Laves Phase Formation in High Entropy Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results
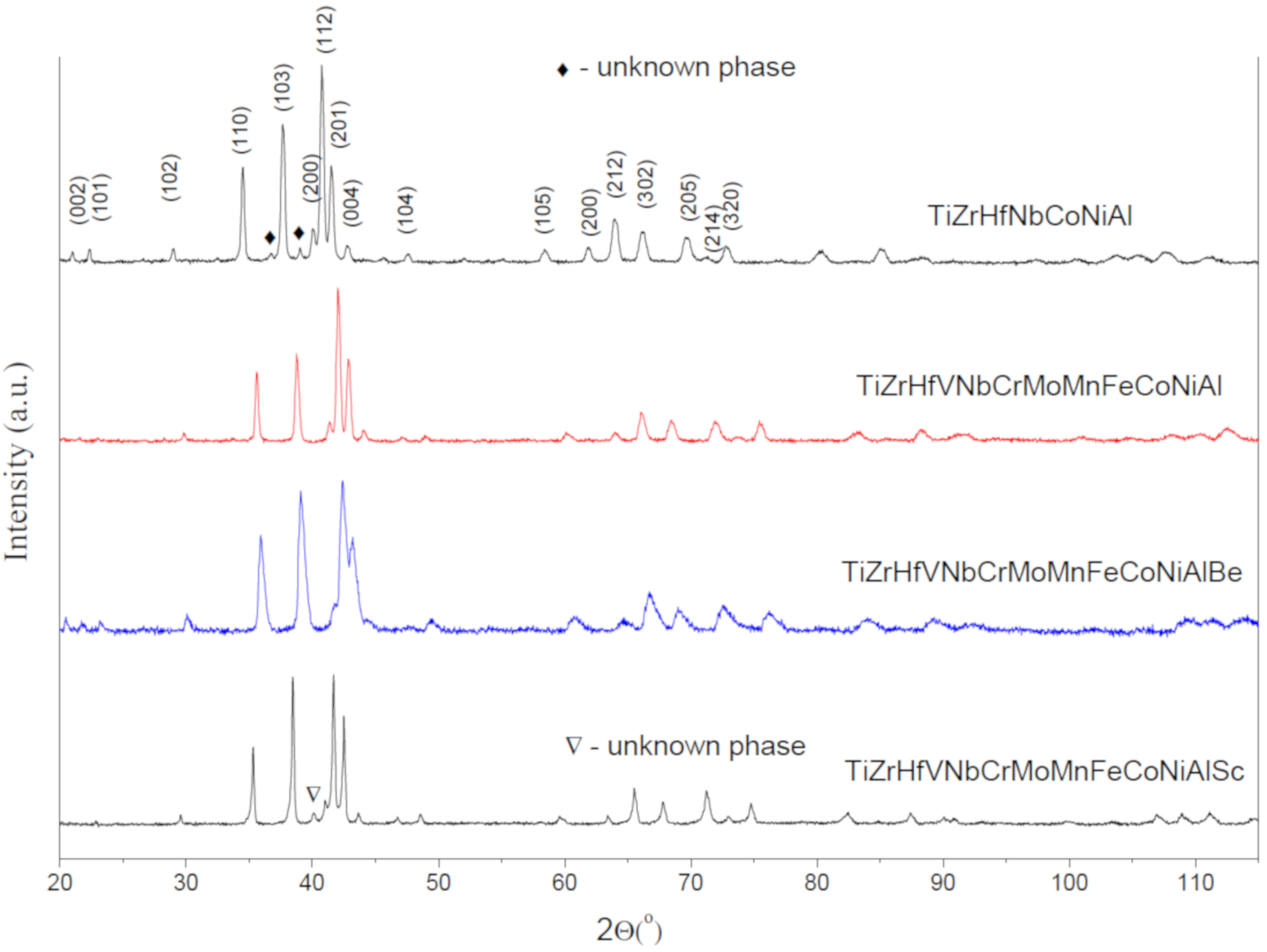
3.1. X-ray Diffraction Analysis
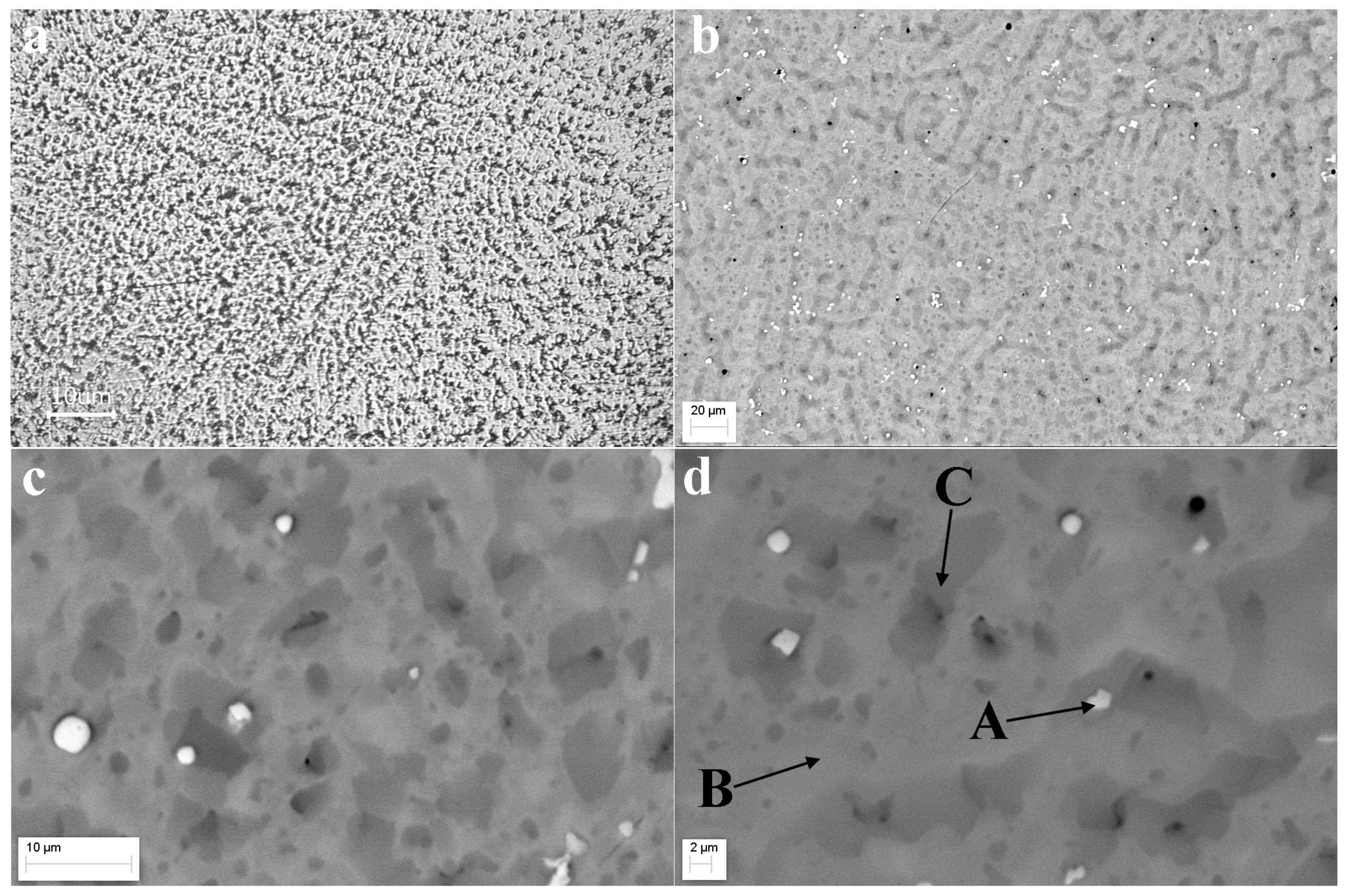
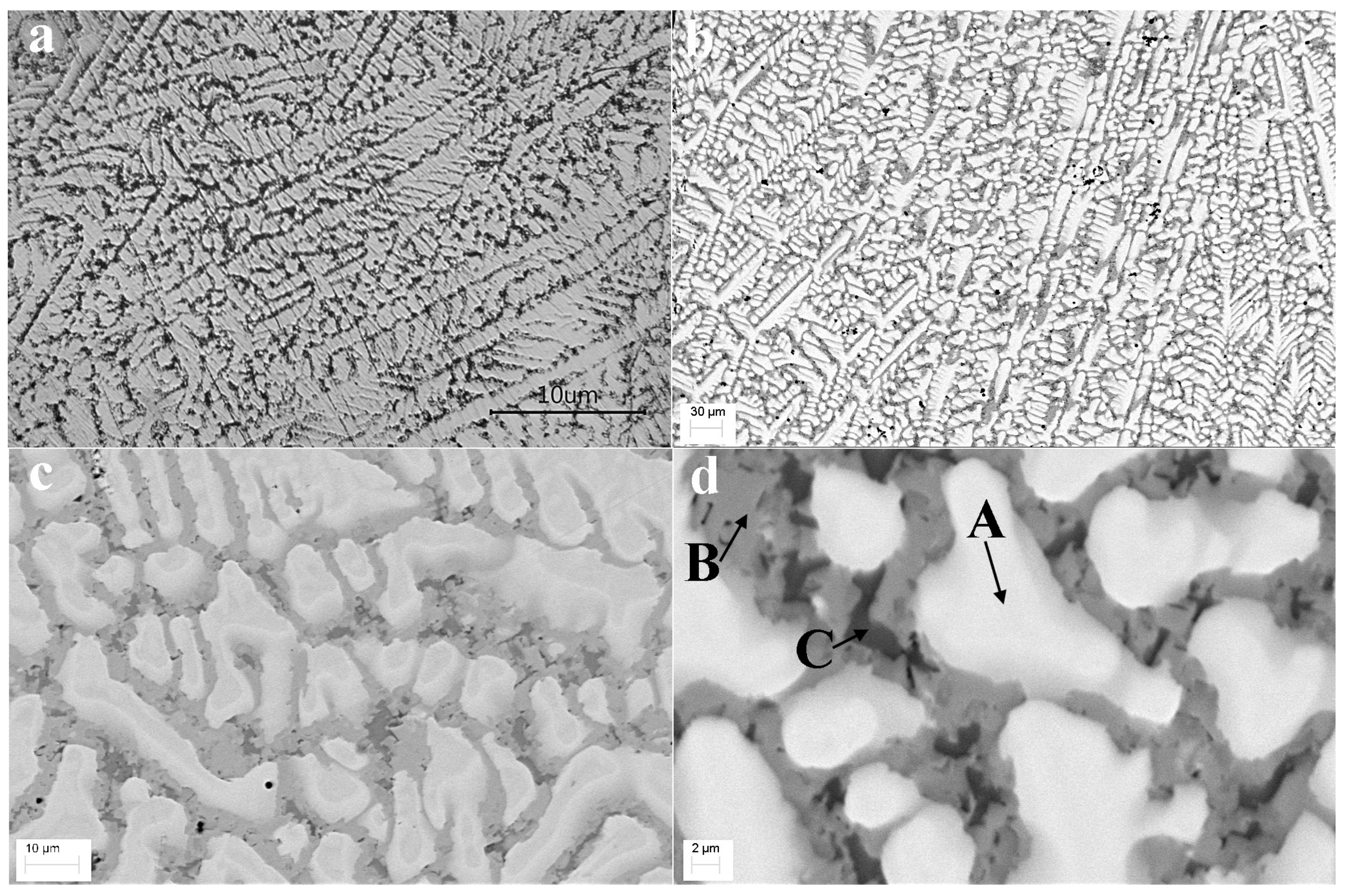
3.2. Optical and SEM Analysis
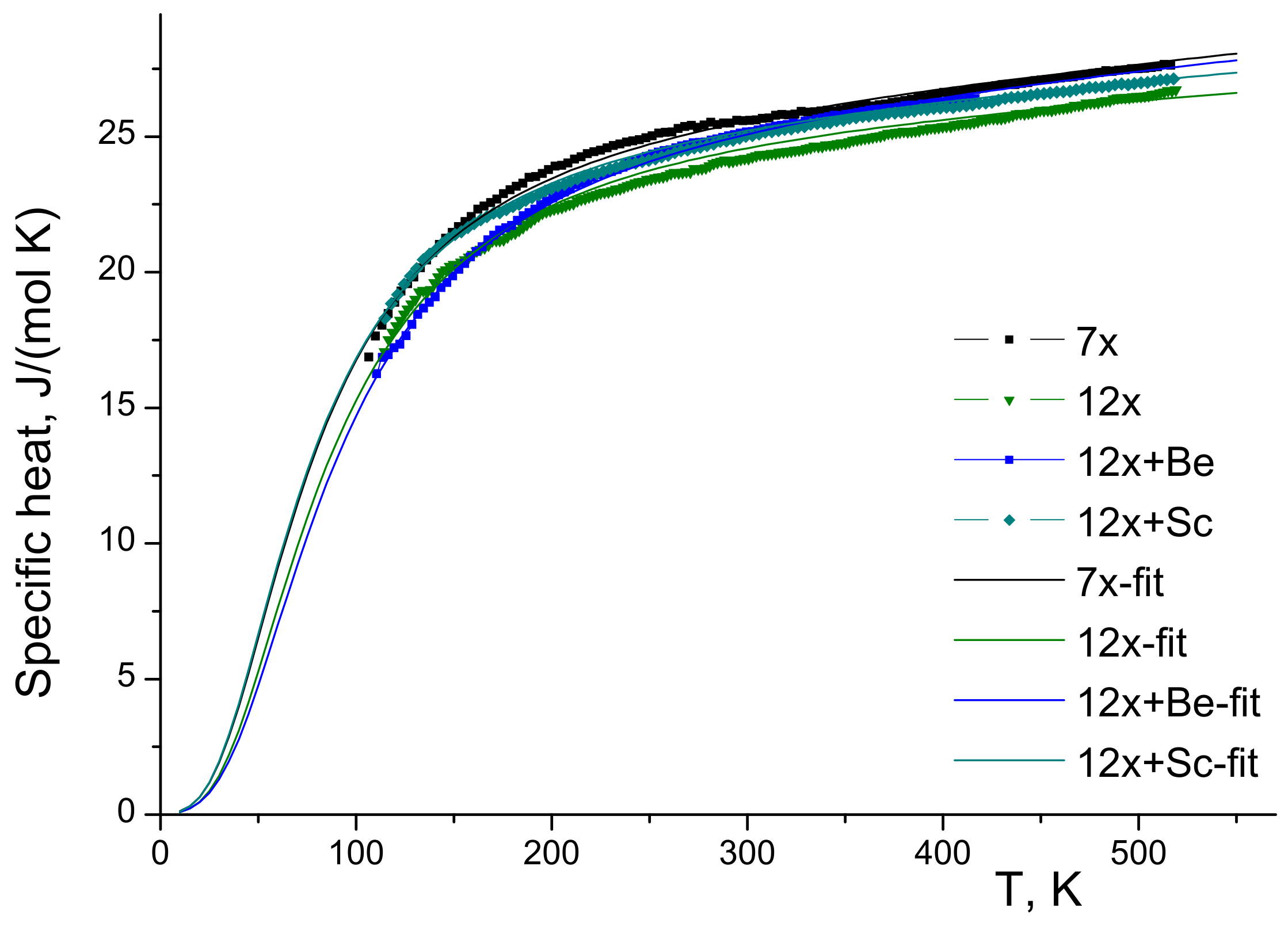
3.3. Specific Heat
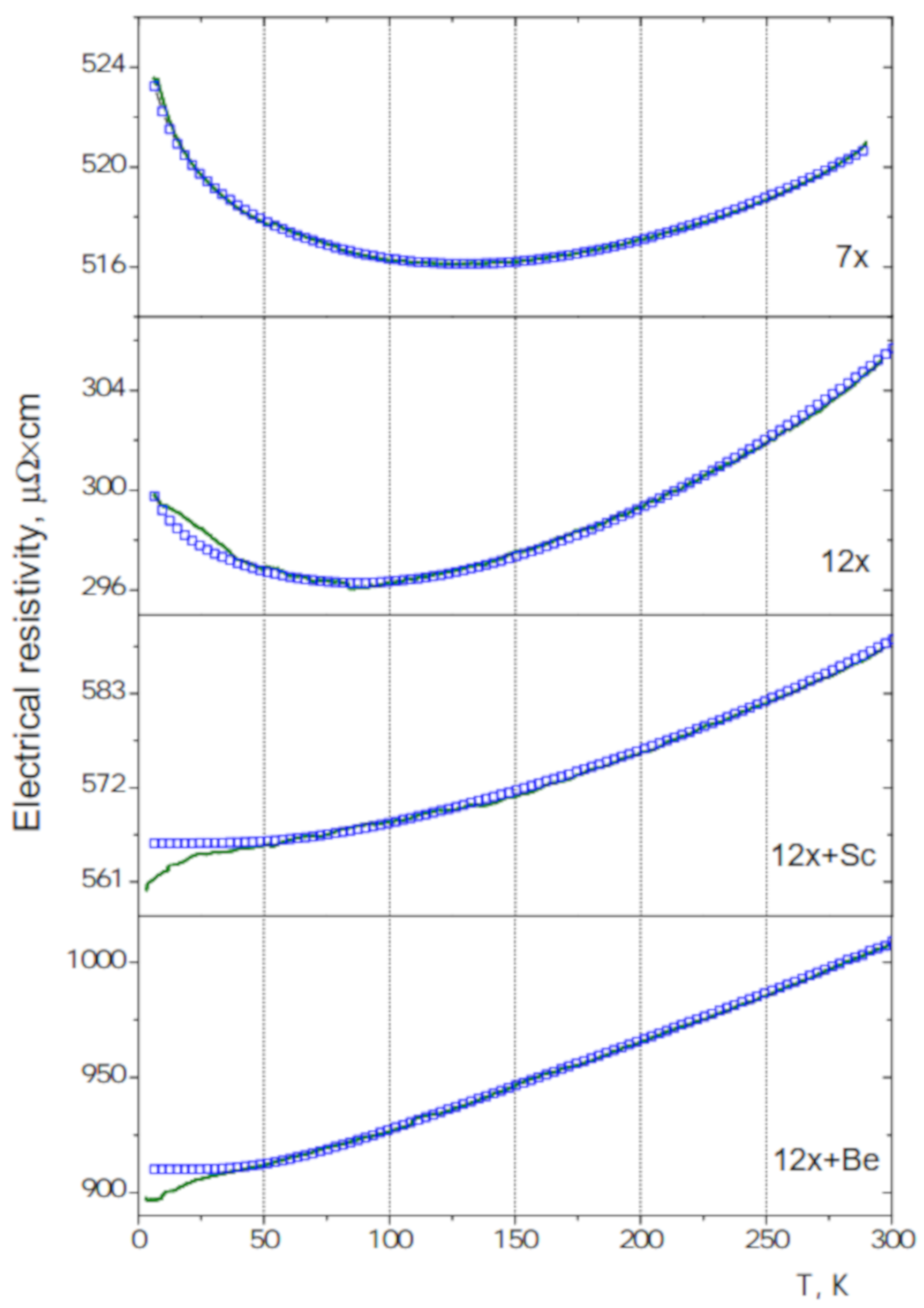
3.4. Electrical Resistivity
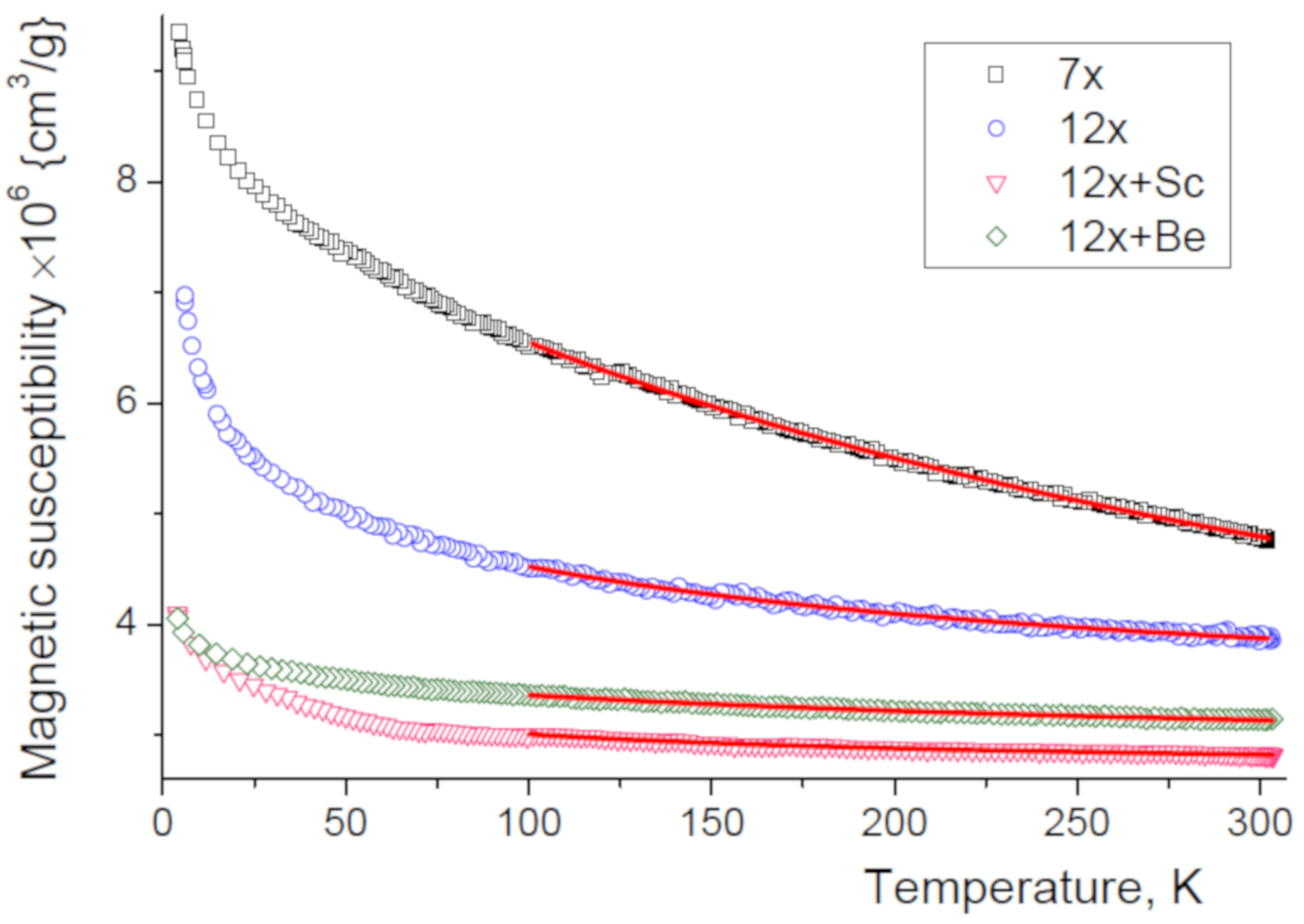
3.5. Magnetic Properties
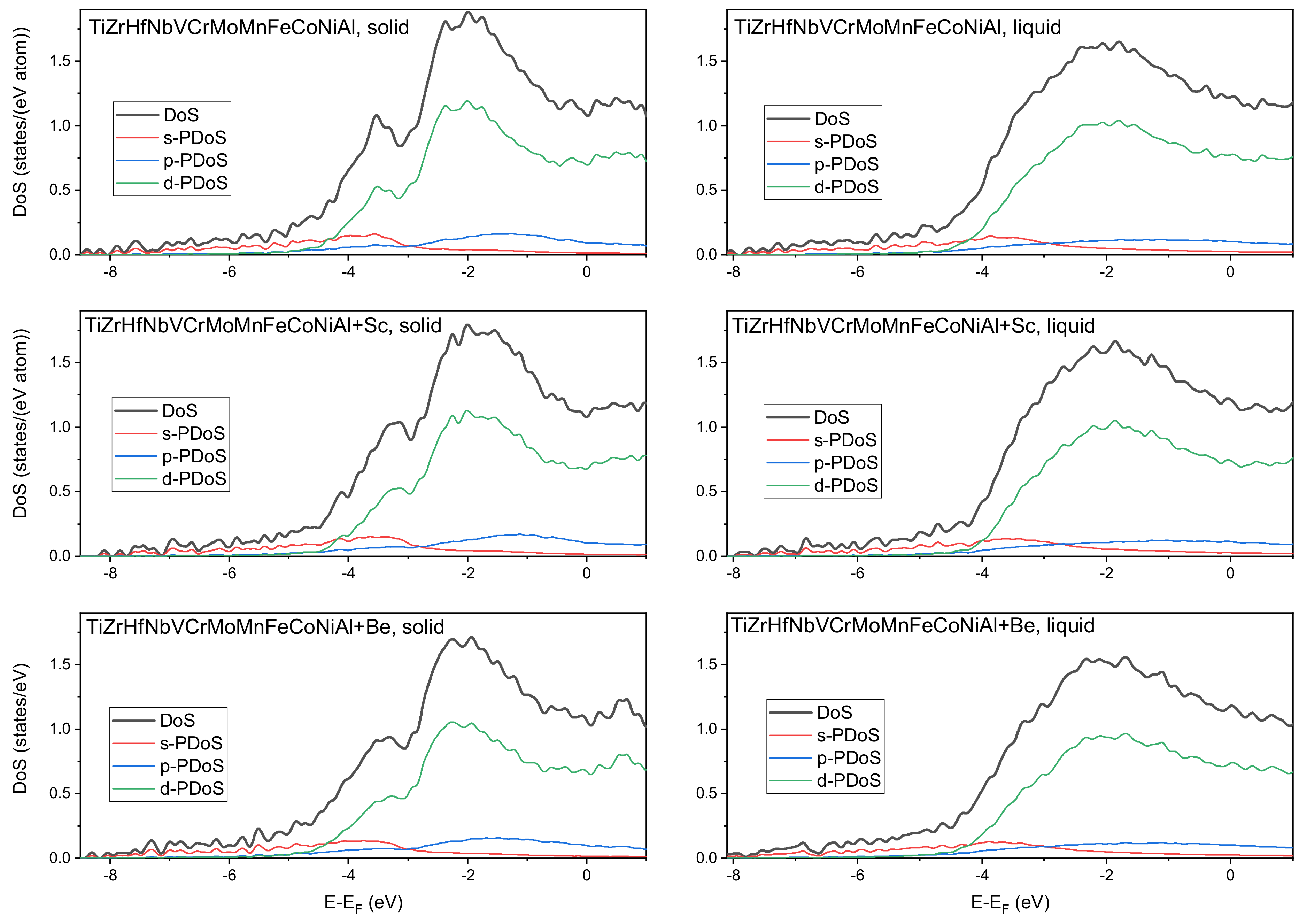
3.6. Ab Initio Calculations
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HEA | high-entropy alloys |
| HELP | high-entropy Laves phases |
| HEIC | high-entropy intermetallic compounds |
| SSS | simple solid solutions |
| VEC | valence electron concentration |
References
- Biswas, K.; Yeh, J.W.; Bhattacharjee, P.P.; DeHosson, J.T. High entropy alloys: Key issues under passionate debate. Scr. Mater. 2020, 188, 54–58. [Google Scholar] [CrossRef]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Miracle, D.; Senkov, O. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Liaw, P.K.; Zhang, Y. Science and technology in high-entropy alloys. Sci. China Mater. 2018, 61, 2–22. [Google Scholar] [CrossRef] [Green Version]
