Abstract
The evolution of inclusions that contain Al, Mg, and Ti was studied through industrial-grade experiments. Field emission scanning electron microscopy, energy dispersive spectrometry, inductively coupled plasma atomic emission spectrometry, and FactSage software were used to analyze the evolution mechanisms of inclusions in Al-killed titanium alloyed interstitial free (IF) steel. The research found that the evolution of inclusions during the smelting process of IF steel is results in ‘large sphere-like SiO2-CaO-FeO-MgO-MnO’ and ‘small cluster spherical FeO-MnO’ change to cluster-like Al2O3 and irregular MgO·Al2O3, then change to Al2O3·TiOx and Al2O3, and finally change to Al2O3. It is difficult for Al2O3·TiOx to stably exist in the IF molten steel. It is the key to extend the holding time properly after Ruhrstahl Heraeus (RH) to ensure the removal of Al2O3 inclusion. With the increase of Mg content, the change path of MgAl2O4 inclusion in IF steel is that Al2O3 changes to MgO·Al2O3, and finally changes to MgO. It is difficult to suppress MgO·Al2O3 spinel formation by controlling the oxygen in the steel, but Ca can modify part of the MgO·Al2O3 spinel inclusions during RH refining. In order to ensure the removal of 6–10 μm inclusions, the holding time is suitable for 19–42 min.
1. Introduction
Ultra-low carbon interstitial-free (IF) steel is widely used in automobile plate production because of its excellent deep draw ability and uniform mechanical properties. With the increasing quality requirements of cold rolling sheet, the control requirements of inclusions in ultra-low carbon steel are increasingly strict [1,2]. In the smelting process of ultra-low carbon IF steel, a certain amount of titanium, niobium, and other elements should be added, and the interstitial atoms, such as carbon and nitrogen, in ultra-low carbon steel should be completely fixed as carbon nitrogen compounds, so as to obtain clean ferritic steel without interstitial atoms [3]. According to the difference in the chemical composition of the inclusions, the inclusions in ultra-low carbon steel can be divided into Al2O3 inclusions, Al2O3-TiOx inclusions, and CaO-Al2O3-MgO inclusions [4,5,6,7]. Such inclusions can easily block immersion nozzles during continuous casting, and can also cause defects in the product. The study shows that Al2O3-TiOx inclusions are closely related to the clogging of the nozzle and the final product defect [8].
Al2O3-TiOx inclusions are easily formed when the content of FeO is high [4], and the inclusions are unstable [6]. The [Al] and [Ti] react with the [O] from the self-dissociation of SiO2 in the slag and form inclusions changing from solid Al2O3 to Al2O3-TiOx inclusions. The inclusion chemistry composition is mainly dependent on the w([Al]) and w([Ti]), while the number of inclusions can be reduced by increasing the ratios of CaO to SiO2 and CaO to Al2O3 in the slag [9]. Rare earth Ce is usually used to modify spinel inclusions in steel [10]. The irregular Al2O3 inclusions will be modified by the rare earth element Ce in Al-killed titanium-alloyed IF steel. The irregular Al2O3 inclusions of 10–15 μm are wrapped by rare earth, and gradually transformed into spherical CeAlO3, Ce2O3, and Ce2O2S inclusions of 5 μm, and finally dispersed into IF slabs [11].
When T[O] in steel is reduced to 0.001%, the spinel inclusions in molten steel become the most important factor affecting the purity of steel [12]. The MgO·Al2O3 spinel will be modified by Ca treatment. The inclusion modification route is Al2O3 change to MgO·Al2O3, and finally change to liquid complex inclusions [13]. Mg migrates to the molten steel and MgAl2O4 spinel inclusion is formed due to a reaction between Mg and Al2O3 inclusions. The spinel inclusion changes entirely into liquid oxide inclusion via the transfer of Ca from slag to metal. The modification reaction is more efficient as the SiO2 content in the slag decreases [14]. When the content of Al in steel is constant, with the increase of Mg content in steel, the inclusions in steel continuously precipitate MgAl2O4 spinel and finally changes into liquid MgO [15]. It is very important to control the content of Mg and Al in the alloy and to prevent the secondary oxidation of molten steel [16].
In summary, the inclusions have an important impact on the blockage of immersion nozzles and product quality of ultra-low carbon steel. In actual production, the molten steel in the basic oxygen furnace (BOF)-Ruhrstahl Heraeus (RH)-continuous casting (CC) process was sampled, and the characteristics, formation and evolution process, formation mechanism, and influencing factors of the inclusions were analyzed in detail to provide a theoretical basis for the control of inclusions in ultra-low carbon steel. Finally, the mechanical properties of DC06 IF steel were tested, and it was confirmed that the improved IF steel met the requirements of relevant steel grades. The experimental results have a complete understanding of the evolution process of inclusions in IF steel, provide guidance for the evolution and control of MgO·Al2O3 spinel inclusions and MgO·TiOx inclusions in steel, and put forward the process parameters to reduce the number and size of inclusions in steel. This is of great significance for improving the control level of non-metallic inclusions in automobile plate steel.
2. Materials and Methods
2.1. Materials
DC06 IF steel was produced by the 260 t BOF-RH-Holding-CC process at the Hanbao steel plant. During tapping, 600 kg lime was added into the BOF, and 680 kg aluminum slag deoxidant was added onto the top slag after tapping. RH vacuum treatment includes decarburization, followed by aluminum deoxidization, then 338 kg Ti-Fe containing 70% Ti alloying and keeping RH pure circulation time for 8–10 min. After RH treatment, molten steel remained unstirred in the ladle for 25–45 min before casting. The standard for judging the chemical composition of DC06 IF steel is shown in Table 1.

Table 1.
The standard for judging the chemical composition of IF steel, wt%.
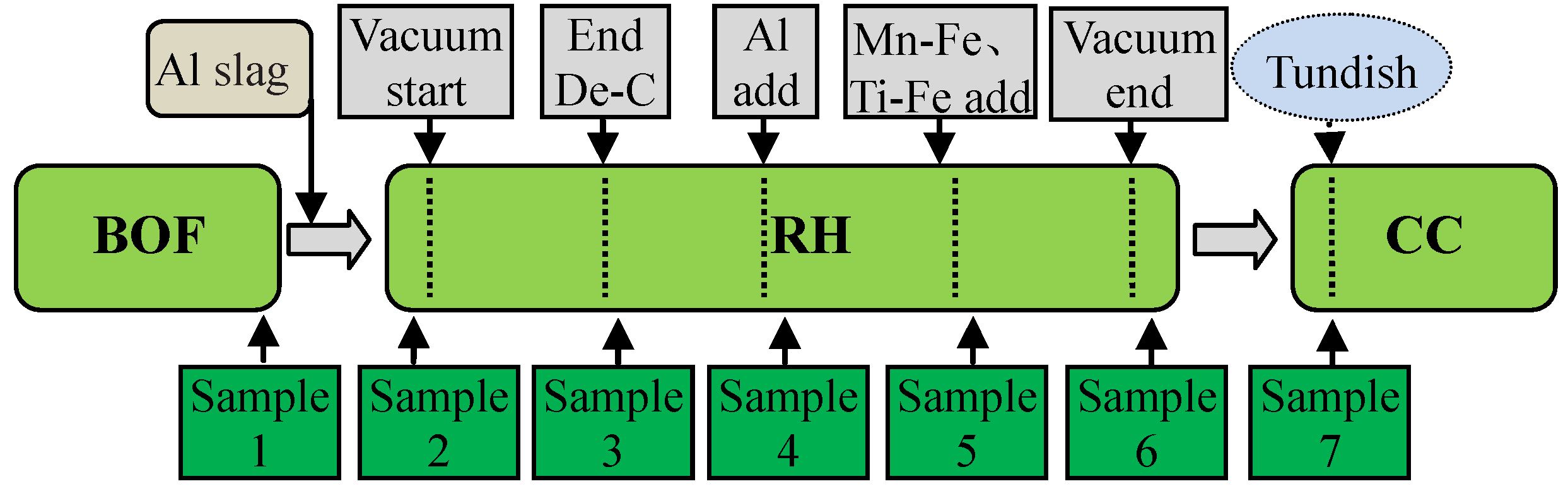
In order to investigate the evolution of inclusions in the IF steel smelting process, samples were taken by samplers of Φ 30 mm × 10 mm during the industrial experiment steelmaking process, and the whole industrial experiments were carried out over 6 heats in total. A schematic diagram of charging and taking specimens during the steelmaking processes is shown in Figure 1.
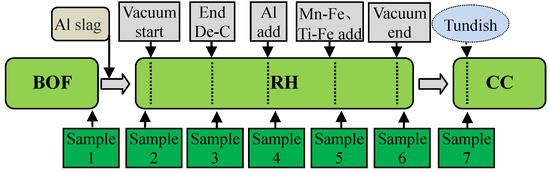
Figure 1.
Schematic of charging and taking specimens during steelmaking processes
2.2. Mechanical Property Experiment
During the tensile experiment, the DC06 IF steel coil transverse sample was taken, and the steel plate was processed into a dumbbell-shaped sample by using the Zwick 2Z50 (Ennepeta, Nordrhein-Westfalen, Germany) sample preparation machine. The initial gauge lengths of the tensile samples l0 and b0 are 80 mm and 20 mm, respectively. Then the XKA714C (BYJC, Beijing, China) automatic numerical control machine was used to mill the edge of the sample to make it meet the standard GB/T228.1-2010. According to the inspection standard GB/T5213-2019, the Zwick automatic tensile testing machine (Model Z150robo Test L, Ennepeta, Nordrhein-Westfalen, Germany) was used to test the tensile test samples to obtain mechanical performance data, whereby 700 sets of tensile tests were carried out on DC06 IF steel, and the average value of mechanical properties was obtained.
