Selective Recovery of Copper from Industrial Sludge by Integrated Sulfuric Leaching and Electrodeposition
Abstract
1. Introduction
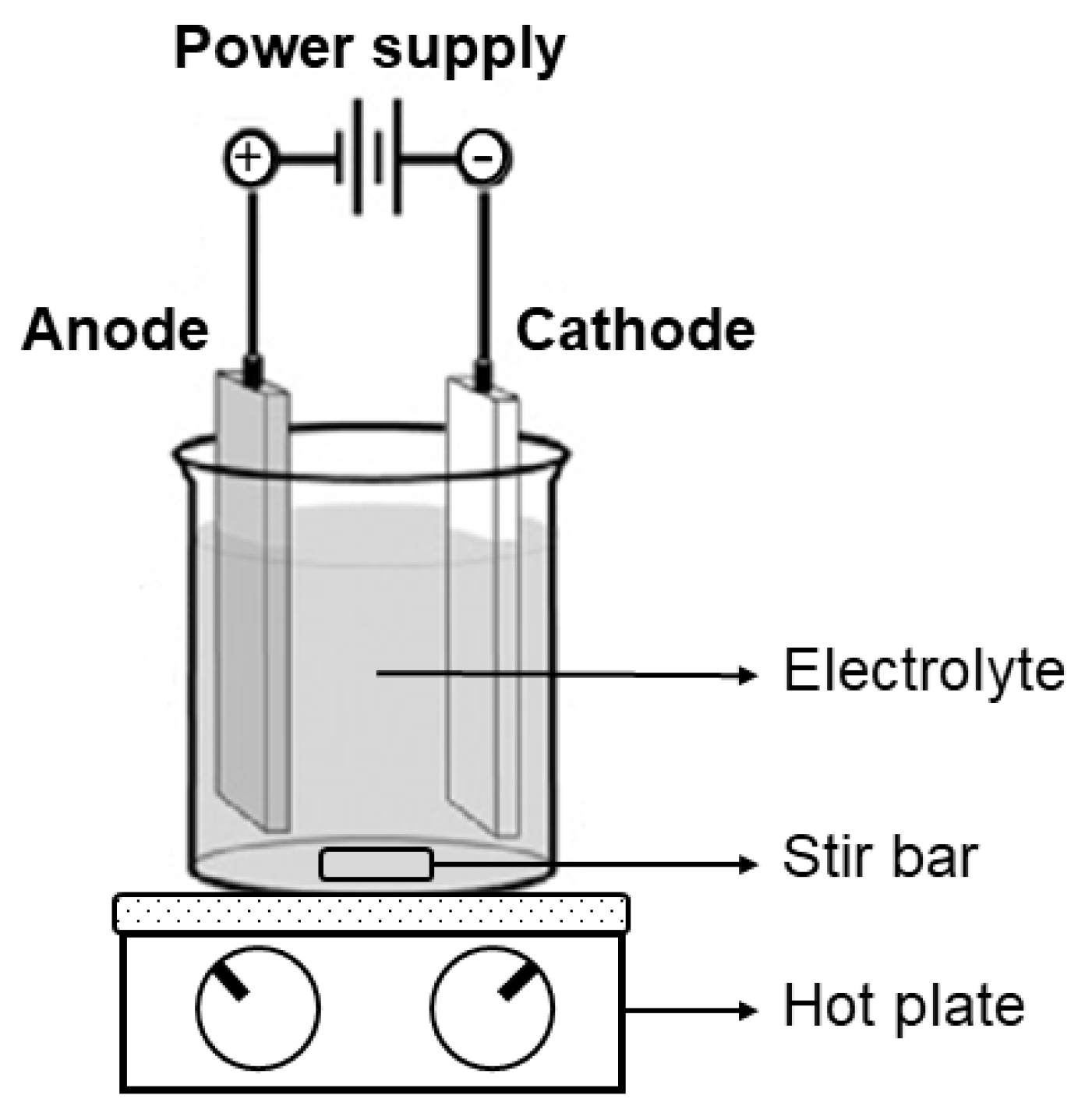
2. Materials and Methods
3. Results
3.1. Integrated Acid Leaching and Electrolytic Deposition of Cu from the Cu-Bearing Sludge
- (i)
