Effect of Dendrite Fraction on the M23C6 Precipitation Behavior and the Mechanical Properties of High Cr White Irons
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Specimen Preparation
2.2. Microstructural Observation and Phase Identification
2.3. Mechanical Properties
2.4. Directional Solidification and Quenching (DSQ)
3. Results and Discussions
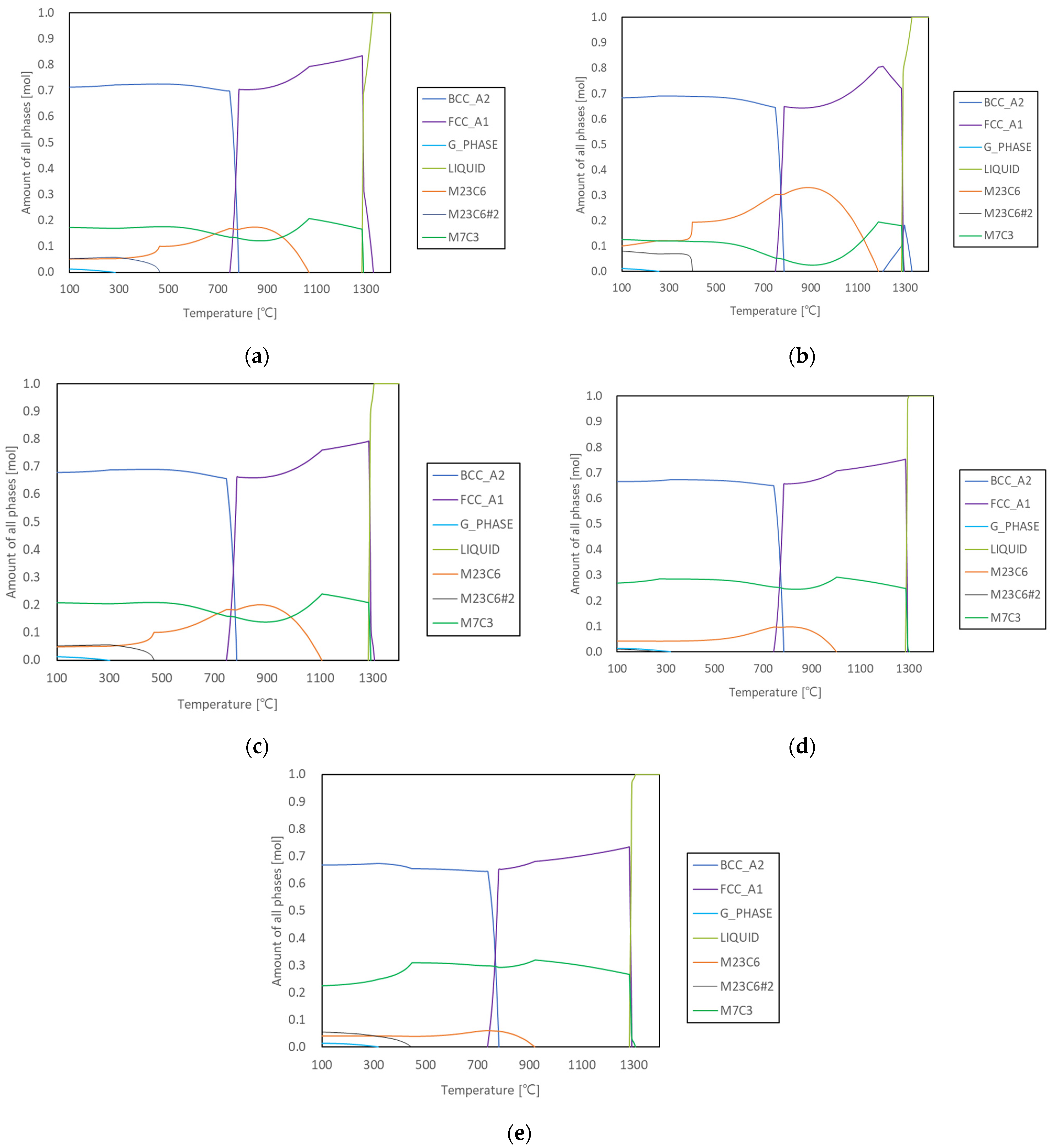
3.1. Prediction of the Microstructure
