Thermally Induced Gradient of Properties on a Superhydrophobic Magnesium Alloy Surface
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Surface Characterization
3. Results
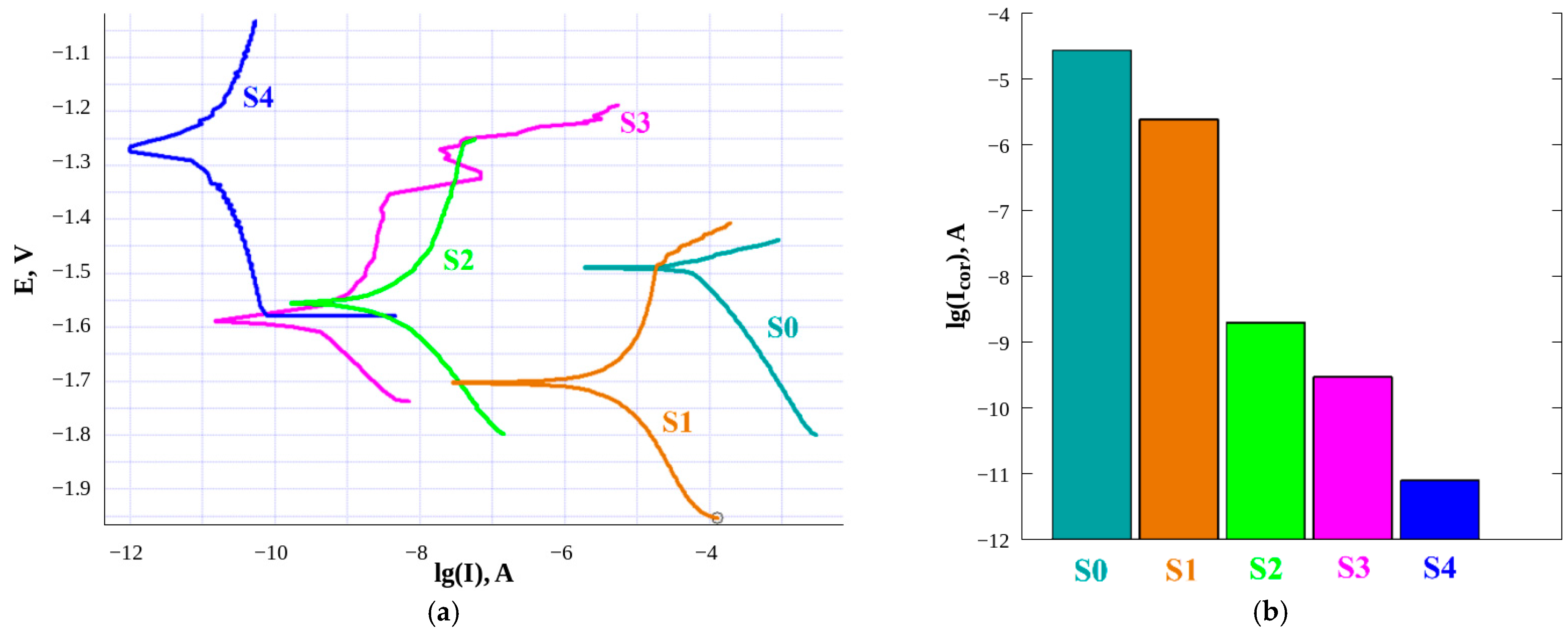
3.1. Selection of the Optimal Regime for Laser Processing
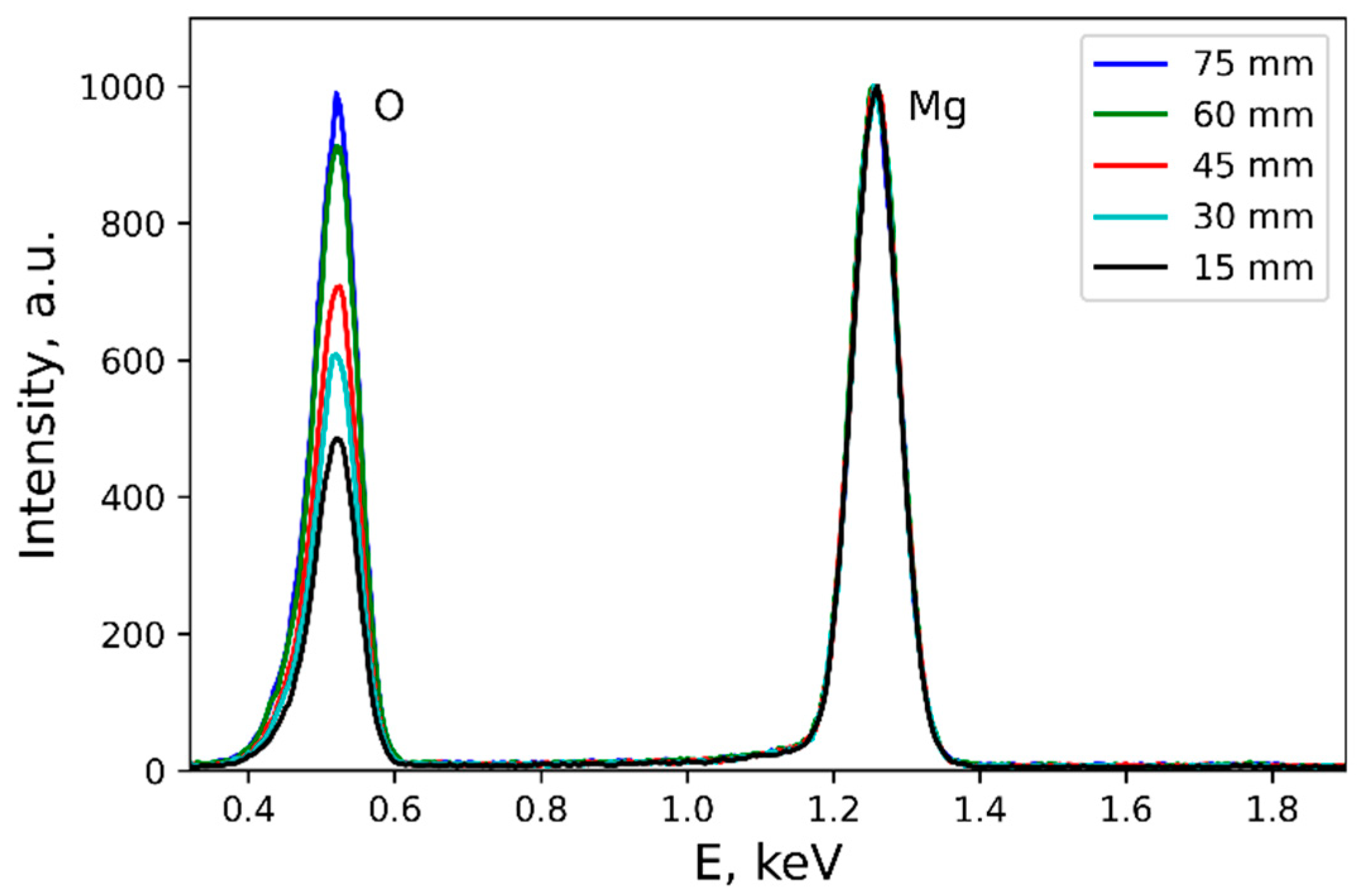
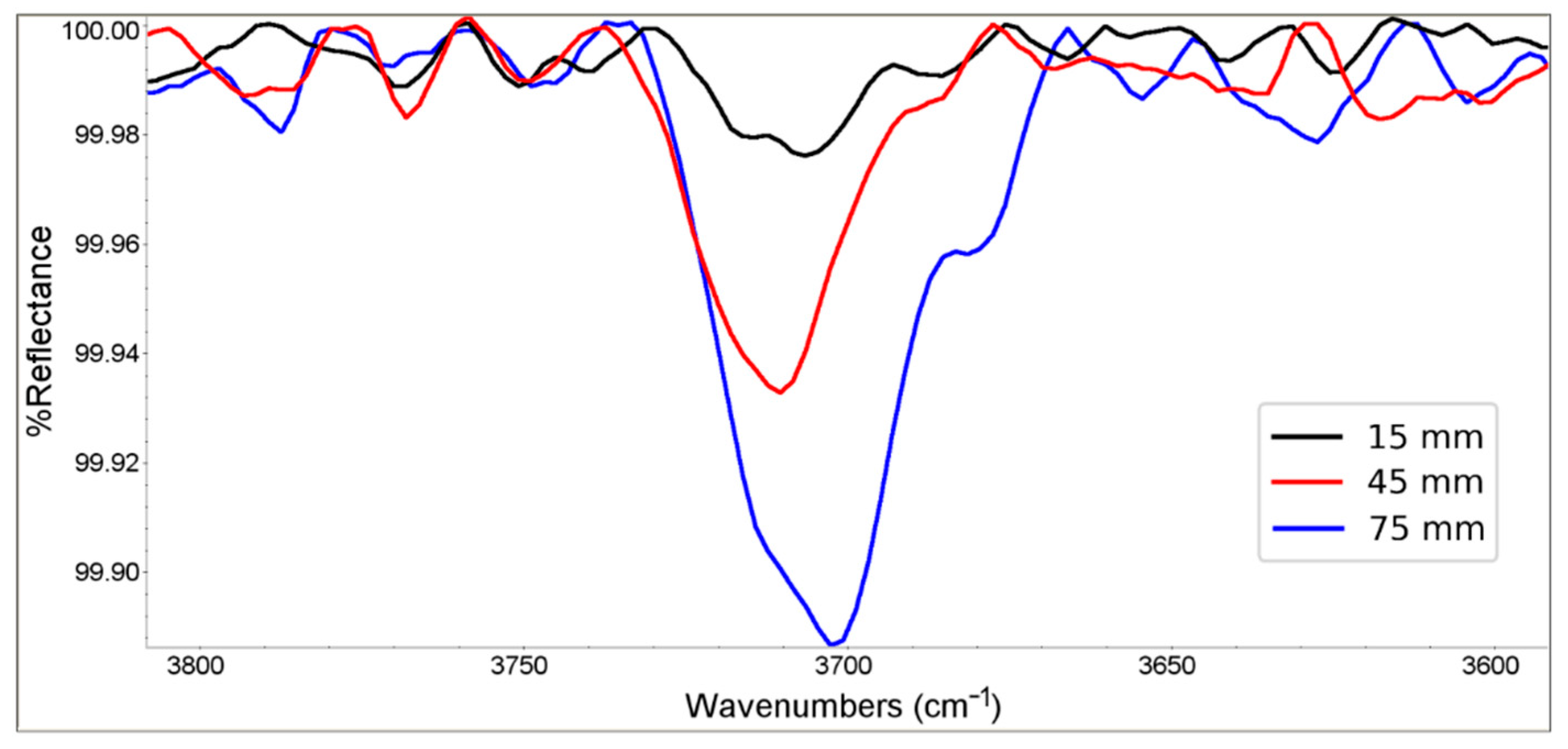
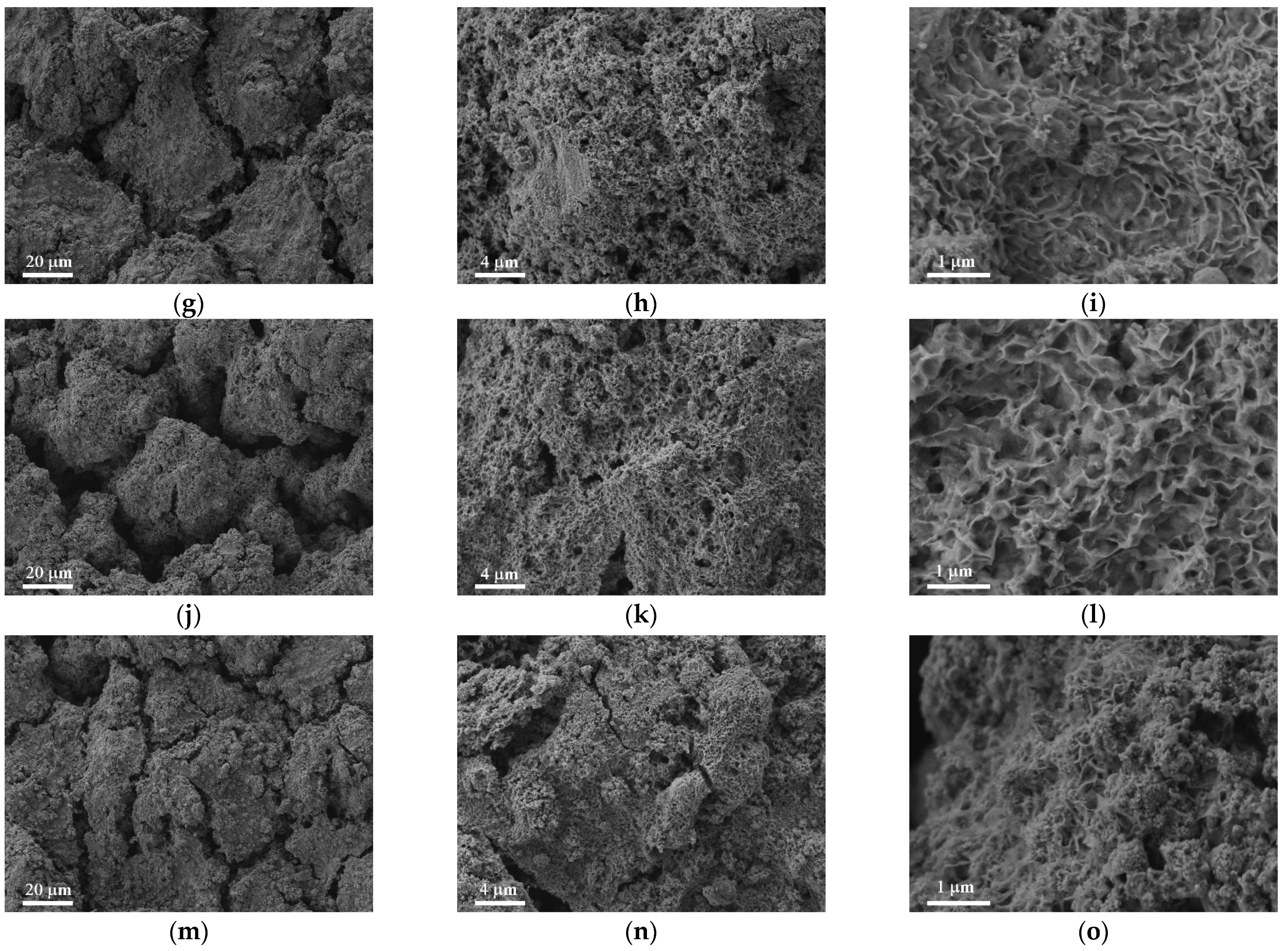
3.2. Mechanism of the Gradient Properties Formation
3.3. The Gradient of the Protective Properties of the Fabricated Superhydrophobic Sample
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erbil, H.Y. Practical Applications of Superhydrophobic Materials and Coatings: Problems and Perspectives. Langmuir 2020, 36, 2493–2509. [Google Scholar] [CrossRef] [PubMed]
- Czyzyk, S.; Dotan, A.; Dodiuk, H.; Kenig, S. Easy-to-Clean Superhydrophobic Coatings Based on Sol-Gel Technology: A Critical Review. Rev. Adhes. Adhes. 2017, 5, 325–360. [Google Scholar] [CrossRef]
- Drelich, J.W.; Boinovich, L.; Chibowski, E.; Della Volpe, C.; Hołysz, L.; Marmur, A.; Siboni, S. Contact angles: History of over 200 years of open questions. Surf. Innov. 2020, 8, 3–27. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A. The prediction of wettability of curved surfaces on the basis of the isotherms of the disjoining pressure. Colloids Surfaces A Physicochem. Eng. Asp. 2011, 383, 10–16. [Google Scholar] [CrossRef]
- Stratakis, E.; Bonse, J.; Heitz, J.; Siegel, J.; Tsibidis, G.; Skoulas, E.; Papadopoulos, A.; Mimidis, A.; Joel, A.-C.; Comanns, P.; et al. Laser engineering of biomimetic surfaces. Mater. Sci. Eng. R: Rep. 2020, 141, 100562. [Google Scholar] [CrossRef]
- Kumar, V.; Verma, R.; Kango, S.; Sharma, V.S. Recent progresses and applications in Laser-based surface texturing systems. Mater. Today Commun. 2020, 101736, 101736. [Google Scholar] [CrossRef]
