Hydrometallurgical Treatment of Waste Printed Circuit Boards: Bromine Leaching
Abstract
1. Introduction
2. Material and Methods
2.1. Material
2.2. Chemical Analysis Methods
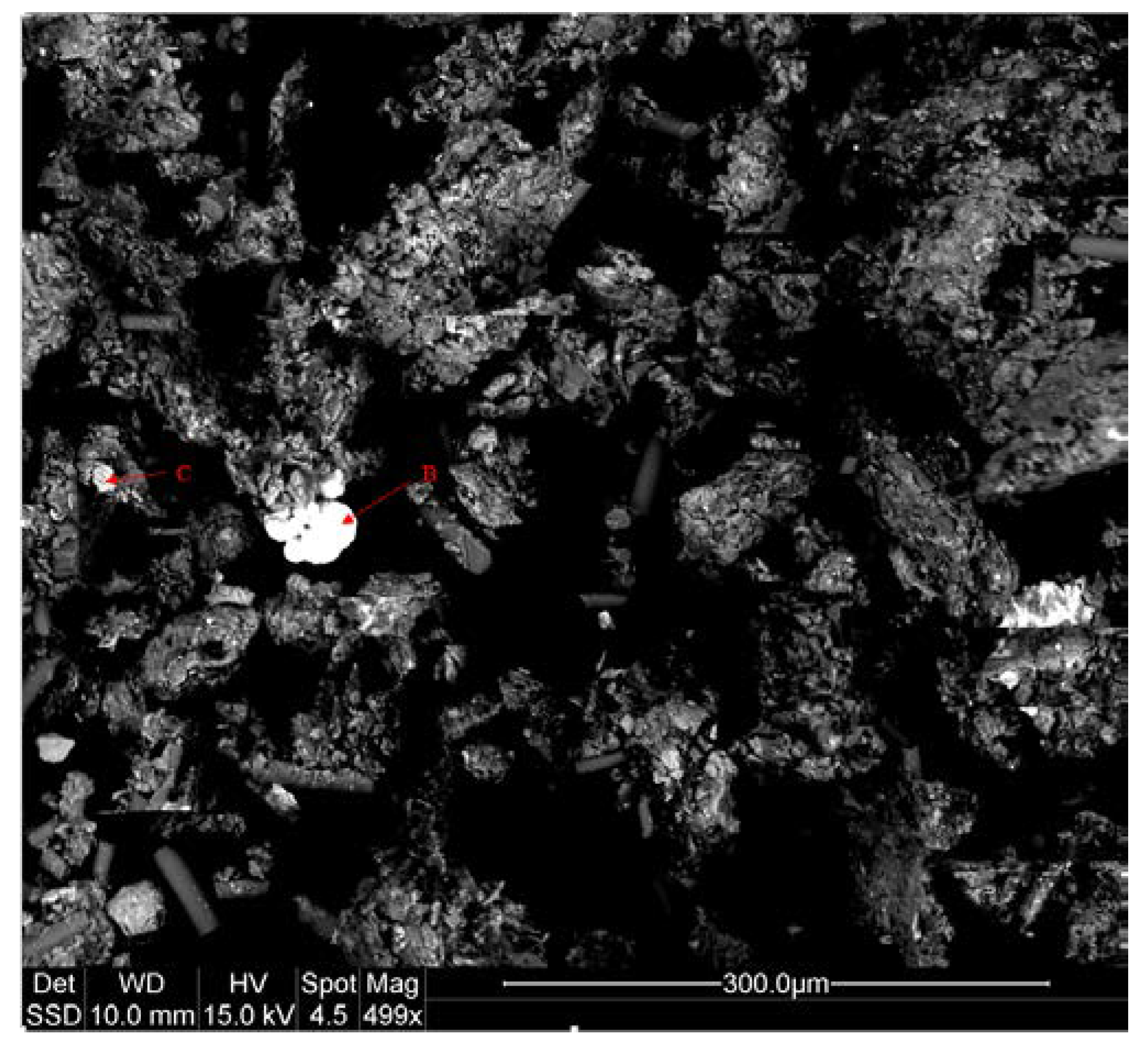
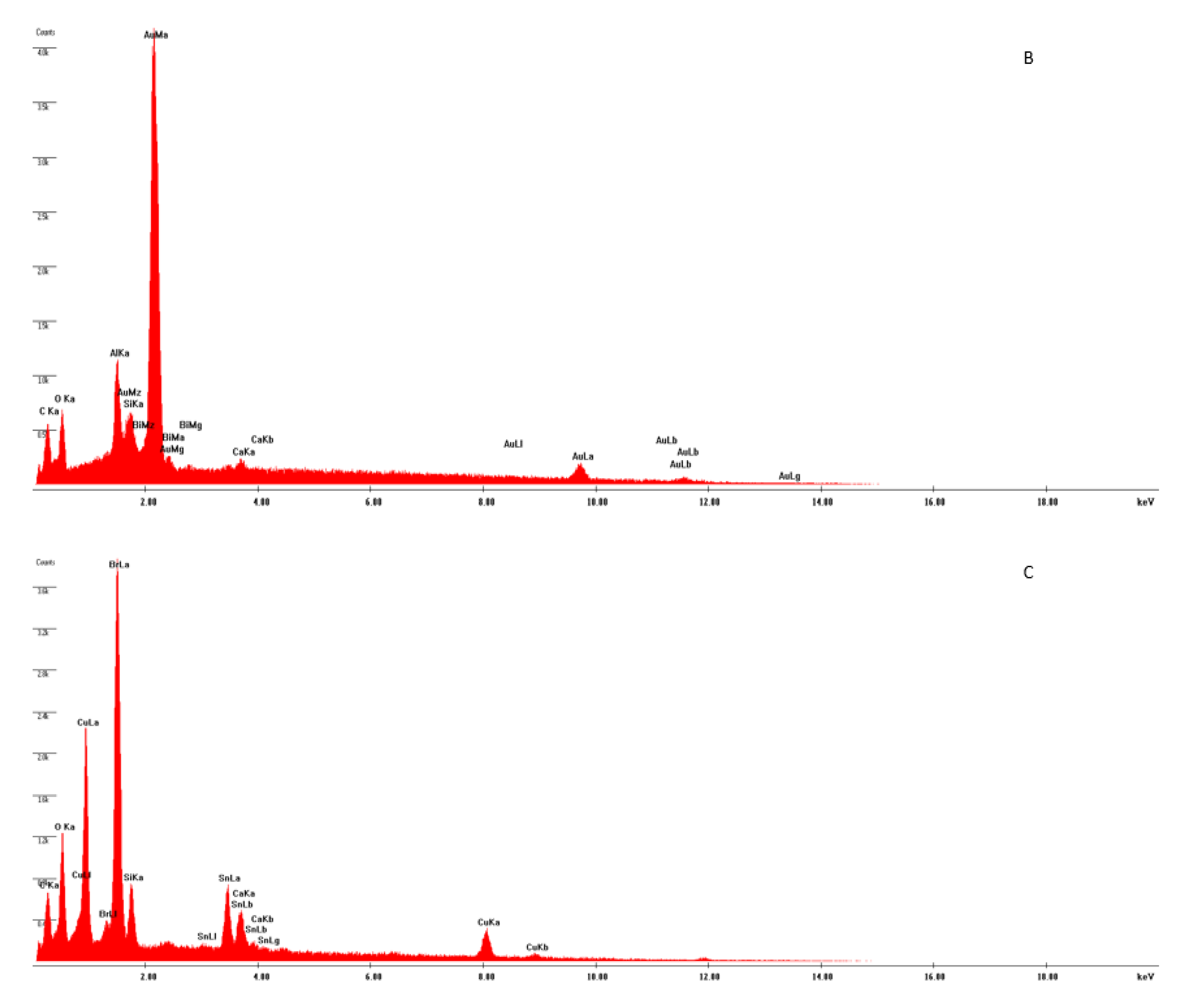
2.3. Mineral Liberation Analysis
