Effects of Primarily Solidified Dendrite and Thermal Treatments on the M23C6 Precipitation Behavior of High-Chromium White Iron
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Specimen Preparation
- Conventional heat treatment of destabilizing and tempering
- -
- 1065 °C for 4 h air cooling (AC) + 500 °C for 4 h (herein: conventional heat treatment);
- -
- 1065 °C for 4 h water quench (WQ) + 500 °C for 4 h (herein: DES + WQ + Temp).
- Modified heat treatment (direct aging (DA)): Each alloy was subjected up to 240 min at the temperature of maximum fraction of M23C6. The DA treatment of each alloy is as follows:
- -
- 2124 alloy: 850 °C for 10, 30, 60, 240, 300 min + AC;
- -
- 2127 alloy: 890 °C for 10, 30, 60, 240, 300 min + AC;
- -
- 2427 alloy: 890 °C for 10, 30, 60, 240, 300 min + AC;
- -
- 2827 alloy: 810 °C for 10, 30, 60, 240, 300 min + AC;
- -
- 2927 alloy: 800 °C for 10, 30, 60, 240, 300 min + AC.
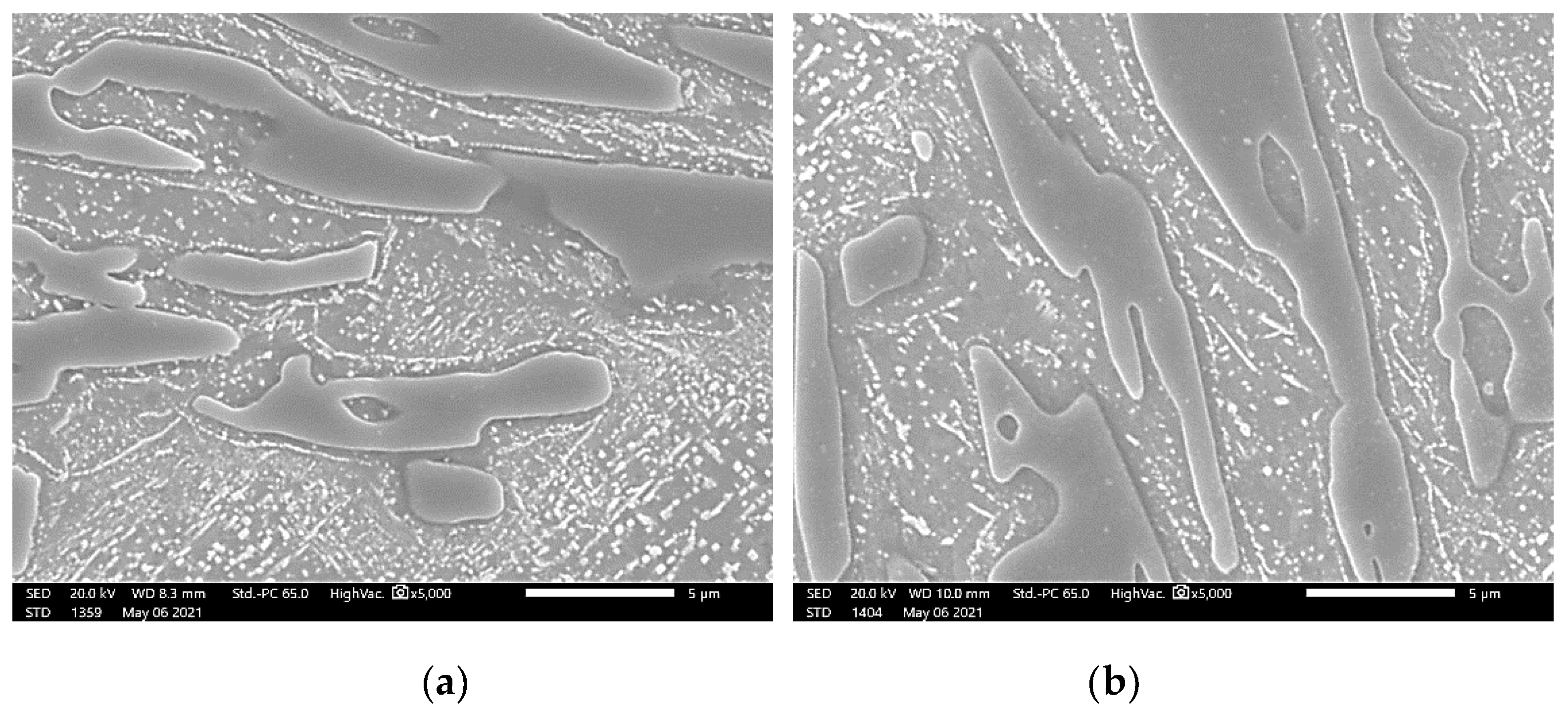
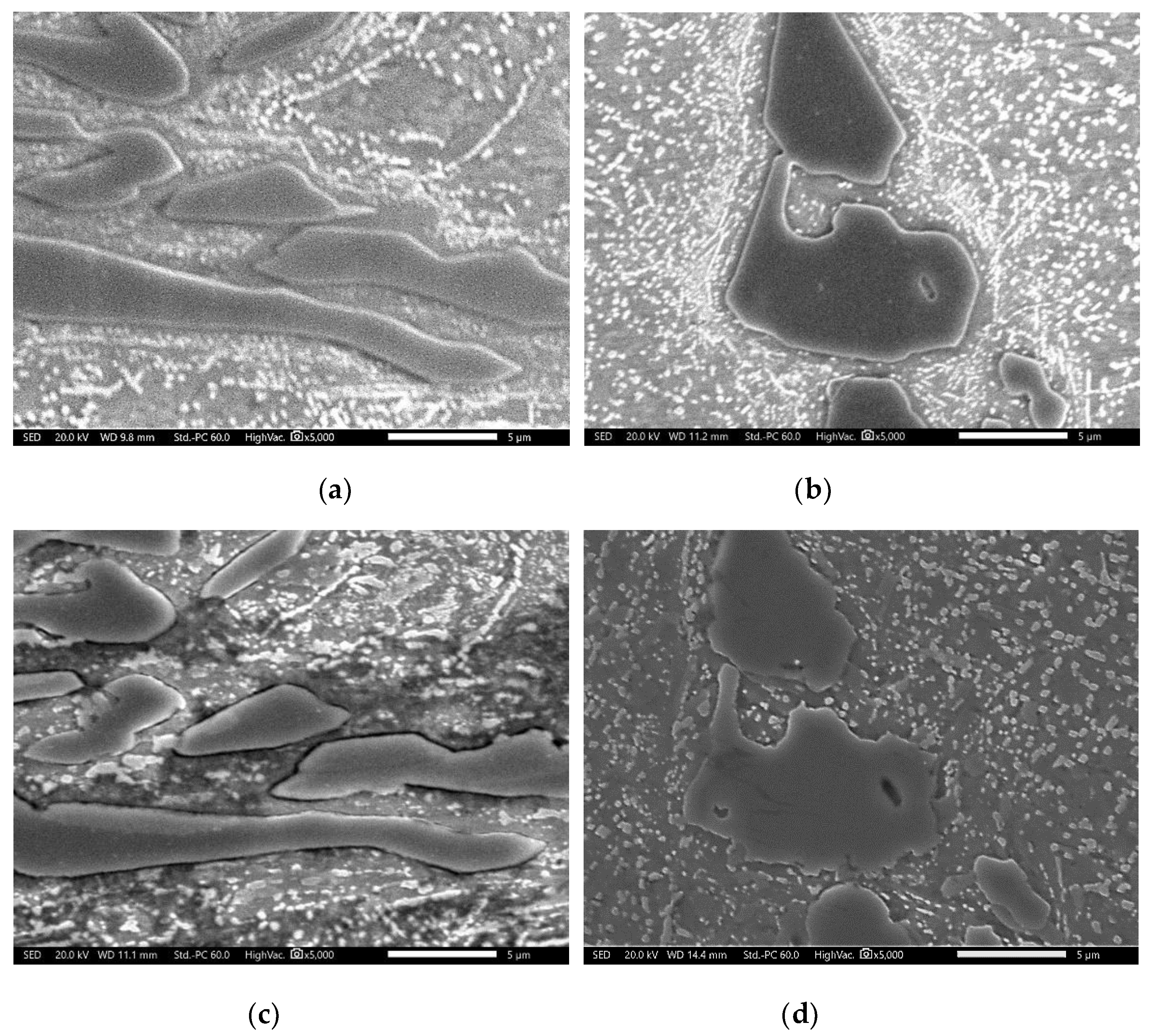
- DA specimens were exposed under 1 × 10−5 torr vacuum for 60 min to observe the movement of the M7C3 carbide interface. Certain points of DA specimens were engraved to observe at the same position before and after the vacuum exposure. The vacuum exposure was carried out in a vacuum furnace (Jungmin 2010), followed by Ar gas fan quenching.
