Gangues and Clays Minerals as Rate-Limiting Factors in Copper Heap Leaching: A Review
Abstract
1. Introduction
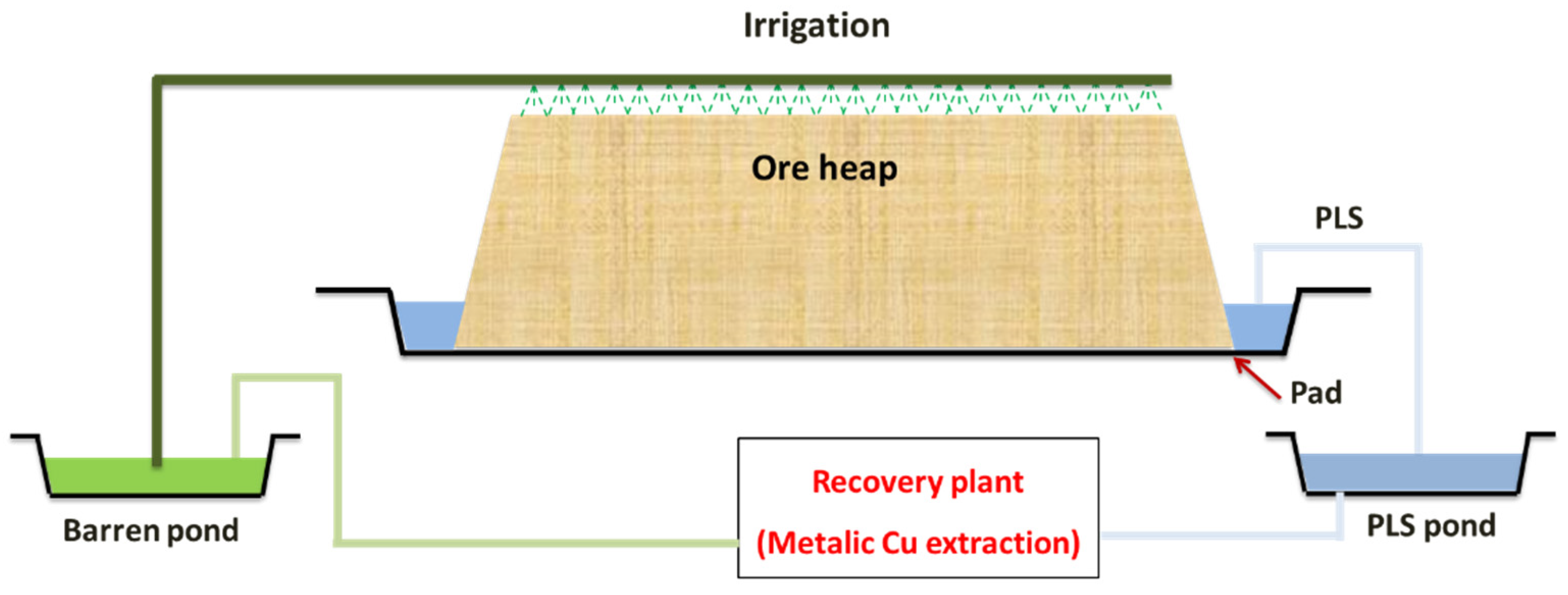
2. Heap Leaching as an Alternative Route in Hydrometallurgy
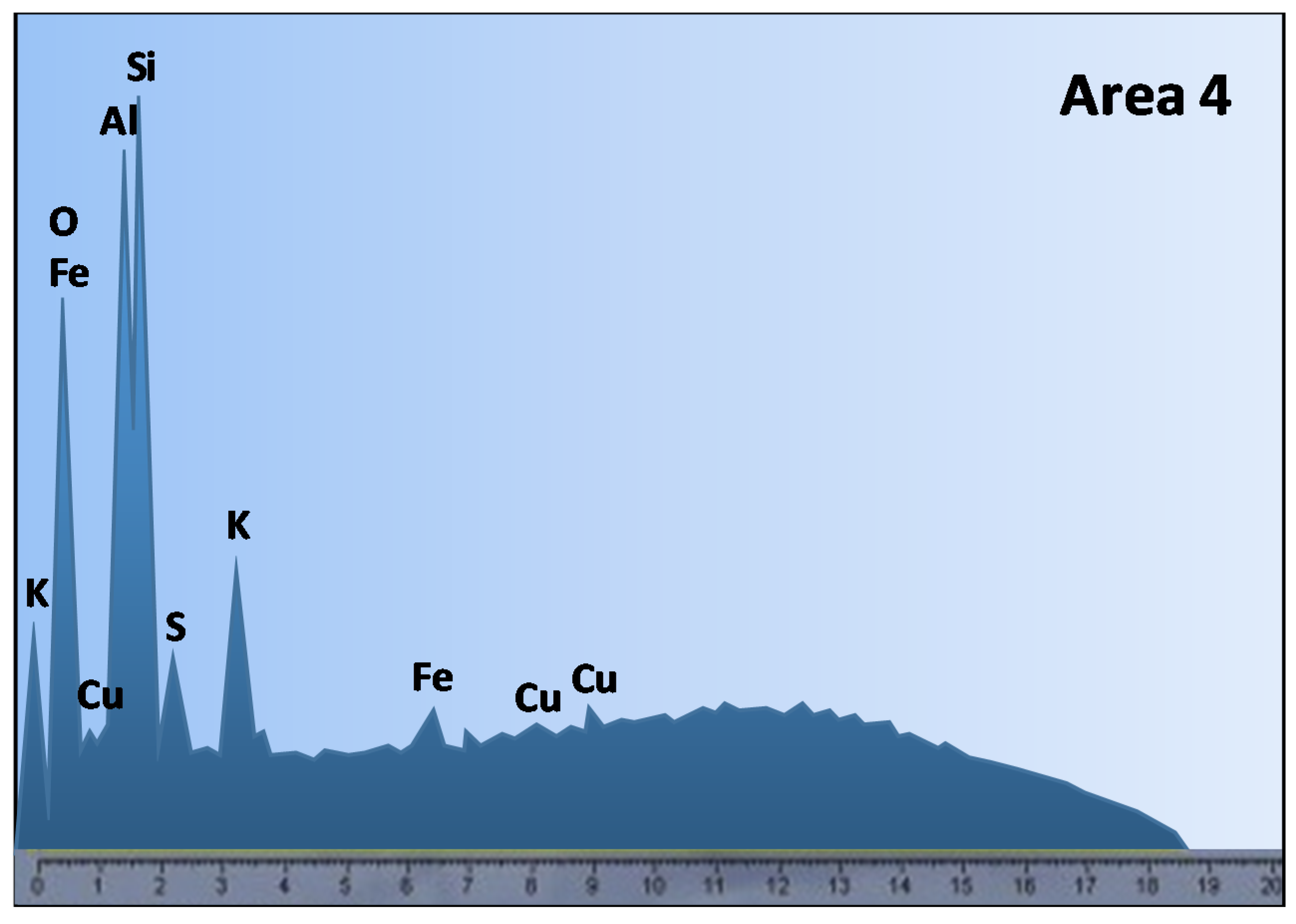
3. Effect of Ore Mineralogy on Copper Heap Leaching Performance
4. Effect of Reactive Gangue on Copper Leaching
4.1. Effect of Iron-Bearing Minerals on Copper Heap Leaching
4.2. Effect of Clay Minerals on Copper Heap Leaching
4.3. Effect of Clay Minerals on Copper Heap Leaching
5. Methods to Reduce Acid Consumption in Heap Leaching
6. Parameters That Are Affected by the Presence of Clays and Reactive Gangues
6.1. Dissolution Rate
6.2. Particle Size
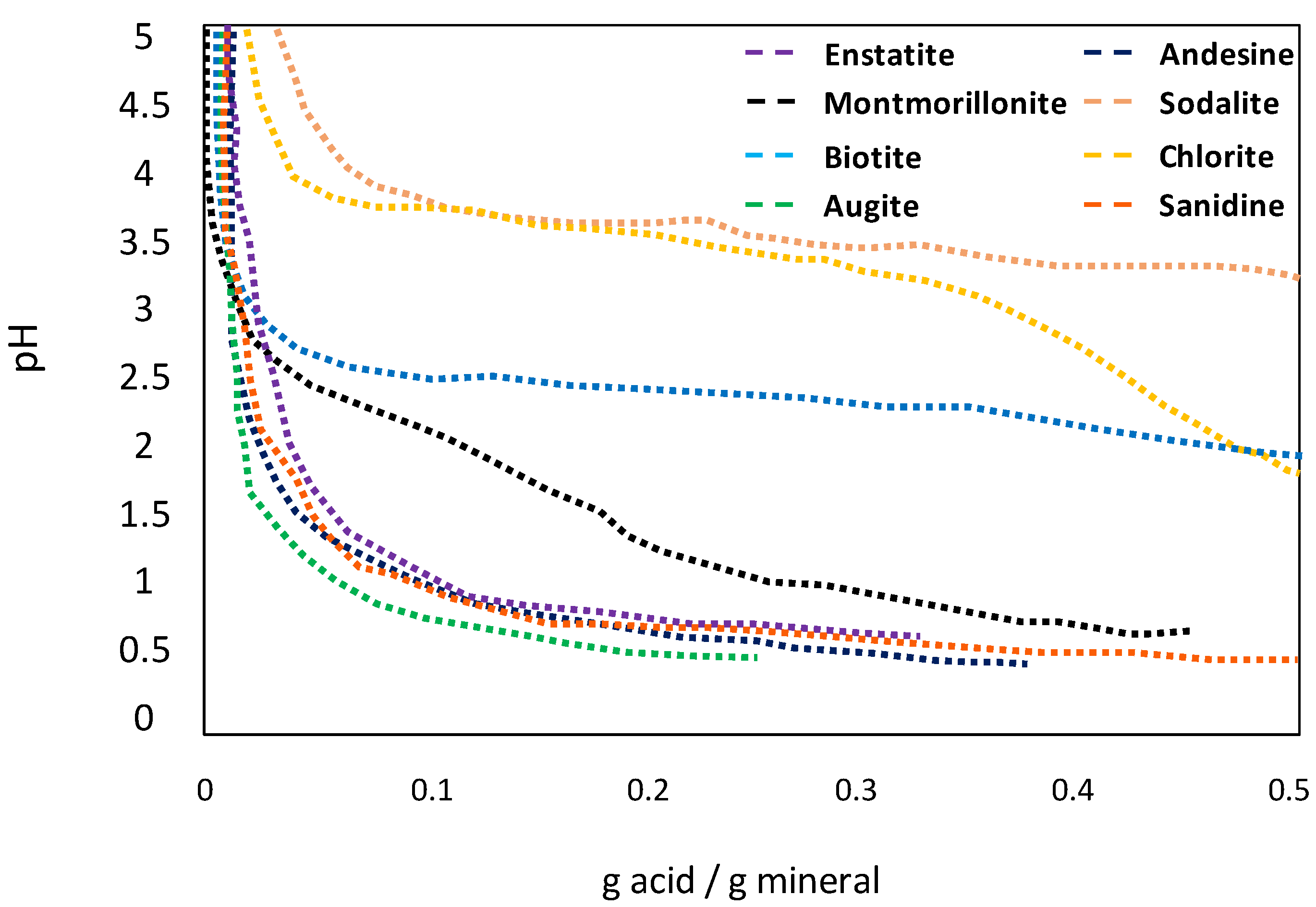
6.3. pH
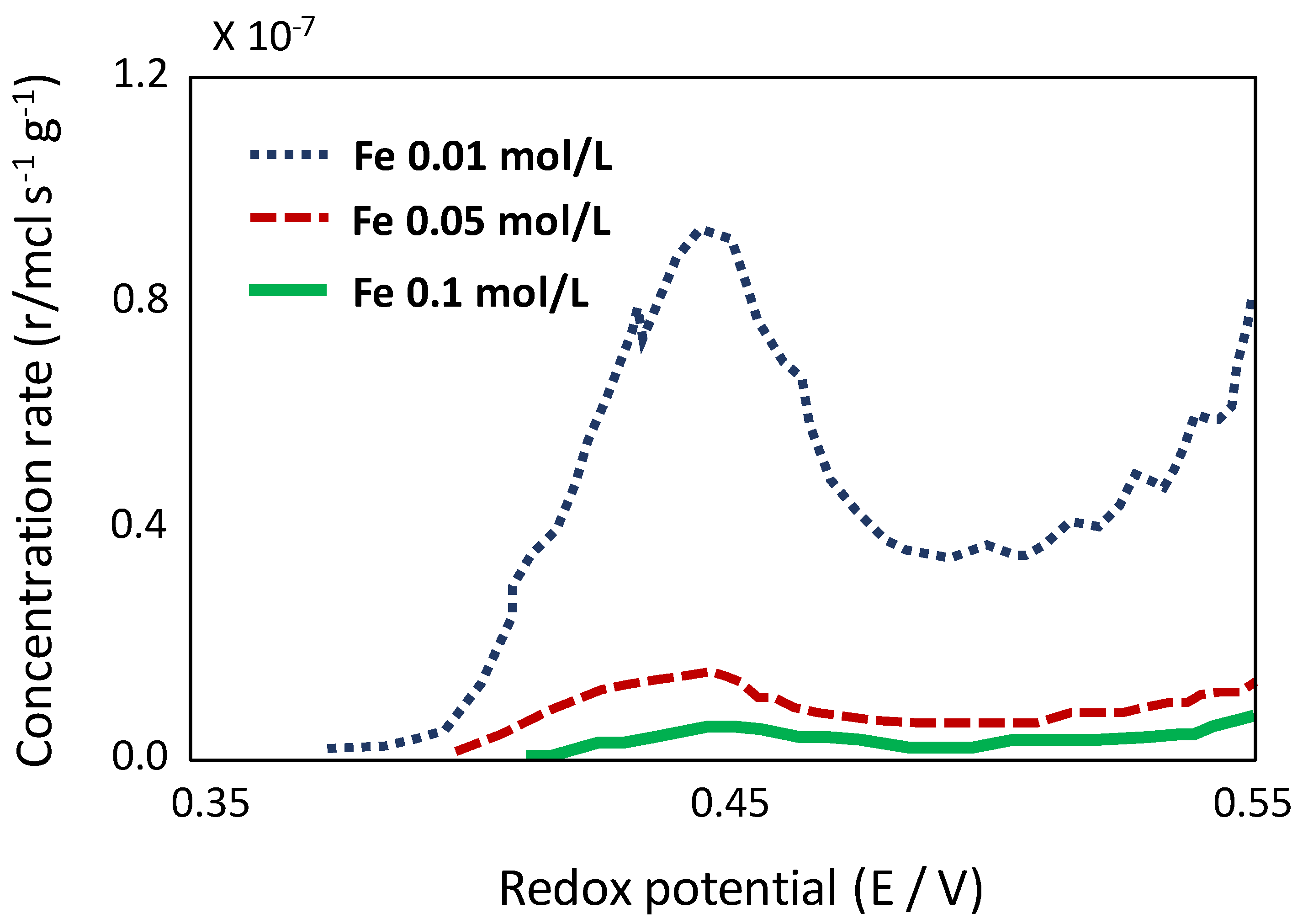
6.4. Redox Potential
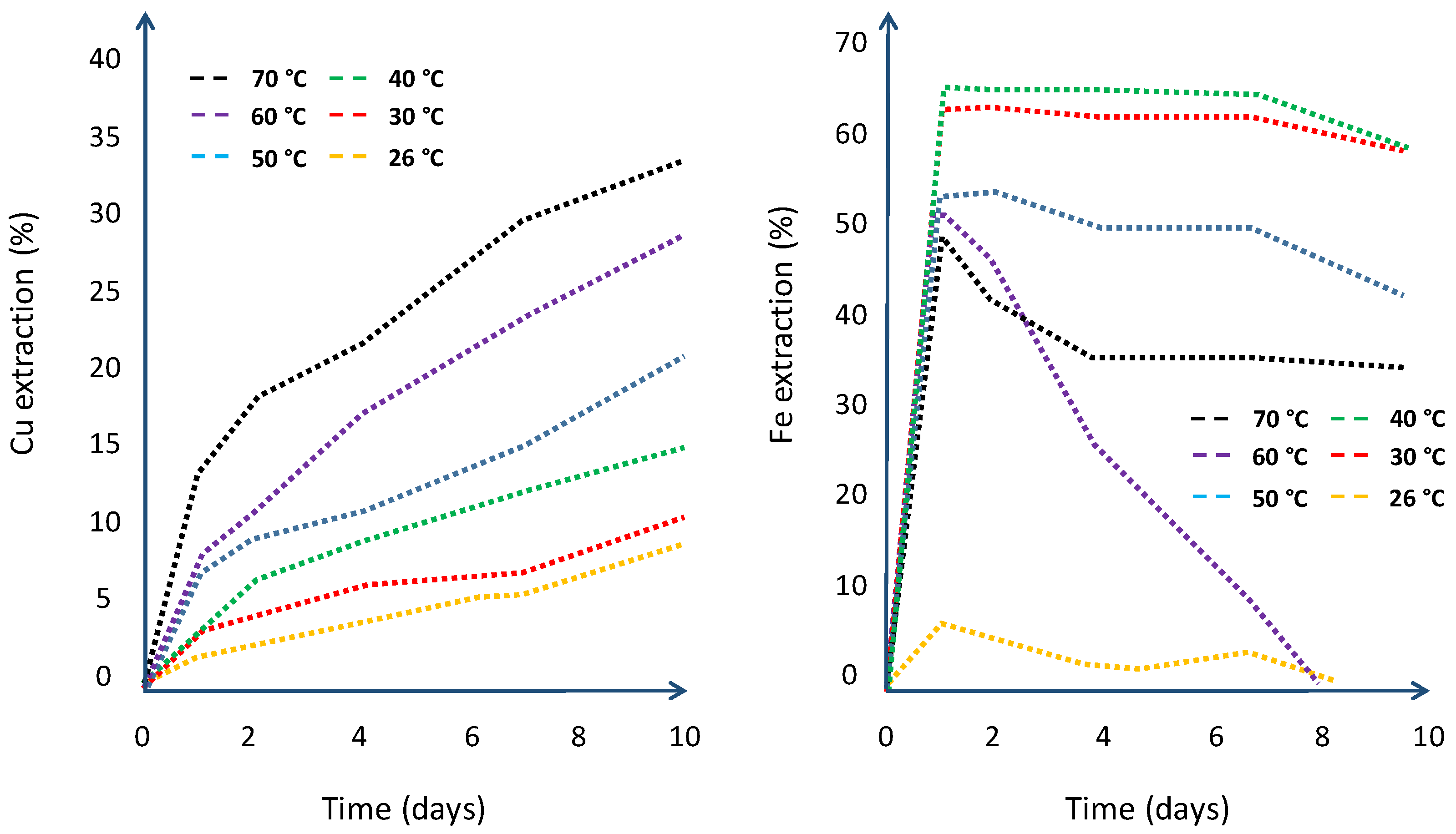
6.5. Temperature
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Toro, N.; Pérez, K.; Saldaña, M.; Salinas-Rodríguez, E.; Hernández, P. Treatment of black copper with the use of iron scrap—Part I. Hem. Ind. Ind. 2020, 74, 237–245. [Google Scholar] [CrossRef]
- Flanagan, D.M. Copper Commodity Summaries 2021; USGS: Reston, VA, USA, 2021.
- Pérez, K.; Toro, N.; Saldaña, M.; Salinas-Rodríguez, E.; Robles, P.; Torres, D.; Jeldres, R.I. Statistical Study for Leaching of Covellite in a Chloride Media. Metals 2020, 10, 477. [Google Scholar] [CrossRef]
- Taboada, M.E.; Hernández, P.C.; Padilla, A.P.; Jamett, N.E.; Graber, T.A. Effects of Fe+2 and Fe+3 in Pretreatment and Leaching on a Mixed Copper Ore in Chloride Media. Metals 2021, 11, 866. [Google Scholar] [CrossRef]
- Rodríguez, M.; Ayala, L.; Robles, P.; Sepúlveda, R.; Torres, D.; Carrillo-Pedroza, F.R.; Jeldres, R.I.; Toro, N. Leaching chalcopyrite with an imidazolium-based ionic liquid and bromide. Metals 2020, 10, 183. [Google Scholar] [CrossRef]
- Bogdanović, G.D.; Petrović, S.; Sokić, M.; Antonijević, M.M. Chalcopyrite leaching in acid media: A review. Metall. Mater. Eng. 2020, 26, 177–198. [Google Scholar] [CrossRef]
- Torres, D.; Ayala, L.; Jeldres, R.I.; Cerecedo-Sáenz, E.; Salinas-Rodríguez, E.; Robles, P.; Toro, N. Leaching Chalcopyrite with High MnO2 and Chloride Concentrations. Metals 2020, 10, 107. [Google Scholar] [CrossRef]
- Saldaña, M.; Rodríguez, F.; Rojas, A.; Pérez, K.; Angulo, P. Development of an empirical model for copper extraction from chalcocite in chloride media. Hem. Ind. 2020, 74, 285–292. [Google Scholar] [CrossRef]
- Saldaña, M.; Neira, P.; Flores, V.; Robles, P.; Moraga, C. A Decision Support System for Changes in Operation Modes of the Copper Heap Leaching Process. Metals 2021, 11, 1025. [Google Scholar] [CrossRef]
- Nikoloski, A.N.; O’Malley, G.P. The acidic ferric sulfate leaching of primary copper sulfides under recycle solution conditions observed in heap leaching. Part 1. Effect of standard conditions. Hydrometallurgy 2018, 178, 231–239. [Google Scholar] [CrossRef]
