Evolution of Inclusions in Steelmaking Process of Rare Earth Steels Containing Arsenic with Alumina Crucibles
Abstract
1. Introduction
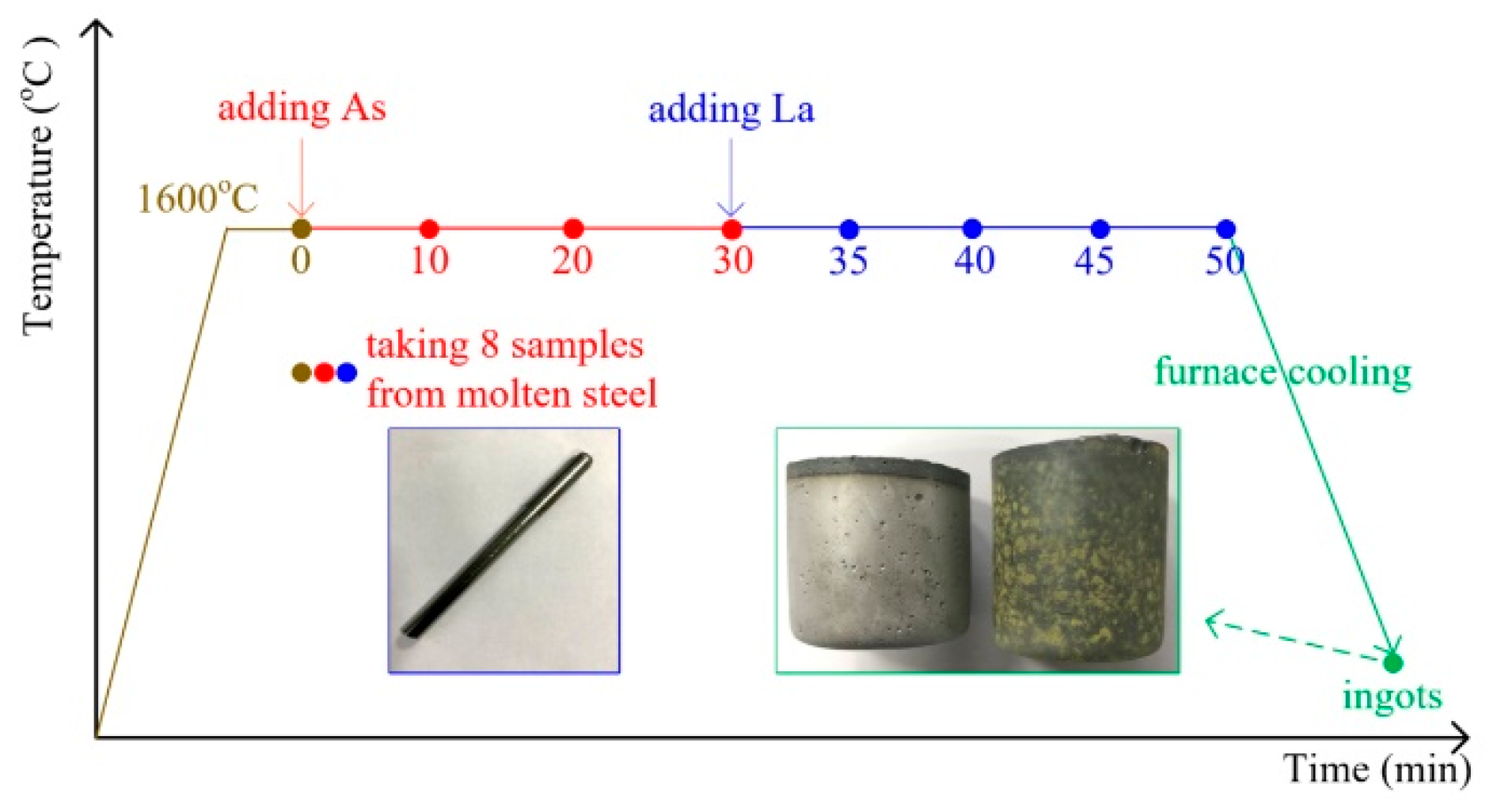
2. Materials and Methods
3. Results and Discussion
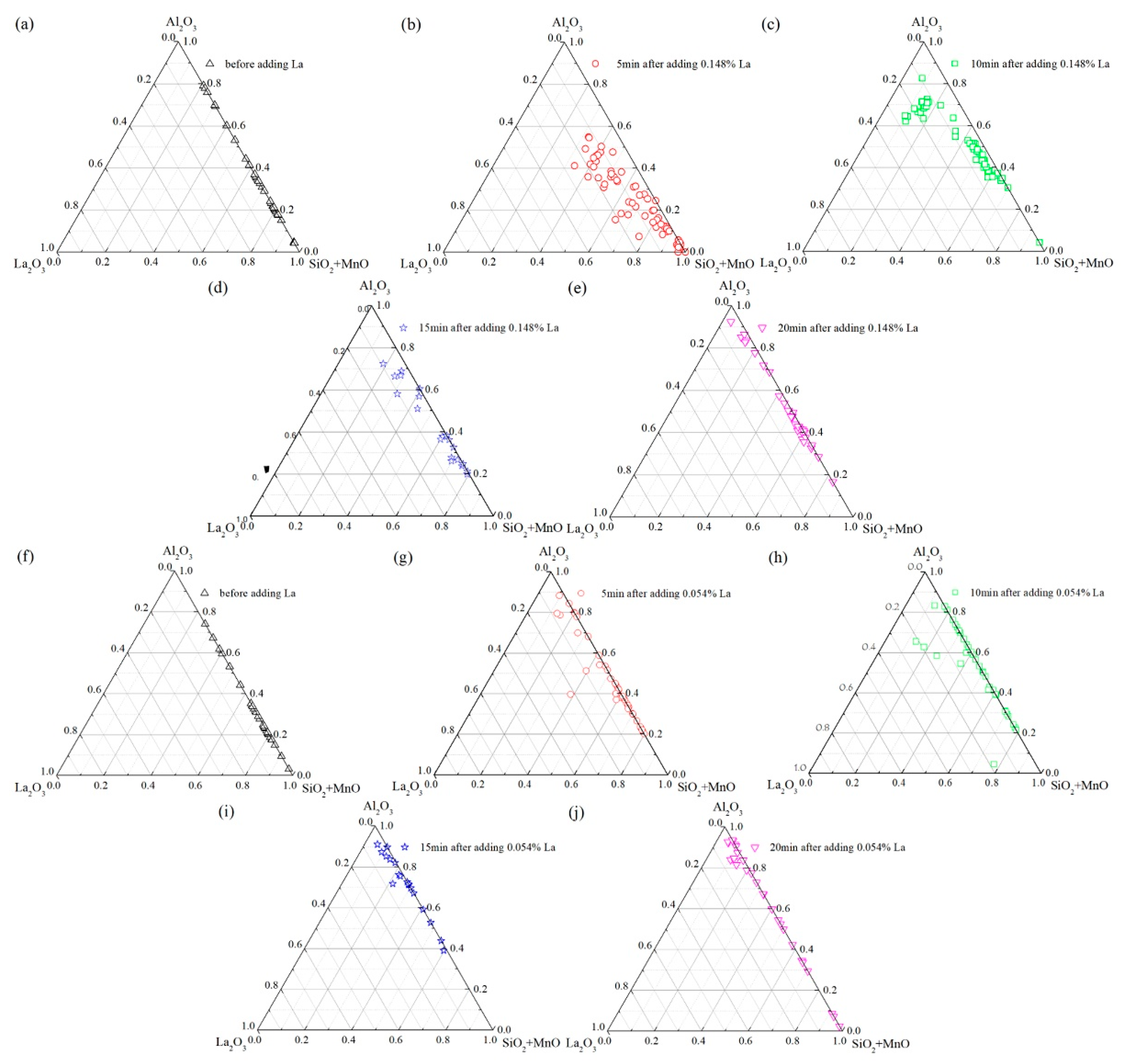
3.1. Evolution of Inclusions in Molten Steel
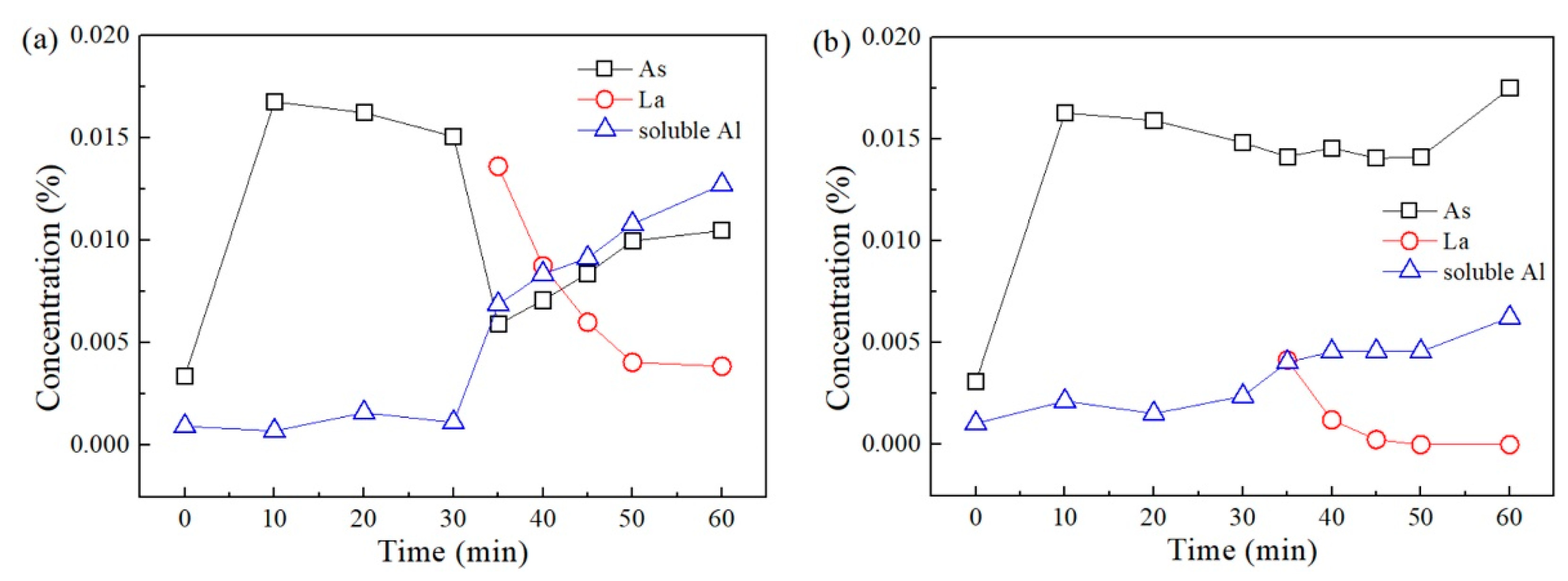
3.2. Changes in Concentrations of Lanthanum, Arsenic, and Acid-soluble Aluminum in Steel
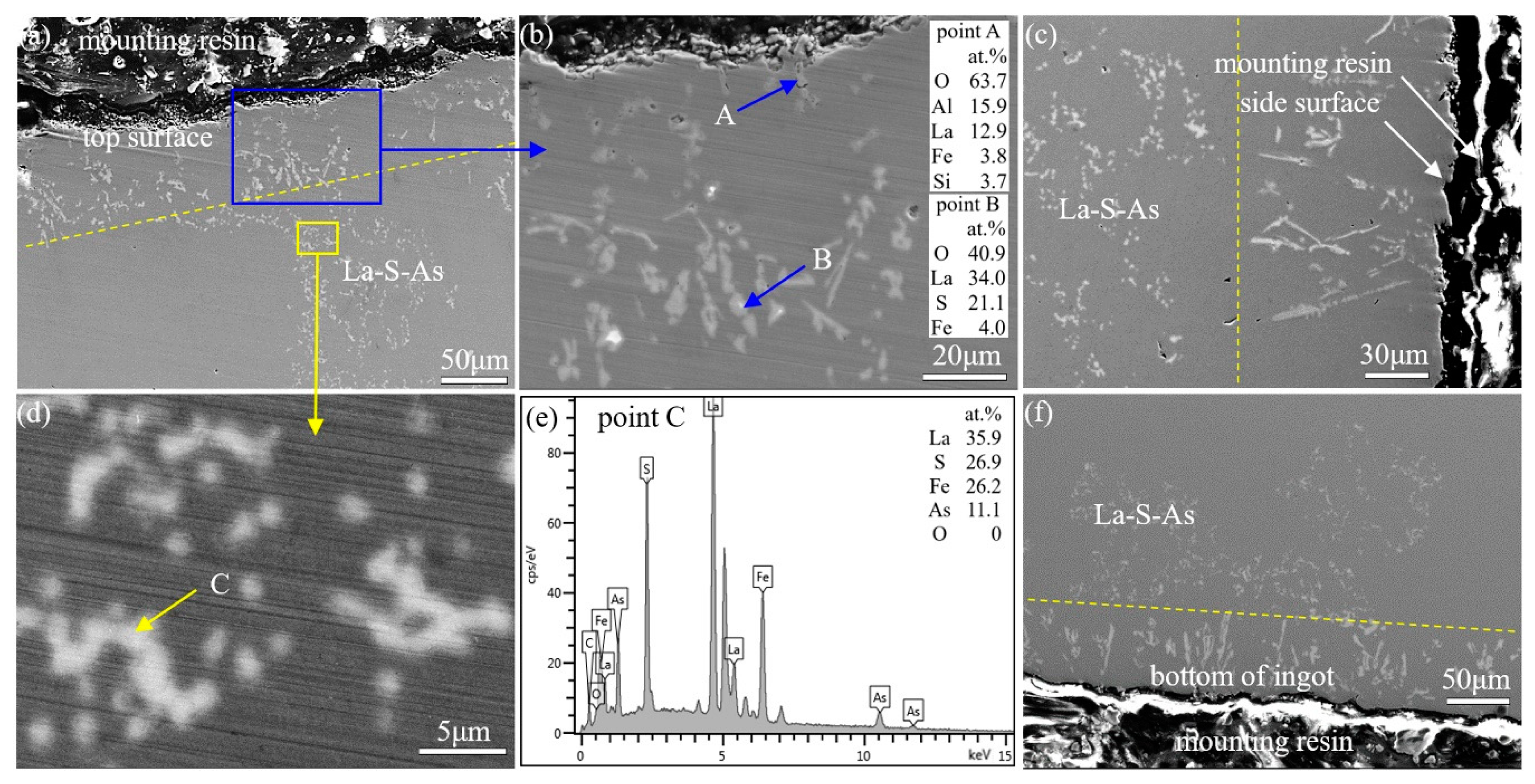
3.3. Types and Distribution of Inclusions in Ingots
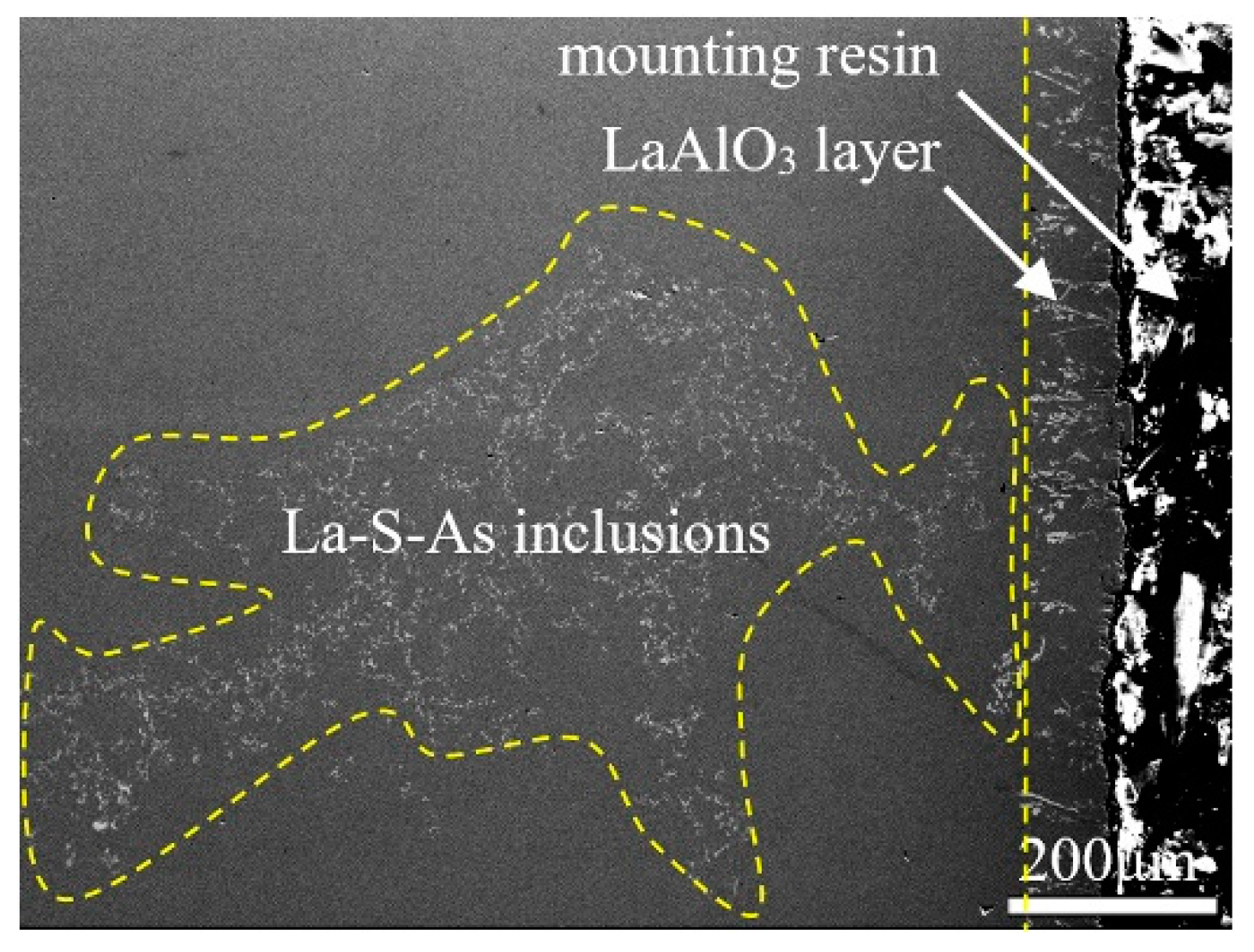
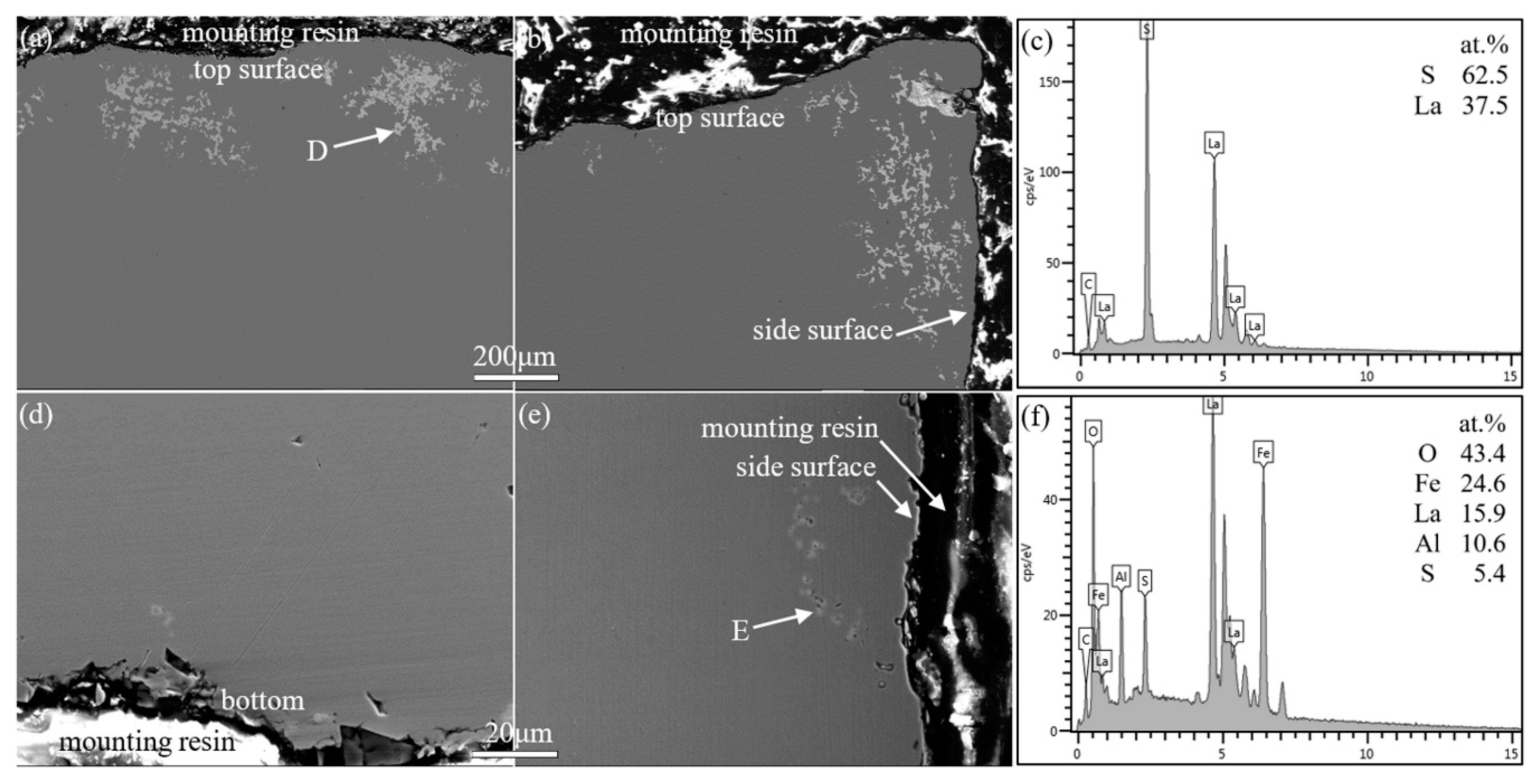
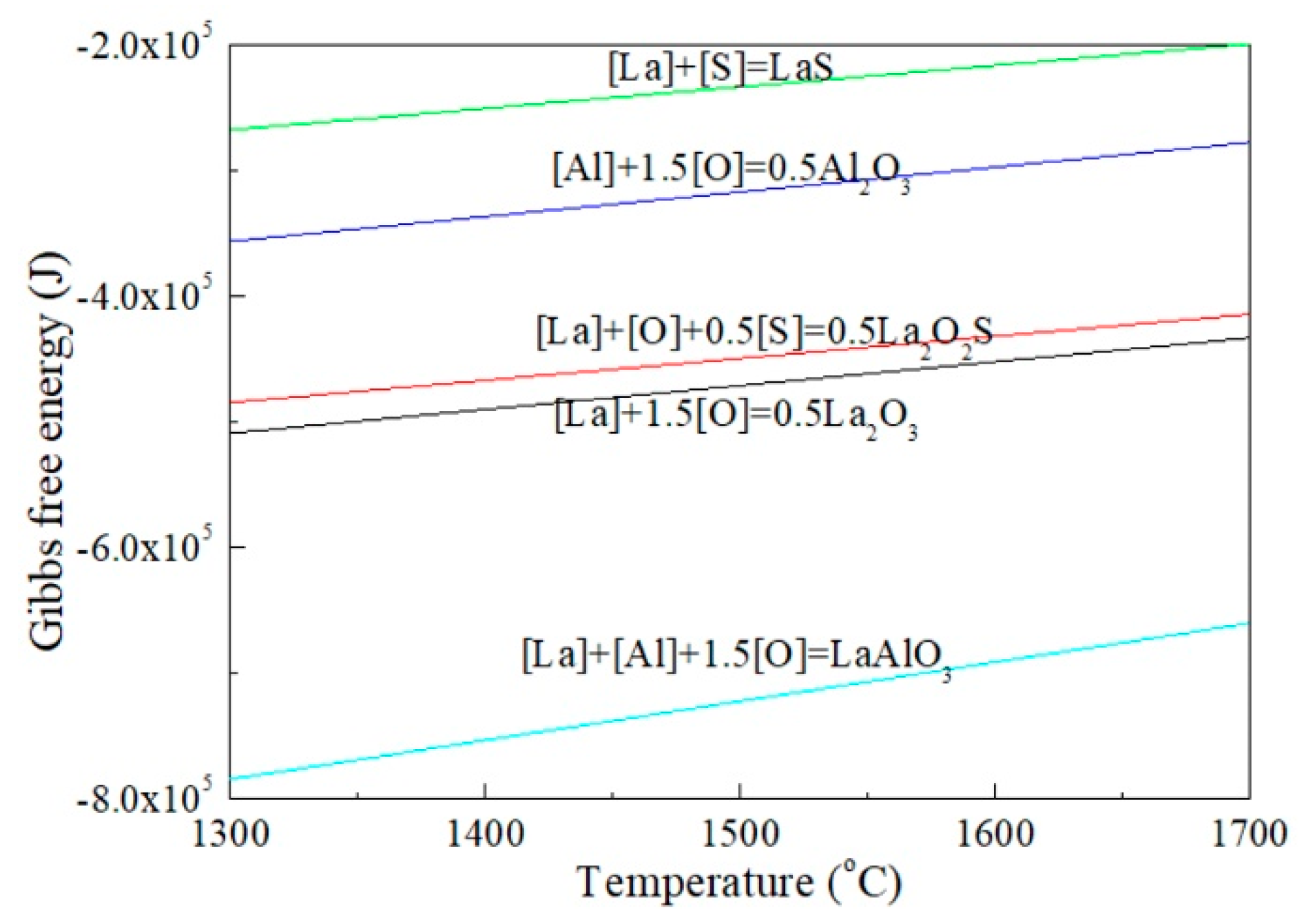
3.4. Effect of Reactions between Lanthanum and Alumina Crucibles on the Existing State of Arsenic
3.5. Evolution of the Existing State of Arsenic in the Smelting and Solidification Process
