Effect of Rotating Magnetic Field on Microstructure in AlCuSi Alloys
Abstract
:1. Introduction
1.1. Rosettes
1.2. Spheroids
1.3. Dendrites
1.4. Eutectics
1.5. Intermetallics
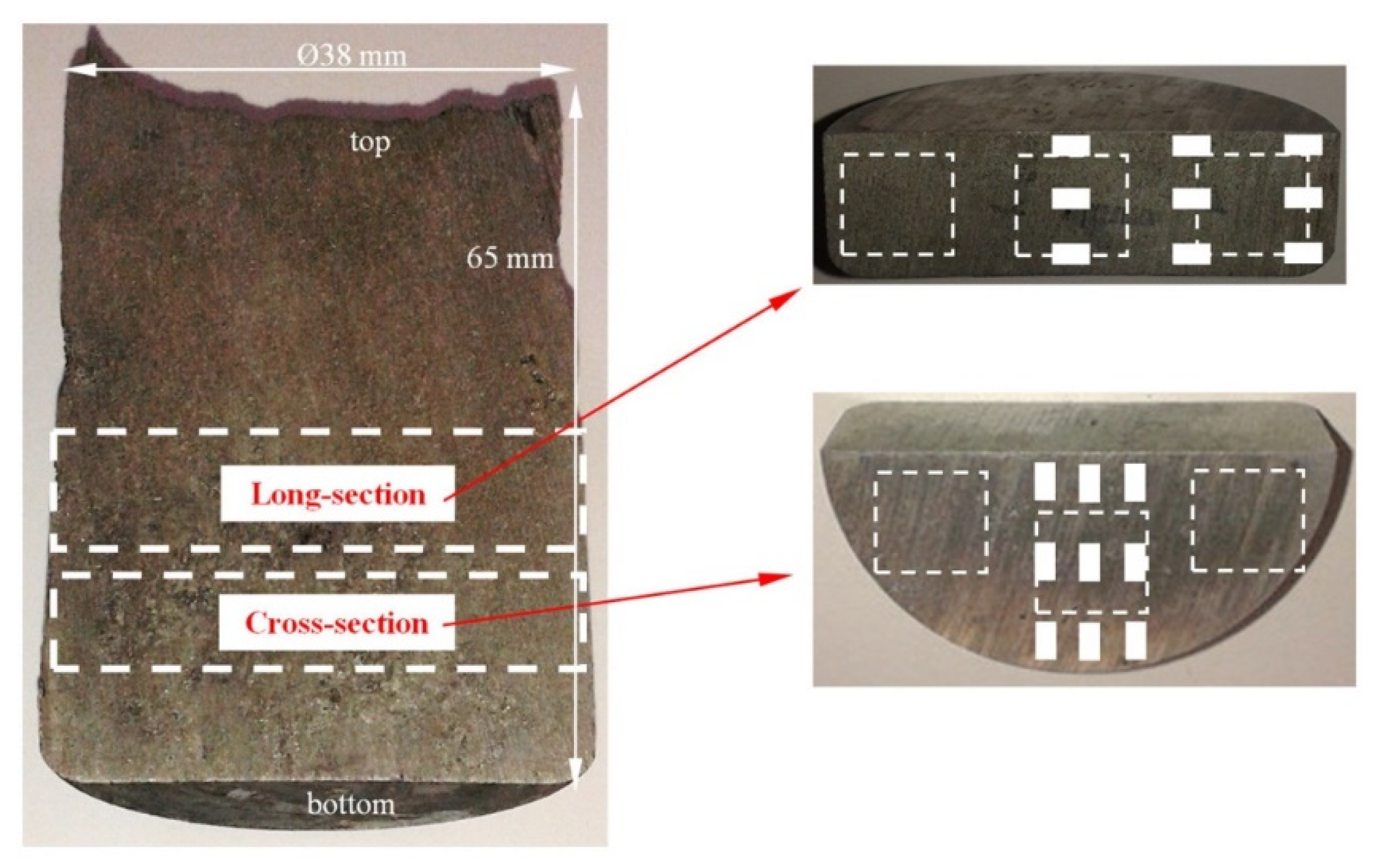
2. Materials and Methods
3. Results
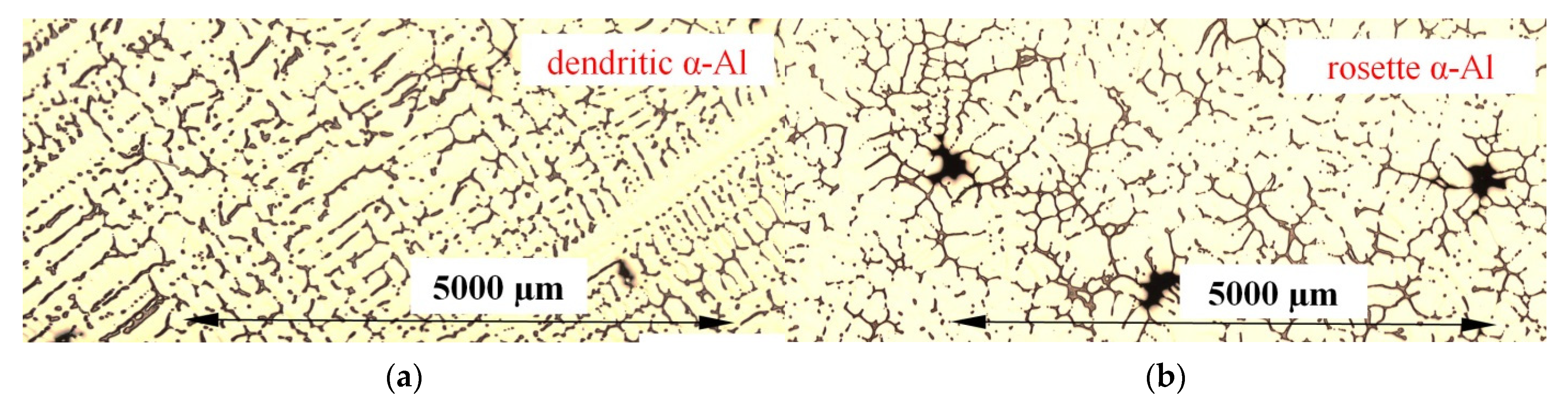
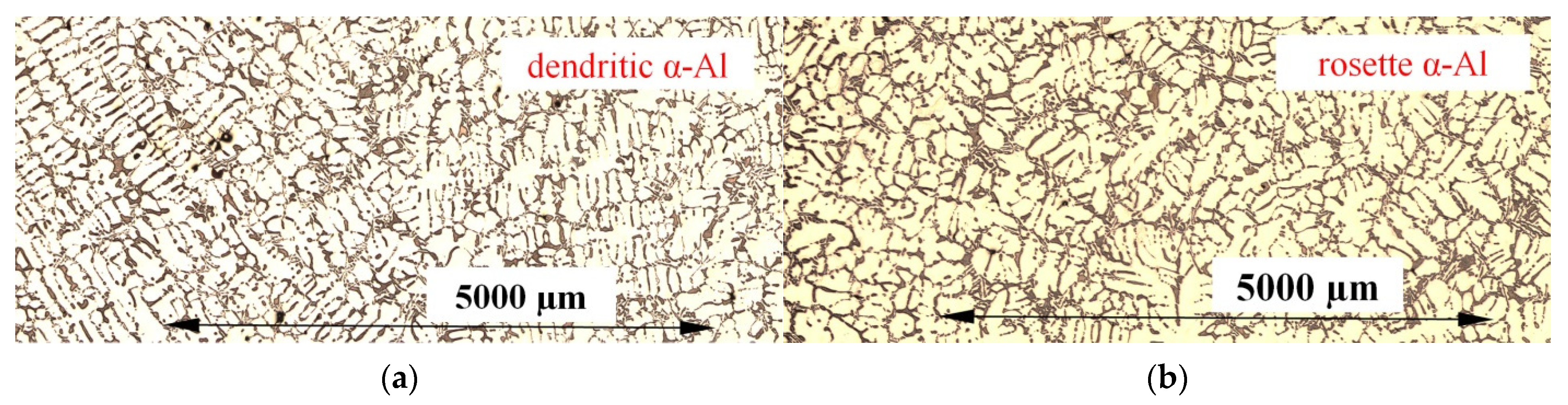
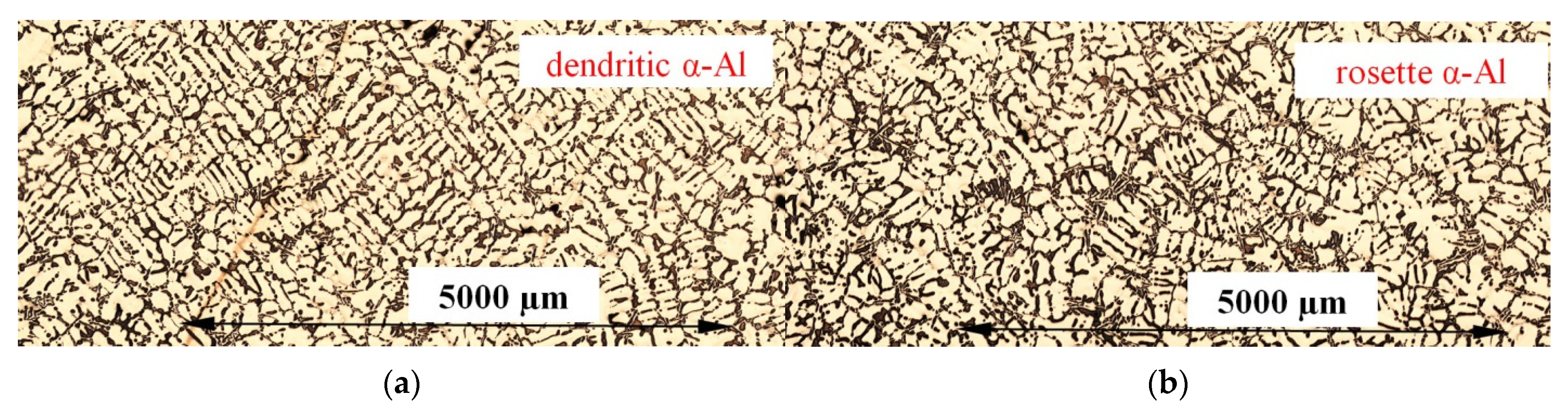
3.1. Microstructure
3.2. Parameters Characterising the Microstructure
3.3. Precipitation Sequence
4. Discussion
4.1. Rosettes
4.2. Spheroids
4.3. Dendrites
4.4. Eutectics
4.5. Intermetallics
4.6. Solidification by Stirring
5. Conclusions
- The forced convection generated by electric coils led to the formation of rosettes with smaller dendrites instead of equiaxed dendrites, as an effect of the rotation of the dendrite tip and ripened arms of the deformed dendrites. Precipitating minor spheroids were found as part of deformed dendritic crystals.
- Stirring shortened the solidification time and secondary dendrite arm spacing λ2 for almost all alloys. The exponents in the formula for λ2 calculations should take different values from those determined in previous research.
- For almost all the studied alloys, Sv decreased (in the range between −23% and −41%) under fluid flow, which means that the dendrites or rosettes are more oval and larger under the influence of stirring. The calculation of Sv based on solidification time requires an accurate coefficient.
