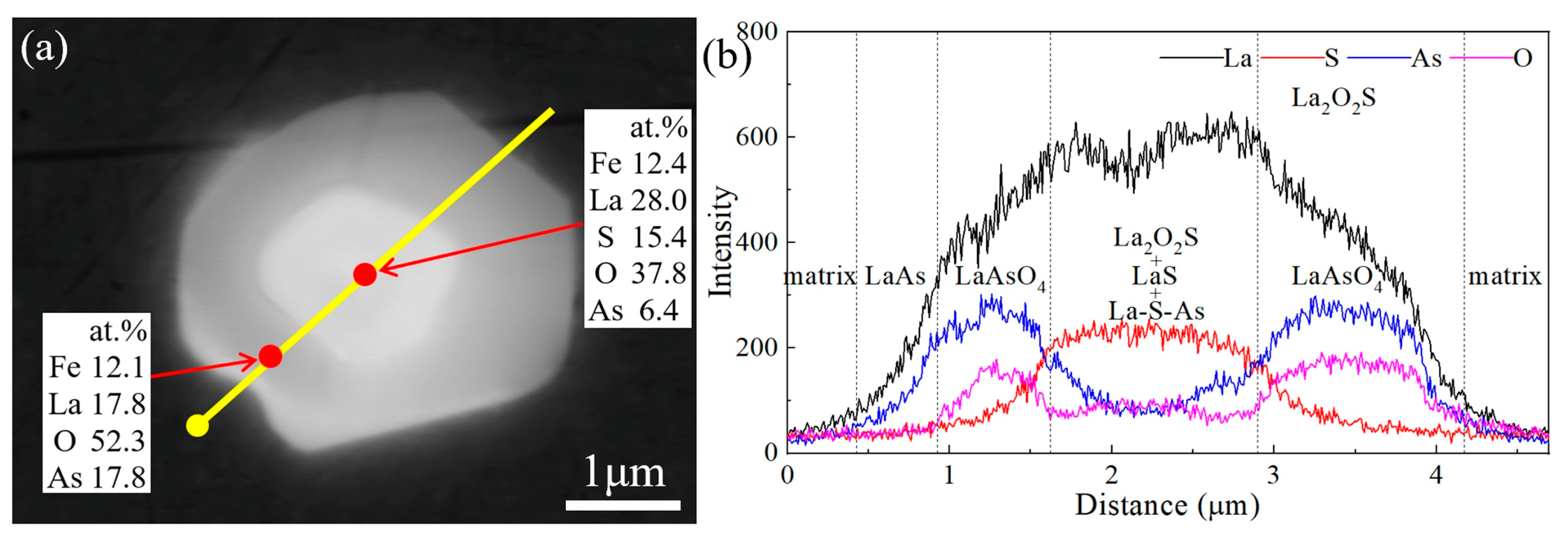
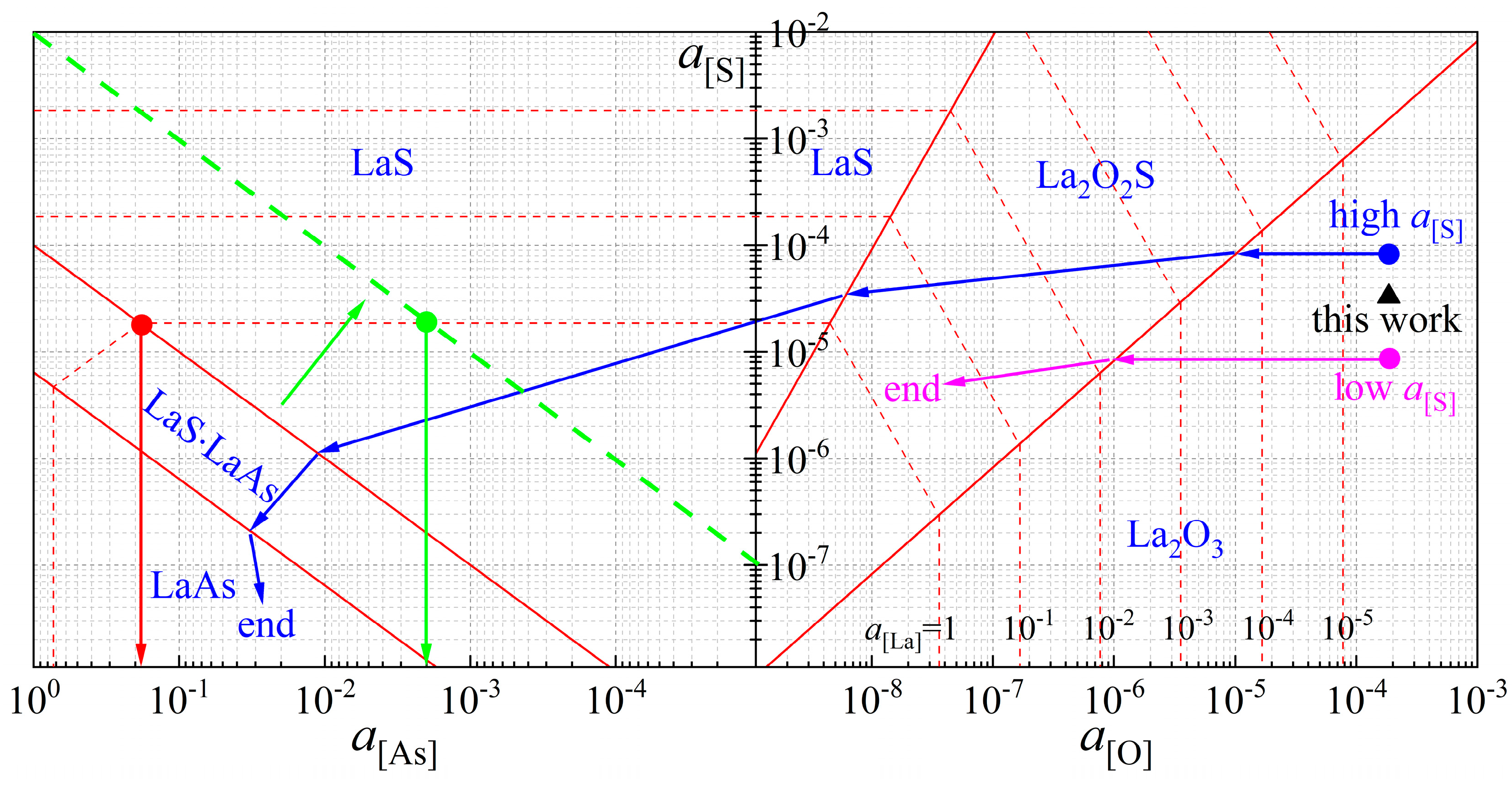
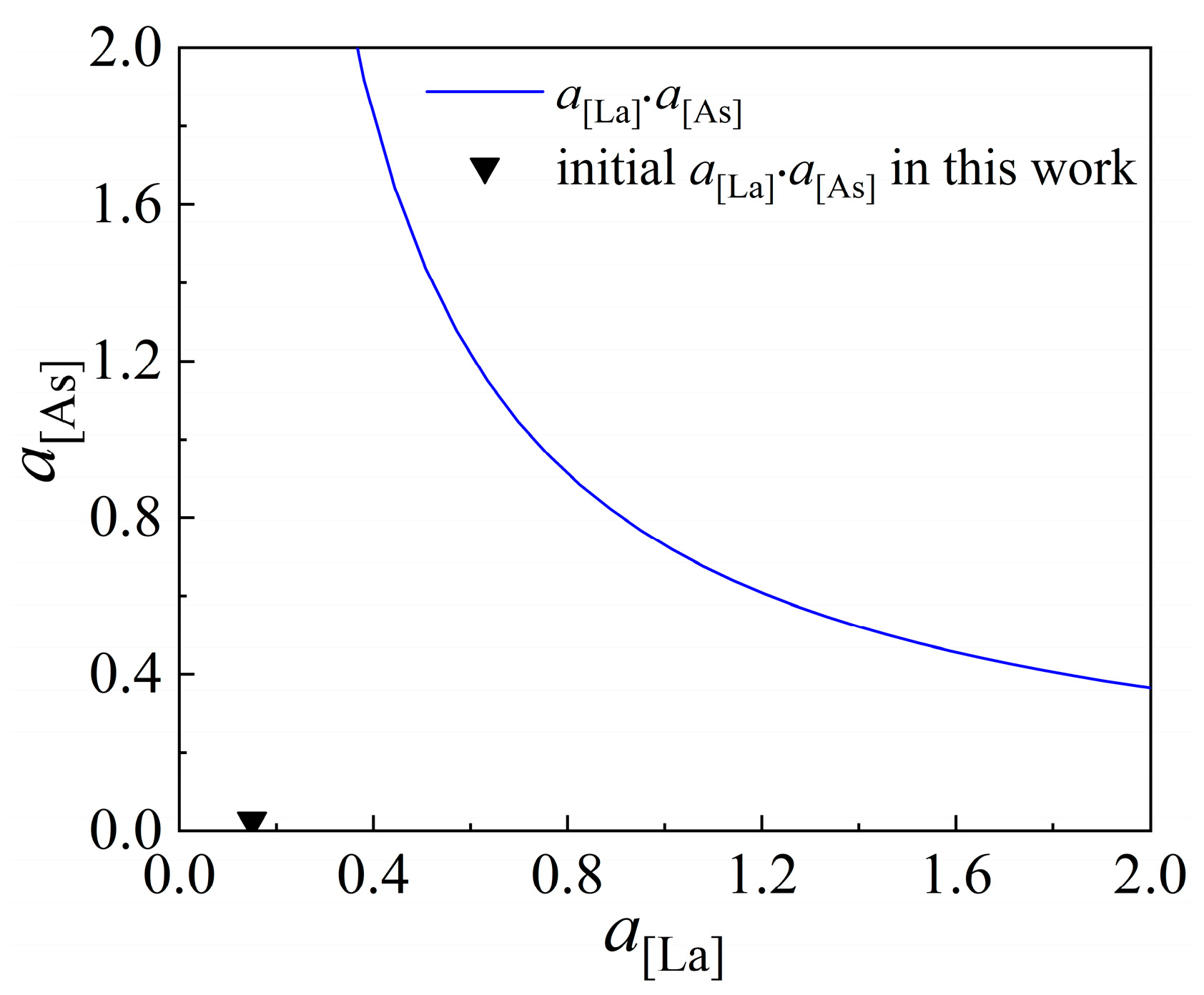
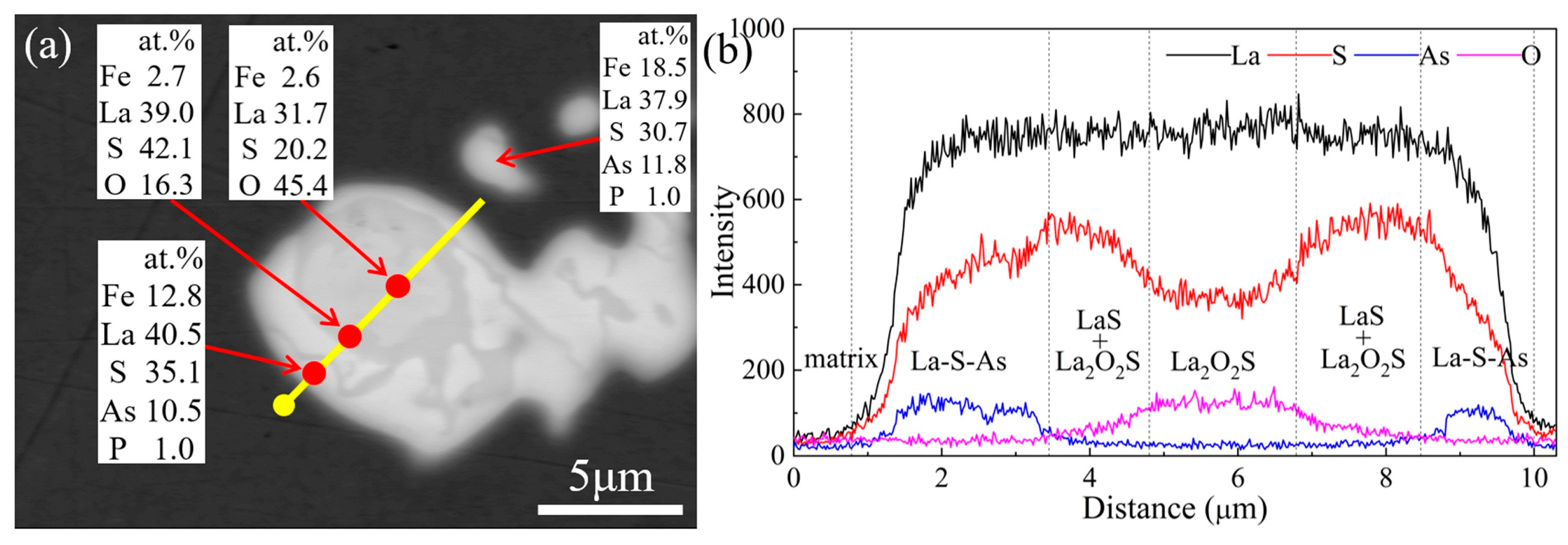
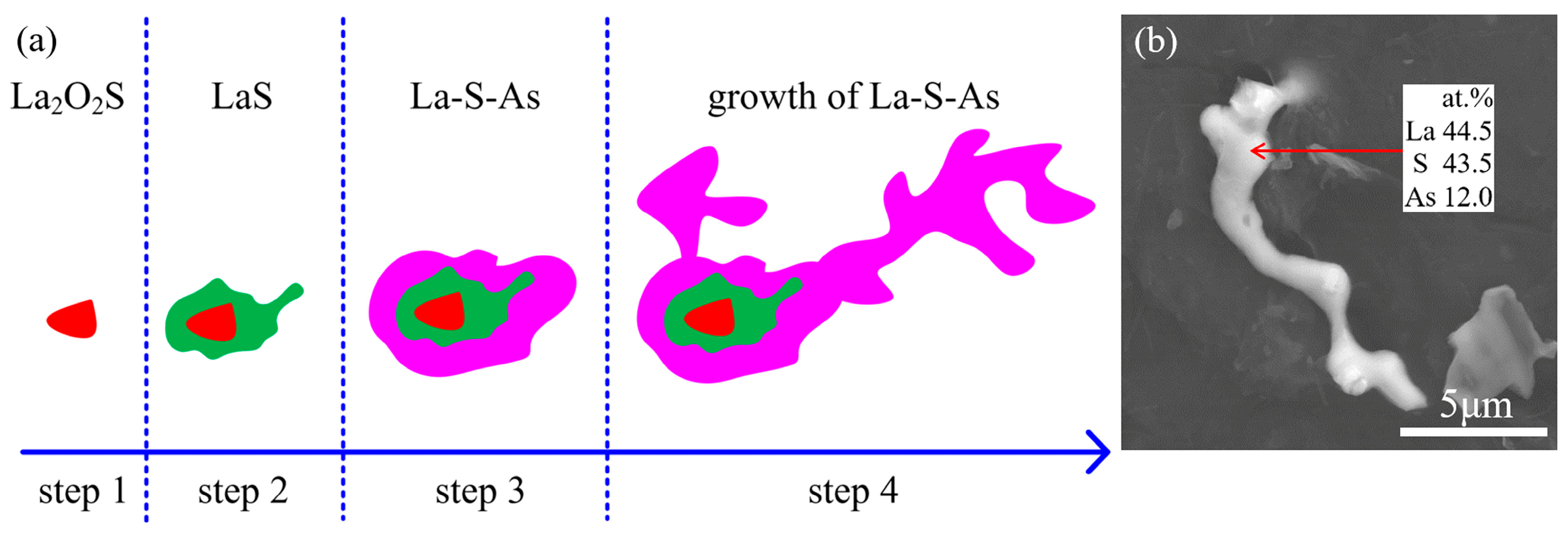
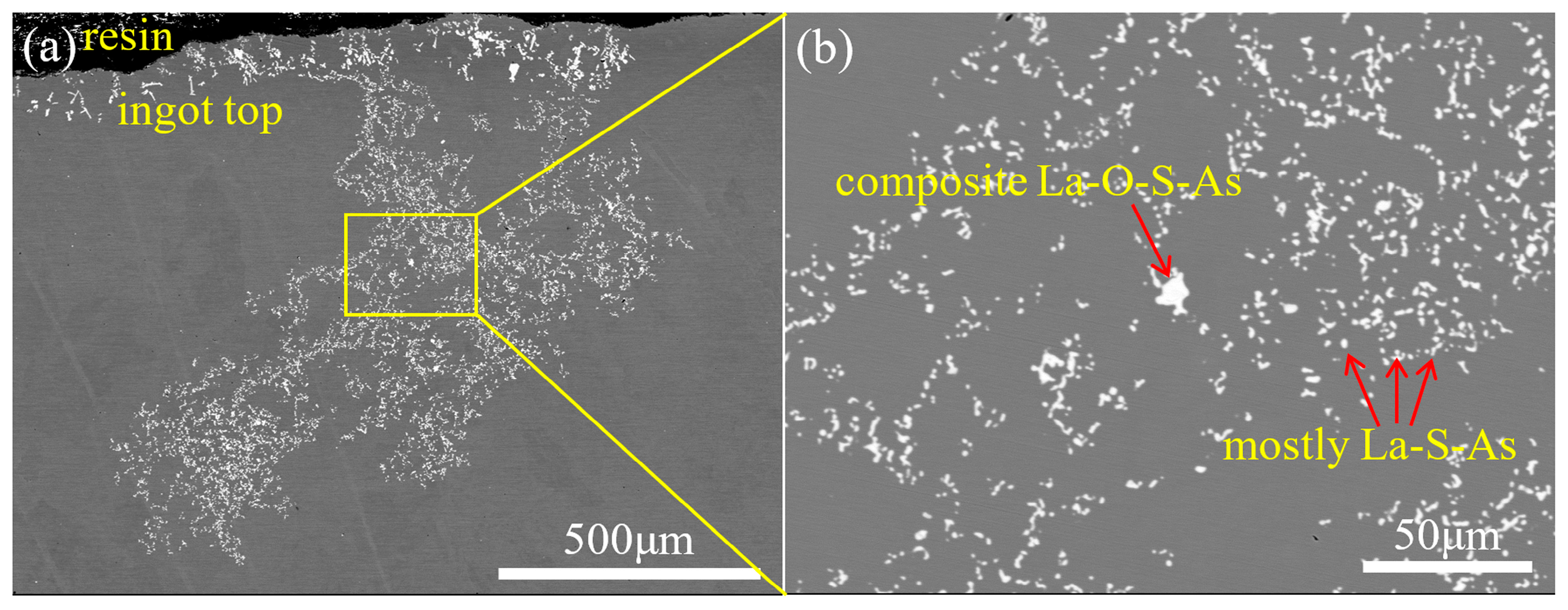
1. Introduction
The residual element arsenic is an essential factor that harms the quality and performance of steel products. The oxidation potential of arsenic is weaker than that of iron, and it is challenging to remove arsenic from steel in the steelmaking process. Arsenic prefers to enrich and form low melting point phases through solidification segregation and interface segregation [
1,
2,
3,
4], resulting in a decrease of hot workability and processability of steel [
5]. In the 1990s, the average concentration of arsenic in 48 steel grades of five special steel mills in China reached 100 ppm, according to the statistical data derived from 303 heats of alloy steel [