- Praveen, S.; Kim, H.S. High-Entropy Alloys: Potential Candidates for High-Temperature Applications—An Overview. Adv. Eng. Mater. 2018, 20, 1700645. [Google Scholar] [CrossRef]
- Tsai, M.H.; Yeh, J.W. High-Entropy Alloys: A Critical Review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Pouraliakbar, H.; Shim, S.H.; Kim, Y.K.; Rizi, M.S.; Noh, H.; Hong, S.I. Microstructure evolution and mechanical properties of (CoCrNi)90(AlTiZr)5(CuFeMo)5 multicomponent alloy: A pathway through multicomponent alloys toward new superalloys. J. Alloys Compd. 2021, 860, 158412. [Google Scholar] [CrossRef]
- Steurer, W. Single-phase high-entropy alloys—A critical update. Mater. Charact. 2020, 162, 110179. [Google Scholar] [CrossRef]
- Zhou, N.; Jiang, S.; Huang, T.; Qin, M.; Hu, T.; Luo, J. Single-phase high-entropy intermetallic compounds (HEICs): Bridging high-entropy alloys and ceramics. Sci. Bull. 2019, 64, 856–864. [Google Scholar] [CrossRef] [Green Version]
- Yao, K.; Liu, L.; Ren, J.; Guo, Y.; Liu, Y.; Cao, Y.; Feng, R.; Wu, F.; Qi, J.; Luo, J.; et al. High-entropy intermetallic compound with ultra-high strength and thermal stability. Scr. Mater. 2021, 194, 113674. [Google Scholar] [CrossRef]
