Phase Field Simulation of AA6XXX Aluminium Alloys Heat Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Al-Mg Phase Diagrams
2.2. Phase-Field Modelling
2.3. Annealing Treatment Simulation
2.4. Isothermal Artificial Ageing Simulation
2.5. Yield Strength and Hardness estimation
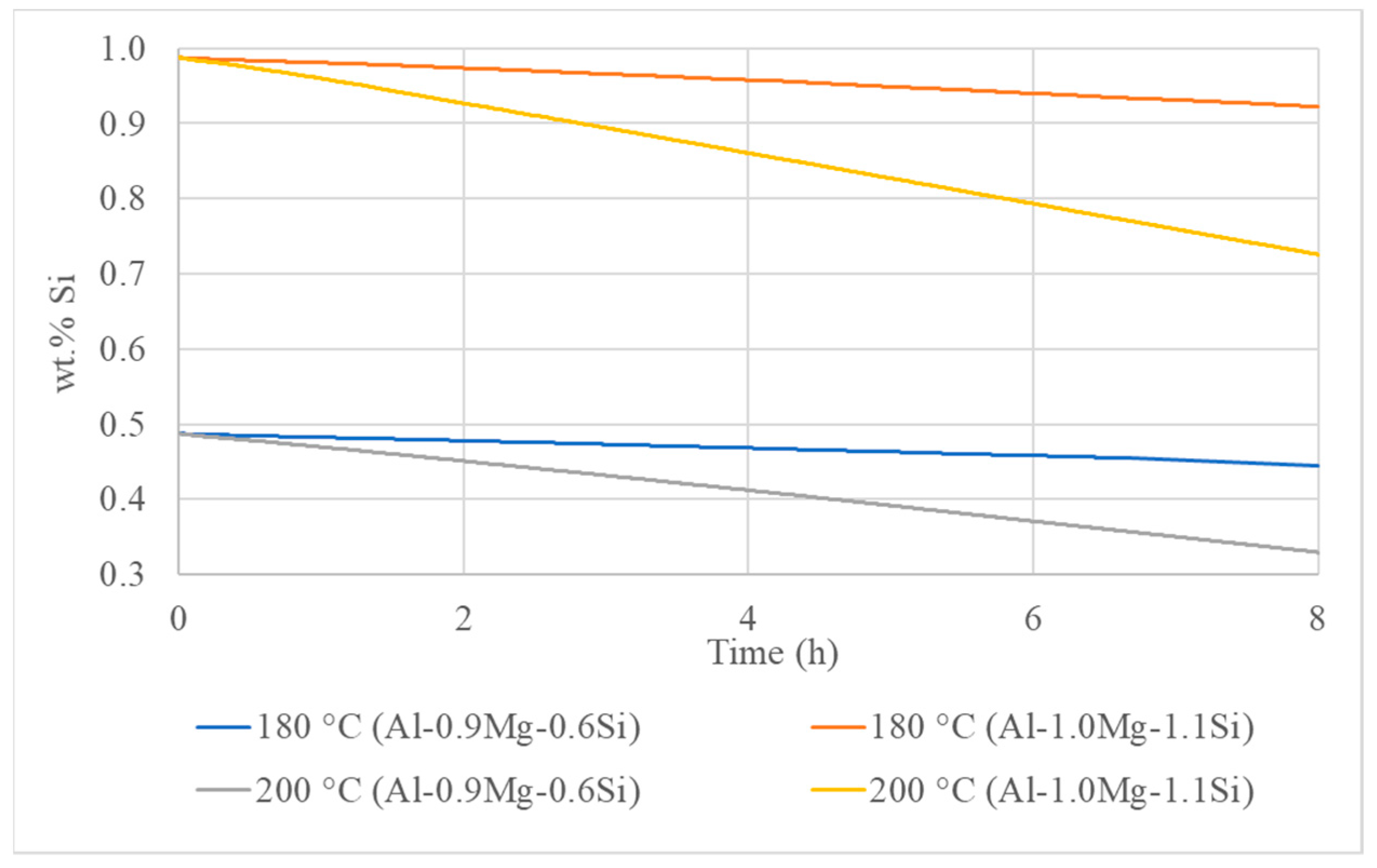
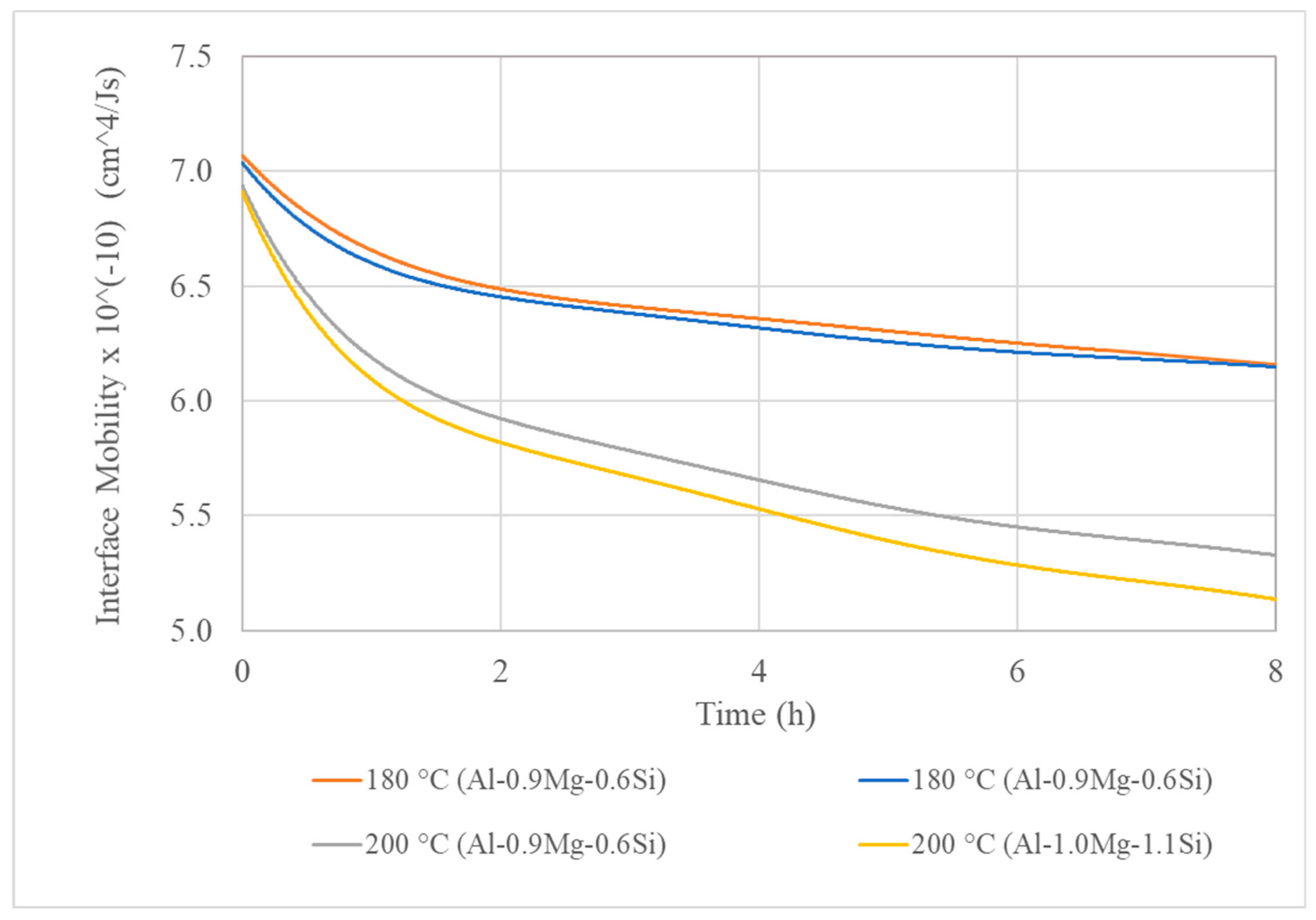
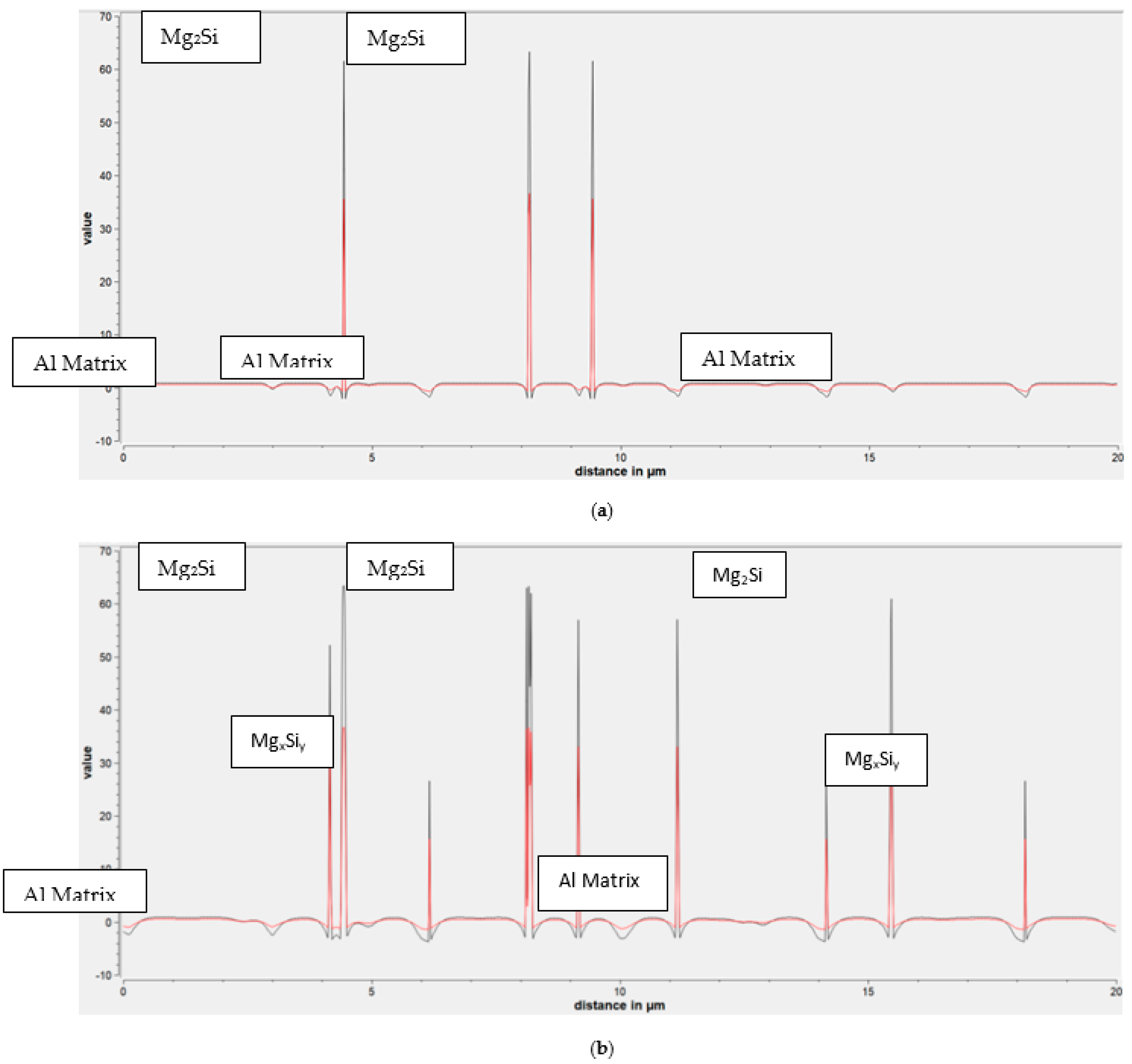
3. Results
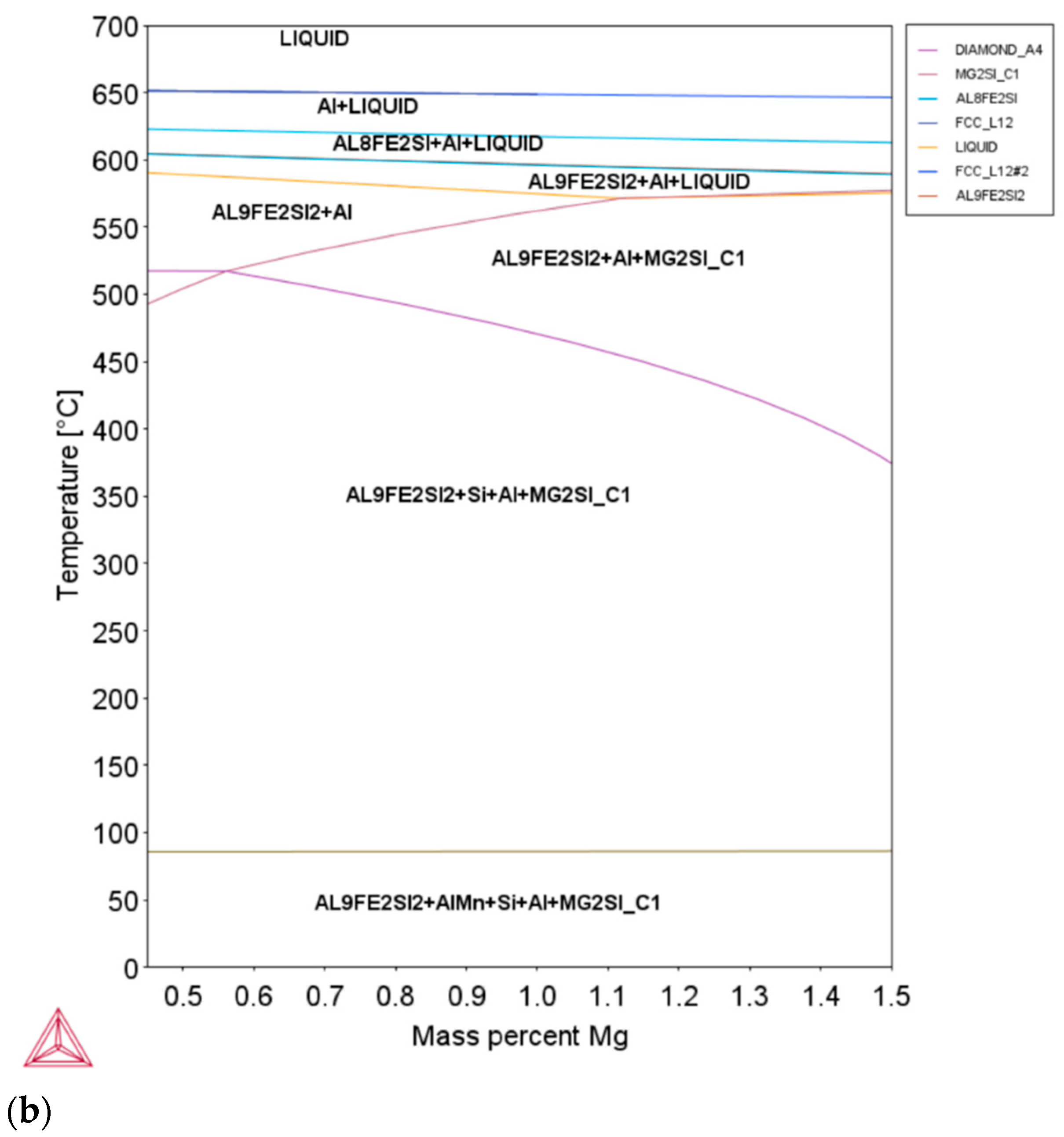
3.1. Al-Mg Phase Diagrams
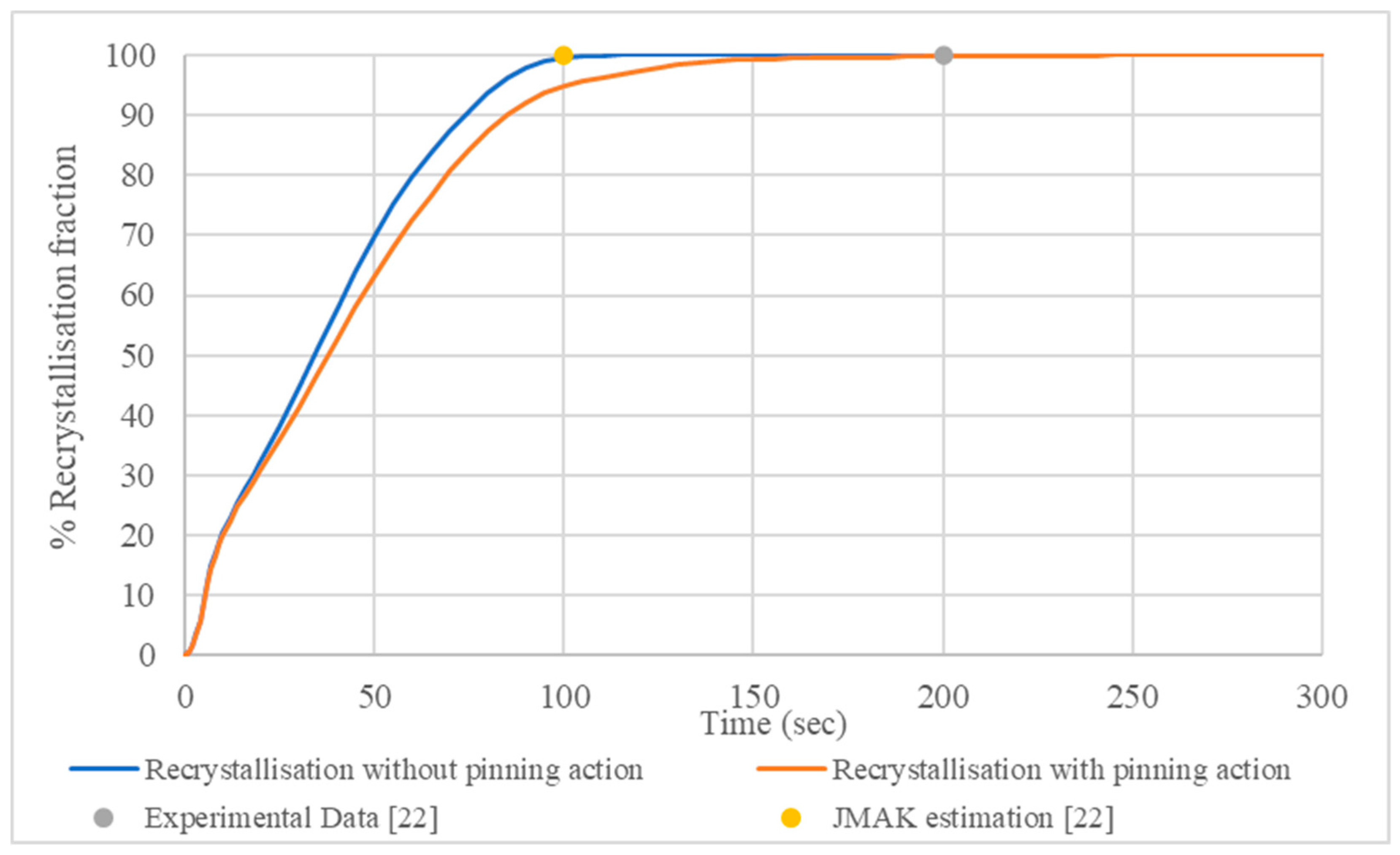
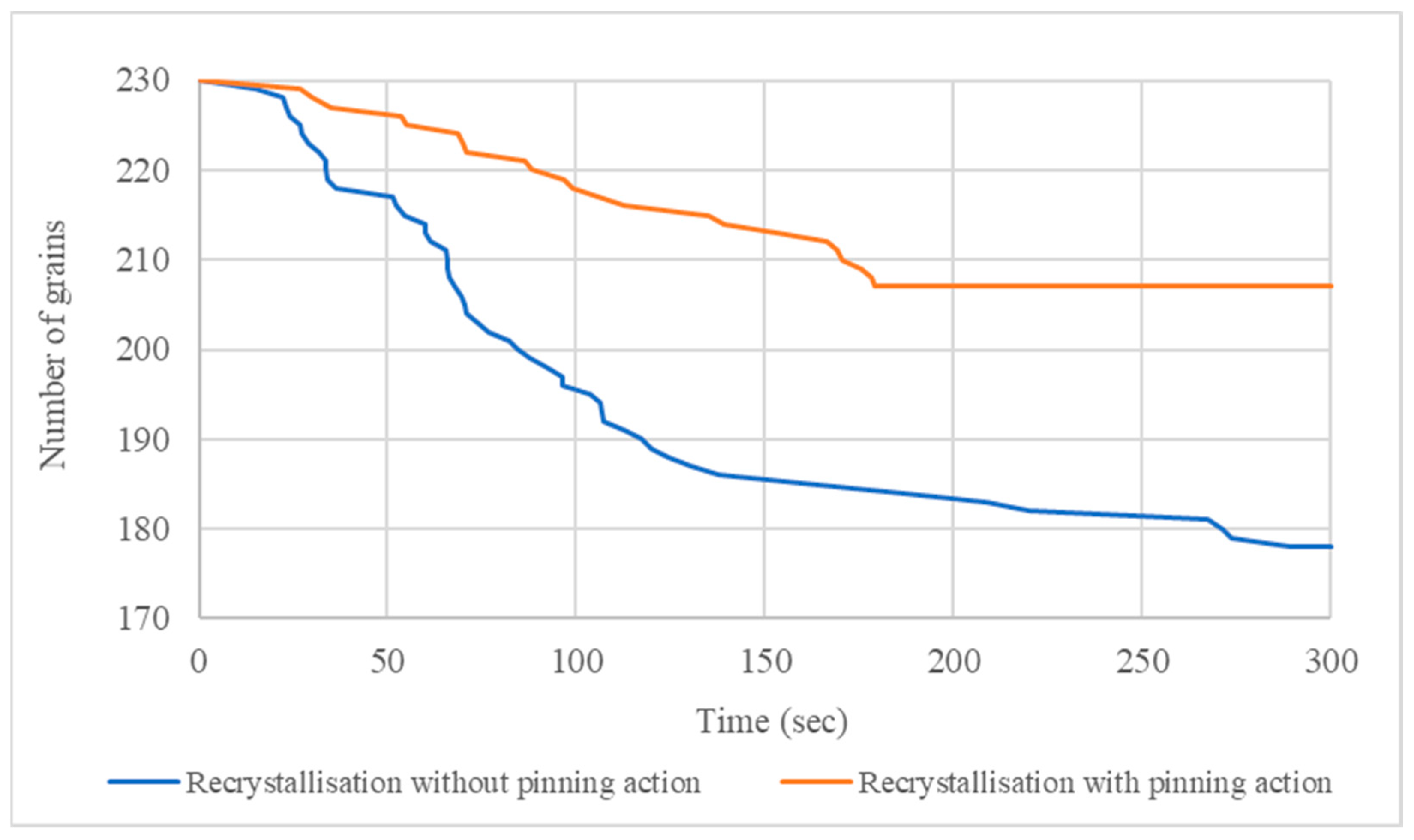
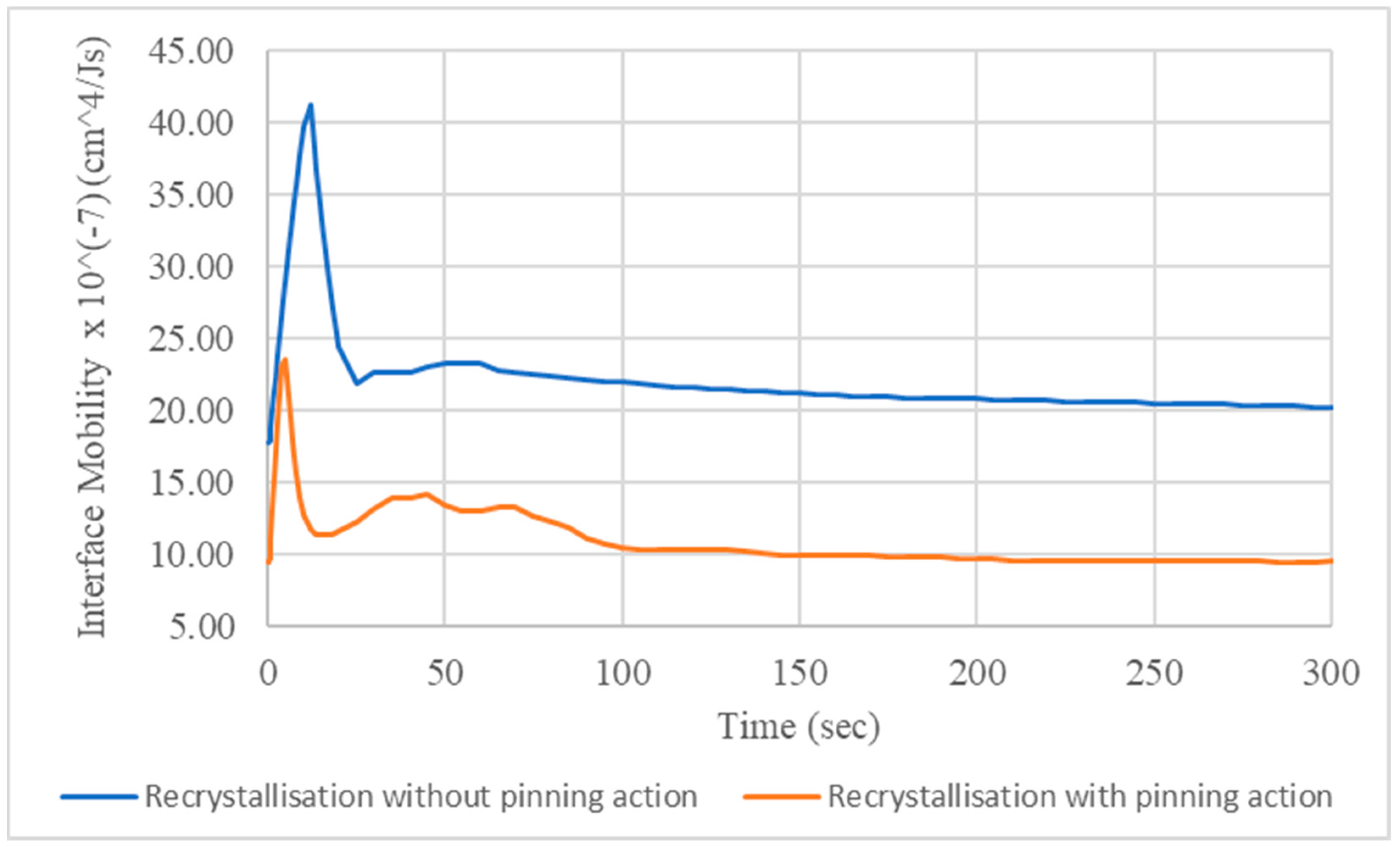
3.2. Recrystallisation and Grain Growth Simulation
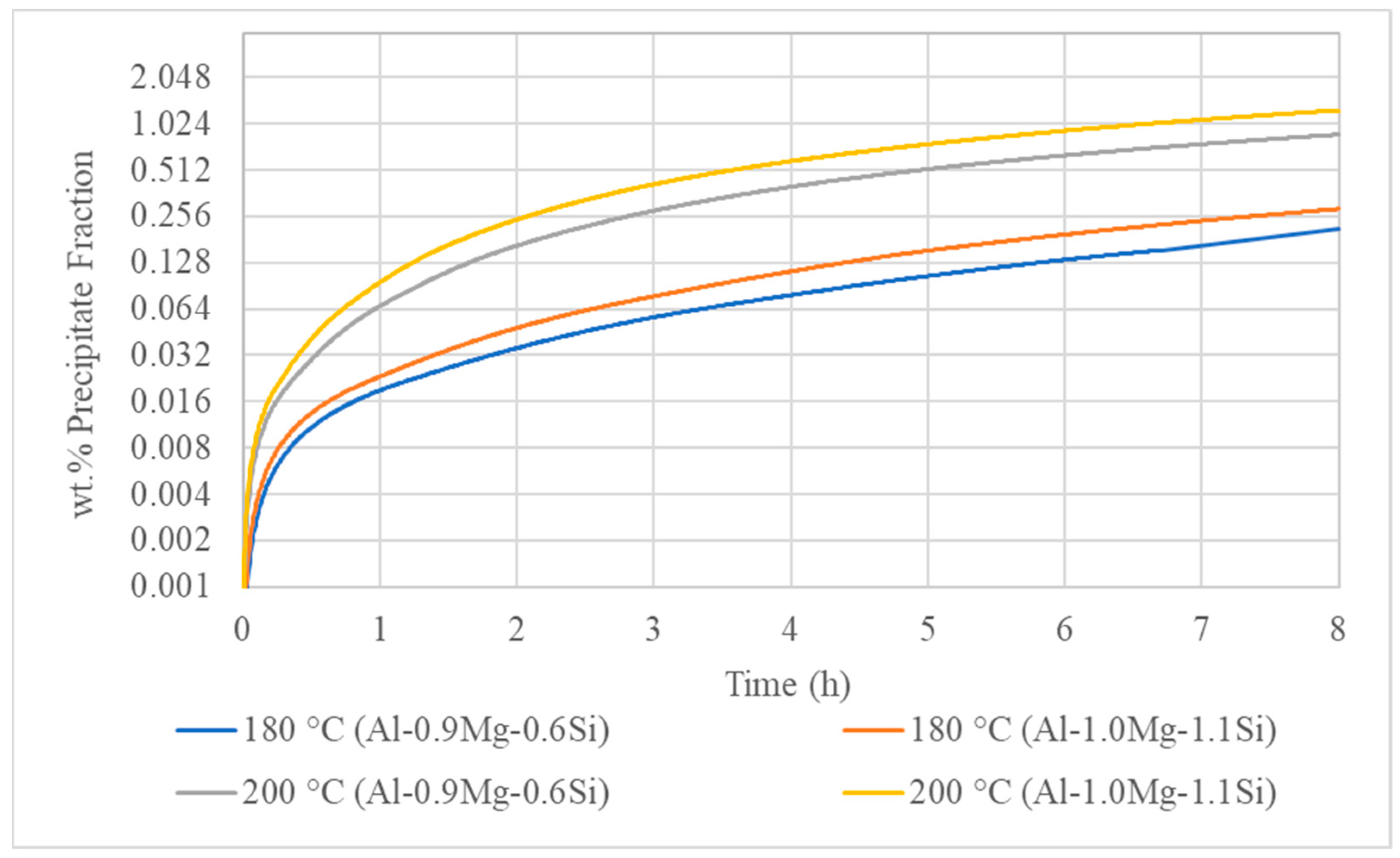
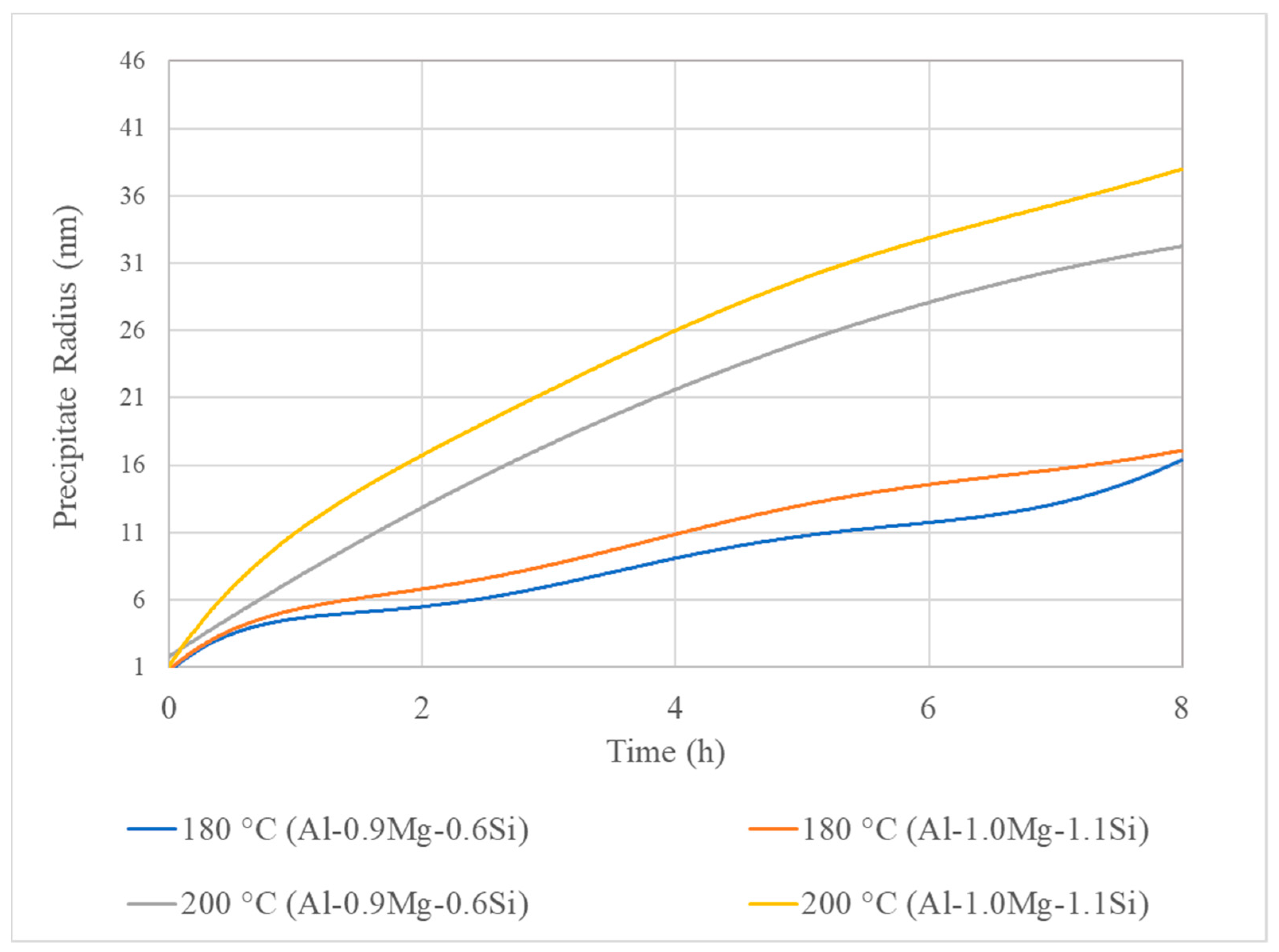
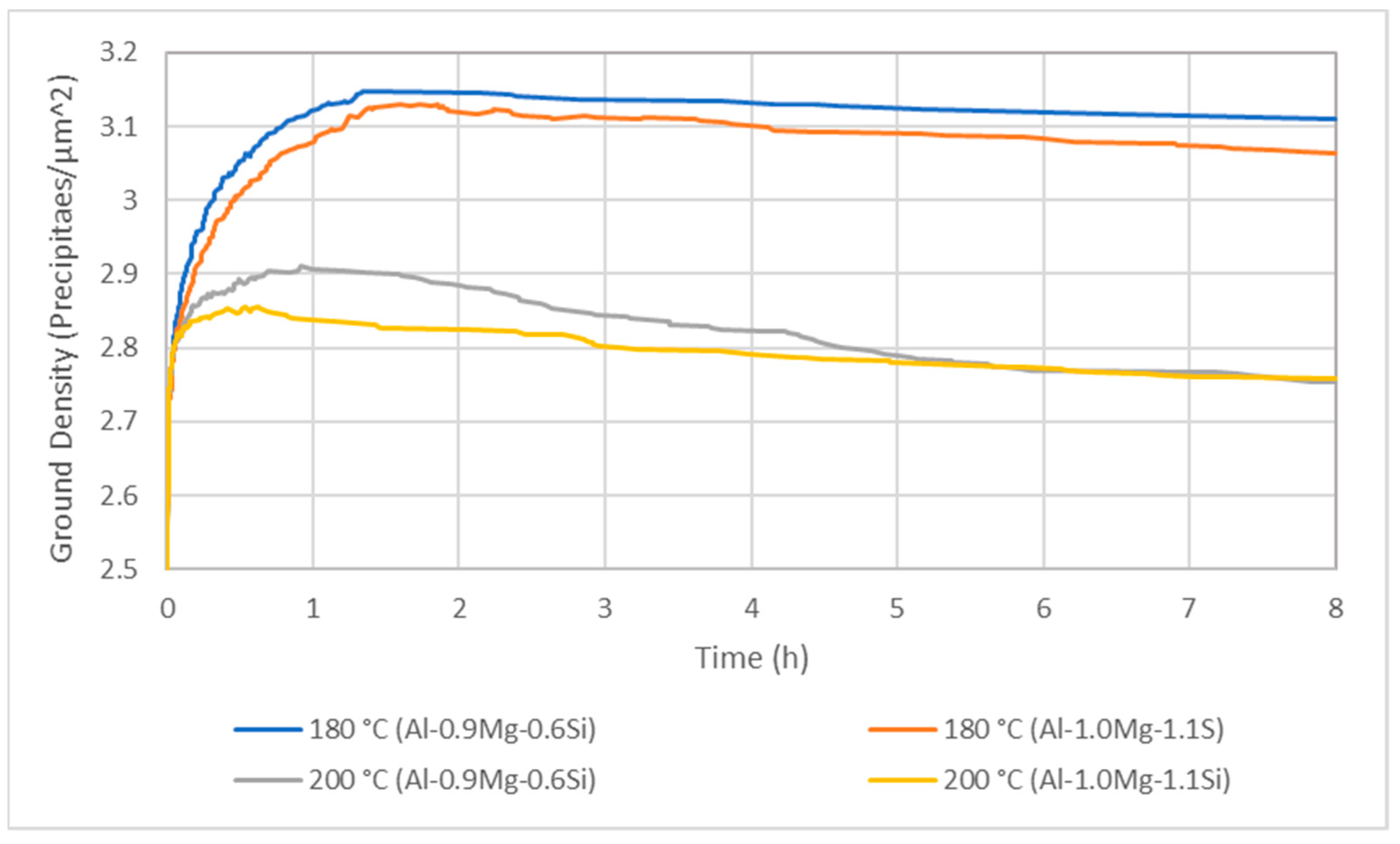
3.3. Isothermal Artificial Ageing Simulation
4. Discussion
5. Conclusions
- The 400 °C/5 min annealing simulation accurately predicted the recrystallisation kinetics proving a slight impact of secondary nanoparticles on the deceleration of recrystallisation mechanism and the average radius of recrystallized grains. This deceleration is explained by the lower values of the interface mobility.
- The ageing simulation predicted the under-ageing condition for the 180 °C-8 h treatment and the peak ageing condition for the 200 °C-8 h ageing simulations.
- For lower ageing temperatures, the interface mobility has more significant impact on the precipitation mechanism. On the contrary, the rise of temperature results in severe increase of diffusion mechanism, causing the coarsening of precipitate particles, which nucleate and grow both on the interior of Al matrix phase grains and the interfaces and triple junctions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bahrami, A. Modeling of Precipitation Sequence and Ageing Kinetics in Al-Mg-Si Alloys. Ph.D. Thesis, Delft University of Technology, Delft, Poland, 2010. [Google Scholar]