2.3. Methods of Chemical Analysis
Each steel sample on the cross-sectional had been ground and polished by SiC papers and w1.5 diamond suspensions. The sample should be ground and polished in the way of transverse and longitudinal intersections, and inclusions of each steel sample were detected using FEI Nova NanoSEM400 (FEI, Hillsboro, OR, USA) scanning electron microscopy (SEM) coupled with energy-dispersive X-ray spectroscopy (EDS, FEI, Hillsboro, USA). The number, size, and chemical composition of inclusions were analyzed automatically using an ASPEX scanning electron microscope (ASPEX SEM, FEI, PA, USA). The concentration of the Al, Ti, and Ca in steel was determined by IRIS advantage radial inductively coupled plasma atomic emission spectrometry (ICP-AES, Thermo Elemental, MA, USA). The [O] concentration in steel was measured online by a MSO-3690oxygen sensor (MINCO, Harland, WI, USA).
3. Results and Discussion
3.1. Morphology and Evolution of Typical Non-Metallic Inclusions in Molten Steel
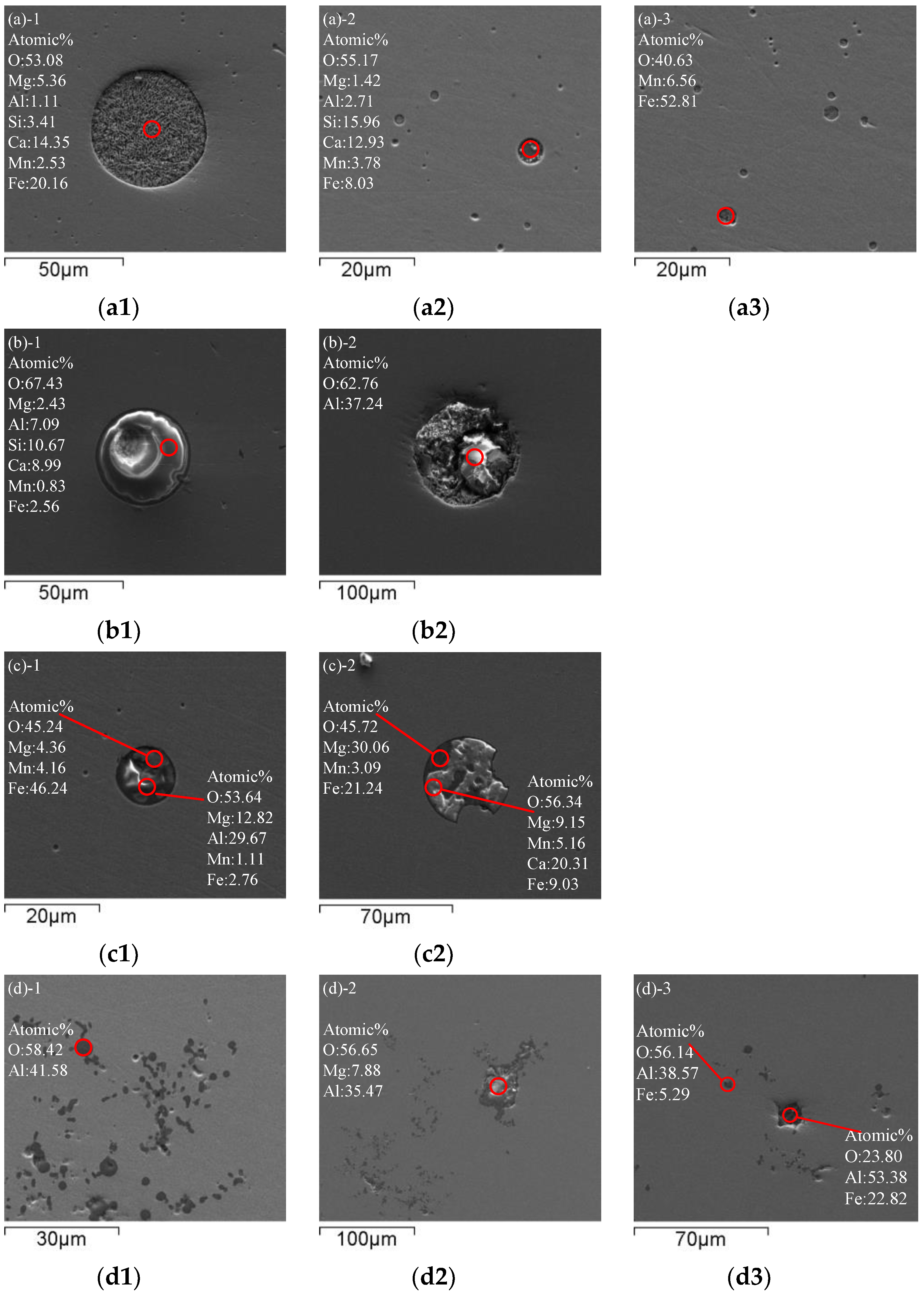
Figure 2 shows the morphology and evolution of typical inclusions in molten steel. Sample 1 was taken from BOF before tapping. The morphology of inclusions before tapping is shown in Figure 2a. Inclusions in BOF steel are spherical. The inclusions in the BOF process are SiO2-CaO-FeO-MgO-MnO type multi-phase composite inclusions. Due to the deep decarburization by oxygen blowing in BOF, the molten steel has strong oxidizability. Therefore, there are many cluster spherical FeO-MnO inclusions below 5 μm in the molten steel, and other large sphere-like inclusions below 50 μm are SiO2-CaO-FeO-MgO-MnO.
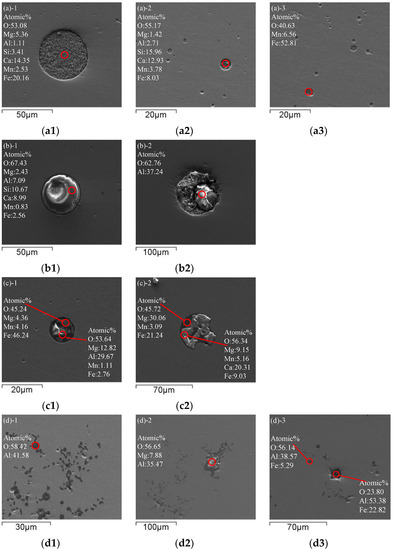
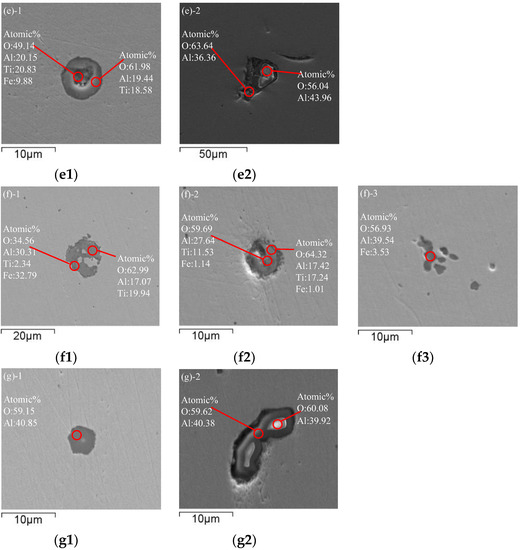
Figure 2.
Typical inclusions in molten steel: (a) Before tapping; (b) Before vacuum start; (c) After decarburization; (d) After adding aluminum for 2 min; (e) After adding titanium for 2 min; (f) After RH treatment; (g) From tundish.
Sample 2 was taken from ladle before vacuum start. Figure 2b shows typical inclusions in molten steel before vacuum start during RH refining. The spherical and lump inclusions in the steel further grow up and float up, and many CaO-SiO2-FeO-Al2O3-MgO-MnO inclusions are in the 50 μm size. Due to the addition of Al slag modifier, large particles of Al2O3 above 50 μm appear in the steel.
Sample 3 was taken from ladle after decarburization. After decarburization by RH vacuum refining, typical inclusions in molten steel are shown in Figure 2c. Irregular FeO-MnO-MgO and MgO-Al2O3 inclusions combine to form approximately 15 μm spherical inclusions or irregular FeO-MnO-MgO and CaO-Al2O3-FeO-SiO2 inclusions combine to form approximately 50 μm nearly spherical inclusions. Large inclusions have been partially removed, and the single Al2O3 has been combined with other inclusions to form complex inclusions.
Sample 4 was taken from ladle after adding aluminum. Typical inclusions in molten steel after adding aluminum for 2 min are shown in Figure 2d. After decarburization in the RH refining process, aluminum is added to the molten steel for deep deoxidization. Due to the reaction between aluminum and oxygen, a large number of cluster-like or coral-like Al2O3 inclusions are generated by reaction (1). In addition, the inclusions in the steel aggregate and grow, and the lump MgO·Al2O3 spinel inclusions are wrapped by compact coral-like Al2O3 inclusions. Other Ca-based and Si-based inclusions are relatively rare after floating and removal.