- Forced convection causes a decrease in the length of β-Al5FeSi and an increase in the number density in alloys, where β platelets precipitate after other phases (e.g α-Al). What is completely new is that, in the alloys where iron-rich phases precipitate first and grow initially alone in the liquid alloy, melt stirring causes an increase in length and a decrease in number density.
- Melt stirring causes a decrease in the length of Mn-rich phases and an increase in the number density in all the studied alloys, both in alloys where Mn-phases form as the first precipitates and also when they grow as second or third precipitates.
- Melt flow changed the eutectic spacing λE depending on the alloy composition. In Fe-containing alloys, an increase was noticed, while in Mn-containing alloys, λE decreased. The presence of Mn in the alloy increased λAl2Cu only weakly.
- For all the studied alloys it was found, that forced convection appears to have no effect on the spacing of Al2Cu eutectics precipitating below the solidus temperature, and vice versa, Al2Cu does not determine fluid flow and phases occurring during solidification processes.
- There was a decrease in the length of β-Al5FeSi and Mn-phases caused by stirring occurred in equiaxed solidification without remelting, probably through mechanical fragmentation, modified solute distribution and additional nucleation sites. The increase in the β platelet length due to solidification in fully liquid alloys probably results from modified solute distribution and lack of obstacles.
- The application of electromagnetically induced melt flow and the efficiency in microstructure modification depends on the phase growth sequence, the precipitating phases, and the chemical composition of the alloys.