- Dijkstra, R. Economical abatement of high-strength SO2 off-gas from a smelter. J. S. Afr. Inst. Min. Metall. 2017, 117, 1003–1007. [Google Scholar] [CrossRef]
- Toro, N.; Briceño, W.; Pérez, K.; Cánovas, M.; Trigueros, E.; Sepúlveda, R.; Hernández, P. Leaching of pure chalcocite in a chloride media using sea water and waste water. Metals 2019, 9, 780. [Google Scholar] [CrossRef]
- Rodríguez, F.; Moraga, C.; Castillo, J.; Gálvez, E.; Robles, P.; Toro, N. Submarine Tailings in Chile—A Review. Metals 2021, 11, 780. [Google Scholar] [CrossRef]
- Dinelli, E.; Tateo, F. Different types of fine-grained sediments associated with acid mine drainage in the Libiola Fe–Cu mine area (Ligurian Apennines, Italy). Appl. Geochem. 2002, 17, 1081–1092. [Google Scholar] [CrossRef]
- Doula, M.; Ioannou, A.; Dimirkou, A. Copper Adsorption and Si, Al, Ca, Mg, and Na Release from Clinoptilolite. J. Colloid Interface Sci. 2002, 245, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef]
- White, A.F.; Brantley, S.L. The effect of time on the weathering of silicate minerals: Why do weathering rates differ in the laboratory and field? Chem. Geol. 2003, 202, 479–506. [Google Scholar] [CrossRef]
- Galhardi, J.A.; Bonotto, D.M. Hydrogeochemical features of surface water and groundwater contaminated with acid mine drainage (AMD) in coal mining areas: A case study in southern Brazil. Environ. Sci. Pollut. Res. 2016, 23, 18911–18927. [Google Scholar] [CrossRef] [PubMed]
- Shim, M.J.; Choi, B.Y.; Lee, G.; Hwang, Y.H.; Yang, J.-S.; O’Loughlin, E.J.; Kwon, M.J. Water quality changes in acid mine drainage streams in Gangneung, Korea, 10 years after treatment with limestone. J. Geochem. Explor. 2015, 159, 234–242. [Google Scholar] [CrossRef]
- Anawar, H.M. Sustainable rehabilitation of mining waste and acid mine drainage using geochemistry, mine type, mineralogy, texture, ore extraction and climate knowledge. J. Environ. Manag. 2015, 158, 111–121. [Google Scholar] [CrossRef]
- Albanese, S.; De Vivo, B.; Lima, A.; Frattasio, G.; Kříbek, B.; Nyambe, I.; Majer, V. Prioritising environmental risk at the regional scale by a GIS aided technique: The Zambian Copperbelt Province case study. J. Geochem. Explor. 2014, 144, 433–442. [Google Scholar] [CrossRef]
- Velásquez-Yévenes, L.; Torres, D.; Toro, N. Leaching of chalcopyrite ore agglomerated with high chloride concentration and high curing periods. Hydrometallurgy 2018, 181, 215–220. [Google Scholar] [CrossRef]
- Turan, M.D.; Orhan, R.; Turan, M.; Nizamoğlu, H. Use of Ammonia Salts in Selective Copper Extraction from Tailings. Mining, Metall. Explor. 2020, 37, 1349–1356. [Google Scholar] [CrossRef]
- Turan, M.D.; Sarı, Z.A.; Nizamoğlu, H. Pressure leaching of chalcopyrite with oxalic acid and hydrogen peroxide. J. Taiwan Inst. Chem. Eng. 2021, 118, 112–120. [Google Scholar] [CrossRef]
- Ghorbani, Y.; Franzidis, J.-P.; Petersen, J. Heap leaching technology—Current state, innovations and future directions: A review. Miner. Process. Extr. Metall. Rev. 2015, 37, 73–119. [Google Scholar] [CrossRef]