[Al] + [O] = (Al2O3)inclusion
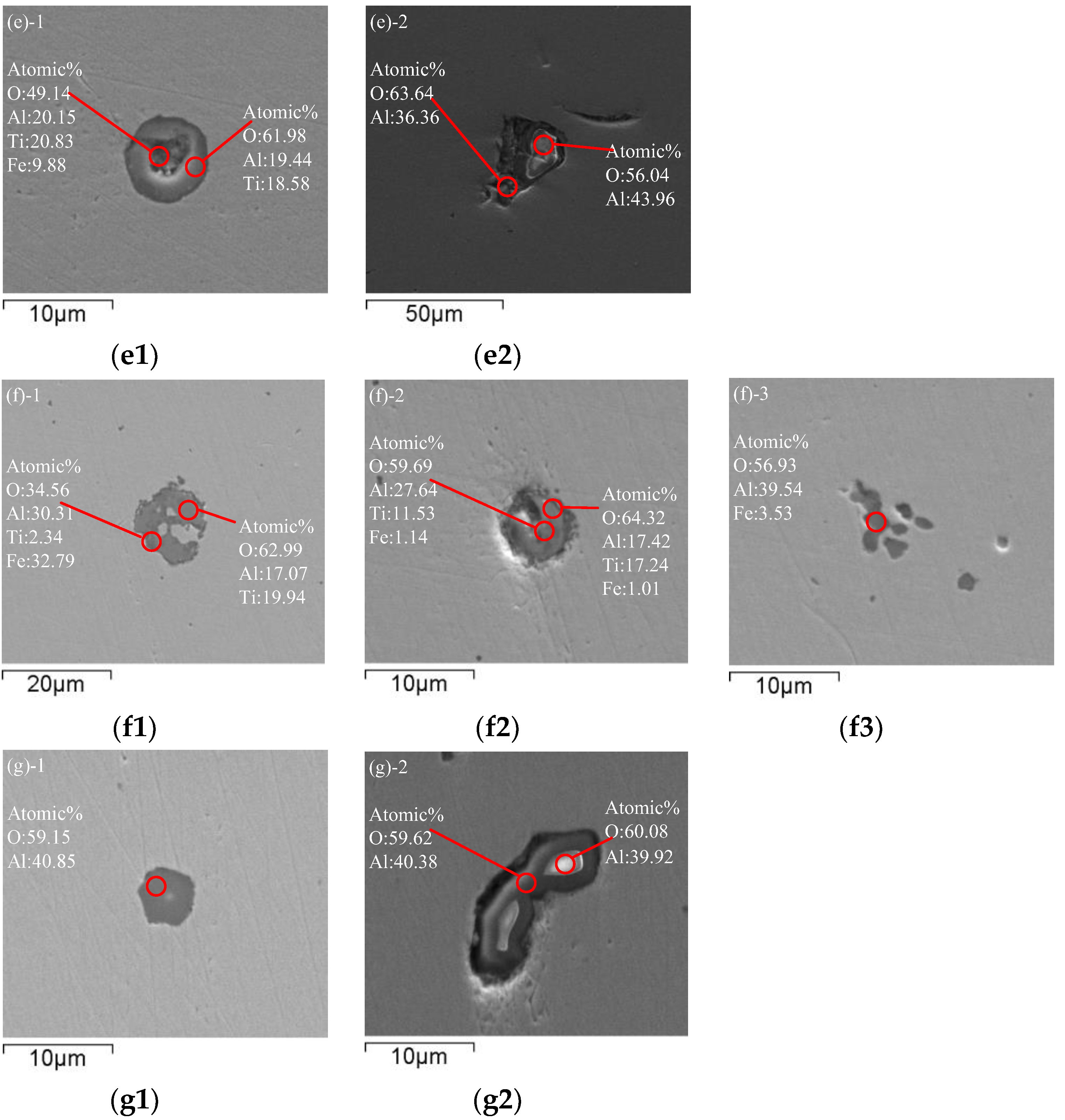
Sample 5 was taken from ladle after adding titanium alloy. Figure 2e shows typical inclusions in molten steel after RH refining and adding titanium alloy. After adding aluminum for deoxidization during RH refining, a large number of cluster-like Al2O3 inclusions are generated in the steel. With the floating of Al2O3 inclusions, the total oxygen content in the steel is greatly reduced. Subsequently, after adding Ti-Fe alloying, some [Ti] reacts with [Al] and [O] by reaction (2) in the steel to form Al2O3·TiOx inclusions below 10 μm, and a part of [Ti] reacts with [O] to generate TiOx, and the TiOx inclusion wraps around the outside of the Al2O3 inclusions to produce irregular lump Al2O3·TiOx inclusions by reaction (3).
[Ti] + [Al] + [O] → (Al2O3·TiOX)inclusion
[Ti] + [O] + (Al2O3)inclusion → (Al2O3·TiOX)inclusion
Sample 6 was taken from ladle after RH treatment. After RH refining is completed, typical inclusions in molten steel are shown in Figure 2f. At this time, large-scale Al2O3·TiOx inclusions in the molten steel above 20 μm have floated and removed, but there are still newly generated Al2O3·TiOx inclusions below 20 μm in the molten steel, and Al2O3·TiOx grows with Al2O3 as the core and wraps Al2O3, and the inclusions are mainly Al2O3·TiOx, except for a few clusters of Al2O3 in the molten steel.
Sample 7 was taken from tundish during continuous casting. Figure 2g shows typical inclusions in the molten steel of tundish. After the molten steel remains unstirred in the ladle for 25–45 min during the holding process, most of the large-particle Al2O3 inclusions have been removed by floating. Since the molten steel is sufficiently homogenized, the Al2O3·TiOx inclusions cannot stably exist in the steel, so the Al2O3·TiOx inclusions have decomposed into stable Al2O3 inclusions before entering the continuous casting mold, and Ti re-enters the molten steel. It can be proven from Figure 2g that the inclusions in the final molten steel are mainly Al2O3. Therefore, it is key to ensure the quality of IF steel to extend the holding time properly after RH to ensure the removal of Al2O3 inclusions.
3.2. Thermodynamics of Evolution and Control of Typical Oxide Inclusions during Refining
The predominance area diagram of Fe-Al-(Mg, Ti)-O system was calculated by using the thermodynamic software FactSage 7.0 (ThermFact Inc., Montreal, QC, Canada). The evolution and control of typical Fe-Al-(Mg, Ti)-O system inclusions in IF steel smelting process are analyzed by using these diagrams.
3.2.1. Typical Spinel Inclusions in Molten Steel before RH Refining
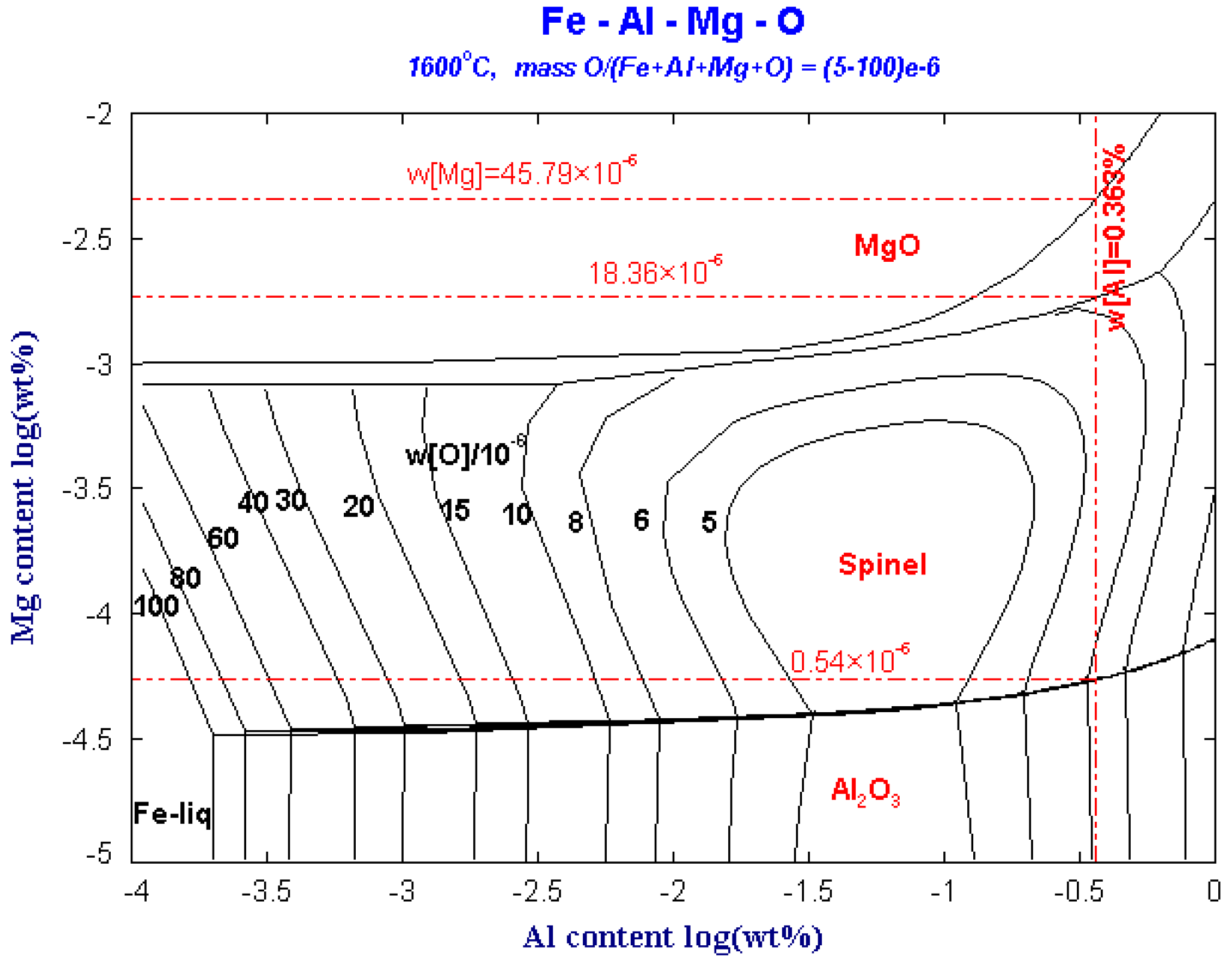
Figure 3 shows the predominance area diagram of the Fe-Al-Mg-O system with different oxygen contents at 1600 °C. The Fe-Al-Mg-O system is used to study the MgO, MgAl2O4 spinel and Al2O3 phase diagrams obtained from the change of Al content from 1 × 10−6 to 1% and the change of Mg content from 0.1 × 10−6 to 100 × 10−6. When the Mg content is lower than the critical line of the Al2O3 and MgO·Al2O3 phases, an Al2O3 phase is formed. At this time, the [Mg] content in the molten steel is very low, and it is dissolved in the molten steel in the form of elemental Mg. The Mg content is higher than the boundary line of MgO·Al2O3 and MgO, and Mg exists as MgO inclusions phase.
