A Comparative Study on the Tribological Properties of a Cobalt-Free Superaustenitic Stainless Steel at Elevated Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Heat Treatment
2.2. Microstructural Characterization
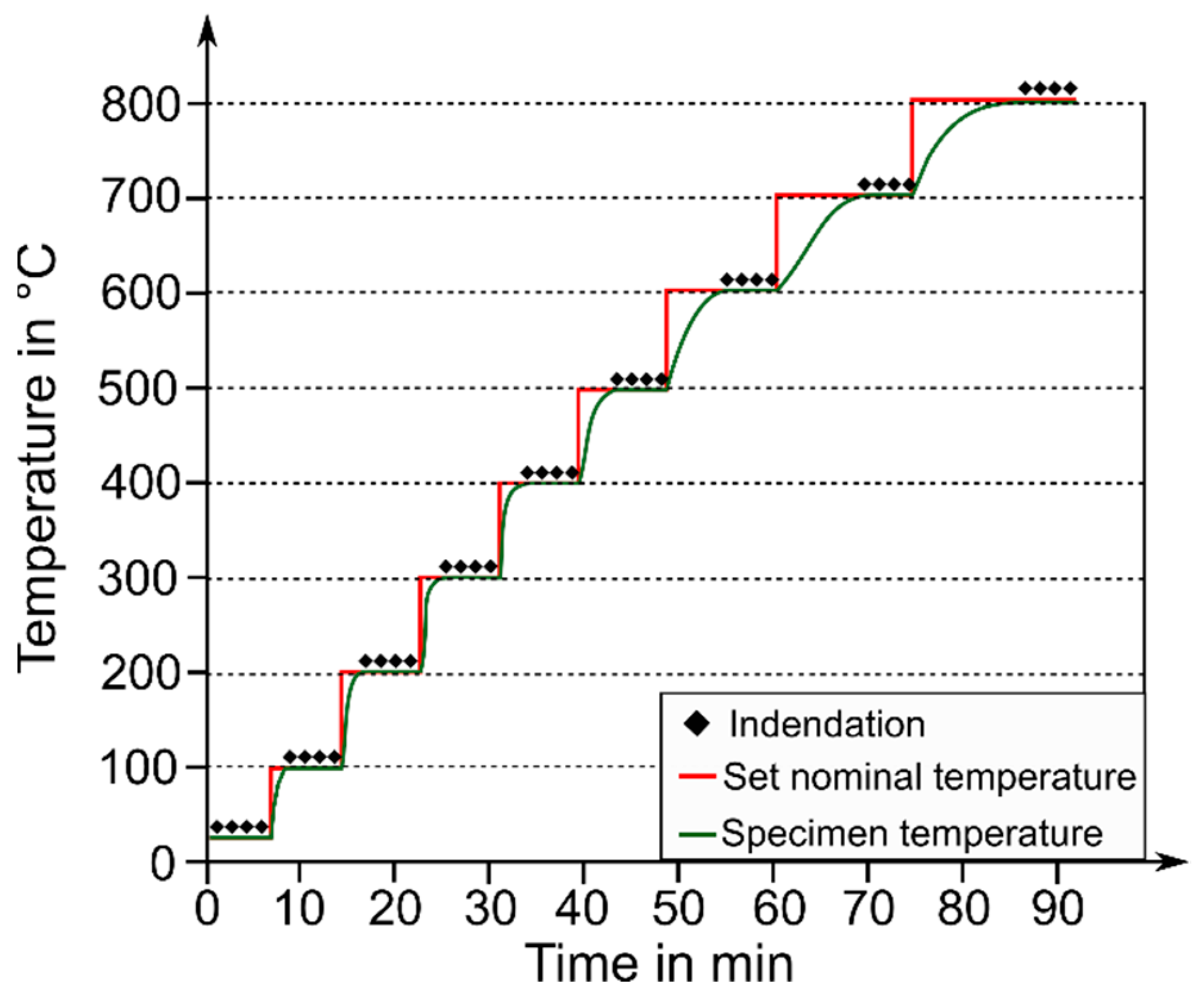