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ohmori, A.; Matsuzaki, A.; Amano, K. Effect of Cu, Sn, and Sb on cold forgeability of a 0.5% carbon steel. ISIJ Int. 1997, 37, 302–304. [Google Scholar] [CrossRef][Green Version]
- Nagasaki, C.; Kihara, J. Effect of copper and tin on hot ductility of ultra-low and 0.2% carbon steels. ISIJ Int. 1997, 37, 523–530. [Google Scholar] [CrossRef]
- Matsuoka, H.; Osawa, K.; Ono, M.; Ohmura, M. Influence of Cu and Sn on hot ductility of steels with various C content. ISIJ Int. 1997, 37, 255–262. [Google Scholar] [CrossRef]
- Imai, N.; Komatsubara, N.; Kunishige, K. Effect of Cu, Sn and Ni on hot workability of hot-rolled mild steel. ISIJ Int. 1997, 37, 217–223. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, J.; Liang, D.; Liu, P. Distribution of arsenic on micro-interfaces in a kind of Cr, Nb and Ti microalloyed low carbon steel produced by a compact strip production process. Mater. Chem. Phys. 2011, 130, 524–530. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, Z.; Xu, J. Grain boundary segregation of minor arsenic and nitrogen at elevated temperatures in a microalloyed steel. Int. J. Miner. Metall. Mater. 2012, 19, 399–403. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, J.; Xu, J. Macroscopic distribution of residual elements As, S, and P in steel strips produced by compact strip production (CSP) process. Metall. Mater. Trans. A 2012, 43, 2509–2513. [Google Scholar] [CrossRef]
- Sun, G.; Song, B.; Yang, L.; Tao, S.; Yang, Y. Effect of manganese sulfide on the precipitation behavior of tin in steel. Int. J. Miner. Metall. Mater. 2014, 21, 654–659. [Google Scholar] [CrossRef]
- Sun, G.; Song, B.; Tao, S.; Cai, Z. Effect of manganese sulphide size on the precipitation of tin heterogeneous nucleation in as-cast steel. High Temp. Mater. Proc. 2015, 34, 251–256. [Google Scholar] [CrossRef]
