New Bactericide Orthodonthic Archwire: NiTi with Silver Nanoparticles
Abstract
1. Introduction
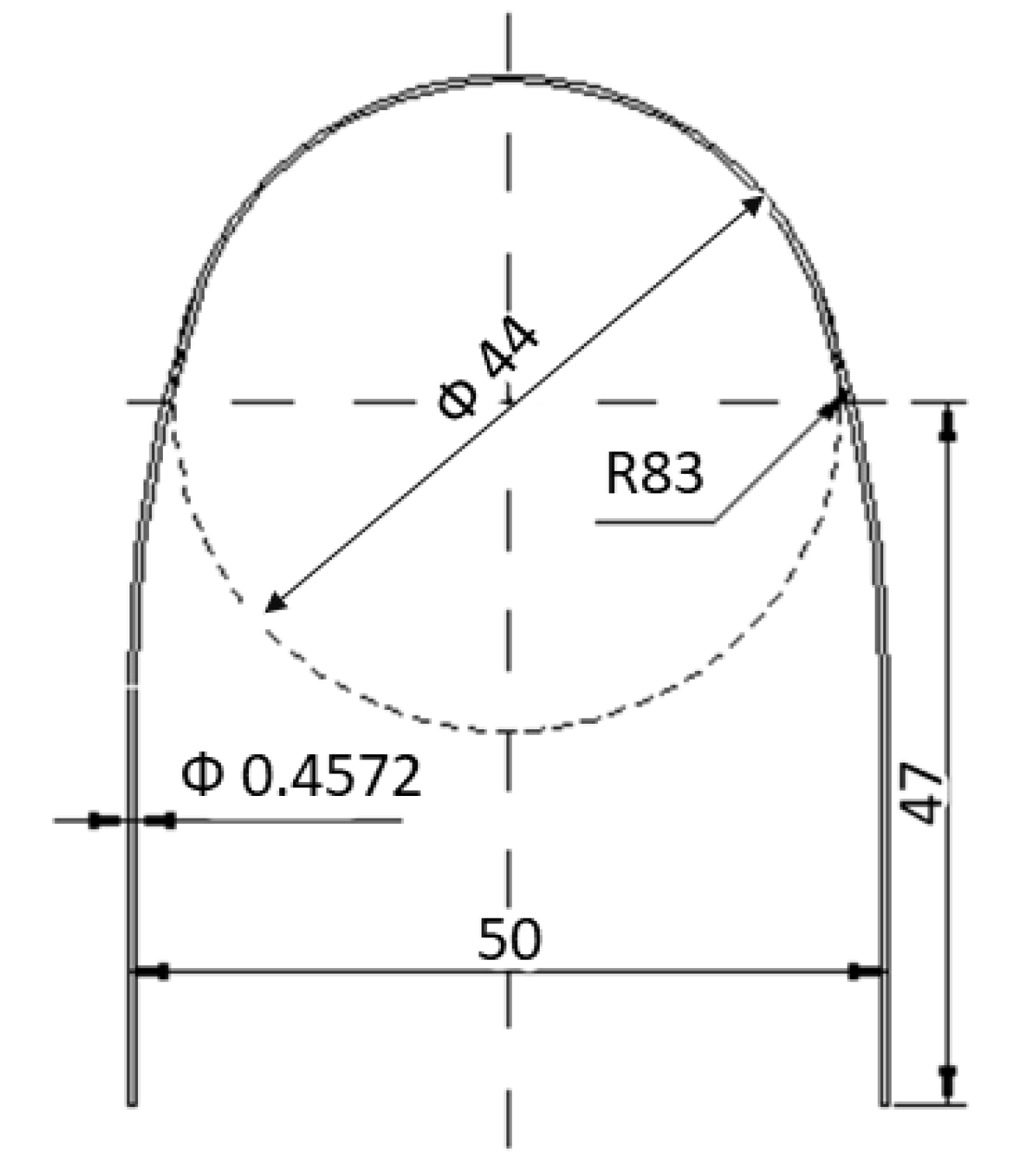
2. Materials and Methods
2.1. Material
2.2. Silver Nanoparticles Electrodeposition
- a.
- Nickel-titanium samples are cleaned by ultrasonication in ethylic alcohol, double distilled water, and acetone for 10 min each. Samples were dried with argon gas. This process removes accumulated contaminants.
- b.
- Samples are treated in acid solution (HNO3 60%, NH4HF2, and ultrapure distilled water) for 10 s to clean the contamination.
- c.
- Samples are rinsed in distilled water eliminating constituent particles not removed by the previous treatment or product from previous treatment solution.
- d.