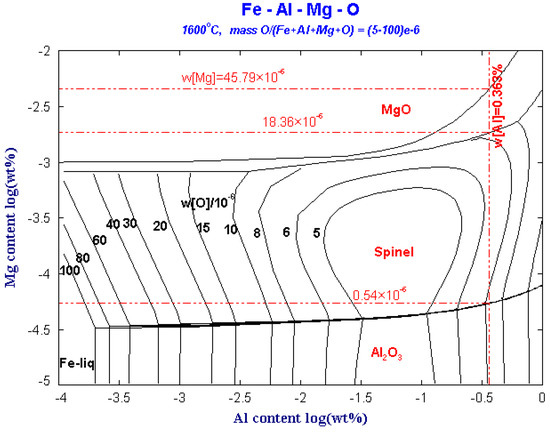
Figure 3.
Predominance area diagram of Fe-Al-Mg-O system with different oxygen contents at 1600 °C.
The steel samples were taken before RH, after RH, and in the tundish, and the samples were taken from six heats industrial experiment. The chemical compositions of Al, Ti, and Ca elements in the steel are analyzed, and the average compositions are shown in Table 2.

Table 2.
The average chemical compositions of Al, Ti, and Ca elements in the steel, wt%
The ladle slag is modified by Al slag modifier after tapping. Although the Al in the molten steel is about 0.363%, the oxygen in the molten steel is still high. When the content of Mg in the molten steel is 0.54 × 10−6, MgAl2O4 can stably exist in the steel. When the Mg content in the steel is greater than 18.36 × 10−6, Mg will coexist as MgAl2O4 and MgO inclusions. When the Mg content in the steel is larger than 45.79 × 10−6, Mg exists only as MgO inclusion. Therefore, as long as the Mg concentration in the molten steel is (0.54–45.79) × 10−6, the MgAl2O4 spinel inclusions will exist in the molten steel.
3.2.2. Typical Mg-Al Spinel Inclusions in Molten Steel after RH Refining
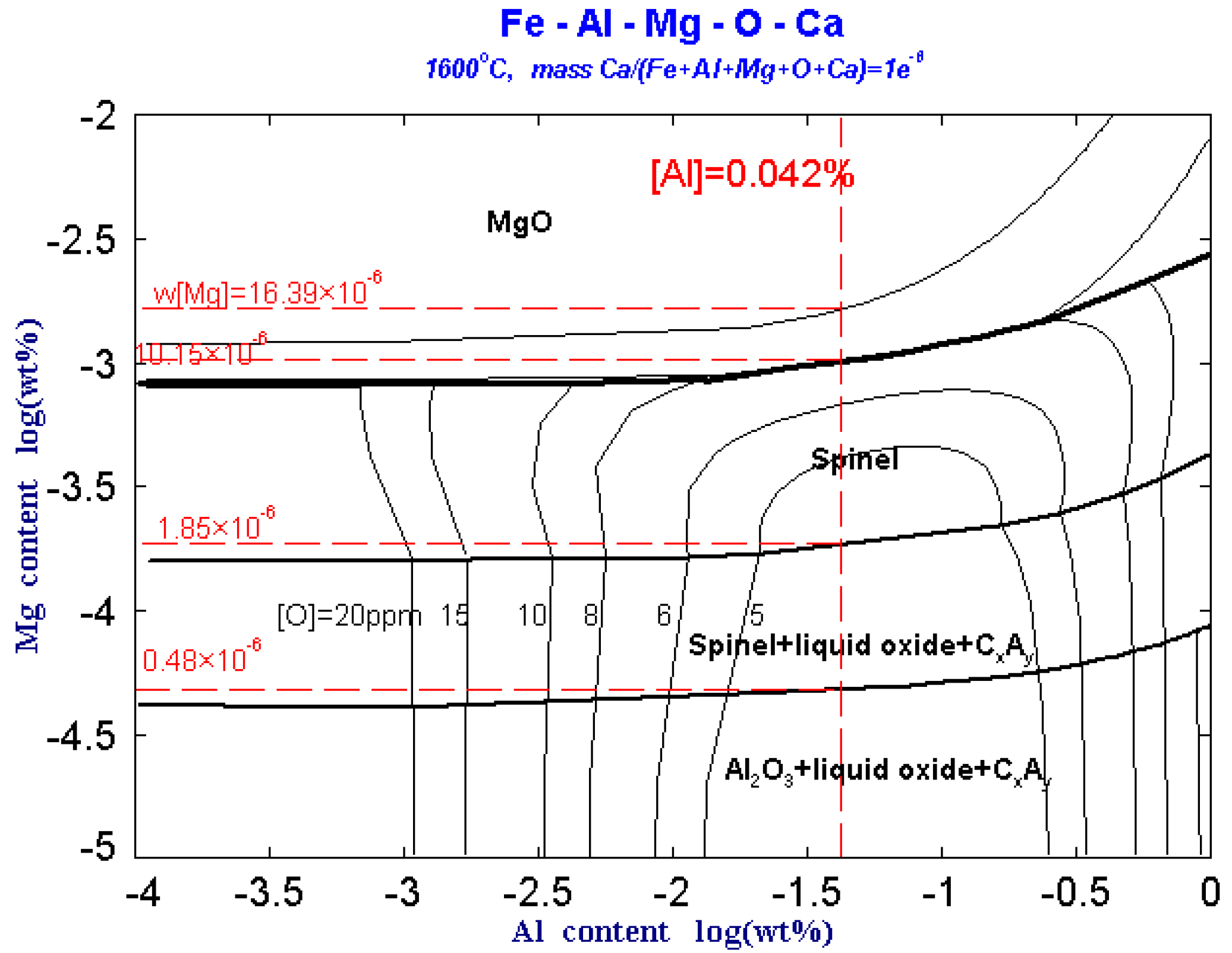
Figure 4 shows the predominance area diagram of Fe-Al-Mg-Ca-O system with 1 × 10−6 Ca and different oxygen contents at 1600 °C. As can be seen from Figure 4, when the content of [Mg] is fixed, the type of inclusions generated in the molten steel is always fixed within a certain range regardless of the change in the [Al] content. Therefore, in the Fe-Al-Mg-O-Ca molten steel system, the type of inclusions is mainly affected by the [Mg] content. In addition, as the [O] content in the molten steel increases, the range of Al corresponding to the formation of MgAl2O4 spinel and Al2O3 phases increases. In other words, the probability of forming brittle inclusions such as MgO·Al2O3 spinel and Al2O3 increases with increase the [O] content in the steel. However, it is difficult to suppress MgO·Al2O3spinel formation by controlling the oxygen in the steel.
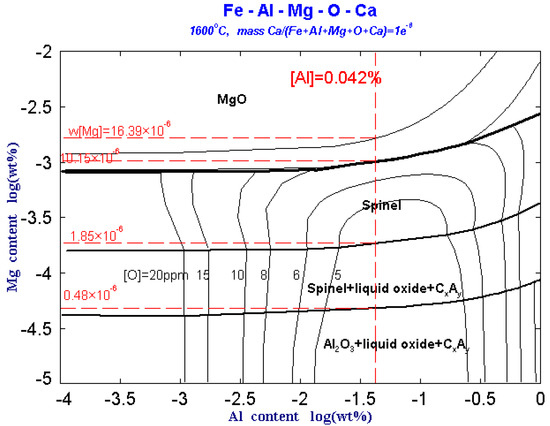
Figure 4.
Predominance area diagram of Fe-Al-Mg-Ca-O system with 1 × 10−6 Ca and different oxygen contents at 1600 °C.
In the Figure 4, ‘liquid oxide’ represents CaO-Al2O3-MgO series liquid inclusions, and ‘CxAy’ represents (CaO)x(Al2O3)y. When 1 × 10−6 [Ca] is added to the molten steel, the MgO·Al2O3 spinel area is significantly reduced, a part of the MgO·Al2O3 spinel area is replaced by the ‘liquid oxide’, and the original Al2O3 phase area is also replaced by a part of the ‘liquid oxide’.