2.4. Leaching Tests
3. Result and Discussions
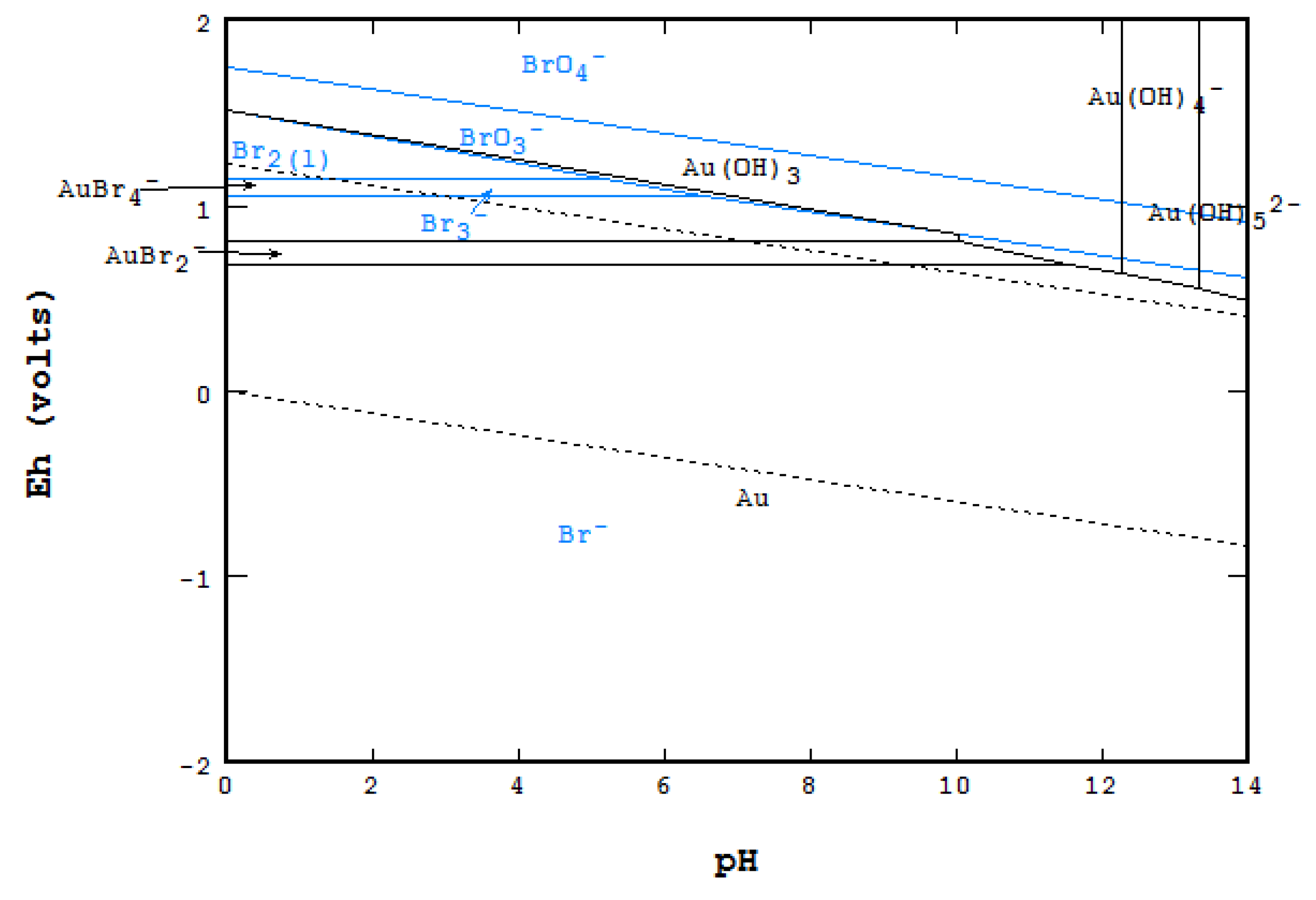
3.1. Sample Characterization
3.2. Bromine Leaching Tests
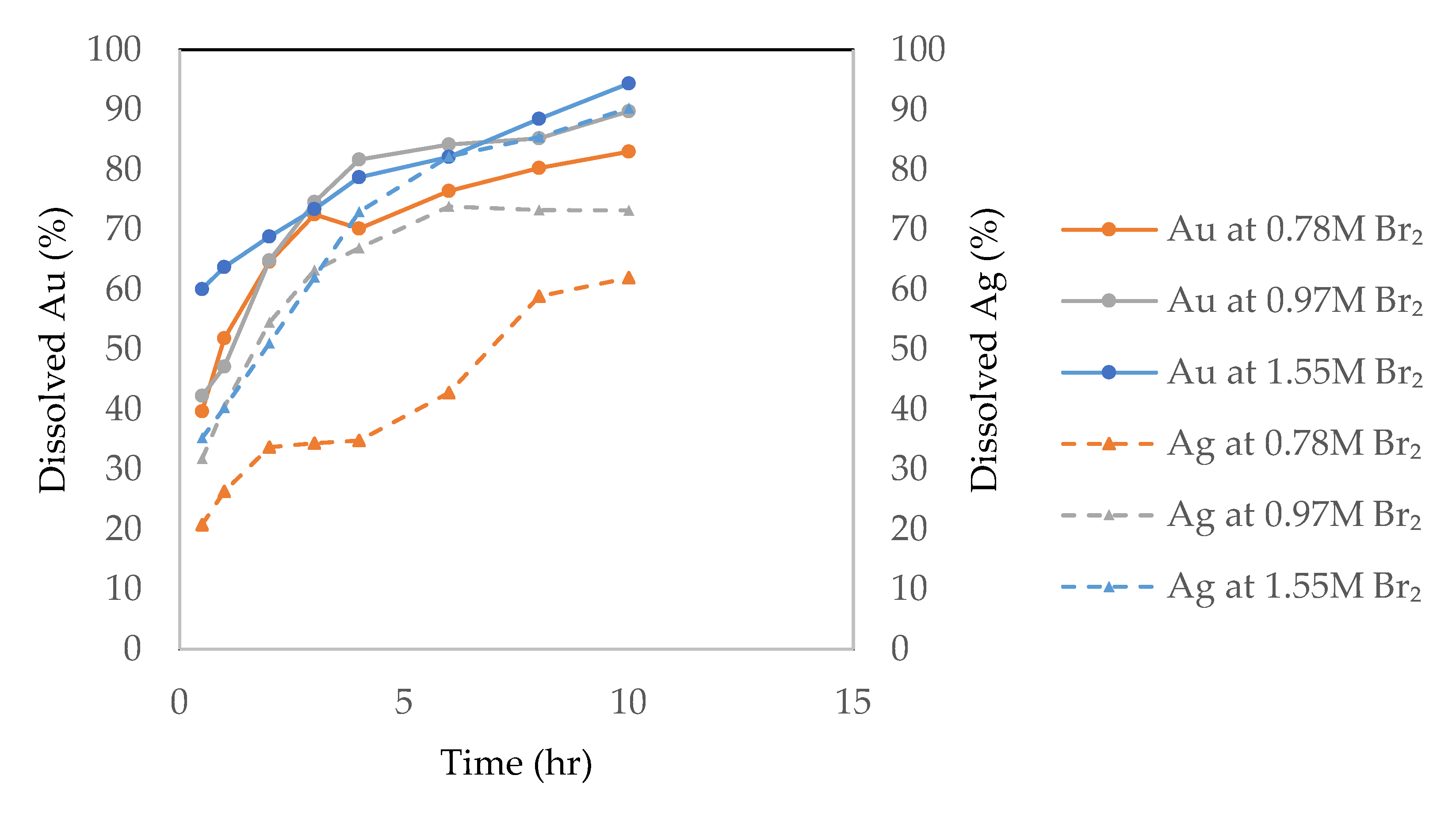
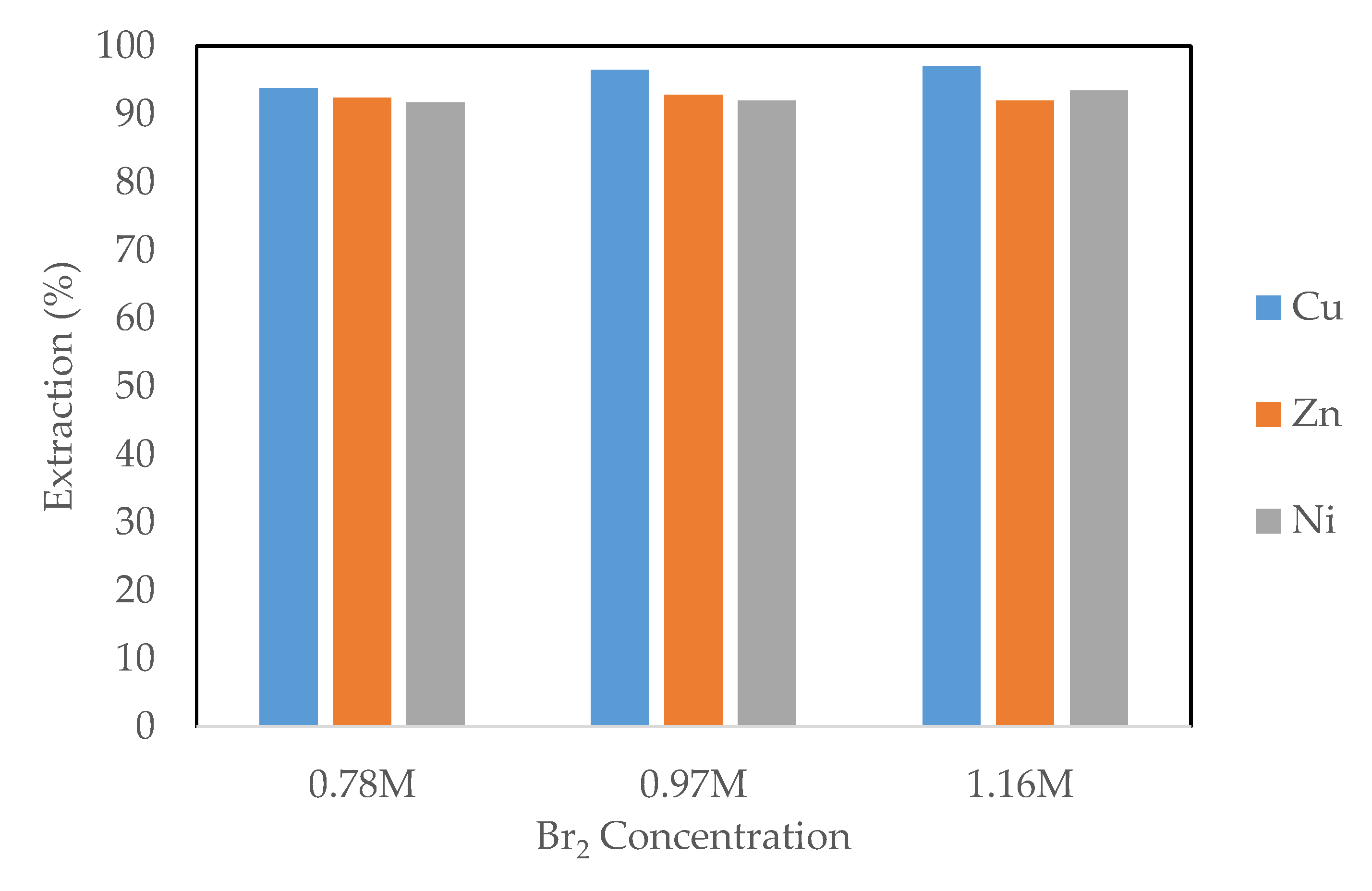
3.2.1. Effect of Liquid Bromine
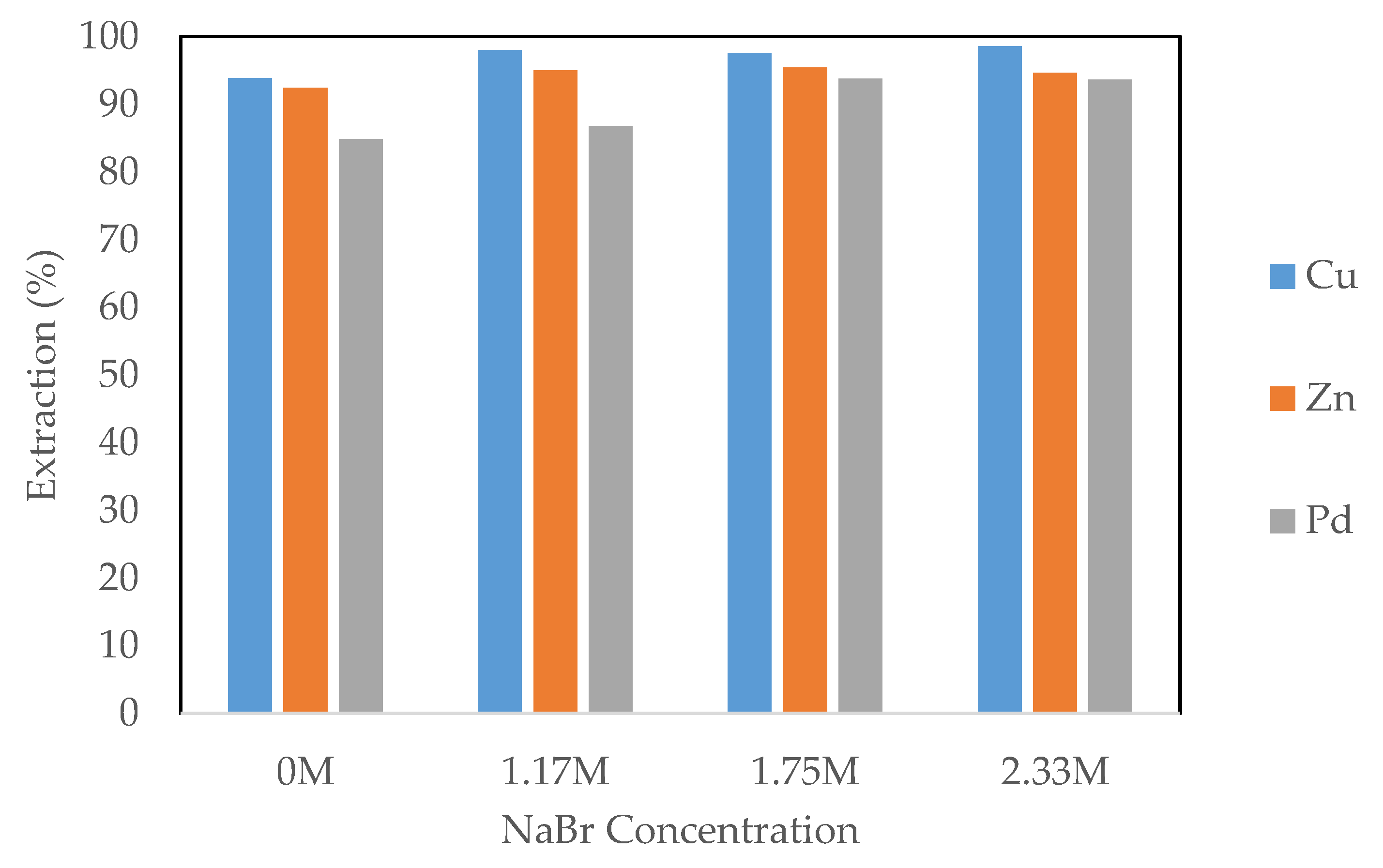