- Darmanin, T.; Guittard, F. Recent advances in the potential applications of bioinspired superhydrophobic materials. J. Mater. Chem. A 2014, 2, 16319–16359. [Google Scholar] [CrossRef]
- Simpson, J.T.; Hunter, S.R.; Aytug, T. Superhydrophobic materials and coatings: A review. Rep. Prog. Phys. 2015, 78, 086501. [Google Scholar] [CrossRef]
- Conde, J.J.; Ferreira-Aparicio, P.; Chaparro, A.M. Anti-corrosion coating for metal surfaces based on superhydrophobic electrosprayed carbon layers. Appl. Mater. Today 2018, 13, 100–106. [Google Scholar] [CrossRef]
- Ma, L.; Wang, L.; Li, C.; Guo, J.; Shrotriya, P.; Deng, C.; Zhao, J. Hybrid Nanosecond Laser Processing and Heat Treatment for Rapid Preparation of Super-Hydrophobic Copper Surface. Metals 2019, 9, 668. [Google Scholar] [CrossRef]
- Li, X.; Shi, T.; Li, B.; Zhang, C.-W.; Zhong, B.; Lv, Y.; Zhang, Q. One-Step Preparation of Super-Hydrophobic Micro-Nano Dendrites on Al Alloy for Enhanced Corrosion Resistance. Metals 2018, 8, 960. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M.; Modestov, A.D.; Domantovsky, A.G.; Emelyanenko, K.A. Not simply repel water: The diversified nature of corrosion protection by superhydrophobic coatings. Mendeleev Commun. 2017, 27, 254–256. [Google Scholar] [CrossRef]
- Ferrari, M.; Benedetti, A. Superhydrophobic surfaces for applications in seawater. Adv. Colloid Interface Sci. 2015, 222, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Gule, N.P.; Begum, N.M.; Klumperman, B. Advances in biofouling mitigation: A review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 535–555. [Google Scholar] [CrossRef]
- Esmeryan, K.D.; Avramova, I.A.; Castano, C.E.; Ivanova, I.A.; Mohammadi, R.; Radeva, E.I.; Stoyanova, D.S.; Vladkova, T.G. Early stage anti-bioadhesion behavior of superhydrophobic soot based coatings towards Pseudomonas putida. Mater. Des. 2018, 160, 395–404. [Google Scholar] [CrossRef]
