T.; Murai, H.; Sugino, H.; Nakajima, T.; Nono, M.; Kawakita, K.; Kimura, A. SCC susceptibility of solution-annealed 316L SS in hydrogenated hot water below 288 °C. Corros. Sci. 2018, 145, 1–9. [Google Scholar] [CrossRef]
- Juang, H.K.; Altstetter, C. Effect of pH and chloride contents on stress corrosion cracking of austenitic stainless steels at room temperature. Corrosion 1990, 46, 881–887. [Google Scholar] [CrossRef]
- Prosek, T.; Gac, A.L.; Thierry, D.; Manchet, S.L.; Lojewski, C.; Fanica, A.; Johansson, E.; Canderyd, C.; Dupoiron, F.; Snauwaert, T.; et al. Low-Temperature stress corrosion cracking of austenitic and duplex stainless steels under chloride deposits. Corrosion 2014, 70, 1052–1063. [Google Scholar] [CrossRef]
- Chang, S.C.; Liu, J.Y.; Juang, H.K. Environment-assisted cracking of Fe-32% Mn-9% Al alloys in 3.5% sodium chloride solution. Corrosion 1995, 51, 399–406. [Google Scholar] [CrossRef]
- Tjong, S.C.; Wu, C.S. The microstructure and stress corrosion cracking behaviour of precipitation-hardened Fe-8.7Al-29.7Mn-1.04C alloy in 20% NaCl solution. Mater. Sci. Eng. 1986, 80, 203–211. [Google Scholar] [CrossRef]
- Ronevich, J.A.; Kim, S.K.; Speer, J.G.; Matlock, D.K. Hydrogen effects on cathodically charged twinning-induced plasticity steel. Scr. Mater. 2012, 66, 956–959. [Google Scholar] [CrossRef]
- Choi, H.; Kim, S.; Sung, H.; Kim, S.-J.; Kim, S. Effect of Cr and N on stress corrosion cracking behavior of Fe-18Mn steel. Korean, J. Met. Mater. 2019, 57, 624–631. [Google Scholar] [CrossRef]
- Ryu, J.H.; Kim, S.K.; Lee, C.S.; Suh, D.-W.; Bhadeshia, H.K.D.H. Effect of aluminium on hydrogen-induced fracture behaviour in austenitic Fe–Mn–C steel. Proc. R. Soc. A Math. Phys. Eng. Sci. 2013, 469, 20120458. [Google Scholar] [CrossRef]
- Han, D.K.; Kim, Y.M.; Han, H.N.; Bhadeshia, H.K.D.H.; Suh, D.-W. Hydrogen and aluminium in high-manganese twinning-induced plasticity steel. Scr. Mater. 2014, 80, 9–12. [Google Scholar] [CrossRef]
- Han, D.K.; Lee, S.K.; Noh, S.J.; Kim, S.-K.; Suh, D.-W. Effect of aluminium on hydrogen permeation of high-manganese twinning-induced plasticity steel. Scr. Mater. 2015, 99, 45–48. [Google Scholar] [CrossRef]
- Song, E.J.; Bhadeshia, H.K.D.H.; Suh, D.-W. Interaction of aluminium with hydrogen in twinning-induced plasticity steel. Scr. Mater. 2014, 87, 9–12. [Google Scholar] [CrossRef]
- Koyama, M.; Akiyama, E.; Tsuzaki, K. Effects of static and dynamic strain aging on hydrogen embrittlement in TWIP steels containing Al. ISIJ Int. 2013, 53, 1268–1274. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Q.; Venezuela, J.; Zhang, M.; Wang, J.; Atrens, A. A review of the influence of hydrogen on the mechanical properties of DP, TRIP, and TWIP advanced high-strength steels for auto construction. Corros. Rev. 2016, 34, 127–152. [Google Scholar] [CrossRef]
- Timmerscheidt, T.; Dey, P.; Bogdanovski, D.; von Appen, J.; Hickel, T.; Neugebauer, J.; Dronskowski, R. The role of κ-carbides as hydrogen traps in high-Mn steels. Metals 2017, 7, 264. [Google Scholar] [CrossRef]