- Karati, A.; Nagini, M.; Ghosh, S.; Shabadi, R.; Pradeep, K.G.; Mallik, R.C.; Murty, B.S.; Varadaraju, U.V. Ti2NiCoSnSb—A new half-Heusler type high-entropy alloy showing simultaneous increase in Seebeck coefficient and electrical conductivity for thermoelectric applications. Sci. Rep. 2019, 9, 5331. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, A.; Breitung, B.; Hahn, H. High entropy oxides: The role of entropy, enthalpy and synergy. Scr. Mater. 2020, 187, 43–48. [Google Scholar] [CrossRef]
- Mishra, S.S.; Mukhopadhyay, S.; Yadav, T.P.; Mukhopadhyay, N.K.; Srivastava, O.N. Synthesis and characterization of hexanary Ti-Zr-V-Cr-Ni-Fe high-entropy Laves phase. J. Mater. Res. 2019, 34, 807–818. [Google Scholar] [CrossRef]
- Yadav, T.P.; Mukhopadhyay, S.; Mishra, S.S.; Mukhopadhyay, N.K.; Srivastava, O.N. Synthesis of a single phase of high-entropy Laves intermetallics in the Ti-Zr-V-Cr-Ni equiatomic alloy. Philos. Mag. Lett. 2017, 97, 494–503. [Google Scholar] [CrossRef]
- Mishra, S.; Yadav, T.; Srivastava, O.; Mukhopadhyay, N.; Biswas, K. Formation and stability of C14 type Laves phase in multi component high-entropy alloys. J. Alloys Compd. 2020, 832, 153764. [Google Scholar] [CrossRef]
- Gorban, V.F.; Krapivka, N.A.; Firstov, S.A. High-entropy alloys: Interrelations between electron concentration, phase composition, lattice parameter, and properties. Phys. Met. Metallogr. 2017, 118, 970–981. [Google Scholar] [CrossRef]