For the IF steel containing 0.042% Al after RH refining in this experiment, when the content of [Mg] in the steel is less than 0.48 × 10−6, Mg is dissolved in the molten steel and the inclusions in the molten steel are Al2O3 and CxAy type calcium aluminates. The overall area ratio is larger than that without Ca. When content of [Mg] in steel increases to 0.48 × 10−6, MgO·Al2O3 spinel inclusions begin to generate in the molten steel and it also contains Al2O3 and CxAy type calcium aluminates. When the content of [Mg] in the molten steel increases to 1.85 × 10−6, the Al-containing inclusions in the molten steel all exist as MgO·Al2O3 spinel. As the content of [Mg] in the molten steel increases to 10.15 × 10−6, the inclusions in the molten steel include MgO inclusions besides MgO·Al2O3 spinel. When the content of [Mg] in the molten steel increases to 16.39 × 10−6, the Mg-containing inclusions in the molten steel all exist as MgO.
3.2.3. Typical Al-Ti Inclusions in Molten Steel after RH Refining
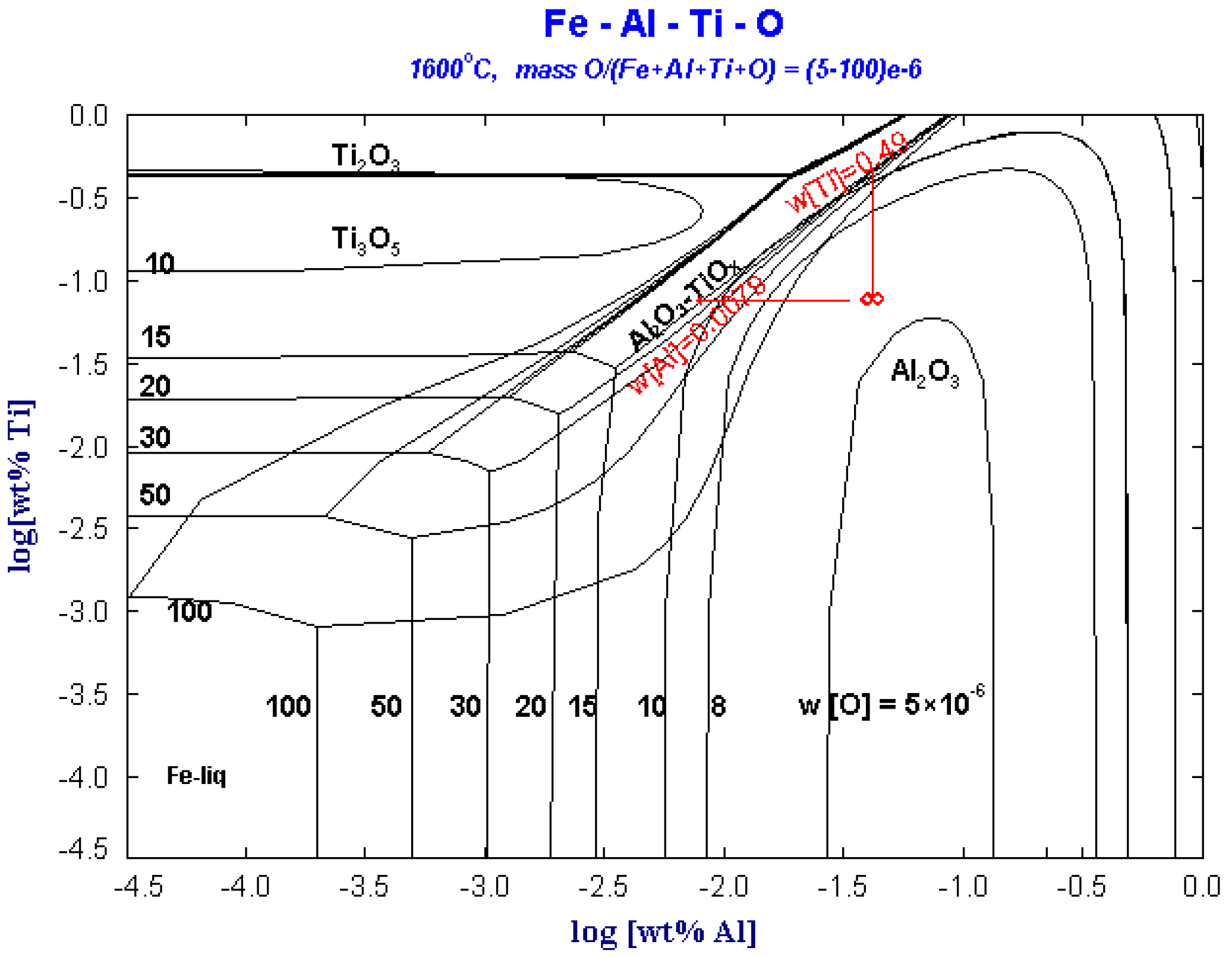
Figure 5 shows the predominance area diagram of Fe-Al-Ti-O system with different oxygen contents at 1600 °C. It can be seen from the figure that Al2O3 can remain stable in the molten steel. When the content of [O] in steel is less than 10 × 10−6, in addition to the [Al] and [Ti] dissolved in the molten steel, other trace Al and Ti in the steel are stable in the form of Al2O3 and Ti3O5, and Al2O3·TiOx will not be stable in the molten steel.
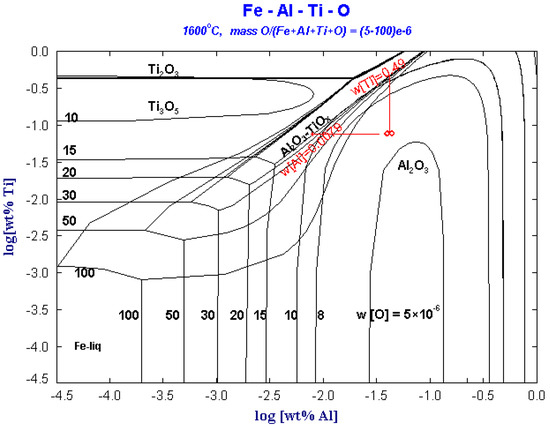
Figure 5.
Predominance area diagram of Fe-Al-Ti-O system with different oxygen contents at 1600 °C.
When the [O] content in steel increases to 10 × 10−6, the stable region of Al2O3 increases. However, Ti exists as Ti2O3 instead of Ti3O5. As the dissolved [O] in steel increases above 15 × 10−6, Al2O3 may react with Ti or TiOx by reaction (3) or (4) to form Al2O3·TiOx.
[Ti] + (Al2O3)inclusion → (Al2O3·TiOX)inclusion + [Al]
It can be seen from the phase diagram that Al2O3·TiOx may exist in steel in three situations: (1) when the dissolved [O] in the molten steel is up to 15 × 10−6. (2) When the local Ti concentration in the molten steel is up to 0.49% during the titanium alloying adjusting process. (3) When the local Al concentration in the molten steel is as low as 0.0079%. As the dissolved oxygen in ultra-low carbon steel is low, the Al-Ti inclusions in the ultra-low carbon steel liquid cannot exist stably. When the composition of Al and Ti in the steel liquid is uniform, the Al-Ti inclusions will react with the Al in the steel continuously to form Al2O3 inclusions.
3.2.4. Typical Al-Mg Inclusions in Tundish Molten Steel
The average [O] content in the six experimental heats steel dropped to 3.9 × 10−6 before Ti adding, but the [O] content increases to 10 × 10−6 after RH refining. In order to understand the transformation process of MgO·Al2O3 spinel, the predominance area diagram of inclusions in Fe-Al-Mg-O system with [O] = 10 ppm and [Ca] = 1 ppm at 1600 °C is shown in Figure 6.
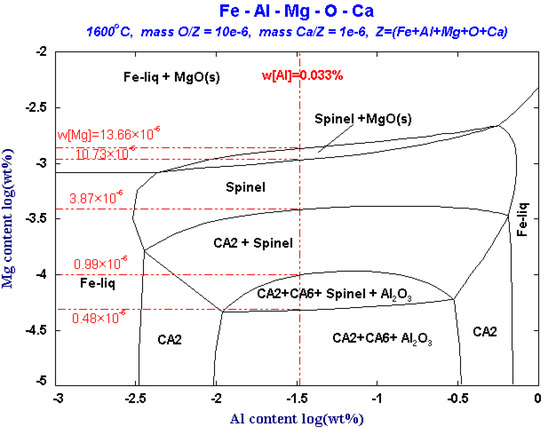
Figure 6.
Phase diagram of inclusions in Fe-Al-Mg-O system with [O] = 10 ppm and [Ca] = 1 ppm at 1600 °C.