- Mukhopadhyay, P. Alloy Designation, Processing, and Use of AA6XXX Series Aluminium Alloys. ISRN Metall. 2012. [Google Scholar] [CrossRef]
- Engler, O.; Hirsch, J. Texture control by thermomechanical processing of AA6xxx Al–Mg–Si sheet alloys for automotive applications—A review. Mater. Sci. Eng. A 2002, 336, 249–262. [Google Scholar] [CrossRef]
- Nisaratanaporn, E. Microstructural Development and Pressure Requirements in 6063 Aluminium Alloy Tube Extrusion. Ph.D. Thesis, Imperial College, London, UK, 1995. [Google Scholar]
- Esmaeili, S.; LIoyd, D.J.; Poole, W.J. Modeling of precipitation hardening for the naturally aged Al-Mg-Si-Cu alloy AA6111. Acta Mater. 2003, 51, 3467–3481. [Google Scholar] [CrossRef]
- Lan, Y.; Pinna, C. Modelling the static recrystallisation texture of FCC metals using a phase field method. Mater. Sci. Forum 2012, 715, 739–744. [Google Scholar] [CrossRef]
- Jou, H.-J.; Lusk, M.T. Comparison of Johnson-Mehl-Avrami-Kologoromov kinetics with a phase-field model for microstructural evolution driven by substructure energy. Phys. Rev. B 1997, 55, 8114–8121. [Google Scholar] [CrossRef]
- Weinkamer, R.; Fratzl, P.; Gupta, H.S.; Penrose, O.; Lebowitz, J.L. Using Kinetic Monte Carlo simulations to study phase separation in Alloys. Phase Transit. 2004, 77, 433–456. [Google Scholar] [CrossRef]
- Myhr, O.R.; Grong, Ø.; Andersen, S.J. Modelling of the age hardening behaviour of Al–Mg–Si alloys. Acta Mater. 2001, 49, 65–75. [Google Scholar] [CrossRef]