- Park, H.J.; Na, Y.S.; Hong, S.H.; Kim, J.T.; Kim, Y.S.; Lim, K.R.; Park, J.M.; Kim, K.B. Phase evolution, microstructure and mechanical properties of equi-atomic substituted TiZrHfNiCu and TiZrHfNiCuM (M = Co, Nb) high-entropy alloys. Met. Mater. Int. 2016, 22, 551–556. [Google Scholar] [CrossRef]
- Kao, Y.F.; Chen, S.K.; Sheu, J.H.; Lin, J.T.; Lin, W.E.; Yeh, J.W.; Lin, S.J.; Liou, T.H.; Wang, C.W. Hydrogen storage properties of multi-principal-component CoFeMnTixVyZrz alloys. Int. J. Hydrogen Energy 2010, 35, 9046–9059. [Google Scholar] [CrossRef]
- Uporov, S.; Ryltsev, R.; Estemirova, S.; Sterkhov, E.; Chtchelkatchev, N. Stable high-entropy TiZrHfNbVCrMoMnFeCoNiAl Laves phase. Scr. Mater. 2021, 193, 108–111. [Google Scholar] [CrossRef]
- Yurchenko, N.; Stepanov, N.; Salishchev, G. Laves-phase formation criterion for high-entropy alloys. Mater. Sci. Technol. 2017, 33, 17–22. [Google Scholar] [CrossRef]
- Zhu, J.H.; Liu, C.T.; Pike, L.M.; Liaw, P.K. A thermodynamic interpretation of the size-ratio limits for laves phase formation. Metall. Mater. Trans. A 1999, 30, 1449–1452. [Google Scholar] [CrossRef]
