Hydrochloric Acid Leaching Behaviors of Copper and Antimony in Speiss Obtained from Top Submerged Lance Furnace
Abstract
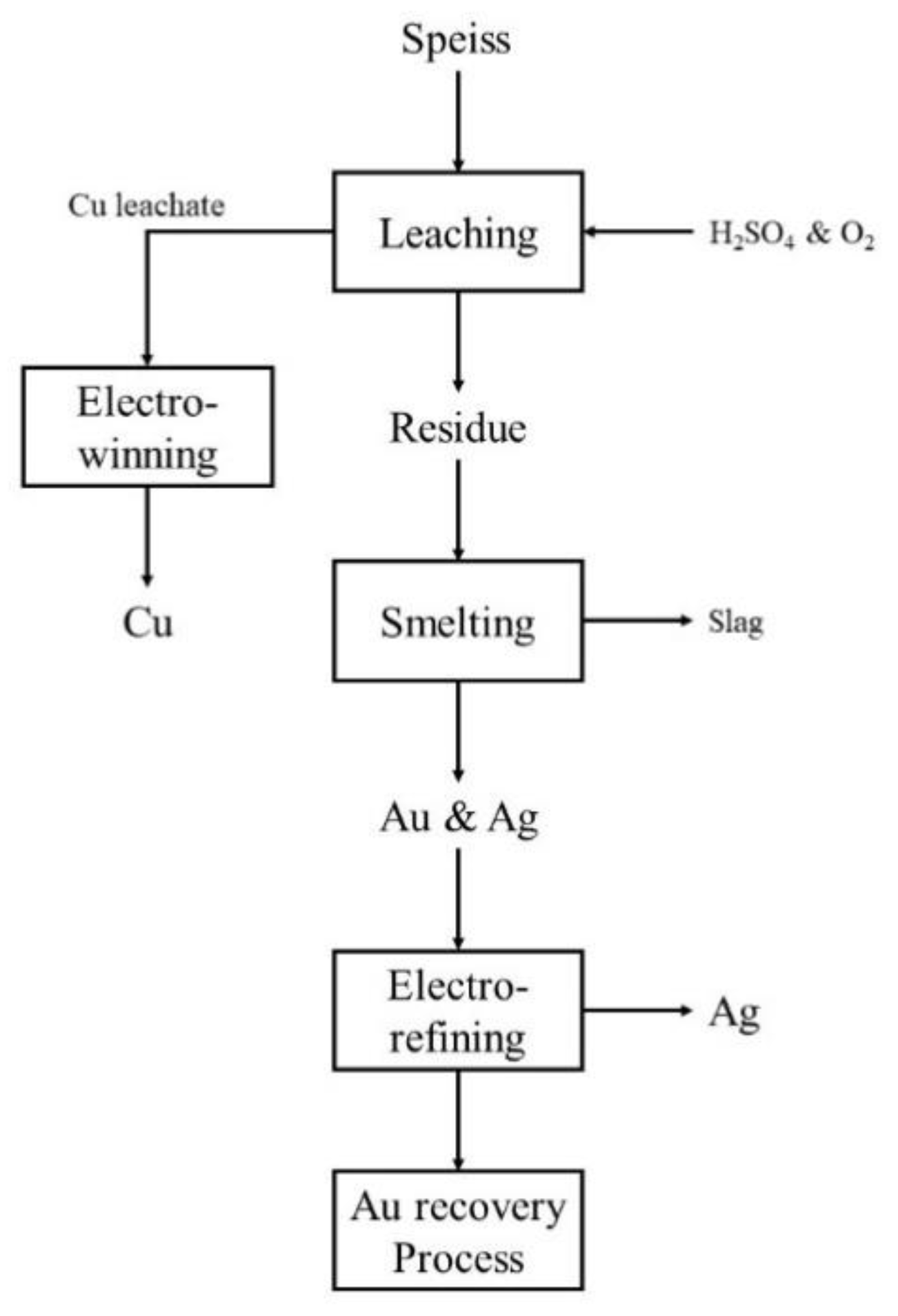
1. Introduction
2. Materials and Methods
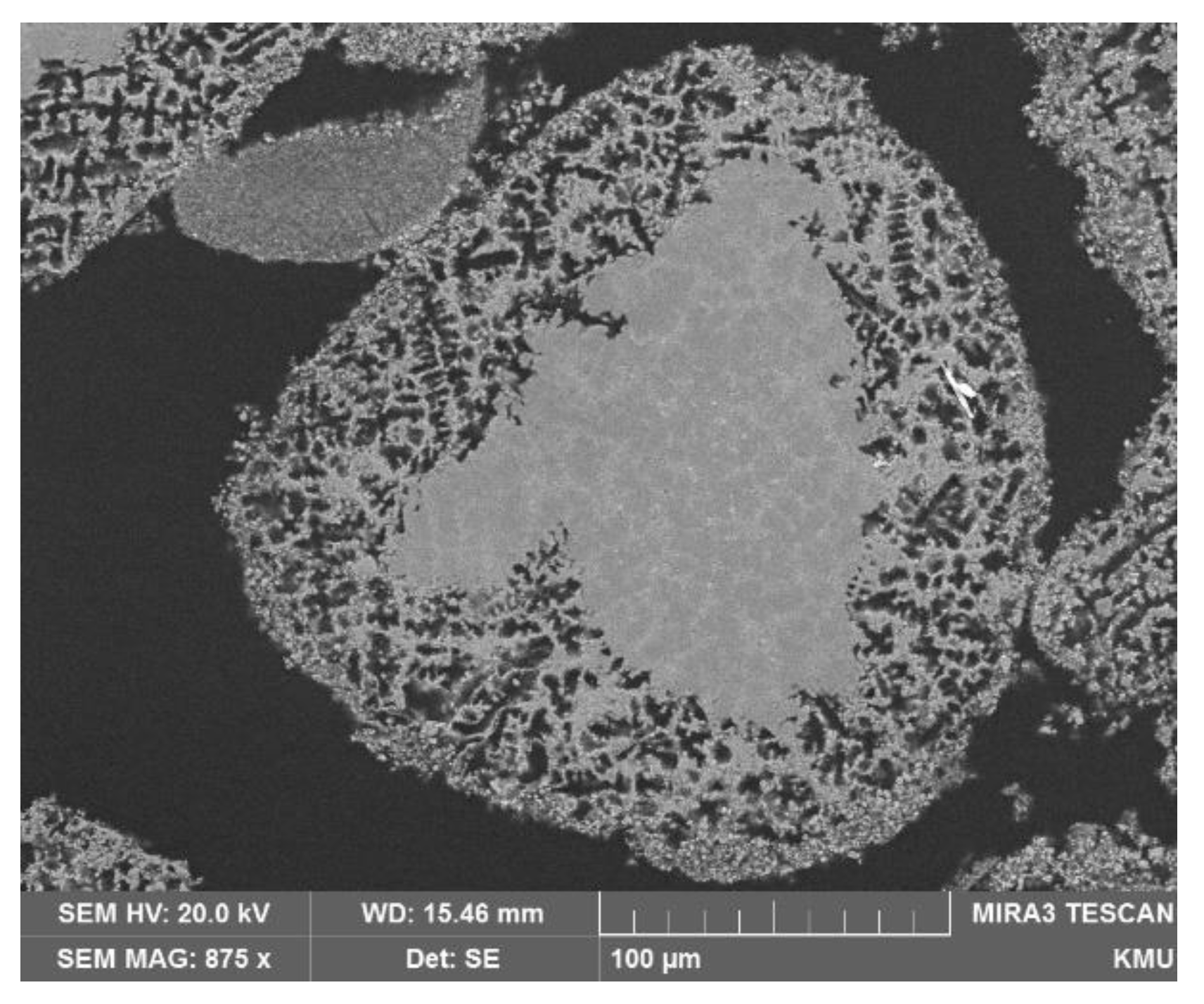
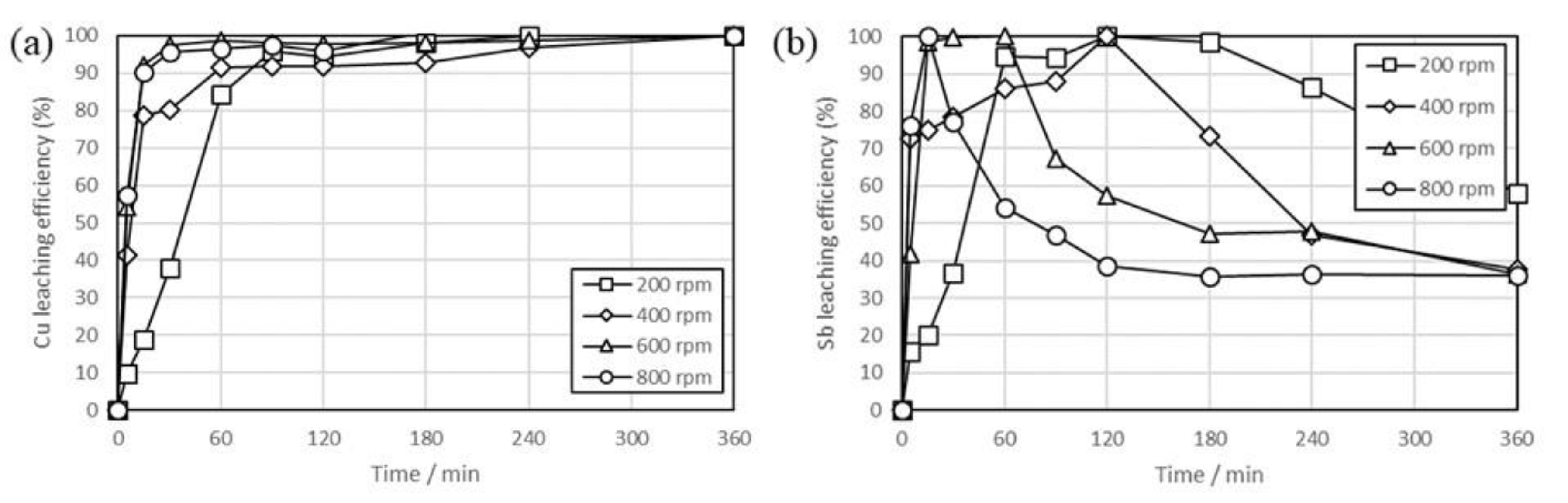
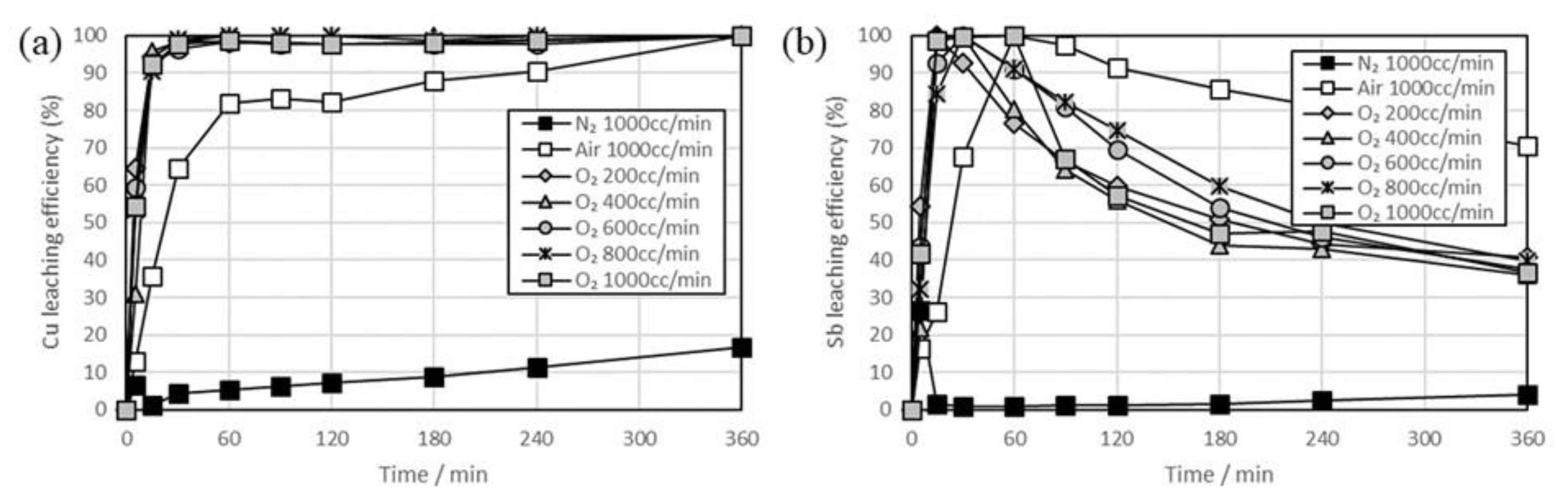
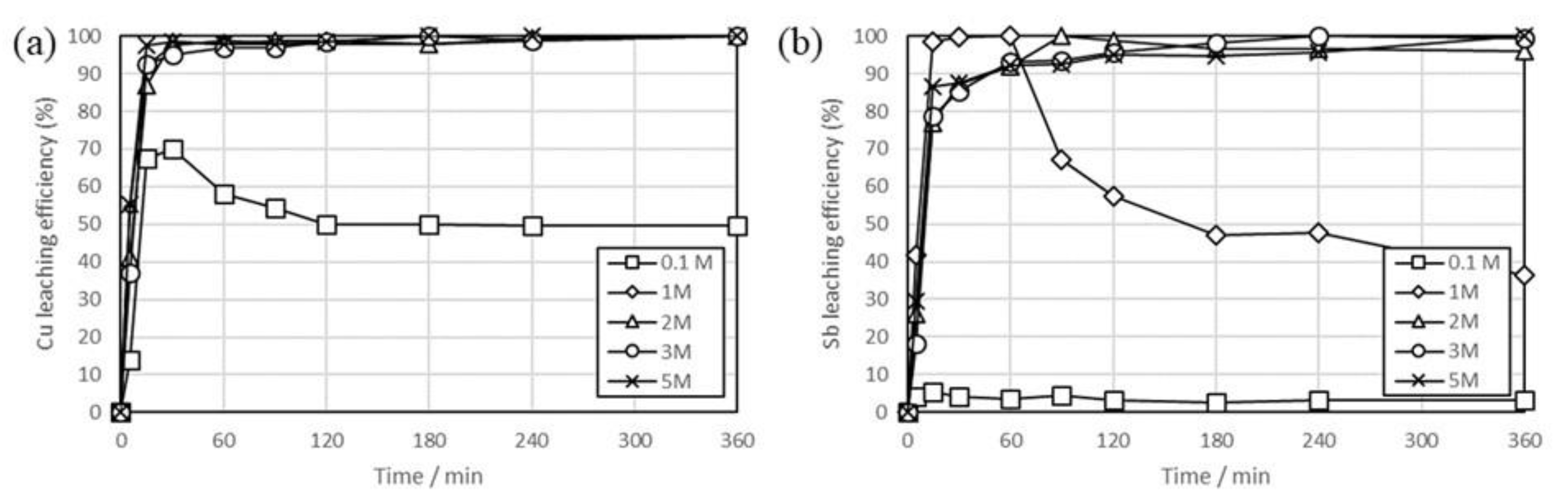
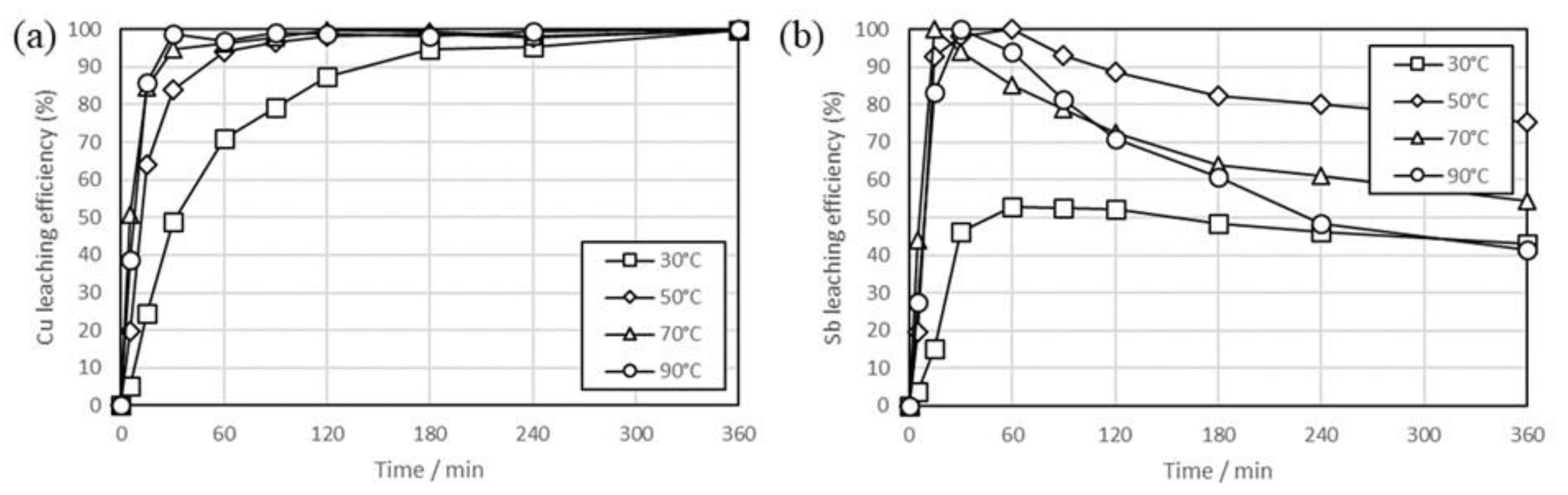
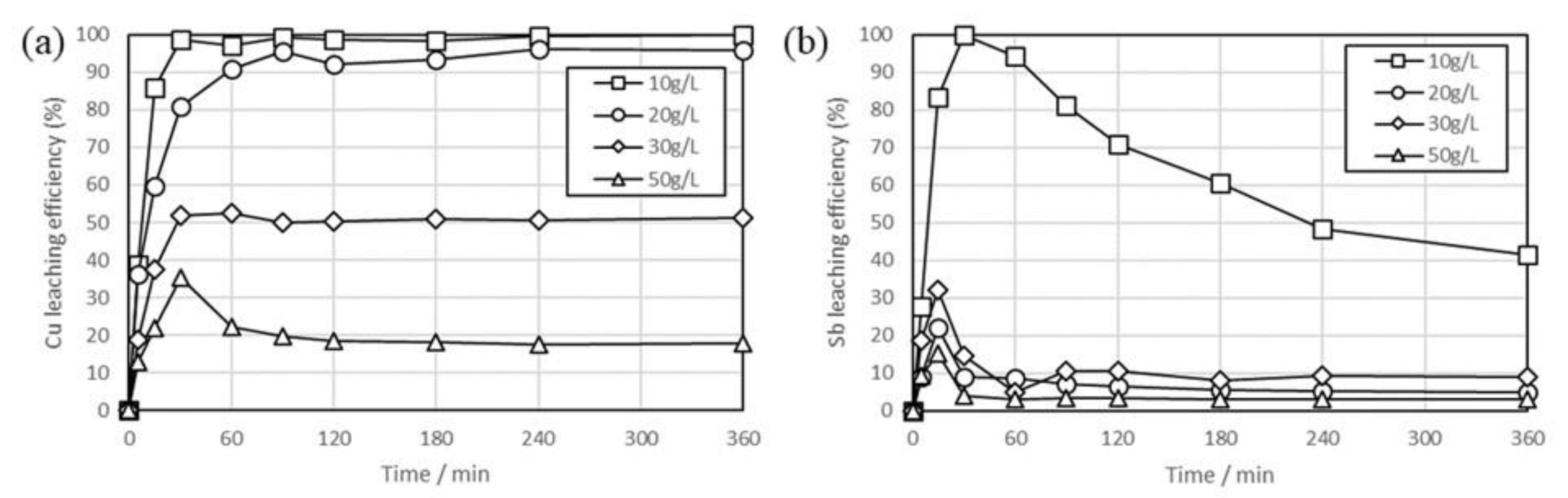
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hoang, J.; Reuter, M.A.; Matusewicz, R.; Hughes, S.; Piret, N. Top submerged lance direct zinc smelting. Miner. Eng. 2009, 22, 742–751. [Google Scholar] [CrossRef]
- Kim, B.S.; Jeong, S.B.; Lee, J.C.; Shin, D.; Moon, N.I. Behaviors of lead and zinc in top submerged lance (TSL) plant at Sukpo zinc refinery. Mater. Trans. 2012, 53, 985–990. [Google Scholar] [CrossRef]
- Sohn, H.S. Current Status of Zinc Smelting and Recycling. J. Korean Inst. Resour. Recycl. 2019, 28, 30–41. [Google Scholar] [CrossRef]