3.2.2. Effect of Sodium Bromide
3.2.3. Effect of Selected Acids
- (1)
- The cost of nitric acid is higher than that of hydrochloric acid.
- (2)
- Nitric acid is required to be removed in order to successfully complete the metal recovery by solvent extraction and electrowinning.
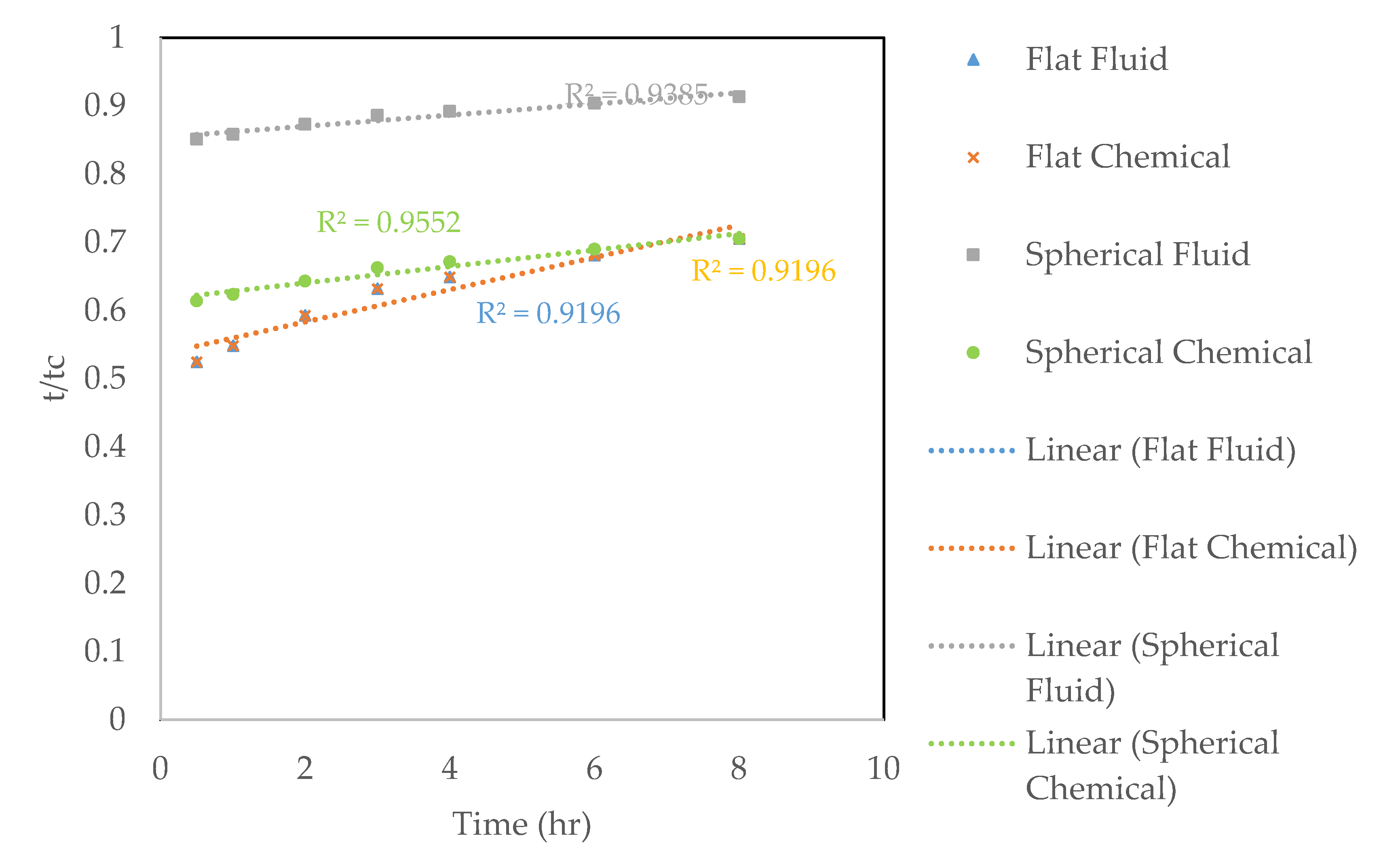
3.3. Leaching Kinetics
- Waste printed circuit boards are heterogeneous, leading to other metallic elements reacting with bromine and also, possibly reacting with gold ions. Thus, the mechanism of gold dissolution in the bromine system becomes more complex.