Funding
Data Availability Statement
Conflicts of Interest
References
- Mondolfo, L.F. Aluminium Alloys: Structure and Properties; Butterworths & Co.: London, UK, 1976. [Google Scholar]
- Glazoff, M.V.; Zolotorevsky, V.S.; Belov, N.A. Casting Aluminum Alloys; Elsevier Science Pub Co.: Amsterdam, The Netherlands, 2007; ISBN 13:978-0080453705. [Google Scholar] [CrossRef]
- Nong, G. (Ed.) Aluminum Alloys; MDPI: Basel, Switzerland, 2018. [Google Scholar]
- Flemings, M. Behavior of metal alloys in the semisolid state. Metall. Mat. Trans. B 1991, 22B, 269–293. [Google Scholar] [CrossRef]
- Pola, A.; Tocci, M.; Kapranos, P. Microstructure and Properties of Semi-Solid Aluminum Alloys: A Literature Review. Metals 2018, 8, 181. [Google Scholar] [CrossRef] [Green Version]
- Modigell, M.; Pola, A.; Tocci, M. Rheological Characterization of Semi-Solid Metals: A Review. Metals 2018, 8, 245. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhou, R.; Li, L.; Xiao, H.; Jiang, Y. Microstructure and Properties of Semi-solid ZCuSn10P1 Alloy Processed with an Enclosed Cooling Slope Channel. Metals 2018, 8, 275. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Xiao, G.; Che, C.; Wang, Y. Microstructure, Mechanical Properties and Wear Behavior of the Rheoformed 2024 Aluminum Matrix Composite Component Reinforced by Al2O Nanoparticles. Metals 2018, 8, 460. [Google Scholar] [CrossRef] [Green Version]
- Beil, W.L.; Brollo, G.L.; Zoqui, E.J. A Continuous Casting Device with Electromagnetic Stirring for Production of SSM Feedstock Using Al-Si Alloys. Mat. Res. 2021, 24, 3. [Google Scholar] [CrossRef]
- Brollo, G.L.; Proni, C.T.W.; Zoqui, E.J. Thixoforming of an Fe-Rich Al-Si-Cu Alloy—Thermodynamic Characterization, Microstructural Evolution, and Rheological Behavior. Metals 2018, 8, 332. [Google Scholar] [CrossRef] [Green Version]
- Eslami, M.; Payandeh, M.; Deflorian, F.; Jarfors, A.E.W.; Zanella, C. Effect of Segregation and Surface Condition on Corrosion of Rheo-HPDC Al–Si Alloys. Metals 2018, 8, 209. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, M.N.; Omar, M.Z.; Al-Zubaidi, S.; Alhawari, K.S.; Abdelgnei, M.A. Microstructure and Mechanical Properties of Thixowelded AISI D2 Tool Steel. Metals 2018, 8, 316. [Google Scholar] [CrossRef] [Green Version]
- Nafisi, S.; Ghomashchi, R. Semi-Solid Processing of Aluminum Alloys; Springer: Berlin, Germany, 2016. [Google Scholar]
- Wang, H.; Davidson, C.J.; St. John, D.H. Semisolid microstructural evolution of AlSi7Mg during partial remelting. Mater. Sci. Eng. A 2004, 368, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Pacheco, M.G. Electromagnetic Processing of Molten Light Alloys Reinforced by Micro/Nanoparticles. Ph.D. Thesis, Universite Grenoble Alpes UGA, Grenoble, France, 13 March 2017. [Google Scholar]
- Das, A.; Ji, S.; Fan, Z. Solidification microstructures obtained by a novel twin screw liquidus casting method. In Proceedings of the 7th International Conference on Demi-Solid Processing of Alloys and Composites, Tsukuba, Japan, 25–27 September 2002; pp. 689–694. [Google Scholar]
- Li, M.; Murakami, Y.; Matsui, I.; Omura, N.; Tada, S. Imposition Time Dependent Microstructure Formation in 7150 Aluminum Alloy Solidified by an Electromagnetic Stirring Technique. Mater. Trans. 2018, 59, 1603–1609. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Zhang, Z.; Mao, W.; Li, B.; Bai, Y.; Xu, J. Numerical and Experimental Study on Melt Treatment for Large-Volume 7075 Alloy by a Modified Annular Electromagnetic Stirring. Materials 2019, 12, 820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, C.K. Microstructure of Semi-Solid Billets Produced by Electromagnetic Stirring and Behavior of Primary Particles during the Indirect Forming Process. Metals 2018, 8, 271. [Google Scholar] [CrossRef] [Green Version]
- Nakato, H.; Oka, M.; Itoyama, S.; Urata, M.; Kawasaki, T.; Hashiguchi, K.; Okano, S. Continous Semi-Solid Casting Process for Aluminum Alloy Billets. Mater. Trans. 2002, 43, 24–29. [Google Scholar] [CrossRef]
- Ferreira, A.F.; Castro, J.A.; Ferreira, L.O. Predicting Secondary-Dendrite Arm Spacing of the Al-4.5wt%Cu Alloy During Unidirectional Solidification. Mater. Res. 2017, 20, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Barros, A.S.; Mahno, I.A.; Souza, F.A.; Mota, C.A.; Moreira, A.L.; Silva, M.A.; Rocha, O.L. Measurements of Microhardness During Transient Horizontal Directional Solidification of Al-Rich Al-Cu Alloys: Effect of Thermal Parameters, Primary Dendrite Arm Spacing and Al2Cu Intermetallic Phase. Met. Mater. Int. 2015, 21, 429–439. [Google Scholar] [CrossRef]
- Lei, Q.; Wang, J.; Misra, A. Mechanical Behavior of Al–Al2Cu–Si and Al–Al2Cu Eutectic Alloys. Crystals 2021, 11, 194. [Google Scholar] [CrossRef]
- Belov, N.A.; Aksenov, A.A.; Eskin, D.G. Iron in Aluminium Alloys—Impurity and Alloying Element, 1st ed.; Taylor and Francis Group: London, UK, 2002. [Google Scholar] [CrossRef]
- Shabestari, S.G. The effect of iron and manganese on the formation of intermetallic compounds in aluminum-silicon alloys. Mater. Sci. Eng. 2004, 383, 289–298. [Google Scholar] [CrossRef]
- Thermo-Calc 4.1—Software package from Thermo-Calc Software AB. Stockholm, Sweden. Available online: www.thermocalc.se (accessed on 4 August 2021).