- Emelyanenko, A.M.; Pytskii, I.S.; Kaminsky, V.V.; Chulkova, E.V.; Domantovsky, A.G.; Emelyanenko, K.A.; Sobolev, V.D.; Aleshkin, A.V.; Boinovich, L.B. Superhydrophobic copper in biological liquids: Antibacterial activity and microbiologically induced or inhibited corrosion. Colloids Surfaces B: Biointerfaces 2020, 185, 110622. [Google Scholar] [CrossRef] [PubMed]
- Boinovich, L.B.; Modin, E.B.; Sayfutdinova, A.R.; Emelyanenko, K.A.; Vasiliev, A.L.; Emelyanenko, A.M. Combination of Functional Nanoengineering and Nanosecond Laser Texturing for Design of Superhydrophobic Aluminum Alloy with Exceptional Mechanical and Chemical Properties. ACS Nano 2017, 11, 10113–10123. [Google Scholar] [CrossRef] [PubMed]
- Emelyanenko, A.M.; Boinovich, L.B.; Bezdomnikov, A.A.; Chulkova, E.V.; Emelyanenko, K.A. Reinforced Superhydrophobic Coating on Silicone Rubber for Longstanding Anti-Icing Performance in Severe Conditions. ACS Appl. Mater. Interfaces 2017, 9, 24210–24219. [Google Scholar] [CrossRef] [PubMed]
- Boinovich, L.B.; Emelyanenko, K.A.; Domantovsky, A.G.; Chulkova, E.V.; Shiryaev, A.A.; Emelyanenko, A.M. Pulsed Laser Induced Triple Layer Copper Oxide Structure for Durable Polyfunctionality of Superhydrophobic Coatings. Adv. Mater. Interfaces 2018, 5, 1801099. [Google Scholar] [CrossRef]
- Sataeva, N.E.; Boinovich, L.B.; Emelyanenko, K.A.; Domantovsky, A.G.; Emelyanenko, A.M. Laser-assisted processing of aluminum alloy for the fabrication of superhydrophobic coatings withstanding multiple degradation factors. Surf. Coatings Technol. 2020, 397, 125993. [Google Scholar] [CrossRef]
- Santos, E.C.; Shiomi, M.; Osakada, K.; Laoui, T. Rapid manufacturing of metal components by laser forming. Int. J. Mach. Tools Manuf. 2006, 46, 1459–1468. [Google Scholar] [CrossRef]