- Yoo, J.K.; Lee, M.; Kim, G.H.; Yoo, K. The Gravity Separation of Speiss and Limestone Granules Using Vibrating Zirconia Ball Bed. J. Korean Inst. Resour. Recycl. 2020, 29, 36–42. [Google Scholar] [CrossRef]
- Brett, C.M.A.; Brett, A.M.O. Electrochemistry; Oxford University Press Inc.: New York, NY, USA, 1993; pp. 416–419. [Google Scholar]
- Yoo, K.; Park, Y.; Choi, S.; Park, I. Improvement of Copper Metal Leaching in Sulfuric Acid Solution by Simultaneous Use of Oxygen and Cupric Ions. Metals 2020, 10, 721. [Google Scholar] [CrossRef]
- Lee, S.; Yoo, K.; Jha, M.K.; Lee, J. Separation of Sn from waste Pb-free Sn-Ag-Cu solder in hydrochloric acid solution with ferric chloride. Hydrometallurgy 2015, 157, 184–187. [Google Scholar] [CrossRef]
- Xing, W.; Lee, S.; Lee, M. Solvent extraction of Li(I) from weak HCl solution with the mixture of neutral extractants containing FeCl3. J. Korean Inst. Resour. Recycl. 2018, 27, 53–58. [Google Scholar] [CrossRef]