- Mao, F.; Bollmann, C.; Brüggemann, T.; Liang, Z.; Jiang, H.; Mohles, V. Modelling of the Age-Hardening Behavior in AA6xxx within a Through-Process Modelling Framework. Mater. Sci. Forum 2017, 877, 640–646. [Google Scholar] [CrossRef]
- Povoden-Karadeniz, E.; Lang, P.; Warczok, P.; Falahati, A.; Jun, W.; Kozeschnik, E. Calphad modeling of metastable phases in the Al–Mg–Si system. Calphad 2013, 43, 94–104. [Google Scholar] [CrossRef]
- Raabe, D. Computational Materials Science: The Simulation of Materials Microstructures and Properties; Wiley-VCH: New York, NY, USA, 1998. [Google Scholar]
- Moelans, N.; Blanpain, B.; Wollants, P. An introduction to phase-field modeling of microstructure evolution. Calphad 2007, 32, 268–294. [Google Scholar] [CrossRef]
- Bhadeshia, H.; Qin, R.S. Phase field method. Mater. Sci. Technol. 2010, 26, 803–811. [Google Scholar]
- Lewis, D.; Warren, J.; Boettinger, W.; Pusztai, T.; Granasy, L. Phase-field models for eutectic solidification. Jom 2004, 56, 34–39. [Google Scholar] [CrossRef]
- Nestler, B.; Choudhury, A. Phase-field modeling of multi-component systems. Curr. Opin. Solid State Mater. Sci. 2011, 15, 93–105. [Google Scholar] [CrossRef]
- Mueller, J.J.; Matlock, D.K.; Speer, J.; De Moor, E.; Moor, D. Accelerated Ferrite-to-Austenite Transformation During Intercritical Annealing of Medium-Manganese Steels Due to Cold-Rolling. Metals 2019, 9, 926. [Google Scholar] [CrossRef]
- Ta, N.; Zhang, L.; Du, Y. Design of the Precipitation Process for Ni-Al Alloys with Optimal Mechanical Properties: A Phase-Field Study. Metall. Mater. Trans. A 2014, 45, 1787–1802. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, Y. Precipitation and Growth Simulation of γ′ Phase in Single Crystal Superalloy DD6 with Multiphase-Field Method and Explicit Nucleation Algorithm. Metals 2020, 10, 1346. [Google Scholar] [CrossRef]