6]. The recycling of scrap steel makes arsenic continuously enriched in steel, and the increasing storage and utilization ratio of scrap steel exacerbate its enrichment. It is expected that the comprehensive utilization ratio of scrap steel will reach 80% by 2050 in China [
7]. Having a massive amount of arsenic-bearing iron ore, China is under high pressure to deal with the problem caused by the enrichment of arsenic.
Steel companies generally adopt the method of mixing high-quality iron ore or molten iron with arsenic-containing raw materials to reduce arsenic concentration in steel products, which is unsustainable [
8]. Therefore, researchers conducted arsenic removal experiments in the sintering, molten iron pretreatment, and refining processes. Sintering reduction [
9], coal-based direct reduction [
10], and microwave heating reduction [
11] have been tried to remove arsenic from iron concentrates in the laboratory. Based on the reduction theory, using calcium to remove arsenic from molten steel is the focus of researchers. Pure calcium metal, various calcium alloys, combined with CaF
2 slags, have been tried to remove arsenic from molten iron or steel [
12,
13,
14,
15,
16,
17]. However, the volatilization of calcium is severe owing to its high vapor pressure, making the effective calcium amount used for arsenic removal difficult to control; moreover, the residual calcium in steel can give rise to the formation of large spherical oxides, detrimental to the properties of steel products.
Rare earth (RE) elements have been used to react with arsenic to form high melting point compounds to remove arsenic from molten steel and to improve the existing state of arsenic in solid steel when arsenic concentration is not high [
2,
3,
5,
18]. It is noted that the arsenic compounds are not the first ones to generate in molten steel after RE additions; instead, the first ones are oxides, oxysulfides, and sulfides of RE [
19,
20,
21,
22]. RE does not react with arsenic until the concentrations of oxygen and sulfur decrease to a certain level [
23], then RE-S-As can generate in molten steel [
24,
25]; further, a series of arsenic compounds, namely RE-As [
3,
18,
26,
27], RE-O-As [
28], RE-S-As [
29], and RE-O-S-As [
28], can generate during the solidification and cooling processes. The thermodynamics and kinetics of the formation of arsenic RE compounds are fundamental issues in the study of using RE to remove and stabilize arsenic in steel.
This work focuses on the formation mechanism of arsenic RE compounds in molten steel. Combined with calculations and laboratory experiments, the effect of heterogeneous nucleation on the removal of arsenic from molten steel by adding RE element lanthanum was investigated.
Author Contributions
Conceptualization and methodology, H.W. and Y.W.; investigation and validation, H.W., P.Y., and S.J.; writing—review and editing, H.W.; funding acquisition, H.W. and Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 51704051.