- Park, I.-J.; Jo, S.Y.; Kang, M.; Lee, S.-M.; Lee, Y.-K. The effect of Ti precipitates on hydrogen embrittlement of Fe–18Mn–0.6C–2Al–xTi twinning-induced plasticity steel. Corros. Sci. 2014, 89, 38–45. [Google Scholar] [CrossRef]
- Haley, D.; Merzlikin, S.V.; Choi, P.; Raabe, D. Atom probe tomography observation of hydrogen in high-Mn steel and silver charged via an electrolytic route. Int. J. Hydrogen Energy 2014, 39, 12221–12229. [Google Scholar] [CrossRef]
- Tsu, I.F.; Perng, T.P. Hydrogen compatibility of Femnal alloys. Metall. Trans. A 1991, 22, 215–224. [Google Scholar] [CrossRef]
- Lovicu, G.; Bottazzi, M.; D’Aiuto, F.; De Sanctis, M.; Dimatteo, A.; Santus, C.; Valentini, R. Hydrogen embrittlement of automotive advanced high-strength steels. Metall. Mater. Trans. A 2012, 43, 4075–4087. [Google Scholar] [CrossRef]
- Michalska, J.; Chmiela, B.; Łabanowski, J.; Simka, W. Hydrogen damage in superaustenitic 904L stainless steels. J. Mater. Eng. Perform. 2014, 23, 2760–2765. [Google Scholar] [CrossRef]
- Robinson, M.J.; Kilgallon, P.J. Hydrogen embrittlement of cathodically protected high-strength, low-alloy steels exposed to sulfate reducing bacteria. Corrosion 1994, 50, 626–635. [Google Scholar] [CrossRef]
- Louthan, M.; Caskey, G.; Donovan, J.; Rawl, D. Hydrogen embrittlement of metals. Mater. Sci. Eng. 1972, 10, 357–368. [Google Scholar] [CrossRef]
- Scott, P.M. A review of environment-sensitive fracture in water reactor materials. Corros. Sci. 1985, 25, 583–606. [Google Scholar] [CrossRef]
- Koyama, M.; Akiyama, E.; Sawaguchi, T.; Ogawa, K.; Kireeva, I.V.; Chumlyakov, Y.I.; Tsuzaki, K. Hydrogen-assisted quasi-cleavage fracture in a single crystalline type 316 austenitic stainless steel. Corros. Sci. 2013, 75, 345–353. [Google Scholar] [CrossRef]
- Beachem, C.D. A new model for hydrogen-assisted cracking (hydrogen “embrittlement”). Metall. Mater. Trans. B 1972, 3, 441–455. [Google Scholar] [CrossRef]
- Koyama, M.; Akiyama, E.; Sawaguchi, T.; Raabe, D.; Tsuzaki, K. Hydrogen-induced cracking at grain and twin boundaries in an Fe–Mn–C austenitic steel. Scr. Mater. 2012, 66, 459–462. [Google Scholar] [CrossRef]
- Koyama, M.; Akiyama, E.; Tsuzaki, K. Hydrogen embrittlement in a Fe–Mn–C ternary twinning-induced plasticity steel. Corros. Sci. 2012, 54, 1–4. [Google Scholar] [CrossRef]
- Koyama, M.; Bashir, A.; Rohwerder, M.; Merzlikin, S.V.; Akiyama, E.; Tsuzaki, K.; Raabe, D. Spatially and kinetically resolved mapping of hydrogen in a twinning-induced plasticity steel by use of scanning Kelvin probe force microscopy. J. Electrochem. Soc. 2015, 162, C638–C647. [Google Scholar] [CrossRef]
- Koyama, M.; Akiyama, E.; Tsuzaki, K.; Raabe, D. Hydrogen-assisted failure in a twinning-induced plasticity steel studied under in situ hydrogen charging by electron channeling contrast imaging. Acta Mater. 2013, 61, 4607–4618. [Google Scholar] [CrossRef]