- Samples are immersed in a reactor containing an electrolyte with silver nitrate (AgNO3) and a coordinating compound sodium thiosulphate (Na2S2O3) at 25 °C and with magnetic stirring. Electric current is passed through the electrolyte and the titanium is made the anode in the electrolytic cell; the platinum electrode is the cathode. A rectangular pulsed potential is applied to the electrode (EI = 0 V, EF = 5 V, ST = 500 ms, SH = 10 mV, PW = 100 ms). The full-cycle period is around 25 s [12,13]. From Figure 2 is shown a diagram of the anodization process.
2.3. Calorimetric Tests
2.4. Mechanical Tests
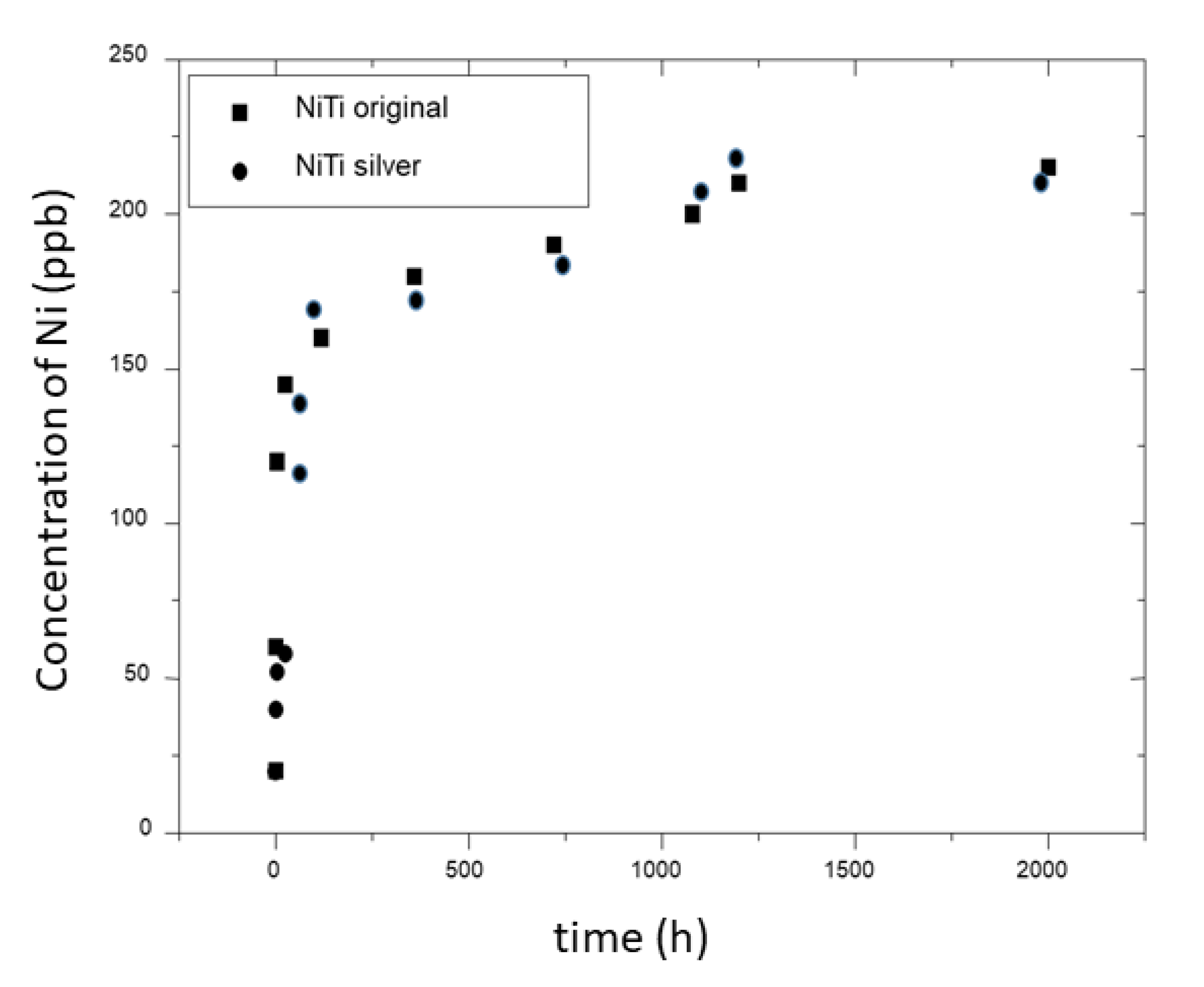
2.5. Nickel Ion Release
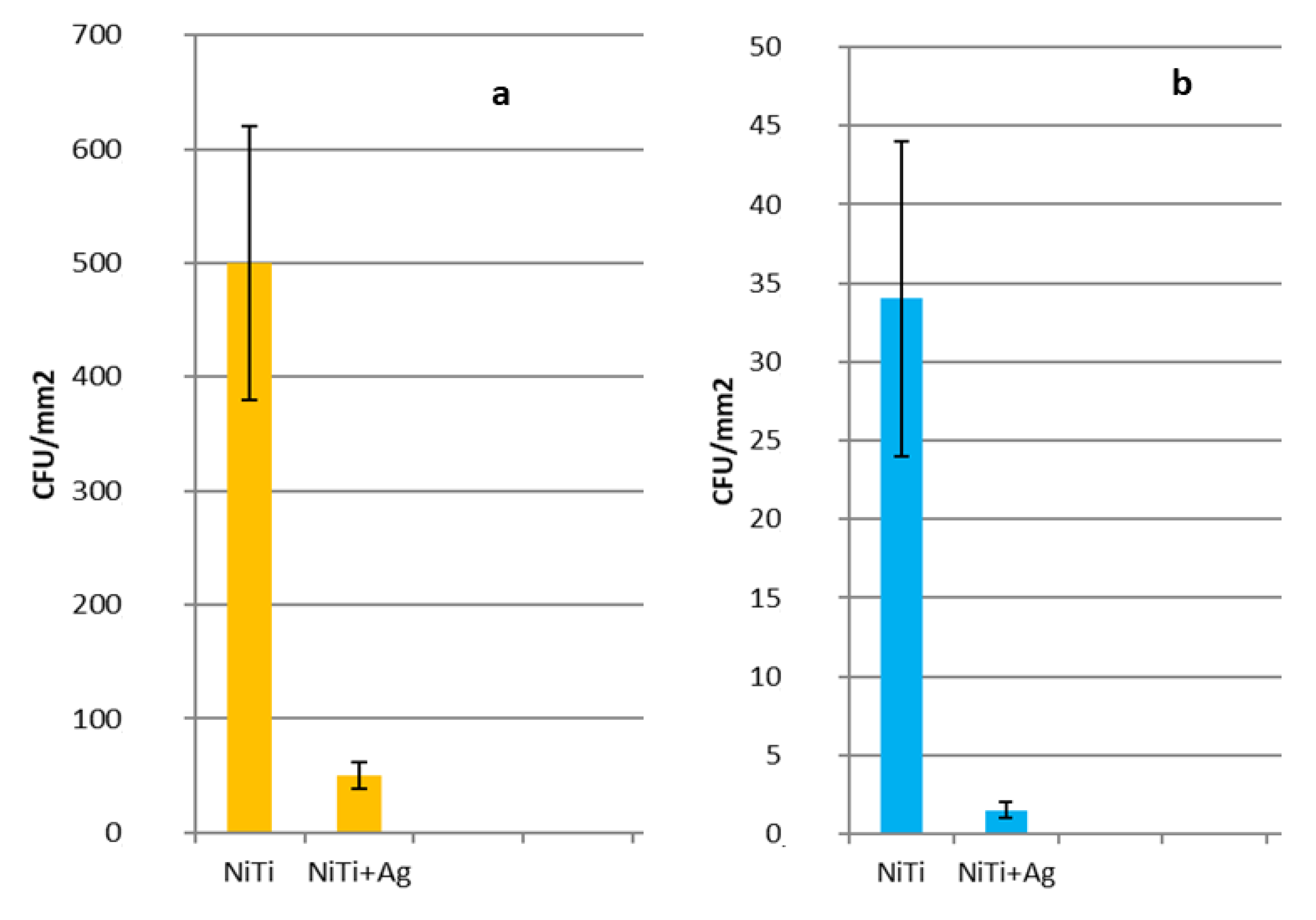
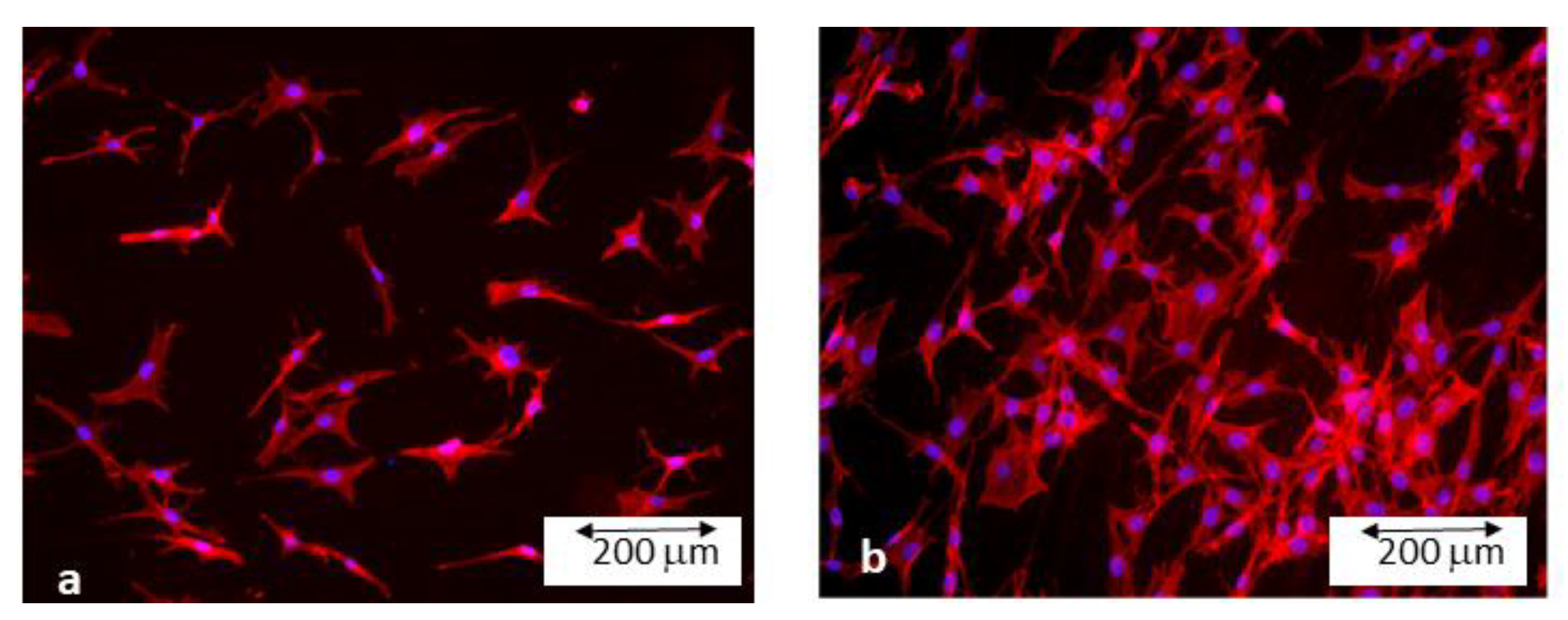
2.6. Bacteria Cultures
2.7. Statistical Analysis
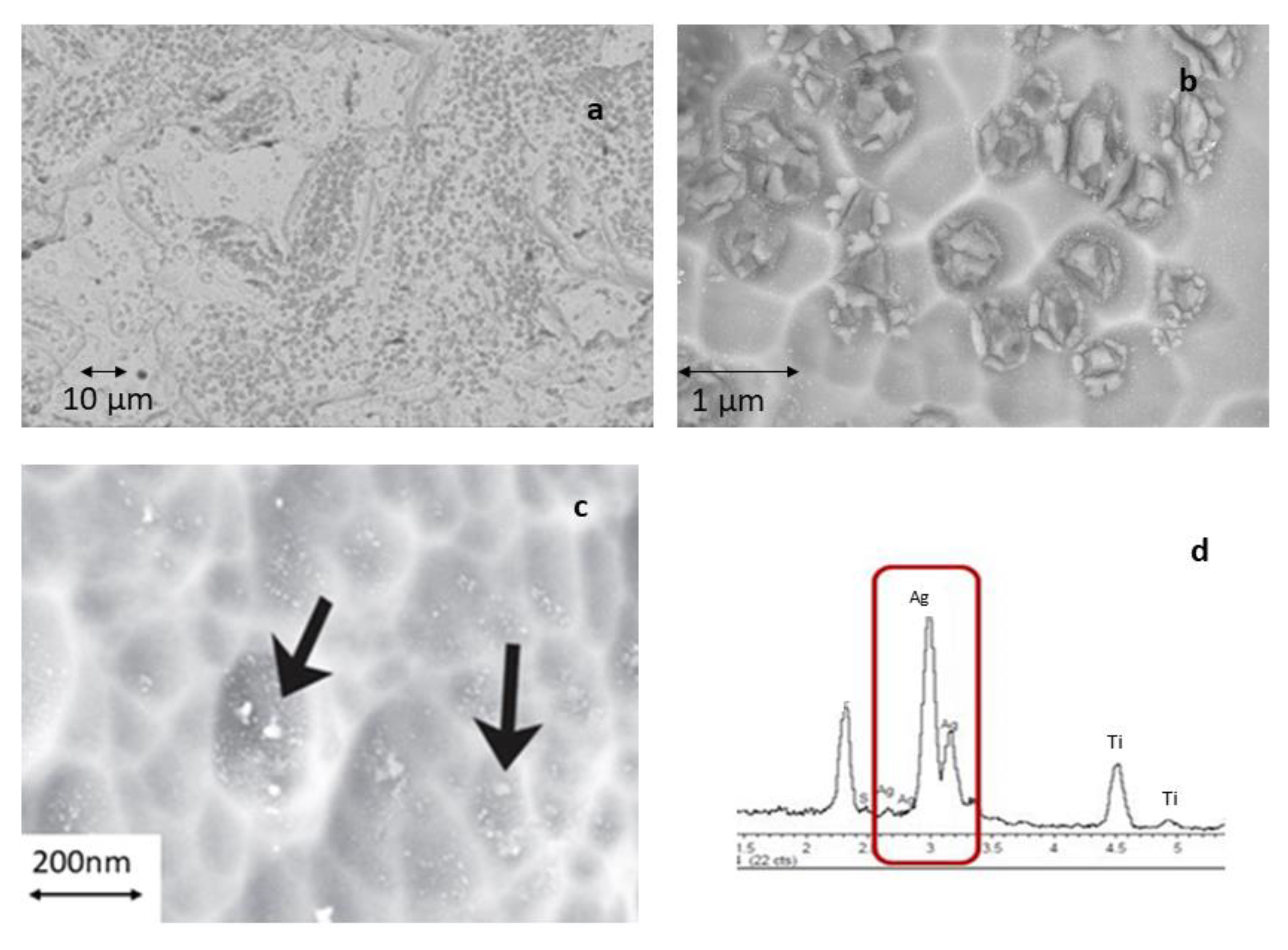
3. Experimental Results and Discussion
4. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Miura, F.; Mogi, M.; Ohura, Y.; Hamanaka, H. The super-elastic property of the Japanese NiTi alloy wire for use in orthodontics. Am. J. Orthod. Dentofac. Orthop. 1986, 90, 1–10. [Google Scholar] [CrossRef]
- Haarsters, J.; Salis-Solio, G.; Bensmann, T. The use of NiTi as a implant material in orthopedics. In Shape Memory in Engineering Aspects of Shape Memory Alloys; Duering, T.W., Melton, K.N., Stöckel, D., Wayman, C.M., Eds.; Butterworth-Heinemann Ed.: London, UK, 1990. [Google Scholar]
- Andreasen, G.F.; Morrow, R.E. Laboratory and clinical analysis of Nitinol wire. Am. J. Orthod. 1978, 73, 142–145. [Google Scholar] [CrossRef]
- Sevilla, P.; Martorell, F.; Libenson, C.; Planell, J.A.; Gil, F.J. Laser welding of NiTi orthodontic archwires for selective force application. J. Mater. Sci. Mater. Med. 2008, 19, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Krousbroeck, R.; Van der Perre, G.; Aernoudt, E.; Mulier, J.C. Shape memory effect in biomedical devices. In Advances in Biomaterials; Winter, G.D., Gibbons, D.F., Plenk, H., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 1982. [Google Scholar]
