Chalcopyrite Leaching with Hydrogen Peroxide and Iodine Species in Acidic Chloride Media at Room Temperature: Technical and Economic Evaluation
Abstract
:1. Introduction
2. Mechanisms
3. Materials and Methods
3.1. Concentrate Characterization
3.2. Procedure of the Leaching Experiments
3.2.1. Effects of Hydrogen Peroxide on the Extraction of Copper during Concentrate Leaching
3.2.2. Effect of Iodide, Hydrogen Peroxide, and Chloride on the Extraction of Copper during Concentrate Leaching
4. Results
4.1. Effect of Hydrogen Peroxide Concentration on Copper Extraction during Concentrate Leaching
4.2. Effects of Iodide, Hydrogen Peroxide, and Chloride on the Extraction of Copper during Concentrate Leaching
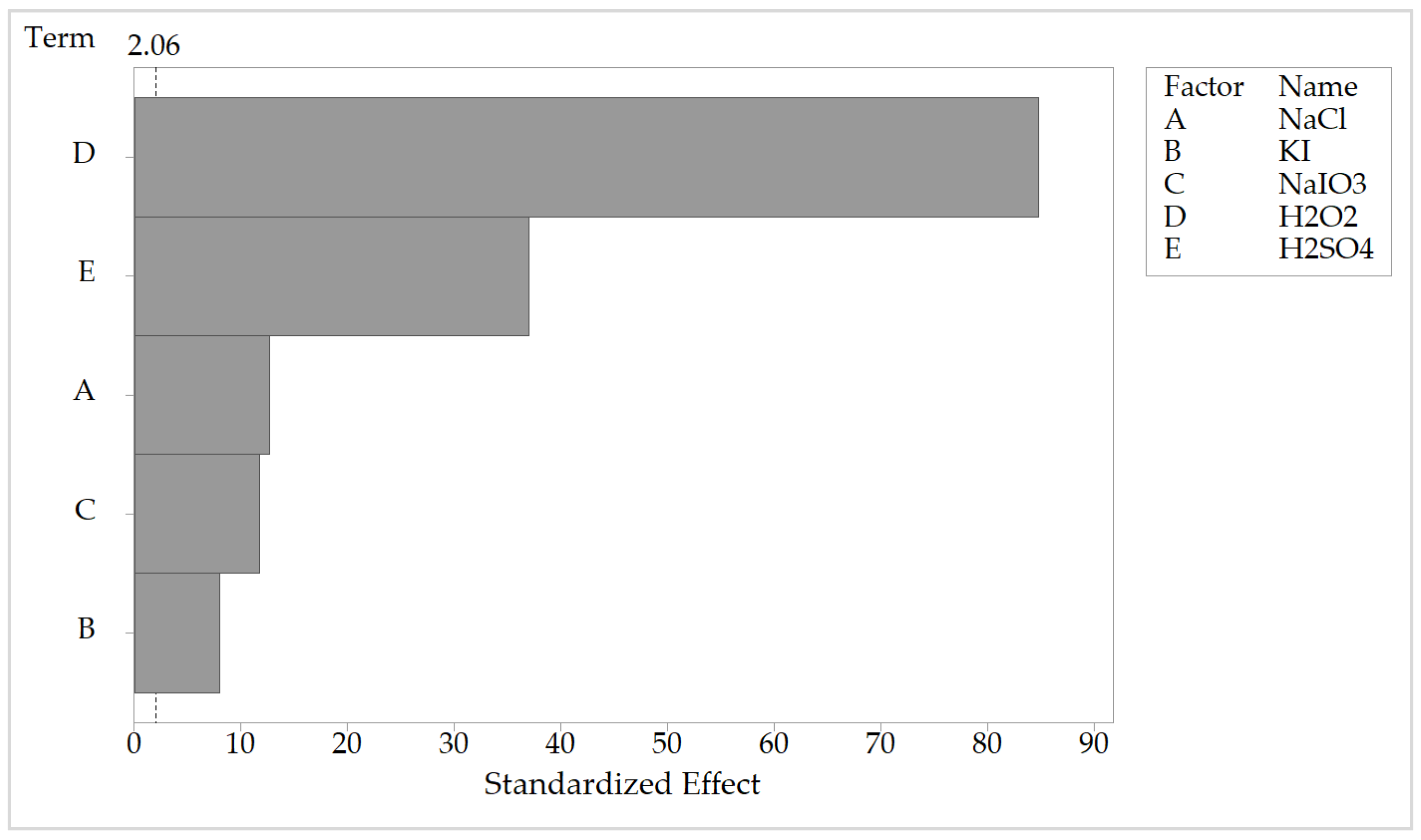
4.2.1. Analysis of the Pareto Chart of Effects
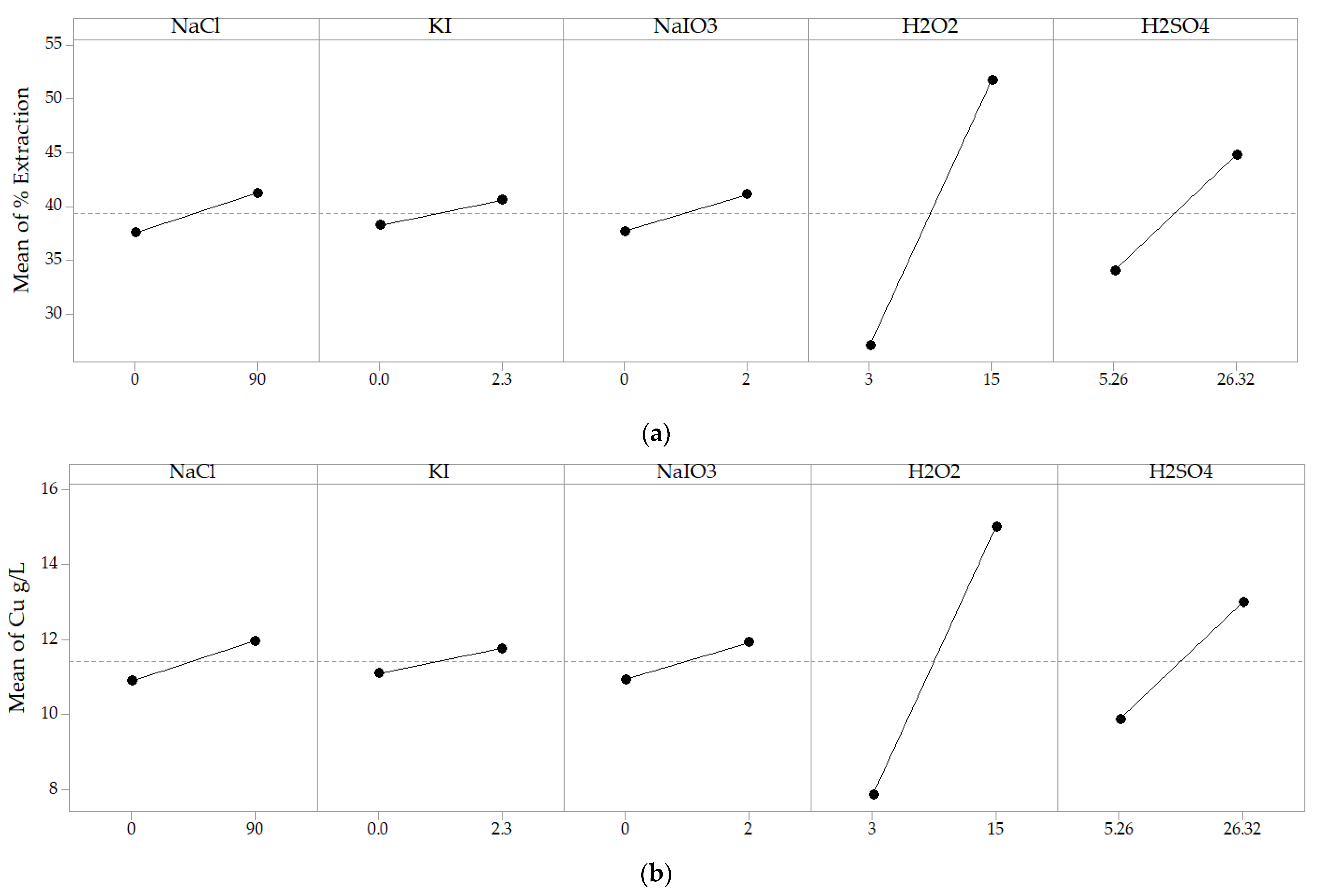
4.2.2. Analysis of the Main Effects
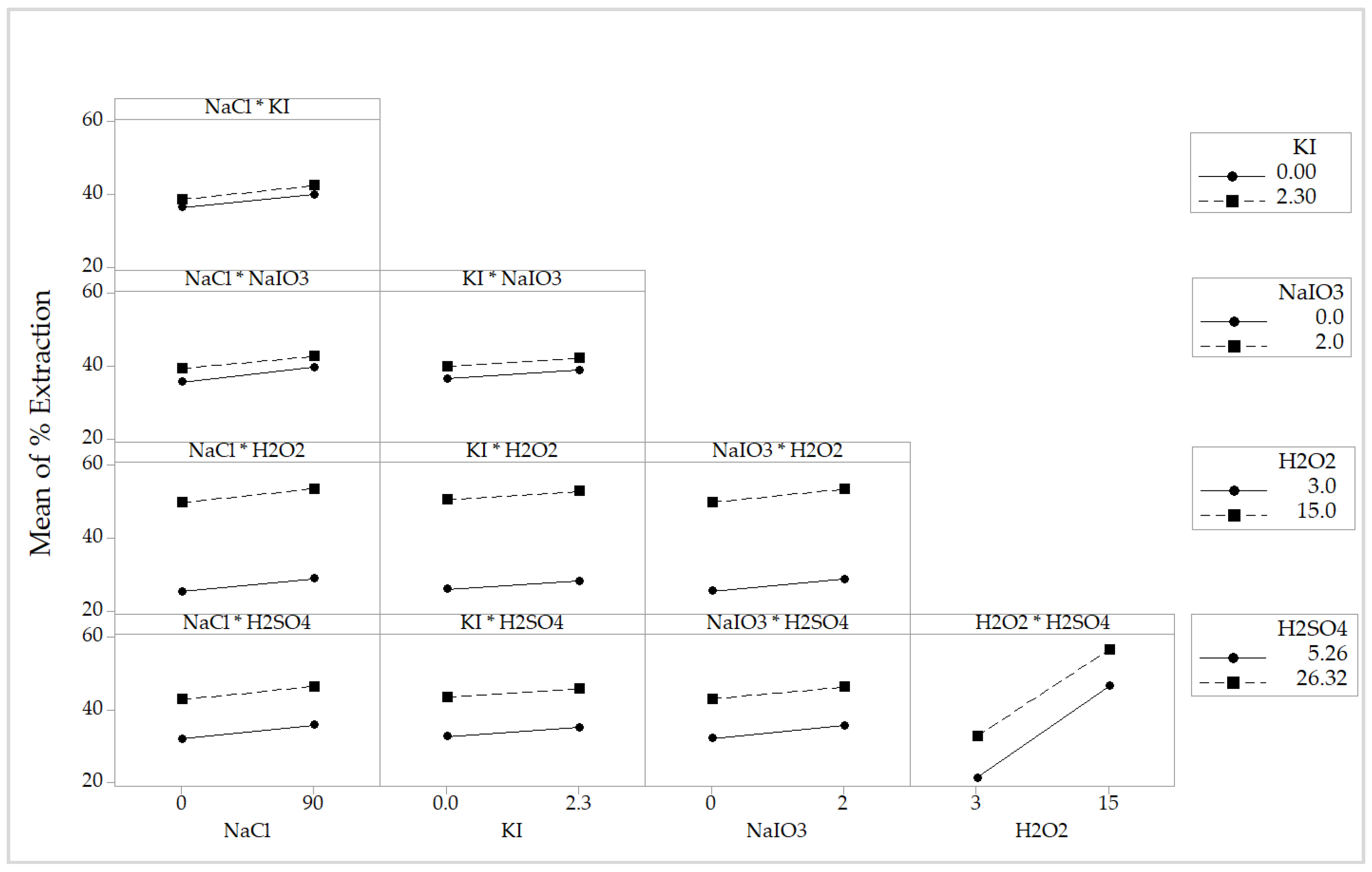
4.2.3. Analysis of the Interaction Plot
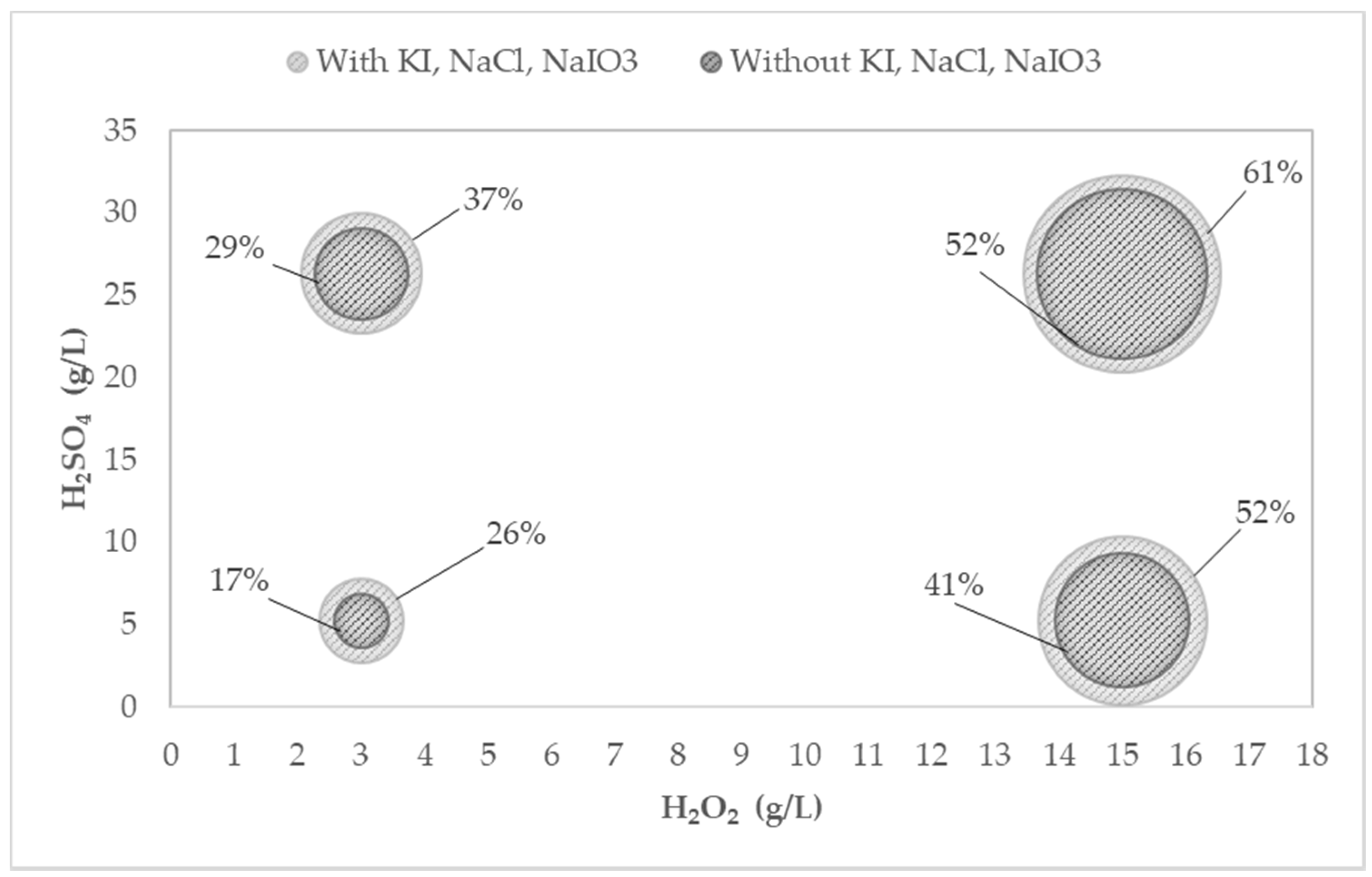
4.2.4. Analysis of the Bubble Chart
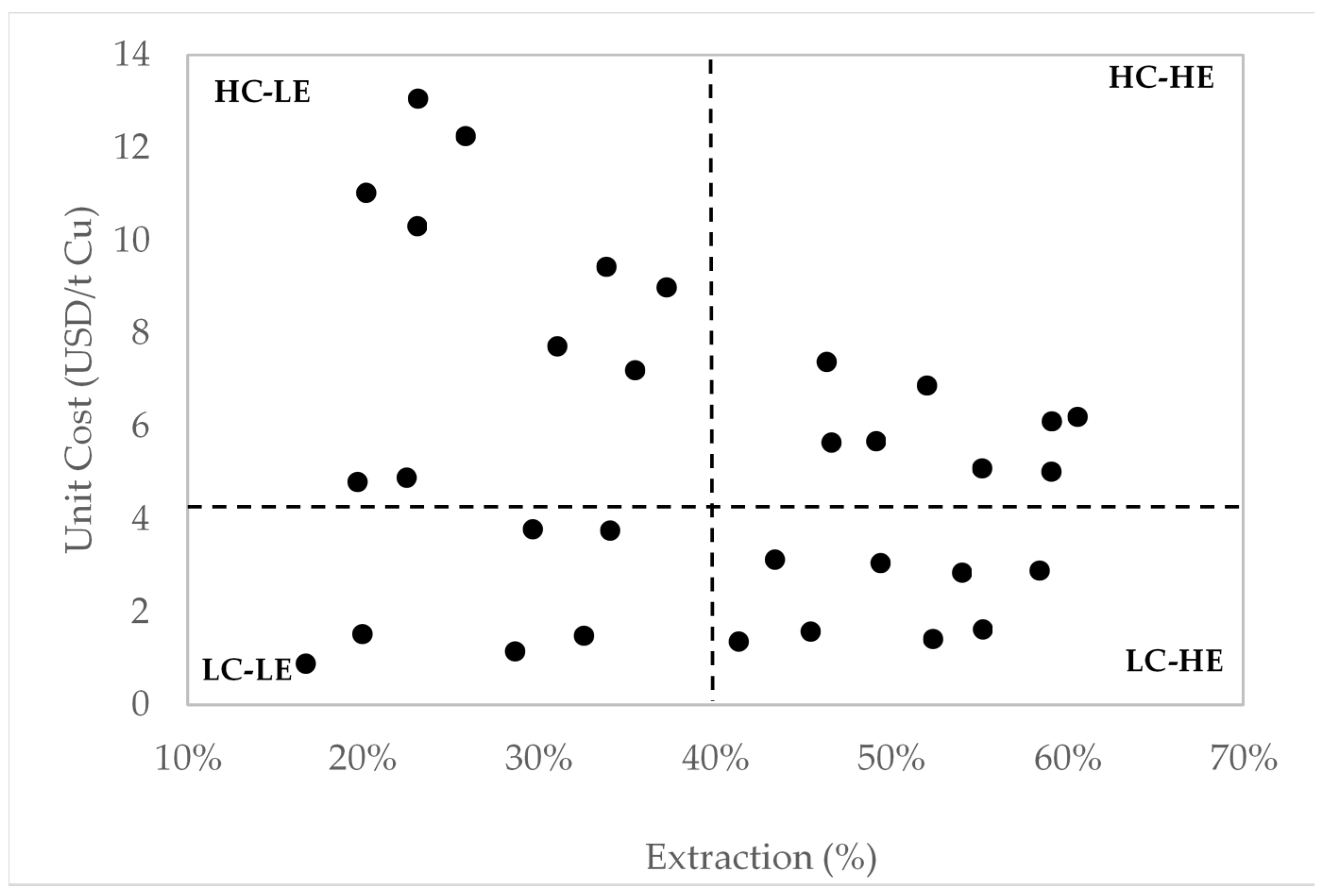
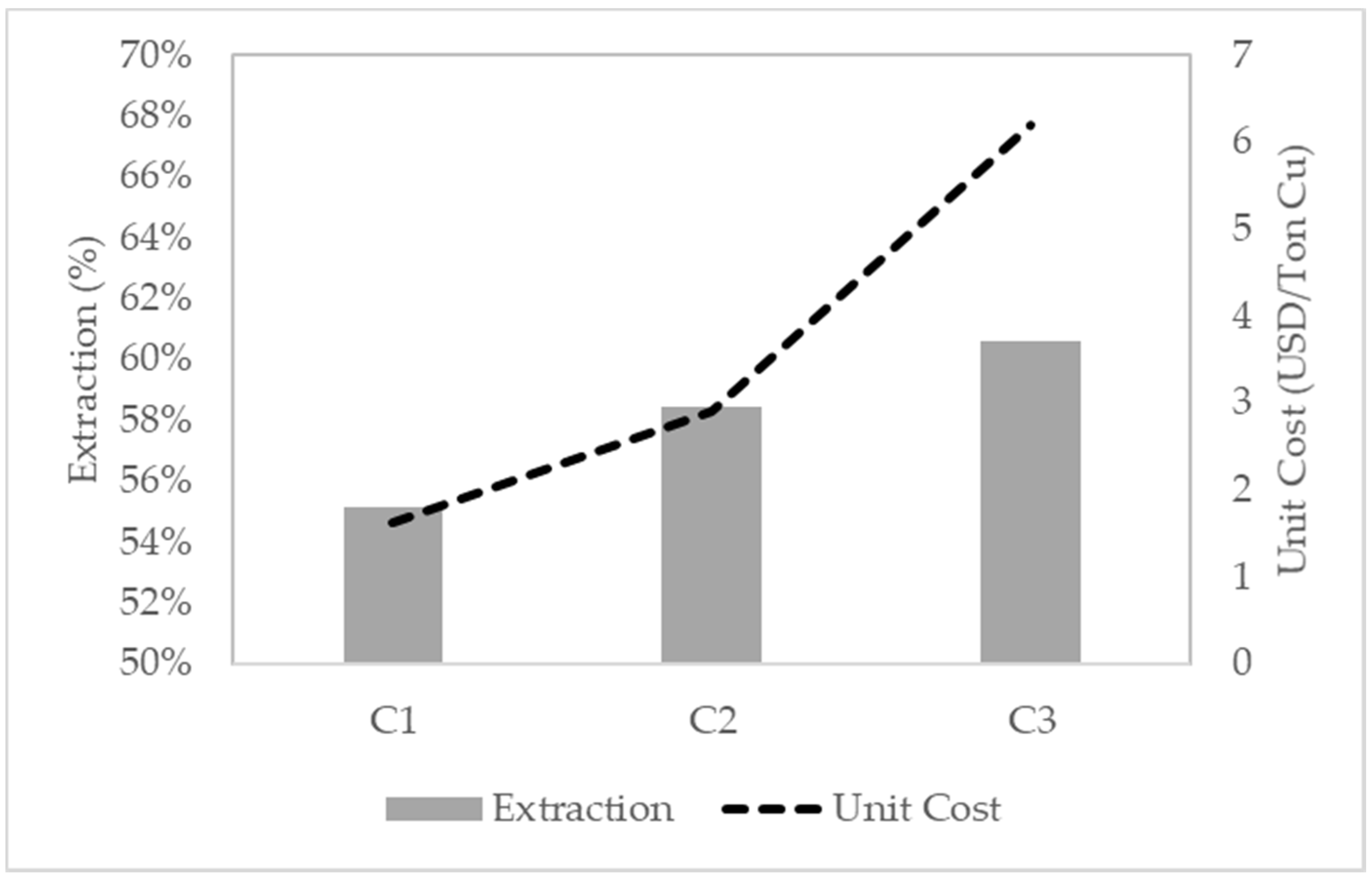
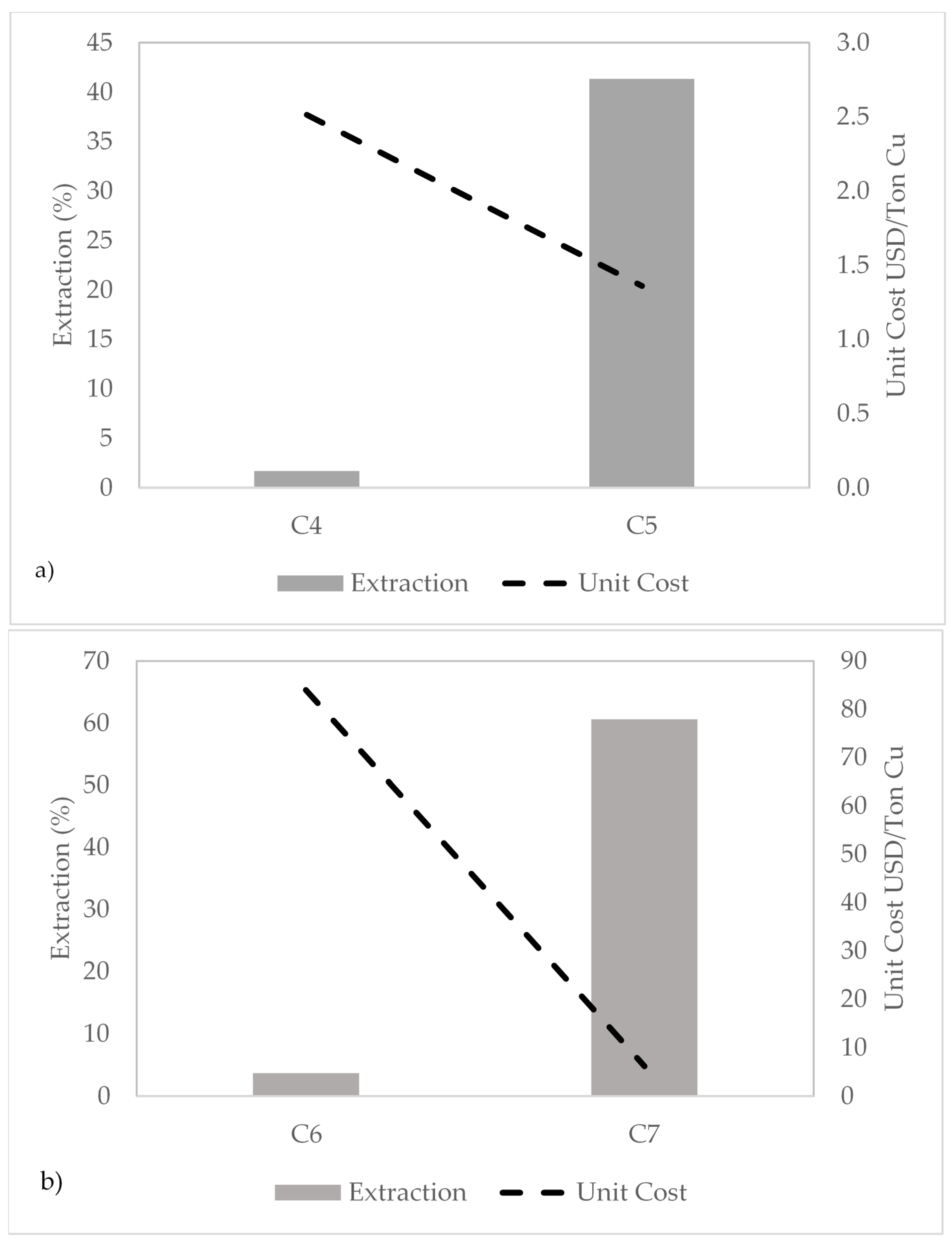
5. Economic Evaluation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Qian, G.; Li, J.; Gerson, A.R. Kinetics and roles of solution and surface species of chalcopyrite dissolution at 650 mV. Geochim. Cosmochim. Acta 2015, 161, 188–202. [Google Scholar] [CrossRef]
- Kartal, M.; Xia, F.; Ralph, D.; Rickard, W.D.A.; Renard, F.; Li, W. Enhancing chalcopyrite leaching by tetrachloroethylene-assisted removal of sulphur passivation and the mechanism of jarosite formation. Hydrometallurgy 2020, 191, 105192. [Google Scholar] [CrossRef]
- Watling, H.R. Chalcopyrite hydrometallurgy at atmospheric pressure: 2. Review of acidic chloride process options. Hydrometallurgy 2014, 146, 96–110. [Google Scholar] [CrossRef]
