Flotation Separation of Chalcopyrite and Molybdenite Assisted by Microencapsulation Using Ferrous and Phosphate Ions: Part I. Selective Coating Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Mineral Samples
2.2. Stability of Fe2+ in the Presence of PO43− vs. pH
2.3. Electrochemical Behaviors of Fe2+ on Chalcopyrite and Molybdenite
2.4. Microencapsulation Treatment
3. Results
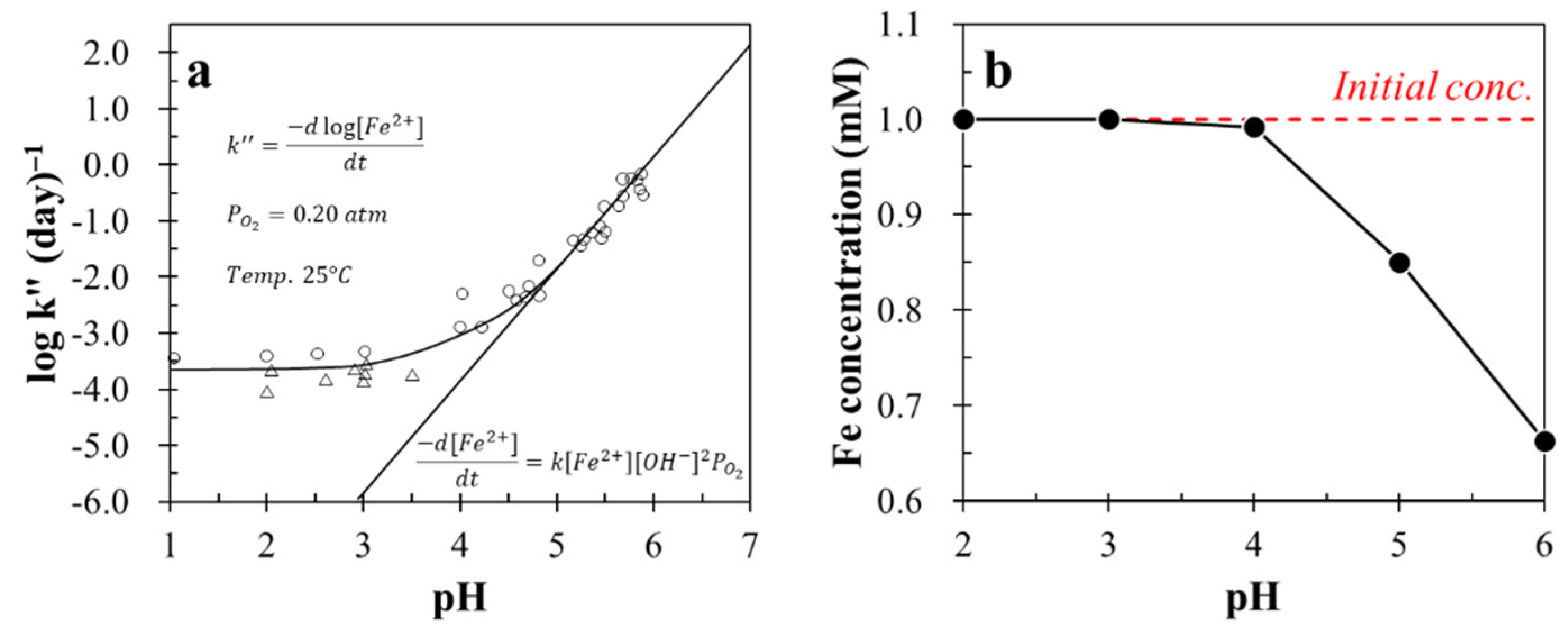
3.1. Stability of Fe2+ in the Presence of PO43− vs. pH
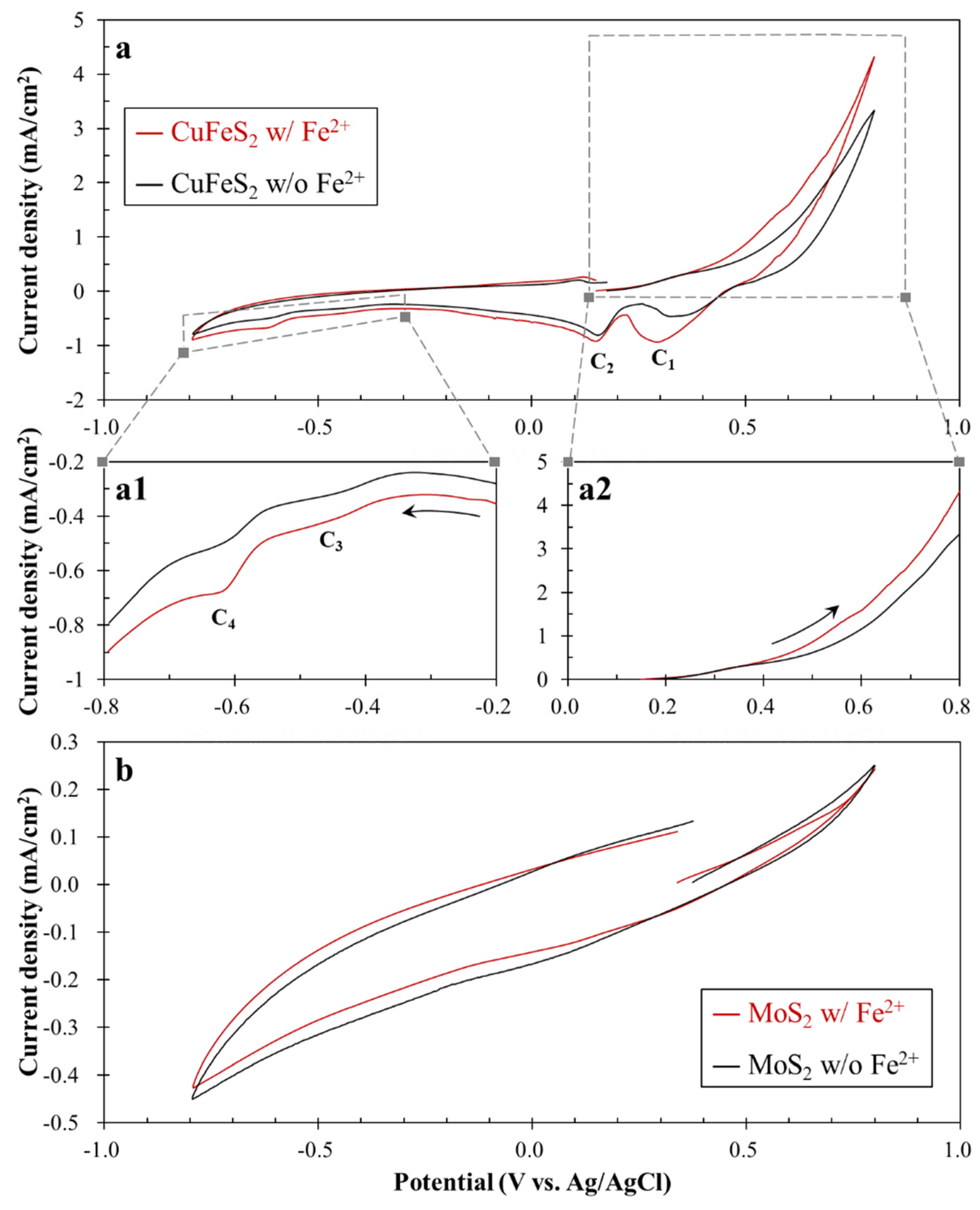