- Arciniegas, M.; Casals, J.; Manero, J.M.; Peña, J.; Gil, F.J. Study of hardness and wear behaviour of NiTi shape memory alloys. J. Alloys Comp. 2008, 460, 213–219. [Google Scholar] [CrossRef]
- Gil, F.J.; Cenizo, M.; Espinar, E.; Rodriguez, A.; Rúperez, E.; Manero, J.M. NiTi superelastic orthodontic wires with variable stress obtained by ageing treatments. Mater. Lett. 2013, 104, 5–7. [Google Scholar] [CrossRef]
- Gil, F.J.; Solano, E.; Peña, J.; Engel, E.; Mendoza, A.; PLanell, J.A. Microstructural. Mechanical and citotoxicity evaluation of different NiTi and NiTiCu shape memory alloys. J. Mater. Sci Mater. Med. 2004, 15, 1181–1185. [Google Scholar] [CrossRef]
- Khouw, F.E.; Goldhaber, P. Changes in vasculature of the periodontium associated with tooth movement in the rhesus monkey and dog. Arch. Oral. Biol. 1970, 15, 1125–1132. [Google Scholar] [CrossRef]
- Nakano, T.; Hotokezaka, H.; Hashimoto, M. Effects of different types of tooth movement and force magnitudes on the amount of tooth movement and root resorption in rats. Angle Orthod 2014, 84, 1079–1085. [Google Scholar] [CrossRef]
- Lang, N.P.; Mombelli, A.; Attström, R. Oral Biofilms and Calculus. In Clinical Periodontology and Implant Dentistry, 5th ed.; Lindhe, J., Lang, N.P., Karring, T., Eds.; Wiley-Blackwell Ed.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Godoy-Gallardo, M.; Rodríguez-Hernández, A.G.; Delgado, L.M.; Manero, J.M.; Javier Gil, F.; Rodríguez, D. Silver deposition on titanium surface by electrochemical anodizing process reduces bacterial adhesion of Streptococcus sanguinis and Lactobacillus salivarius. Clin. Oral. Implants Res. 2014, 26, 1170–1179. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Manzanares-Cespedes, M.C.; Sevilla, P.; Nart, J.; Manzanares, N.; Manero, J.M.; Gil, F.J.; Boyd, S.K.; Rodriguez, D. Evaluation of bone los in antibacterial coated dental implants: An experimental study in dogs. Mater. Sci. Eng. C 2016, 69, 538–545. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Guillem-Marti, J.; Sevilla, P.; Manero, J.M.; Gil, F.J.; Rodriguez, D. Anhydride-functional silane immobilized onto titanium surfaces induces osteoblast cell differentiation and reduces bacterial adhesion and biofilm formation. Mater. Sci. Eng. C 2016, 1, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chu, P.K.; Zhang, Y.; Wu, Z. Antibacterial coatings on titanium implants. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Suárez, C.; Vilar, T.; Gil, F.J.; Sevilla, P. In vitro evaluation of surface topographic changes and nickel release of lingual orthodontic archwires. J. Mater Sci. Mater Med. 2010, 21, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Saburi, T.; Tatsumi, T.; Nenno, S. Effects of heat treatment on mechanical behaviour of TiNi alloys. J. Phys. Colloq. C4 1982, 12, 261–266. [Google Scholar]
- Barrabés, M.; Michiardi, A.; Aparicio, C.; Sevilla, P.; Planell, J.A.; Gil, F. Oxidized nickel-titanium foams for bone reconstructions: Chemical and mechanical characterization. J. Mater. Sci. Mater. Med. 2007, 18, 2123–2129. [Google Scholar] [CrossRef]
- Preetha, A.; Barnejee, R. Comparison of artificial saliva substitutes. Trends Biomater. Artif. Organs 2005, 18, 178–186. [Google Scholar]
- Messer, L.M.; Lucas, L.C. Cytotoxicity of nickel-chromium alloys:bulk alloys compared to multiple ion salts solutions. Dent. Mater. 2000, 16, 207–212. [Google Scholar] [CrossRef]
- Park, H.Y.; Shearer, T.R. In vitro release of nickel and chromium from simulated orthodontic appliances. Am. J. Orthod. 1983, 84, 156–159. [Google Scholar] [CrossRef]
- Sinha, M.; Kaushik, S.; Kaur, P.; Sharma, S.; Singh, T.P. Antimicrobial lactoferrin peptides: The hidden players in the protective function of a multifunctional protein. Int. J. Pept. 2013, 13, 1–13. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Mas-Moruno, C.; Fernández-Calderón, M.C.; Pérez-Giraldo, C.; Manero, J.M.; Albericio, F.; Gil, F.J.; Rodríguez, D. Covalent immobilization of hLf1-11 peptide on a titanium surface reduces bacterial adhesion and biofilm formation. Acta Biomater. 2014, 10, 3522–3534. [Google Scholar] [CrossRef]