- Park, I.; Yoo, K.; Alorro, R.D.; Kim, M.; Kim, S. Leaching of copper from cuprous oxide in aerated sulfuric acid. Mater. Trans. 2017, 58, 1500–1504. [Google Scholar] [CrossRef]
- Kim, E.Y.; Kim, M.S.; Lee, J.C.; Yoo, K.; Jeong, J. Leaching behavior of copper using electro-generated chlorine in hydrochloric acid solution. Hydrometallurgy 2010, 100, 95–102. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.C.; Lee, K.S.; Yoo, K.; Alorro, R.D. Separation of tin, silver and copper from waste Pb-free solder using hydrochloric acid leaching with hydrogen peroxide. Mater. Trans. 2014, 55, 1885–1889. [Google Scholar] [CrossRef]
- Yoo, K.; Lee, J.C.; Lee, K.S.; Kim, B.S.; Kim, M.S.; Kim, S.K.; Pandey, B.D. Recovery of Sn, Ag and Cu from waste Pb-free solder using nitric acid leaching. Mater. Trans. 2012, 53, 2175–2180. [Google Scholar] [CrossRef]
- Yoo, K.; Lee, K.; Jha, M.K.; Lee, J.; Cho, K. Preparation of nano-sized tin oxide powder from waste Pb-free solder by direct nitric acid leaching. J. Nanosci. Nanotechnol. 2016, 16, 11238–11241. [Google Scholar] [CrossRef]