- Ji, Y.; Ghaffari, B.; Li, M.; Chen, L.-Q. Phase-field modeling of θ′ precipitation kinetics in 319 aluminum alloys. Comput. Mater. Sci. 2018, 151, 84–94. [Google Scholar] [CrossRef]
- Steinbach, I.; Pezzolla, F.; Nestler, B.; Seeßelberg, M.; Prieler, R.; Schmitz, G.J.; Rezende, J.L. A phase field concept for multiphase systems. Phys. D Nonlinear Phenom. 1996, 94, 135–147. [Google Scholar] [CrossRef]
- Poletti, M.C.; Bureau, R.; Loidolt, P.; Simon, P.; Mitsche, S.; Spuller, M. Microstructure Evolution in a 6082 Aluminium Alloy during Thermomechanical Treatment. Material 2018, 11, 1319. [Google Scholar] [CrossRef]
- Pedersen, K.O.; Lademo, O.-G.; Berstad, T.; Furu, T.; Hopperstad, O. Influence of texture and grain structure on strain localisation and formability for AlMgSi alloys. J. Mater. Process. Technol. 2008, 200, 77–93. [Google Scholar] [CrossRef]
- Georgakou, A.-G. Phase-Field Simulation of Recrystallization and Grain Growth: Description of MICRESS Software and Application in AI-Mg-Sc-Zr Alloy. Bachelor’s Thesis, University of Volos, Volos, Greece, 2013. [Google Scholar]
- Ratchev, P.; Jessner, P. Role of the Mn-Dispersoids and Mg2Si Particles in the Recrystallization of Automotive 6xxx Alloys. Mater. Sci. Forum 2014, 794, 1227–1232. [Google Scholar] [CrossRef]
- Farè, S.; Lecis, N.; Vedani, M. Aging Behaviour of Al-Mg-Si Alloys Subjected to Severe Plastic Deformation by ECAP and Cold Asymmetric Rolling. J. Met. 2011, 2011, 1–8. [Google Scholar] [CrossRef]