- Huo, L.; Zhang, K.; Ling, J.; Peng, Z.; Huang, X.; Liu, H.; Gu, L. Antimicrobial and DNA-binding activities of the peptide fragments of human lactoferrin and histatin 5 against Streptococcus mutans. Arch. Oral Biol. 2011, 56, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Gallardo, M.; Mas-Moruno, C.; Yu, K.; Manero, J.M.; Gil, F.J.; Kizhakkedathu, J.N.; Rodriguez, D. Antibacterial Properties of hLf1–11 Peptide onto Titanium Surfaces: A Comparison Study Between Silanization and Surface Initiated Polymerization. Biomacromolecules 2015, 16, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Gallardo, M.; Wang, Z.; Shen, Y.; Manero, J.M.; Gil, F.J.; Rodriguez, D.; Haapasalo, M. Antibacterial Coatings on Titanium Surfaces: A Comparison Study Between in Vitro in Single-Species and Multispecies Biofilm. ACS Appl. Mater. Interfaces 2015, 7, 5992–6001. [Google Scholar] [CrossRef] [PubMed]
- Valenti, L.E.; Giacomelli, C.E. Stability of silver nanoparticles: Agglomeration and oxidation in biological relevant conditions. J. Nanopart. Res. 2017, 19, 156. [Google Scholar] [CrossRef]
- Bastiaan, M.; Hiemstra, T. Surface Structure of Silver Nanoparticles as a Model for Understanding the Oxidative Dissolution of Silver Ions. Langmuir 2015, 31, 13361–13372. [Google Scholar]
- Ching-Ming, H.; Yau, S.K.W.; Lok, C.N.; So, M.H.; Che, C.M. Oxidative Dissolution of Silver Nanoparticles by Biologically Relevant Oxidants: A Kinetic and Mechanistic Study. Chem. Asian J. 2010, 2, 2016. [Google Scholar]
- Setcos, J.C.; Babaei-Mahamani, A.; Di Silvio, L.; Mijör, M.A.; Wilson, N.H.F. The safety of nickel containing dental alloys. Dent Mater. 2006, 22, 1163–1168. [Google Scholar] [CrossRef]
- Wiltshire, W.A.; Noble, J. Allergies to dental materials. Vital Autumn 2007, 27, 39. [Google Scholar]
- Huang, H.H. Variation in corrosion resistance of nickel-titanium wires from different manufacturers. Angle Orthod 2005, 75, 661–665. [Google Scholar]
- Huang, H.H.; Chiu, Y.H.; Lee, T.H.; Wu, S.C.; Yang, H.W.; Su, K.H. Ion release from Ni-Ti orthodontic wires in artificial saliva with various acidities. Biomaterials 2003, 20, 3585–3592. [Google Scholar] [CrossRef]