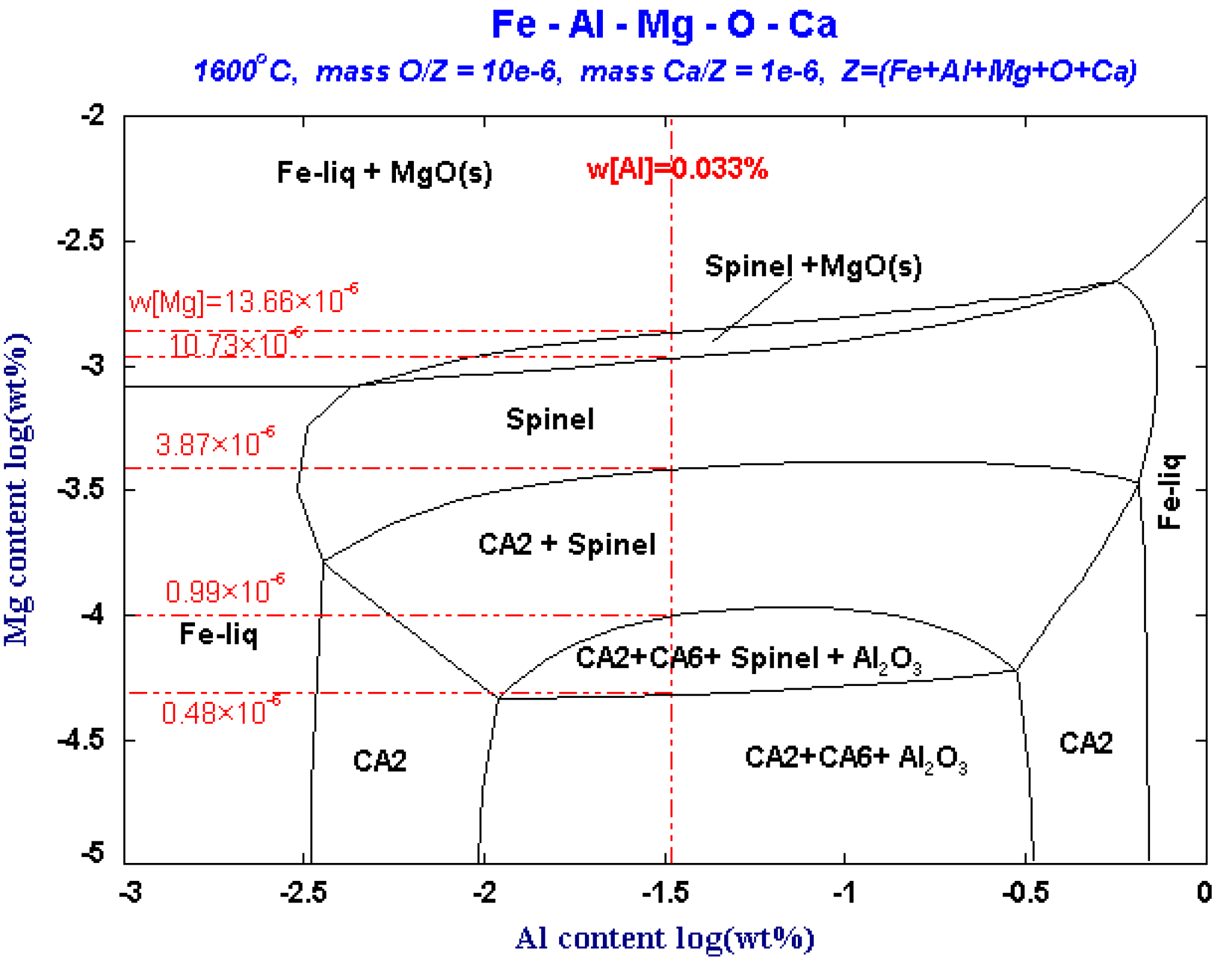
As can be seen from Figure 6, in the Fe-Al-Mg-O system, when the content of [O] and [Al]s in the steel is 10 × 10−6 and 0.033%, respectively, MgO and MgO·Al2O3 and Al2O3 inclusions will be precipitated separately with the difference in Mg content. If [Mg] content is less than 0.48 × 10−6 in the steel, Al2O3, CA2, and CA6 inclusions will be precipitated in the molten steel. If [Mg] content increases to 0.48 × 10−6, that is, the critical line of the Al2O3 and MgO·Al2O3 phases, MgO·Al2O3 spinel begins to precipitate in the molten steel. At this time, [Mg] and Al2O3 inclusions in the molten steel react to form MgO·Al2O3 spinel at the boundary between the Al2O3 phase and the MgO·Al2O3 phase. As the Mg content increase to 0.99 × 10−6, Al2O3 will be transformed to MgO·Al2O3, and the CA6 phase will disappear. When [Mg] content reaches to 3.87 × 10−6, Al2O3 will be completely transformed into MgO·Al2O3 spinel, and the CA2 phase will disappear. When [Mg] content continues to increase to 10.73 × 10−6, MgO begins to precipitate in the molten steel. When the content Mg increases to 13.66 × 10−6, the spinel is completely transformed to MgO. With the increase of Mg content, the change path of inclusions is that Al2O3 change to MgO·Al2O3, and finally change to MgO.
3.3. Change and Control of Inclusions during Refining and Holding Time
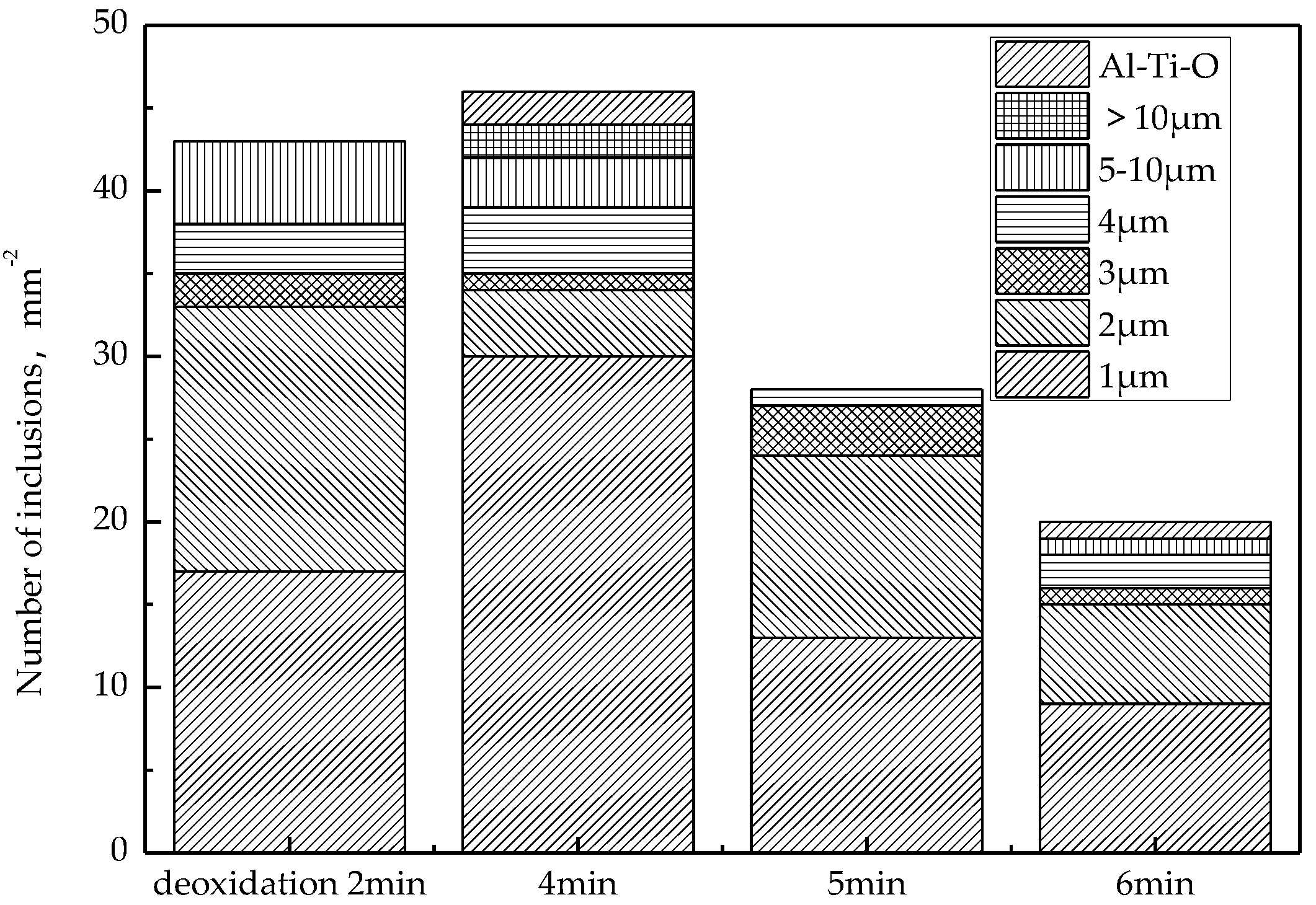
After the RH deoxidization is completed, the histogram of the change in the number of inclusions in the molten steel at different times is shown in Figure 7. The number, size, and chemical composition of inclusions were analyzed automatically by using ASPEX SEM. As can be seen from Figure 7, the number of inclusions reaches a maximum after 4 min of aluminum deoxidization, which indicates that within 4 min, the oxygen in aluminum and steel reacts quickly to form Al2O3 inclusions. When aluminum is added for 4–8 min, the removal rate of inclusions floating up is greater than the rate of generation, and the total number of inclusions in molten steel decreases, especially the number of inclusions larger than 10 μm. Therefore, in order to ensure that the inclusions generated by deoxidization are fully floated and removed, the pure circulation time after alloying should be greater than 8 min.
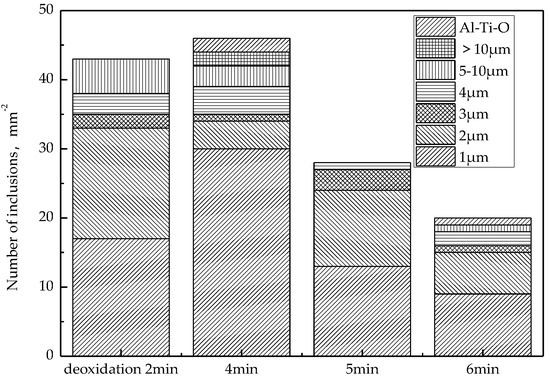
Figure 7.
Change of inclusions after RH deoxidization.