- Niroumand, B.; Xia, K. 3D study of the structure of primary crystals in a rheocast Al-Cu alloy. Mater. Sci. Eng. 2000, 283, 70–75. [Google Scholar] [CrossRef]
- Birol, Y. A357 thixoforming feedstock produced by cooling slope casting. J. Mater. Process. Technol. 2007, 186, 94–101. [Google Scholar] [CrossRef]
- Mullis, A. Growth induced dendritic bending and rosette formation during solidification in a shearing flow. Acta Mater. 1999, 47, 1783–1789. [Google Scholar] [CrossRef]
- Ji, S.; Fan, Z.; Bevis, M.J. Semi-solid processing of engineering alloys by a twin-screw rheomoulding process. Mater. Sci. Eng. 2001, 299, 210–217. [Google Scholar] [CrossRef]
- Das, A.; Ji, S.; Fan, Z. Morphological development of solidification structures under forced fluid flow: A Monte Carlo simulation. Acta Mater. 2002, 50, 4571–4585. [Google Scholar] [CrossRef]
- Birol, Y. Evolution of globular microstructures during processing of aluminum slurries. Trans. Nonferrous Met. Soc. China 2013, 23, 1–6. [Google Scholar] [CrossRef]
- Li, T.; Lin, X.; Huang, W. Morphological evolution during solidification under stirring. Acta Mater. 2006, 54, 4815–4824. [Google Scholar] [CrossRef]
- Martinez., R.A.; Flemings, M.C. Evolution of particle morphology in semisolid processing. Met. Mat. Trans. A. 2005, 36, 2205–2210. [Google Scholar] [CrossRef]
- Kurz, W.D. Fisher. In Fundamentals of Solidification; Trans Tech Public: Bäch, Switzerland, 1992; pp. 85–90. [Google Scholar]
- Dantzig, J.A.; Rappaz, M. Solidification; EPFL Press: Lausanne, Switzerland, 2009; ISBN 9780849382383. [Google Scholar]
- Stefanescu, D. Science and Engineering of Casting and Solidification; Springer: Boston, MA, USA, 2009; ISBN 978-0-387-74609-8. [Google Scholar] [CrossRef]
- Wang, C.Y.; Beckermann, C. Equiaxed Dendritic Solidification with Convection: Part II. Numerical Simulations for an Al-4 Wt Pct Cu Alloy. Metall. Mater. Trans. A 1996, 27A, 2765. [Google Scholar] [CrossRef]
- Mendoza, R.; Alkemper, J.; Voorhees, P. The morphological evolution of dendritic microstructures during coarsening. Metall. Mater. Trans. A 2003, 34, 481–489. [Google Scholar] [CrossRef]
- Hunt, J.D. Pattern formation in solidification. Sci. Technol. Adv. Mater. 2001, 2, 147–155. [Google Scholar] [CrossRef]
- Hunt, J.D.; Lu, S.Z. Numerical modeling of cellular/dendritic array growth: Spacing and structure predictions. Metall. Mater. Trans. A 1996, 27, 611–623. [Google Scholar] [CrossRef]
- Kattamis, T.Z.; Flemings, M.C. Dendrite morphology. Microsegregation and Homogenization of low alloy steel. Trans. Met. Soc. AIME 1965, 233, 992–999. [Google Scholar]
- Rappaz, M.; Boettinger, W. On dendritic solidification of multicomponent alloys with unequal liquid diffusion coefficients. Acta Mater. 1999, 47, 3205–3219. [Google Scholar] [CrossRef]