2.3. Hot Hardness Testing
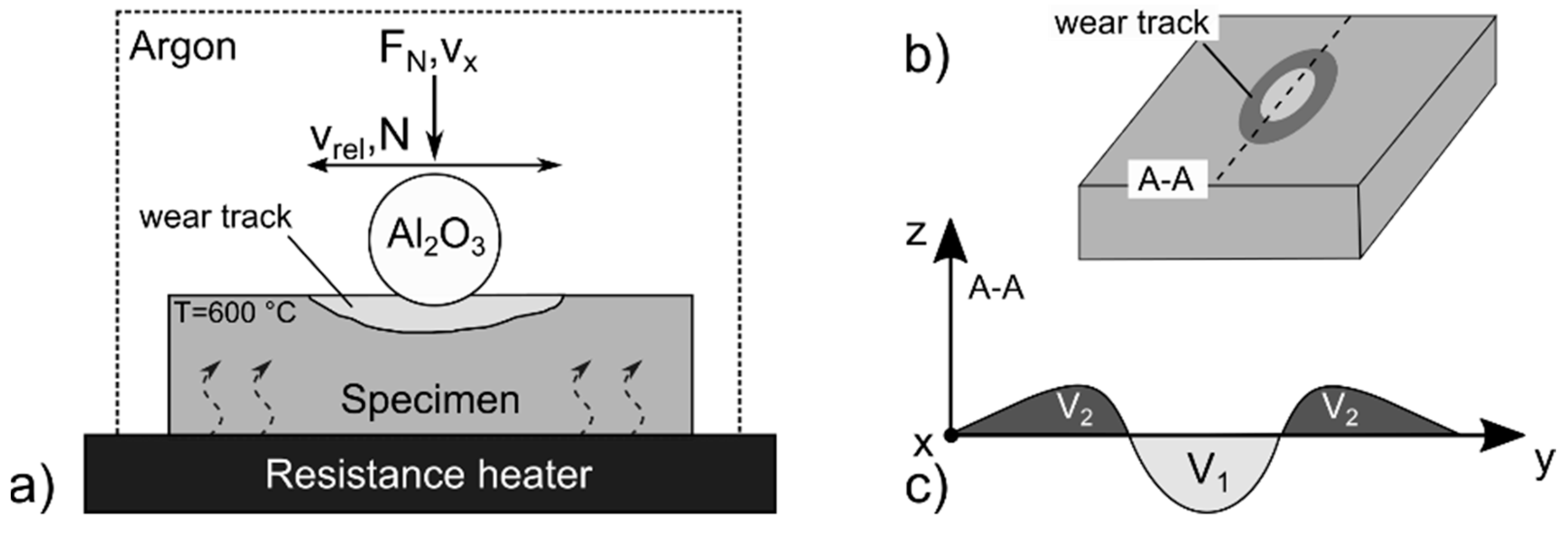
2.4. Hot Wear Testing
3. Results
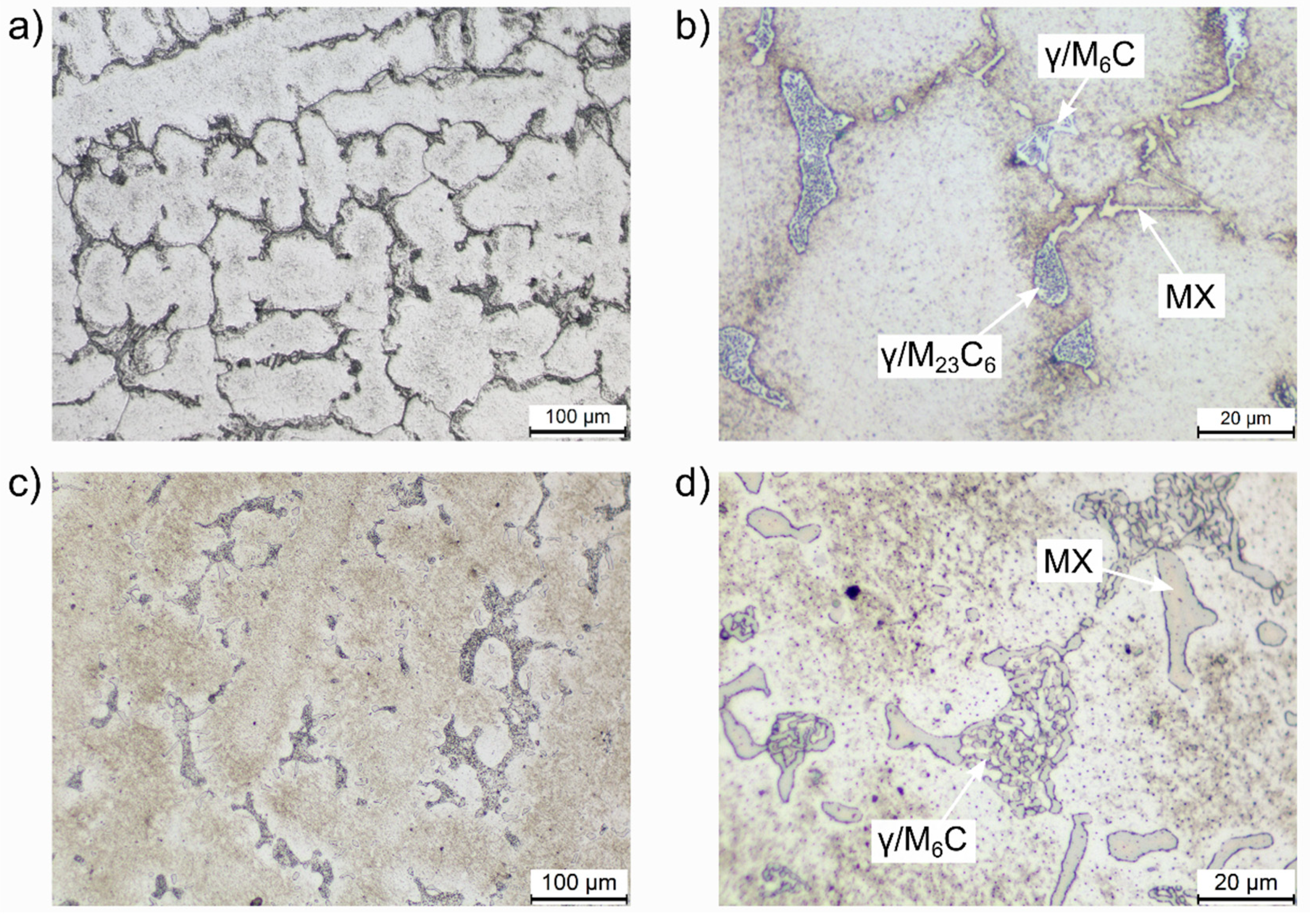
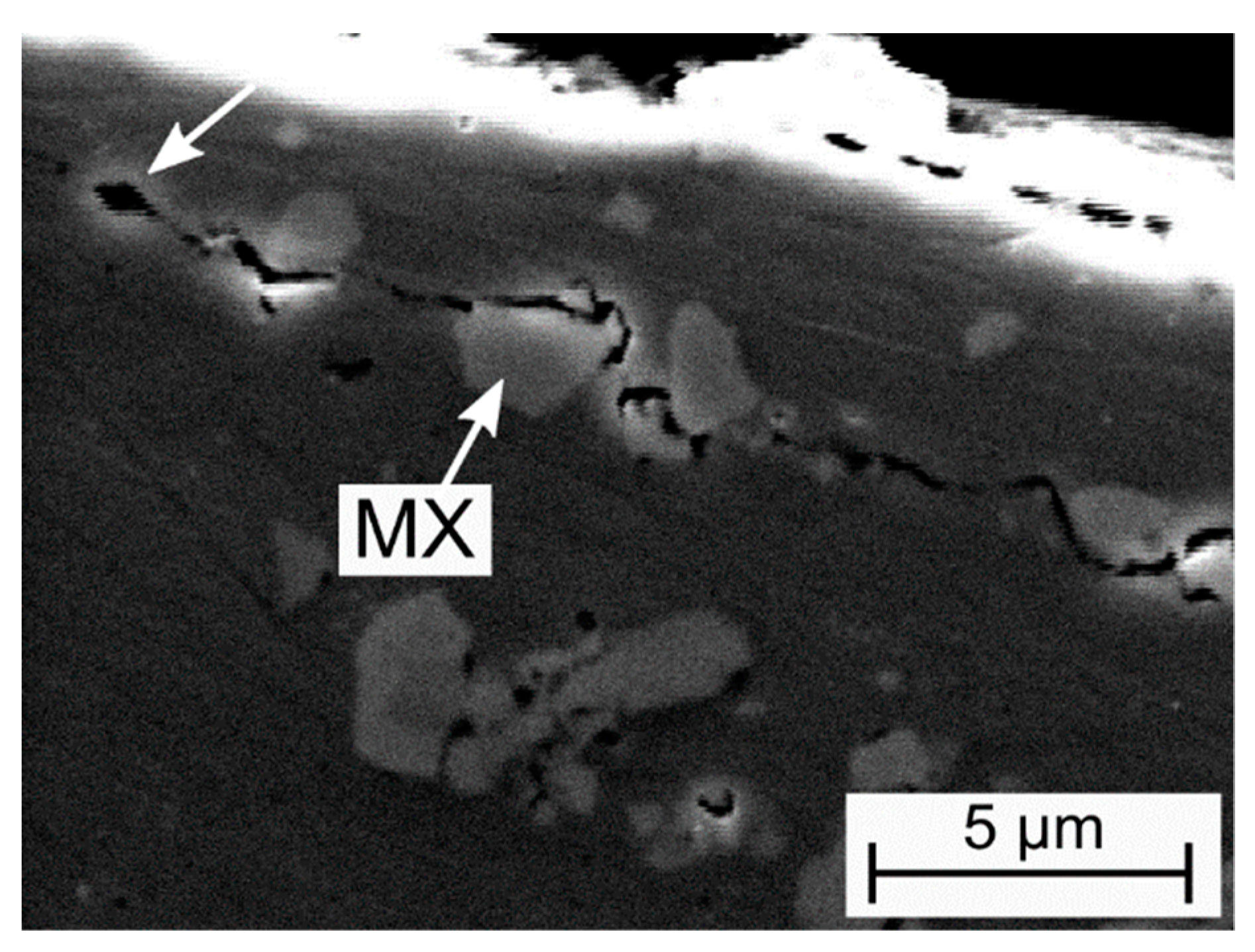
3.1. Microstructural Characterization
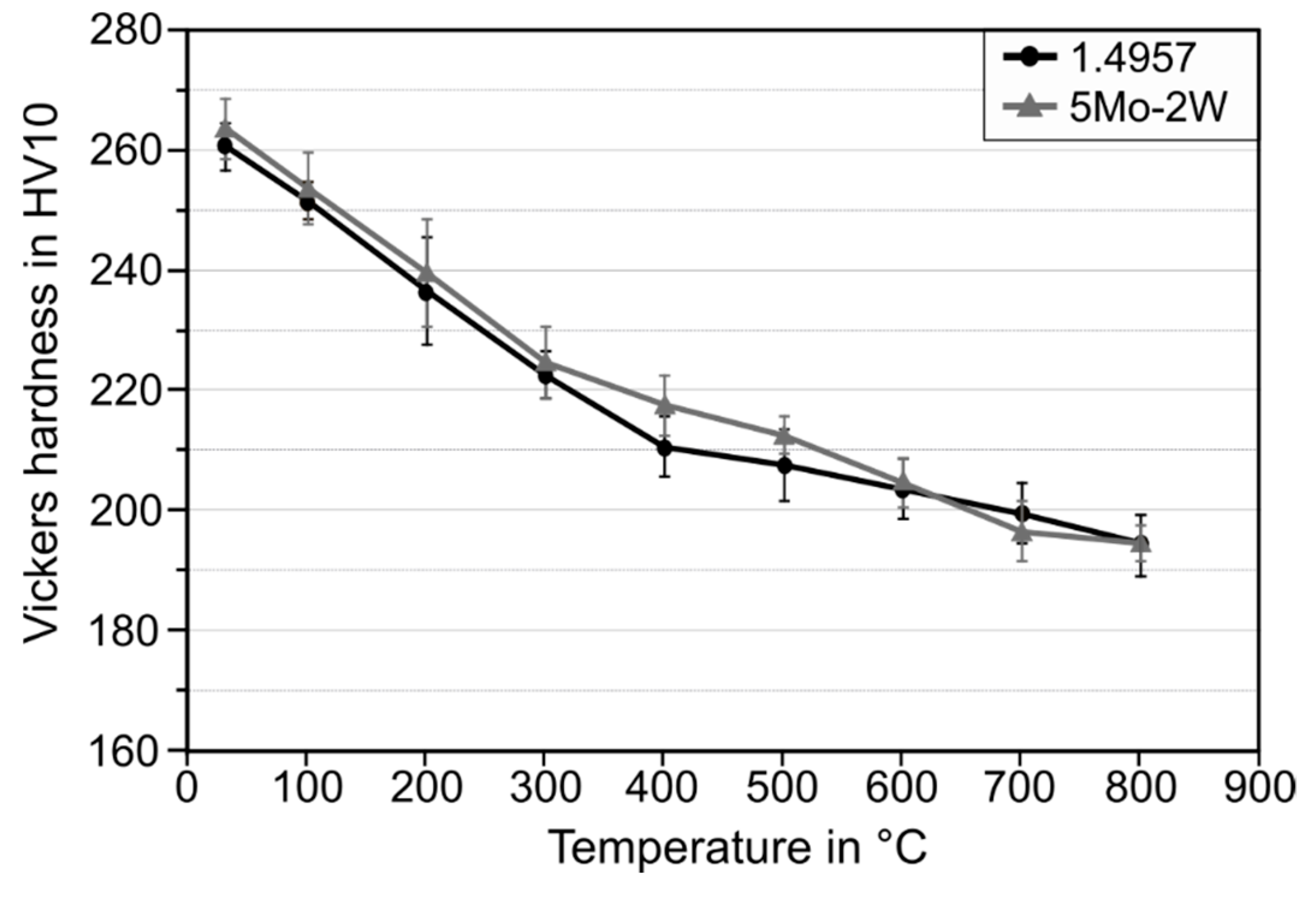
3.2. Hot Hardness
3.3. Sliding Wear
4. Discussion
4.1. Microstructural Evolution
4.2. Effect of the Microstructural Properties on the Hot Hardness
4.3. Correlation between the Hot Hardness and the Hot Wear Behavior
5. Conclusions
- The investigated Co-free steel 5Mo-2W provides an equal hot hardness and a significantly improved sliding wear resistance at 600 °C. As a potential alternative material to commercial SASS, it offers the possibility to save cobalt for future applications.
- The good hot hardness and wear resistance of the steel can be traced back to the absence of an eutectic Cr-rich carbide network as well as the presence of fine-dispersed blocky-globular Nb-rich MX-type carbonitrides which are embedded in a primarily Laves phase strengthened γ-matrix.