- Watling, H.R.; Shiers, D.W.; Li, J.; Chapman, N.M.; Douglas, G.B. Effect of water quality on the leaching of a low-grade copper sulfide ore. Miner. Eng. 2014, 58, 39–51. [Google Scholar] [CrossRef]
- Scheffel, R. Copper Heap Leach Design and Practice. In Mineral Processing Plant Design, Practice, and Control; Chapter 12; Mular, A.L., Halbe, D.N., Barratt, D.J., Eds.; SME: Englewood, CO, USA, 2002; Volume 2, pp. 1571–1605. [Google Scholar]
- Kappes, D.W. Precious metal heap leach design and practice. In Mineral Processing Plant Design, Practice, and Control; SME: Englewood, CO, USA, 2002; Volume 2, pp. 1606–1630. ISBN 0-87335-223-8. [Google Scholar]
- Valencia, J.A.; Méndez, D.A.; Cueto, J.Y.; Cisternas, L.A. Saltpeter extraction and modelling of caliche mineral heap leaching. Hydrometallurgy 2008, 90, 103–114. [Google Scholar] [CrossRef]
- Padilla, G.A.; Cisternas, L.A.; Cueto, J.Y. On the optimization of heap leaching. Miner. Eng. 2008, 21, 673–678. [Google Scholar] [CrossRef]
- Scheffel, R.D. Heap Leaching Design for Success-a Case Study. In Proceedings of the Low-Grade Uranium Dump and Heap Leach Technical Meeting, Vienna, Austria, 29–31 March 2010. [Google Scholar]
- Petersen, J. Heap leaching as a key technology for recovery of values from low-grade ores—A brief overview. Hydrometallurgy 2016, 165, 206–212. [Google Scholar] [CrossRef]
- Robertson, S.W.; van Staden, P.J.; Seyedbagheri, A. Advances in high-temperature heap leaching of refractory copper sulphide ores. J. S. Afr. Inst. Min. Metall. 2012, 112, 1045–1050. [Google Scholar]
- Lizama, H.M.; Jensen, S.E.; Stradling, A.W. Dynamic microbial populations in heap leaching of zinc sulphide ore. Miner. Eng. 2012, 25, 54–58. [Google Scholar] [CrossRef]
- Petersen, J.; Dixon, D.G. Modeling and optimization of heap bioleach processes. In Biomining; Springer: Berlin/Heidelberg, Germany, 2007; pp. 153–176. [Google Scholar]
- Carlsson, E.; Büchel, G. Screening of residual contamination at a former uranium heap leaching site, Thuringia, Germany. Geochemistry 2005, 65, 75–95. [Google Scholar] [CrossRef]
- Mwase, J.M.; Petersen, J.; Eksteen, J.J. A conceptual flowsheet for heap leaching of platinum group metals (PGMs) from a low-grade ore concentrate. Hydrometallurgy 2012, 111–112, 129–135. [Google Scholar] [CrossRef]
- Ilyas, S.; Lee, J.; Chi, R. Bioleaching of metals from electronic scrap and its potential for commercial exploitation. Hydrometallurgy 2013, 131–132, 138–143. [Google Scholar] [CrossRef]
- Saldaña, M.; Toro, N.; Castillo, J.; Hernández, P.; Navarra, A. Optimization of the heap leaching process through changes in modes of operation and discrete event simulation. Minerals 2019, 9, 421. [Google Scholar] [CrossRef]
- Lu, J.; Dreisinger, D.; West-Sells, P. Acid curing and agglomeration for heap leaching. Hydrometallurgy 2017, 167, 30–35. [Google Scholar] [CrossRef]