- Adamczyk-Cieślak, B.; Mizera, J. Ultra-Fine Grain Structures of Model Al-Mg-Si Alloys Produced by Hydrostatic Extrusion. In Proceedings of the International Conference on Advances in Materials and Processing Technologies, Paris, France, 24–27 October 2010. [Google Scholar]
- Kai, X.; Chen, C.; Sun, X.; Wang, C.; Zhao, Y. Hot deformation behavior and optimization of processing parameters of a typical high-strength Al–Mg–Si alloy. Mater. Des. 2016, 90, 1151–1158. [Google Scholar] [CrossRef]
- Werinos, M.; Antrekowitsch, H.; Kozeschnik, E.; Ebner, T.; Moszner, F.; Löffler, J.; Uggowitzer, P.; Pogatscher, S. Ultrafast artificial aging of Al–Mg–Si alloys. Scr. Mater. 2016, 112, 148–151. [Google Scholar] [CrossRef]
- Yang, M.; Wei, H.; Zhang, J.; Zhao, Y.; Jin, T.; Liu, L.; Young, S. Phase-field study on effects of antiphase domain and elastic energy on evolution of γ′ precipitates in nickel-based superalloys. Comput. Mater. Sci. 2017, 129, 211–219. [Google Scholar] [CrossRef]
- Fallah, V.; Korinek, A.; Raeisinia, B.; Gallerneault, M.; Esmaeili, S. Early-Stage Precipitation Phenomena and Composi-tion-dependent Hardening in Al-Mg-Si-(Cu) Alloys. Mater. Sci. Forum 2014, 794–796, 933–938. [Google Scholar] [CrossRef]
- Qiao, J.; Chen, J.; Che, H. Crashworthiness assessment of square aluminum extrusions considering the damage evolution. Thin-Walled Struct. 2006, 44, 692–700. [Google Scholar] [CrossRef]
- Yang, M.; Chen, H.; Orekhov, A.; Lu, Q.; Lan, X.; Li, K.; Zhang, S.; Song, M.; Kong, Y.; Schryvers, D.; et al. Quantified contri-bution of β″ and β′ precipitates to the strengthening of an aged Al–Mg–Si alloy. Mater. Sci. Eng. A 2020, 774, 138776. [Google Scholar] [CrossRef]
- Nandy, S.; Ray, K.K.; Das, D. Process model to predict yield strength of AA6063 alloy. Mater. Sci. Eng. A 2015, 644, 413–424. [Google Scholar] [CrossRef]