Acknowledgments
Many thanks to Chao Deng, from Electron Microscopy Center of Chongqing University, for his great help in the observation with SEM. We also thank Yang Zhou, from the Analytical and Testing Center of Chongqing University, for her great help in the identification of inclusions by EPMA.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xin, W.; Song, B.; Huang, C.; Song, M.; Song, G. Effect of arsenic content and quenching temperature on solidification microstructure and arsenic distribution in iron-arsenic alloys. Int. J. Miner. Metall. Mater. 2015, 22, 704–713. [Google Scholar] [CrossRef]
- Xin, W.; Zhang, J.; Jiang, Y.; Deng, Y.; Meng, Q.; Song, B. Effect of rare earth Ce on the isothermal oxidation behavior in air of arsenic bearing steels. Metall. Res. Technol. 2019, 116, 415. [Google Scholar] [CrossRef]
- Xin, W.; Song, B.; Song, M.; Song, G. Effect of cerium on characteristic of inclusions and grain boundary segregation of arsenic in iron melts. Steel Res. Int. 2015, 86, 1430–1438. [Google Scholar] [CrossRef]
- Wang, H.; Bai, B.; Jiang, S.; Sun, L.; Wang, Y. An in situ study of the formation of rare earth inclusions in arsenic high carbon steels. ISIJ Int. 2019, 59, 1259–1265. [Google Scholar] [CrossRef]
- Xin, W.; Zhang, J.; Luo, G.; Wang, R.; Meng, Q.; Song, B. Improvement of hot ductility of C-Mn steel containing arsenic by rare earth Ce. Metall. Res. Technol. 2018, 115, 419. [Google Scholar] [CrossRef]
- Zhu, D.; Fang, J. Investigation on the current status of harmful elements in high-quality alloy steel. In The Influence and Control of Impurity Elements Tin, Arsenic, Antimony, Lead and Bismuth on the Quality of Alloy Structural Steel; Central Iron & Steel Research Institute: Beijing, China, 1994; pp. 12–21. [Google Scholar]