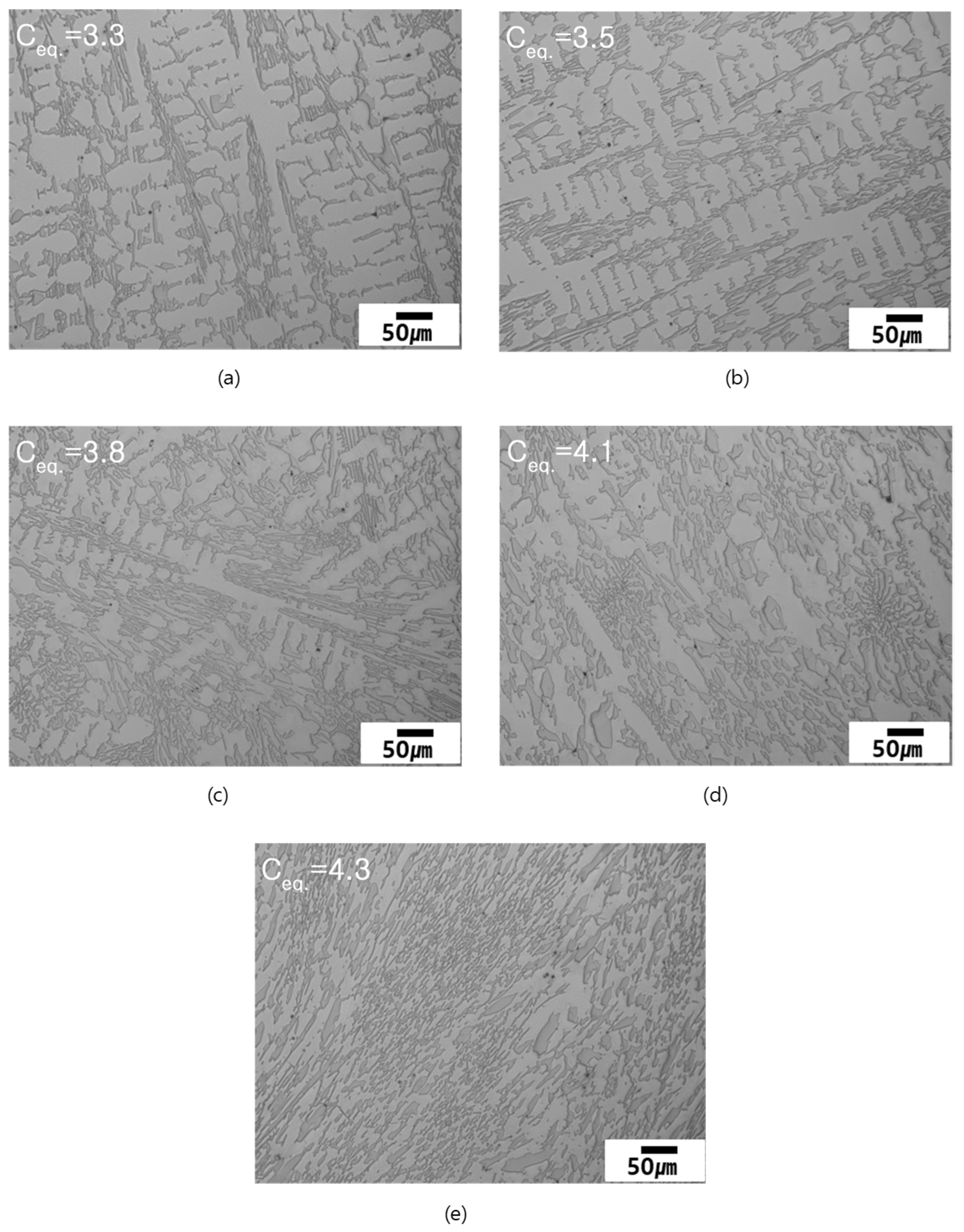
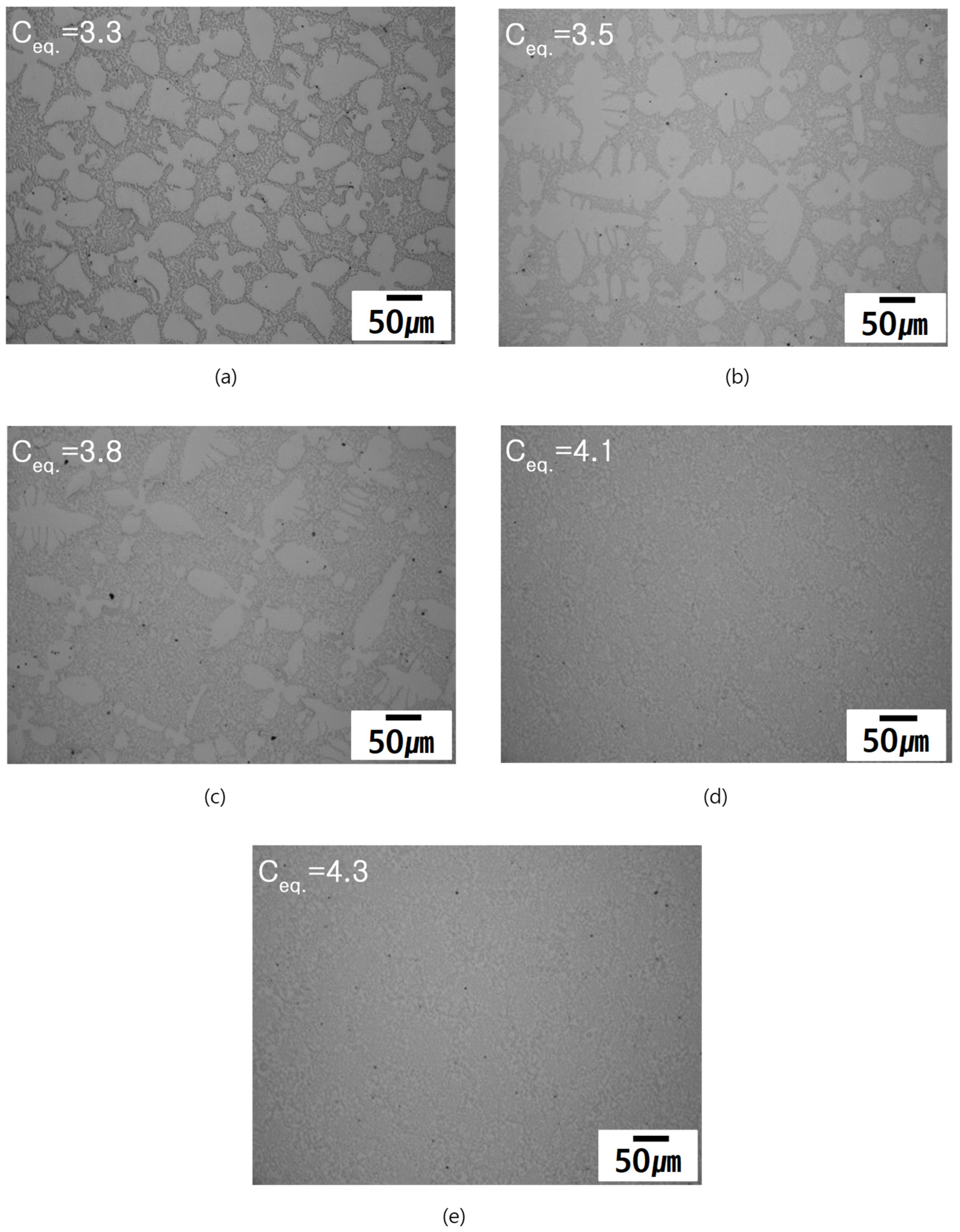
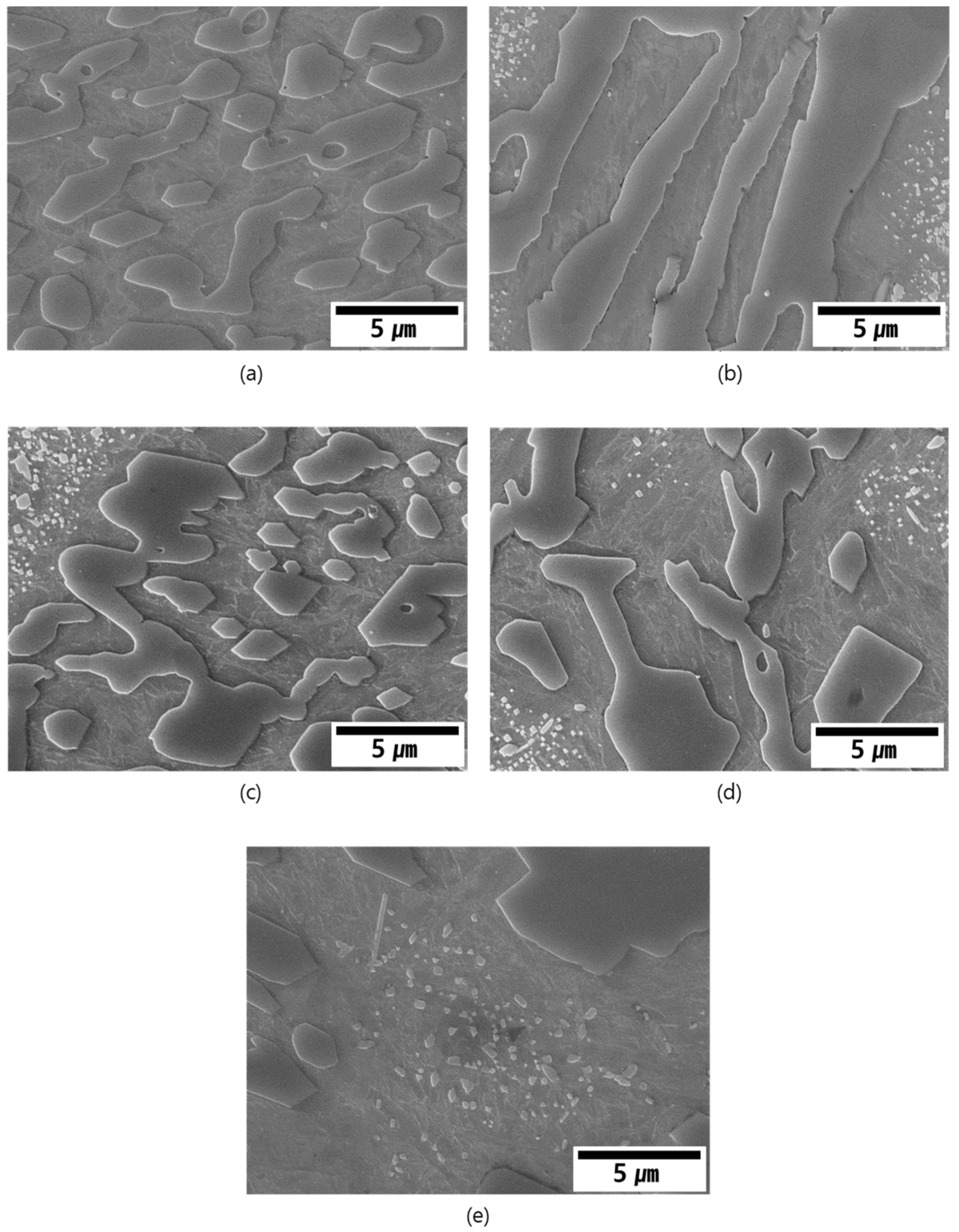
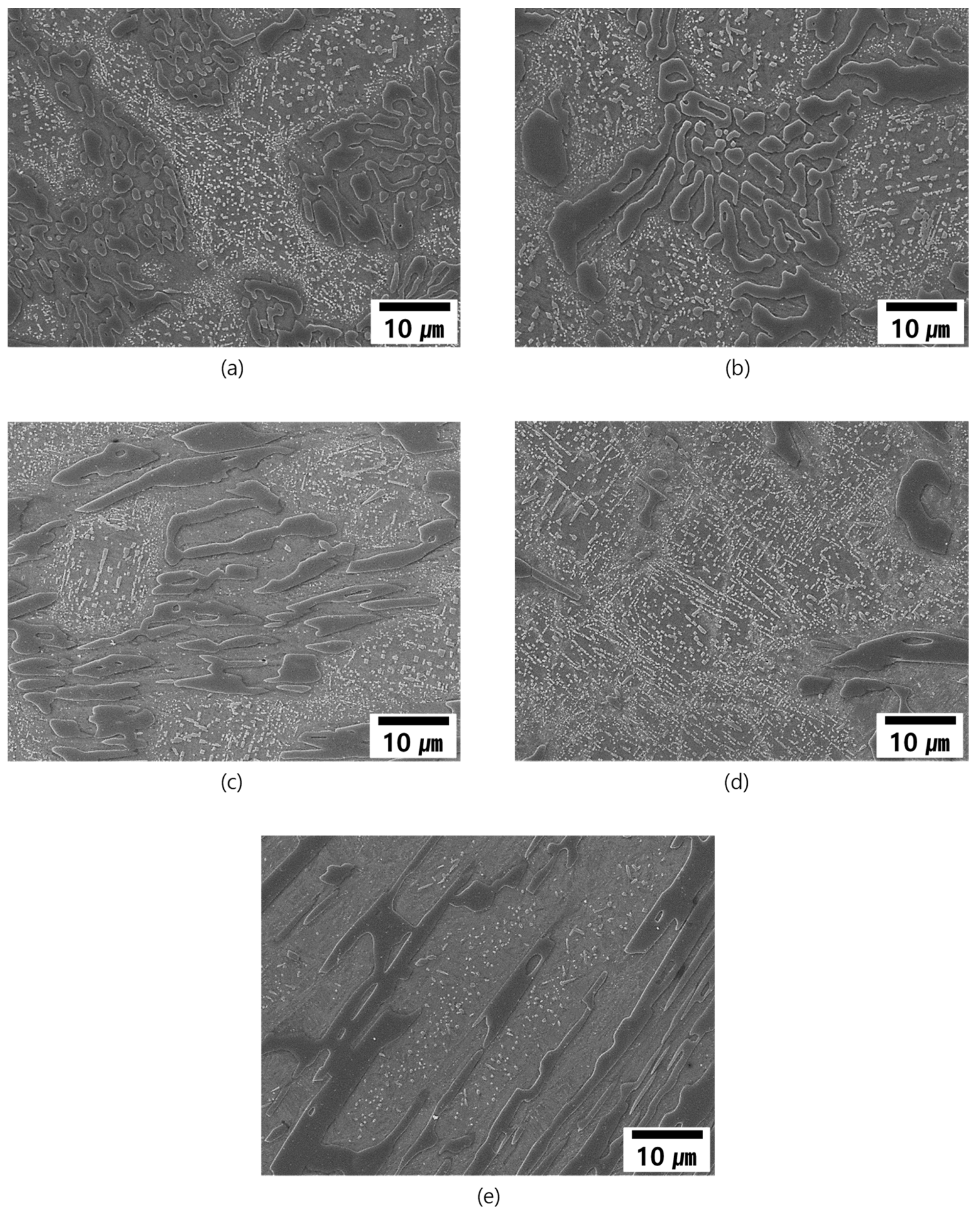
3.2. As-Cast Microstructure
3.3. Solidification Behavior of the Alloys