- Sarafoglou, P.I.; Serafeim, A.; Fanikos, I.A.; Aristeidakis, J.S.; Haidemenopoulos, G.N. Modeling of Microsegregation and Homogenization of 6xxx Al-Alloys Including Precipitation and Strengthening During Homogenization Cooling. Material 2019, 12, 1421. [Google Scholar] [CrossRef]
- Kuijpers, N.C.; Vermolen, F.J.; Vuik, C.; Van der Zwang, S. A Model of the β-AlFeSi to α-Al(FeMn)Si transformation in Al-Mg-Si Alloys. Mater. Trans. 2003, 44, 1448–1456. [Google Scholar] [CrossRef]
- Liang, Y. An Investigation on the Optimum Aging Condition for HFQ-processed AA6082 Aluminium Alloy. Master’s Thesis, University of Birmingham, Birmingham, UK, 2016. [Google Scholar]
- Edwards, G.A.; Stiller, K.; Dunlop, G.L.; Couper, M.J. The precipitation sequence in Al-Mg-Si alloys. Acta Mater. 1998, 46, 3893–3904. [Google Scholar] [CrossRef]
- Herrnring, J.; Kashaev, N.; Klusemann, B. Precipitation Kinetics of AA6082: An Experimental and Numerical Investigation. Mater. Sci. Forum 2018, 941, 1411–1417. [Google Scholar] [CrossRef]
- Wagner, R.; Kampman, R. Materials Science and Technology-A Comprehensive Treatment; Wiley-VCH: Weinheim, Germany, 1991; p. 5. [Google Scholar]
- Du, Q.; Tang, K.; Marioara, C.D.; Andersen, S.J.; Holmedal, B.; Holmestad, R. Modeling over-ageing in Al-Mg-Si alloys by a multi-phase Calphad-coupled Kampmann-Wagner Numerical model. Acta Mater. 2017, 122, 178–186. [Google Scholar] [CrossRef]
- Holmedal, B.; Osmundsen, E.; Du, Q. Precipitation of Non-Spherical Particles in Aluminum Alloys Part I: Generalization of the Kampmann–Wagner Numerical Model. Met. Mater. Trans. A 2016, 47, 581–588. [Google Scholar] [CrossRef]