- Uporov, S.; Ryltsev, R.; Bykov, V.; Estemirova, S.K.; Zamyatin, D. Microstructure, phase formation and physical properties of AlCoCrFeNiMn high-entropy alloy. J. Alloys Compd. 2020, 820, 153228. [Google Scholar] [CrossRef]
- Williamson, G.; Hall, W. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Ohba, T.; Kitano, Y.; Komura, Y. The charge-density study of the Laves phases, MgZn2 and MgCu2. Acta Crystallogr. Sect. C 1984, 40, 1–5. [Google Scholar] [CrossRef]
- Stein, F.; Leineweber, A. Laves phases: A review of their functional and structural applications and an improved fundamental understanding of stability and properties. J. Mater. Sci. 2021, 56, 5321–5427. [Google Scholar] [CrossRef]
- Coey, J.M. Magnetism and Magnetic Materials; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Kresse, G.; Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Zunger, A.; Wei, S.H.; Ferreira, L.G.; Bernard, J.E. Special quasirandom structures. Phys. Rev. Lett. 1990, 65, 353–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van de Walle, A.; Tiwary, P.; de Jong, M.; Olmsted, D.; Asta, M.; Dick, A.; Shin, D.; Wang, Y.; Chen, L.Q.; Liu, Z.K. Efficient stochastic generation of special quasirandom structures. Calphad 2013, 42, 13–18. [Google Scholar] [CrossRef]