- Leaching of Cu-bearing sludge in the H2SO4 solution:Cu(OH)2 + H2SO4 → CuSO4 + 2H2OFe(OH)2 + H2SO4 → FeSO4 + 2H2O2Fe(OH)3 + 3H2SO4 → Fe2(SO4)3 + 6H2OCu + Fe2(SO4)3 → CuSO4 + 2FeSO4
- (ii)
- Electrodeposition of the leaching solution:The ions in the leaching solution: Cu2+, Fe2+, Fe3+, H+, SO42−.The reactions on the cathode:Fe3+ + e− → Fe2+, Eo = 0.77 VCu2+ + 2e− → Cu, Eo = 0.34 V2H+ + 2e− → H2, Eo = 0.00 VFe2+ + 2e− → Fe, Eo = −0.44 VThe reaction on the anode:2H2O → O2 + 4H+ + 4e−, Eo = −1.23 V
3.2. Optimization of Cu Electrodeposition
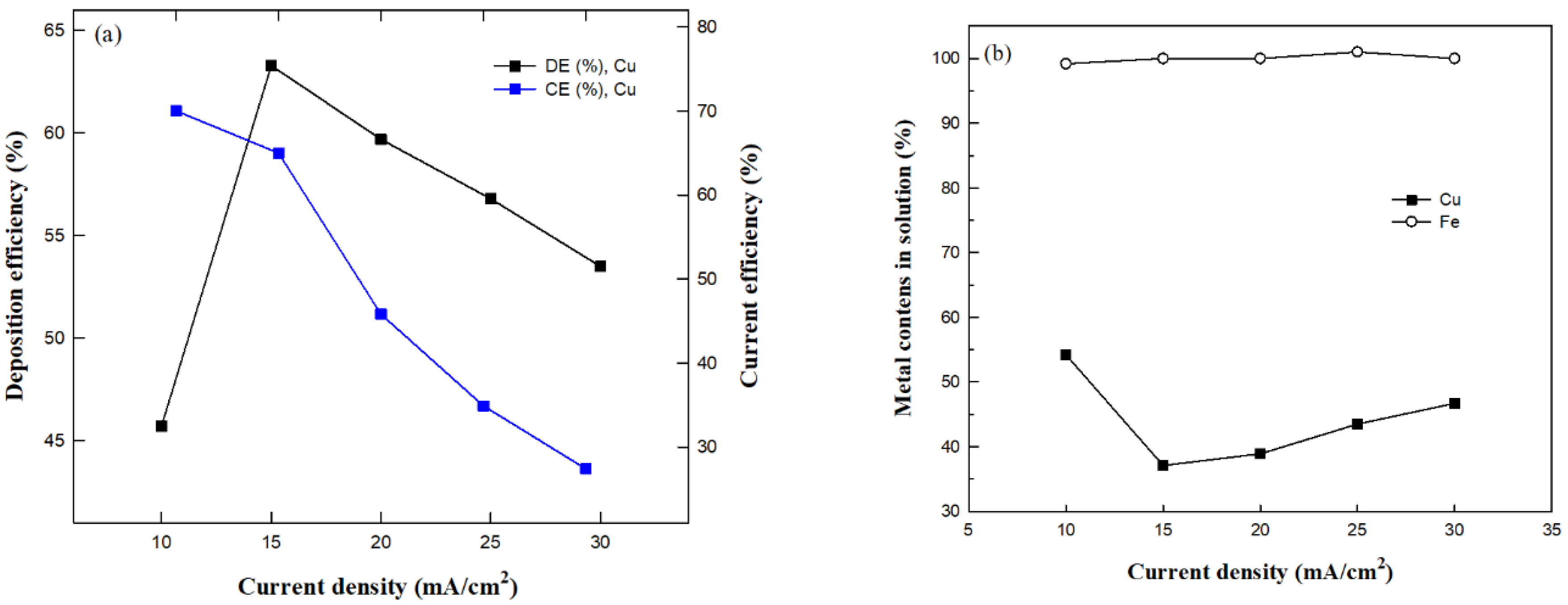
3.2.1. Effect of Current Density
3.2.2. Effect of Temperature
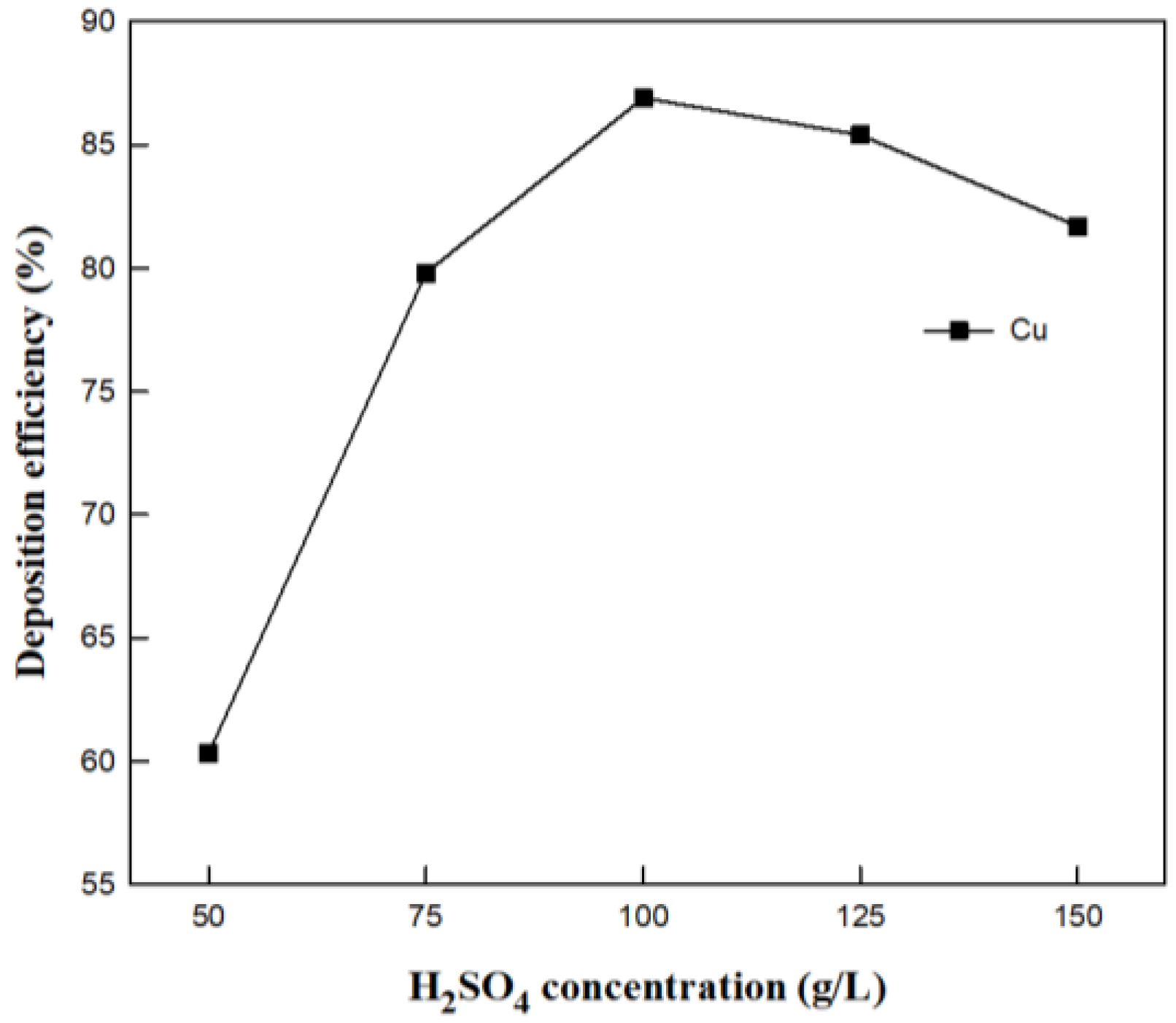