- Peng, H.; Chen, W.; Chen, L.; Guo, D. Effect of rare earth yttrium on hot ductility of 20CrMnTi steel with 0.05% tin. Metall. Res. Technol. 2015, 112, 1–9. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, C. Modification mechanism of spinel inclusions in medium manganese steel with rare earth treatment. Metals 2019, 9, 804. [Google Scholar] [CrossRef]
- Torkamani, H.; Raygan, S.; Mateo, C.G.; Rassizadehghani, J.; Vivas, J.; Palizdar, Y.; San-Martin, D. The influence of La and Ce addition on inclusion modification in cast niobium microalloyed steels. Metals 2017, 7, 377. [Google Scholar] [CrossRef]
- Xu, Y.; Song, S.; Wang, J. Effect of rare earth cerium on the creep properties of modified 9Cr–1Mo heat-resistant steel. Mater. Lett. 2015, 161, 616–619. [Google Scholar] [CrossRef]
- Adabavazeh, Z.; Hwang, W.S.; Su, Y.H. Effect of adding cerium on microstructure and morphology of Ce-based inclusions formed in low-carbon steel. Sci. Rep. 2017, 7, 46503. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Song, B.; Huang, C.; Song, M.; Song, G. Effect of arsenic content and quenching temperature on solidification microstructure and arsenic distribution in iron-arsenic alloys. Int. J. Miner. Metall. Mater. 2015, 22, 704–713. [Google Scholar] [CrossRef]
- Wang, H.; Bai, B.; Jiang, S.; Sun, L.; Wang, Y. An in situ study of the formation of rare earth inclusions in arsenic high carbon steels. ISIJ Int. 2019, 59, 1259–1265. [Google Scholar] [CrossRef]
- Wang, H.; Xiong, L.; Zhang, L.; Wang, Y.; Shu, Y.; Zhou, Y. Investigation of RE-O-S-As inclusions in high carbon steels. Metall. Mater. Trans. B 2017, 48, 2849–2858. [Google Scholar] [CrossRef]