2.2. Microstructural Observation and Phase Identification
3. Results and Discussions
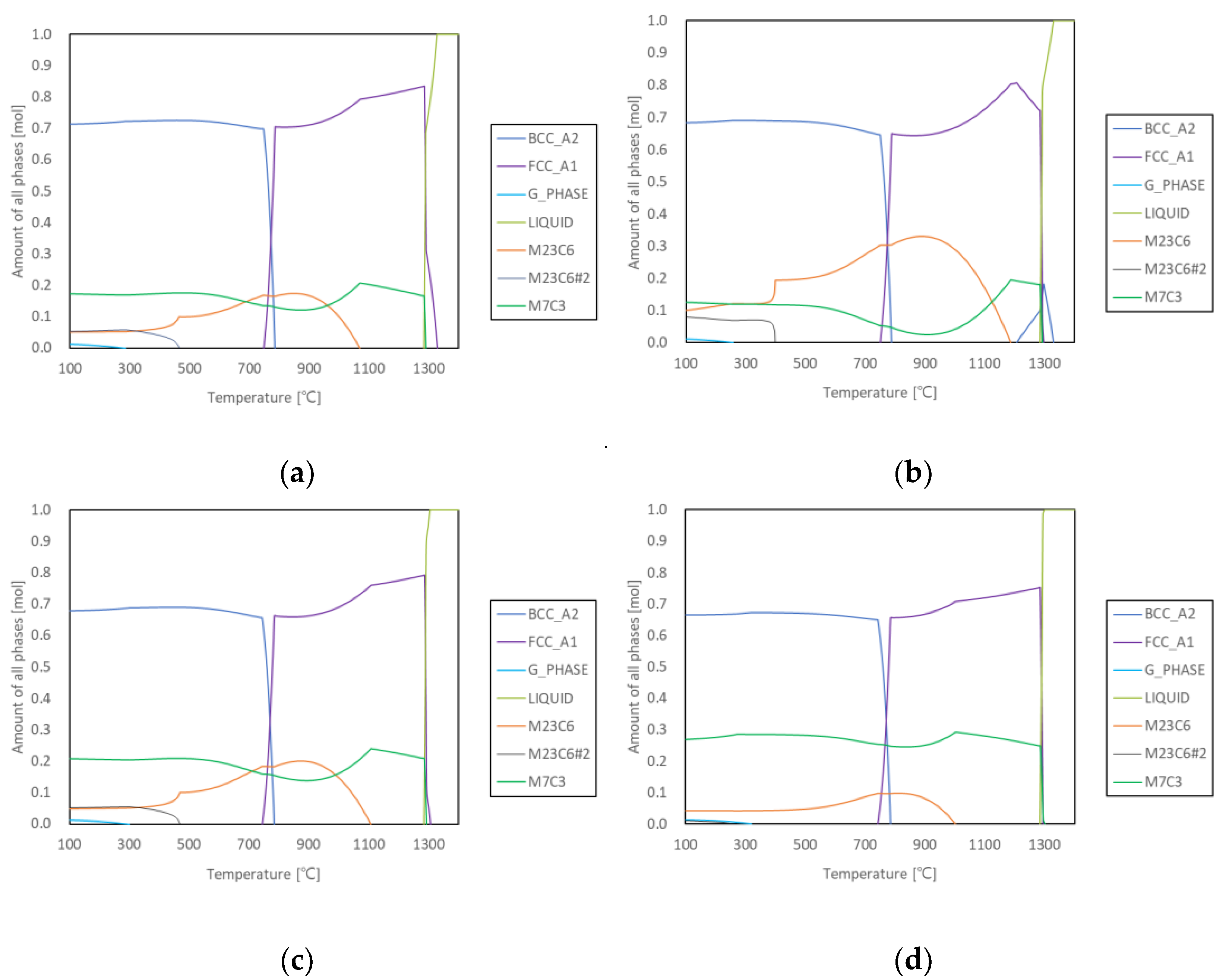
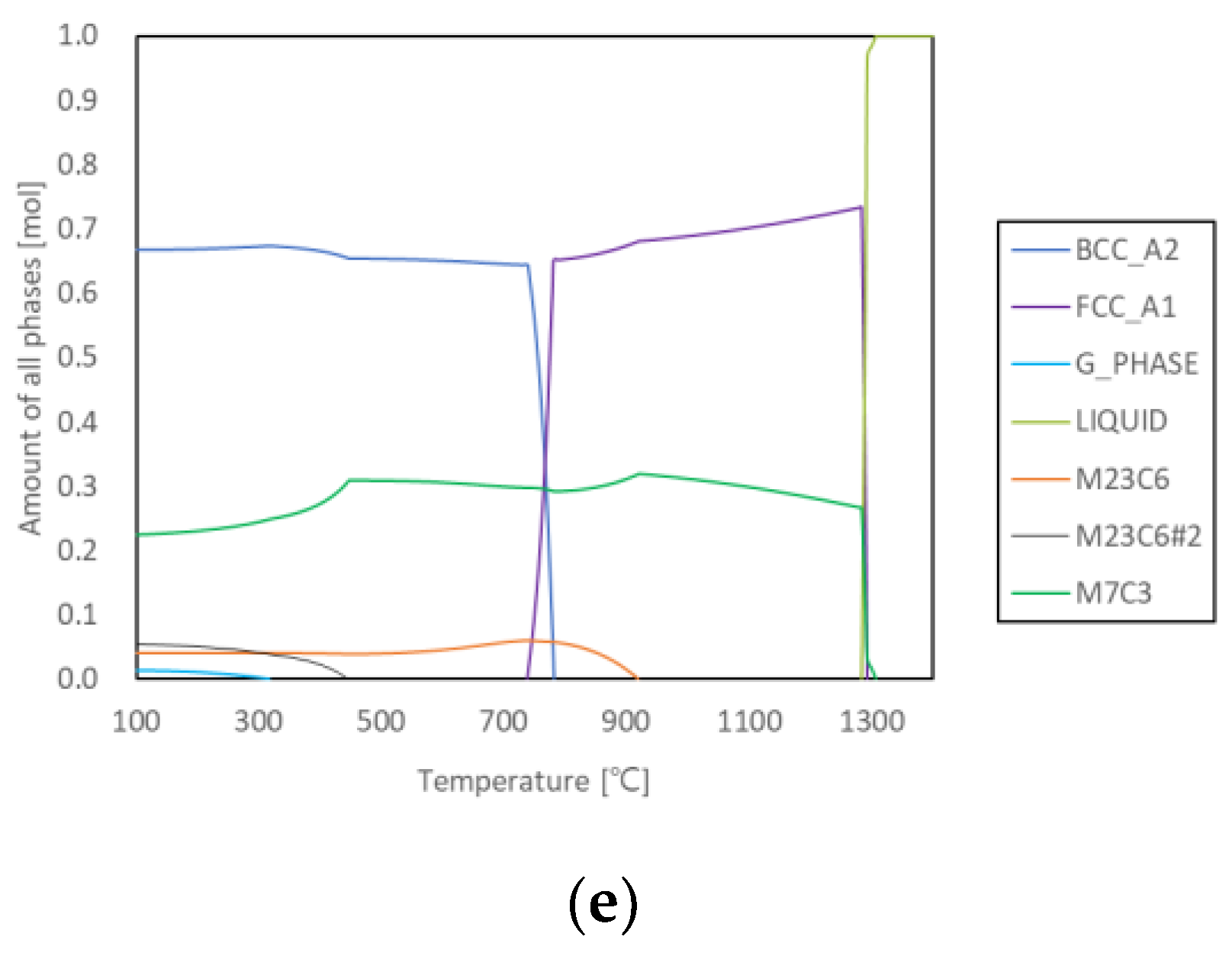
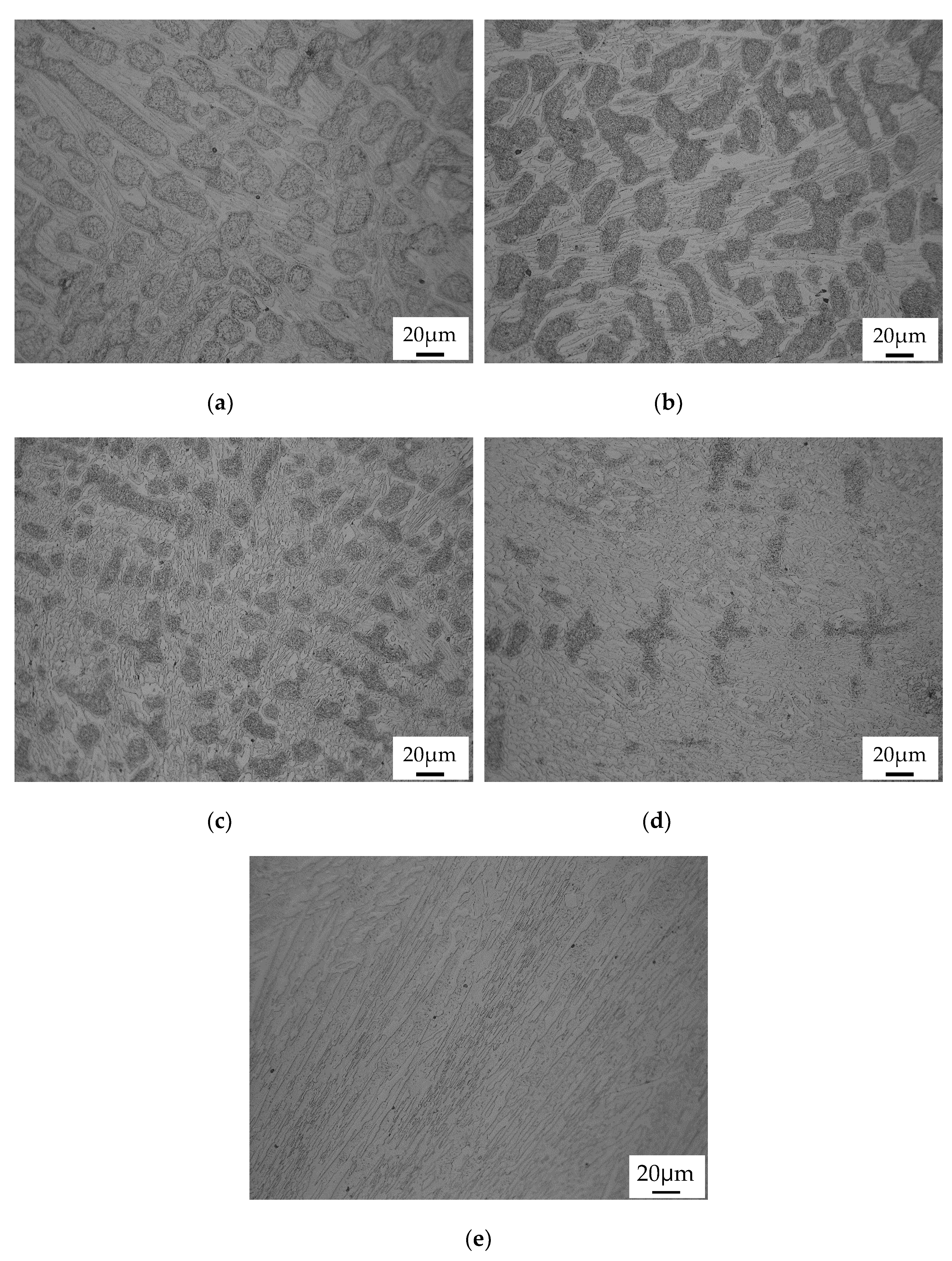