- Schlesinger, M.; King, M.; Sole, K.; Davenport, W. Extractive Metallurgy of Copper, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 9780080967899. [Google Scholar]
- Liu, H.; Xia, J.; Nie, Z.; Ma, C.; Zheng, L.; Hong, C.; Zhao, Y.; Wen, W. Bioleaching of chalcopyrite by Acidianus manzaensis under different constant pH. Miner. Eng. 2016, 98, 80–89. [Google Scholar] [CrossRef]
- Lee, J.; Acar, S.; Doerr, D.L.; Brierley, J.A. Comparative bioleaching and mineralogy of composited sulfide ores containing enargite, covellite and chalcocite by mesophilic and thermophilic microorganisms. Hydrometallurgy 2011, 105, 213–221. [Google Scholar] [CrossRef]
- Ruan, R.; Zou, G.; Zhong, S.; Wu, Z.; Chan, B.; Wang, D. Why Zijinshan copper bioheapleaching plant works efficiently at low microbial activity—Study on leaching kinetics of copper sulfides and its implications. Miner. Eng. 2013, 48, 36–43. [Google Scholar] [CrossRef]
- Ma, L.; Wang, X.; Liu, X.; Wang, S.; Wang, H. Intensified bioleaching of chalcopyrite by communities with enriched ferrous or sulfur oxidizers. Bioresour. Technol. 2018, 268, 415–423. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, C.; Kou, J.; Zhao, H.; Wei, D.; Xing, Y. Enhancing the Leaching of Chalcopyrite Using Acidithiobacillus ferrooxidans under the Induction of Surfactant Triton X-100. Minerals 2018, 9, 11. [Google Scholar] [CrossRef]
- Moraga, C.; Cerecedo-Saenz, E.; González, J.; Robles, P.; Carrillo-Pedroza, F.R.; Toro, N. Comparative Study of MnO2 Dissolution from Black Copper Minerals and Manganese Nodules in an Acid Medium. Metals 2021, 11, 817. [Google Scholar] [CrossRef]
- Jansen, M.; Taylor, A. Overview of gangue mineralogy issues in oxide copper heap leaching. In Proceedings of the ALTA Conference, Perth, WA, Australia, 19–24 May 2003; p. 32. [Google Scholar]
- Ghorbani, Y.; Becker, M.; Petersen, J.; Mainza, A.N.; Franzidis, J.-P. Investigation of the Effect of mineralogy as rate-limiting factors in large particle leaching. Miner. Eng. 2013, 52, 38–51. [Google Scholar] [CrossRef]
- Bouffard, S.C. Review of Agglomeration Practice and Fundamentals in Heap Leaching. Miner. Process. Extr. Metall. Rev. 2005, 26, 233–294. [Google Scholar] [CrossRef]
- Ghomi, M.A.; Mozammel, M.; Moghanni, H.; Shahkar, L. Atmospheric leaching of chalcopyrite in the presence of some polar organic reagents: A comparative study and optimization. Hydrometallurgy 2019, 189, 105120. [Google Scholar] [CrossRef]
- Helle, S.; Kelm, U. Experimental leaching of atacamite, chrysocolla and malachite: Relationship between copper retention and cation exchange capacity. Hydrometallurgy 2005, 78, 180–186. [Google Scholar] [CrossRef]
- Baum, W. The use of a mineralogical data base for production forecasting and troubleshooting in copper leach operations. In Proceedings of the C. 99, Copper 99 Fourth International Conference, Phoenix, AZ, USA, 10–13 October 1999; pp. 393–408. [Google Scholar]