- Koyama, M.; Sawaguchi, T.; Tsuzaki, K. Quasi-cleavage fracture along annealing twin boundaries in a Fe–Mn–C austenitic steel. ISIJ Int. 2012, 52, 161–163. [Google Scholar] [CrossRef]
- Koyama, M.; Sawaguchi, T.; Tsuzaki, K. Premature fracture mechanism in an Fe-Mn-C austenitic steel. Metall. Mater. Trans. A 2012, 43, 4063–4074. [Google Scholar] [CrossRef]
- Koyama, M.; Akiyama, E.; Tsuzaki, K. Effect of hydrogen content on the embrittlement in a Fe–Mn–C twinning-induced plasticity steel. Corros. Sci. 2012, 59, 277–281. [Google Scholar] [CrossRef]
- Koyama, M.; Springer, H.; Merzlikin, S.V.; Tsuzaki, K.; Akiyama, E.; Raabe, D. Hydrogen embrittlement associated with strain localization in a precipitation-hardened Fe–Mn–Al–C light weight austenitic steel. Int. J. Hydrogen Energy 2014, 39, 4634–4646. [Google Scholar] [CrossRef]
- Koyama, M.; Akiyama, E.; Lee, Y.-K.; Raabe, D.; Tsuzaki, K. Overview of hydrogen embrittlement in high-Mn steels. Int. J. Hydrogen Energy 2017, 42, 12706–12723. [Google Scholar] [CrossRef]
- Chin, K.-G.; Kang, C.-Y.; Shin, S.Y.; Hong, S.; Lee, S.; Kim, H.S.; Kim, K.; Kim, N.J. Effects of Al addition on deformation and fracture mechanisms in two high manganese TWIP steels. Mater. Sci. Eng. A 2011, 528, 2922–2928. [Google Scholar] [CrossRef]
- Chun, Y.S.; Park, K.-T.T.; Lee, C.S. Delayed static failure of twinning-induced plasticity steels. Scr. Mater. 2012, 66, 960–965. [Google Scholar] [CrossRef]
- Park, I.-J.; Jeong, K.-H.; Jung, J.-G.; Lee, C.S.; Lee, Y.-K. The mechanism of enhanced resistance to the hydrogen delayed fracture in Al-added Fe–18Mn–0.6C twinning-induced plasticity steels. Int. J. Hydrogen Energy 2012, 37, 9925–9932. [Google Scholar] [CrossRef]
- Song, S.W.; Kwon, Y.J.; Lee, T.; Lee, C.S. Effect of Al addition on low-cycle fatigue properties of hydrogen-charged high-Mn TWIP steels. Mater. Sci. Eng. A 2016, 677, 421–430. [Google Scholar] [CrossRef]
- Noh, H.-S.; Kang, J.-H.; Kim, S.-J. Effect of grain size on hydrogen embrittlement in stable austenitic high-Mn TWIP and high-N stainless steels. Int. J. Hydrogen Energy 2019, 44, 25076–25090. [Google Scholar] [CrossRef]
- Jung, J.-K.; Lee, O.-Y.; Park, Y.-K.; Kim, D.-E.; Jin, K.-G. Hydrogen embrittlement behavior of high Mn TRIP/TWIP steels. Korean J. Mater. Res. 2008, 18, 394–399. [Google Scholar] [CrossRef]
- Ronevich, J.A.; de Cooman, B.C.; Speer, J.G.; de Moor, E.; Matlock, D.K. Hydrogen effects in prestrained transformation induced plasticity steel. Metall. Mater. Trans. A 2012, 43, 2293–2301. [Google Scholar] [CrossRef]
- Suh, D.-W. Critical assessment 2: Hydrogen induced fracture in austenitic, high-manganese TWIP steel. Mater. Sci. Technol. 2014, 30, 1131–1134. [Google Scholar] [CrossRef]
- Grajcar, A.; Krukiewiez, W.; Kołodziej, S. Corrosion behavior of plastically deformed high-Mn austenitic steels. J. Achiev. Mater. Manuf. Eng. 2010, 43, 228–235. [Google Scholar]
- Grajcar, A.; Kolodziej, S.; Krukiewiez, W. Corrosion resistance of high-manganese austenitic steels. Arch. Mater. Sci. Eng. 2010, 41, 77–84. [Google Scholar]