- Parson, N.C.; Yiu, H.L. The effect of heat treatment on the microstructure and properties of 6000 series alloy extrusion ingots. Miner. Met. Mater. Soc. 1989, 40, 18. [Google Scholar]
- Camero, S.; Puchi, E.S.; Gonzalez, G. Effect of 0.1% vanadium on precipitation behavior and mechanical properties of Al-6063 commercial alloy. J. Mater. Sci. 2006, 41, 7361–7373. [Google Scholar] [CrossRef]
- Polmer, I. Light Alloys: From Traditional Alloys to Nanocrystals, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Eastering, K.; Porter, D. Phase Transformations in Metals; Chapman & Hall: Hong Kong, China, 1992. [Google Scholar]





| Element | Mg | Si | Fe | Mn | Al |
|---|---|---|---|---|---|
| 1st case (wt.%) | 0.9 | 0.6 | 0.35 | 0.1 | 98.05 (bal.) |
| 2nd case (wt.%) | 1.0 | 1.1 | 0.35 | 0.1 | 97.45 (bal.) |
| Parameter | Value |
|---|---|
| Annealing Conditions | 400 °C/5 min [22] |
| Interface Energy | 0.32 J/m2 [22] |
| Interface Mobility | 3.8 × 10−5 cm4/Js [24] |
| Shear Modulus Equation | (84.8–4.06 × 10−2 × T)/(2 × (1 + v)) [22] |
| Poisson Ration (v) | 0.33 [22] |
| Burger Vector | 0.286 nm [22] |
| Dislocation Density (pi) | 0.5 × 1014 1/m2 [22] |
| Energy Threshold | 4.42 × 10−2 MPa |
| Pinning Force | 0.18 1/μm [25] |
| Miller Indices (hkl) and (uvw) | 001 and 100 respectively |
| Number of Cells (x × z) | 500 × 500 |
| Cell Dimension | 0.5 μm |
| Maximum Rotation Angle | 15° |
| Prefactor of Interfacial Energy for Low-angle Grain Boundaries | 0.2 |
| Prefactor of Interfacial Mobility for Low-angle Grain Boundaries | 0.1 |
| Parameter | Value |
|---|---|
| Ageing Conditions | 220 °C-4 h [29] |
| Number of Cells (x × z) | 500/800/1100/1400/1700/2000 |
| Cell Dimension (nm) | 40/25/18.2/14.3/11.8/10 |
| Composition wt.% | Al-0.9Mg-0.6Si |