- Baum, W.; Ausburn, K. Daily process mineralogy: A metallurgical tool for optimized copper leaching. In Proceedings of the 5th International Seminar on Process Hydrometallurgy, Santiago, Chile, 10–12 July 2013; pp. 49–56. [Google Scholar] [CrossRef]
- Rich, R. Inorganic Reactions in Water; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 978-3-540-73961-6. [Google Scholar]
- Harmer, S.L.; Thomas, J.E.; Fornasiero, D.; Gerson, A.R. The evolution of surface layers formed during chalcopyrite leaching. Geochim. Cosmochim. Acta 2006, 70, 4392–4402. [Google Scholar] [CrossRef]
- Ahonen, L.; Tuovinen, O.H. Bacterial leaching of complex sulfide ore samples in bench-scale column reactors. Hydrometallurgy 1995, 37, 1–21. [Google Scholar] [CrossRef]
- Phuong Thao, N.T.; Tsuji, S.; Jeon, S.; Park, I.; Tabelin, C.B.; Ito, M.; Hiroyoshi, N. Redox potential-dependent chalcopyrite leaching in acidic ferric chloride solutions: Leaching experiments. Hydrometallurgy 2020, 194, 105299. [Google Scholar] [CrossRef]
- Hernández, P.C. Estudio del Equilibrio Sólido-Líquido de Sistemas Acuosos de Minerales de Cobre Con Agua de Mar, Aplicado a Procesos de Lixiviación. Ph.D. Thesis, Universidad De Antofagasta, Antofagasta, Chile, 2013. [Google Scholar]
- Chetty, D. Acid-Gangue Interactions in Heap Leach Operations: A Review of the Role of Mineralogy for Predicting Ore Behaviour. Minerals 2018, 8, 47. [Google Scholar] [CrossRef]
- Youlton, B.J.; Kinnaird, J.A. Gangue–reagent interactions during acid leaching of uranium. Miner. Eng. 2013, 52, 62–73. [Google Scholar] [CrossRef]
- SERNAGEOMIN. Guía Metadológica sobre Drenaje ácido en la Industria Minera; SERNAGEOMIN: Av. Santa María, Chile, 2002.
- SERNAGEOMIN. Guía metodológica para la Estabilidad Química de Faenas e Instalaciones Mineras; SERNAGEOMIN: Av. Santa María, Chile, 2015.
- Toro Villarroel, N.R. Optimización de Parámetros Para la Extracción de Elementos Desde Minerales en Medios Ácido; Universidad Politécnica de Cartagena: Cartagena, Spain,, 2020. [Google Scholar]
- Sequeira, M.; Leite, L. Alternative conceptions and history of science in physics teacher education. Sci. Educ. 1991, 75, 45–56. [Google Scholar] [CrossRef]
- Li, J.; McFarlane, A.; Klauber, C.; Smith, P. Leaching and solution chemistry. In Clays in the Minerals Processing Value Chain; Grafe, M., Klauber, C., McFarlane, A.J., Robinson, D.J., Eds.; Cambridge University Press: Cambridge, UK, 2017; pp. 111–141. [Google Scholar]
- Pérez, K.; Toro, N.; Campos, E.; González, J.; Jeldres, R.I.; Nazer, A.; Rodriguez, M.H. Extraction of Mn from Black Copper Using Iron Oxides from Tailings and Fe2+ as Reducing Agents in Acid Medium. Metals 2019, 9, 1112. [Google Scholar] [CrossRef]
- Seyedbagheri, A.; Van Staden, P.; McLaren, C. A Study of Acid-Gangue Reactions In Heap-Leach Operations. In Proceedings of the V International Copper Hydrometallurgy Workshop, Antofagasta, Chile, 13–15 May 2009; pp. 68–76. Available online: https://gecamin.com/english/conferences-and-seminars-2009 (accessed on 16 August 2021).