- Colla, V.; de Sanctis, M.; Dimatteo, A.; Lovicu, G.; Valentini, R. Prediction of continuous cooling transformation diagrams for dual-phase steels from the intercritical region. Metall. Mater. Trans. A 2011, 42, 2781–2793. [Google Scholar] [CrossRef]
- Fu, H.; Wang, W.; Zhao, H.; Jin, F.; Li, J. Study of hydrogen-induced delayed fracture in high-Mn TWIP/TRIP steels during in situ electrochemical hydrogen-charging: Role of microstructure and strain rate in crack initiation and propagation. Corros. Sci. 2020, 162, 108191. [Google Scholar] [CrossRef]
- Sheng, Z.; Altenbach, C.; Prahl, U.; Zander, D.; Bleck, W. Effect of cutting method on hydrogen embrittlement of high-Mn TWIP steel. Mater. Sci. Eng. A 2019, 744, 10–20. [Google Scholar] [CrossRef]
- Wang, D.; Lu, X.; Deng, Y.; Guo, X.; Barnoush, A. Effect of hydrogen on nanomechanical properties in Fe-22Mn-0.6C TWIP steel revealed by in-situ electrochemical nanoindentation. Acta Mater. 2019, 166, 618–629. [Google Scholar] [CrossRef]
- Otto, M.; John, D.; Schmidt-Juergensen, R.; Springub, B.; Cornelissen, M.; Berkhout, B.; Bracke, L.; Patel, J. HSD-Steels Optimized High Strength and High Ductility Austenitic Steel. In Proceedings of the 2nd International Conference Super-High Strength Steels, Peschiera del Garda, Italy, 17–20 October 2010. [Google Scholar]
- Shin, S.H.; Hong, S.; Kim, H.S.; Lee, S.; Kim, N.J. Tensile Properties and Cup Formability of High Mn and Al-Added TWIP Steels. In Proceedings of the 2nd International Conference Super-High Strength Steels, Peschiera del Garda, Italy, 17–20 October 2010; pp. 1–9. [Google Scholar]
- Xu, C.; Zhang, Y.; Liu, W.; Jin, Y.; Wen, L.; Sun, D. Influence of laser-welding on microstructure and corrosion properties of twinning-induced plasticity (TWIP) steel. Materials 2020, 13, 4315. [Google Scholar] [CrossRef]
- Monrrabal, G.; Jiménez, J.A.; Ress, J.; Fajardo, S.; Bastidas, J.M.; Llorente, I.; Bastidas, D.M. Corrosion behaviour of resistance-spot-welded high-Mn austenitic TWIP steel. Corros. Eng. Sci. Technol. 2020, 55, 1–10. [Google Scholar] [CrossRef]
- Herms, E.; Olive, J.; Puiggali, M. Hydrogen embrittlement of 316L type stainless steel. Mater. Sci. Eng. A 1999, 272, 279–283. [Google Scholar] [CrossRef]
- West, A.J.; Louthan, M.R. Dislocation transport and hydrogen embrittlement. Metall. Trans. A 1979, 10, 1675–1682. [Google Scholar] [CrossRef]
- Whiteman, M.B.; Troiano, A.R. Hydrogen embrittlement of austenitic stainless steels. Corrosion 1965, 21, 53–56. [Google Scholar] [CrossRef]
- Michler, T.; Marchi, C.S.; Naumann, J.; Weber, S.; Martin, M. Hydrogen environment embrittlement of stable austenitic steels. Int. J. Hydrogen Energy 2012, 37, 16231–16246. [Google Scholar] [CrossRef]
- Müllner, P. On the ductile to brittle transition in austenitic steel. Mater. Sci. Eng. A 1997, 234–236, 94–97. [Google Scholar] [CrossRef]