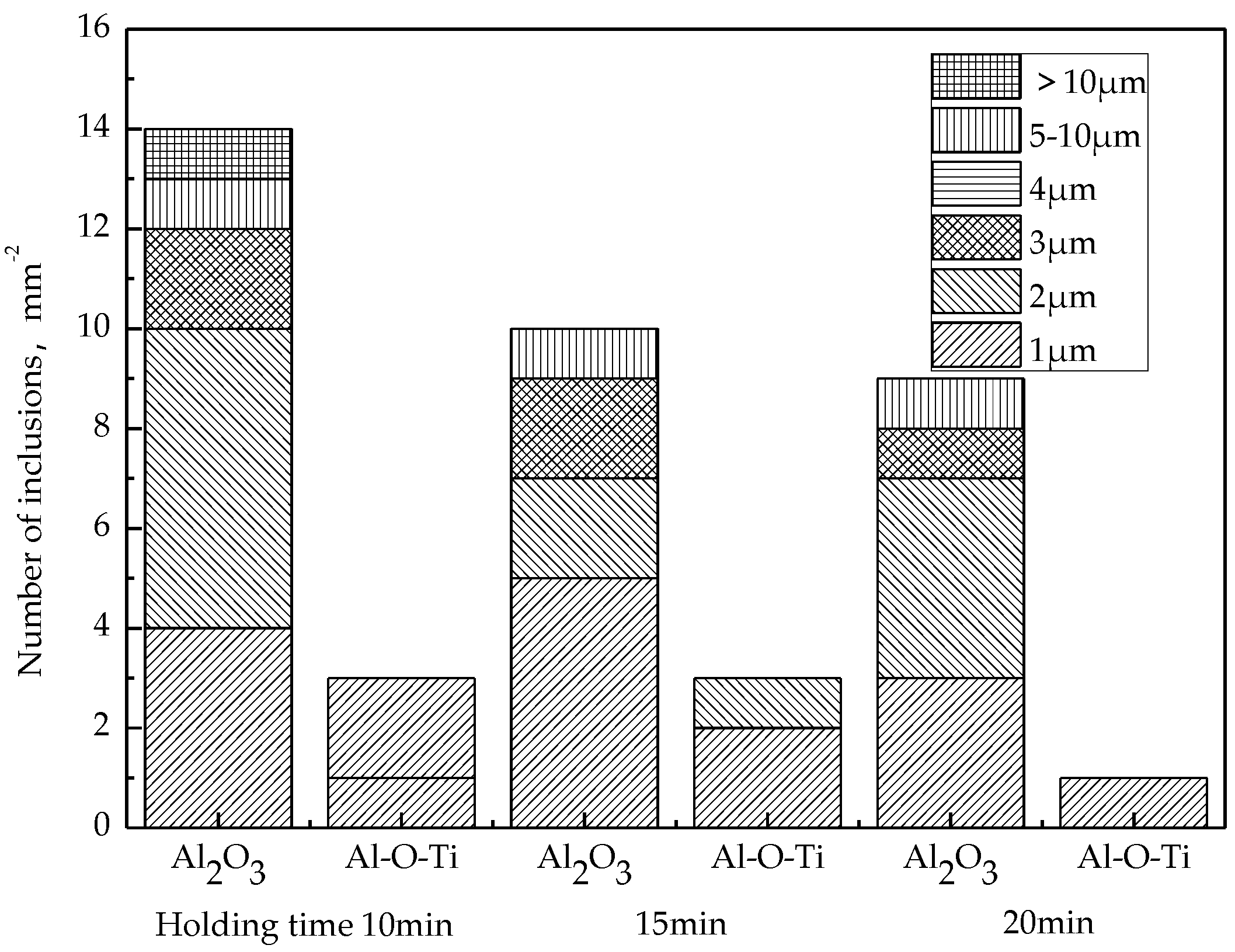
After RH refining, the change of the number of inclusions in the molten steel with holding time is shown in Figure 8. It can be seen that the number of inclusions in the molten steel decreases with the extension of the holding time within 20 min, especially the number of large particle inclusions above 10 μm decreases to 1 mm−2, and Al2O3·TiOx also decreases to 1 mm−2.
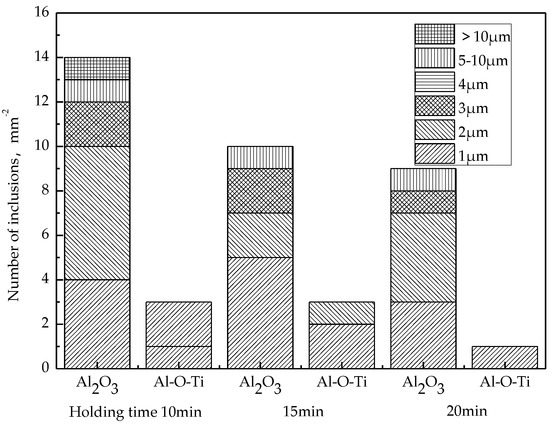
Figure 8.
Change of inclusions during holding time.
Inclusions float in the molten steel in the way of Stokes to the surface of the ladle. If the time required to reach the top of the molten steel is less than the average residence time of the molten steel, the inclusions can be floated to the top of the molten steel and removed from the molten steel [17].
The floating velocity of inclusions in the still molten steel can be calculated using the Stokes settlement Formula (5).
where Vs is the floating velocity of the inclusions, cm/s; g is the acceleration of gravity, m/s2; d is the diameter of the inclusions, mm; ρ1 is the density of the molten steel, ρ1 = 7.0 g/cm3; ρ2 is the density of the inclusions, ρ2 = 3.5 g/cm3; η is molten steel viscosity, η = 0.05 g/cm·s at 1600 °C.
Vs = gd2(ρ1 − ρ2)/(18η)
It can be seen from the above formula that the floating speed of inclusions is proportional to the square of the diameter of the inclusions, so large inclusions are easy to float. Substituting the data gives:
Vs = 0.00381 × d2
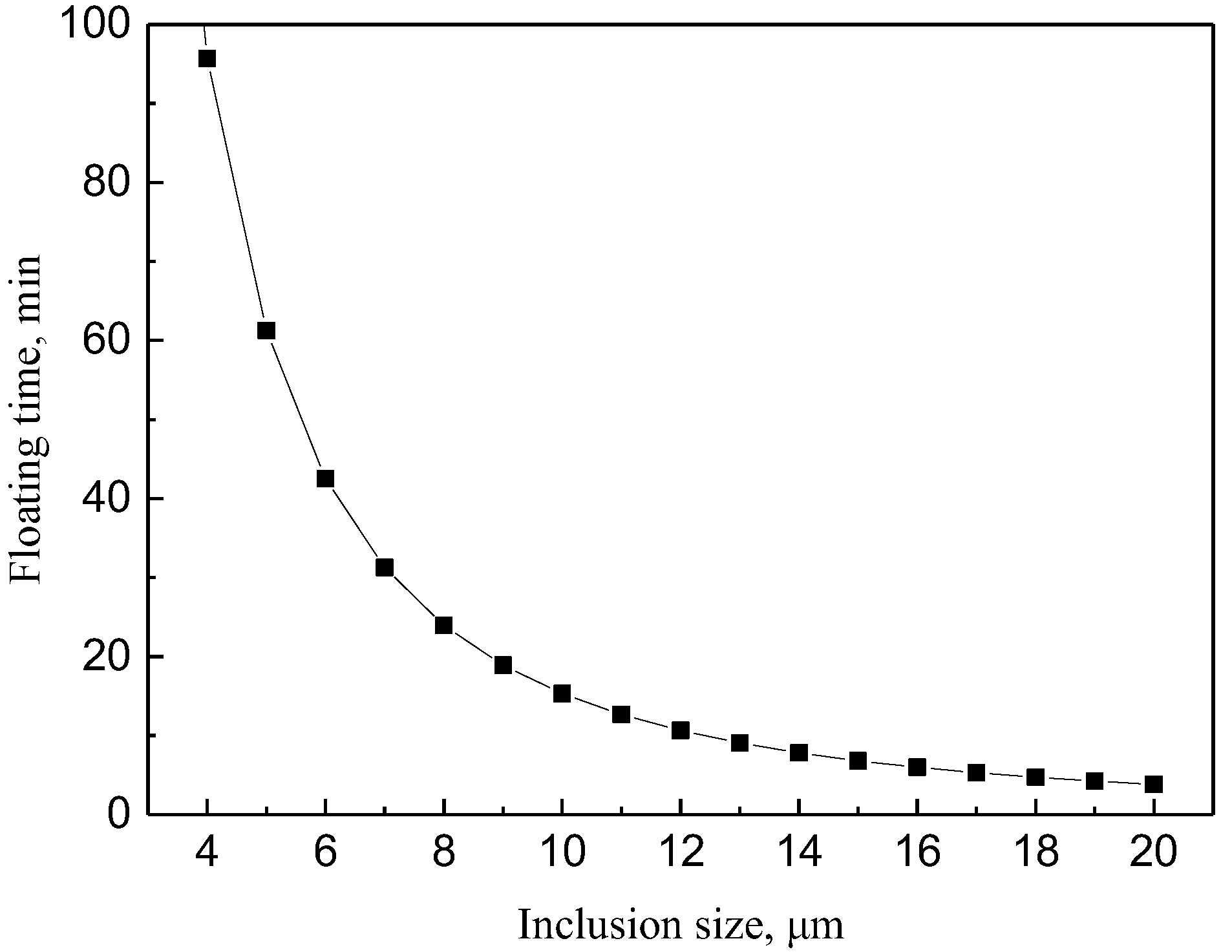
The depth of molten steel in this ladle is about 3.5 m, and the floating time of inclusions can be calculated by Equation (7), and the result is shown in Figure 9.