- Free, M.L. Understanding acid consumption and its relationship with copper recovery, conference. In Proceedings of the SME Annual Meeting, Phoenix, AZ, USA, 28 February–3 March 2010; Volume 1, pp. 74–77. [Google Scholar]
- Terry, B. The acid decomposition of silicate minerals part I. Reactivities and modes of dissolution of silicates. Hydrometallurgy 1983, 10, 135–150. [Google Scholar] [CrossRef]
- Bouffard, S.C. Agglomeration for heap leaching: Equipment design, agglomerate quality control, and impact on the heap leach process. Miner. Eng. 2008, 21, 1115–1125. [Google Scholar] [CrossRef]
- Das, T.; Ayyappan, S.; Chaudhury, G. Factors affecting bioleaching kinetics of sulfide ores using acidophilic microorganisms. Biometals 1999, 12, 1–10. [Google Scholar] [CrossRef]
- Ramachandra, M.; Crawford, D.L.; Hertel, G. Characterization of an extracellular lignin peroxidase of the lignocellulolytic actinomycete Streptomyces viridosporus. Appl. Environ. Microbiol. 1988, 54, 3057–3063. [Google Scholar] [CrossRef]
- Sui, C.C.; Brienne, S.H.R.; Ramachandra Rao, S.; Xu, Z.; Finch, J.A. Metal ion production and transfer between sulphide minerals. Miner. Eng. 1995, 8, 1523–1539. [Google Scholar] [CrossRef]
- Arslan, V. A study on the dissolution kinetics of iron oxide leaching from clays by oxalic acid. Physicochem. Probl. Miner. Process. 2021, 57, 97–111. [Google Scholar] [CrossRef]
- Watling, H.R. The bioleaching of sulphide minerals with emphasis on copper sulphides—A review. Hydrometallurgy 2006, 84, 81–108. [Google Scholar] [CrossRef]
- Parker, A.; Klauber, C.; Kougianos, A.; Watling, H.R.; van Bronswijk, W. An X-ray photoelectron spectroscopy study of the mechanism of oxidative dissolution of chalcopyrite. Hydrometallurgy 2003, 71, 265–276. [Google Scholar] [CrossRef]
- Klauber, C.; Parker, A.; van Bronswijk, W.; Watling, H. Sulphur speciation of leached chalcopyrite surfaces as determined by X-ray photoelectron spectroscopy. Int. J. Miner. Process. 2001, 62, 65–94. [Google Scholar] [CrossRef]
- Bartlett, R.W. Solution Mining: Leaching and Fluid Recovery of Materials; Routledge: Abingdon, UK, 1992; ISBN 978-2881245466. [Google Scholar]