- Salinas, K.E.; Herreros, O.; Torres, C.M. Leaching of primary copper sulfide ore in chloride-ferrous media. Minerals 2018, 8, 312. [Google Scholar] [CrossRef] [Green Version]
- Cochilco. Proyección de la Producción de Cobre en Chile 2019–2030; Cochilco: Santiago, Chile, 2019. [Google Scholar]
- Cochilco. Sulfuros Primarios: Desafíos y Oportunidades; Cochilco: Santiago, Chile, 2017; DEPP 17/20; pp. 1–40. [Google Scholar]
- Watling, H.R. The bioleaching of sulphide minerals with emphasis on copper sulphides—A review. Hydrometallurgy 2006, 84, 81–108. [Google Scholar] [CrossRef]
- Dreisinger, D. Copper leaching from primary sulfides: Options for biological and chemical extraction of copper. Hydrometallurgy 2006, 83, 10–20. [Google Scholar] [CrossRef]
- Faris, N.; Ram, R.; Chen, M.; Tardio, J.; Pownceby, M.I.; Jones, L.A.; McMaster, S.; Webster, N.A.S.; Bhargava, S. The effect of thermal pre-treatment on the dissolution of chalcopyrite (CuFeS2) in sulfuric acid media. Hydrometallurgy 2017, 169, 68–78. [Google Scholar] [CrossRef]
- Ram, R.; Beiza, L.; Becker, M.; Pownceby, M.I.; Chen, M.; Yang, Y.; Yang, S.; Petersen, J. Study of the leaching and pore evolution in large particles of a sulfide ore. Hydrometallurgy 2020, 192, 1–40. [Google Scholar] [CrossRef]
- Schlesinger, M.E.; King, M.J.; Sole, K.C.; Davenport, W.G. Extractive Metallurgy of Copper; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 9780080967899. [Google Scholar]
- Hernández, P.C.; Dupont, J.; Herreros, O.O.; Jimenez, Y.P.; Torres, C.M. Accelerating copper leaching from sulfide ores in acid-nitrate-chloride media using agglomeration and curing as pretreatment. Minerals 2019, 9, 250. [Google Scholar] [CrossRef] [Green Version]
- Baba, A.; Ayinla, K.; Adekola, F.; Ghosh, M.; Ayanda, O.; Bale, R.; Sheik, A.; Pradhan, S. A Review on Novel Techniques for Chalcopyrite Ore Processing. Int. J. Min. Eng. Miner. Process. 2012, 1, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Watling, H.R. Chalcopyrite hydrometallurgy at atmospheric pressure: 1. Review of acidic sulfate, sulfate-chloride and sulfate-nitrate process options. Hydrometallurgy 2013, 140, 163–180. [Google Scholar] [CrossRef]