3.4. Heat Treated Microstructure
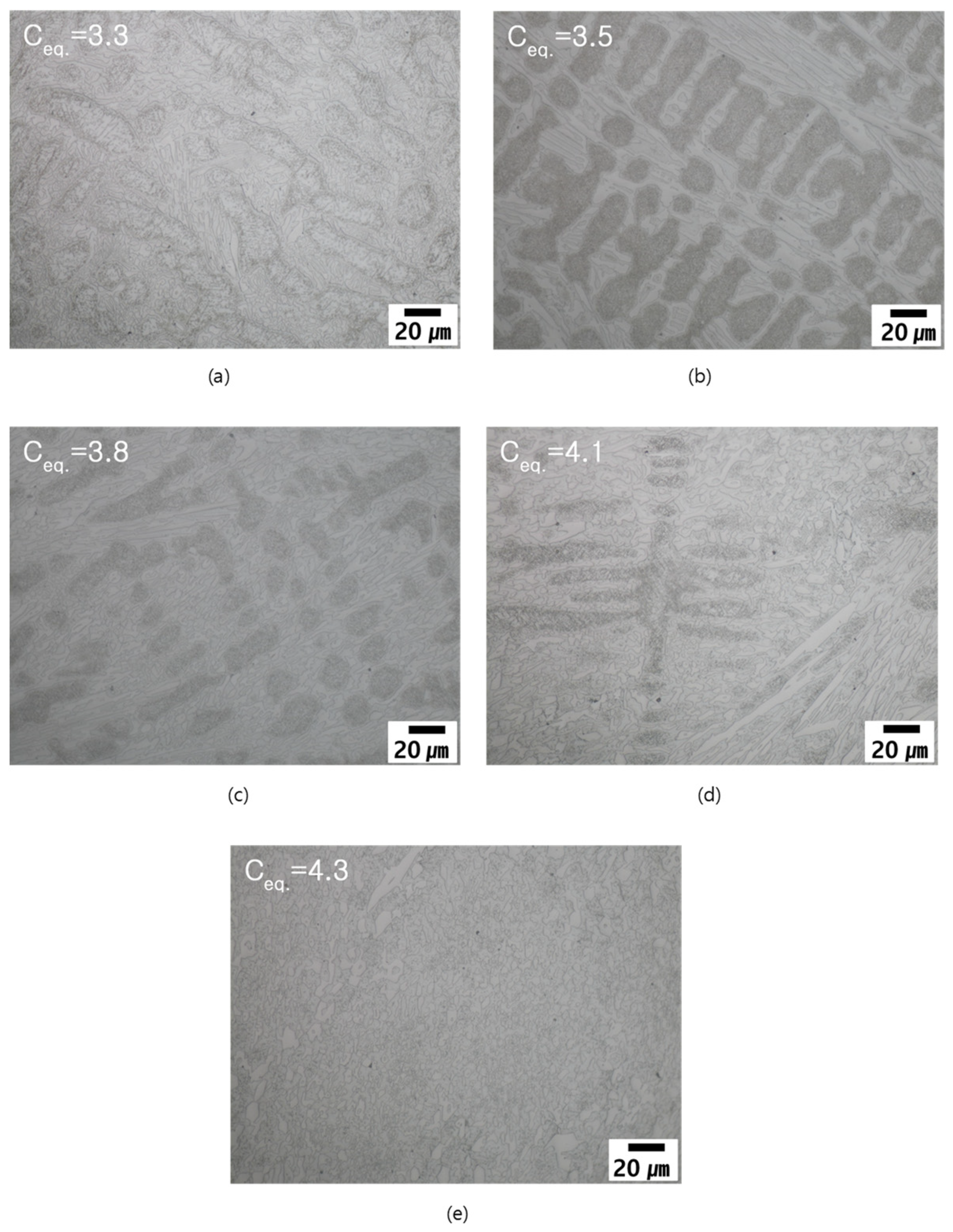
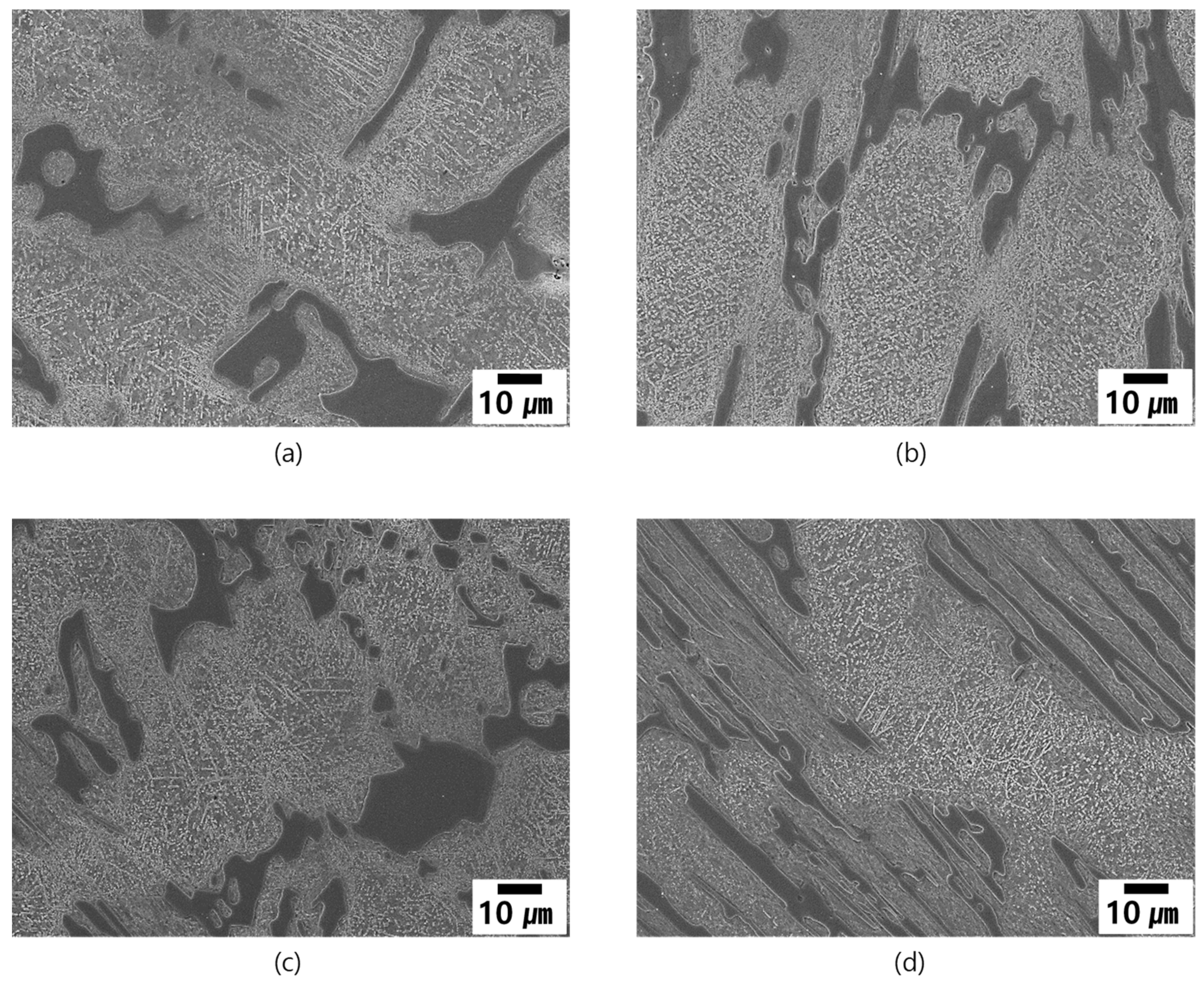
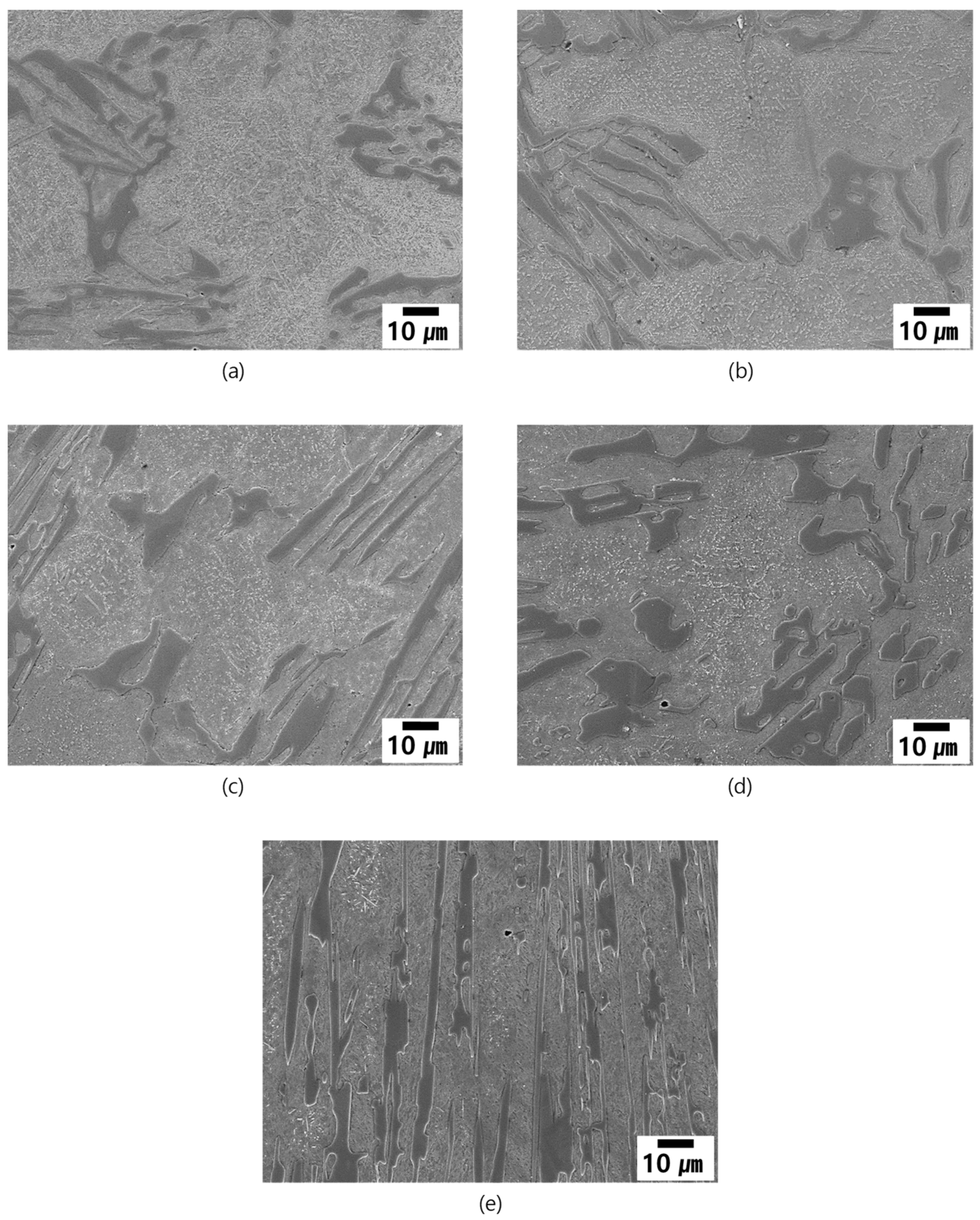
3.4.1. Microstructural Evolution during Heat Treatment
- Destabilizing and low-temperature aging1065 °C for 4 h AC (Air cooling to room temperature) + 500 °C for 4 h (below: DES + Age)1065 °C for 4 h WQ (water quench) + 500 °C for 4 h (below: DES + WQ + Age);
- Destabilizing and low-temperature tempering1065 °C for 4 h AC + 250 °C for 4 h (below: DES + Temper);
- Modified destabilizing and low-temperature tempering1065 °C for 1 h AC + 250 °C for 1 h (below: MDES + Temper).