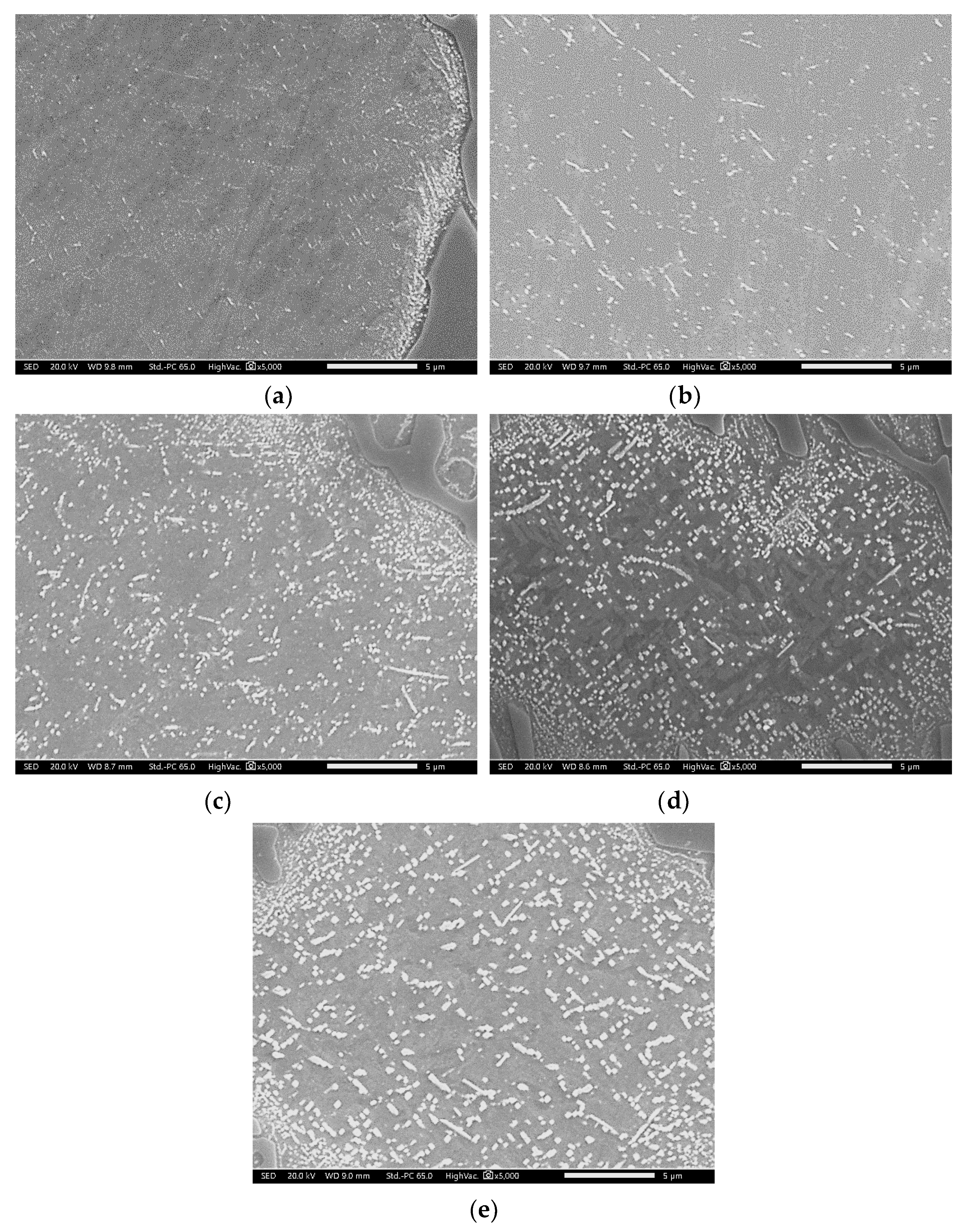
3.1. Prediction and As-Cast Microstructure
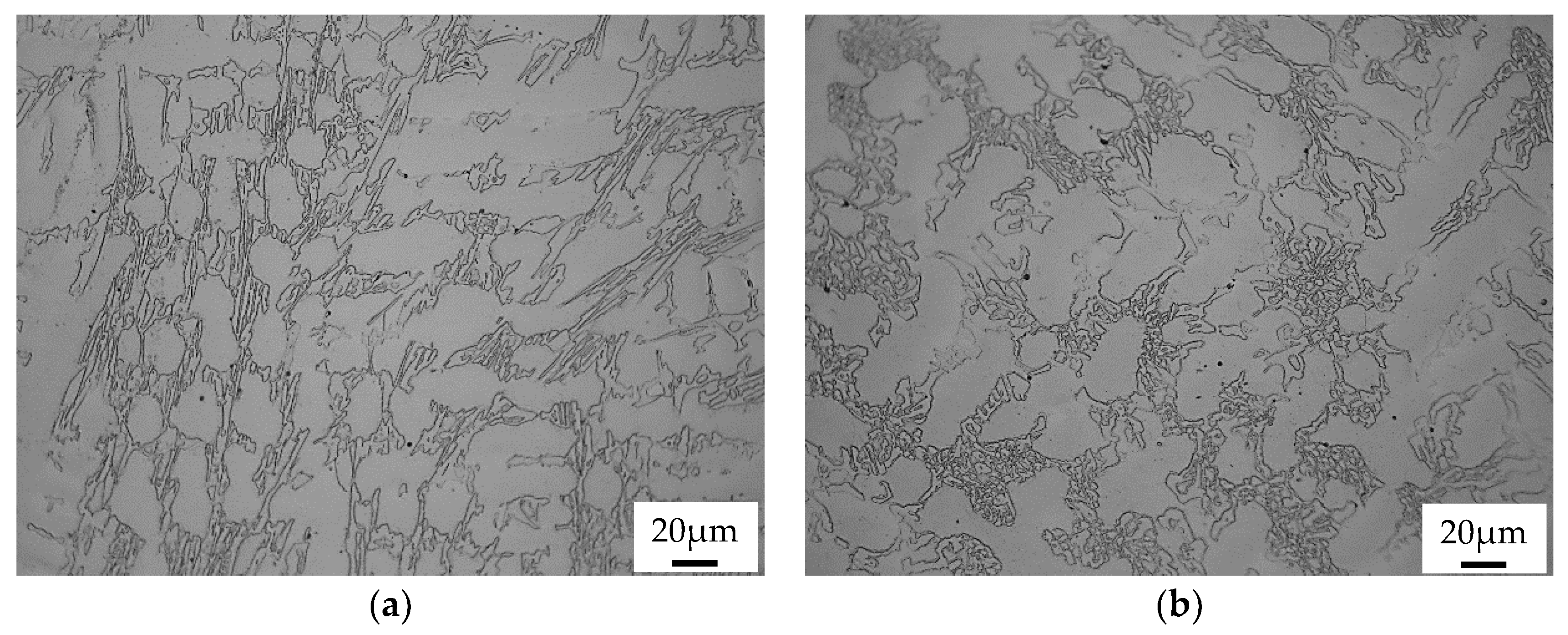
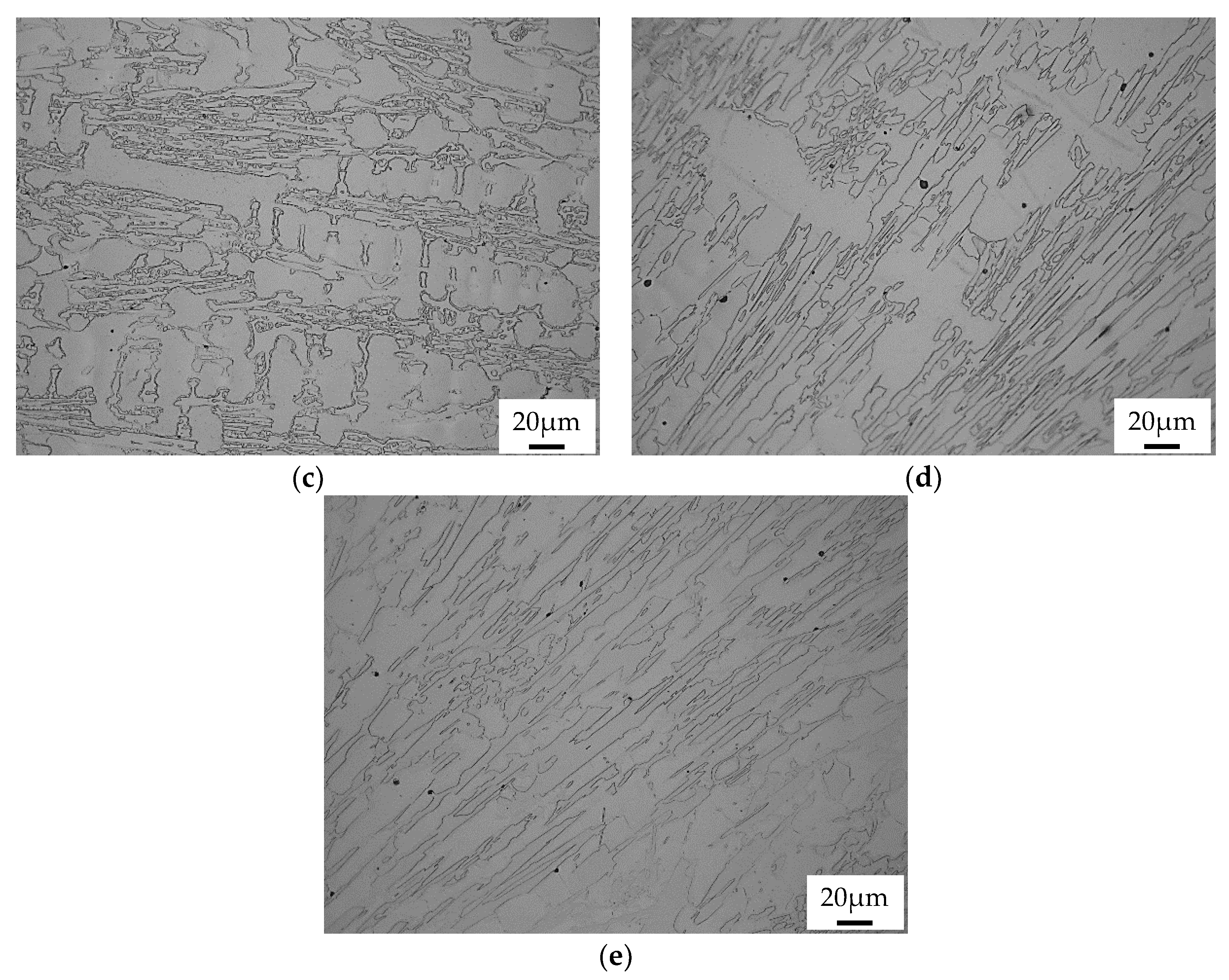