3.2.3. Effect of H2SO4 Concentration
3.2.4. Effect of Total Cu Concentration in the Electrolyte
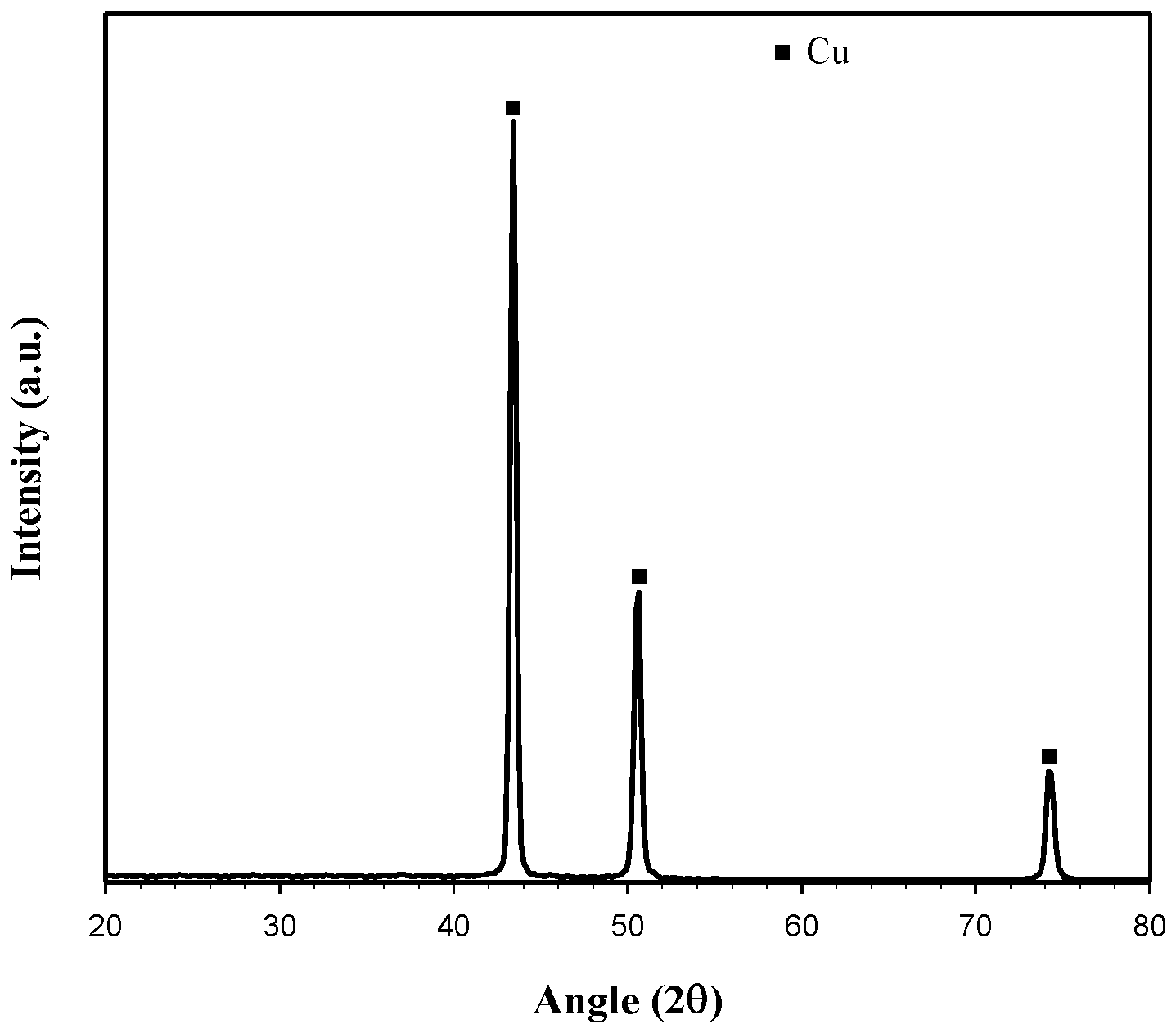
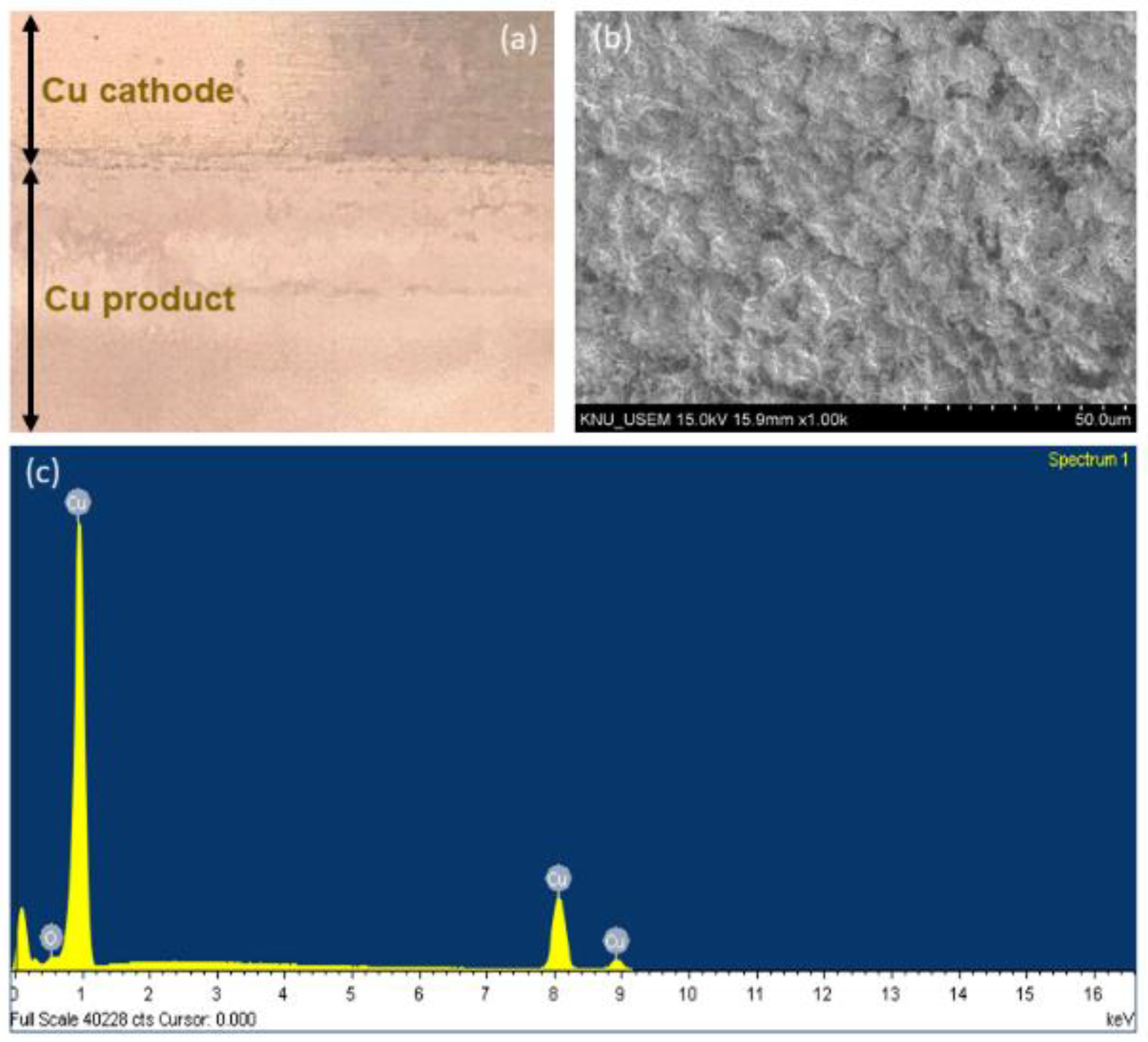
3.3. Characterization of Cu Deposited on the Cathode
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Printed Circuit Board Recycling Methods, Handout 10, Workshop Materials on WEEE Management in Taiwan; United States Environmental Protection Agency: Washington, DC, USA. Available online: chromeextension://oemmndcbldboiebfnladdacbdfmadadm/https://www.epa.gov/sites/production/files/2014-05/documents/handout-10-circuitboards.pdf (accessed on 1 December 2020).