- Carter, L.; Martin, C.; Withers, P.J.; Attallah, M.M. The influence of the laser scan strategy on grain structure and cracking behaviour in SLM powder-bed fabricated nickel superalloy. J. Alloys Compd. 2014, 615, 338–347. [Google Scholar] [CrossRef]
- Son, Y.; Yeo, J.; Moon, H.; Lim, T.W.; Hong, S.; Nam, K.H.; Yoo, S.; Grigoropoulos, C.P.; Yang, D.-Y.; Ko, S.H. Nanoscale Electronics: Digital Fabrication by Direct Femtosecond Laser Processing of Metal Nanoparticles. Adv. Mater. 2011, 23, 3176–3181. [Google Scholar] [CrossRef]
- Yeo, J.; Kim, G.; Hong, S.; Kim, M.S.; Kim, D.; Lee, J.; Lee, H.B.; Kwon, J.; Suh, Y.D.; Kang, H.W.; et al. Flexible supercapacitor fabrication by room temperature rapid laser processing of roll-to-roll printed metal nanoparticle ink for wearable electronics application. J. Power Sources 2014, 246, 562–568. [Google Scholar] [CrossRef]
- Malinauskas, M.; Žukauskas, A.; Hasegawa, S.; Hayasaki, Y.; Mizeikis, V.; Buividas, R.; Juodkazis, S. Ultrafast laser processing of materials: From science to industry. Light. Sci. Appl. 2016, 5, e16133. [Google Scholar] [CrossRef] [PubMed]
- Boinovich, L.B.; Emelyanenko, A.M.; Emelyanenko, K.A.; Modin, E.B. Modus Operandi of Protective and Anti-icing Mechanisms Underlying the Design of Longstanding Outdoor Icephobic Coatings. ACS Nano 2019, 13, 4335–4346. [Google Scholar] [CrossRef] [PubMed]
- Ocaña, J.L.; Huerta-Murillo, D.; Lasagni, A.F.; Aguilar-Morales, A.I.; Alamri, S.; Cardoso, J.T.; García-Beltrán, A.; Cordovilla, F.; Angulo, I. Modification of Ti6Al4V surface properties by combined DLW-DLIP hierarchical micro-nano structuring. Adv. Opt. Technol. 2020, 9, 121–130. [Google Scholar] [CrossRef]
- Garcia-Giron, A.; Romano, J.-M.; Batal, A.; Dashtbozorg, B.; Dong, H.; Solanas, E.M.; Angos, D.U.; Walker, M.; Penchev, P.; Dimov, S.S. Durability and Wear Resistance of Laser-Textured Hardened Stainless Steel Surfaces with Hydrophobic Properties. Langmuir 2019, 35, 5353–5363. [Google Scholar] [CrossRef]