- Steinbach, S.; Ratke, L. The influence of fluid flow on the microstructure of directionally solidified AlSi-base alloys. Metall. Mater. Trans. A 2007, 38, 1388–1394. [Google Scholar] [CrossRef]
- Mortensen, A. On the rate of dendrite arm coarsening. Metall. Mater. Trans. A 1991, 22, 569–574. [Google Scholar] [CrossRef]
- Voorhees, P.W.; Glicksman, M.E. Ostwald ripening during liquid phase sintering—Effect of volume fraction on coarsening kinetics. Metall. Mater. Trans. A 1984, 15, 1081–1089. [Google Scholar] [CrossRef]
- Bouchard, D.; Kirkaldy, J.S. Prediction of dendrite arm spacing in unsteady- and steady-state heat flow. Metall. Mater. Trans. B 1997, 28, 651–663. [Google Scholar] [CrossRef]
- Bellon, B.; Boukellal, A.K.; Isensee, T.; Wellborn, O.M.; Trumble, K.P.; Krane, M.J.M.; Titus, M.S.; Tourret, D.; Llorca, J. Multiscale prediction of microstructure length scale in metallic alloy casting. Acta Mater. 2021, 207, 116686. [Google Scholar] [CrossRef]
- Mullis, A.M. The effects of fluid flow on the secondary arm coarsening during dendritic solidification. J. Mater. Sci. 2003, 38, 2517–2523. [Google Scholar] [CrossRef]
- Diepers, H.J.; Beckerman, C.; Steinbach, I. Simulation of convection and ripening in a binary alloy mush using the phase field method. Acta Mater. 1999, 47, 3663–3678. [Google Scholar] [CrossRef] [Green Version]
- Kasperovich, G.; Genau, A.; Ratke, L. Mushy zone coarsening in an AlCu30 alloy accelerated by a rotating magnetic field. Metall. Mater. Trans. A 2011, 42, 1657–1666. [Google Scholar] [CrossRef]
- Ratke, L.; Thieringer, W.K. The influence of particle motion on Ostwald ripening in liquids. Acta Mater. 1985, 33, 1793–1802. [Google Scholar] [CrossRef]
- Marsh, S.P.; Glicksman, M.E. Overview of geometric effects on coarsening of mushy zones. Metall. Mater. Trans. A 1996, 27, 557–567. [Google Scholar] [CrossRef]
- Loué, W.R.; Suéry, M. Microstructural evolution during partial remelting of AlSi7Mg alloys. Mater. Sci. Eng. A 1995, 203, 1–13. [Google Scholar] [CrossRef]
- Jackson, K.A.; Hunt, J.D. Lamellar and rod eutectic growth. Trans. AIME 1966, 236, 1129–1142. [Google Scholar]
- Sous, S. Instationäre Erstarrung Eutektischer Al-Si Legierungen. Ph.D. Thesis, RWTH, Aachen, Germany, 2000. [Google Scholar]
- Mikolajczak, P.; Ratke, L. Intermetallic phases and microstructure in AlSi alloys influenced by fluid flow. Miner. Met. Soc. TMS 2011, 10, 9781118062173. [Google Scholar] [CrossRef]
- Mikolajczak, P.; Ratke, L. Thermodynamic assessment of mushy zone in directional solidification. Arch. Foundry Eng. 2015, 15, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Mikolajczak, P. Microstructural Evolution in AlMgSi Alloys during Solidification under Electromagnetic Stirring. Metals 2017, 7, 89. [Google Scholar] [CrossRef] [Green Version]