- The diverse presence of gold in the waste printed circuit boards (coating on board surfaces or gold particles in the waste printed circuit boards) also led to the difference of the leaching mechanisms.
- The metals that are more active than gold may reduce gold from gold–bromine complex ions to gold metal, which may further change the leaching mechanism.
- There is also a possibility of a mechanism change as the leaching proceeds.
- In terms of the chemical reaction, three steps may be involved:
- (1)
- Adsorption of bromine and bromide on the gold surface to form AuBr2 [32],
- (2)
- Oxidation of AuBr2− to produce a stable species, AuBr4−,
- (3)
- Copper cementation to reduce AuBr4− to Au0.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Widmer, R.; Oswald-Krapf, H.; Sinha-Khetriwal, D.; Schnellmann., M.; Böni, H. Global perspectives on e-waste. Environ. Impact Assess. Rev. 2005, 25, 436–458. [Google Scholar] [CrossRef]
- Tuncuk, A.; Stazi, V.; Akcil, A.; Yazici, E.Y.; Deveci, H. Aqueous metal recovery techniques from e-scrap: Hydrometallurgy in recycling. Miner. Eng. 2012, 25, 28–37. [Google Scholar] [CrossRef]
- Schluep, M.; Hagelueken, C.; Kuehr, R.; Magalini, F.; Maurer, C.; Meskers, C.; Mueller, E.; Wang, F. Recycling-from E-Waste to Resources; Oktoberdruck AG;: Berlin, Germany, 2009; p. 6, United Nationals Environment Programme & United Nations University. [Google Scholar]
- Vasile, C.; Brebu, M.A.; Totolin, M.; Yanik, J.; Karayildirim, T.; Darie, H. Feedstock recycling from the printed circuit boards of used computers. Energy Fuels 2008, 22, 1658–1665. [Google Scholar] [CrossRef]
- Hino, T.; Agawa, R.; Moriya, Y.; Nishida, M.; Tsugita, Y.; Araki, T. Techniques to separate metal from waste printed circuit boards from discarded personal computers. J. Mater. Cycles Waste Manag. 2009, 11, 42–54. [Google Scholar] [CrossRef]
- Birloaga, I.; Michelis, I.D.; Ferella, F.; Buzatu, M.; Vegliò, F. Study on the influence of various factors in the hydrometallurgical processing of waste printed circuit boards for copper and gold recovery. Waste Manag. 2013, 33, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Xu, Z.; Wen, J.; Yang, L. Factors influencing bioleaching copper from waste printed circuit boards by acidithiobacillus ferrooxidants. Hydrometallurgy 2009, 97, 29–32. [Google Scholar] [CrossRef]
- Oishi, T.; Koyama, K.; Alam, S.; Tanaka, M.; Lee., L.C. Recovery of high purity copper cathode from printed circuit boards using ammoniacal sulfate or chloride solutions. Hydrometallurgy 2007, 89, 82–88. [Google Scholar] [CrossRef]
- Behnamfard, A.; Salarirad, M.M.; Veglio, F. Process development for recovery of copper and precious metals from waste printed circuit boards with emphasize on palladium and gold leaching and precipitation. Waste Manag. 2013, 33, 2354–2363. [Google Scholar] [CrossRef] [PubMed]
- Boardsort.com Current Payout Rates-as of 12/15/2017. Available online: http://broadsort.com/payout.php (accessed on 15 December 2017).
- Gold, InvestmentMine, Commodity and Metal Prices. Available online: http://www.infomine.com/investment/gold/ (accessed on 1 December 2017).
- Silver, InvestmentMine, Commodity and Metal Prices. Available online: http://www.infomine.com/investment/silver/ (accessed on 1 December 2017).
- Palladium, InvestmentMine, Commodity and Metal Prices. Available online: http://www.infomine.com/investment/palladium/ (accessed on 1 December 2017).
- Copper, InvestmentMine, Commodity and Metal Prices. Available online: http://www.infomine.com/investment/copper/ (accessed on 1 December 2017).
- Tin, InvestmentMine, Commodity and Metal Prices. Available online: http://www.infomine.com/investment/tin/ (accessed on 1 December 2017).
- Lead, InvestmentMine, Commodity and Metal Prices. Available online: http://www.infomine.com/investment/lead/ (accessed on 1 December 2017).
- Zinc, InvestmentMine, Commodity and Metal Prices. Available online: http://www.infomine.com/investment/zinc/ (accessed on 1 December 2017).
- Nickel, InvestmentMine, Commodity and Metal Prices. Available online: http://www.infomine.com/investment/zinc/ (accessed on 1 December 2017).
- Scrap Iron Prices, SCRAP Metal Pricer. Available online: https://www.scrapmetalpricer.com/ (accessed on 3 December 2017).
- Cui, H.; Anderson, C. Literature review of hydrometallurgical recycling of printed circuit boards (PCBs). J. Adv. Chem. Eng. 2016, 6, 142. [Google Scholar]
- Yazici, E.Y.; Deveci, H. Ferric sulfate leaching of metals from waste printed circuit boards. Int. J. Miner. Process. 2014, 133, 39–45. [Google Scholar] [CrossRef]
- Yazici, E.Y.; Deveci, H. Cupric chloride leaching (HCl-CuCl2-NaCl) of metals from waste printed circuit boards (WPCBs). Int. J. Miner. Process. 2015, 134, 89–96. [Google Scholar] [CrossRef]
- Zhu, P.; Chen, Y.; Wang, L.Y.; Zhou, M. Treatment of waste printed circuit board by green solvent using ionic liquid. Waste Manag. 2012, 32, 1914–1918. [Google Scholar] [CrossRef] [PubMed]
- Zhang Y. Liu, S.; Xie, H.; Zeng, X.; Li, J. Current status on leaching precious metals from waste printed circuit boards. Procedia Environ. Sci. 2012, 16, 560–568. [Google Scholar] [CrossRef]
- Petter, P.M.H.; Veit, H.M.; Bernardes, A.M. Evaluation of gold and silver leaching from printed circuit board of cellphones. Waste Manag. 2014, 34, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Kelsall, G.H.; Welham, N.J.; Diaz, M.A. Thermodynamics of Cl-H2O, Br-H2O, I-H2O, Au-Cl-H2O, Au-Br-H2O and Au-I-H2O systems at 298K. J. Electroanal. Chem. 1993, 361, 13–24. [Google Scholar] [CrossRef]
- Sahin, M.; Akcil, A.; Erust, C.; Altynbek, S.; Gahan, C.; Tuncuk, A. A potential alternative for precious metal recovery from e-waste: iodine leaching. Sep. Sci. Technol. 2015, 50, 2587–2595. [Google Scholar] [CrossRef]
- Batnasan, A.; Haga, K.; Shibayama, A. Recovery of valuable metals from waste printed circuit boards by using iodine- iodide leaching and precipitation. In Rare Metal Technology 2018; Kim., H., Wesstrom, B., Alam, S., Ouchi, T., Azimi, G., Neelameggham, N.R., Wang, S., Guan, X., Eds.; TMS: Pittsburgh, PA, USA, 2018; pp. 131–142. [Google Scholar]
- Lyday, P.A. Bromine, Mineral Yearbook; U.S. Geological Survey: Reston, VA, USA, 2002.