- Pauliuk, S.; Wang, T.; Müller, D.B. Moving toward the circular economy: The role of stocks in the Chinese steel cycle. Environ. Sci. Technol. 2011, 46, 148–154. [Google Scholar] [CrossRef]
- Björkman, B.; Samuelsson, C. Recycling of steel. In Handbook of Recycling; Elsevier: Oxford, UK, 2014; pp. 65–83. [Google Scholar]
- Lv, Q.; Zhang, S.; Hu, X. Experimental study on removal arsenic in iron ore with arsenic sintering process. Iron Steel 2010, 45, 7–11. [Google Scholar]
- Jiang, M.; Sun, T.; Qin, X.; Ji, Y.; Wang, Z. Study on removing arsenic and tin from As-Sn bearing iron concentrates by direct reduction roasting of coal. Min. Metall. Eng. 2011, 31, 86–89. [Google Scholar]
- Wang, L.; Haichuan, W.; Xu, F.; Chen, P. Experimental study of microwave treating high arsenic ore. Metall. Eng. 2015, 2, 29–35. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tokumitsu, N.; Harashima, K.; Segawa, K. Refining of 18% Cr-8% Ni steel with Ca-CaF2 solution. Trans. ISIJ 1976, 16, 623–627. [Google Scholar] [CrossRef]
- Kitamura, K.; Takenouchi, T.; Iwanami, Y. Removal of impurities from molten steel by CaC2. Tetsu–to–Hagané 1985, 71, 220–227. [Google Scholar] [CrossRef][Green Version]
- Li, W.; Bao, Y.; Wang, M.; Lin, L. Experimental study on dearsenization of molten steel with different Ca alloy. Chin. J. Eng. 2016, 38, 484–493. [Google Scholar]
- Fu, B.; Xue, Z.; Wu, G.; Wu, L.; Wu, Y.; Ke, C. Experimental study on the dearsenization of hot metal with CaC2–CaF2 slag. Chin. J. Proc. Eng. 2010, 10, 146–149. [Google Scholar]