- Debernardi, G.; Carlesi, C. Chemical-electrochemical approaches to the study passivation of chalcopyrite. Miner. Process. Extr. Metall. Rev. 2013, 34, 10–41. [Google Scholar] [CrossRef]
- Dutrizac, J.E. Elemental sulphur formation during the ferric sulphate leaching of chalcopyrite. Can. Metall. Q. 1989, 28, 337–344. [Google Scholar] [CrossRef]
- Liebhafsky, H.A. The Catalytic Decomposition of Hydrogen Peroxide by the iodine iodide couple II and II. The rate of Oxidation in Neutral, and in Acid, solution of Hydrigen Peroxide by Iodine. J. Am. Chem. Soc. 1932, 54, 3499–3508. [Google Scholar] [CrossRef]
- Liebhafsky, H.A.; Mohammad, A. The Kinetics of the Reduction, in Acid Solution, of Hydrogen Peroxide by Iodide Ion. J. Am. Chem. Soc. 1933, 55, 3977–3986. [Google Scholar] [CrossRef]
- Kolar-Anić, L.; Schmitz, G. Mechanism of the Bray-Liebhafsky reaction: Effect of the oxidation of iodous acid by hydrogen peroxide. J. Chem. Soc. Faraday Trans. 1992, 88, 2343–2349. [Google Scholar] [CrossRef]
- Sharma, K.; Noyes, R. Oscillations in Chemical Systems. A Detailed Molecular Mechanism for the Bray-Liebhafsky Reaction of Iodate and Hydrogen Peroxide. J. Am. Chem. Soc. 1976, 98, 4345–4361. [Google Scholar] [CrossRef]
- Milenković, M.C.; Stanisavljev, D.R. The kinetics of iodide oxidation by hydrogen peroxide in acid solution. Russ. J. Phys. Chem. A 2011, 85, 2279–2282. [Google Scholar] [CrossRef]
- Bray, W.C.; Liebhafsky, H.A. Reactions involving hydrogen peroxide, iodine and iodate ion. I. Introduction. J. Am. Chem. Soc. 1931, 53, 38–44. [Google Scholar] [CrossRef]
- Schmitz, G. Kinetics and mechanism of the iodate-iodide reaction and other related reactions. Phys. Chem. Chem. Phys. 1999, 1, 1909–1914. [Google Scholar] [CrossRef]