- Networks of coarse eutectic carbides favor the formation of severely deformed mechanically mixed layers (MML) that worsen the resistance against sliding wear at elevated temperature.
- Cobalt influences the precipitation kinetics of intermetallic phases during aging but has no impact on the global hot hardness of SASS.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davis, J.R. Heat-Resistant Materials; ASM International: Materials Park, OH, USA, 1997; pp. 200–220. [Google Scholar]
- Haynes International. Multimet Alloy; Publ. No. H-3010 B. Haynes International: Kokomo, IN, USA, 2017. Available online: https://www.haynesintl.com/docs/default-source/pdfs/new-alloy-brochures/high-temperature-alloys/brochures/multimet.pdf?sfvrsn=817229d4_22 (accessed on 20 August 2020).
- European Commission. Study on the Review of the List of Critical Raw Materials—Criticality Assessments—Final Report; European Union: Luxembourg, 2017. [Google Scholar] [CrossRef]
- Tkaczyk, A.H.; Bartl, A.; Amato, A.; Lapkovskis, V.; Petranikova, M. Sustainability evaluation of essential critical raw materials: Cobalt, niobium, tungsten and rare earth elements. J. Phys. D Appl. Phys. 2018, 51, 203001. [Google Scholar] [CrossRef]
- Harf, F.H. The response of cobalt-free Udimet 700 type alloy to modified heat treatments. J. Mater. Sci. 1986, 21, 2497–2508. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.H.; Li, M.; Godlewski, L.A.; Zindel, J.W.; Feng, Q. Effective design of new austenitic cast steels for ultra-high temperature automotive exhaust components through combined CALPHAD and experimental approaches. Mater. Sci. Eng. A 2017, 683, 195–206. [Google Scholar] [CrossRef]
- Attarian, M.; Taheri, A.K. Microstructural evolution in creep aged of directionally solidified heat resistant HP-Nb steel alloyed with tungsten and nitrogen. Mater. Sci. Eng. A 2016, 659, 104–118. [Google Scholar] [CrossRef]
- Voicu, R.; Andrieu, E.; Poquillon, D.; Furtado, J.; Lacaze, J. Microstructure evolution of HP40-Nb alloys during aging under air at 1000 °C. Mater. Charact. 2009, 60, 1020–1027. [Google Scholar] [CrossRef]
- Peng, B.; Zhang, H.; Hong, J.; Gao, J.; Wang, Q.; Zhang, H. Effect of aging on the impact toughness of 25Cr–20Ni–Nb–N steel. Mater. Sci. Eng. A 2010, 527, 1957–1961. [Google Scholar] [CrossRef]
- Shi, S.; Lippold, J.C. Microstructure evolution during service exposure of two cast, heat-resisting stainless steels—HP-Nb modified and 20–32Nb. Mater. Charact. 2008, 59, 1029–1040. [Google Scholar] [CrossRef]
- Chai, G.; Biström, M.; Olaison, M.; Forsberg, U. Creep and LCF Behaviors of Newly Developed Advanced Heat Resistant Austenitic Stainless Steel for A-USC. Procedia Eng. 2013, 55, 232–239. [Google Scholar] [CrossRef]
- Zhu, C.Z.; Yuan, Y.; Zhang, P.; Yang, Z.; Zhou, Y.L.; Huang, J.Y.; Yin, H.F.; Dang, Y.Y.; Zhao, X.B.; Lu, J.T.; et al. A modified HR3C Austenitic Heat Resistant Steel for Ultra-supercritical Power Plants Applications Beyond 650 °C. Metall. Mater. Trans. A 2018, 49A, 434–438. [Google Scholar] [CrossRef]