- Karaman, I.; Sehitoglu, H.; Maier, H.; Chumlyakov, Y. Competing mechanisms and modeling of deformation in austenitic stainless steel single crystals with and without nitrogen. Acta Mater. 2001, 49, 3919–3933. [Google Scholar] [CrossRef]
- Pérez, P.; Pérez, F.J.; Gómez, C.; Adeva, P. Oxidation behaviour of an austenitic Fe-30Mn-5Al-0.5C alloy. Corros. Sci. 2002, 44, 113–127. [Google Scholar] [CrossRef]
- Park, S.H.; Chung, I.S.; Kim, T.W. Characterization of the high-temperature oxidation behaviour of Fe-25Mm-5Al-0.5C alloy. Oxid. Met. 1998, 49, 349–371. [Google Scholar] [CrossRef]
- Tomaszewicz, P.; Wallwork, G.R. The oxidation of Fe-Al alloys containing chromium, nickel or manganese. Corrosion 1984, 40, 152–157. [Google Scholar] [CrossRef]
- Erhart, H.; Wang, R.; Rapp, R.A. In situ SEM study of the high-temperature oxidation of an Fe-Mn-Al-Si alloy. Oxid. Met. 1984, 21, 81–88. [Google Scholar] [CrossRef]
- Kao, C.H.; Wan, C.M. Effect of carbon on the oxidation of Fe-5.5Al-0.55 C alloy. J. Mater. Sci. 1987, 22, 3203–3208. [Google Scholar] [CrossRef]
- Kao, C.H.; Wan, C.M. Effect of temperature on the oxidation of Fe-7.5Al-0.65C alloy. J. Mater. Sci. 1988, 23, 1943–1947. [Google Scholar] [CrossRef]
- Pérez, P.; Garcés, G.; Pérez, F.J.; Gómez, C.; Adeva, P. Influence of chromium additions on the oxidation resistance of an austenitic Fe-30Mn-5Al alloy. Oxid. Met. 2002, 57, 339–361. [Google Scholar] [CrossRef]
- Lins, V.D.F.C.; Freitas, M.A.; Silva, E.S.D.P. Oxidation kinetics of an Fe–31.8Mn–6.09Al–1.60Si–0.40C alloy at temperatures from 600 to 900 °C. Corros. Sci. 2004, 46, 1895–1907. [Google Scholar] [CrossRef]
- Razmpoosh, M.H.; Biro, E.; Chen, D.L.; Goodwin, F.; Zhou, Y. Liquid metal embrittlement in laser lap joining of TWIP and medium-manganese TRIP steel: The role of stress and grain boundaries. Mater. Charact. 2018, 145, 627–633. [Google Scholar] [CrossRef]
- Saha, D.C.; Han, S.; Chin, K.G.; Choi, I.; Park, Y.-D. Weldability evaluation and microstructure analysis of resistance-spot-welded high-Mn steel in automotive applications. Steel Res. Int. 2012, 83, 352–357. [Google Scholar] [CrossRef]
- Zeytin, H.K.; Emre, H.E.; Kaçar, R. Properties of resistance spot-welded TWIP steels. Metals 2017, 7, 14. [Google Scholar] [CrossRef]
- Rossini, M.; Spena, P.R.; Cortese, L.; Matteis, P.; Firrao, D. Investigation on dissimilar laser welding of advanced high strength steel sheets for the automotive industry. Mater. Sci. Eng. A 2015, 628, 288–296. [Google Scholar] [CrossRef]
- Razmpoosh, M.H.; Shamanian, M.; Esmailzadeh, M. The microstructural evolution and mechanical properties of resistance spot welded Fe–31Mn–3Al–3Si TWIP steel. Mater. Des. 2015, 67, 571–576. [Google Scholar] [CrossRef]
- Roncery, L.M.; Weber, S.; Theisen, W. Welding of twinning-induced plasticity steels. Scr. Mater. 2012, 66, 997–1001. [Google Scholar] [CrossRef]
- Zambrano, O.A.; Aguilar, Y.; Valdés, J.; Rodríguez, S.A.; Coronado, J.J. Effect of normal load on abrasive wear resistance and wear micromechanisms in FeMnAlC alloy and other austenitic steels. Wear 2016, 348–349, 61–68. [Google Scholar] [CrossRef]