- Nafisi, S.; Emad, D.; Shehata, T.; Ghomashchi, R. Effects of electromagnetic stirring and superheat on the microstructural characteristics of Al-Si-Fe alloy. Mater. Sci. Eng. A 2006, 432, 71–83. [Google Scholar] [CrossRef]
- Fang, X.; Shao, G.; Liu, Y.Q.; Fan, Z. Effects of intensive forced melt convection on the mechanical properties of Fe containing Al-Si based alloys. Mater. Sci. Eng. A 2007, 445–446, 65–72. [Google Scholar] [CrossRef]
- Steinbach, S.; Euskirchen, N.; Witusiewicz, V.; Sturz, L.; Ratke, L. Fluid flow effects on intermetallic phases in Al-cast alloys. Trans. Indian Inst. Met. 2007, 60, 137–141. [Google Scholar] [CrossRef]
- Mikolajczak, P.; Ratke, L. Effect of stirring induced by rotating magnetic field on β-Al5FeSi intermetallic phases during directional solidification in AlSi alloys. Int. J. Cast Met. Res. 2013, 26, 339–353. [Google Scholar] [CrossRef]

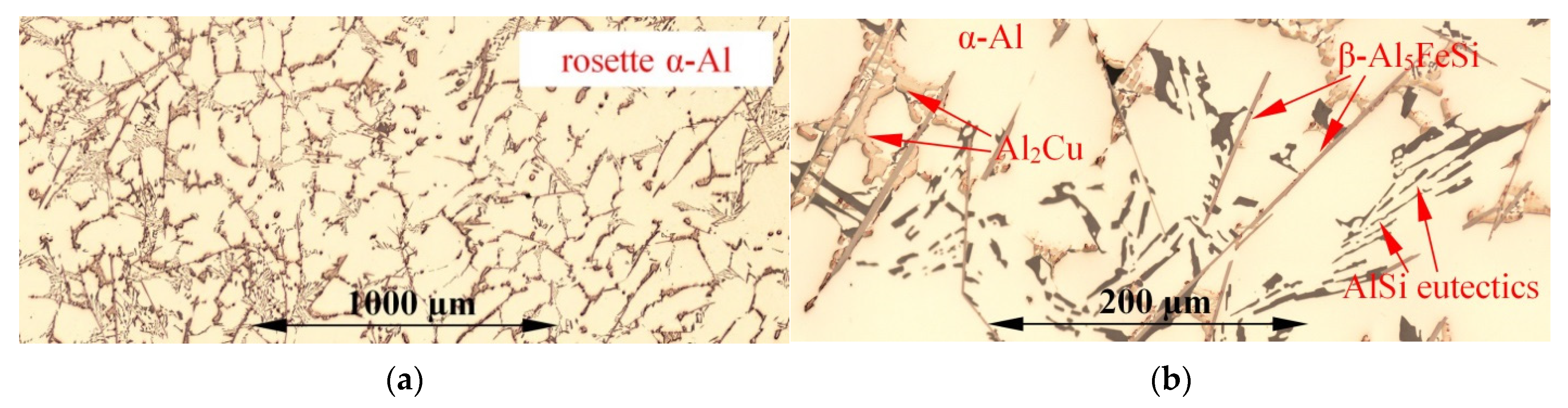
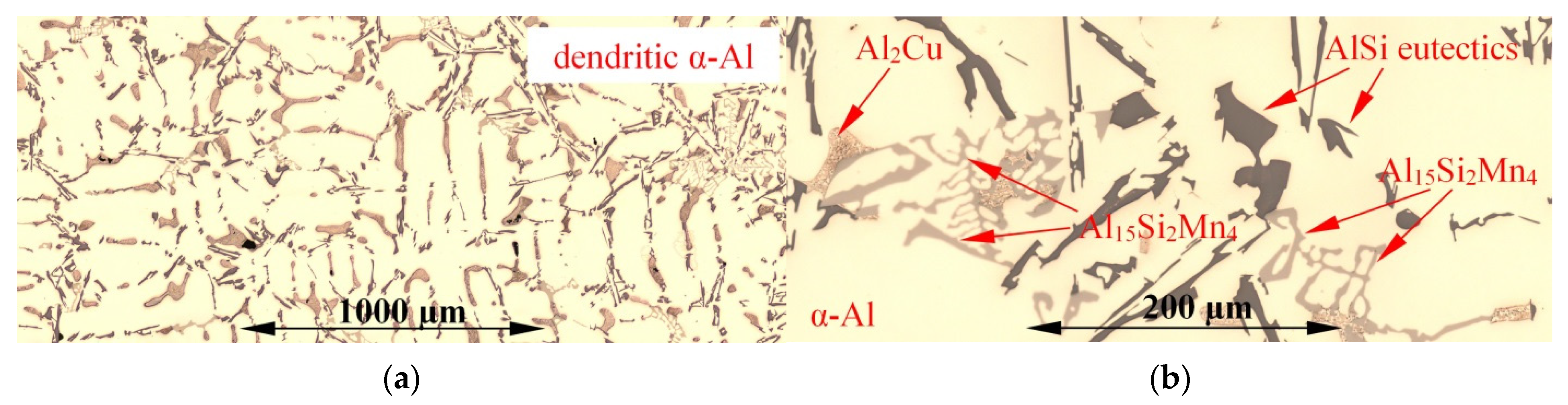


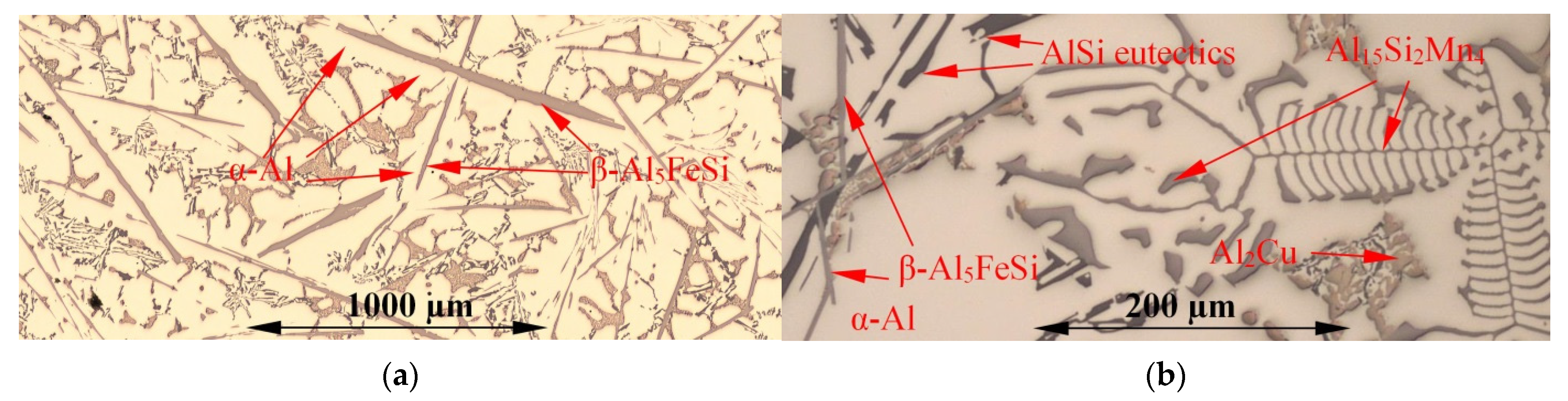
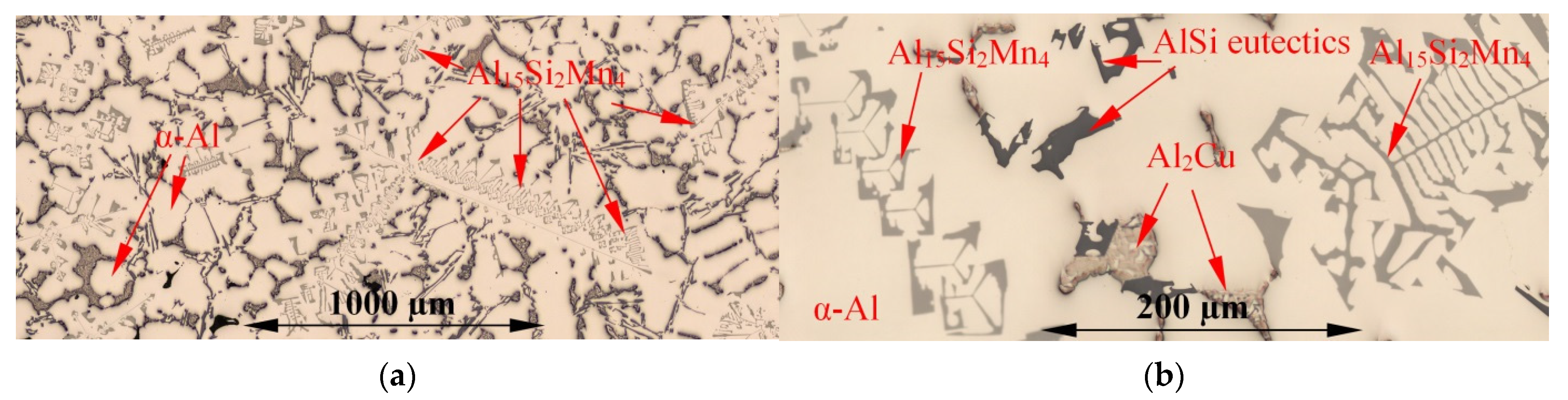
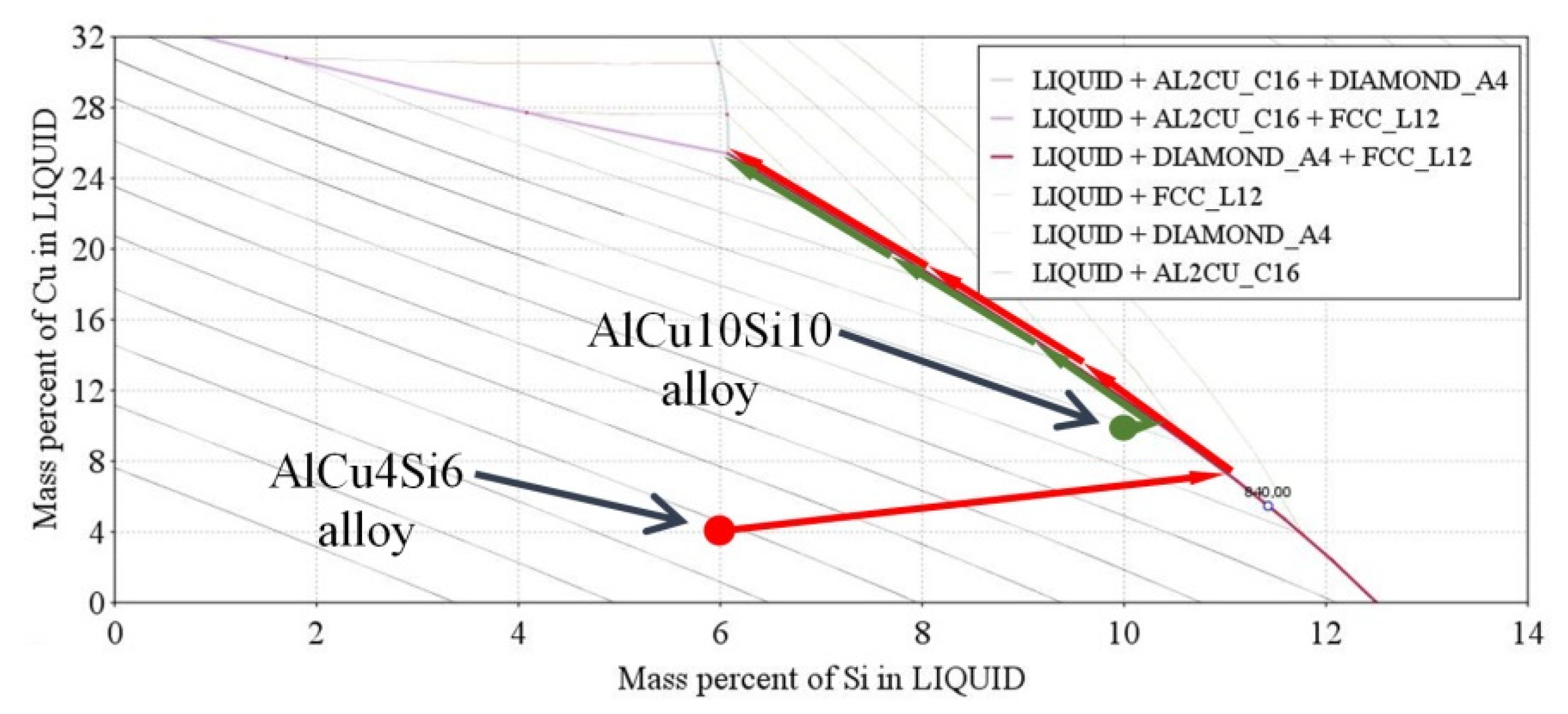
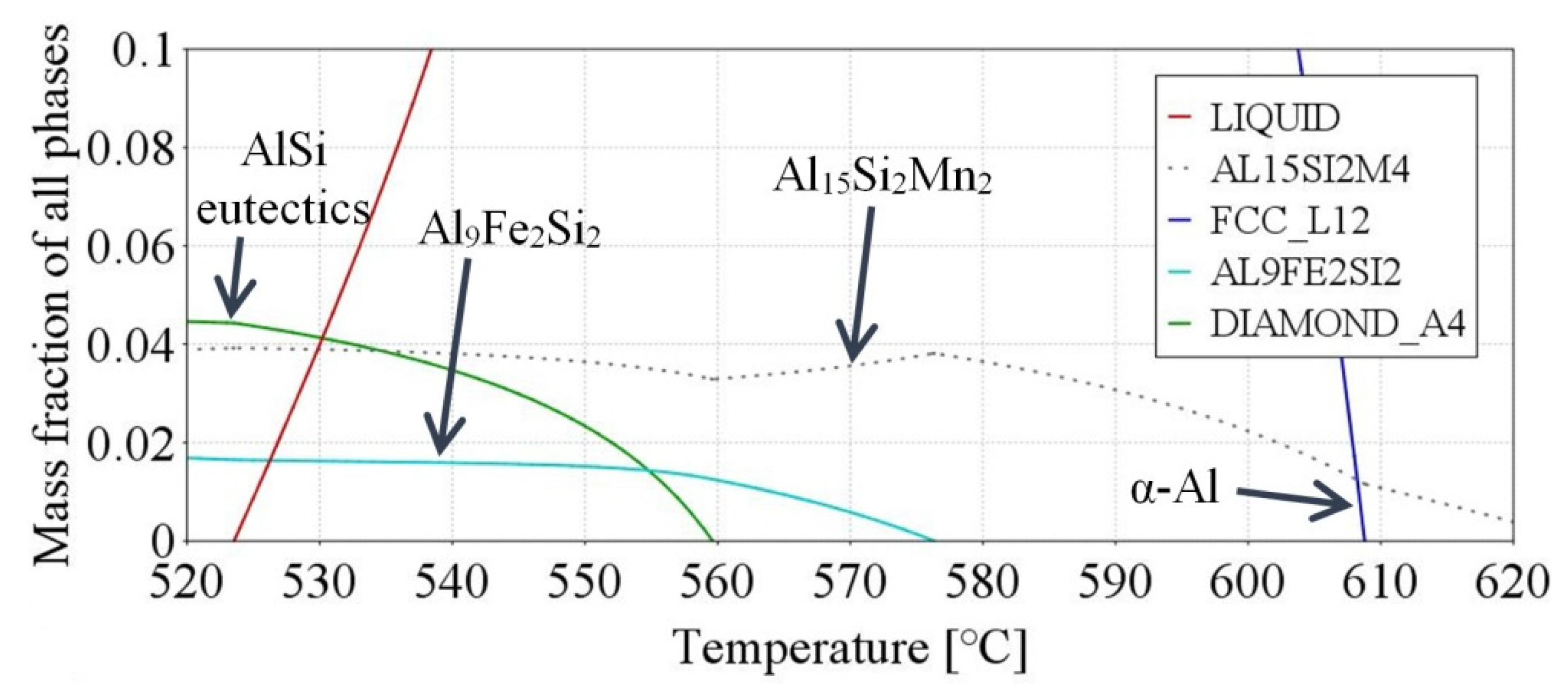

| Aluminium Alloys | RMF [mT] {Solid. Time [s]} | Microstructure Parameters | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Dendrites | Fe-Phases (β-Al5FeSi) | Mn-Phases | AlSi Eutectics | Al2Cu | |||||
| λSDAS [µm] | Sv [µm−1] | Lβ [µm] | nβ [mm−2] | LMn [µm] | nMn [mm−2] | λEut [µm] | λAl2Si [µm] | ||
| AlCu4 | 0{552} | 232 [16.4] (34/660) | 0.027 [0.002] | - | - | - | - | - | 2.53 [0.2] (2.0–2.8) |
| 11{471} | 145 [4.4] (26/242) (−37%) | 0.016 [0.002] (−41%) | - | - | - | - | - | 2.27 [0.2] (1.7–2.7) (−10.3%) | |
| AlCu4Si6 | 0{547} | 99 [5.0] (20/292) | 0.044 [0.002] | - | - | - | - | 39 [4.7] | 5.72 [0.5] (4.8–7.3) |
| 11{463} | 88 [6.5] (21/235) (−11%) | 0.027 [0.001] (−38%) | - | - | - | - | 38 [3.9] (−2.5%) | 8.05 [1.5] (5.4–11.8) (52.7%) | |
| AlCu4Si6Fe1 | 0{627} | 79 [10.3] (20/238) | 0.050 [0.003] | 115 [7.1] (936) | 71 | - | - | 12.4 [2.0] | 9.07 [0.6] (8.1–11.3) |
| 11{548} | 80 [7.1] (22/201) (1%) | 0.032 [0.001] (−36%) | 77 [5.9] (1484) (−33%) | 113 (59%) | - | - | 14.3 [1.9] (15.3%) | 7.45 [1.0] (5.1–10.0) (−17.9%) | |
| AlCu4Si6Mn0.65 | 0{689} | 100 [6.7] (30/294) | 0.043 [0.001] | - | - | 189 [8.7] (122) | 0.17 | 32.7 [4.3] | 5.87 [0.3] (5.2–6.3) |
| 11{612} | 80 [5.8] (28/250) (−20%) | 0.032 [0.002] (−26%) | - | - | 109 [6.5] (188) (−42%) | 0.26 (53%) | 30.5 [2.9] (−6.7%) | 6.62 [0.7] (5.6–8.8) (12.8%) | |
| AlCu4Si6Fe1Mn0.65 | 0{671} | 85 [8.7] (27/293) | 0.047 [0.001] | 80 [5.5] (276) | 21 | 315 [17.3] (129) | 0.18 | 14.3 [2.0] | 8.41 [1.3] (7.2–13.7) |
| 11{608} | 79 [6.0] (36/295) (−7%) | 0.036 [0.002] (−23%) | 75 [5.6] (912) (−6%) | 69 (229%) | 323 [20.3] (53) (3%) | 0.08 (−56%) | 13.2 [2.1] (−7.7%) | 8.59 [0.7] (6.7–10.0) (2.1%) | |