- Liang, C.J.; Li, J.Y. Recovery of gold in iodine-iodide system—A review. Sep. Sci. Technol. 2019, 54, 1055–1066. [Google Scholar] [CrossRef]
- Konyratbekova, S.S.; Baikonurova, A.; Ussoltseva, G.A.; Erust, C.; Akcil, A. Thermodynamic and kinetic of iodine-iodide leaching in gold hydrometallurgy. Trans. Nonferrous Met. Soc. China 2015, 25, 3774–3783. [Google Scholar] [CrossRef]
- Baghalha, M. The leaching kinetics of an oxide gold ore with iodide/iodine solutions. Hydrometallurgy 2012, 113, 42–50. [Google Scholar] [CrossRef]
- Hernández, P.C.; Taboada, M.E.; Herreros, O.O.; Torres, C.M.; Ghorbani, Y. Chalcopyrite dissolution using seawater-based acidic media in the presence of oxidants. Hydrometallurgy 2015, 157, 325–332. [Google Scholar] [CrossRef]
- Granata, G.; Miura, A.; Liu, W.; Pagnanelli, F.; Tokoro, C. Iodide-assisted leaching of chalcopyrite in acidic ferric sulfate media. Hydrometallurgy 2019, 186, 244–251. [Google Scholar] [CrossRef]
- Winarko, R.; Dreisinger, D.B.; Miura, A.; Tokoro, C.; Liu, W. Kinetic modelling of chalcopyrite leaching assisted by iodine in ferric sulfate media. Hydrometallurgy 2020, 197, 105481. [Google Scholar] [CrossRef]
- Manabe, M. Method of Leaching Copper Sulfide Ore with the Use of Lodine. U.S. Patent 8,163,063B2, 24 April 2012. [Google Scholar]
- Sato, K.; Manabe, M. Method of Leaching Copper Ore. U.S. Patent 2013/0239752A1, 19 September 2013. [Google Scholar]
- Kawashiro, S.; Miura, A. Method of Leaching Copper from Sulfide Ore and Method of Evaluating Iodine Loss Content of Column Leaching Test of the Copper Sulfide Ore. W.O. Patent 2016/148305, 22 September 2016. [Google Scholar]
- Baral, A.; Sarangi, C.K.; Tripathy, B.C.; Bhattacharya, I.N.; Subbaiah, T. Copper electrodeposition from sulfate solutions—Effects of selenium. Hydrometallurgy 2014, 146, 8–14. [Google Scholar] [CrossRef]