- Gopinath, V.M.; Arulvel, S. A review on the steels, alloys/high entropy alloys, composites and coatings used in high temperature wear applications. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Krell, J.; Röttger, A.; Ziesing, U.; Theisen, W. Influence of precipitation hardening on the high-temperature sliding wear resistance of an aluminium alloyed iron-nickel base alloy. Tribol. Int. 2020, 148, 106342. [Google Scholar] [CrossRef]
- EN ISO 6507-1:2018. Metallic Materials—Vickers Hardness Test—Part 1: Test Method; ICS 77.040.10; ISO: Geneva, Switzerland, 2018. [Google Scholar]
- Berns, H. Hartlegierungen und Hartverbundwerkstoffe; Springer: Berlin/Heidelberg, Germany, 1998; p. 69. [Google Scholar] [CrossRef]
- Rigney, D.A. Transfer, mixing and associated chemical and mechanical processes during the sliding of ductile materials. Wear 2000, 245, 1–9. [Google Scholar] [CrossRef]
- Durand-Charre, M. Microstructure of Steels and Cast Irons; Springer: Berlin/Heidelberg, Germany, 2004; pp. 108–116. [Google Scholar] [CrossRef]
- Winter, K. Independently Controlled Carbon and Nitrogen Potential: A New Approach to Carbonitriding Process. J. Mater. Eng. Perform. 2013, 22, 1945–1956. [Google Scholar] [CrossRef]
- Sourmail, T. Precipitation in creep resistant austenitic stainless steels. Mater. Sci. Technol. 2001, 17, 1–14. [Google Scholar] [CrossRef]
- Gulyaev, A.P.; Kupalova, I.K. Effect of cobalt on the structure and properties of high-speed steels. Met. Sci. Heat Treat. 1970, 12, 666–671. [Google Scholar] [CrossRef]
- Sha, W. Steels: From Materials Science to Structural Engineering; Springer: London, UK, 2013; pp. 96–97. [Google Scholar] [CrossRef]
- Kondrat’ev, S.Y.; Svyatisheva, E.V.; Anastasiadi, G.P.; Petrov, S.N. Structure of strengthening particles of niobium carbide in Fe–Cr–Ni cast refractory alloys. Phys. Met. Metallogr. 2017, 118, 659–670. [Google Scholar] [CrossRef]
- Gavriljuk, V.G.; Berns, H. High Nitrogen Steels—Structure, Properties, Manufacture, Applications; Springer: Berlin/Heidelberg, Germany, 1999; p. 111. [Google Scholar] [CrossRef]
- Imai, Y.; Masumoto, T. Minor Phases in the Gamma-Type Fe-Co-Cr-Ni Base Heat-Resisting Alloy, LCN-155 Aged and Effect of Additional Elements on These Phases. Tetsu Hagane 1961, 47, 1689–1696. [Google Scholar] [CrossRef]
- Brockway, L.O.; Bigelow, W.C. Development of Procedures for the identification of minor phases in heat-resistant alloys by electron diffraction. Eng. Res. Inst. 1953, 33, 616–623. [Google Scholar]
- Bürgel, R.; Maier, J.; Niendorf, T. Handbuch Hochtemperatur-Werkstofftechnik—Grundlagen, Werkstoffbeanspruchungen, Hochtemperaturlegierungen und Beschichtungen, 4th ed.; Vieweg+Teubner: Wiesbaden, Germany, 2011; pp. 375–377. [Google Scholar]
- Babakr, A.M.; Al-Ahmari, A.; Al-Jumayiah, K.; Habiby, F. Sigma Phase Formation and Embrittlement of Cast Iron-Chromium-Nickel (Fe-Cr-Ni) Alloys. J. Miner. Mater. Charact. Eng. 2008, 7, 127–145. [Google Scholar] [CrossRef]
- Berns, H.; Theisen, W. Ferrous Materials: Steel and Cast Iron, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2008; p. 7. [Google Scholar] [CrossRef]