- Oganov, A.R.; Glass, C.W. Crystal structure prediction using ab initio evolutionary techniques: Principles and applications. J. Chem. Phys. 2006, 124, 244704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oganov, A.R.; Valle, M. How to quantify energy landscapes of solids. J. Chem. Phys. 2009, 130, 104504. [Google Scholar] [CrossRef] [PubMed]
- Kamaeva, L.; Ryltsev, R.; Ladyanov, V.; Chtchelkatchev, N. Viscosity, undercoolability and short-range order in quasicrystal-forming Al-Cu-Fe melts. J. Mol. Liq. 2020, 299, 112207. [Google Scholar] [CrossRef]
- Lindquist, B.A.; Jadrich, R.B.; Truskett, T.M. Communication: From close-packed to topologically close-packed: Formation of Laves phases in moderately polydisperse hard-sphere mixtures. J. Chem. Phys. 2018, 148, 191101. [Google Scholar] [CrossRef] [PubMed] [Green Version]

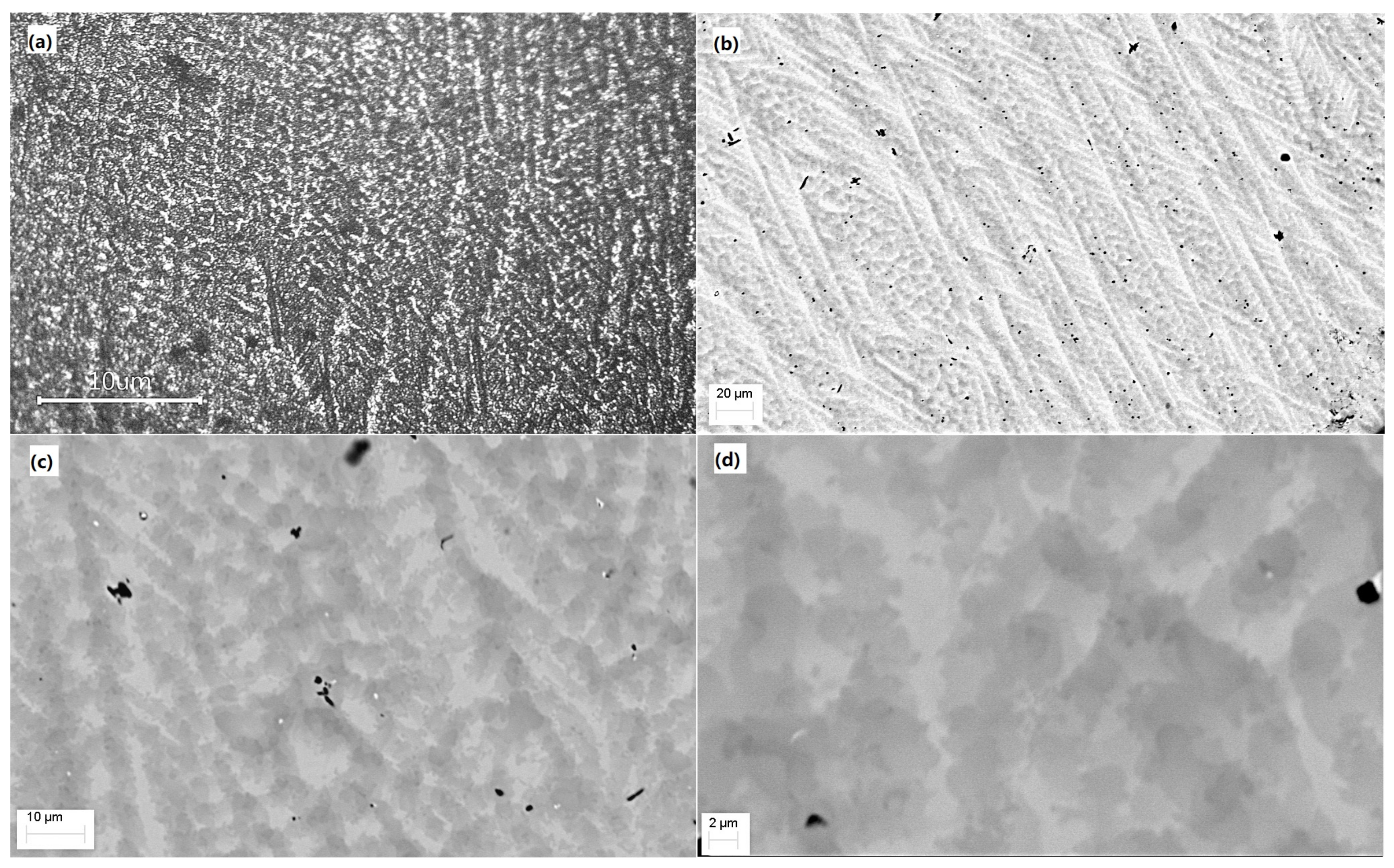
| Parameter | 7x | 12x | 12x + Sc | 12x + Be |
|---|---|---|---|---|
| a, Å | 5.1923(3) | 5.03234(6) | 5.07770(2) | 4.9835(9) |
| c, Å | 8.436(1) | 8.2041(2) | 8.2778(5) | 8.1298(4) |
| c/a | 1.625 | 1.630 | 1.630 | 1.631 |
| V, Å | 196.96(5) | 179.930(4) | 184.78(1) | 174.859(9) |
| (%) | 6.7 | 3.3 | 10.3 | 3 |
| (%) | 8.5 | 4.4 | 14.0 | 5 |
| (%) | 7.4 | 6.7 | 8.4 | 7 |
| (%) | 2.5 | 1.3 | 4.5 | 1.01 |
| 2.6 | 2.6 | 1.9 | 3.9 |
| Region | Ti | Zr | Hf | Nb | Co | Ni | Al |