- Barnard, K.R.; Kelly, N.J. Effect of long term exposure of aliphatic ELIXORE 205 diluent to acidic and oxidising conditions on copper extraction. Hydrometallurgy 2017, 169, 362–371. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Hughes, C.A.; Barnard, K.R.; Larcombe, K. Manganese in copper solvent extraction and electrowinning. Hydrometallurgy 2000, 58, 135–150. [Google Scholar] [CrossRef]
- Clancy, M.; Bettles, C.J.; Stuart, A.; Birbilis, N. The influence of alloying elements on the electrochemistry of lead anodes for electrowinning of metals: A review. Hydrometallurgy 2013, 131, 144–157. [Google Scholar] [CrossRef]
- Li, G.; Wang, C.; Zhong, S.; Xie, H.; Chen, H. Identification and regeneration of degradation products from phenolic hydroxyoxime-based extractant in long-term copper solvent extraction plant. Hydrometallurgy 2019, 183, 112–117. [Google Scholar] [CrossRef]
- Liu, X.; Qiu, G.; Hu, Y. Degradation of Lix984N and its effect on interfacial emulsion. J Cent. South Univ. Technol 2006, 13, 668–672. [Google Scholar] [CrossRef]
- Lu, J.; Dreisinger, D. Solvent extraction of copper from chloride solution I: Extraction isotherms. Hydrometallurgy 2013, 137, 13–17. [Google Scholar] [CrossRef]
- Lu, J.; Dreisinger, D. Solvent extraction of copper from chloride solution II: Cuprous oxidation by oxygen coupled with simultaneous cupric solvent extraction. Hydrometallurgy 2013, 138, 48–53. [Google Scholar] [CrossRef]
- Yáñez, H.; Ardiles, L.; del Río, C. High Chloride in PLS and their Impact on Copper Solvent Extraction. In Proceedings of the Hydroprocess, Santiago, Chile, 21–23 June 2017. [Google Scholar]
- Ruiz, M.C.; Gonzalez, I.; Salgado, J.; Padilla, R. Extraction of copper from sulfate-chloride solutions by using hydroxyoxime extractants. In Applications of Process Engineering Principles in Materials Processing, Energy and Environmental Technologies; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2017; pp. 161–168. [Google Scholar] [CrossRef]