- Yin, S.; Wang, L.; Wu, A.; Free, M.L.; Kabwe, E. Enhancement of copper recovery by acid leaching of high-mud copper oxides: A case study at Yangla Copper Mine, China. J. Clean. Prod. 2018, 202, 321–331. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, W.; Granata, G. Effects of grain size gradation on the porosity of packed heap leach beds. Hydrometallurgy 2018, 179, 238–244. [Google Scholar] [CrossRef]
- Heinen, H.J. Enhancing Percolation Rates in Heap Leaching of Gold-Silver Ores; University of Michigan Library: Ann Arbor, MI, USA, 1979. [Google Scholar]
- McClelland, G.E. Agglomerated and unagglomerated heap leaching behaviour is compared in production heaps. Min. Eng. 1986, 38, 503. [Google Scholar]
- Torres, D.; Pérez, K.; Trigueros, E.; Jeldres, R.I.; Salinas-Rodríguez, E.; Robles, P.; Toro, N. Reducing-Effect of Chloride for the Dissolution of Black Copper. Metals 2020, 10, 123. [Google Scholar] [CrossRef]
- Miller, P.C.; Rhodes, M.K.; Winby, R.; Pinches, A.; van Staden, P.J. Commercialization of bioleaching for base-metal extraction. Min. Metall. Explor. 1999, 16, 42–50. [Google Scholar] [CrossRef]
- Dorado, A.D.; Solé, M.; Lao, C.; Alfonso, P.; Gamisans, X. Effect of pH and Fe(III) ions on chalcopyrite bioleaching by an adapted consortium from biogas sweetening. Miner. Eng. 2012, 39, 36–38. [Google Scholar] [CrossRef][Green Version]
- Viramontes-Gamboa, G.; Rivera-Vasquez, B.F.; Dixon, D.G. The Active-to-passive Transition of Chalcopyrite. ECS Trans. 2019, 2, 165–175. [Google Scholar] [CrossRef]
- Vilcáez, J.; Yamada, R.; Inoue, C. Effect of pH reduction and ferric ion addition on the leaching of chalcopyrite at thermophilic temperatures. Hydrometallurgy 2009, 96, 62–71. [Google Scholar] [CrossRef]
- Córdoba, E.M.; Muñoz, J.A.; Blázquez, M.L.; González, F.; Ballester, A. Leaching of chalcopyrite with ferric ion. Part I: General aspects. Hydrometallurgy 2008, 93, 81–87. [Google Scholar] [CrossRef]
- Holden, P.; Foster, L.; Neilan, B.; Berra, G.; Vu, Q. Characterisation of novel salt tolerant iron-oxidising bacteria. In Biohydrometallurgy: Fundamentals, Technology and Sustainable Development, Part A; Ciminelli, V.S.T., Garcia, O., Jr., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 283–290. [Google Scholar]