- Astudillo, A.P.C.; Soriano, G.A.F.; Osorio, G.M.B.; Sthepa, H.S.; Ramos, J.; Durán, J.F.; Alcázar, G.A.P. Comparative study of the mechanical and tribological properties of a Hadfield and a Fermanal steel. Hyperfine Interact. 2017, 238, 56. [Google Scholar] [CrossRef]
- Zambrano, O.A.; Valdés, J.; Rodriguez, L.A.; Reyes, D.; Snoeck, E.; Rodríguez, S.A.; Coronado, J.J. Elucidating the role of κ-carbides in Fe Mn Al C alloys on abrasion wear. Tribol. Int. 2019, 135, 421–431. [Google Scholar] [CrossRef]
- Mujica, L.; Jácome, L.A.; Aghajani, A.; Theisen, W.; Weber, S. Subsurface characterization of high-strength high-interstitial austenitic steels after impact wear. Wear 2018, 402–403, 137–147. [Google Scholar] [CrossRef]
- Hamada, A.S.; Sahu, P.; Porter, D.A. Indentation property and corrosion resistance of electroless nickel–phosphorus coatings deposited on austenitic high-Mn TWIP steel. Appl. Surf. Sci. 2015, 356, 1–8. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, Y.; Qin, X. Some aspects of high manganese twinning-induced plasticity (TWIP) steel, a review. Acta Metall. Sin. 2013, 26, 1–15. [Google Scholar] [CrossRef]
- Allam, T.; Guo, X.; Sevsek, S.; Lipińska-Chwałek, M.; Hamada, A.S.; Ahmed, E.; Bleck, W. Development of a Cr-Ni-V-N medium manganese steel with balanced mechanical and corrosion properties. Metals 2019, 9, 705. [Google Scholar] [CrossRef]
- Hermawan, H.; Mantovani, D. Process of prototyping coronary stents from biodegradable Fe–Mn alloys. Acta Biomater. 2013, 9, 8585–8592. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, P.; Hariharan, A.; Kini, A.; Kürnsteiner, P.; Raabe, D.; Jägle, E.A. Steels in additive manufacturing: A review of their microstructure and properties. Mater. Sci. Eng. A 2020, 772, 138633. [Google Scholar] [CrossRef]
- Chou, D.-T.; Wells, D.; Hong, D.; Lee, B.; Kuhn, H.; Kumta, P.N. Novel processing of iron–manganese alloy-based biomaterials by inkjet 3-D printing. Acta Biomater. 2013, 9, 8593–8603. [Google Scholar] [CrossRef] [PubMed]
- Niendorf, T.; Brenne, F.; Hoyer, P.; Schwarze, D.; Schaper, M.; Grothe, R.; Wiesener, M.; Grundmeier, G.; Maier, H.J. Processing of new materials by additive manufacturing: Iron-based alloys containing silver for biomedical applications. Metall. Mater. Trans. A 2015, 46, 2829–2833. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, W.; Yang, Y.; Pan, H.; He, C.; Qi, F.; Xie, D.; Liang, H. Selective laser melted Fe-Mn bone scaffold: Microstructure, corrosion behavior and cell response. Mater. Res. Express 2020, 7, 015404. [Google Scholar] [CrossRef]
- Hermawan, H.; Dubé, D.; Mantovani, D. Degradable metallic biomaterials: Design and development of Fe-Mn alloys for stents. J. Biomed. Mater. Res. Part. A 2009, 93, 1–11. [Google Scholar] [CrossRef]
- Heiden, M.; Kustas, A.; Chaput, K.; Nauman, E.; Johnson, D.; Stanciu, L. Effect of microstructure and strain on the degradation behavior of novel bioresorbable iron-manganese alloy implants. J. Biomed. Mater. Res. Part. A 2015, 103, 738–745. [Google Scholar] [CrossRef] [PubMed]