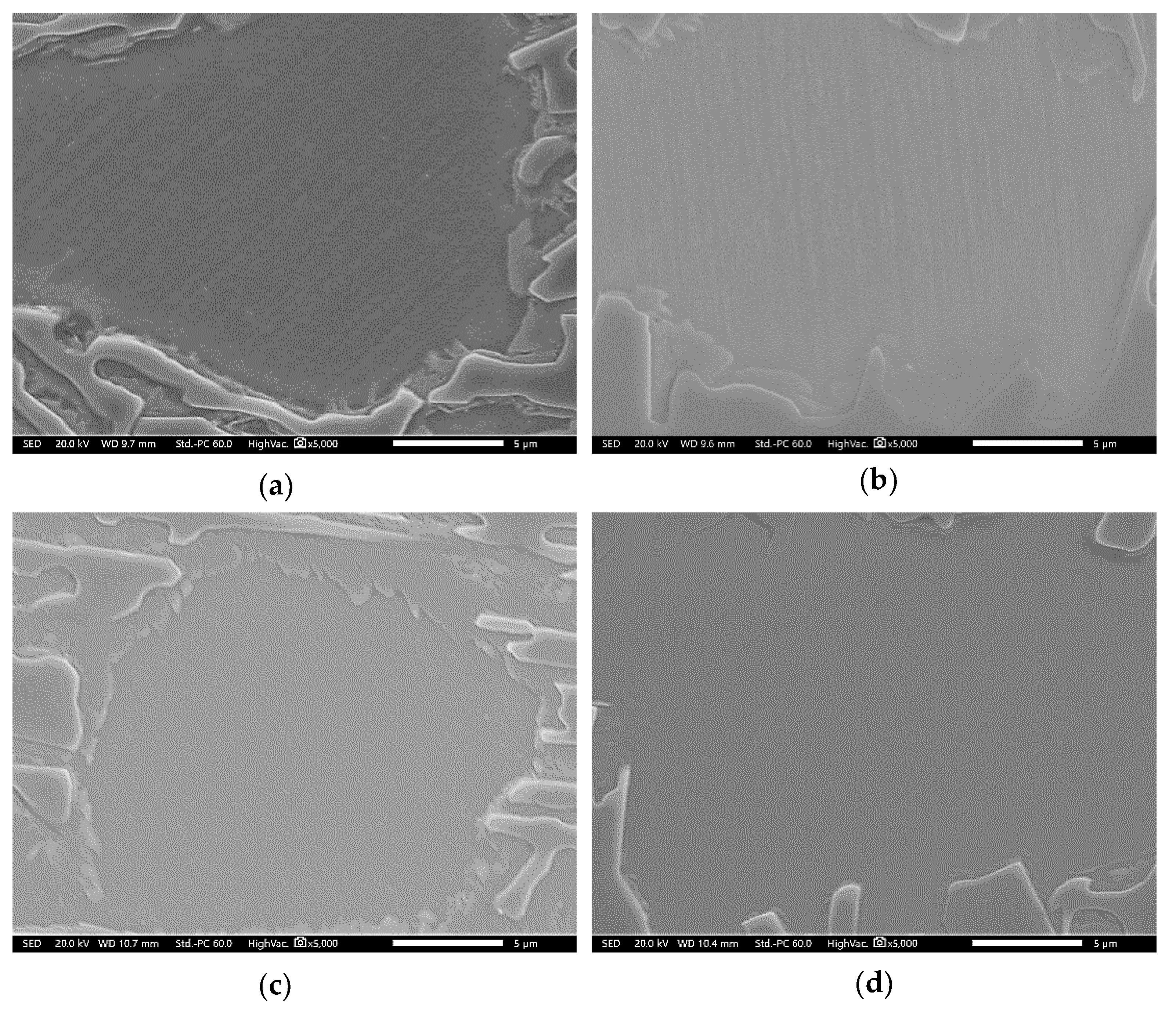
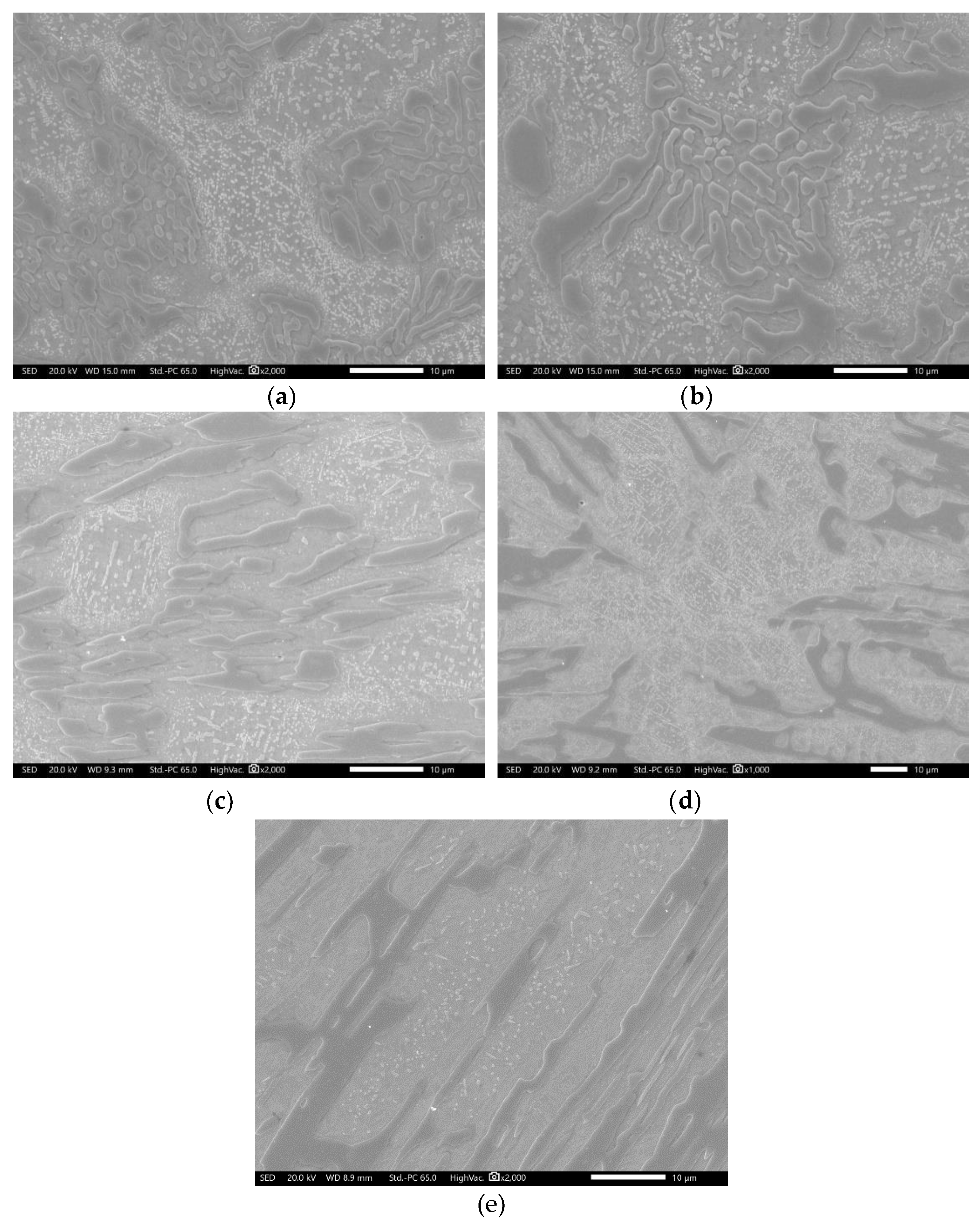
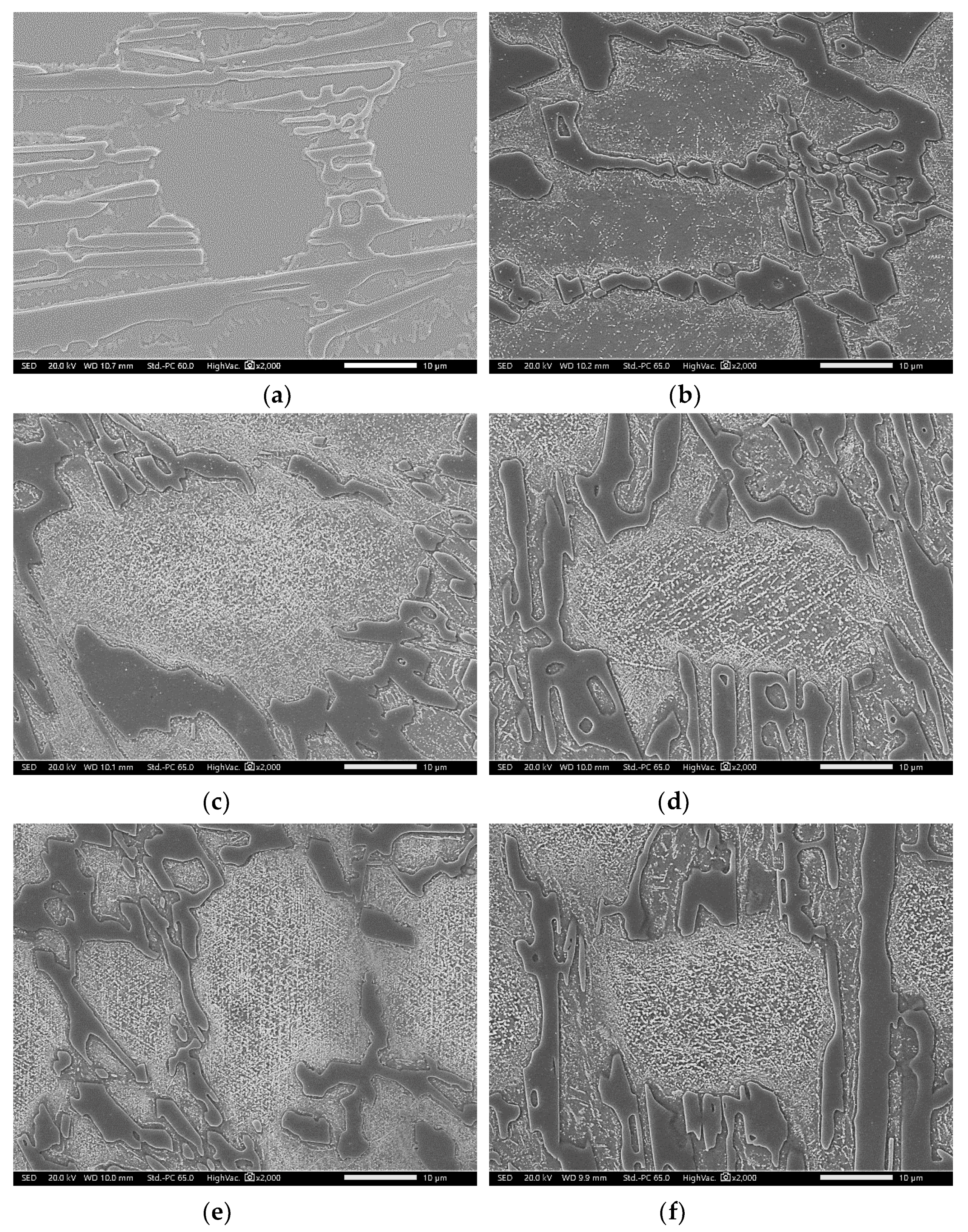
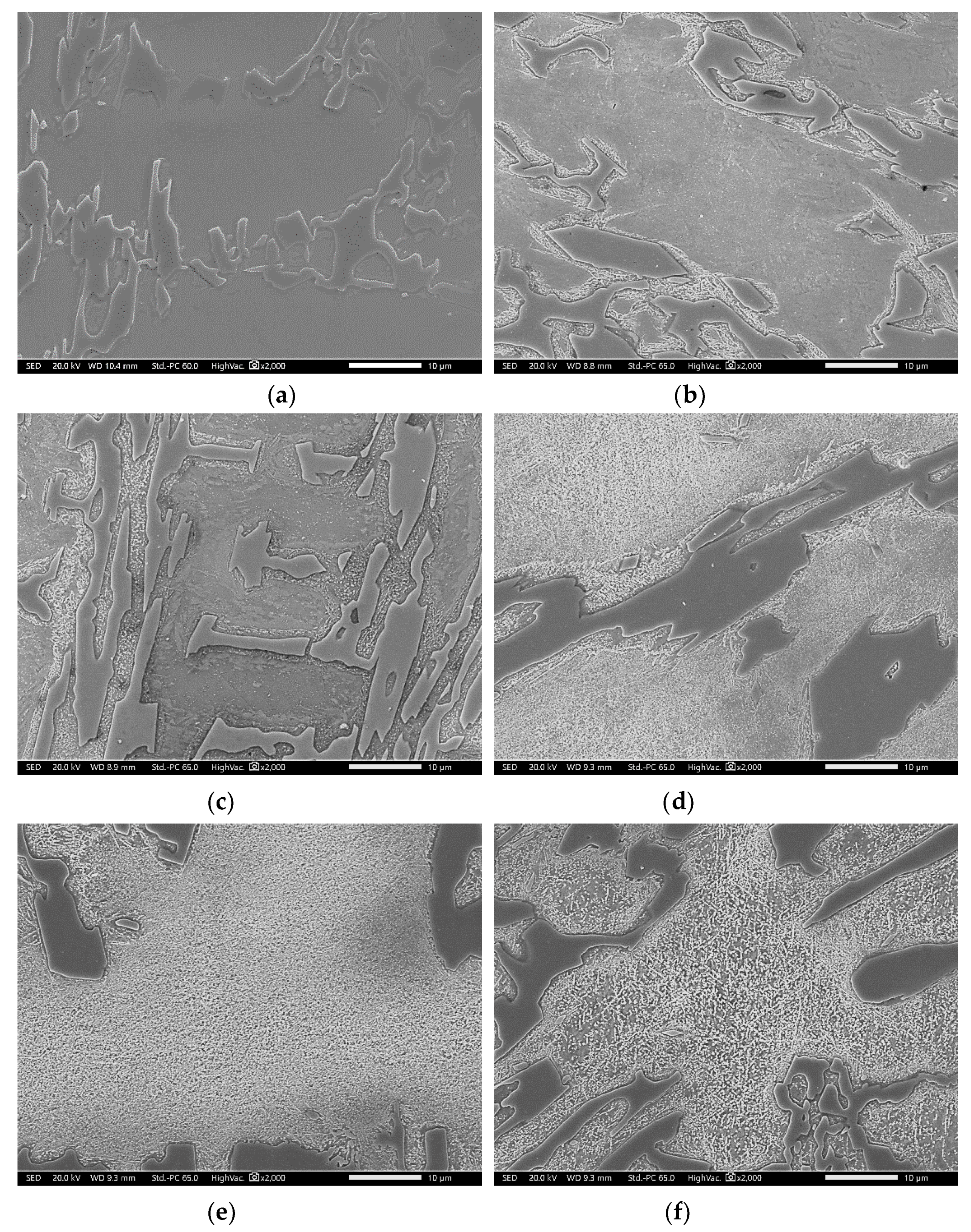
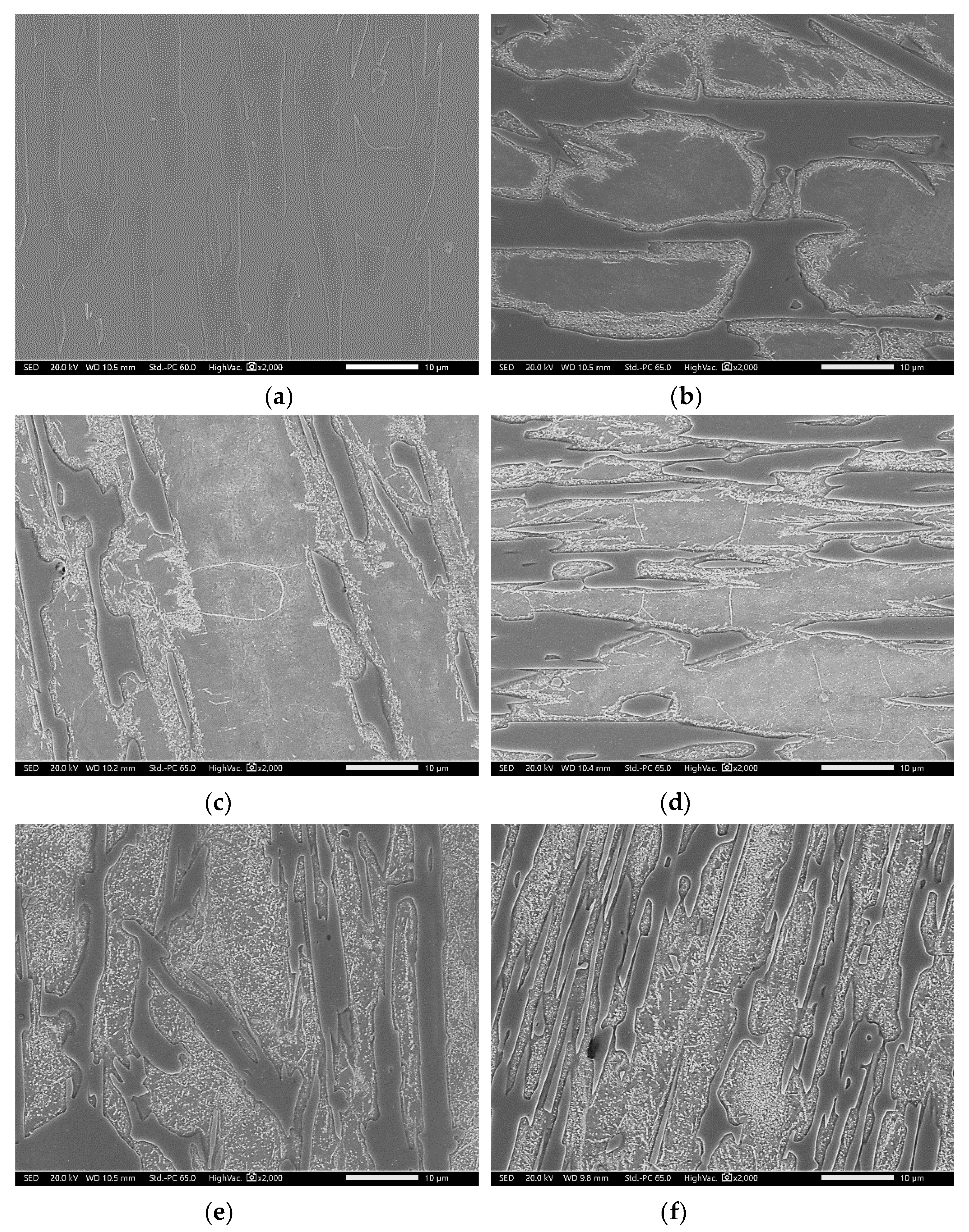