|---|---|---|---|---|---|---|---|
| A | 13.93 | 16.44 | 15.70 | 14.93 | 14.08 | 14.36 | 10.56 |
| B | 16.28 | 13.44 | 14.75 | 12.23 | 14.11 | 14.30 | 14.89 |
| C | 12.73 | 14.10 | 13.87 | 17.56 | 15.78 | 11.80 | 14.16 |
| Element | White (A) | Gray (B) | Dark (C) |
|---|---|---|---|
| Ti | 5.35 | 6.94 | 11.65 |
| Zr | 19.87 | 8.94 | 8.65 |
| Hf | 46.37 | 9.22 | 6.84 |
| Nb | - | 9.89 | 8.80 |
| V | 3.79 | 8.59 | 8.46 |
| Cr | 3.55 | 9.19 | 7.10 |
| Mo | - | 9.46 | 8.04 |
| Mn | 2.28 | 5.06 | 4.66 |
| Fe | 4.27 | 9.19 | 7.91 |
| Co | 4.15 | 8.02 | 8.86 |
| Ni | 5.14 | 7.36 | 10.41 |
| Al | 5.26 | 8.15 | 8.61 |
| Element | White (A) | Gray (B) | Dark (C) |
|---|---|---|---|
| Ti | 4.79 | 12.20 | 6.26 |
| Zr | 7.91 | 8.59 | 5.26 |
| Hf | 10.47 | 5.59 | 3.82 |
| Nb | 10.21 | 7.34 | 1.72 |
| V | 8.39 | 6.33 | 1.09 |
| Cr | 11.73 | 4.56 | 0.92 |
| Mo | 9.76 | 4.41 | 0.69 |
| Mn | 6.74 | 5.87 | 1.04 |
| Fe | 9.74 | 6.57 | 1.45 |
| Co | 6.51 | 10.25 | 7.27 |
| Ni | 2.73 | 10.51 | 34.68 |
| Al | 7.52 | 8.44 | 3.92 |
| Sc | 3.52 | 9.34 | 31.87 |
| Sample | , | , K | , mJ/(mol ×) | A, | C, | D, | , K |
|---|---|---|---|---|---|---|---|
| 7x | 515.4 | 310 | 64 | 120 | |||
| 12x | 295.4 | 340 | 39 | 80 | |||
| 12x + Sc | 565.1 | 305 | 51 | - | - | ||