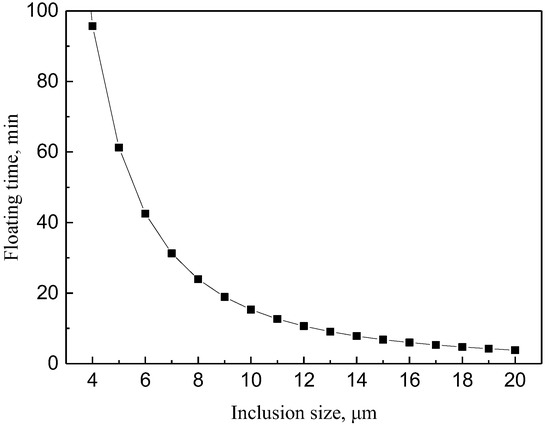
Figure 9.
The relationship between the particle size and the time of inclusion floating up.
t = 350/(0.00381 × d2)
It can be seen from Figure 9 that inclusions with particle size less than 6 μm need about 42 min to float up. When smaller inclusions aggregate into inclusions with a particle size greater than 9 μm, they can float up and remove within about 19 min, so the holding time should be 19–42 min.
3.4. Mechanical Properties of IF Steel
The results of the DC06 IF steel mechanical properties test and requirements (GB/T 5213-2019) are shown in Table 3. According to Table 3, the yield strength Rp0.2, tensile strength Rm, elongation after fracture A80, tensile strain hardening index N90, and plastic strain ratio R90 of DC06 IF steel are 138.7 MPa, 304.7 MPa, 45.3%, 0.24, and 2.91, respectively. Compared with standard values of GB/T5213-2019, the mechanical properties of DC06 IF steel meet the requirements.

Table 3.
Mechanical properties of DC06 IF steel.
4. Conclusions
- The IF steel inclusions in the BOF process are large sphere-like SiO2-CaO-FeO-MgO-MnO multi-phase composite inclusions below 50 μm and cluster spherical FeO-MnO inclusions below 5 μm. With the addition of Al-slag modifier and Al deoxidizer, a large amount of cluster-like or coral-like Al2O3 inclusions are formed. Moreover, MgO·Al2O3 spinel inclusions reduce gradually. Al2O3·TiOx inclusions begin to form after Ti addition, and Al2O3-type inclusions increase slightly. During the holding process, the inclusions are removed and the number of Al2O3 inclusions and Al2O3·TiOx inclusions in the steel is greatly reduced. In addition, Al2O3·TiOx inclusions are larger in size compared to Al2O3 inclusions. After the molten steel remains unstirred in the ladle during the holding process, most of the large-particle Al2O3 inclusions have been removed by floating. In the tundish, the inclusions of Al2O3·TiOx in the molten steel disappear, and only Al2O3 inclusions remain in the end. It is key to extend the holding time properly after RH to ensure the removal of Al2O3 inclusion.
- The predominance area diagram of Fe-Al-Mg-O system show that, when the Mg concentration in the molten steel before RH is (0.54–45.79) × 10−6, the MgAl2O4 spinel inclusions will exist in the molten steel. The type of inclusions is mainly affected by the [Mg] content, and it is difficult to suppress MgO·Al2O3 spinel formation by controlling the oxygen in the steel. When Ca content in steel is 1 × 10−6, Ca can modify part of the MgO·Al2O3 spinel inclusions during RH refining, and the amount of Mg required to form spinel is reduced to (0.48–16.39) × 10−6. With the increase of Mg content, the change path of inclusions in IF steel is that Al2O3 change to MgO·Al2O3, and finally change to MgO.
- Al2O3·TiOx will not be stable in the IF molten steel, except for three situations: (1) when the dissolved [O] in the molten steel is up to 15 × 10−6. (2) When the local Ti concentration in the molten steel is up to 0.49% during the titanium alloying adjusting process. (3) When the local Al concentration in the molten steel is as low as 0.0079%.
- In order to ensure the removal of 6–10 μm inclusions, the holding time is suitable for 19–42min.
- The yield strength Rp0.2, tensile strength Rm, elongation after fracture A80, tensile strain hardening index N90, and plastic strain ratio R90 of DC06 IF steel are 138.7 MPa, 304.7 MPa, 45.3%, 0.24, and 2.91, respectively. The mechanical properties of DC06 IF steel meet the requirements.
Author Contributions
R.C.: project administration, conceptualization, investigation, methodology, data curation, writing—original draft preparation. D.C.: Investigation. Q.F.: writing—review and editing. R.L.: project administration. J.L.: investigation, project administration. J.Z.: formal analysis. W.D.: visualization, drawing. H.Z.: funding acquisition, methodology, writing—review and editing. H.N.: conceptualization, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the financial support provided by the Open Youth Fund of State Key Laboratory of Refractories and Metallurgy, Wuhan University of Science and Technology (Grant No. 2018QN03) and and the National Natural Science Foundation of China (51774217).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tian, Y.; An, L.Q.; Jian, L.Z.; Mao, Y.; Guo, M.; Wang, P.; Li, Z.; Wen, T.G. Process analysis and practice of clean IF steel production. J. Iron Steel Res. Int. 2011, 18, 399–405. [Google Scholar]
- Yuan, P.; Li, H.B.; Chen, B.; Zhu, K.R.; Pei, X.W.; Jiang, J.R. Control technology of surface sliver defects for automobile steel sheets. J. Iron Steel Res. 2017, 29, 800–806. [Google Scholar]
- Sui, Y.F.; Sun, G.D.; Zhao, Y.; Wang, C.G.; Guo, M.; Zhang, M. Evolution of titaniferous inclusions in IF steelmaking. J. Univ. Sci. Technol. Beijing 2014, 36, 1174–1182. [Google Scholar]
- Wang, R.; Bao, Y.P.; Li, Y.H.; Li, T.Q.; Chen, D. Effect of slag composition on steel cleanliness in interstitial-free steel. J. Iron Steel Res. Int. 2017, 24, 579–585. [Google Scholar] [CrossRef]
- Shu, H.F.; Liu, L.; Liu, X.H.; Zhang, X.F. Investigation on variation of inclusions and total oxygen in IF steel after RH deoxidization. J. Iron Steel Res. Int. 2011, 18, 347–351. [Google Scholar]
- Qin, Y.M.; Wang, X.H.; Li, L.P.; Huang, F.X. Effect of Oxidizing Slag on Cleanliness of IF Steel during Ladle Holding Process. Steel Res. Int. 2015, 86, 1037–1045. [Google Scholar] [CrossRef]
- Yuan, P.; Zhang, J.; Liu, D.Z.; Li, H.B.; Zhu, K.R.; Chen, B. Formation mechanism and control technology of Al-Ti inclusions in ultra low carbon steel. Iron Steel 2018, 53, 24–30. [Google Scholar]
- Park, D.C.; Jung, I.H.; Rhee, P.C.H.; Lee, H.G. Reoxidation of Al-Ti containing steels by CaO-Al2O3-MgO-SiO2 slag. ISIJ Int. 2004, 44, 1669–1678. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, L.F.; Zhang, Y. Modeling reoxidation behavior of Al–Ti-containing steels by CaO–Al2O3–MgO–SiO2 slag. J. Iron Steel Res. Int. 2018, 25, 146–156. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, C.J. Modification mechanism of spinel inclusions in medium manganese steel with rare earth treatment. Metals 2019, 9, 804. [Google Scholar] [CrossRef]
- Wang, R.; Bao, Y.P.; Yan, Z.J.; Li, D.Z.; Kang, Y. Comparison between the surface defects caused by Al2O3 and TiN inclusions in interstitial-free steel auto sheets. Int. J. Miner. Metall. Mater. 2019, 26, 178–185. [Google Scholar] [CrossRef]
- Todoroki, H.; Mizuno, K. Effect of silica in slag on inclusion compositions in 304 stainless steel deoxidized with aluminum. ISIJ Int. 2004, 44, 1350–1357. [Google Scholar] [CrossRef]
- Yang, S.F.; Li, J.S.; Wang, Z.F.; Li, J.; Lin, L. Modification of MgO·Al2O3 spinel inclusions in Al-killed steel by Ca treatment. Int. J. Miner. Metall. Mater. 2011, 18, 18–23. [Google Scholar] [CrossRef]
- Shin, J.H.; Par, J.H. Modification of Inclusions in Molten Steel by Mg-Ca Transfer from Top Slag: Experimental Confirmation of the ‘Refractory-Slag-Metal-Inclusion (ReSMI)’ Multiphase Reaction Model. Metall. Mater. Trans. B 2017, 48, 1–6. [Google Scholar] [CrossRef]
- Cao, L.; Wang, G.C.; Yuan, X.H.; Jin, P.L.; Sridhar, S. Thermodynamics and agglomeration behavior on spinel inclusion in Al-deoxidized steel coupling with Mg treatment. Metals 2019, 9, 900. [Google Scholar] [CrossRef]
- Chu, Y.P.; Chen, Z.Y.; Liu, N.; Zhang, L.F. Formation and control of spinel inclusions in high-speed heavy rail steel. Iron steel 2020, 55, 38–46. [Google Scholar]
- Wang, Y.; Cui, H.; Wang, W.; Yang, H. Effect of RH circulation time and holding time on cleanliness of IF steel. J. Iron Steel Res. 2017, 29, 649–653. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).