| AlCu10Si10 | 0{724} | 56 [7.5] (33/306) | 0.066 [0.002] | - | - | - | - | 24.1 [3.2] | 13.70 [1.2] (10.9–17.3) |
| 11{637} | 53 [4.8] (25/171) (−5%) | 0.043 [0.003] (−35%) | - | - | - | - | 29.7 [4.7] (23.2%) | 12.38 [1.6] (8.5–13.8) (−9.6%) | |
| AlCu10Si10Fe1 | 0{709} | 66 [7.2] (31/239) | 0.047 [0.005] | 524 [46.3] (231) | 4.2 | - | - | 17.5 [3.0] | 13.08 [1.6] (10.1–17.6) |
| 11{607} | 57 [4.2] (28/220) (−13%) | 0.071 [0.008] (51%) | 642 [43.4] (208) (23%) | 3.7 (−12%) | - | - | 18.9 [2.6] (8.0%) | 15.38 [1.4] (13–21.3) (17.6%) | |
| AlCu4Si6Fe2 | 0{673} | No dendrites | 0.056 [0.004] | 66 [7.2] (3990) | 303 | - | - | 14.6 [1.8] | 6.04 [0.9] (4.4–9.0) |
| 11{597} | No dendrites | 0.041 [0.004] (−27%) | 116 [15.6] (1146) (76%) | 87 (−71%) | - | - | 19.3 [2.3] (32.2%) | 7.92 [1.3] (3.6–11.0) (31.1%) | |
| AlCu4Si6Mn2 | 0{705} | 94 [4.7] (27/223) | 0.036 [0.004] | - | - | 268 [15.2] (205) | 0.27 | 29.5 [3.6] | 7.90 [0.6] (6.4–9.0) |
| 11{611} | 85 [4.8] (32/285) (−9%) | 0.026 [0.003] (−28%) | - | - | 216 [14.2](−19%) | 0.16(−41%) | 26.3 [2.5] (−10.8%) | 9.55 [1.0] (6.5–12.4) (20.9%) | |
| Alloy | Reaction | Temperature Range of Reaction | Mass Fraction of Solid Phases [%] (the Rest Is Liquid Alloy) at the Temperature [°C] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Temperature | α-Al | β-Al9Fe2Si2 | β-Al8Fe2Si | Al15Si2Mn4 | Eut Si | Al2Cu | |||
| AlCu4 | L → α-Al + L | 649.8–571.6 | 571.6 | 100 | - | - | - | - | 0.0 |
| α-Al → α-Al + Al2Cu | 571.6–20.0 | 20 | 92.60 | - | - | - | - | 7.40 | |
| AlCu4Si6 | L → α-Al + L | 611.8–562.9 | 562.9 | 51.99 | - | - | - | 0.0 | 0.0 |
| L → L + α-Al + Si | 562.9–526.3 | 526.3 | 94.98 | - | - | - | 5.02 | 0.0 | |
| α-Al + Si → α-Al + Al2Cu + Si | 507.3–20.0 | 20 | 86,60 | - | - | - | 6.00 | 7.40 | |
| AlCu4Si6Fe1 | L → α-Al + L | 608.9–601.1 | 601.1 | 13.55 | 0.0 | - | - | 0.0 | 0.0 |
| L → α-Al + β-Al9Fe2Si2 | 601.1–560.4 | 560.4 | 52.9 | 3.05 | - | - | 0.0 | 0.0 | |
| L → α-Al + β-Al9Fe2Si2 + Si | 560.4–525.0 | 525.0 | 91.75 | 3.71 | - | - | 4.54 | 0.0 | |
| α-Al + β-Al9Fe2Si2 + Si | 525.0–20.0 | 20 | 83.22 | 3.71 | - | - | 5.67 | 7.40 | |
| AlCu4Si6Mn0.65 | L → α-Al + L | 610.9–593.6 | 593.6 | 26.66 | - | - | 0.0 | 0.0 | 0.0 |
| L → α-Al + Al15Si2Mn4 | 593.6–561.5 | 561.5 | 51.91 | - | - | 1.07 | 0.0 | 0.0 | |
| L → α-Al + Al15Si2Mn4 + Si | 561.5–524.1 | 524.1 | 93.16 | - | - | 2.01 | 4.83 | 0.0 | |
| α-Al + Al15Si2Mn4+ Si → α-Al + Al2Cu + Al15Si2Mn4 + Si | 509.4–20.0 | 20 | 84.60 | - | - | 2.17 | 5.83 | 7.40 | |
| AlCu4Si6Fe1Mn0.65 | L → Al15Si2Mn4 + L | 625.1–608.8 | 608.8 | 0.0 | 0.0 | - | 1.16 | 0.0 | 0.0 |
| L → Al15Si2Mn4 + L + α-Al | 608.8–576.3 | 576.3 | 41.31 | 0.0 | - | 3.81 | 0.0 | 0.0 | |