- Moon, G.; Yoo, K. Separation of Cu, Sn, Pb from photovoltaic ribbon by hydrochloric acid leaching with stannic ion followed by solvent extraction. Hydrometallurgy 2017, 171, 123–127. [Google Scholar] [CrossRef]
- Chen, W.S.; Chen, Y.J.; Yueh, K.C. Separation of Valuable Metal from Waste Photovoltaic Ribbon through Extraction and Precipitation. J. Korean Inst. Resour. Recycl. 2020, 29, 69–77. [Google Scholar] [CrossRef]
- Kang, Y.H.; Hyun, S.K. Study on the Preparation of Copper Sulfate by Copper Powder using Cation Membrane Electrowinning Prepared from Waste Cupric Chloride Solution. J. Korean Inst. Resour. Recycl. 2019, 28, 62–72. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, J.C.; Yoo, K. Leaching of tin from waste Pb-free solder in hydrochloric acid solution with stannic chloride. Hydrometallurgy 2016, 165, 143–147. [Google Scholar] [CrossRef]
- Jeon, S.; Yoo, K.; Alorro, R.D. Separation of Sn, Bi, Cu from Pb-free solder paste by ammonia leaching followed by hydrochloric acid leaching. Hydrometallurgy 2017, 169, 26–30. [Google Scholar] [CrossRef]