- Tokunaga, A.; Tsuruta, T. Enhancement of condensation heat transfer on a microstructured surface with wettability gradient. Int. J. Heat Mass Transf. 2020, 156, 119839. [Google Scholar] [CrossRef]
- Zhang, Q.; Dong, J.; Peng, M.; Yang, Z.; Wan, Y.; Yao, F.; Zhou, J.; Ouyang, C.; Deng, X.; Wan, Y. Laser-induced wettability gradient surface on NiTi alloy for improved hemocompatibility and flow resistance. Mater. Sci. Eng. C 2020, 111, 110847. [Google Scholar] [CrossRef]
- Ge, P.; Wang, S.; Zhang, J.; Yang, B. Micro-/nanostructures meet anisotropic wetting: From preparation methods to applications. Mater. Horiz. 2020, 7, 2566–2595. [Google Scholar] [CrossRef]
- Ju, J.; Zheng, Y.; Jiang, L. Bioinspired One-Dimensional Materials for Directional Liquid Transport. Accounts Chem. Res. 2014, 47, 2342–2352. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ran, T.; Gan, Y.; Zhou, J.; Zhang, Y.; Zhang, L.; Zhang, D.; Jiang, L. Ultrafast water harvesting and transport in hierarchical microchannels. Nat. Mater. 2018, 17, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chao, P.H.-S.; Hanet, S.; Van Dam, R.M. Performing multi-step chemical reactions in microliter-sized droplets by leveraging a simple passive transport mechanism. Lab Chip 2017, 17, 4342–4355. [Google Scholar] [CrossRef]
- Gray-Munro, J.; Campbell, J. Mimicking the hierarchical surface topography and superhydrophobicity of the lotus leaf on magnesium alloy AZ31. Mater. Lett. 2017, 189, 271–274. [Google Scholar] [CrossRef]