- Dong, Y.; Shi, Z.; Zhang, L.; Peng, Y.; Hong, Y. Study on dearsenization of molten iron. Iron Steel 1984, 19, 1–7. [Google Scholar]
- Chang, L.; Shi, X.; Wang, J.; Zhou, L.; Luo, L. Research on dearsenication by reduction process in hot metal. Iron Steel Vanadium Titan. 2014, 35, 113–117. [Google Scholar]
- Huang, C.; Song, B.; Xin, W.; Jia, S.; Yang, Y. Influence of rare earth La on hot ductility of low carbon steel containing As and Sn. Heat Treat. Met. 2015, 40, 1–6. [Google Scholar]
- Vahed, A.; Kay, D.A.R. Thermodynamics of rare earths in steelmaking. Metall. Trans. B 1976, 7, 375–383. [Google Scholar] [CrossRef]
- Lu, W.K.; McLean, A. Thermodynamic behaviour of rare-earth elements in molten steel. Ironmak. Steelmak. 1974, 1, 228–233. [Google Scholar]
- Han, Q.; Dong, Y.; Feng, X.; Xiang, C.; Yang, S. Equilibria between rare earth elements and sulfur in molten iron. Metall. Mater. Trans. B 1985, 16, 785–792. [Google Scholar] [CrossRef]
- Katsumata, A.; Todoroki, H. Effect of rare earth metal on inclusion composition in molten stainless steel. Iron Steelmak. 2002, 29, 51–57. [Google Scholar]
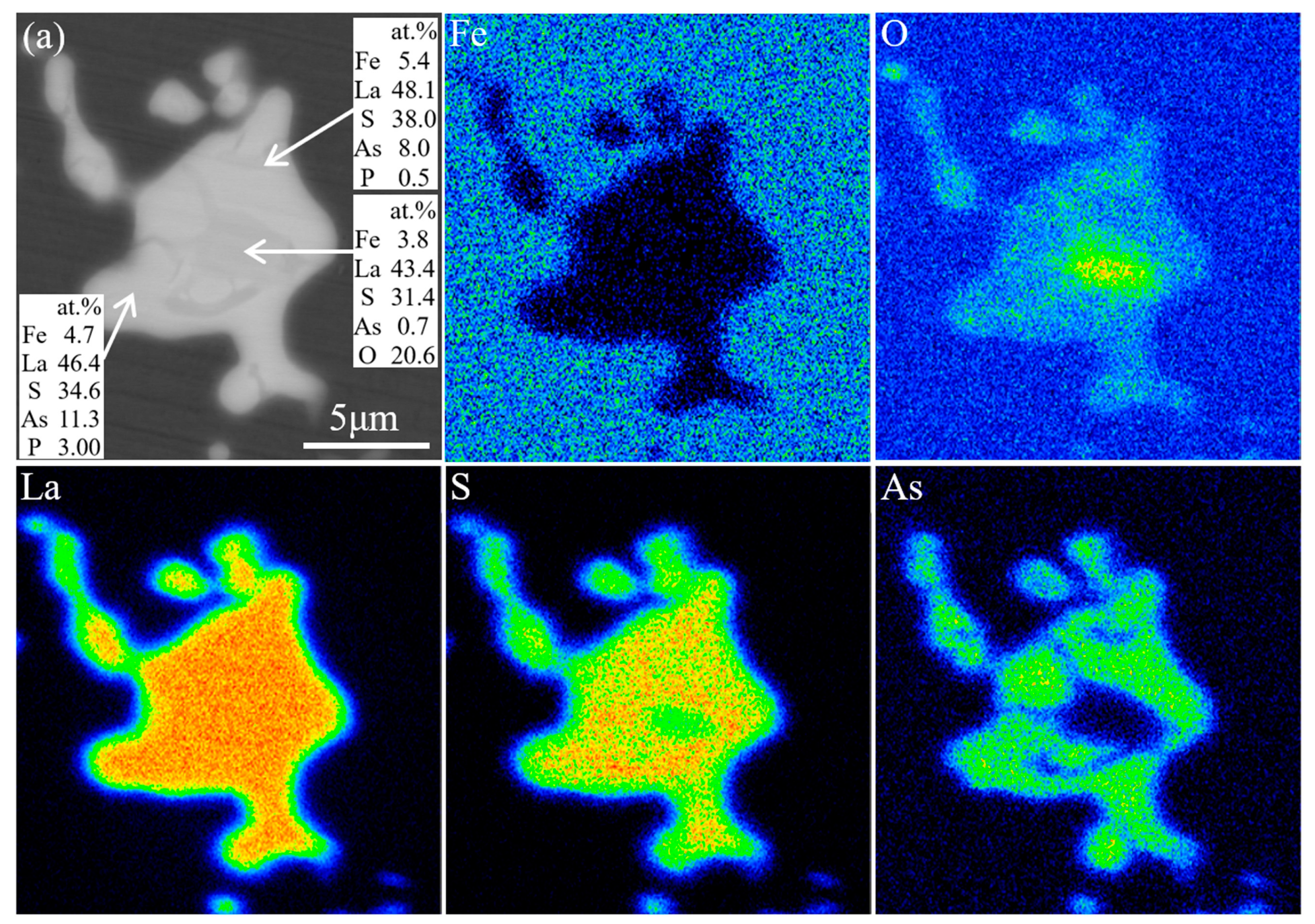
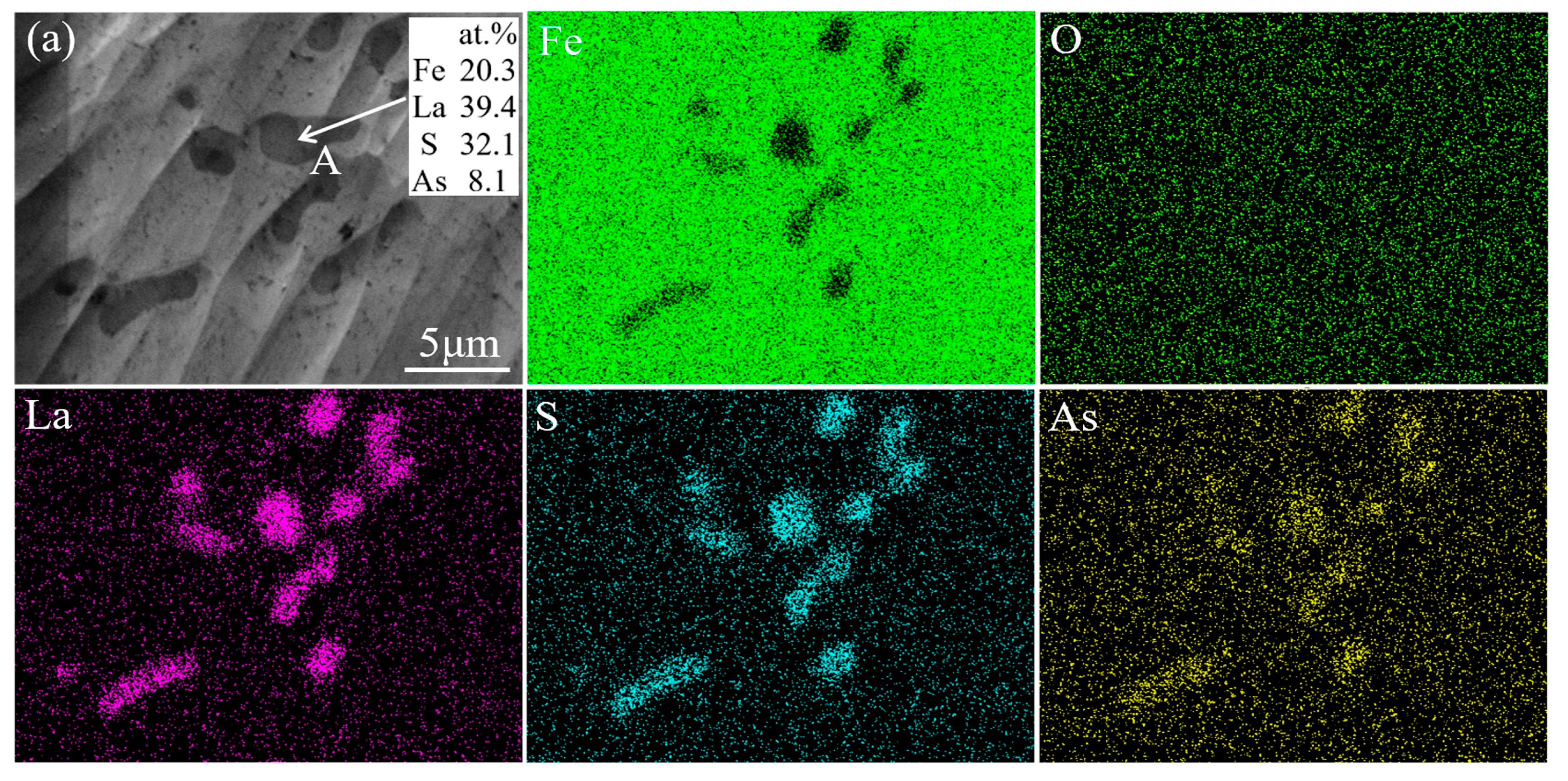
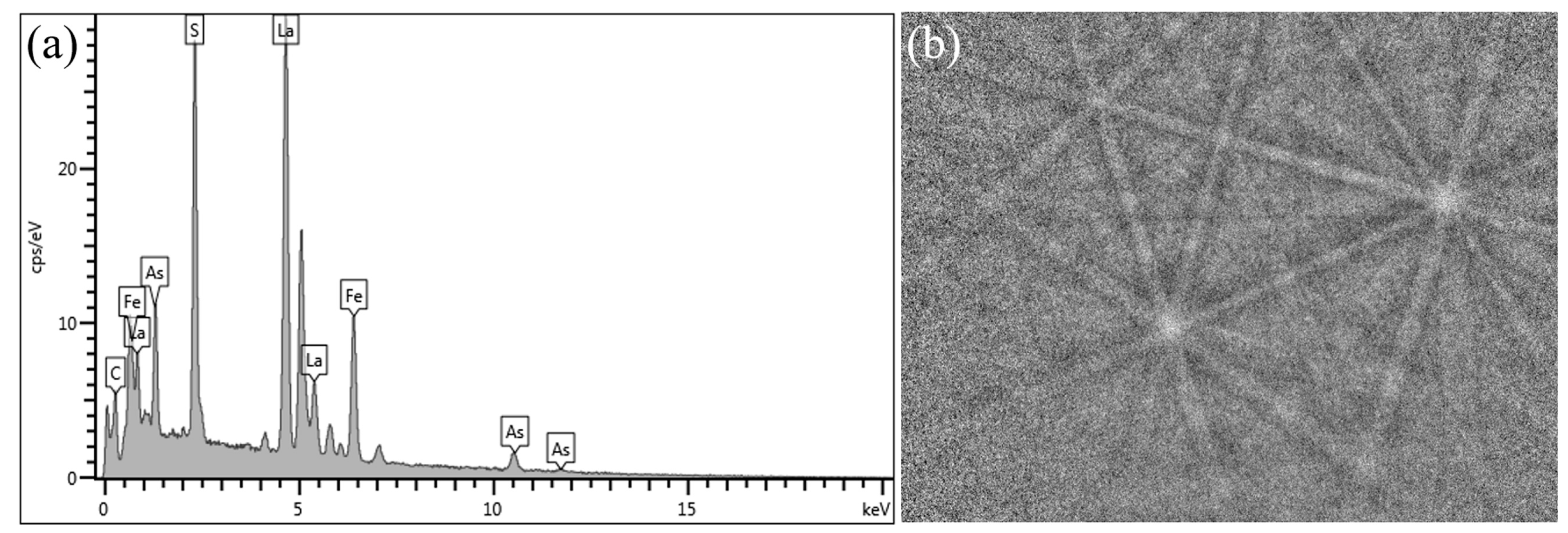
- Wang, H.; Xiong, L.; Zhang, L.; Wang, Y.; Shu, Y.; Zhou, Y. Investigation of RE-O-S-As inclusions in high carbon steels. Metall. Mater. Trans. B 2017, 48, 2849–2858. [Google Scholar] [CrossRef]
- Wang, H.; Yu, P.; Jiang, S.; Bai, B.; Sun, L.; Wang, Y. Evolution of inclusions in steelmaking process of rare earth steels containing arsenic with alumina crucibles. Metals 2020, 10, 275. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, S.; Yu, P.; Bai, B.; Sun, L.; Wang, Y. Distribution of arsenic inclusions in rare earth steel ingots. Metals 2020, 10, 146. [Google Scholar] [CrossRef]