- Zhutaeva, G.V.; Shumilova, N.A. Silver. In Standard Potentials in Aqueous Solution; Bard, A.J., Parsons, R., Jordan, J., Eds.; International Union of Pure and Applied Chemistry, Marcel Dekker, Inc., CRC Press: Boca Raton, FL, USA, 1985; p. 297. [Google Scholar]
- Dadgar, A. Extraction and recovery of gold from concentrates by bromine process. In Precious Metals ’89; Jha, M.C., Hill, S.D., Eds.; The Minerals, Metals & Materials Society: Pennsylvania, PA, USA, 1988; pp. 227–240. [Google Scholar]
- Pesic, B.; Sergent, R.H. Reaction mechanism of gold dissolution with bromine. Metall. Mater. Trans. B 1993, 24, 419–431. [Google Scholar] [CrossRef]
- Apodaca, L.E. Sulfur, Mineral Commodities; U.S. Geological Survey: Reston, VA, USA, 2018.
- Gupta, C.K.; Mukherjee, T.K. Leaching with acid. In Hydrometallurgy in Extraction Processes; Gupta, C.K., Mukherjee, T.K., Eds.; CRC Press: Boca Raton, FL, USA, 1990; Volume I, p. 59. [Google Scholar]
- Zhang, W.; BAS, A.D.; Ghali, E.; Choi, Y. Passive behavior of gold in sulfuric acid medium. Trans. Nonferrous Met. Soc. China 2015, 25, 2037–2046. [Google Scholar] [CrossRef]


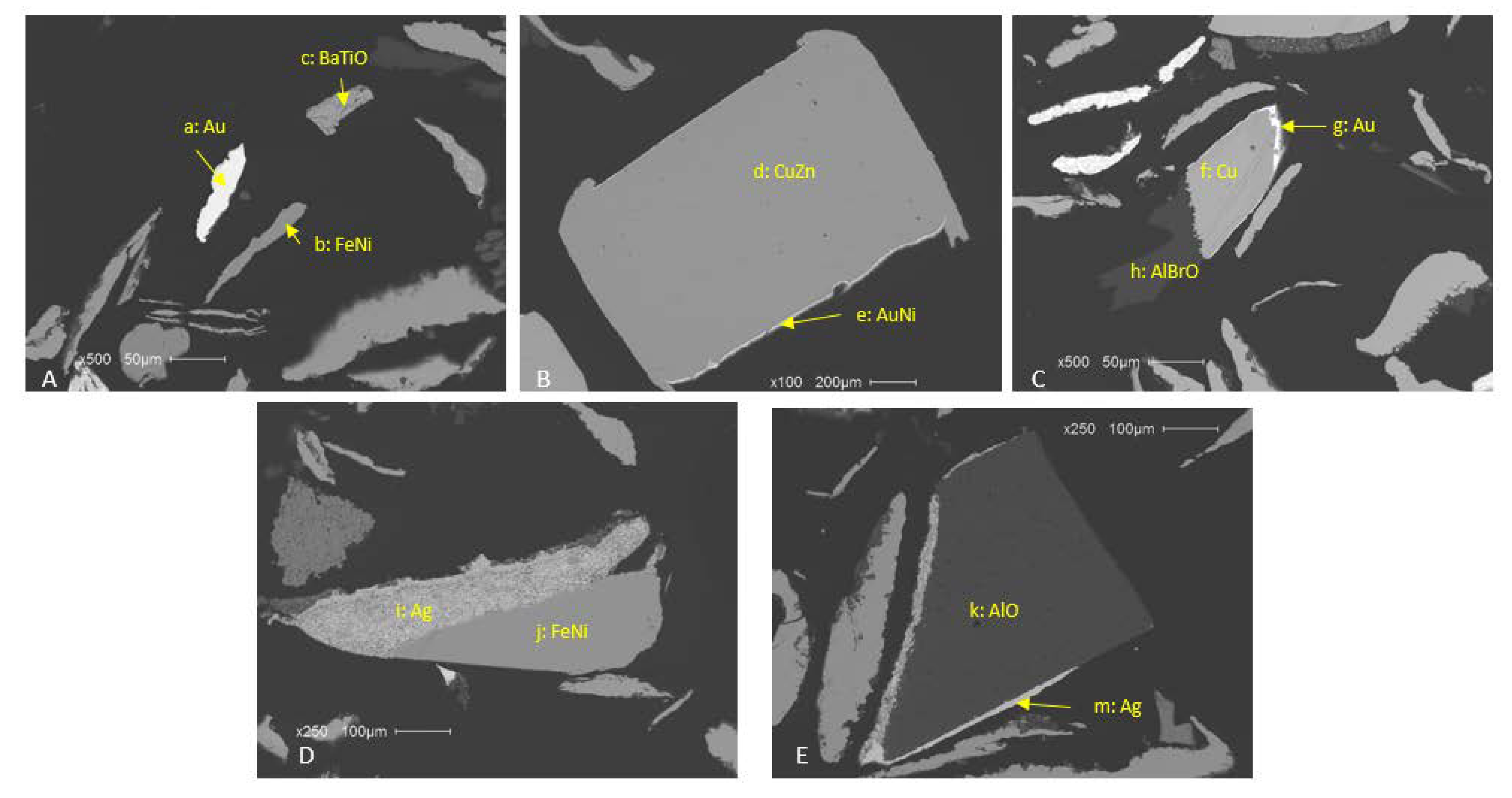
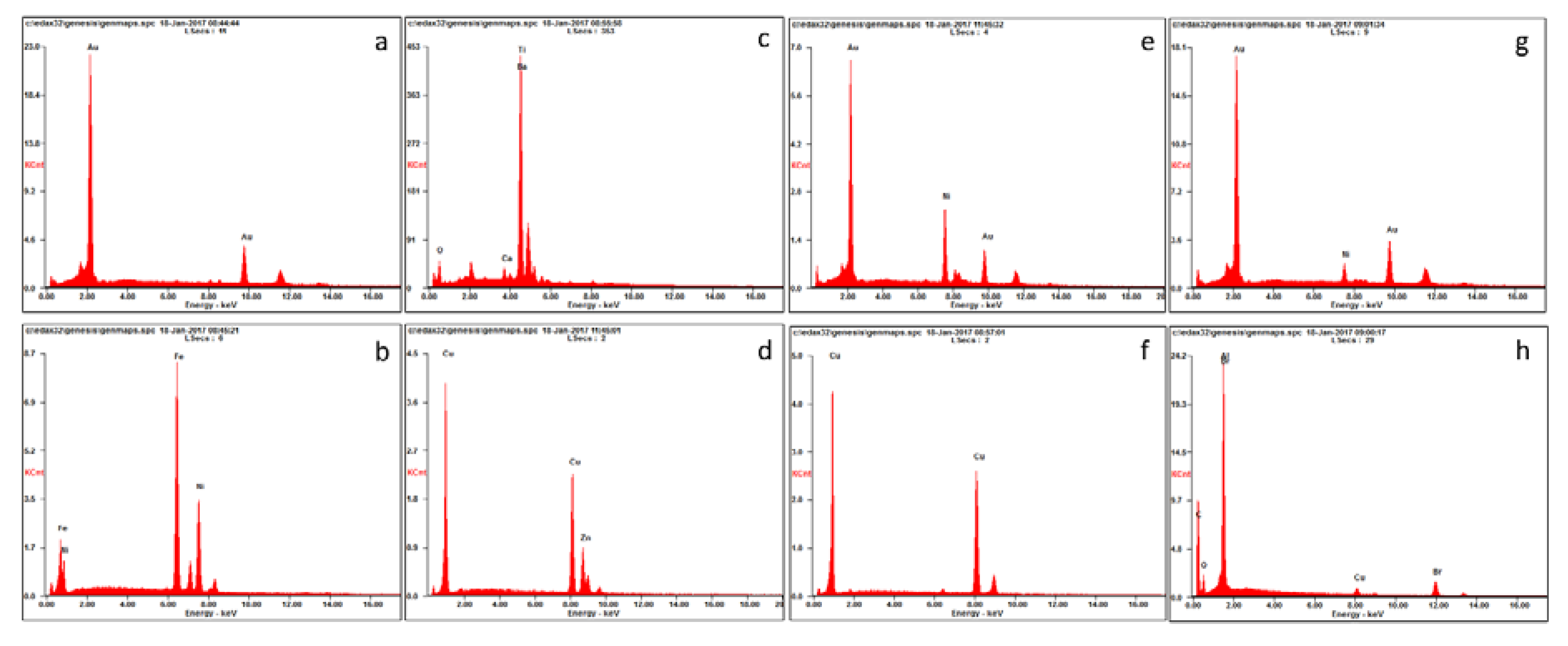
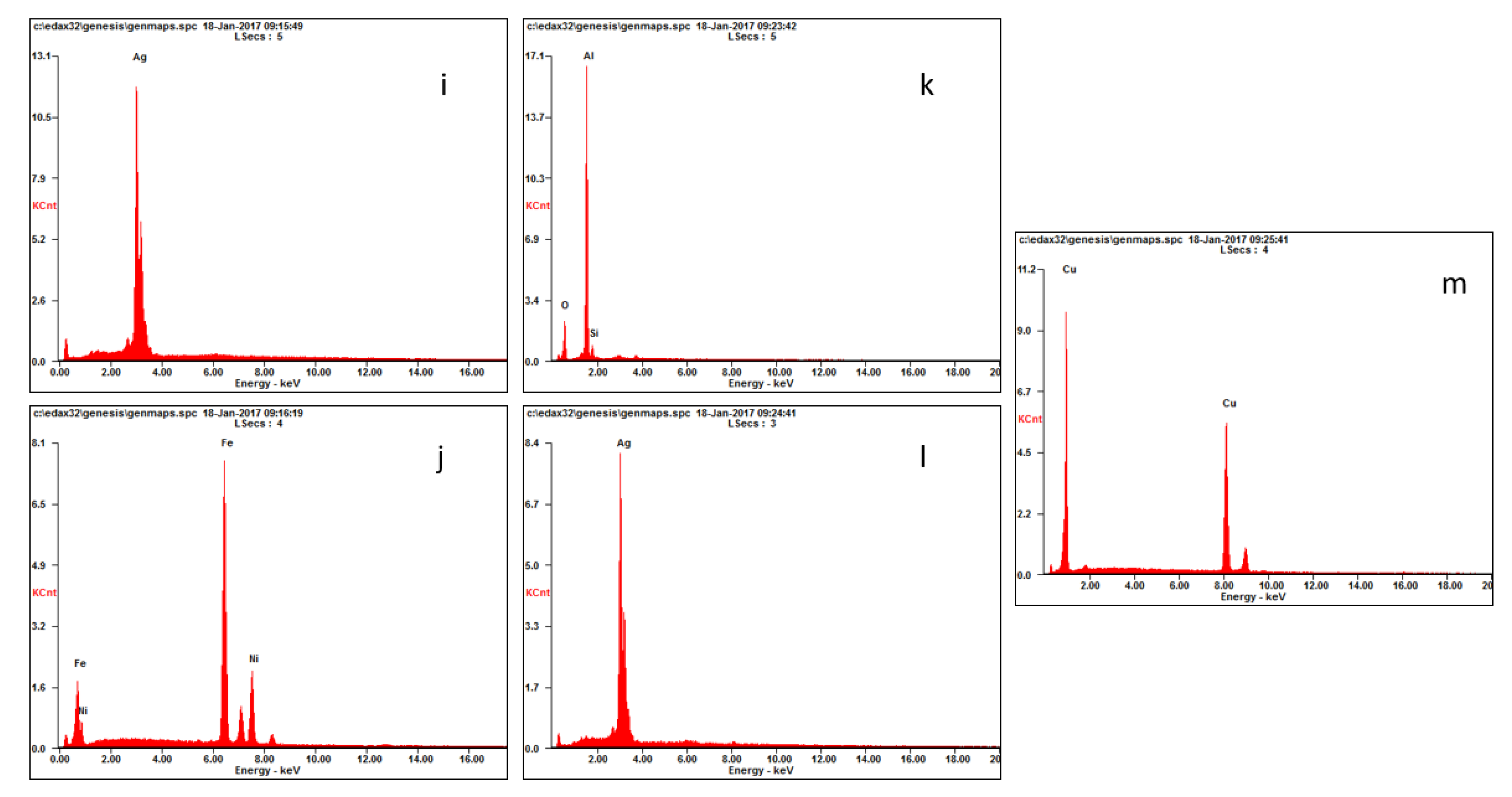

| Material | Element | Vasile et al. 2008 [4] | Hino et al. 2009 [5] | Birloaga et al. 2013 [6] | Yang et al. 2009 [7] | Oishi et al. 2007 [8] | Behnamfard et al. 2013 [9] | Average |
|---|---|---|---|---|---|---|---|---|
| Organic epoxy resin | C (wt. %) | 24.7 | 18.1 | - | - | - | - | 21.4 |
| H (wt. %) | 1.38 | 1.8 | - | - | - | - | 1.59 | |
| N (wt. %) | 0.85 | 0.32 | - | - | - | - | 0.59 | |
| Br (wt. %) | 4.94 | 5.07 | - | - | - | - | 5.01 | |
| Sb (wt. %) | - | 0.45 | - | - | 0.16 | 0.37 | 0.33 | |
| Inorganic glass fiber | - | 37.6 | - | - | - | - | 37.6 | |
| Cu (wt. %) | 13.8 | 14.6 | 30.6 | 25.1 | 26 | 19.2 | 21.5 | |
| Metallic elements | Fe (wt. %) | 1.97 | 4.79 | 15.21 | 0.66 | 3.4 | 1.13 | 4.53 |
| Sn (wt. %) | - | 5.62 | 7.36 | 1.86 | 4.9 | 0.69 | 4.09 | |
| Ni (wt. %) | 0.17 | 1.65 | 1.58 | 0.0024 | 1.5 | 0.17 | 0.85 | |
| Zn (wt. %) | - | - | 1.86 | 0.04 | 2.6 | 0.84 | 1.34 | |
| Pb (wt. %) | - | 2.96 | 6.70 | 0.80 | 3.0 | 0.39 | 2.77 | |