- Li, Y.; Sun, M.; Jiang, Z.; Chen, C.; Chen, K.; Huang, X.; Sun, S.; Li, H. Inclusion modification in C104Cr saw wire steel with different cerium content. Metals 2019, 9, 54. [Google Scholar] [CrossRef]
- Xin, W.; Song, B.; Song, M.; Song, G. Effect of cerium on characteristic of inclusions and grain boundary segregation of arsenic in iron melts. Steel Res. Int. 2015, 86, 1430–1438. [Google Scholar] [CrossRef]
- Kwon, S.K.; Park, J.S.; Park, J.H. Influence of refractory-steel interfacial reaction on the formation behavior of inclusions in Ce-containing stainless steel. ISIJ Int. 2015, 55, 2589–2596. [Google Scholar] [CrossRef]
- Kwon, S.K.; Kong, Y.M.; Park, J.H. Effect of Al deoxidation on the formation behavior of inclusions in Ce-added stainless steel melts. Met. Mater. Int. 2014, 20, 959–966. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, T.; Liu, C.; Jiang, M. Thermodynamic and experimental studies on Al addition of 253MA steel. Metals 2019, 9, 433. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, S.; Yu, P.; Bai, B.; Sun, L.; Wang, Y. Distribution of arsenic inclusions in rare earth steel ingots. Metals 2020, 10, 146. [Google Scholar] [CrossRef]
- Hino, M.; Ito, K. Thermodynamic Data for Steelmaking; Tohoku University Press: Sendai, Japan, 2010; pp. 249–257. [Google Scholar]
- Mikhailov, G.G.; Makrovets, L.A.; Smirnov, L.A. Thermodynamic modelling of rare-earth elements-oxygen interaction. Mater. Sci. Forum 2016, 843, 39–45. [Google Scholar] [CrossRef]


| Elements | C | Si | Mn | P | S | Al | O | As |
|---|---|---|---|---|---|---|---|---|
| Concentrations | 0.79 | 0.21 | 0.61 | 0.011 | 0.015 | 0.001 | 0.0024 | 0.005 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Yu, P.; Jiang, S.; Bai, B.; Sun, L.; Wang, Y. Evolution of Inclusions in Steelmaking Process of Rare Earth Steels Containing Arsenic with Alumina Crucibles. Metals 2020, 10, 275. https://doi.org/10.3390/met10020275
Wang H, Yu P, Jiang S, Bai B, Sun L, Wang Y. Evolution of Inclusions in Steelmaking Process of Rare Earth Steels Containing Arsenic with Alumina Crucibles. Metals. 2020; 10(2):275. https://doi.org/10.3390/met10020275
Chicago/Turabian StyleWang, Hongpo, Peng Yu, Silu Jiang, Bin Bai, Lifeng Sun, and Yu Wang. 2020. "Evolution of Inclusions in Steelmaking Process of Rare Earth Steels Containing Arsenic with Alumina Crucibles" Metals 10, no. 2: 275. https://doi.org/10.3390/met10020275
APA StyleWang, H., Yu, P., Jiang, S., Bai, B., Sun, L., & Wang, Y. (2020). Evolution of Inclusions in Steelmaking Process of Rare Earth Steels Containing Arsenic with Alumina Crucibles. Metals, 10(2), 275. https://doi.org/10.3390/met10020275