- Liao, M.-I.; Shih, X.-H.; Ma, H. Secondary copper resource recycling and reuse: A waste input–output model. J. Clean. Prod. 2019, 239. [Google Scholar] [CrossRef]
- Gherghel, A.; Teodosiu, C.; De Gisi, S. A review on wastewater sludge valorisation and its challenges in the context of circular economy. J. Clean. Prod. 2019, 228, 244–263. [Google Scholar] [CrossRef]
- Xie, F.; Cai, T.; Yang, M.; Li, H.; Li, C.; Huang, Z.; Yuan, G. Recovery of Cu and Fe from Printed Circuit Board waste sludge by ultrasound: Evaluation of industrial application. J. Clean. Prod. 2009, 17, 1494–1498. [Google Scholar] [CrossRef]
- Kim, S.; Kim, Y.; Kim, S.; Lee, J. Analyses of Physical Properties of Copper-contained Sludge Pelletized for Applied Pyro-metallurgical Process. J. Korean Inst. Resour. Recycl. 2019, 28, 31–39. [Google Scholar]
- Wu, C.H.; Kuo, C.Y.; Lo, S.L. Removal of Metals from Industrial Sludge by Extraction with Different Acids. J. Environ. Sci Health A Tox Hazard. Subst Environ. Eng. 2004, 39, 2205–2219. [Google Scholar] [CrossRef]
- Kuo, C.Y.; Wu, C.H.; Lo, S.L. Leaching efficiency of copper from industrial sludge with traditional and microwave acid extraction. J. Hazard. Mater. B 2005, 120, 249–256. [Google Scholar] [CrossRef]
- Wu, C.H.; Kuo, C.Y.; Lo, S.L. Recovery of heavy metals from industrial sludge using various acid extraction approaches. Water Sci Technol. 2009, 59, 289–293. [Google Scholar] [CrossRef]
- Huang, Z.; Xie, F.; Ma, Y. Ultrasonic recovery of copper and iron through simultaneous utilization of Printed Circuit Boards (PCB) spent acid etching solution and PCB waste sludge. J. Hazard. Mater. 2011, 185, 155–156. [Google Scholar] [CrossRef]
- Thawornchaisit, U.; Juthaisong, K.; Parsongjeen, K.; Phoengchan, P. Optimizing acid leaching of copper from the wastewater treatment sludge of a printed circuit board industry using factorial experimental design. J. Mater. Cycles Waste Manag. 2019, 21, 1291–1299. [Google Scholar] [CrossRef]
- Gunarathne, V.; Rajapaksha, A.U.; Vithanage, M.; Adassooriya, N.; Cooray, A.; Liyanage, S.; Athapattu, B.; Rajakaruna, N.; Igalavithana, A.D.; Hou, D.; et al. Heavy metal dissolution mechanisms from electrical industrial sludge. Sci. Total Environ. 2019, 696. [Google Scholar] [CrossRef]
- Hu, S.H.; Tsai, M.S.; Yen, F.S.; Onlin, T. Recovery of copper-contaminated sludge in a two-stage leaching process. Environ. Prog. 2006, 25, 72–78. [Google Scholar] [CrossRef]
- Hu, S.H.; Hu, S.C.; Fu, Y.P. Resource Recovery of Copper Contaminated Sludge with Jarosite Process and Selective Precipitation. Environ. Prog. Sustain. Energy 2012, 31, 379–385. [Google Scholar] [CrossRef]
- Tu, Y.-J.; Chang, C.-K.; You, C.-F.; Lou, J.-C. Recycling of Cu powder from industrial sludge by combined acid leaching, chemical exchange and ferrite process. J. Hazard. Mater. 2010, 181, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, M.E.; King, M.J.; Sole, K.C.; Davenport, W.G. Extractive Metallurgy of Copper, 5th ed.; Elsevier: Oxford, UK, 2011. [Google Scholar]
- Kim, E.-Y.; Kim, M.-S.; Lee, J.-C.; Jeong, J.; Pandey, B.D. Leaching kinetics of copper from waste printed circuit boards by electro-generated chlorine in HCl solution. Hydrometallurgy 2011, 107, 124–132. [Google Scholar] [CrossRef]