3.2. Electrochemical Behavior of Fe2+ on Chalcopyrite and Molybdenite
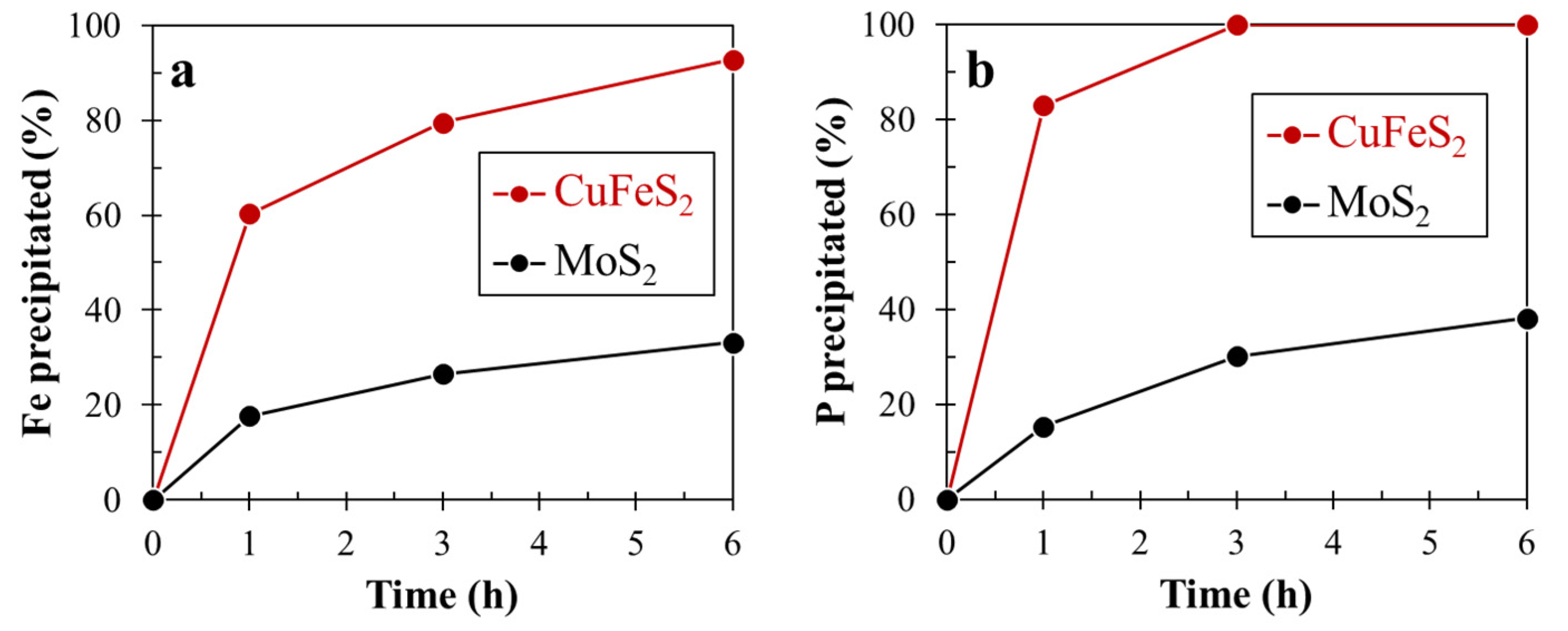
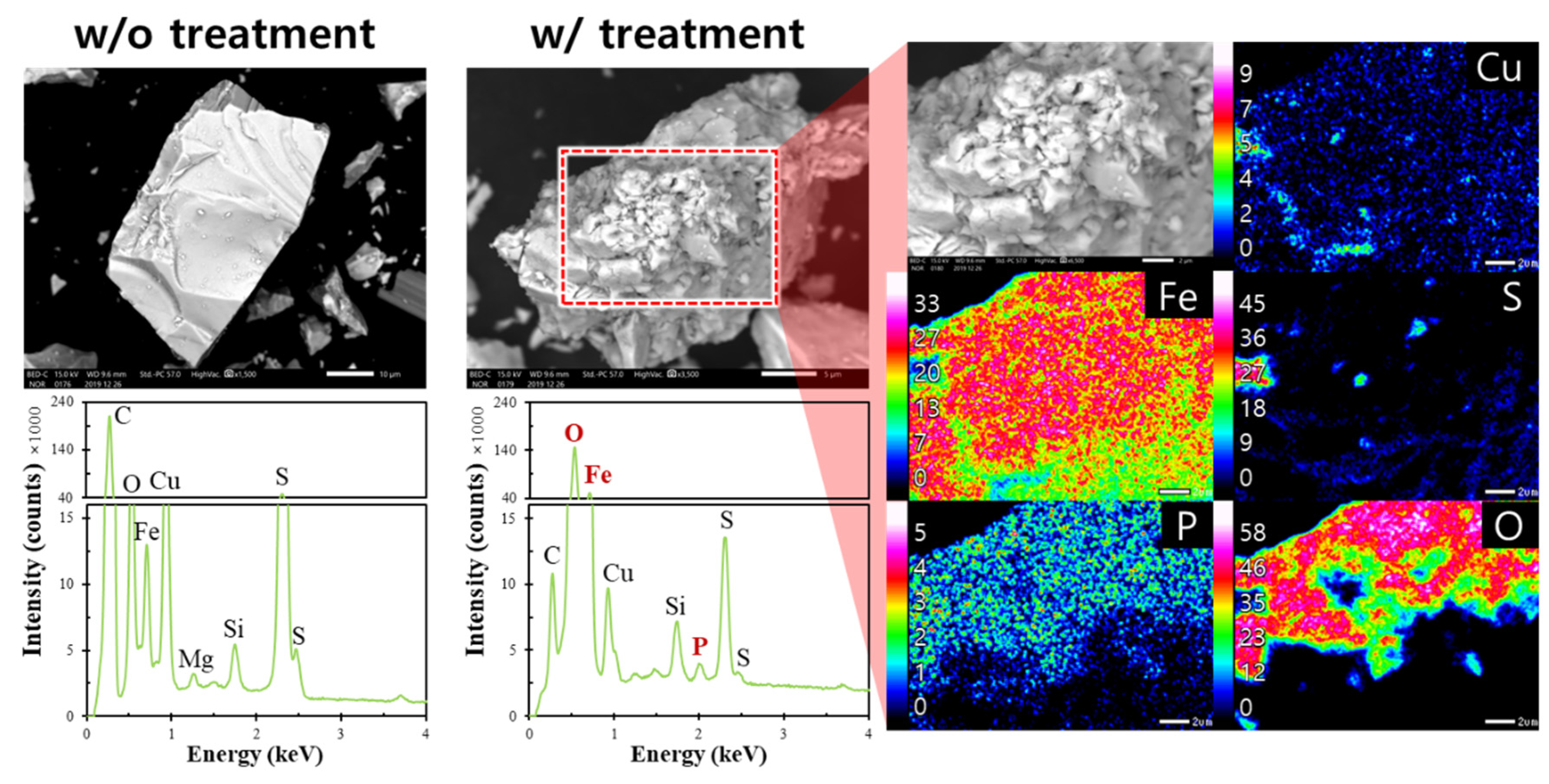
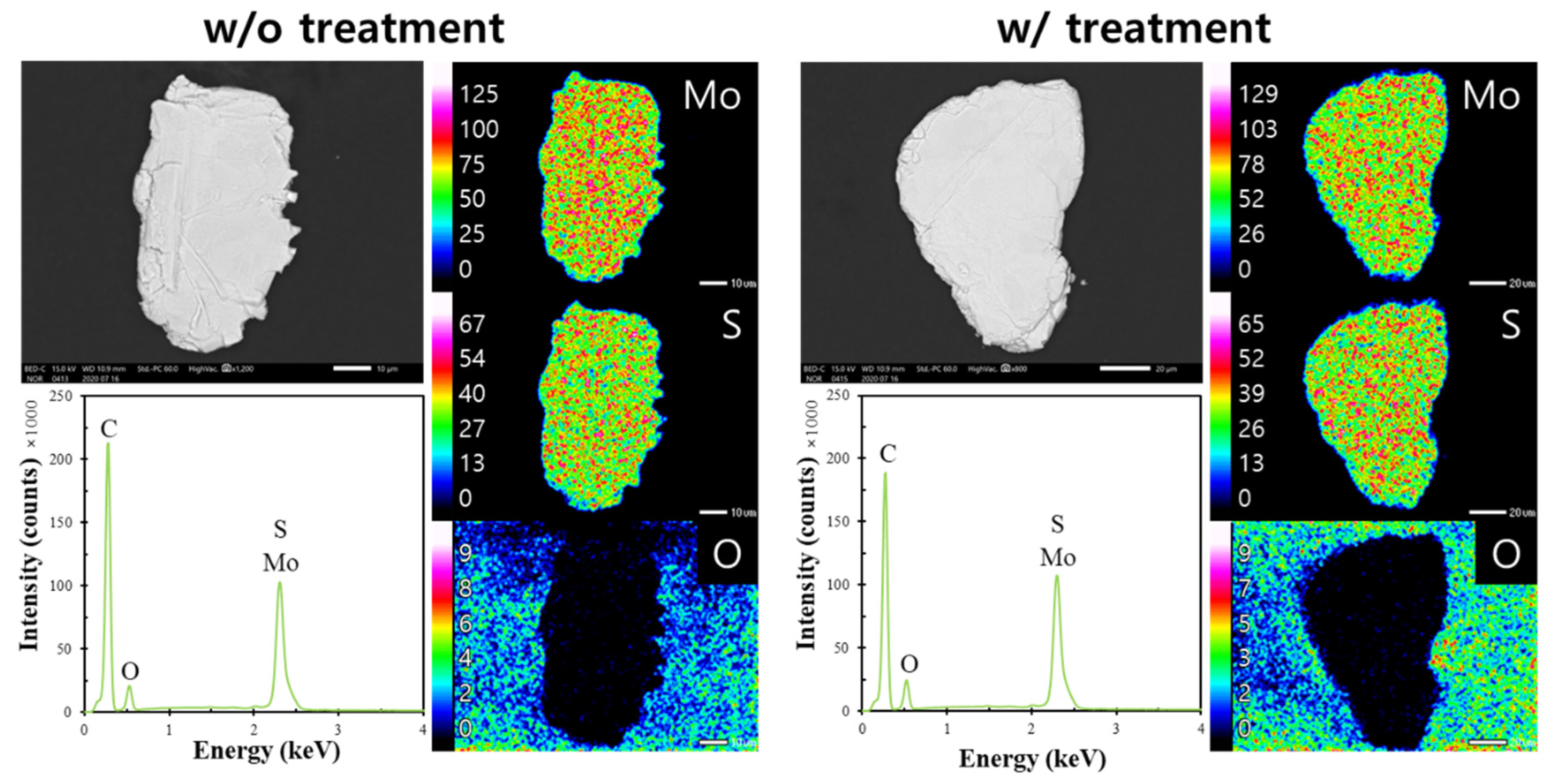
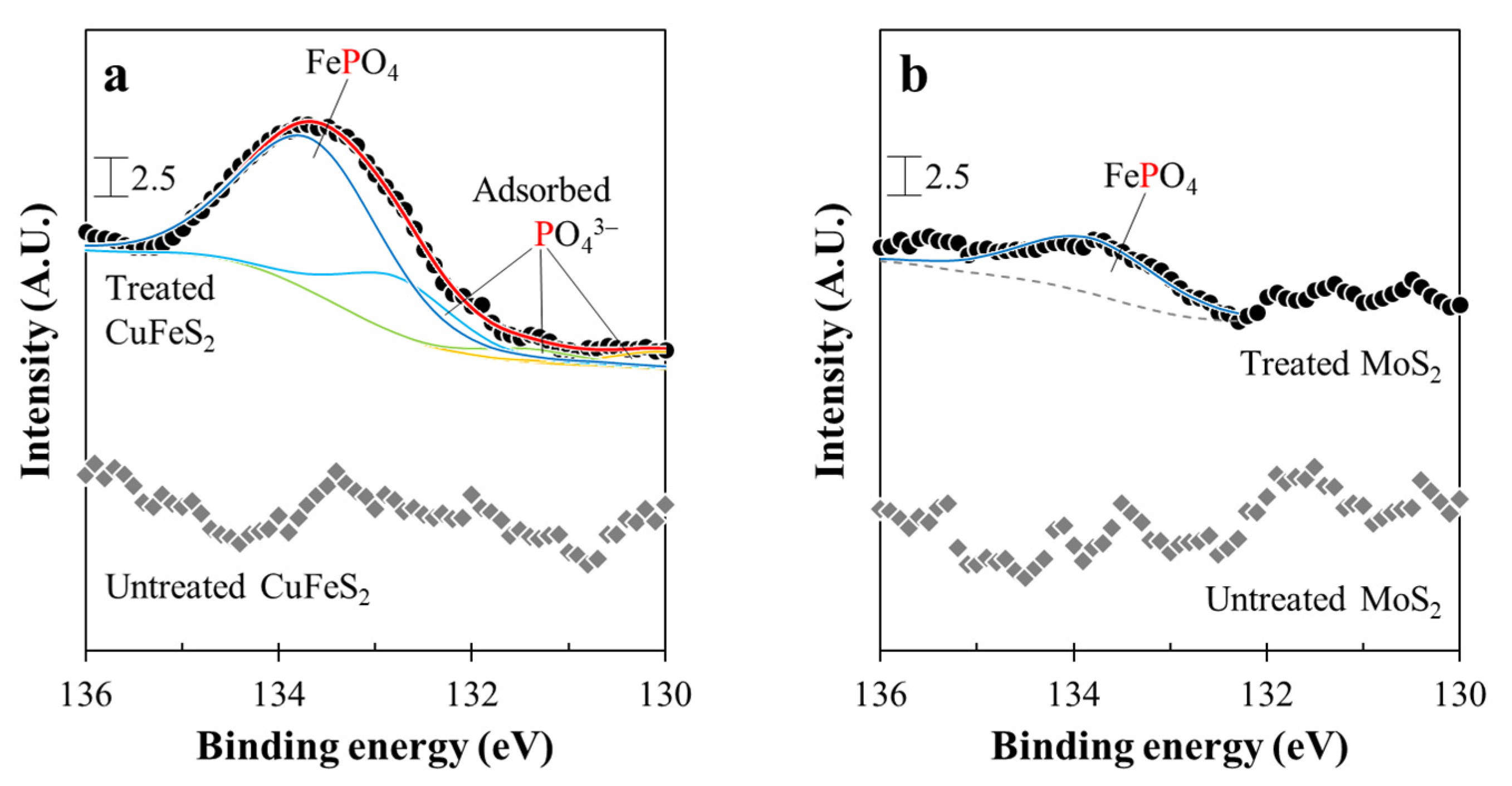
3.3. Microencapsulation Treatment for Chalcopyrite and Molybdenite
4. Conclusions
- In the presence of phosphate ion, Fe2+ was stable at pH ≤ 4, above which, however, Fe2+ became unstable due to its rapid oxidation to Fe3+, which was then precipitated as FePO4 and/or FeO(OH). Thus, microencapsulation