- Wang, J.; Li, N.; Rao, G.; Han, E.H.; Ke, W. Stress corrosion cracking of Ni-Ti in artificial saliva. Dent Mater. 2007, 23, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Michiardi, A.; Aparicio, C.; Planell, J.A.; Gil, F.J. New oxidation treatment of NiTi shape memory alloys to obtain Ni-free surfaces and to improve biocompatibility. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 77B, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Schultz, J.C.; Connelly, E.; Glesene, L.; Warshaw, E.M. Cutaneous and oral eruption from oral exposure to nickel dental braces. Dermatitis 2004, 15, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [PubMed]
- Khalandi, B.; Asadi, N.; Milani, M.; Davaran, S.; Jafari, A.; Abadi, N.; Abasi, E.; Akbarzadeh, A. A Review on Potential Role of Silver Nanoparticles and Possible Mechanisms of their Actions on Bacteria. Drug Res. 2017, 67, 70–76. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The Bactericidal Effect of Silver Nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Kaur, A.; Kumar, R. Enhanced bactericidal efficacy of polymer stabilized silver nanoparticles in conjugation with different classes of antibiotics. RSC Adv. 2019. [Google Scholar] [CrossRef]
- Lara, H.H.; Garza-Treviño, E.N.; Ixtepan-Turrent, L.; Singh, D.K. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J. Nanobiotechnol. 2011. [Google Scholar] [CrossRef]
- Shatan, A.B.; Venclíková, K.; Zasońska, B.A.; Patsula, V.; Pop-Georgievski, O.; Petrovský, E.; Horák, D. Antibacterial Silver-Conjugated Magnetic Nanoparticles: Design, Synthesis and Bactericidal Effect. Pharm. Res. 2019, 36, 147. [Google Scholar] [CrossRef]
- Tian, X.; Jiang, X.; Welch, C.; Croley, T.R.; Wong, T.Y.; Chen, C.; Fan, S.; Chong, Y.; Li, R.; Ge, C.; et al. Bactericidal Effects of Silver Nanoparticles on Lactobacilli and the Underlying Mechanism. ACS Appl. Mater. Interfaces 2018, 10, 8443–8450. [Google Scholar] [CrossRef]
- Rangasamy, S.; Purushothaman, B.; Song, J.M. The Application of Bactericidal Silver Nanoparticles in Wound Treatment. Rev. Nanomater. Nanotechnol. 2015. [Google Scholar] [CrossRef]