| Parameter | Value |
|---|---|
| Microstructure dimension | 20 × 20 μm2 |
| Interface energy (Al phase/Al phase-Mg2Si) | 0.26 J/m2 [1]/0.18 J/m2 [33] |
| Interface mobility (Al phase/Al phase-Mg2Si) | 3.2 × 10−13 m4/Js/2 × 10−13 m4/Js |
| Matrix Phase molar volume | 10.1 × 10−6 m3/mol |
| Mg2Si Phase molar volume | 12.9 × 10−6 m3/mol |
| Parameter | Value |
|---|---|
| kSi | 66.3 MPa/wt%3 [1] |
| kMg | 29.0 MPa/wt%3 [1] |
| Δσgb | 16 MPa [1] |
| Taylor Factor M | 3.1 [1] |
| rc | 5 nm [9] |
| GAl | 26.5 GPa [33] |
| GMg2Si | 37.4 GPa [33] |
| Γ = (GAlb2)/2 | 1.1025 × 10−14 GPa × m2 [33] |
| Simulation | Mg Diffusion Coefficient (cm2/s) | Si Diffusion Coefficient (cm2/s) |
|---|---|---|
| 180 °C (Al-0.9Mg-0.6Si) | 2.40 × 10−15 | 3.77 × 10−15 |
| 180 °C (Al-1.0Mg-1.1Si) | 2.35 × 10−15 | 3.66 × 10−15 |
| 200 °C (Al-0.9Mg-0.6Si) | 9.08 × 10−15 | 1.42 × 10−14 |
| 200 °C (Al-1.0Mg-1.1Si) | 9.14 × 10−15 | 1.37 × 10−14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baganis, A.; Bouzouni, M.; Papaefthymiou, S. Phase Field Simulation of AA6XXX Aluminium Alloys Heat Treatment. Metals 2021, 11, 241. https://doi.org/10.3390/met11020241
Baganis A, Bouzouni M, Papaefthymiou S. Phase Field Simulation of AA6XXX Aluminium Alloys Heat Treatment. Metals. 2021; 11(2):241. https://doi.org/10.3390/met11020241
Chicago/Turabian StyleBaganis, Antonis, Marianthi Bouzouni, and Spyros Papaefthymiou. 2021. "Phase Field Simulation of AA6XXX Aluminium Alloys Heat Treatment" Metals 11, no. 2: 241. https://doi.org/10.3390/met11020241
APA StyleBaganis, A., Bouzouni, M., & Papaefthymiou, S. (2021). Phase Field Simulation of AA6XXX Aluminium Alloys Heat Treatment. Metals, 11(2), 241. https://doi.org/10.3390/met11020241