- Ruiz, M.C.; González, I.; Rodriguez, V.; Padilla, R. Solvent Extraction of Copper from Sulfate–Chloride Solutions Using LIX 84-IC and LIX 860-IC. Miner. Process. Extr. Metall. Rev. 2021, 42, 1–8. [Google Scholar] [CrossRef]
- Ruiz, M.C.; Risso, J.; Seguel, J.; Padilla, R. Solvent extraction of copper from sulfate-chloride solutions using mixed and modified hydroxyoxime extractants. Miner. Eng. 2020, 146, 106109. [Google Scholar] [CrossRef]
- Shakibania, S.; Mahmoudi, A.; Mokmeli, M.; Rashchi, F. The effect of chloride ions on copper solvent extraction from sulfate-chloride medium using LIX 984N. Miner. Eng. 2020, 156, 106498. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, W.; Pranolo, Y.; Cheng, C.Y. Separation and recovery of copper, nickel, cobalt and zinc in chloride solutions by synergistic solvent extraction. Hydrometallurgy 2012, 127, 1–7. [Google Scholar] [CrossRef]
- Fletcher, A.W.; Sudderth, R.B.; Olafson, S.M. Combining sulfate electrowinning with chloride leaching. JOM 1991, 43, 57–59. [Google Scholar] [CrossRef]
- Kuwano, K.; Abe, A.; Manabe, M.; Miura, A. Method for Processing Acidic Solution that Contains Iodide Ions and Iron Ions. U.S. Patent 8,865,119B2, 21 October 2015. [Google Scholar]
- Kuwano, K.; Abe, A.; Manabe, M.; Miura, A. Method of Leaching Copper Sulfide Ore. U.S Patent 2011/0229385, 22 September 2011. [Google Scholar]
- Hernández, P.; Dorador, A.; Martínez, M.; Toro, N.; Castillo, J.; Ghorbani, Y. Use of seawater/brine and caliche’s salts as clean and environmentally friendly sources of chloride and nitrate ions for chalcopyrite concentrate leaching. Minerals 2020, 10, 477. [Google Scholar] [CrossRef]
- Minitab 18 Support Effects Plots for Analyze Factorial Design. Available online: https://support.minitab.com/en-us/minitab/18/help-and-how-to/modeling-statistics/doe/how-to/factorial/analyze-factorial-design/interpret-the-results/all-statistics-and-graphs/effects-plots/ (accessed on 15 July 2020).