- Zhang, J.; Dou, S. Study on interaction between cerium and arsenic. Adv. Mater. Res. 2011, 194, 1231–1234. [Google Scholar] [CrossRef]
- Wang, L.; Lin, Q.; Ji, J.; Lan, D. New study concerning development of application of rare earth metals in steels. J. Alloy. Compd. 2006, 384–386. [Google Scholar] [CrossRef]
- Li, Y.; Sun, M.; Jiang, Z.; Chen, C.; Chen, K.; Huang, X.; Sun, S.; Li, H. Inclusion modification in C104Cr saw wire steel with different cerium content. Metals 2019, 9, 54. [Google Scholar] [CrossRef]
- Wu, C.; Cheng, G.; Tian, J.; Xie, Y. Effect of rare earths on 09Cr2AlMoRE steel with residual elements arsenic and tin. J. Chin. Soc. Rare Earths 2013, 31, 597–604. [Google Scholar]
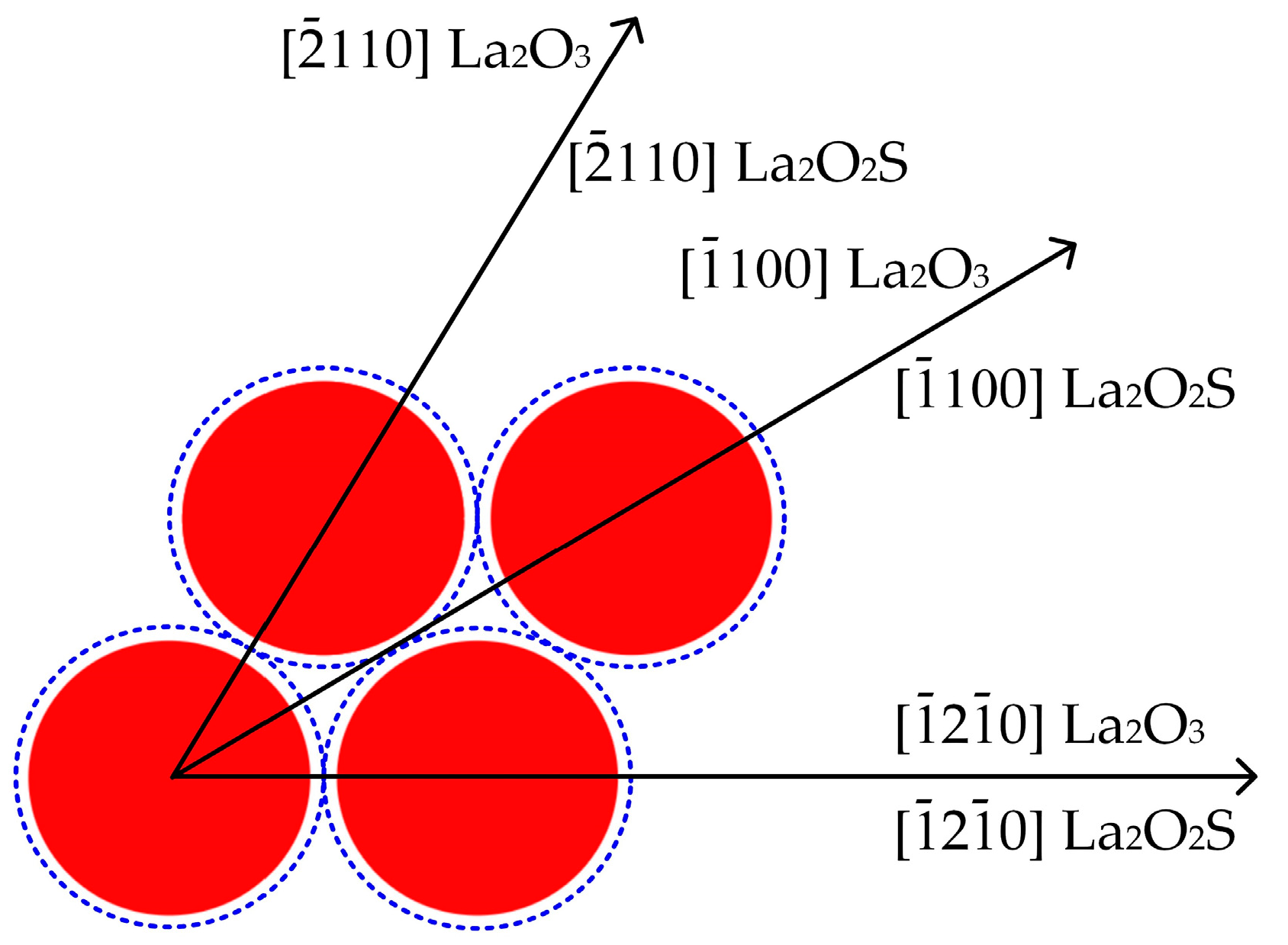
- Bramfitt, B.L. The effect of carbide and nitride additions on the heterogeneous nucleation behavior of liquid iron. Metall. Trans. 1970, 1, 1987–1995. [Google Scholar] [CrossRef]
- Villars, P. Pauling file. In Inorganic Solid Phases, SpringerMaterials (Online Database); Springer: Heidelberg, Germany, 2012. [Google Scholar]
- Li, W.; Liu, Q.; Ye, W.; Zhang, C. Kinetics of rare-earth on the low carbon steel containing arsenic. Chin. J. Eng. 1983, 61–67. [Google Scholar]
- Hino, M.; Ito, K. Thermodynamic Data for Steelmaking; Tohoku University Press: Sendai, Japan, 2010; pp. 249–257. [Google Scholar]
- Wang, L.; Du, T. Study on equilibrium of [La]-[S]-[O] system in steel. Rare Earth 1986, 2, 25–32. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).