| 12x + Be | 910.0 | 360 | 62 | - | - |
| Sample | () | (K) | () | (eV) |
|---|---|---|---|---|
| 7x | −329 | 1.23 | 0.8 | |
| 12x | −130 | 0.43 | 2.3 | |
| 12x + Sc | −60 | 0.17 | 1.9 | |
| 12x + Be | −195 | 0.29 | 1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryltsev, R.; Gaviko, V.; Estemirova, S.; Sterkhov, E.; Cherepanova, L.; Yagodin, D.; Chtchelkatchev, N.; Dubinin, N.; Uporov, S. Laves Phase Formation in High Entropy Alloys. Metals 2021, 11, 1962. https://doi.org/10.3390/met11121962
Ryltsev R, Gaviko V, Estemirova S, Sterkhov E, Cherepanova L, Yagodin D, Chtchelkatchev N, Dubinin N, Uporov S. Laves Phase Formation in High Entropy Alloys. Metals. 2021; 11(12):1962. https://doi.org/10.3390/met11121962
Chicago/Turabian StyleRyltsev, Roman, Vasiliy Gaviko, Svetlana Estemirova, Evgenii Sterkhov, Lubov Cherepanova, Denis Yagodin, Nikolay Chtchelkatchev, Nikolay Dubinin, and Sergey Uporov. 2021. "Laves Phase Formation in High Entropy Alloys" Metals 11, no. 12: 1962. https://doi.org/10.3390/met11121962
APA StyleRyltsev, R., Gaviko, V., Estemirova, S., Sterkhov, E., Cherepanova, L., Yagodin, D., Chtchelkatchev, N., Dubinin, N., & Uporov, S. (2021). Laves Phase Formation in High Entropy Alloys. Metals, 11(12), 1962. https://doi.org/10.3390/met11121962