- Shiers, D.W.; Collinson, D.M.; Kelly, N.J.; Watling, H.R. Copper extraction from chalcopyrite: Comparison of three non-sulfate oxidants, hypochlorous acid, sodium chlorate and potassium nitrate, with ferric sulfate. Miner. Eng. 2016, 85, 55–65. [Google Scholar] [CrossRef]
- Ntengwe, F. The Leaching of Dolomitic-Copper Ore Using Sulphuric Acid Under Controlled Conditions. Open Miner. Process. J. 2010, 3, 60–67. [Google Scholar] [CrossRef]
| Mineral | Amount (w/w%) |
|---|---|
| Digenite | 3.30 |
| Covellite | 0.80 |
| Chalcopyrite | 63.70 |
| Enargite | 1.20 |
| Tennantite | 0.02 |
| Bornite | 6.40 |
| Pyrite | 16.40 |
| Molybdenite | 0.30 |
| Hematite | 0.10 |
| Limonite | 0.04 |
| Gangue | 7.30 |
| Sphalerite | 0.20 |
| Pyrargyrite | 0.10 |
| Magnetite | 0.10 |
| Reagent | Leaching Solution | |||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | |||||
| (M) | (g/L) | (M) | (g/L) | (M) | (g/L) | (M) | (g/L) | |
| NaCl | 0.00 | 0.00 | 1.54 | 90.00 | 0.00 | 0.00 | 1.54 | 90.00 |
| NaIO3 | 0.00 | 0.00 | 0.03 | 6.20 | 0.00 | 0.00 | 0.03 | 6.20 |
| KI | 0.00 | 0.00 | 0.04 | 6.50 | 0.00 | 0.00 | 0.04 | 6.50 |
| H2O2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.09 | 3.00 | 0.44 | 15.00 |
| H2SO4 | 0.05 | 5.30 | 0.27 | 26.30 | 0.05 | 5.30 | 0.27 | 26.30 |
| Factor | Low Level | High Level | ||
|---|---|---|---|---|
| (M) | (g/L) | (M) | (g/L) | |
| NaCl | 0.00 | 0.00 | 1.54 | 90.00 |
| NaIO3 | 0.00 | 0.00 | 0.01 | 2.00 |
| KI | 0.00 | 0.00 | 0.01 | 2.30 |
| H2O2 | 0.09 | 3.00 | 0.44 | 15.00 |
| H2SO4 | 0.05 | 5.30 | 0.27 | 26.30 |
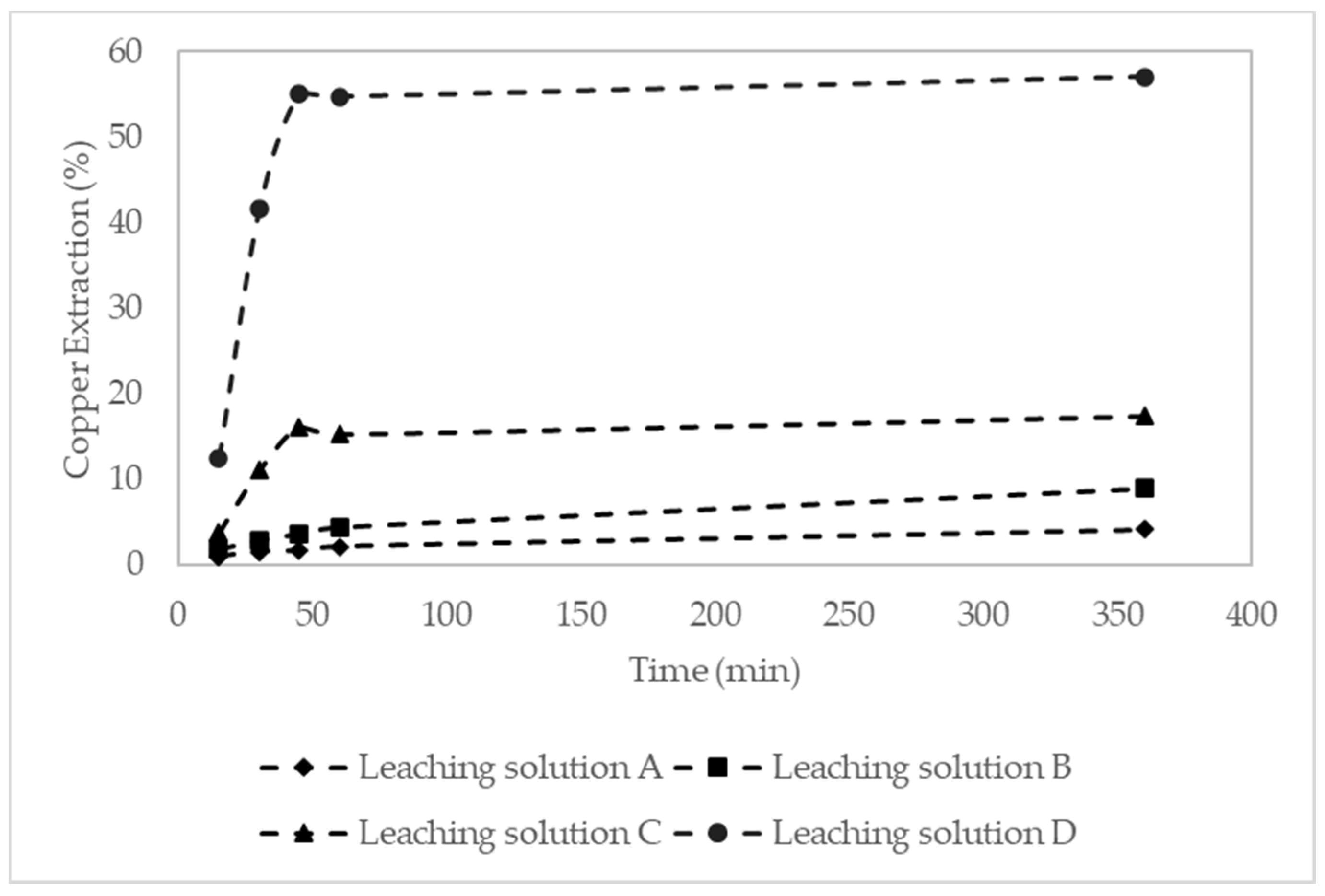
| Leaching Time (h) | Cu Extraction (%) | |||
|---|---|---|---|---|
| Leaching Solution A | Leaching Solution B | Leaching Solution C | Leaching Solution D | |
| 0.25 | 1 | 1.9 | 3.8 | 12.5 |
| 0.5 | 1.6 | 3 | 11.1 | 41.6 |
| 0.75 | 1.7 | 3.7 | 16.2 | 55.1 |
| 1 | 2.2 | 4.5 | 15.3 | 54.8 |
| 6 | 4.2 | 9 | 17.4 | 57.1 |
| 24 | 6.2 | 12 | 16 | 58.7 |
| 48 | 7.1 | 15.3 | 15.5 | 58.5 |
| 96 | 9.3 | 19.4 | 17.3 | 60.2 |
| Test | NaCl (g/L) | NaIO3 (g/L) | KI (g/L) | H2O2 (g/L) | H2SO4 (g/L) | Cu Extraction (%) | Cu (g/L) |
|---|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 3 | 5.3 | 16.6 | 4.8 |
| 2 | 90 | 0 | 0 | 15 | 26.3 | 55.1 | 16.0 |
| 3 | 90 | 0 | 2.3 | 3 | 5.3 | 22.4 | 6.5 |
| 4 | 90 | 2 | 0 | 3 | 5.3 | 23.0 | 6.7 |
| 5 | 90 | 2 | 0 | 15 | 26.3 | 59.1 | 17.1 |
| 6 | 90 | 2 | 2.3 | 3 | 26.3 | 37.2 | 10.8 |
| 7 | 0 | 2 | 2.3 | 15 | 5.3 | 46.3 | 13.4 |
| 8 | 90 | 0 | 2.3 | 3 | 26.3 | 34.0 | 9.9 |
| 9 | 0 | 0 | 2.3 | 3 | 26.3 | 29.6 | 8.6 |
| 10 | 90 | 2 | 0 | 15 | 5.3 | 49.0 | 14.2 |
| 11 | 90 | 0 | 0 | 3 | 5.3 | 19.8 | 5.8 |
| 12 | 0 | 2 | 0 | 15 | 26.3 | 55.2 | 16.0 |
| 13 | 90 | 0 | 2.3 | 15 | 5.3 | 49.4 | 14.3 |
| 14 | 0 | 2 | 2.3 | 3 | 26.3 | 33.8 | 9.8 |
| 15 | 0 | 2 | 2.3 | 15 | 26.3 | 59.1 | 17.1 |
| 16 | 0 | 2 | 2.3 | 3 | 5.3 | 23.1 | 6.7 |
| 17 | 0 | 0 | 2.3 | 3 | 5.3 | 19.6 | 5.7 |
| 18 | 0 | 2 | 0 | 15 | 5.3 | 46.6 | 13.5 |
| 19 | 90 | 0 | 0 | 15 | 5.3 | 45.4 | 13.2 |
| 20 | 0 | 2 | 0 | 3 | 5.3 | 20.1 | 5.8 |
| 21 | 0 | 2 | 0 | 3 | 26.3 | 31.0 | 9.0 |