3.2. Conventional Heat Treatment
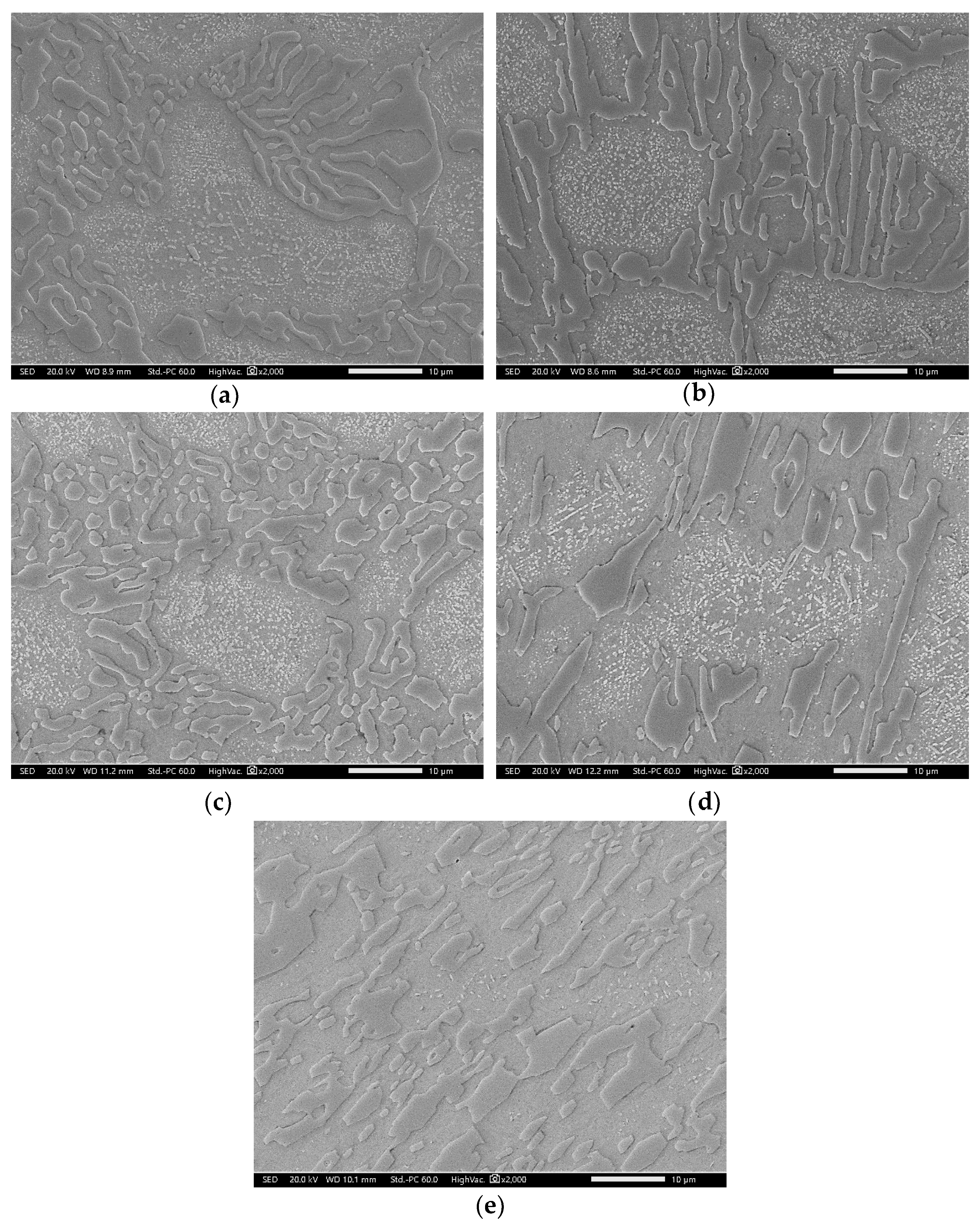
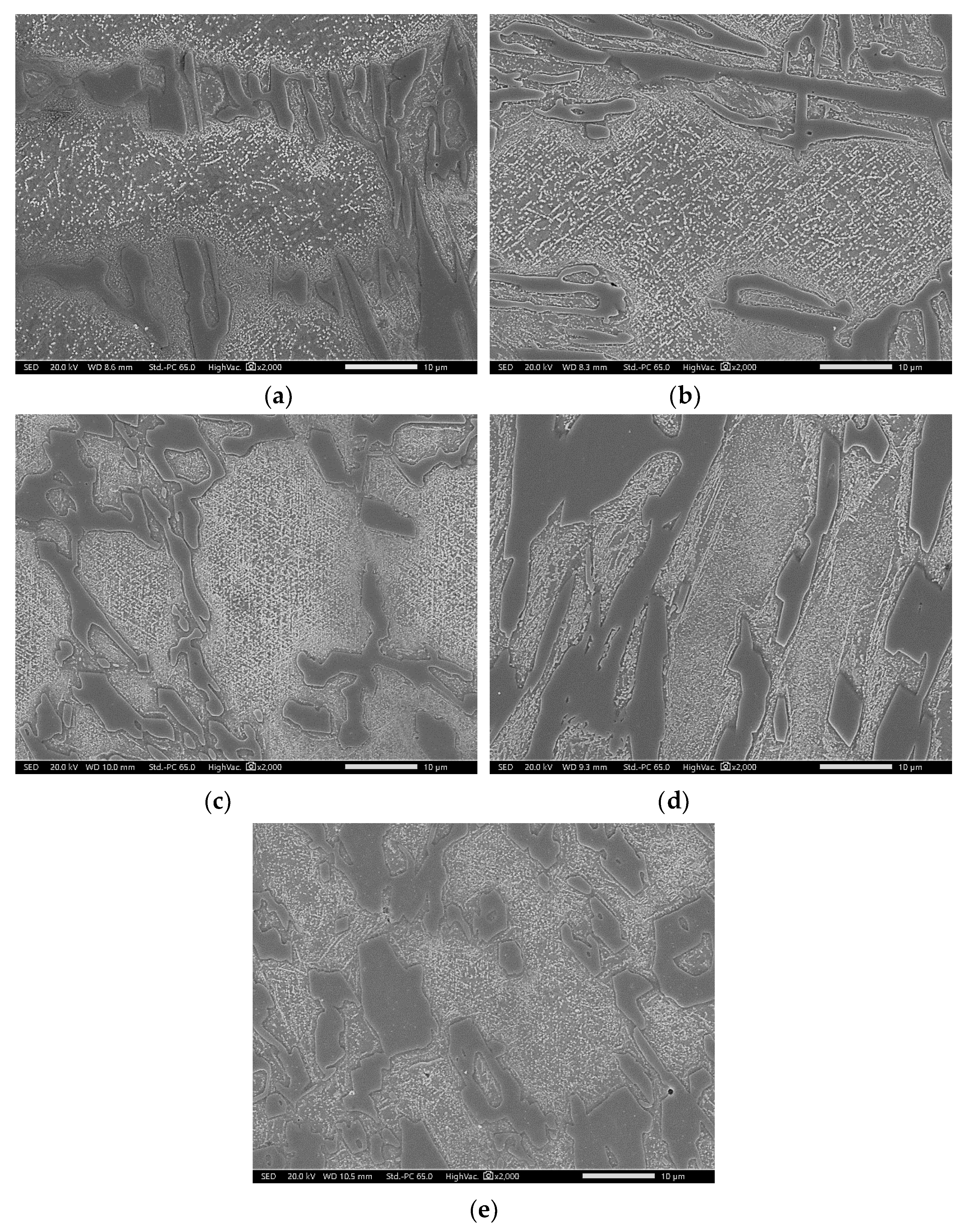
3.3. Modified Heat-Treated (DA-Treated) Microstructure