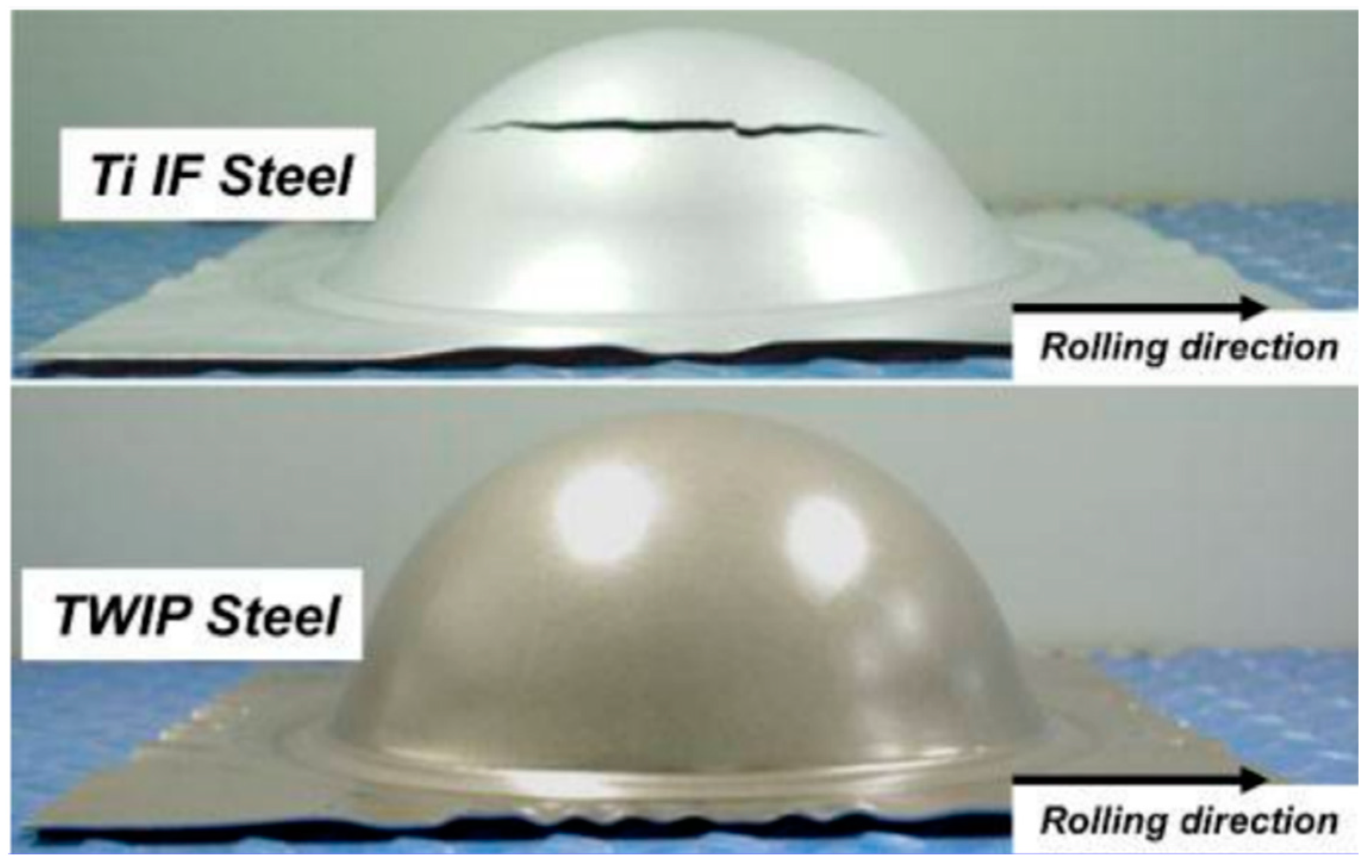
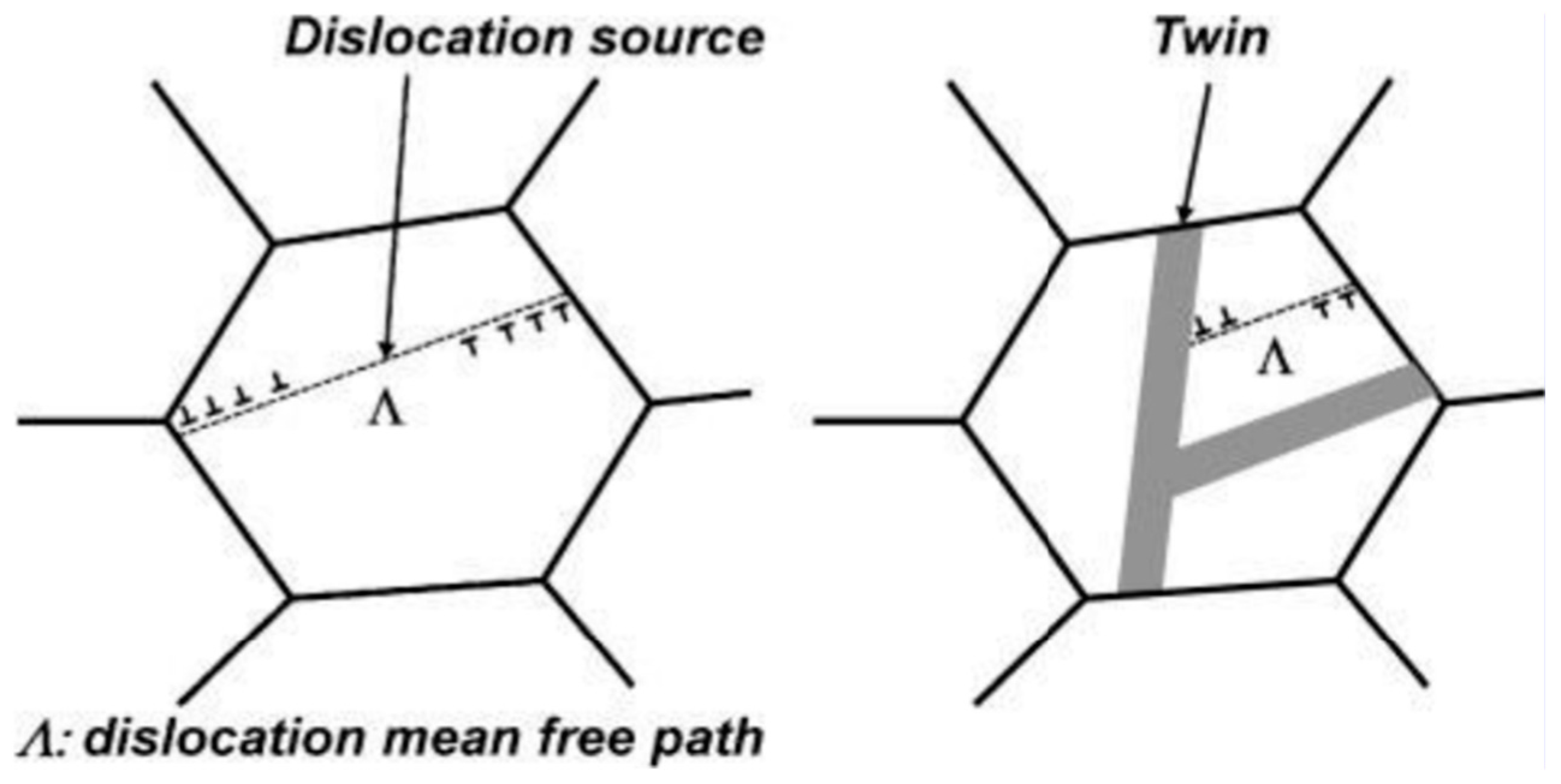
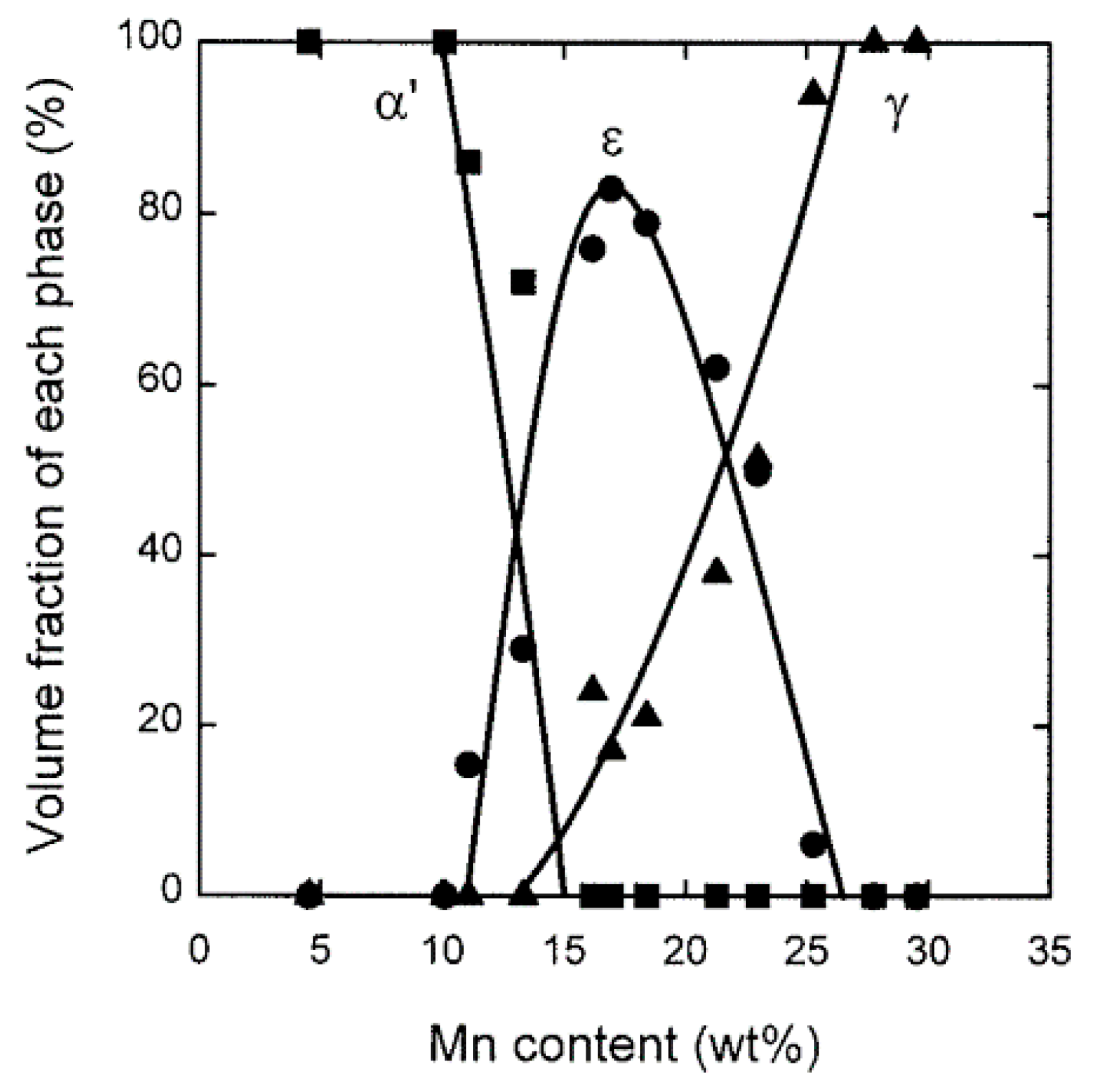
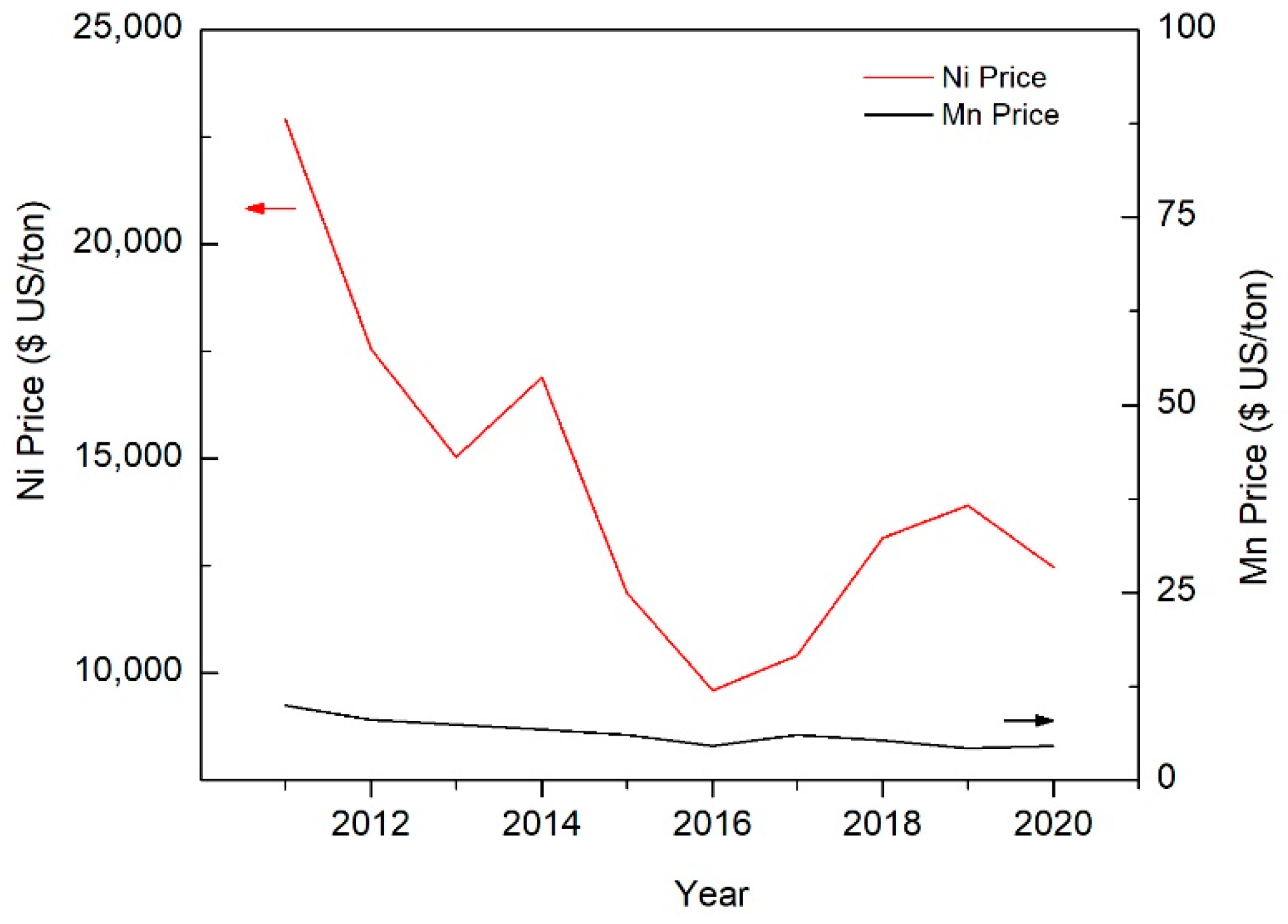
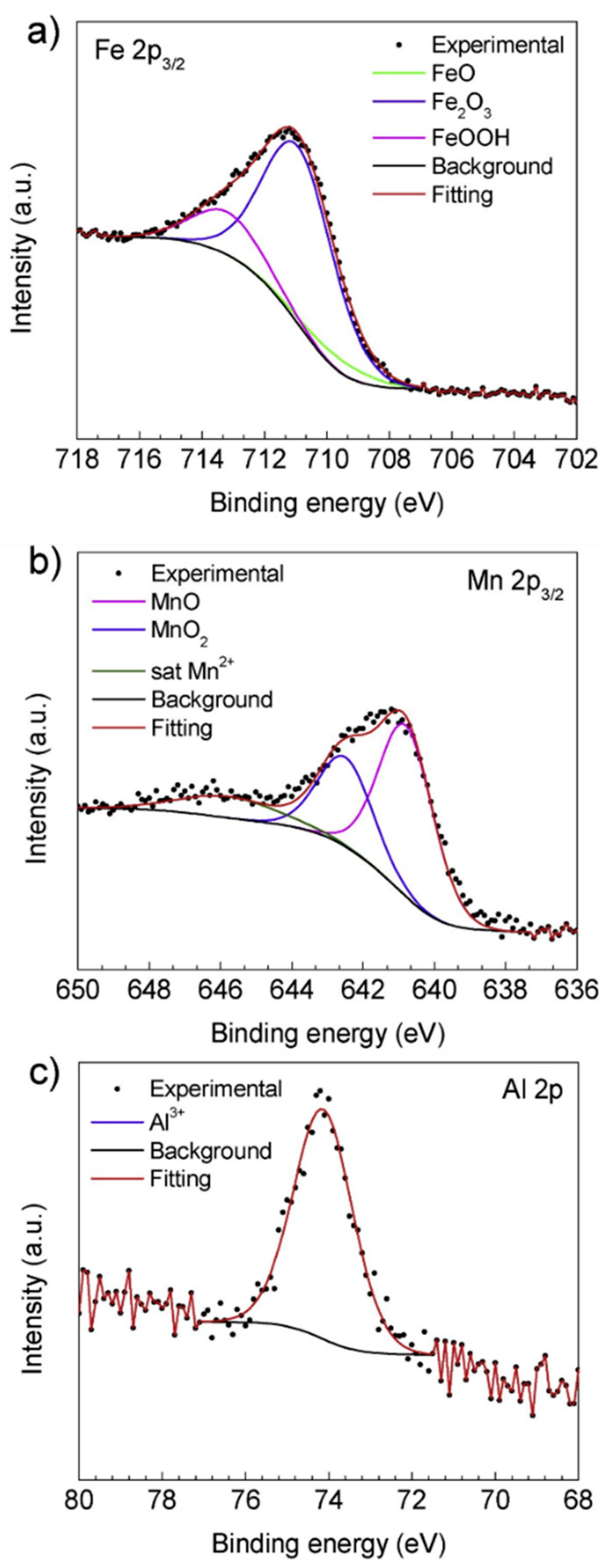
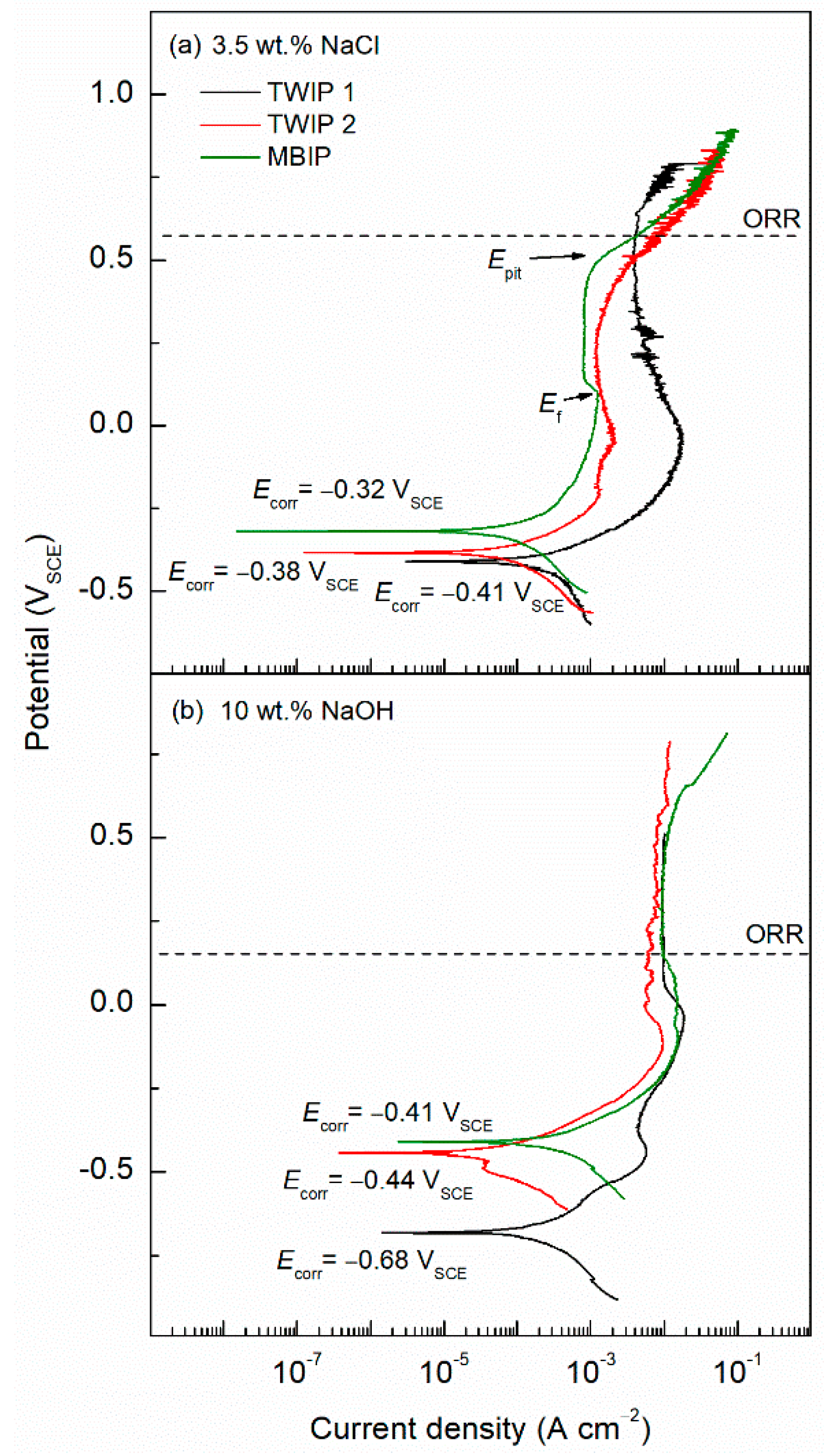
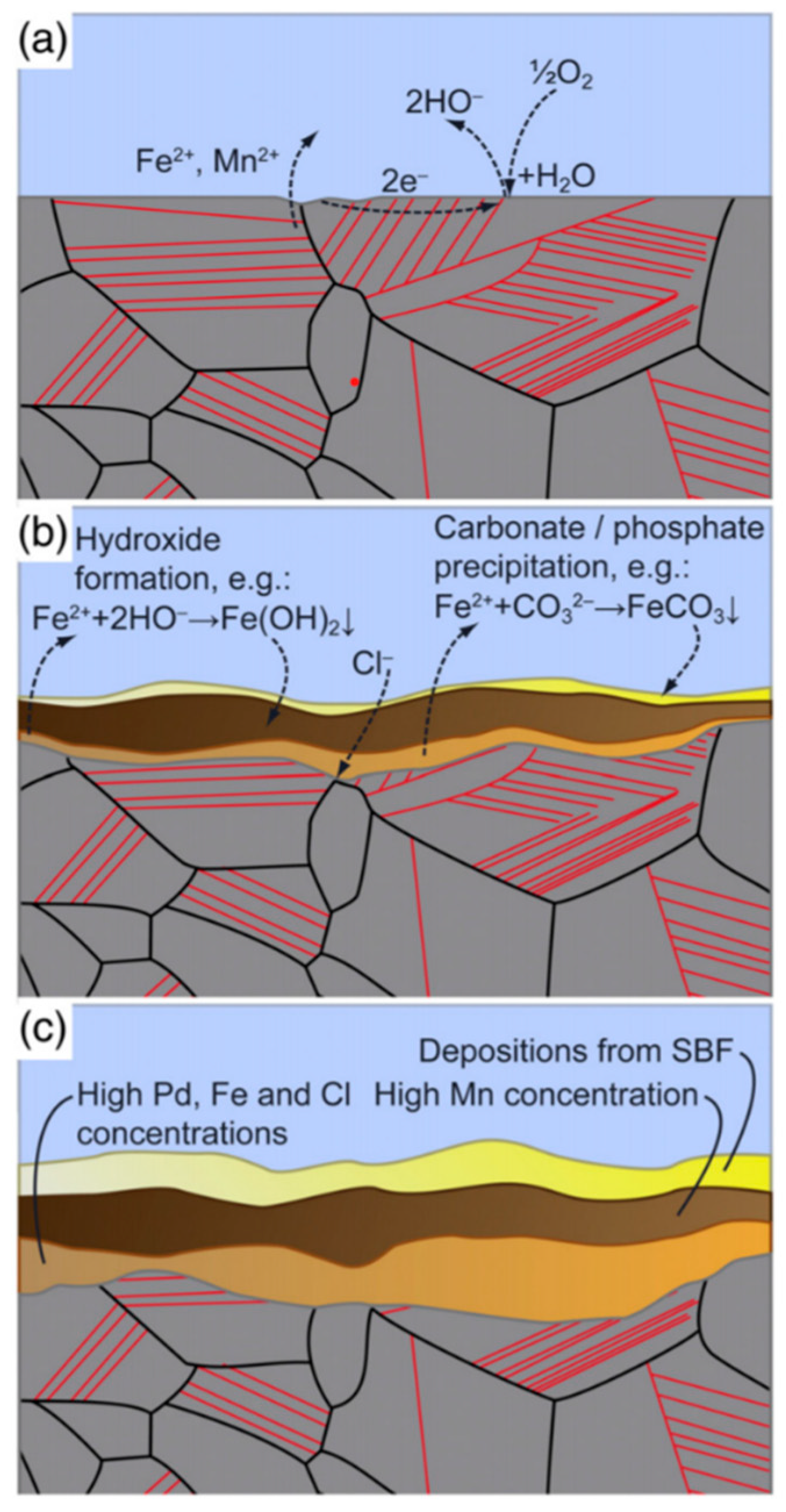

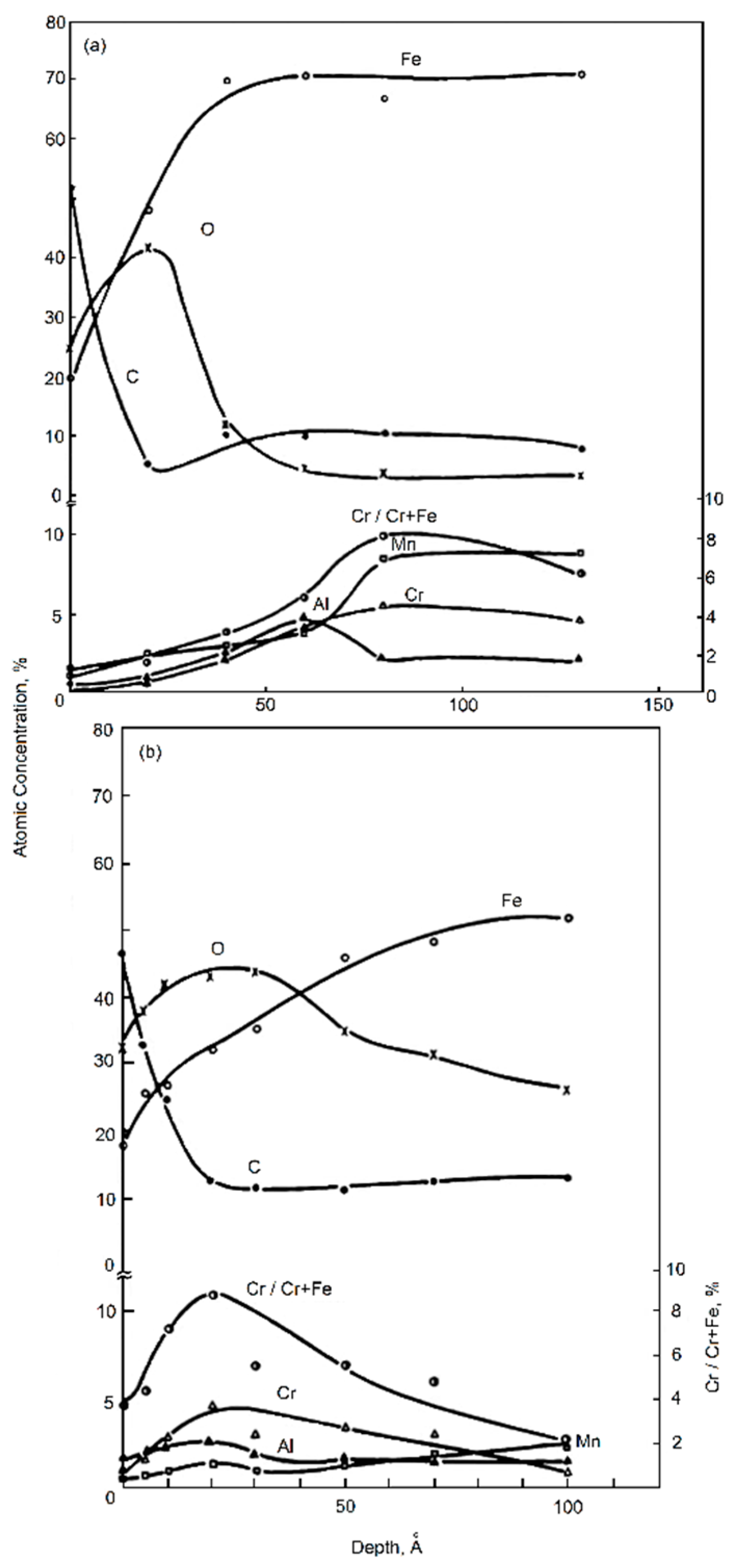
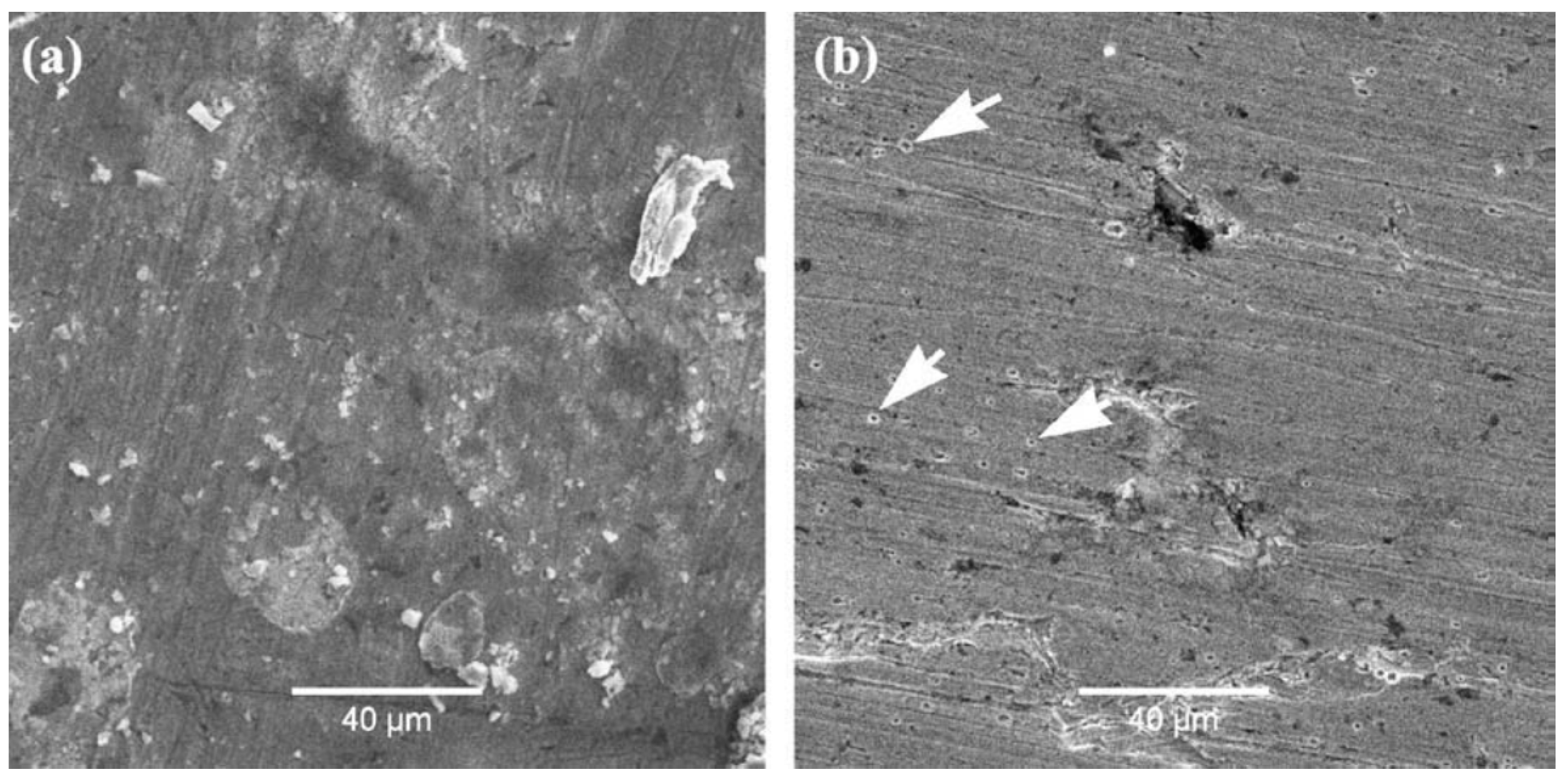
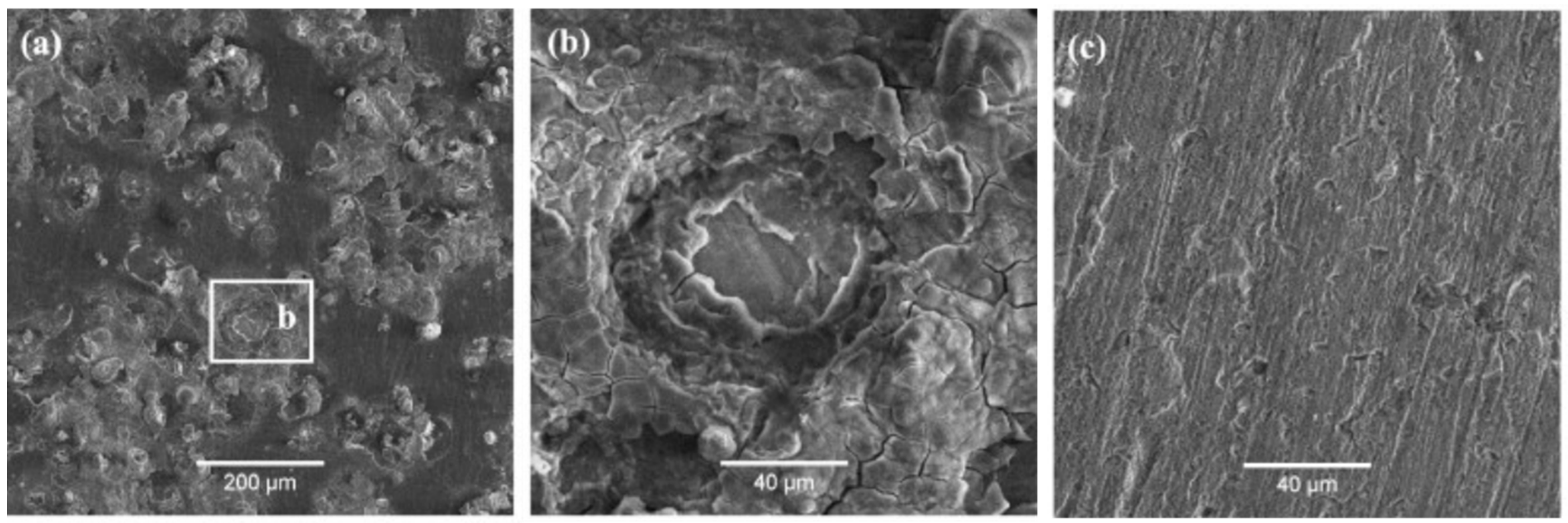
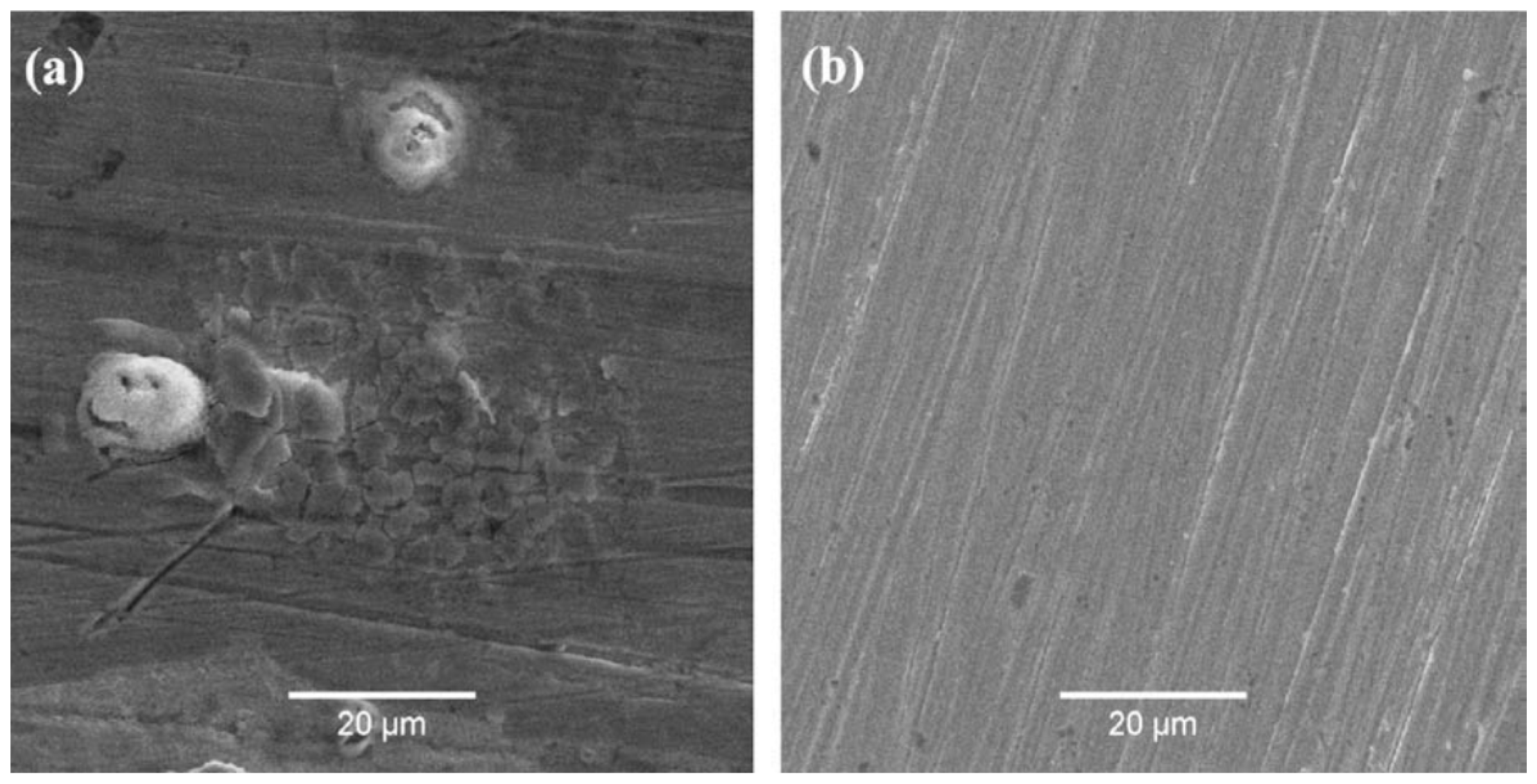
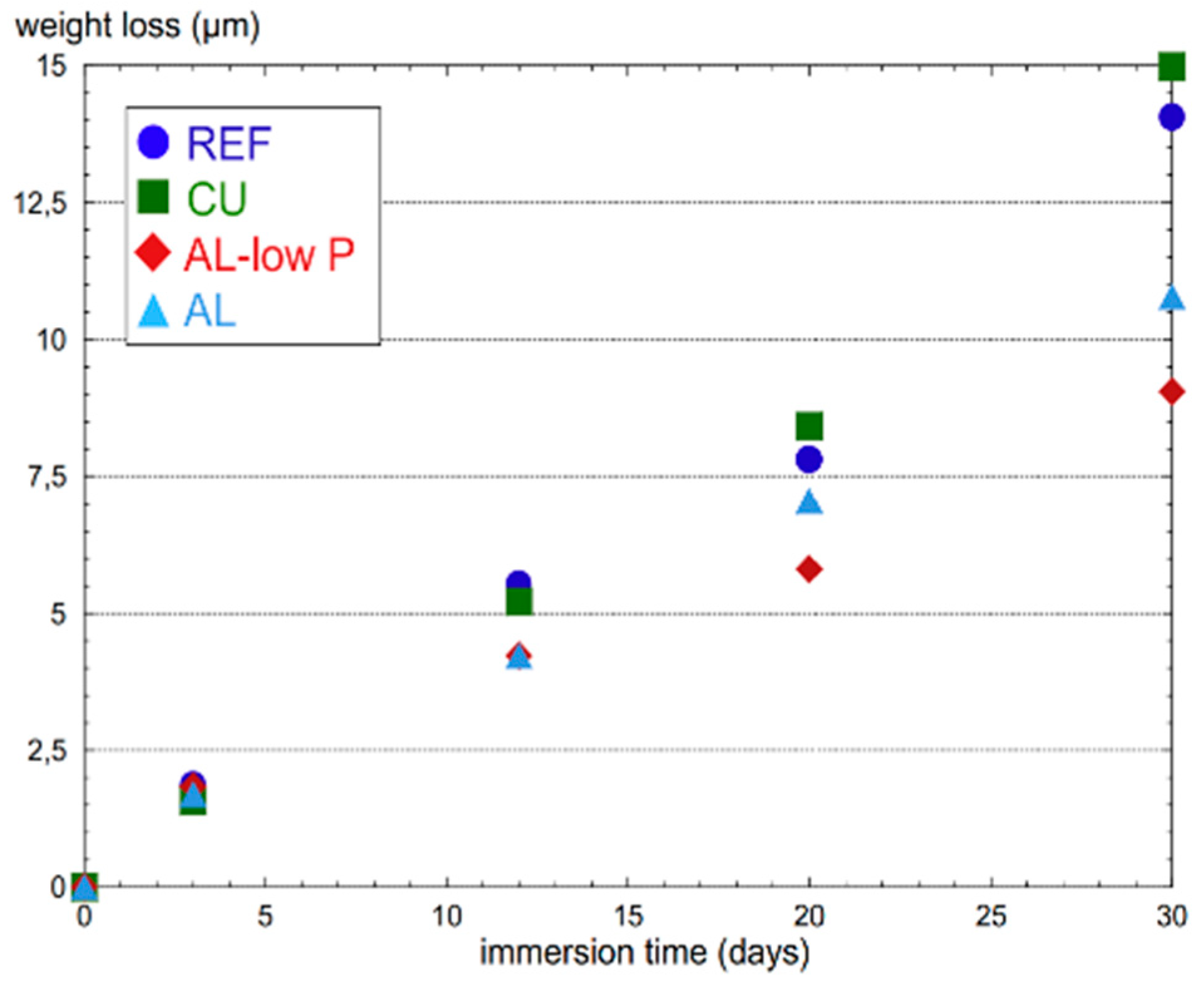



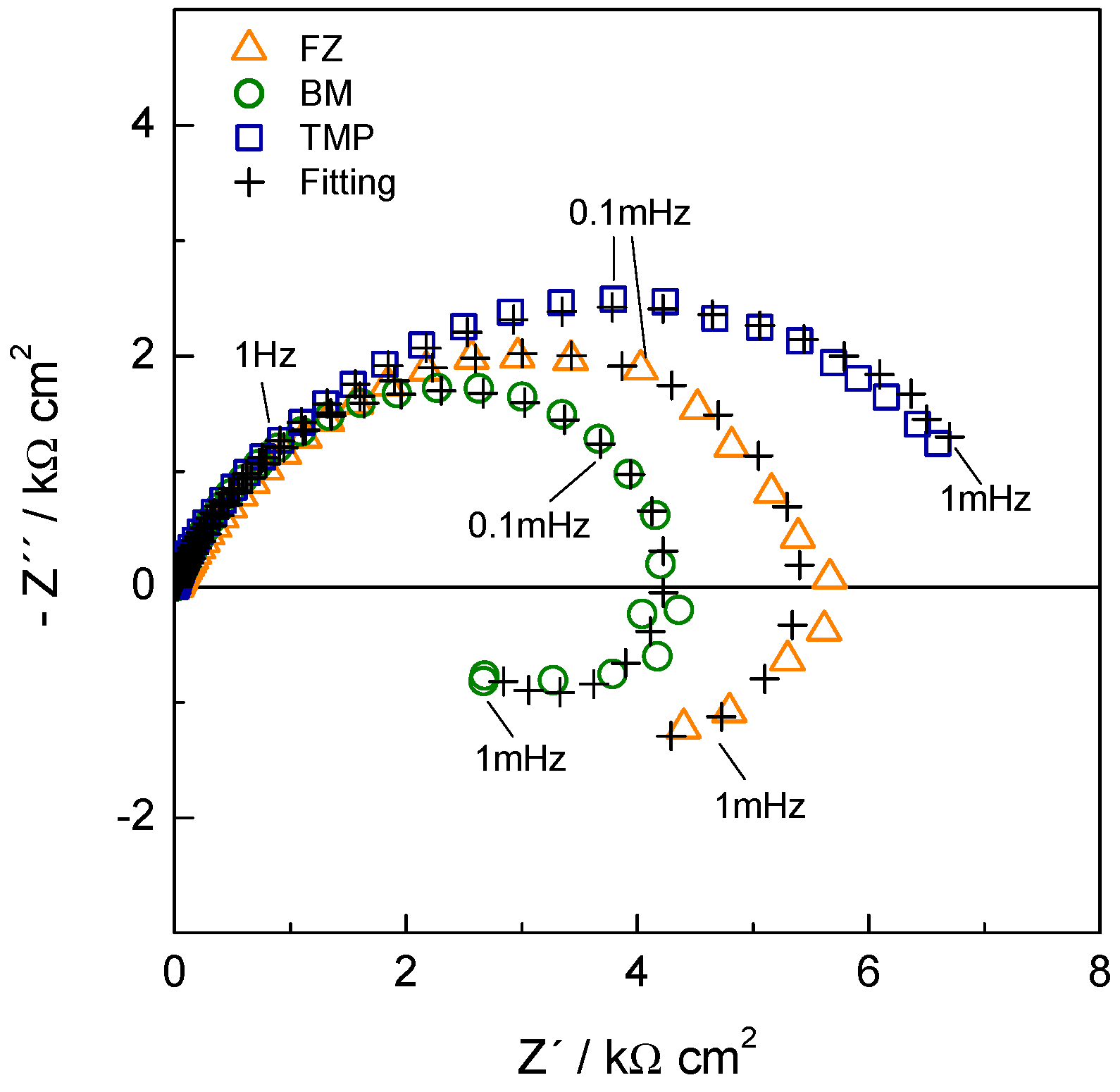
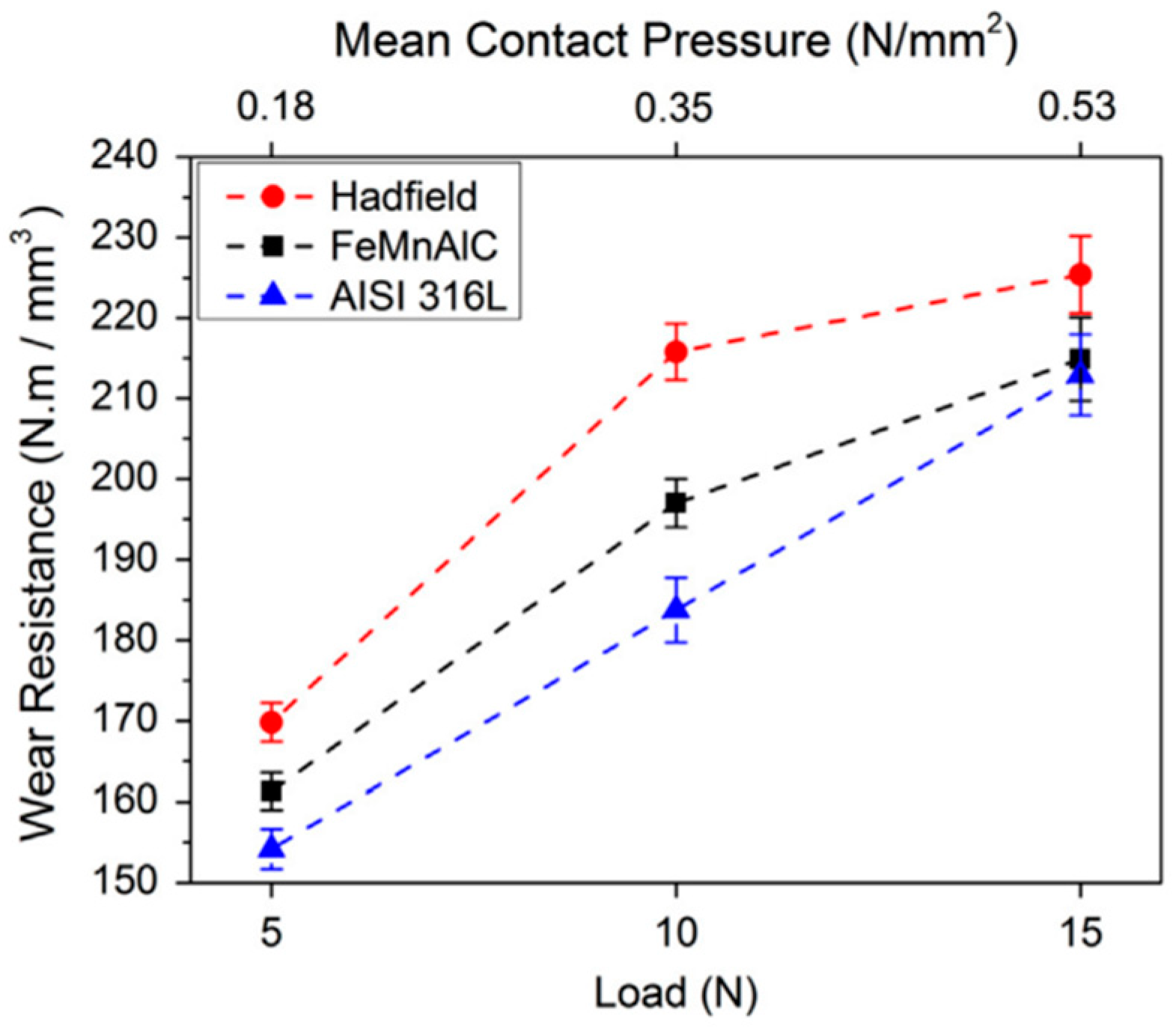
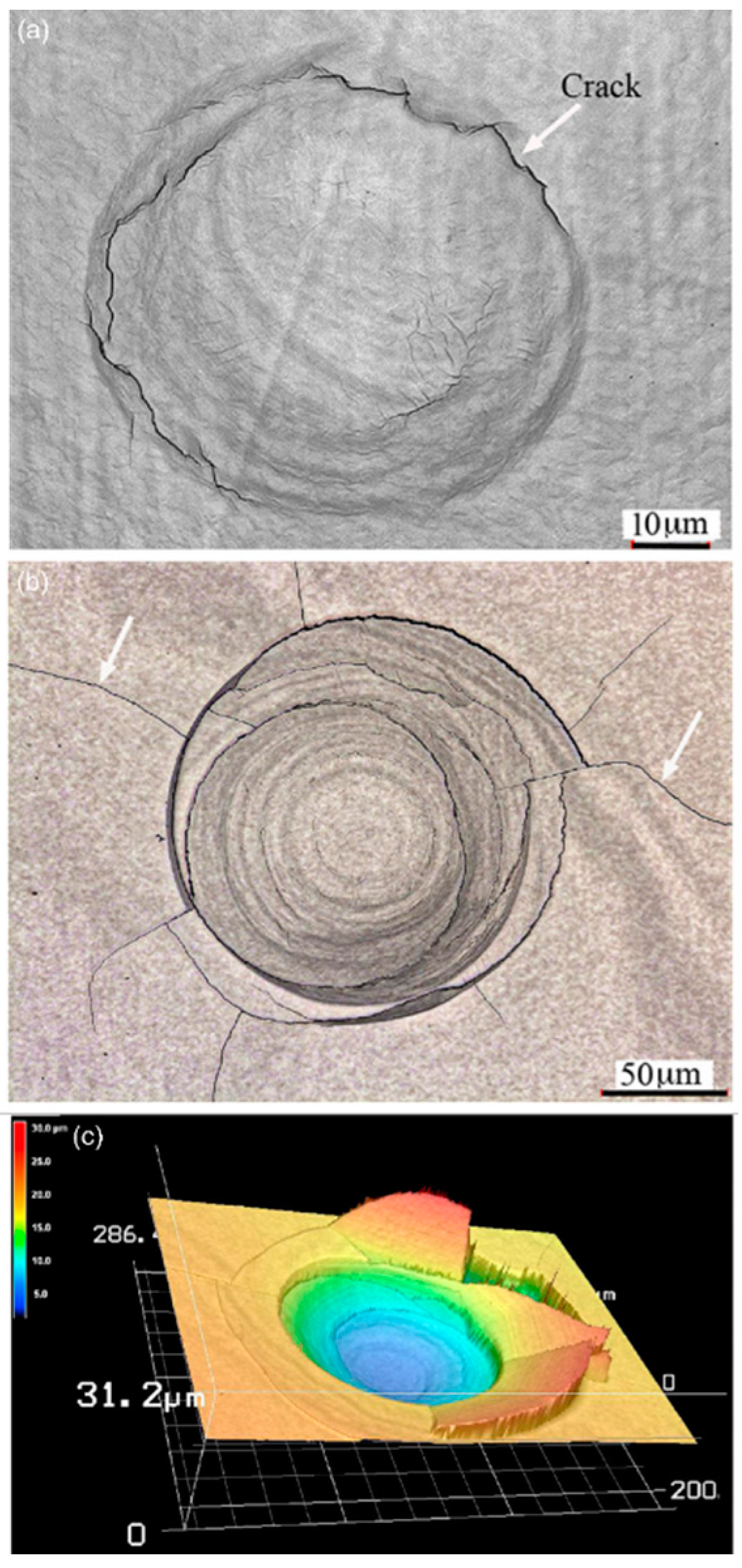
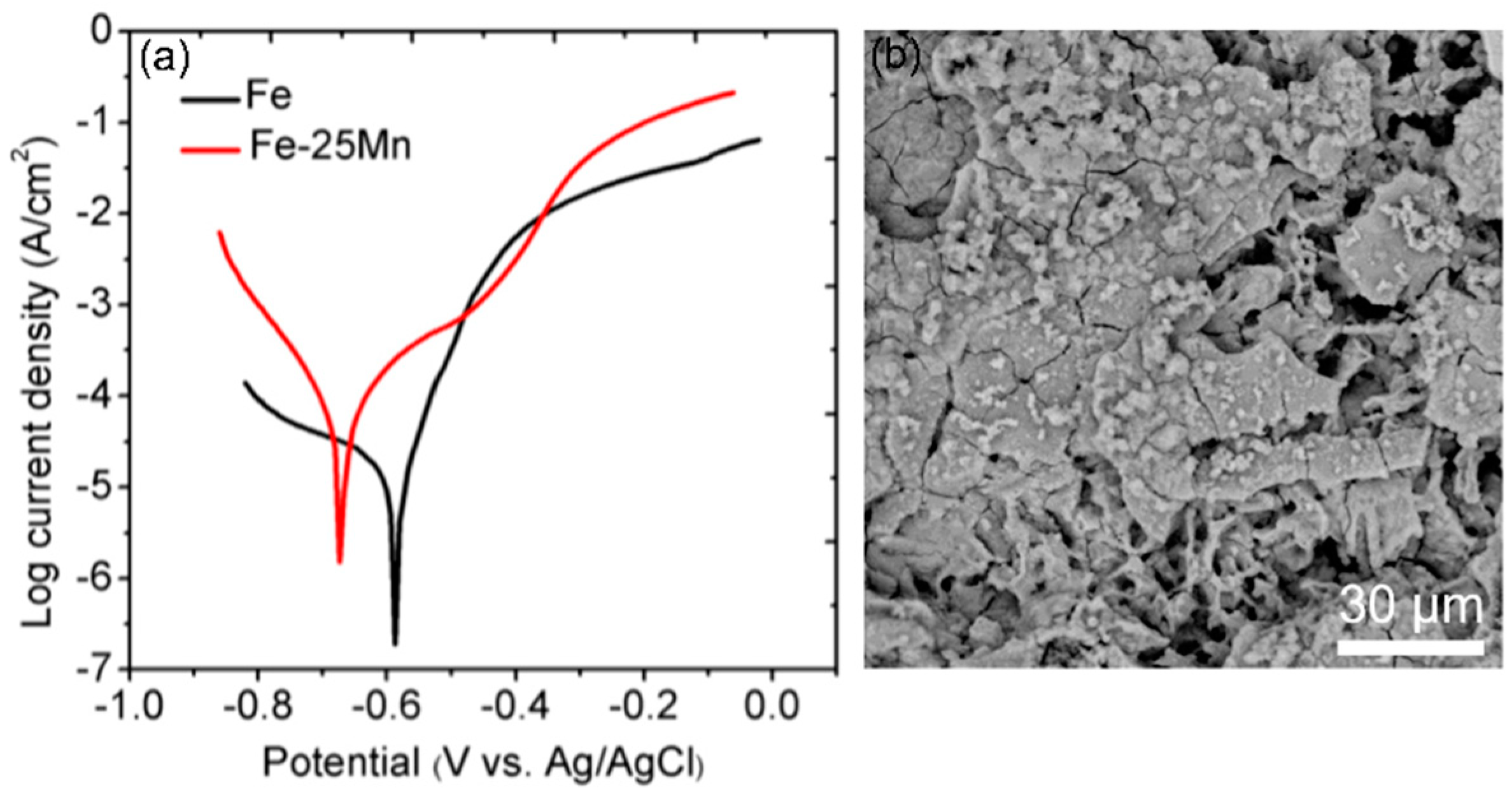
| TWIP Grade | Yield Strength (MPa) | Ultimate Tensile Strength (MPa) | Total Elongation (%) | Automotive Application |
|---|---|---|---|---|
| TWIP 500/900 | 500 | 900 | 50 | A-pillar, wheelhouse, front side member |
| TWIP 500/980 | 500 | 980 | 50 | Wheel, lower control arm, front and rear bumper beams, B-pillar, wheel rim |
| TWIP 600/900 | 600 | 900 | 45 | Floor cross-member, wheelhouse |
| TWIP 750/1000 | 750 | 1000 | 35 | Door impact beam |
| TWIP 950/1200 | 950 | 1200 | 20 | Door impact beam |
| Alloy | Electrolyte | vcorr, µm year−1 | Reference |
|---|---|---|---|
| Fe-25Mn-5Al-0.22C | 5% HNO3 | 1090 | [82] |
| Fe-25Mn-8Al-0.20C | 5% HNO3 | 835 | [82] |
| Fe-25Mn-5Al-0.22C | 10% HNO3 | 1450 | [82] |
| Fe-25Mn-8Al-0.20C | 10% HNO3 | 974 | [82] |
| Fe-25Mn-5Al-0.22C | 30% HNO3 | 1740 | [82] |
| Fe-25Mn-8Al-0.20C | 30% HNO3 | 1508 | [82] |
| Fe-25Mn-5Al-0.22C | 50% HNO3 | 2969 | [82] |
| Fe-25Mn-8Al-0.20C | 50% HNO3 | 742 | [82] |
| Fe-30Mn-8Al-1C | 10% HCl | 126 × 103 | [97] |
| Fe-25Mn-5Al-0.15C | 10% HCl | 74,160 | [97] |
| Fe-25Mn-5Al-0.15C | 30% HNO3 | 345,307 | [61] |
| Fe-25Mn-5Al-0.15C | 10% HCl | 74,160 | [61] |
| Fe-25Mn-5Al-0.15C | 30% NaOH | 88.1 | [61] |
| Fe-25Mn-5Al-0.15C | 10% Na2SO4 + HNO3 | 212 × l03 | [61] |
| Fe-25Mn-5Al-0.15C | 3.5% NaCl | 133.3 | [61] |
| Fe-19.8Mn-7.1Al-(0.76−0.99)C | 1N NaOH | 81.2 | [62] |
| Fe-23.3Mn-7.1Al-(0.76−0.99)C | 1N H2SO4 | 69.6 | [62] |
| Fe-30.5Mn-7.1Al-(0.76−0.99)C | Borate buffer pH 8.4 | 232 | [62] |
| Fe-28Mn-9Al | Mine water (pH 3.5) | 150 | [66] |
| Fe-30Mn-14Al | Salt water | 190 | [66] |
| Fe-30Mn-9Al-1C | 1N H2SO4 | 195 × 103 | [66] |
| Fe-24.8Mn-7.3Al-0.90C | Artificial seawater | 33.2 | [67] |
| Fe-24.4Mn-9.2Al-0.40C | Artificial seawater | 48.3 | [67] |
| Fe-28Mn-10Al-1C | 3.5% NaCl | 11.6 | [69] |
| Fe-24Mn-9Al-0.40C | 3.5% NaCl | 580 | [70] |
| Fe-30.7Mn-13.03Al-0.44C | 3.5% NaCl | 570 | [70] |
| Fe-26.6Mn-9.29Al-0.43C | 3.5% NaCl | 200 | [70] |
| Fe-30.5Mn-8.68Al-1.85C | 3.5% NaCl | 912 | [71] |
| Fe-30Mn-9Al | 1M Na2SO4 | 34.8 | [73] |
| Fe-30Mn-9Al-1.8C | 3.5% NaCl | 0.9 | [99] |
| Alloy | Electrolyte | vcorr, µm year−1 | Reference |
|---|---|---|---|
| Fe-30Mn-4Al-14Cr | 5% HNO3 | 174 | [82] |
| Fe-30Mn-8Al-16Cr | 5% HNO3 | 17.4 | [82] |