- Mhaske, A.R.; Shetty, P.C.; Bhat, N.S.; Ramachandra, C.S.; Laxmikanth, S.M.; Nagarahalli, K.; Tekale, P.D. Antiadherent and antibacterial properties of stainless steel and NiTi orthodontic wires coated with silver against Lactobacillus acidophilus—An in vitro study. Prog. Orthod. 2015, 16, 40. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Schluesener, H.J. Nanosilver: A nanoproduct in medical application. Toxicol. Appl. Pharmacol. Lett. 2008, 176, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Namasivayam, S.K.; Ganesh, S.; Avimanyu, B. Evaluation of anti-bacterial activity of silver nanoparticles synthesized from Candida glabrata and Fusarium oxysporum. Int. J. Med. Res. 2011, 1, 131–136. [Google Scholar]
- El-Kheshen, A.A.; El-Rab, S.F.G. Effect of reducing and protecting agents on size of silver nanoparticles and their anti-bacterial activity. Schol. Res. Librar. 2012, 4, 53–65. [Google Scholar]
- Graves, J.L., Jr.; Tajkarimi, M.; Cunningham, Q.; Campbell, A.; Nonga, H.; Harrison, S.H. Rapid evolution of silver nanoparticle resistance in Escherichia coli. Front. Genet. 2015, 6, 42. [Google Scholar] [CrossRef]
- Panpaliya, N.P.; Prasanna, T.D.; Yogesh, J.K.; Mahesh, V.D.; Shrikant, B.K.; Ayesha, G.S.; Ulka, R.M. In vitro evaluation of antimicrobial property of silver nanoparticles and chlorhexidine against five different oral pathogenic bacteria. S Dent J. 2019, 31, 76–83. [Google Scholar] [CrossRef]
- Besinis, A.; Hadi, S.D.; Le, H.R.; Tredwin, C.; Handy, R.D. Antibacterial activity and biofilm inhibition by surface modified titanium alloy medical implants following application of silver, titanium dioxide and hydroxyapatite nanocoatings. Nanotoxicology 2017, 11, 327–338. [Google Scholar] [CrossRef]
- Besinis, A.; De Peralta, T.; Handy, R.D. Inhibition of biofilm formation and antibacterial properties of a silver nano-coating on human dentine. Nanotoxicology 2014, 8, 745–754. [Google Scholar] [CrossRef]
- Hernández-Gómora, A.; Lara-Carrillo, E.; Robles-Navarro, J. Biosynthesis of silver nanoparticles on orthodontic elastomeric modules: Evaluation of mechanical and antibacterial properties. Molecules 2017, 22, 1407. [Google Scholar] [CrossRef]