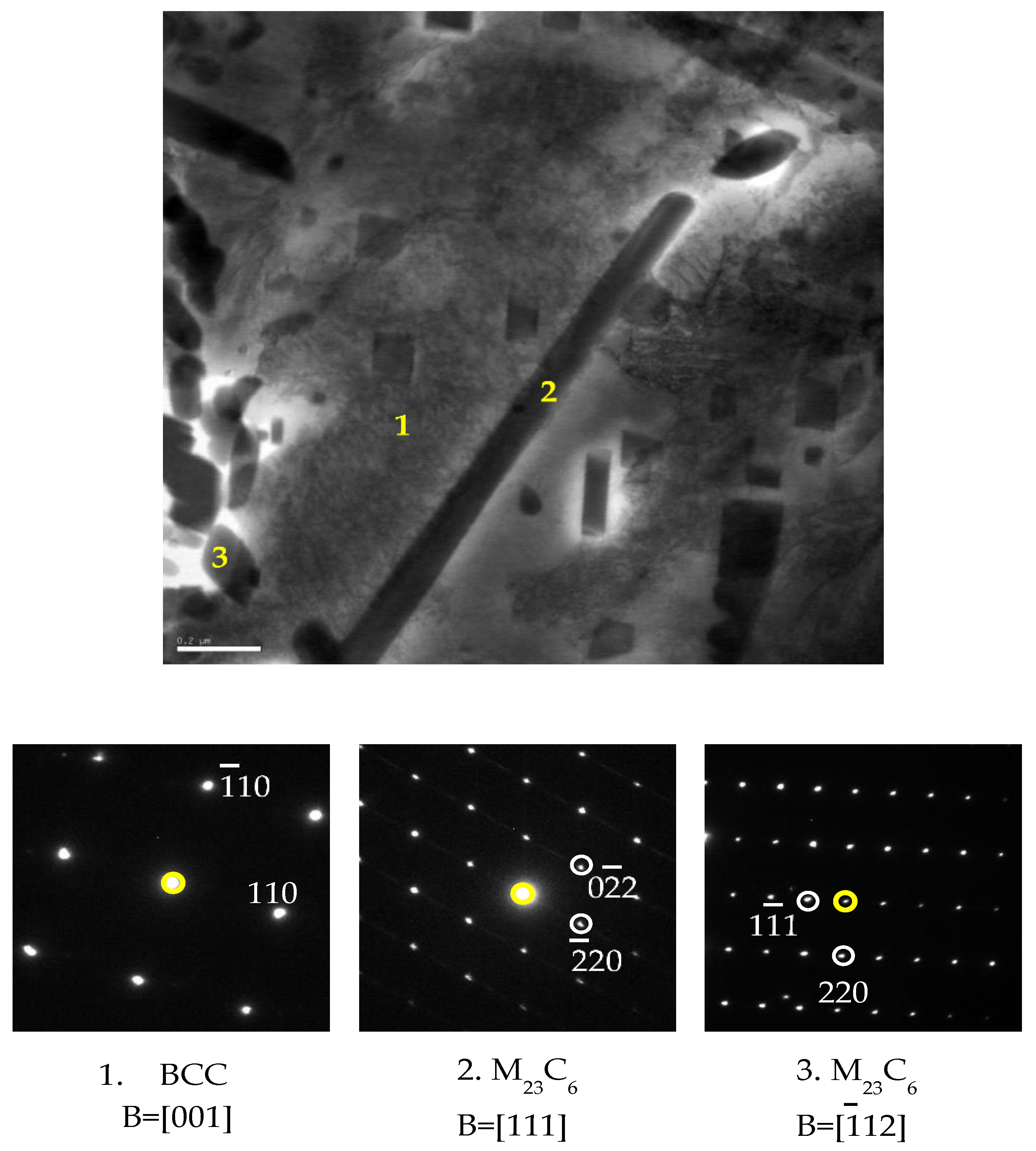
3.3.1. Basic Background of M23C6 Precipitation
3.3.2. Effect of Direct Aging (DA) on the M23C6 Precipitation
4. Summary
- It was found that destabilization of austenite during conventional heat treatment releases saturated solute elements, C and Cr, to form M23C6 in the dendrite austenite; however, little M23C6 precipitation occurred in eutectic austenite.