| Fe-30Mn-4Al-14Cr | 10% HNO3 | 232 | [82] |
| Fe-30Mn-8Al-16Cr | 10% HNO3 | 40.6 | [82] |
| Fe-30Mn-4Al-14Cr | 30% HNO3 | 522 | [82] |
| Fe-30Mn-8Al-16Cr | 30% HNO3 | 92.8 | [82] |
| Fe-30Mn-4Al-14Cr | 50% HNO3 | 60.3 | [82] |
| Fe-30Mn-8Al-16Cr | 50% HNO3 | 1.2 | [82] |
| Fe-24Mn-3Al-7Cr | 1M Na2SO4 | 23.4 | [103] |
| Fe-24Mn-3Al-7Cr | 10% HNO3 | 0.3 × 103 | [103] |
| Fe-24Mn-3Al-7Cr | 30% HNO3 | 0.3 × 103 | [103] |
| Fe-24Mn-3Al-7Cr | 50% HNO3 | 110 | [103] |
| Fe-24Mn-3Al-7Cr | 10% NaOH | 10.4 | [103] |
| Fe-24Mn-3Al-7Cr | 30% NaOH | 23.2 | [103] |
| Fe-24Mn-3Al-7Cr | 50% NaOH | 11.6 | [103] |
| Fe-24Mn-3Al-7Cr | 10% HCl | 0.1 × 103 | [103] |
| Fe-24Mn-3Al-7Cr | 3.5% NaCl | 98.6 | [103] |
| Fe-28Mn-9Al-(0−8)Cr-1.8C | 3.5% NaCl | 34.8 | [102] |
| Fe-21Mn-10Al-6Cr | 3.5% NaCl | 10.4 | [69] |
| Fe-27Mn-9Al-3Cr | 3.5% NaCl | 92.8 | [70] |
| Fe-27Mn-9Al-6Cr | 3.5% NaCl | 92.8 | [70] |
| Fe-26.4Mn-2.74Al-1.13Cr-0.32C | 3.5% NaCl | 783.3 | [100] |
| Alloy | Electrolyte | vcorr, µm year−1 | Reference |
|---|---|---|---|
| Fe-15Mn-3Al-3Si | 0.5M H2SO4 | 127.6 | [50] |
| Fe-29Mn-3.5Al-0.5Si-0.5C | 3.5% NaCl | 30.5 | [127] |
| Fe-29Mn-3.5Al-0.5Si-0.5C | 3.5% NaCl | 147.2 | [127] |
| Fe-30Mn-1Al-1Si-1C | 1N H2SO4 | 0.2 × l03 | [66] |
| Fe-32.7Mn-6.59Al-1.26Si-0.25C | 1N H2SO4 | 98,493 | [109] |
| Fe-32.7Mn-8.54Al-1.3Si-0.54C | 1N H2SO4 | 0.3 × l03 | [109] |
| Fe-18Mn-2Al-2Si-0.07C | 3.5% NaCl | 39.2 | [134] |
| Fe-30Mn-3Al-1.5Si-0.06C | 3.5% NaCl | 263 | [120] |
| Fe-17.3Mn-3.10Al-0.38Si-0.24C | 3.5% NaCl | 366 | [78] |
| Fe-20.6Mn-3.5Al-2.92Si-0.29C | 3.5% NaCl | 46.3 | [78] |
| Fe-30Mn-3Al-1.5Si-0.06C | 0.1M NaOH | 22.9 | [120] |
| Fe-30Mn-3Al-1.5Si-0.06C | 0.1M H2SO4 | 25,921 | [120] |
| Fe-30Mn-10Al-1Si-1C | 3.5% NaCl | 20 | [66] |
| Fe-30.5Mn-7.5Al-1.5Si-1C | 3.5% NaCl | 50 | [66] |
| Fe-30.5Mn-7.5Al-1Si-1C | 3.5% NaCl | 350 | [66] |
| Fe-30.5Mn-7.5Al-0.5Si-1C | 3.5% NaCl | 230 | [66] |
| Fe-33Mn-9Al-1.4Si-0.9C | 3.5% NaCl | 3.775 × l03 | [112] |
| Fe-29Mn-8.6Al-2.7Si-0.7C | 3.5% NaCl | 2.573 × l03 | [112] |
| Fe-24Mn-7.3Al-2Si-1C | 3.5% NaCl | 2.354 × l03 | [112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastidas, D.M.; Ress, J.; Bosch, J.; Martin, U. Corrosion Mechanisms of High-Mn Twinning-Induced Plasticity (TWIP) Steels: A Critical Review. Metals 2021, 11, 287. https://doi.org/10.3390/met11020287
Bastidas DM, Ress J, Bosch J, Martin U. Corrosion Mechanisms of High-Mn Twinning-Induced Plasticity (TWIP) Steels: A Critical Review. Metals. 2021; 11(2):287. https://doi.org/10.3390/met11020287
Chicago/Turabian StyleBastidas, David M., Jacob Ress, Juan Bosch, and Ulises Martin. 2021. "Corrosion Mechanisms of High-Mn Twinning-Induced Plasticity (TWIP) Steels: A Critical Review" Metals 11, no. 2: 287. https://doi.org/10.3390/met11020287
APA StyleBastidas, D. M., Ress, J., Bosch, J., & Martin, U. (2021). Corrosion Mechanisms of High-Mn Twinning-Induced Plasticity (TWIP) Steels: A Critical Review. Metals, 11(2), 287. https://doi.org/10.3390/met11020287