- Ishizaki, T.; Shimada, Y.; Tsunakawa, M.; Lee, H.; Yokomizo, T.; Hisada, S.; Nakamura, K. Rapid Fabrication of a Crystalline Myristic Acid-Based Superhydrophobic Film with Corrosion Resistance on Magnesium Alloys by the Facile One-Step Immersion Process. ACS Omega 2017, 2, 7904–7915. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M.; Pashinin, A.S.; Gnedenkov, S.V.; Egorkin, V.S.; Sinebryukhov, S.L. Mg alloy treatment for superhydrophobic anticorrosion coating formation. Surf. Innov. 2013, 1, 162–172. [Google Scholar] [CrossRef]
- Yeganeh, M.; Mohammadi, N. Superhydrophobic surface of Mg alloys: A review. J. Magnes. Alloy. 2018, 6, 59–70. [Google Scholar] [CrossRef]
- Joo, J.; Kim, D.; Moon, H.-S.; Kim, K.; Lee, J. Durable anti-corrosive oil-impregnated porous surface of magnesium alloy by plasma electrolytic oxidation with hydrothermal treatment. Appl. Surf. Sci. 2020, 509, 145361. [Google Scholar] [CrossRef]
- Ishizaki, T.; Kumagai, S.; Tsunakawa, M.; Furukawa, T.; Nakamura, K. Ultrafast fabrication of superhydrophobic surfaces on engineering light metals by single-step immersion process. Mater. Lett. 2017, 193, 42–45. [Google Scholar] [CrossRef]
- Gupta, M.; Sharon, N.M.L. Magnesium, Magnesium Alloys, and Magnesium Composites; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Magnesium Technology; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2006.
- Seitz, J.-M.; Durisin, M.; Goldman, J.; Drelich, J.W. Recent Advances in Biodegradable Metals for Medical Sutures: A Critical Review. Adv. Heal. Mater. 2015, 4, 1915–1936. [Google Scholar] [CrossRef] [PubMed]
- Kamrani, S.; Fleck, C. Biodegradable magnesium alloys as temporary orthopaedic implants: A review. BioMetals 2019, 32, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Emel’Yanenko, A.M.; Boinovich, L.B. Analysis of wetting as an efficient method for studying the characteristics of coatings and surfaces and the processes that occur on them: A review. Inorg. Mater. 2011, 47, 1667–1675. [Google Scholar] [CrossRef]