| Au (ppm) | - | 205 | 238 | - | - | 130 | 191 | |
| Ag (ppm) | - | 450 | 688 | - | 630 | 704 | 618 | |
| Pd (ppm) | - | 220 | - | - | - | 27 | 124 |
| Metal | Market Price Per Unit (USD) | Unit | Average Composition (%) | Market Value Per Tonne of WPCBs (USD) |
|---|---|---|---|---|
| Gold | 41.5 | g | 0.0191 | 7927 |
| Silver | 0.54 | g | 0.0618 | 334 |
| Palladium | 32.5 | g | 0.0124 | 4030 |
| Copper | 7 | kg | 21.5 | 1505 |
| Tin | 20 | kg | 4.09 | 818 |
| Fe | 9.5 | kg | 4.53 | 429 |
| Lead | 2 | kg | 2.77 | 55 |
| Zinc | 3 | kg | 1.34 | 40 |
| Nickel | 11 | kg | 0.85 | 94 |
| Total market value per tonne of printed circuit boards (USD) | 15,231 | |||
| Reagent | Pros | Cons |
|---|---|---|
| Sulfuric acid | Highly selective, low reagent cost, well established process for copper ore | At elevated temperature, corrosive |
| Chloride | Fast kinetics at room temperature, high solubility and activity of base metals, low toxicity | Excessive corrosion, difficul telectrowinning of copper, poor quality of copper |
| Aqua regia | Fast kinetics, effective | High reagent cost, highly corrosive, low selectivity |
| Ionic liquids | Thermally stable, environmentally friendly | High cost, excessive dosage |
| Reagent | Pros | Cons |
|---|---|---|
| Cyanide | Highly effective, low reagent dosage and cost | Difficult to process wastewater, environmental risk, low kinetics |
| Thiourea | Less toxic, high reaction rate, less interference ions | Poorer stability, high consumption, more expensive than cyanide, downstream metal recovery |
| Thiosulfate | High selectivity, non-toxic and non-corrosive, fast leaching rate | High consumption of reagent, downstream metal recovery |
| Halide | High leaching rate, high selectivity, relatively healthy and safe except for bromine | Highly corrosive for chlorine, high consumption for iodine |
| Aqua regia | Fast kinetics, low reagent dosage | Strongly oxidative and corrosive, difficult to deal with downstream |
| Element | Fe (%) | Ni (%) | Cu (%) | Zn (%) | Pb (%) | Sn (%) | Sb (%) | Pd (ppm) | Ag (ppm) | Au (ppm) |
|---|---|---|---|---|---|---|---|---|---|---|
| Content | 6.3 | 0.5 | 24.1 | 3.3 | 1.1 | 3.8 | 0.3 | 20 | 513 | 145 |
| Standard deviation | 0.7 | 0.06 | 1.9 | 0.3 | 0.05 | 0.4 | 0.03 | 2.6 | 46 | 18 |