- The amount of M23C6 precipitation during destabilization was closely related to that of the primarily solidified dendrites, which means that it depended on chemical composition.
- The alloy whose solidification began with delta ferrite had a relatively small fraction of M7C3, but precipitation of M23C6 within dendrite was very active.
- M23C6 precipitation was caused by the following two phenomena:
- (1)
- Destabilization of dendritic austenite, which released saturated solute elements, C and Cr, to form M23C6 in the austenitic matrix. Additionally, destabilization of eutectic austenite occurred at a relatively low temperature.
- (2)
- The reaction between eutectic M7C3 carbide and austenite with the following reaction:M7C3 + γ → M23C6 + γ*
- Direct aging at the temperature of maximum M23C6 fraction (at which M7C3 is expected to be metastable) developed uniform precipitation of M23C6 across the materials. M23C6 precipitation during conventional heat treatment preferentially occurred within the primarily solidified dendrites and along the periphery of M7C3, whereas direct aging resulted in both dendrite and eutectic austenites.
- During direct aging, both the destabilization in dendritic and eutectic austenites and the reaction between M7C3 and austenite simultaneously occurred.
5. Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, J.R. Metallurgy and Properties of High-Alloy White Irons. In ASM Specialty Handbook Cast Irons, 1st ed.; ASM International: Novelty, OH, USA, 1996; pp. 111–122. [Google Scholar]
- Smith, W.F. Cast Irons. In Structure and Properties of EngineeringAalloys, 1st ed.; Brown, J.V., Maisel, J.W., Eds.; MaGraw-Hill: New York, NY, USA, 1981; pp. 322–323. [Google Scholar]
- Dupin, P.; Saverna, J.; Schissler, J.M. Structural Study of Chromium white Cast Irons. AFS Trans. 1982, 90, 711–718. [Google Scholar]
- Tabrett, C.P.; Sare, I.R.; Ghomashchi, M.R. Microstructure-property relationship in high chromium white iron alloys. Int. Mater. Rev. 1996, 41, 59–82. [Google Scholar] [CrossRef]
- Oh, J.S.; Song, Y.G.; Choi, B.G.; Bhamornsut, C.; Nakkuntod, R.; Jo, C.Y.; Lee, J.H. Effect of dendrite fraction on the M23C6 precipitation behavior and the mechanical properties of High Cr white irons. Metals 2021, 11, 1576. [Google Scholar] [CrossRef]
- Pearce, J.T.H. Abrasive Wear Behavior of Alloy Cast Irons. Br. Foundrym. 1985, 78, 13–23. [Google Scholar]
- Ohide, T.; Ohira, G. Solidification of high chromium alloyed cast iron. Br. Foundrym. 1983, 76, 7–14. [Google Scholar]
- Powell, G.L.F.; Laird, G. Structure, nucleation, growth and morphology of secondary carbides in high chromium and Cr-Ni white cast irons. J. Mater. Sci. 1992, 27, 29–35. [Google Scholar] [CrossRef]
- Pearce, J.T.H. High chromium cast irons to resist abrasive wear. Foundryman 2002, 95, 156. [Google Scholar]
- Kibble, K.A.; Pearce, J.T.H. An examination of the effects of annealing heat treatment on secondary carbide formation in 25% Cr high chromium irons. Cast Met. 1995, 8, 123. [Google Scholar] [CrossRef]
- Wiengmoon, A.; Chairuangsri, T.; Pearce, J.T.H. A microstructural study of detabilised 30 wt%Cr-2.3 wt%C high chromium cast iron. ISIJ Int. 2004, 44, 396–403. [Google Scholar] [CrossRef]
- Pearce, J.T.H.; Elwell, D.W.L. Duplex nature of eutectic carbides in heat treated 30% chromium cast iron. J. Mater. Sci. Lett. 1986, 5, 1063. [Google Scholar] [CrossRef]




| Designation | Composition (wt.%) | |||||
|---|---|---|---|---|---|---|
| C | Si | Mn | Cr | Ni | Mo | |
| 2124 | 2.12 | 0.66 | 0.65 | 24.05 | 0.85 | 0.86 |
| 2127 | 2.13 | 0.71 | 0.67 | 27.00 | 0.89 | 0.86 |
| 2427 | 2.43 | 0.68 | 0.70 | 27.06 | 0.86 | 0.85 |
| 2827 | 2.78 | 0.70 | 0.70 | 27.34 | 0.87 | 0.85 |
| 2927 | 2.95 | 0.67 | 0.71 | 26.94 | 0.92 | 0.82 |
| Alloy | M23C6 Precipitation Initiation | M23C6 Peak Precipitation | |||
|---|---|---|---|---|---|
| T (°C) | M7C3 Fraction (mol.%) | T (°C) | M23C6 Fraction (mol.%) | M7C3 Fraction (mol.%) | |
| 2124 | 1060 | 19.8 | 850 | 17.3 | 12.2 |
| 2127 | 1180 | 18.8 | 890 | 33.0 | 2.5 |
| 2427 | 1100 | 23.4 | 870 | 20.1 | 14.0 |
| 2827 | 1000 | 29.1 | 810 | 9.8 | 24.6 |
| 2927 | 920 | 31.9 | 739 | 6.0 | 29.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.-G.; Oh, J.-S.; Choi, B.-G.; Jo, C.-Y.; Lee, J.-H. Effects of Primarily Solidified Dendrite and Thermal Treatments on the M23C6 Precipitation Behavior of High-Chromium White Iron. Metals 2021, 11, 1690. https://doi.org/10.3390/met11111690
Song Y-G, Oh J-S, Choi B-G, Jo C-Y, Lee J-H. Effects of Primarily Solidified Dendrite and Thermal Treatments on the M23C6 Precipitation Behavior of High-Chromium White Iron. Metals. 2021; 11(11):1690. https://doi.org/10.3390/met11111690
Chicago/Turabian StyleSong, Young-Gy, Jun-Seok Oh, Baig-Gyu Choi, Chang-Yong Jo, and Je-Hyun Lee. 2021. "Effects of Primarily Solidified Dendrite and Thermal Treatments on the M23C6 Precipitation Behavior of High-Chromium White Iron" Metals 11, no. 11: 1690. https://doi.org/10.3390/met11111690
APA StyleSong, Y.-G., Oh, J.-S., Choi, B.-G., Jo, C.-Y., & Lee, J.-H. (2021). Effects of Primarily Solidified Dendrite and Thermal Treatments on the M23C6 Precipitation Behavior of High-Chromium White Iron. Metals, 11(11), 1690. https://doi.org/10.3390/met11111690