- Rawlings, D.E.; Tributsch, H.; Hansford, G.S. Reasons why ‘Leptospirillum’-like species rather than Thiobacillus ferrooxidans are the dominant iron-oxidizing bacteria in many commercial processes for the biooxidation of pyrite and related ores. Microbiology 1999, 145, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Hiroyoshi, N.; Hirota, M.; Hirajima, T.; Tsunekawa, M. A case of ferrous sulfate addition enhancing chalcopyrite leaching. Hydrometallurgy 1997, 47, 37–45. [Google Scholar] [CrossRef]
- Third, K.; Cord-Ruwisch, R.; Watling, H. The role of iron-oxidizing bacteria in stimulation or inhibition of chalcopyrite bioleaching. Hydrometallurgy 2000, 57, 225–233. [Google Scholar] [CrossRef]
- Hansford, G.; Vargas, T. Chemical and electrochemical basis of bioleaching processes. Hydrometallurgy 2001, 59, 135–145. [Google Scholar] [CrossRef]


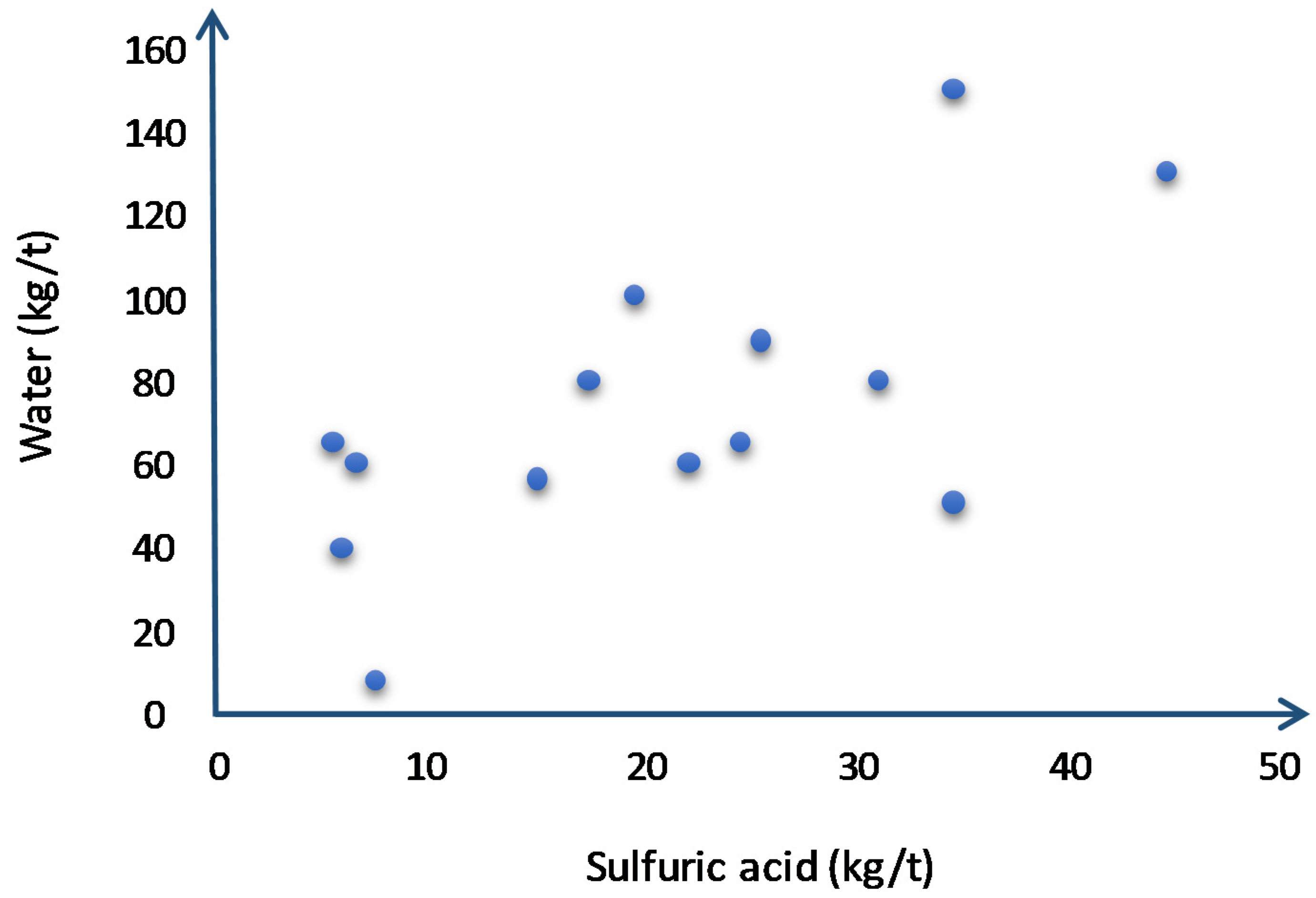
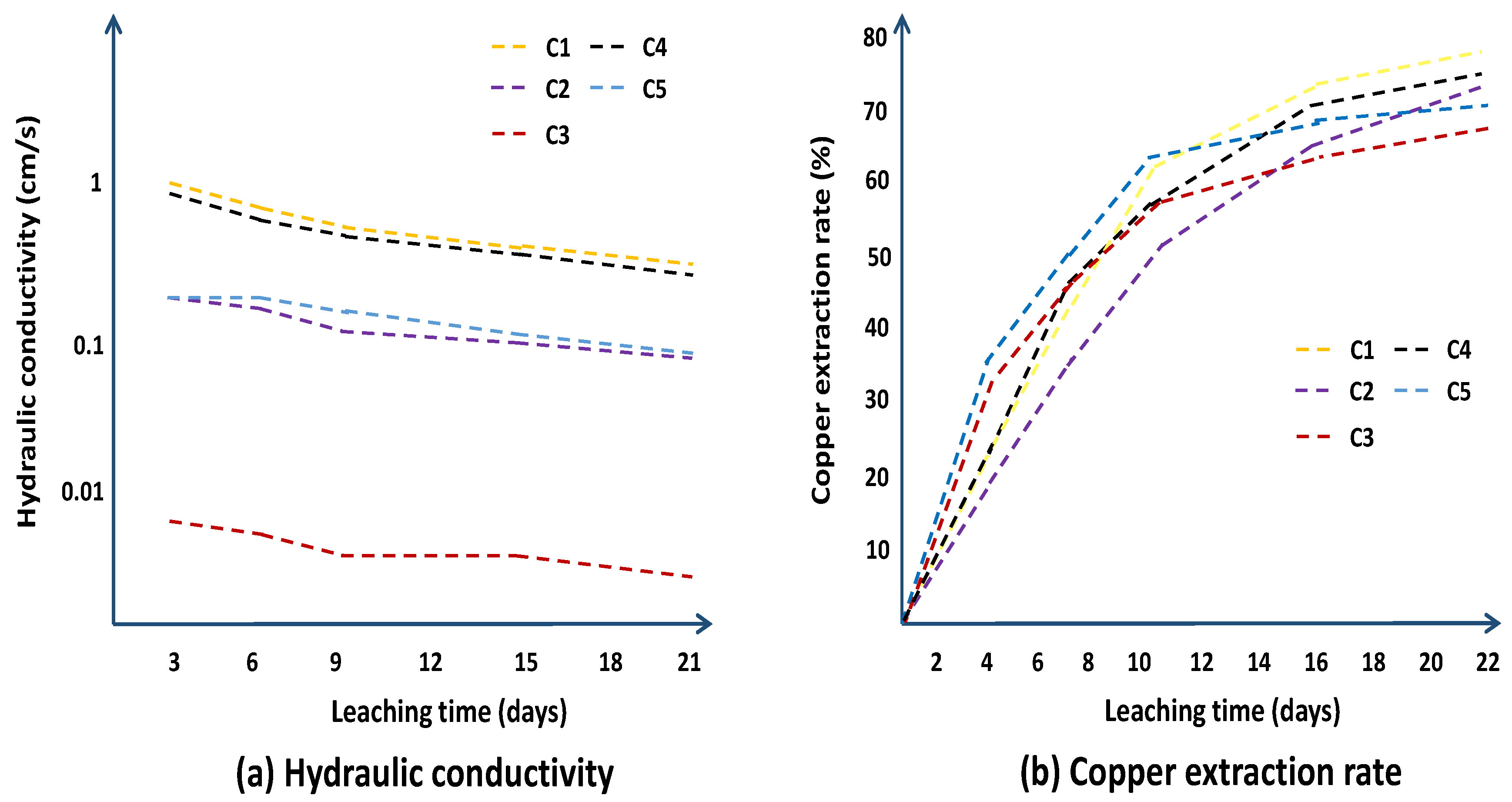
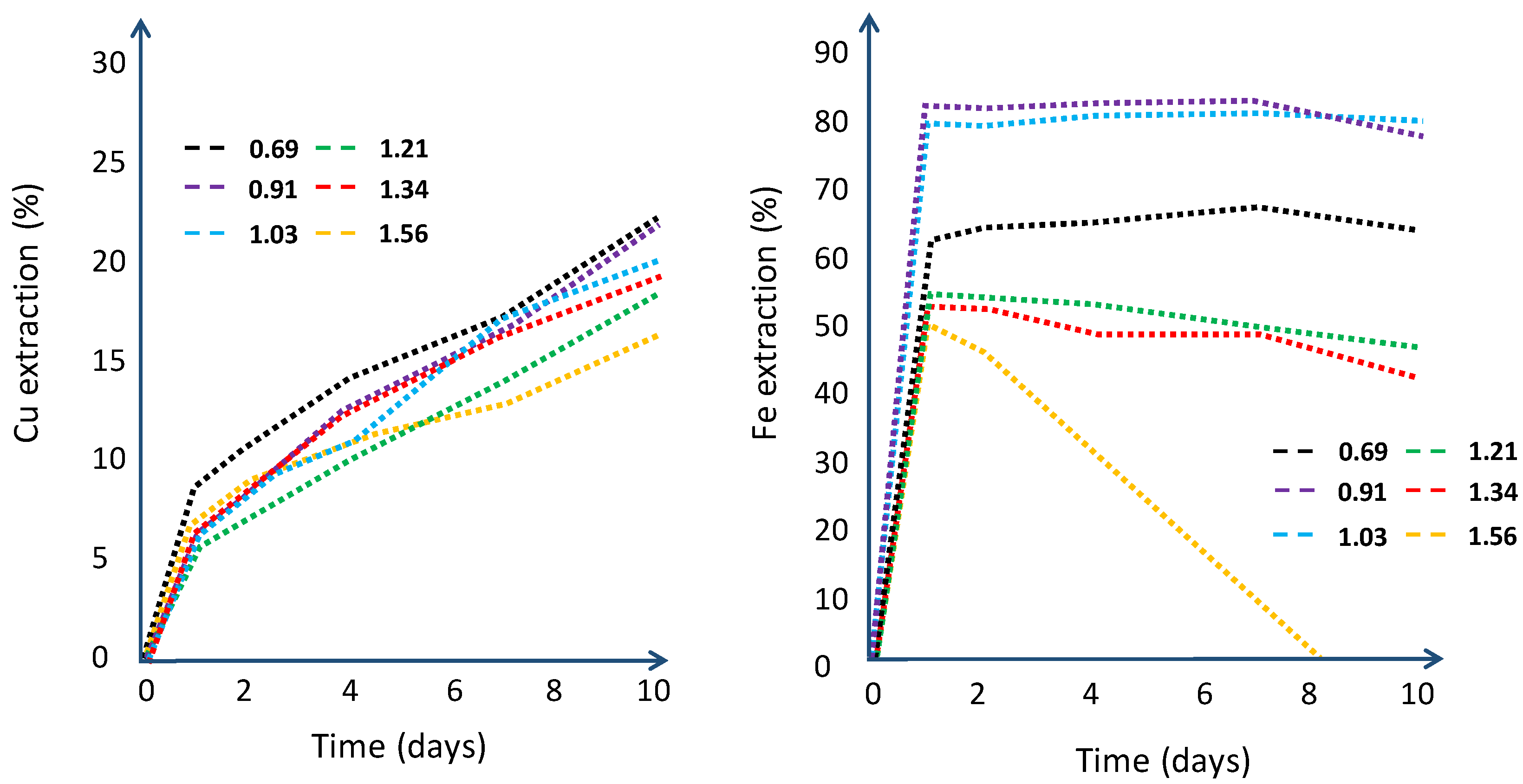
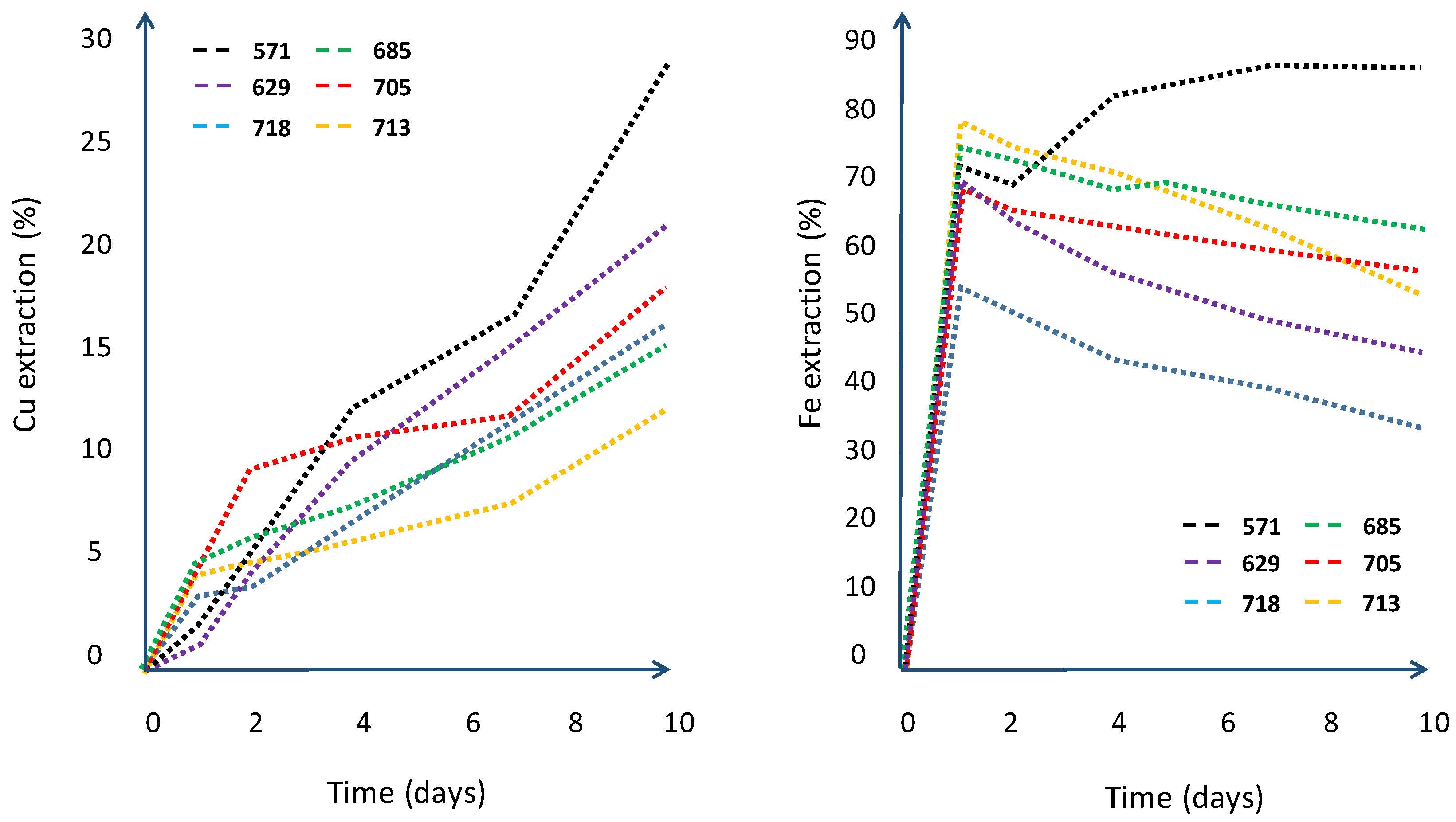
| Column | |||||
|---|---|---|---|---|---|
| Parameters | C1 | C2 | C3 | C4 | C5 |
| Concentration of sulfuric acid (g/L) | 25 | 30 | 35 | 40 | 45 |
| Irrigation rate (L/m2·h−1) | 40 | 60 | 20 | 60 | 20 |
| Particle size distribution (mm) | +5 | 1~5 | −1 | +5 | 1~5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toro, N.; Ghorbani, Y.; Turan, M.D.; Robles, P.; Gálvez, E. Gangues and Clays Minerals as Rate-Limiting Factors in Copper Heap Leaching: A Review. Metals 2021, 11, 1539. https://doi.org/10.3390/met11101539
Toro N, Ghorbani Y, Turan MD, Robles P, Gálvez E. Gangues and Clays Minerals as Rate-Limiting Factors in Copper Heap Leaching: A Review. Metals. 2021; 11(10):1539. https://doi.org/10.3390/met11101539
Chicago/Turabian StyleToro, Norman, Yousef Ghorbani, Mehmet Deniz Turan, Pedro Robles, and Edelmira Gálvez. 2021. "Gangues and Clays Minerals as Rate-Limiting Factors in Copper Heap Leaching: A Review" Metals 11, no. 10: 1539. https://doi.org/10.3390/met11101539
APA StyleToro, N., Ghorbani, Y., Turan, M. D., Robles, P., & Gálvez, E. (2021). Gangues and Clays Minerals as Rate-Limiting Factors in Copper Heap Leaching: A Review. Metals, 11(10), 1539. https://doi.org/10.3390/met11101539