- Takeyama, M. Novel Concept of Austenitic Heat Resistant Steels Strengthened by Intermetallics. Mater. Sci. Forum 2007, 539, 3012–3017. [Google Scholar] [CrossRef]
- Fischer, A. Sliding abrasion of metallic materials above 550 °C, Society of Tribologists (JSLE). In Proceedings of the Japan International Tribology Conference, Nagoya, Japan, 29 October–1 November 1990; pp. 221–226. [Google Scholar]
- Varga, M.; Rojacz, H.; Winkelmann, H.; Mayer, H.; Badisch, E. Wear reducing effects and temperature dependence of tribolayer formation in harsh environment. Tribol. Int. 2013, 65, 190–199. [Google Scholar] [CrossRef]
- Zum Gahr, K.-H. Microstructure and Wear of Materials; Elsevier: Amsterdam, The Netherlands, 1987; pp. 90–130. [Google Scholar]
- Winkelmann, H.; Badisch, E.; Kirchgaßner, M.; Danninger, H. Wear mechanisms at high temperatures. Part 1: Wear mechanisms of different Fe-based alloys at elevated temperatures. Tribol. Lett. 2009, 34, 155–166. [Google Scholar] [CrossRef]
- Winkelmann, H.; Badisch, E.; Varga, M.; Danninger, H. Wear mechanisms at high temperatures. Part 3: Changes of the wear mechanism in the continuous impact abrasion test with increasing testing temperature. Tribol. Lett. 2010, 37, 419–429. [Google Scholar] [CrossRef]
- Das, A. Revisiting Stacking Fault Energy of Steels. Metall. Mater. Trans. A 2016, 47, 748–768. [Google Scholar] [CrossRef]
- Vitos, L.; Nilsson, J.-O.; Johansson, B. Alloying effects on the stacking fault energy in austenitic stainless steels from first-principles theory. Acta Mater. 2006, 54, 3821–3826. [Google Scholar] [CrossRef]
- Lu, S.; Hu, Q.-M.; Johansson, B.; Vitos, L. Stacking fault energies of Mn, Co and Nb alloyed austenitic stainless steels. Acta Mater. 2011, 59, 5728–5734. [Google Scholar] [CrossRef]




| Steel | C | Cr | Ni | Co | Mo | Nb | W | Mn | Si | P | S | N | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.4957 | 0.22 | 19.8 | 19.1 | 18.8 | 2.7 | 0.8 | 2.4 | 1.2 | 1.3 | 0.02 | 0.01 | 0.14 | bal. |
| 5Mo-2W | 0.21 | 21.1 | 25.0 | 0.1 | 5.5 | 1.8 | 1.8 | 1.9 | 1.3 | 0.02 | 0.01 | 0.23 | bal. |
| Steel | V1 | V2 | ΔV(V1−V2) |
|---|---|---|---|
| 1.4957 | 194.6 ± 31.9 | 15.2 ± 1.5 | 179.4 |
| 5Mo-2W | 40.4 ± 9.4 | 11.1 ± 0.8 | 29.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van gen Hassend, F.; Weber, S. A Comparative Study on the Tribological Properties of a Cobalt-Free Superaustenitic Stainless Steel at Elevated Temperature. Metals 2020, 10, 1123. https://doi.org/10.3390/met10091123
van gen Hassend F, Weber S. A Comparative Study on the Tribological Properties of a Cobalt-Free Superaustenitic Stainless Steel at Elevated Temperature. Metals. 2020; 10(9):1123. https://doi.org/10.3390/met10091123
Chicago/Turabian Stylevan gen Hassend, Frederic, and Sebastian Weber. 2020. "A Comparative Study on the Tribological Properties of a Cobalt-Free Superaustenitic Stainless Steel at Elevated Temperature" Metals 10, no. 9: 1123. https://doi.org/10.3390/met10091123
APA Stylevan gen Hassend, F., & Weber, S. (2020). A Comparative Study on the Tribological Properties of a Cobalt-Free Superaustenitic Stainless Steel at Elevated Temperature. Metals, 10(9), 1123. https://doi.org/10.3390/met10091123