- Lim, Y.; Kwon, O.; Lee, J.; Yoo, K. The ammonia leaching of alloy produced from waste printed circuit boards smelting process. Geosyst. Eng. 2013, 16, 216–224. [Google Scholar] [CrossRef]
- Karlsson, T.Y. Studies on the Recovery of Secondary Antimony Compounds from Waste. Ph.D. Thesis, Chalmers University of Technology, Göteborg, Sweden, 2017; pp. 5–7. [Google Scholar]
- Awe, S.A.; Sundkvist, J.E.; Bolin, N.J.; Sandström, Å. Process flowsheet development for recovering antimony from Sb-bearing copper concentrates. Miner. Eng. 2013, 49, 45–53. [Google Scholar] [CrossRef]
- Awe, S.A.; Sandström, Å. Selective leaching of arsenic and antimony from a tetrahedrite rich complex sulphide concentrate using alkaline sulphide solution. Miner. Eng. 2010, 23, 1227–1236. [Google Scholar] [CrossRef]
- Guo, X.Y.; Xin, Y.T.; Wang, H.; Tian, Q. Leaching kinetics of antimony-bearing complex sulfides ore in hydrochloric acid solution with ozone. Trans. Nonferrous Met. Soc. China 2017, 27, 2073–2081. [Google Scholar] [CrossRef]