| Phase | Formula | Coarse | Fine | −40 mesh |
|---|---|---|---|---|
| Copper | Cu | 70.4 | 65.5 | 69.0 |
| FeMnZnO | Fe3.5MnZn0.5O3 | 1.13 | 6.33 | 6.65 |
| BrCO | (CH2)40BrO6 | 0.67 | 2.15 | 2.82 |
| SnBi | Sn0.8Bi0.2 | 3.82 | 2.78 | 1.53 |
| BaTiO | BaTiO3 | 0.06 | 1.67 | 1.65 |
| PbSn | Pb0.8Sn0.2 | 1.18 | 1.21 | 1.21 |
| Carbon_Mix | C10BaSO4 | 0.11 | 0.52 | 0.52 |
| BrSbCO | (CH2)40BrO6Sb0.3 | 0.04 | 0.15 | 0.17 |
| NdTiO | NdTiO3 | 1.83 | 0.14 | 0.11 |
| Fe60Ni40 | Fe0.6Ni0.4 | 1.10 | 0.58 | 0.13 |
| Metal | 5 v/v% (0.9 M) H2SO4 Extraction (%) | 15 v/v% (2.7 M) H2SO4 Extraction (%) | 5 v/v% (2.0 M) HCl Extraction (%) | 5 v/v% (1.2 M) HNO3 Extraction (%) |
|---|---|---|---|---|
| Ni | 95.8 | 93.4 | 95.2 | 94.6 |
| Cu | 97.6 | 96.8 | 97.9 | 97.8 |
| Zn | 97.2 | 91.7 | 92.5 | 95.8 |
| Sn | 98.5 | 99.2 | 96.8 | 97.1 |
| Pd | 88.7 | 91.6 | 90.0 | 96.4 |
| Ag | 90.0 | 84.8 | 96.5 | 97.2 |
| Au | 91.0 | 71.4 | 95.6 | 93.6 |
| Metal | Average Extraction (%) | Standard Deviation |
|---|---|---|
| Ni | 95.21 | 1.81 |
| Cu | 97.88 | 0.56 |
| Zn | 92.50 | 5.36 |
| Sn | 96.79 | 0.44 |
| Pb | 97.61 | 0.29 |
| Pd | 90.04 | 5.41 |
| Ag | 96.52 | 0.79 |
| Au | 95.59 | 2.05 |
| Br2 (mol/L) | NaBr (mol/L) | Cu (mol/L) | Rate (mmol/L∙min) |
|---|---|---|---|
| 0.388 | 0 | 0.156 | 0.00434 |
| 0.776 | 0 | 0.156 | 0.00635 |
| 1.94 | 0 | 0.156 | 0.00751 |
| 1.94 | 0 | 0.261 | 0.00609 |
| 1.94 | 0.583 | 0.156 | 0.01244 |
| 1.94 | 1.166 | 0.156 | 0.01386 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, H.; Anderson, C. Hydrometallurgical Treatment of Waste Printed Circuit Boards: Bromine Leaching. Metals 2020, 10, 462. https://doi.org/10.3390/met10040462
Cui H, Anderson C. Hydrometallurgical Treatment of Waste Printed Circuit Boards: Bromine Leaching. Metals. 2020; 10(4):462. https://doi.org/10.3390/met10040462
Chicago/Turabian StyleCui, Hao, and Corby Anderson. 2020. "Hydrometallurgical Treatment of Waste Printed Circuit Boards: Bromine Leaching" Metals 10, no. 4: 462. https://doi.org/10.3390/met10040462
APA StyleCui, H., & Anderson, C. (2020). Hydrometallurgical Treatment of Waste Printed Circuit Boards: Bromine Leaching. Metals, 10(4), 462. https://doi.org/10.3390/met10040462