| 22 | 0 | 0 | 0 | 3 | 26.3 | 28.6 | 8.3 |
| 23 | 0 | 0 | 0 | 15 | 26.3 | 52.4 | 15.2 |
| 24 | 0 | 0 | 0 | 15 | 5.3 | 41.3 | 12.0 |
| 25 | 90 | 2 | 2.3 | 15 | 5.3 | 52.0 | 15.1 |
| 26 | 90 | 2 | 2.3 | 15 | 26.3 | 60.6 | 17.6 |
| 27 | 90 | 0 | 2.3 | 15 | 26.3 | 58.4 | 16.9 |
| 28 | 0 | 0 | 2.3 | 15 | 26.3 | 54.0 | 15.6 |
| 29 | 90 | 2 | 0 | 3 | 26.3 | 35.4 | 10.3 |
| 30 | 90 | 2 | 2.3 | 3 | 5.3 | 25.8 | 7.5 |
| 31 | 90 | 0 | 0 | 3 | 26.3 | 32.5 | 9.4 |
| 32 | 0 | 0 | 2.3 | 15 | 5.3 | 43.3 | 12.6 |
| Reagent | Price (USD/t) |
|---|---|
| H2SO4 98% w/w | 250 |
| H2O2 50% w/w | 1000 |
| NaIO3 | 30,000 |
| KI | 10,000 |
| NaCl | 50 |
| Subsets | NaCl (g/L) | NaIO3 (g/L) | KI (g/L) | H2O2 (g/L) | H2SO4 (g/L) | Unit Cost (US$/t Cu) |
|---|---|---|---|---|---|---|
| HC-LE | 90 | 2 | 2.3 | 3 | 26.3 | 9.0 |
| 0 | 2 | 2.3 | 3 | 26.3 | 9.4 | |
| 0 | 2 | 2.3 | 3 | 5.3 | 13.1 | |
| 90 | 2 | 2.3 | 3 | 5.3 | 12.3 | |
| 0 | 2 | 0 | 3 | 5.3 | 11.0 | |
| 90 | 2 | 0 | 3 | 5.3 | 10.3 | |
| 0 | 2 | 0 | 3 | 26.3 | 7.7 | |
| 90 | 2 | 0 | 3 | 26.3 | 7.2 | |
| 0 | 0 | 2.3 | 3 | 5.3 | 4.8 | |
| 90 | 0 | 2.3 | 3 | 5.3 | 4.9 | |
| HC-HE | 0 | 2 | 2.3 | 15 | 26.3 | 6.1 |
| 90 | 2 | 2.3 | 15 | 26.3 | 6.2 | |
| 90 | 2 | 2.3 | 15 | 5.3 | 6.9 | |
| 0 | 2 | 2.3 | 15 | 5.3 | 7.4 | |
| 90 | 2 | 0 | 15 | 26.3 | 5.0 | |
| 0 | 2 | 0 | 15 | 26.3 | 5.1 | |
| 90 | 2 | 0 | 15 | 5.3 | 5.7 | |
| 0 | 2 | 0 | 15 | 5.3 | 5.7 | |
| LC-LE | 90 | 0 | 2.3 | 3 | 26.3 | 3.8 |
| 0 | 0 | 2.3 | 3 | 26.3 | 3.8 | |
| 90 | 0 | 0 | 3 | 26.3 | 1.5 | |
| 0 | 0 | 0 | 3 | 26.3 | 1.2 | |
| 0 | 0 | 0 | 3 | 5.3 | 0.9 | |
| 90 | 0 | 0 | 3 | 5.3 | 1.5 | |
| LC-HE | 0 | 0 | 2.3 | 15 | 5.3 | 3.1 |
| 0 | 0 | 2.3 | 15 | 26.3 | 2.8 | |
| 90 | 0 | 2.3 | 15 | 5.3 | 3.1 | |
| 90 | 0 | 2.3 | 15 | 26.3 | 2.9 | |
| 0 | 0 | 0 | 15 | 5.3 | 1.4 | |
| 0 | 0 | 0 | 15 | 26.3 | 1.4 | |
| 90 | 0 | 0 | 15 | 5.3 | 1.6 | |
| 90 | 0 | 0 | 15 | 26.3 | 1.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moraga, G.A.; Jamett, N.E.; Hernández, P.C.; Graber, T.A.; Taboada, M.E. Chalcopyrite Leaching with Hydrogen Peroxide and Iodine Species in Acidic Chloride Media at Room Temperature: Technical and Economic Evaluation. Metals 2021, 11, 1567. https://doi.org/10.3390/met11101567
Moraga GA, Jamett NE, Hernández PC, Graber TA, Taboada ME. Chalcopyrite Leaching with Hydrogen Peroxide and Iodine Species in Acidic Chloride Media at Room Temperature: Technical and Economic Evaluation. Metals. 2021; 11(10):1567. https://doi.org/10.3390/met11101567
Chicago/Turabian StyleMoraga, Germán A., Nathalie E. Jamett, Pía C. Hernández, Teófilo A. Graber, and María E. Taboada. 2021. "Chalcopyrite Leaching with Hydrogen Peroxide and Iodine Species in Acidic Chloride Media at Room Temperature: Technical and Economic Evaluation" Metals 11, no. 10: 1567. https://doi.org/10.3390/met11101567
APA StyleMoraga, G. A., Jamett, N. E., Hernández, P. C., Graber, T. A., & Taboada, M. E. (2021). Chalcopyrite Leaching with Hydrogen Peroxide and Iodine Species in Acidic Chloride Media at Room Temperature: Technical and Economic Evaluation. Metals, 11(10), 1567. https://doi.org/10.3390/met11101567