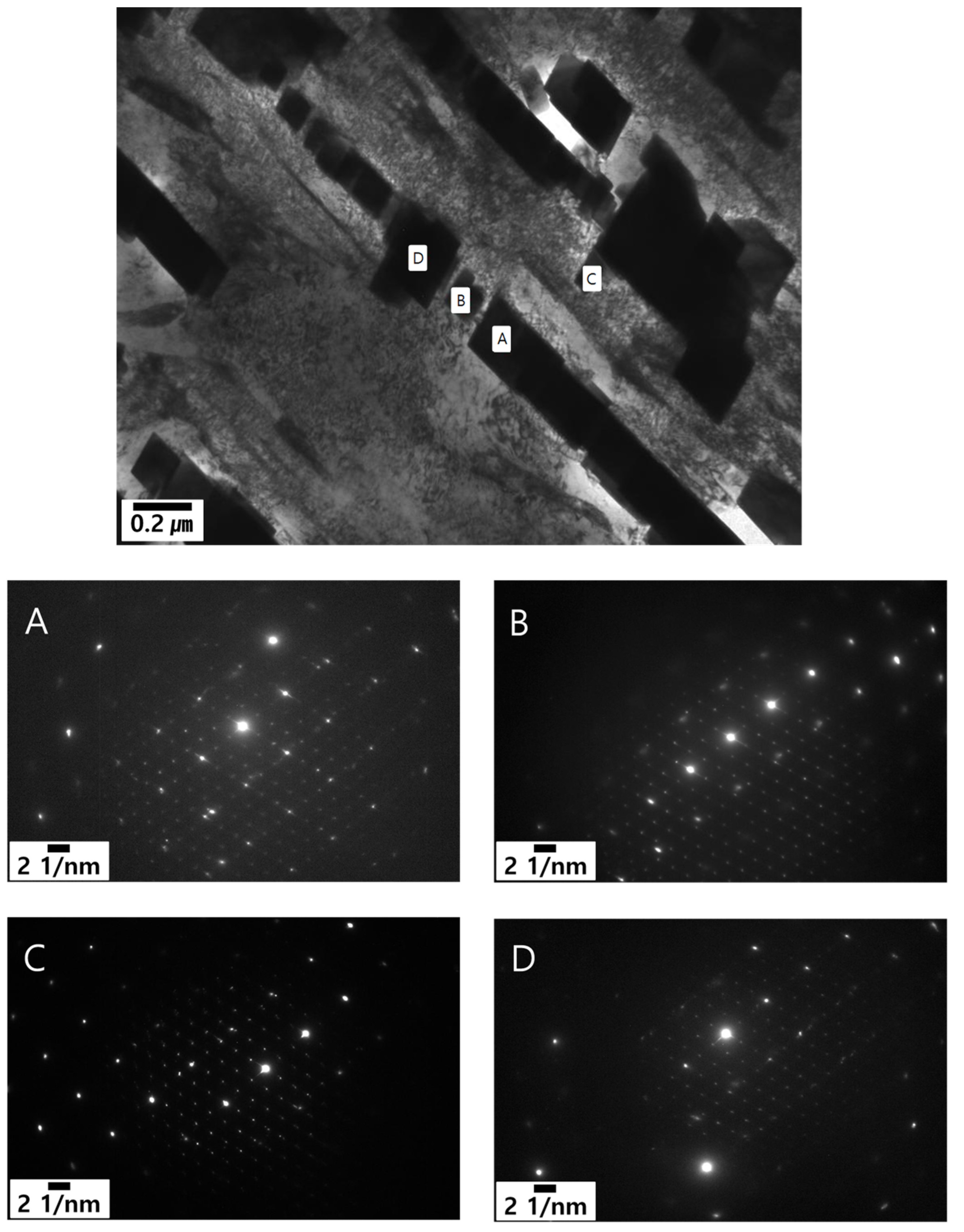
- Formation of M23C6 as a shell of M7C3 in the eutectic structure is a peritectoid type reaction between M7C3 and austenite matrix.
- M7C3–M23C6 transition and precipitation of M23C6 from the adjacent austenite matrix.
3.4.2. Effect of Heat Treatment on M23C6 Precipitation
- Effect of low temperature aging (DES + Age) and tempering (DES + Temper).
- 2.
- Effect of cooling condition
4. Mechanical Properties
4.1. Hardness
4.2. Wear Resistance
5. Conclusions
- With increasing Ceq the fraction of the primarily solidified dendrites decreased. The measured and calculated fractions of the primarily solidified dendrites have similar propensity to Ceq;
- Eutectic reaction occurred with the ratio of M7C3:austenite = 1:2.76, which was predicted by ThermoCalc calculation;
- It was found that destabilization of austenite during conventional heat treatment releases the saturated solute elements C and Cr to form M23C6 in the primary (austenite) dendrite, however little or no M23C6 precipitation occurred within austenite in eutectic;
- The amount of M23C6 precipitation during destabilization is closely related to that of the primarily solidified dendrites which means that it is very sensitive to chemical composition;
- The mechanical properties such as hardness and wear resistance of the alloys are closely related to the fractions of M7C3 and M23C6.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, W.F. Cast Irons. Structure and Properties of Engineering Alloys, 1st ed.; Brown, J.V., Maisel, J.W., Eds.; McGraw-Hill: New York, NY, USA, 1981; pp. 322–323. [Google Scholar]
- Davis, J.R. Metallurgy and properties of high-alloy white irons. In ASM Specialty Handbook Cast Irons, 1st ed.; ASM International: Russell Township, OH, USA, 1996; pp. 112–116. [Google Scholar]
- ASTm standards. Standard Test Method for Linearly Reciprocating Ball-on-Flat Sliding Wear. In ASTM Standard G133-05; ASTM International: West Conshohocken, PA, USA, 2003. [Google Scholar]
- Pearce, J.T.H. Wear of Abrasion Resisting Materials. Ph.D. Thesis, University of Aston, Birmingham, UK, 1982. [Google Scholar]
- Ohide, T.; Ohira, G. Solidification of high chromium alloyed cast iron. Br. Foundrym. 1983, 76, 7–14. [Google Scholar]
- Flemings, M.C. Solidification Processing; McGraw-Hill: New York, NY, USA, 1974; pp. 77–78. [Google Scholar]
- Tabrett, C.P.; Sare, I.R.; Ghomashchi, M.R. Microstructure-property relationships in high chromium white iron alloys. Int. Mater. Rev. 1996, 41, 59–82. [Google Scholar] [CrossRef]
- Powell, G.L.F.; Laird, G. Structure, nucleation, growth and morphology of secondary carbides in high chromium and Cr-Ni white cast irons. J. Mater. Sci. 1992, 27, 29–35. [Google Scholar] [CrossRef]
- Wiengmoon, A.; Chairuangsri, T.; Pearce, J.T.H. A microstructural study of destabilised 30 wt.% Cr-2.3 wt.% C high chromium cast iron. ISIJ Int. 2004, 44, 396–403. [Google Scholar] [CrossRef]





| Designation | Composition | |||||
|---|---|---|---|---|---|---|
| C | Si | Mn | Cr | Ni | Mo | |
| 2124 | 2.12 | 0.66 | 0.65 | 24.05 | 0.85 | 0.86 |
| 2127 | 2.13 | 0.71 | 0.67 | 27.00 | 0.89 | 0.86 |