- Emelyanenko, A.; Boinovich, L. The role of discretization in video image processing of sessile and pendant drop profiles. Colloids Surfaces A: Physicochem. Eng. Asp. 2001, 189, 197–202. [Google Scholar] [CrossRef]
- Fishburn, J.; Withford, M.; Coutts, D.; Piper, J. Study of the fluence dependent interplay between laser induced material removal mechanisms in metals: Vaporization, melt displacement and melt ejection. Appl. Surf. Sci. 2006, 252, 5182–5188. [Google Scholar] [CrossRef]
- Fishburn, J.; Withford, M.; Coutts, D.W.; Piper, J.A. Study of the interplay of vaporisation, melt displacement and melt ejection mechanisms under multiple pulse irradiation of metals. Appl. Surf. Sci. 2006, 253, 662–667. [Google Scholar] [CrossRef]
- Stoian, R.; Colombier, J.-P. Advances in ultrafast laser structuring of materials at the nanoscale. Nanophotonics 2020, 9, 4665–4688. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 84th ed.; Lide, D.R., Ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Tan, Q.; Yin, Y.; Mo, N.; Zhang, M.; Atrens, A. Recent understanding of the oxidation and burning of magnesium alloys. Surf. Innov. 2019, 7, 71–92. [Google Scholar] [CrossRef]
- Buchanan, R.A.; Caspers, H.H.; Murphy, J. Lattice Vibration Spectra of Mg (OH)_2 and Ca(OH)_2. Appl. Opt. 2008, 2, 1147–1150. [Google Scholar] [CrossRef]
- Hexter, R.M. On the Infrared Absorption Spectra Crystalline Brucite [Mg (OH)_2] and Portlandite [Ca(OH)_2]. J. Opt. Soc. Am. 2008, 48, 770–772. [Google Scholar] [CrossRef]
- Tan, Q.; Atrens, A.; Mo, N.; Zhang, M.-X. Oxidation of magnesium alloys at elevated temperatures in air: A review. Corros. Sci. 2016, 112, 734–759. [Google Scholar] [CrossRef]
- Czerwinski, F. Oxidation Characteristics of Magnesium Alloys. JOM 2012, 64, 1477–1483. [Google Scholar] [CrossRef]
- Ma, S.; Xing, F.; Ta, N.; Zhang, L. Kinetic modeling of high-temperature oxidation of pure Mg. J. Magnes. Alloy. 2020, 8, 819–831. [Google Scholar] [CrossRef]