- Beauchesne, I.; Drogui, P.; Seyhi, B.; Mercier, G.; Blais, J.-F. Simultaneous Electrochemical Leaching and Electrodeposition of Heavy Metals in a Single-Cell Process for Wastewater Sludge Treatment. J. Environ. Eng. 2014, 140. [Google Scholar] [CrossRef]
- Lee, H.; Bae, M.; Lee, E.; Mishra, B. Copper Extraction from Flue Dust of Electronic Waste by Electrowinning and Ion Exchange Process. JOM 2019, 71, 2360–2367. [Google Scholar] [CrossRef]
- Lister, T.E.; Wang, P.; Anderko, A. Electrorecycling of Critical and Value Metals from Mobile Electronics. In Proceedings of the Conference of Metallurgists (COM 2014), Vancouver, BC, Canada, 28 September–1 October 2014. [Google Scholar]
- Liu, X.; Tan, Q.; Li, Y.; Xu, Z.; Chen, M. Copper recovery from waste printed circuit boards concentrated metal scraps by electrolysis. Front. Environ. Sci. Eng. 2017, 11, 10. [Google Scholar] [CrossRef]
- Agrawal, A.; Kumari, S.; Bagchi, D.; Kumar, V.; Pandey, B.D. Recovery of copper powder from copper bleed electrolyte of an Indian copper smelter by electrolysis. Miner. Eng. 2007, 20, 95–97. [Google Scholar] [CrossRef]
- Li, P.P.; Peng, C.S.; Li, F.M.; Song, S.X.; Juan, A.O. Copper and Nickel Recovery from Electroplating Sludge by the Process of Acid-leaching and Electro-depositing. Int. J. Environ. Res. 2011, 5, 797–804. [Google Scholar]
- Chen, T.-C.; Priambodo, R.; Huang, R.-L.; Huang, Y.-H. The Effective Electrolytic Recovery of Dilute Copper from Industrial Wastewater. J. Waste Manag. 2013, 2013. [Google Scholar] [CrossRef]
- Sun, Z.; Cao, H.; Venkatesan, P.; Jin, W.; Xiao, Y.; Sietsma, J.; Yang, Y. Electrochemistry during efficient copper recovery from complex electronic waste using ammonia based solutions. Front. Chem. Sci. Eng. 2017, 11, 308–316. [Google Scholar] [CrossRef]
- Guimarães, Y.F.; Santos, I.D.; Dutra, A.J. Direct recovery of copper from printed circuit boards (PCBs) powder concentrate by a simultaneous electroleaching—Electrodeposition process. Hydrometallurgy 2014, 149, 63–70. [Google Scholar] [CrossRef]
- Wei, X.; Liu, D.; Huang, W.; Huang, W.; Lei, Z. Simultaneously enhanced Cu bioleaching from E-wastes and recovered Cu ions by direct current electric field in a bioelectrical reactor. Bioresour. Technol. 2019, 298. [Google Scholar] [CrossRef] [PubMed]
- Trinh, H.B.; Kim, S.; Lee, J. Selective Copper Recovery by Acid Leaching from Printed Circuit Board Waste Sludge. Metals 2020, 10, 293. [Google Scholar] [CrossRef]
- Bard, A.J.; Parsons, R.; Jordan, J. Standard Potential in Aqueous Solutions; Marcel Dekker: New York, NY, USA, 1985. [Google Scholar]
- Zhang, S.; Li, Y.; Wang, R.; Xu, Z.; Wang, B.; Chen, S.; Chen, M. Superfine copper powders recycled from concentrated metal scraps of waste printed circuit boards by slurry electrolysis. J. Clean. Prod. 2017, 152, 1–6. [Google Scholar] [CrossRef]
- Haccuria, E.; Ninga, P.; Cao, H.; Venkatesan, P.; Jina, W.; Yang, Y.; Sun, Z. Effective treatment for electronic waste—Selective recovery of copper by combining electrochemical dissolution and deposition. J. Clean. Prod. 2017, 152, 150–156. [Google Scholar] [CrossRef]