- Choi, S.; Yoo, K.; Alorro, R.D. Hydrochloric acid leaching behavior of metals from non-magnetic fraction of Pb dross. Geosyst. Eng. 2019, 22, 347–354. [Google Scholar] [CrossRef]
- Elomaa, H.; Seisko, S.; Lehtola, J.; Lundström, M. A study on selective leaching of heavy metals vs. iron from fly ash. J. Mater. Cycles Waste Manag. 2019, 21, 1004–1013. [Google Scholar] [CrossRef]
- Bae, E.; Yoo, K. Leaching behavior of valuable metals from by-product generated during purification of zinc electrolyte. Geosyst. Eng. 2016, 19, 312–316. [Google Scholar] [CrossRef]
- Oh, J.; Yoo, K.; Bae, M.; Kim, S.; Alorro, R.D. The adsorption behaviors of gold ions in simulated leachate using magnetite. J. Korean Soc. Miner. Energy Resour. Eng. 2019, 56, 79–85. [Google Scholar] [CrossRef]
- Lee, S.; Yoo, K.; Lee, J. Preparation of Cu2O Powder in NaOH solution Using CuCl Obtained from Spent Printed Circuit Boards Etchant. J. Korean Soc. Miner. Energy Resour. Eng. 2018, 55, 194–199. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chae, S.; Yoo, K.; Tabelin, C.B.; Alorro, R.D. Hydrochloric Acid Leaching Behaviors of Copper and Antimony in Speiss Obtained from Top Submerged Lance Furnace. Metals 2020, 10, 1393. https://doi.org/10.3390/met10101393
Chae S, Yoo K, Tabelin CB, Alorro RD. Hydrochloric Acid Leaching Behaviors of Copper and Antimony in Speiss Obtained from Top Submerged Lance Furnace. Metals. 2020; 10(10):1393. https://doi.org/10.3390/met10101393
Chicago/Turabian StyleChae, Sujin, Kyoungkeun Yoo, Carlito Baltazar Tabelin, and Richard Diaz Alorro. 2020. "Hydrochloric Acid Leaching Behaviors of Copper and Antimony in Speiss Obtained from Top Submerged Lance Furnace" Metals 10, no. 10: 1393. https://doi.org/10.3390/met10101393
APA StyleChae, S., Yoo, K., Tabelin, C. B., & Alorro, R. D. (2020). Hydrochloric Acid Leaching Behaviors of Copper and Antimony in Speiss Obtained from Top Submerged Lance Furnace. Metals, 10(10), 1393. https://doi.org/10.3390/met10101393