| Sample | Line Density, mm−1 | Linear Scanning Rate, mm/s | Pulse Duration, ns | Repetition Rate, kHz | Fluence, J/cm2 |
|---|---|---|---|---|---|
| S1 | 400 | 100 | 200 | 20 | 76 |
| S2 | 400 | 100 | 14 | 286 | 5.3 |
| S3 | 400 | 100 | 8 | 500 | 3.0 |
| S4 | 400 | 100 | 4 | 1000 | 1.5 |
| Sample | Contact Angle, ° | Roll-Off Angle, ° |
|---|---|---|
| S0 | 43.1 ± 1.3 | No roll-off |
| S1 | 170.7 ± 0.7 | 3.1 ± 0.6 |
| S2 | 171.2 ± 0.7 | 4.3 ± 1.1 |
| S3 | 171.5 ± 1.0 | 3.8 ± 0.5 |
| S4 | 170.9 ± 0.3 | 2.5 ± 0.5 |
| Time, h | Corrosion Current, A cm−2 | |
|---|---|---|
| l = 15 mm | l = 75 mm | |
| 1 | 5.0 × 10−9 | 1.0 × 10−11 |
| 16 | 1.2 × 10−7 | 5.2 × 10−10 |
| 20 | 1.5 × 10−6 | 3.4 × 10−9 |
| 24 | 1.7 × 10−6 | 3.8 × 10−9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emelyanenko, K.A.; Domantovsky, A.G.; Chulkova, E.V.; Emelyanenko, A.M.; Boinovich, L.B. Thermally Induced Gradient of Properties on a Superhydrophobic Magnesium Alloy Surface. Metals 2021, 11, 41. https://doi.org/10.3390/met11010041
Emelyanenko KA, Domantovsky AG, Chulkova EV, Emelyanenko AM, Boinovich LB. Thermally Induced Gradient of Properties on a Superhydrophobic Magnesium Alloy Surface. Metals. 2021; 11(1):41. https://doi.org/10.3390/met11010041
Chicago/Turabian StyleEmelyanenko, Kirill A., Alexander G. Domantovsky, Elizaveta V. Chulkova, Alexandre M. Emelyanenko, and Ludmila B. Boinovich. 2021. "Thermally Induced Gradient of Properties on a Superhydrophobic Magnesium Alloy Surface" Metals 11, no. 1: 41. https://doi.org/10.3390/met11010041
APA StyleEmelyanenko, K. A., Domantovsky, A. G., Chulkova, E. V., Emelyanenko, A. M., & Boinovich, L. B. (2021). Thermally Induced Gradient of Properties on a Superhydrophobic Magnesium Alloy Surface. Metals, 11(1), 41. https://doi.org/10.3390/met11010041