| 2427 | 2.43 | 0.68 | 0.70 | 27.06 | 0.86 | 0.85 |
| 2827 | 2.78 | 0.70 | 0.70 | 27.34 | 0.87 | 0.85 |
| 2927 | 2.95 | 0.67 | 0.71 | 26.94 | 0.92 | 0.82 |
| Alloy | 2124 (Ceq:3.3) | 2127 (Ceq:3.5) | 2427 (Ceq:3.8) | 2827 (Ceq:4.1) | 2927 (Ceq:4.3) |
|---|---|---|---|---|---|
| Solidification leading phase and temperature (°C) | (8.8%) 1320 | *,1 (5.9%) 1320 | (5.4%) 1300 | M7C3 (0.1%) 1300 | M7C3 (1.6%) 1300 |
| Phase fraction (:L:M7C3) and eutectic reaction beginning temperature (°C) | 32.1:67.6:0.3 at 1290 | 37.6*,2:57.7:4.7 at 1290 | 39.1:52.5:8.4 at 1290 | 38.5:48.4:13.1 at 1290 | 26.7:62.0:11.3 at 1290 |
| Phase fraction (:M7C3) at final freezing temperature (°C) | 83.4:16.6 at 1284 | 81.9*,3:18.1 at 1285 | 79.1:20.9 at 1285 | 75.2:24.8 at 1285 | 73.4:26.6 at 1284 |
| Primarily solidified dendrite fraction | 37.6 | 31.9 | 21.4 | 6.8 | 0 |
| Alloy | 2124 (Ceq:3.3) | 2127 (Ceq:3.5) | 2427 (Ceq:3.8) | 2827 (Ceq:4.1) | 2927 (Ceq:4.3) |
|---|---|---|---|---|---|
| Measured dendrite fraction (vol.%) | 48.4 | 42.2 | 32.4 | 9.3 | 0 |
| ThermoCalc prediction (mol.%) | 37.6 | 31.9*,1 | 21.4 | 6.8 | 0 |
| Alloy | M23C6 Precipitation Initiation | M23C6 Peak Precipitation | |||
|---|---|---|---|---|---|
| T (°C) | M7C3 Fraction | T (°C) | M23C6 Fraction | M7C3 Fraction | |
| 2124 | 1060 | 19.8 | 850 | 17.3 | 12.2 |
| 2127 | 1180 | 18.8 | 890 | 33.0 | 2.5 |
| 2427 | 1100 | 23.4 | 870 | 20.1 | 14.0 |
| 2827 | 1000 | 29.1 | 810 | 9.8 | 24.6 |
| 2927 | 920 | 31.9 | 739 | 6.0 | 29.7 |
| Alloy | 2124 | 2127 | 2427 | 2827 | 2927 |
|---|---|---|---|---|---|
| As-Cast | 48.0 | 46.8 | 48.0 | 50.3 | 49.8 |
| DES + Age | 60.0 | 59.3 | 59.7 | 59.3 | 60.5 |
| DES + WQ + Age | 52.3 | 57.7 | 57.7 | 58.3 | 61.3 |
| DES + Temper | 58.3 | 60.0 | 59.7 | 60.3 | 62.2 |
| MDES + Temper | 57.3 | 58.3 | 59.2 | 60.3 | 62.0 |
| Alloy | 2124 | 2127 | 2427 | 2827 | 2927 |
|---|---|---|---|---|---|
| DES + Age | 0.352 | 0.367 | 0.338 | 0.306 | 0.297 |
| MDES + Temper | 0.239 | 0.281 | 0.201 | 0.271 | 0.256 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.-S.; Song, Y.-G.; Choi, B.-G.; Bhamornsut, C.; Nakkuntod, R.; Jo, C.-Y.; Lee, J.-H. Effect of Dendrite Fraction on the M23C6 Precipitation Behavior and the Mechanical Properties of High Cr White Irons. Metals 2021, 11, 1576. https://doi.org/10.3390/met11101576
Oh J-S, Song Y-G, Choi B-G, Bhamornsut C, Nakkuntod R, Jo C-Y, Lee J-H. Effect of Dendrite Fraction on the M23C6 Precipitation Behavior and the Mechanical Properties of High Cr White Irons. Metals. 2021; 11(10):1576. https://doi.org/10.3390/met11101576
Chicago/Turabian StyleOh, Jun-Seok, Young-Gy Song, Baig-Gyu Choi, Chalothorn Bhamornsut, Rujeeporn Nakkuntod, Chang-Yong Jo, and Je-Hyun Lee. 2021. "Effect of Dendrite Fraction on the M23C6 Precipitation Behavior and the Mechanical Properties of High Cr White Irons" Metals 11, no. 10: 1576. https://doi.org/10.3390/met11101576
APA StyleOh, J.-S., Song, Y.-G., Choi, B.-G., Bhamornsut, C., Nakkuntod, R., Jo, C.-Y., & Lee, J.-H. (2021). Effect of Dendrite Fraction on the M23C6 Precipitation Behavior and the Mechanical Properties of High Cr White Irons. Metals, 11(10), 1576. https://doi.org/10.3390/met11101576