| L → Al15Si2Mn4 + L + α-Al + β-Al9Fe2Si2 | 576.3–559.6 | 559.6 | 51.94 | 1.25 | - | 3.29 | 0.0 | 0.0 | |
| L → Al15Si2Mn4 + α-Al + β-Al9Fe2Si2 + Si | 559.6–523.5 | 523.5 | 90.00 | 1.65 | - | 3.92 | 4.43 | 0.0 | |
| Al15Si2Mn4 + α-Al + β-Al9Fe2Si2 + Si → Al15Si2Mn4 + α-Al + β-Al9Fe2Si2 + Si + Al2Cu | 513.1–20 | 20 | 81.22 | 3.71 | - | 2.17 | 5.50 | 7.40 | |
| AlCu10Si10 | L → α-Al + L | 560.7–556.9 | 556.9 | 3.40 | - | - | - | 0.0 | 0.0 |
| L → L + α-Al + Si | 556.9–521.6 | 521.6 | 65.35 | - | - | - | 7.71 | 0.0 | |
| L → α-Al + Si+ Al2Cu | 521.6–521.5 | 521.5 | 78.97 | - | - | - | 9.18 | 11.85 | |
| α-Al + Si+ Al2Cu | 521.5–20.0 | 20 | 71.51 | - | - | - | 10.00 | 18.49 | |
| AlCu10Si10Fe1 | L → β-Al9Fe2Si2 + L | 610.0–559.6 | 559.6 | 0.0 | 2.51 | - | - | 0.0 | 0.0 |
| L → β-Al9Fe2Si2 + α-Al + L | 559.6–554.8 | 554.8 | 4.19 | 2.70 | - | - | 0.0 | 0.0 | |
| L → β-Al9Fe2Si2 + α-Al + L+ Si | 554.8–521.6 | 521.6 | 61.30 | 3.67 | - | - | 7.18 | 0.0 | |
| β-Al9Fe2Si2 + α-Al + L+ Si → β-Al9Fe2Si2 + α-Al + Si + Al2Cu | 521.6–521.5 | 521.5 | 75.4 | 3.69 | - | - | 8.71 | 12.2 | |
| β-Al9Fe2Si2 + α-Al + Si + Al2Cu | 521.5–20.0 | 20 | 68.12 | 3.71 | - | - | 9.67 | 18.5 | |
| AlCu4Si6Fe2 | L → β-Al8Fe2Si + L | 638.2–608.0 | 608.0 | 0.0 | 0.0 | 2.19 | - | 0.0 | 0.0 |
| L → β-Al8Fe2Si + α-Al + L | 608.0–603.5 | 603.5 | 9.14 | 0.0 | 2.80 | - | 0.0 | 0.0 | |
| L → β-Al9Fe2Si2 + L + α-Al | 603.5–603.2 | 603.2 | 12.19 | 3.63 | 0.0 | - | 0.0 | 0.0 | |
| L → β-Al9Fe2Si2 + L + α-Al | 603.2–558.8 | 558.8 | 53.87 | 6.89 | 0.0 | - | 0.0 | 0.0 | |
| L → β-Al9Fe2Si2 + α-Al + Si | 558.8–523.8 | 523.8 | 88.51 | 7.43 | 0.0 | - | 4.06 | 0.0 | |
| α-Al + β-Al9Fe2Si2 + Si → α-Al + Al2Cu + β-Al9Fe2Si2 + Si | 514.9–20.0 | 20 | 79.85 | 7.42 | 0.0 | - | 5.34 | 7.39 | |
| AlCu4Si6Mn2 | L → Al15Si2Mn4 + L | 661.1–610.9 | 610.9 | 0.0 | - | - | 3.43 | 0.0 | 0.0 |
| L → Al15Si2Mn4 + α-Al + L | 610.9–560.2 | 560.2 | 51.18 | - | - | 5.67 | 0.0 | 0.0 | |
| L → Al15Si2Mn4 + α-Al + Si | 560.2–522.6 | 522.6 | 89.08 | - | - | 6.53 | 4.38 | 0.0 | |
| α-Al + Al15Si2Mn4 + Si → α-Al + Al2Cu + Al15Si2Mn4 + Si | 514.1–20.0 | 20 | 80.43 | - | - | 6.69 | 5.48 | 7.40 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikolajczak, P. Effect of Rotating Magnetic Field on Microstructure in AlCuSi Alloys. Metals 2021, 11, 1804. https://doi.org/10.3390/met11111804
Mikolajczak P. Effect of Rotating Magnetic Field on Microstructure in AlCuSi Alloys. Metals. 2021; 11(11):1804. https://doi.org/10.3390/met11111804
Chicago/Turabian StyleMikolajczak, Piotr. 2021. "Effect of Rotating Magnetic Field on Microstructure in AlCuSi Alloys" Metals 11, no. 11: 1804. https://doi.org/10.3390/met11111804
APA StyleMikolajczak, P. (2021). Effect of Rotating Magnetic Field on Microstructure in AlCuSi Alloys. Metals, 11(11), 1804. https://doi.org/10.3390/met11111804