- Fuentes-Aceituno, J.C.; Lapidus, G.T. A kinetic-mechanistic study of the hydrogen evolution reaction in sulfuric acid solutions with different electrode materials. J. N. Mater. Electrochem. Syst. 2012, 15, 225–231. [Google Scholar] [CrossRef]
- Panda, B.; Das, S.C. Electrowinning of copper from sulfate electrolyte in presence of sulfurous acid. Hydrometallurgy 2001, 59, 55–67. [Google Scholar] [CrossRef]
- Oishi, T.; Koyama, K.; Konishi, H.; Tanaka, M.; Lee, J.-C. Influence of ammonium salt on electrowinning of copper from ammoniacal alkaline solutions. Electrochim. Acta 2007, 53, 127–132. [Google Scholar] [CrossRef]





| Metals | UOM | Composition |
|---|---|---|
| Cu | % | 18.34 |
| Fe | 29.35 | |
| Ca | 6.24 | |
| Pb | 2.06 | |
| Sn | 1.72 | |
| Mg | ppm | 7134 |
| Al | 2277 | |
| Zn | 1165 | |
| Na | 1053 | |
| Si | 0 |
| Cu Resources | Processes | Processing Conditions | Deposit Character | CE (%) | EC (kWh/kg) | References |
|---|---|---|---|---|---|---|
| Cu primary ores | Electrodeposition (Commercial process) | Cell voltage: 1.8–2.1 V Current density: 23–40 mA/cm2 Electrolyte: Cu 37–55 g/L; H2SO4 > 150 g/L; 40–50 °C | Cu-sheet | 85–95 | 1.8–2.5 | [15] |
| Cu bleed electrolyte | Electrodeposition | Cell voltage: 2.53 V Current density: 70 mA/cm2 Electrolyte (g/L): Cu 38.13 g/L, H2SO4 90 g/L | Cu-powder | 88 | 2.3 | [21] |
| Industrial wastewater | Electrodeposition | Current density: 58.5 mA/cm2 Electrolyte: Cu 36 g/L, Fe 100 g/L | Cu-sheet | NI | 32.39 | [23] |
| Electronic waste | Electro-leaching and electrodeposition | Electrolytic cell: 1.6 V Current density: 35 mA/cm2 Electrolyte: Cu 40 g/L; NH3 12%; (NH4)2SO4 155 g/L; 40 °C | Cu-sheet | 62.7 | 2.33 | [30] |
| Simulated Cu solution | Electrodeposition | Current density: 15 mA/cm2 Electrolyte: Cu 20 g/L; H2SO4 30 g/L; 50 °C | Cu-sheet | 94–98 | 1.3–1.4 | [32] |
| Cu-containing ammoniacal solution | Electrodeposition | Current density: 20 mA/cm2 Electrolyte: Cu 0.03 M; NH3 5.0 M; NH4Cl 4.0 M; Cu2O 0.5 M | Cu-sheet | ~90% | 0.5 | [33] |
| Cu sludge | Acid leaching and electrodeposition | Electrolytic cell: ~1.6 V Current density: 15 mA/cm2 Electrolyte: Cu 20.0 g/L; H2SO4 100 g/L; 45 °C | Cu-sheet | ~81% | 1.7 | The present work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trinh, H.B.; Lee, J.; Kim, S.; Lee, J.-c.; Aceituno, J.C.F.; Oh, S. Selective Recovery of Copper from Industrial Sludge by Integrated Sulfuric Leaching and Electrodeposition. Metals 2021, 11, 22. https://doi.org/10.3390/met11010022
Trinh HB, Lee J, Kim S, Lee J-c, Aceituno JCF, Oh S. Selective Recovery of Copper from Industrial Sludge by Integrated Sulfuric Leaching and Electrodeposition. Metals. 2021; 11(1):22. https://doi.org/10.3390/met11010022
Chicago/Turabian StyleTrinh, Ha Bich, Jaeryeong Lee, Seunghyun Kim, Jae-chun Lee, Juan Carlos Fuentes Aceituno, and Seokhoon Oh. 2021. "Selective Recovery of Copper from Industrial Sludge by Integrated Sulfuric Leaching and Electrodeposition" Metals 11, no. 1: 22. https://doi.org/10.3390/met11010022
APA StyleTrinh, H. B., Lee, J., Kim, S., Lee, J.-c., Aceituno, J. C. F., & Oh, S. (2021). Selective Recovery of Copper from Industrial Sludge by Integrated Sulfuric Leaching and Electrodeposition. Metals, 11(1), 22. https://doi.org/10.3390/met11010022