- Yin, I.X.; Yu, O.Y.; Zhao, I.S. Developing biocompatible silver nanoparticles using epigallocatechin gallate for dental use. Arch. Oral Biol. 2019, 102, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Cristóbal, L.F.; Holguín-Meráz, C.; Zaragoza-Contreras, E.A.; Martínez-Martínez, R.E.; Donohue-Cornejo, A.; Loyola-Rodríguez, J.P.; Cuevas-González, J.C.; Reyes-López, S. Antimicrobial and Substantivity Properties of Silver Nanoparticles against Oral Microbiomes Clinically Isolated from Young and Young-Adult Patients. J. Nanomat. 2019, 2019, 14. [Google Scholar] [CrossRef]

| Chemical Product | Composition (g/dm3) |
|---|---|
| K2HPO4 | 0.22 |
| KCI | 1.19 |
| KSCN | 0.29 |
| Na2HPO4 | 0.26 |
| NaCl | 0.69 |
| NaHCO3 | 1.49 |
| Urea | 1.49 |
| Lactic acid | up to pH = 6.8 |
| Alloy | Ms | Mf | As | Af |
|---|---|---|---|---|
| NiTi | 26.2 ± 0.3 | 16.3 ± 0.4 | 20.4 ± 0.5 | 32.4 ± 1.7 |
| NiTi + Ag | 26.5 ± 2.4 | 16.9 ± 1.2 | 19.4 ± 1.7 | 33.2 ± 4.5 |
| Archwire | σβ→SIM (MPa) | σSIM→β (MPa) | ||
|---|---|---|---|---|
| 20 °C | 37 °C | 20 °C | 37 °C | |
| NiTi | 230 ± 15 | 321 ± 20 | 58 ± 10 | 212 ± 15 |
| NiTi + Ag | 237 ± 12 | 333 ± 22 | 65 ± 32 | 216 ± 10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil, F.J.; Espinar-Escalona, E.; Clusellas, N.; Fernandez-Bozal, J.; Artes-Ribas, M.; Puigdollers, A. New Bactericide Orthodonthic Archwire: NiTi with Silver Nanoparticles. Metals 2020, 10, 702. https://doi.org/10.3390/met10060702
Gil FJ, Espinar-Escalona E, Clusellas N, Fernandez-Bozal J, Artes-Ribas M, Puigdollers A. New Bactericide Orthodonthic Archwire: NiTi with Silver Nanoparticles. Metals. 2020; 10(6):702. https://doi.org/10.3390/met10060702
Chicago/Turabian StyleGil, F. Javier, Eduardo Espinar-Escalona, Nuria Clusellas, Javier Fernandez-Bozal, Montserrat Artes-Ribas, and Andreu Puigdollers. 2020. "New Bactericide Orthodonthic Archwire: NiTi with Silver Nanoparticles" Metals 10, no. 6: 702. https://doi.org/10.3390/met10060702
APA StyleGil, F. J., Espinar-Escalona, E., Clusellas, N., Fernandez-Bozal, J., Artes-Ribas, M., & Puigdollers, A. (2020). New Bactericide Orthodonthic Archwire: NiTi with Silver Nanoparticles. Metals, 10(6), 702. https://doi.org/10.3390/met10060702