treatment using Fe2+ and PO43− is recommended to be conducted at pH 4 in order to achieve the selective Fe2+ oxidation on chalcopyrite surface.
- The CV results indicated that Fe2+ oxidation can occur on the chalcopyrite surface, but not on the molybdenite surface, due to their different electrical properties.
- After microencapsulation treatment using Fe2+ and PO43−, SEM-EDX and XPS analyses confirmed that chalcopyrite was more coated with FePO4 than molybdenite.
Author Contributions
Funding
Conflicts of Interest
References
- Bulatovic, S.M. 12-Flotation of Copper Sulfide Ores. In Handbook of Flotation Reagents; Bulatovic, S.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 235–293. ISBN 978-0-444-53029-5. [Google Scholar]
- Ayuso, R.A.; Barton, M.D.; Blakely, R.J.; Bodnar, R.J.; Dilles, J.H.; Gray, F.; Graybeal, F.T.; Mars, J.L.; McPhee, D.K.; Seal II, R.R.; et al. Porphyry Copper Deposit Model: Chapter B in Mineral Deposit Models for Resource Assessment; Scientific Investigations Report; U.S. 2010-5070-B; Geological Survey: Reston, VA, USA, 2010. [Google Scholar]
- Miki, H.; Hirajima, T.; Muta, Y.; Suyantara, G.P.W.; Sasaki, K. Effect of Sodium Sulfite on Floatability of Chalcopyrite and Molybdenite. Minerals 2018, 8, 172. [Google Scholar] [CrossRef]
- Johnston, A.; Meadows, D.G.; Cappuccitti, F. Copper Mineral Processing. In SME Mineral Processing & Extractive Metallurgy Handbook; Society for Mining, Metallurgy & Exploration (SME): Englewood, CO, USA, 2019; Volume 2, pp. 1615–1641. [Google Scholar]
- Amelunxen, P.; Schmitz, C.; Hill, H.; Goodweiler, N.; Andres, J. Molybdenum. In SME Mineral Processing & Extractive Metallurgy Handbook; Society for Mining, Metallurgy & Exploration (SME): Englewood, CO, USA, 2019; Volume 2, pp. 1891–1916. [Google Scholar]
- Sutherland, K.L.; Wark, I.W. Depressants. In Principles of Flotation; Australasian Institute of Mining and Metallurgy: Melbourne, Australia, 1955; pp. 113–153. [Google Scholar]
- Park, I.; Hong, S.; Jeon, S.; Ito, M.; Hiroyoshi, N. A Review of Recent Advances in Depression Techniques for Flotation Separation of Cu–Mo Sulfides in Porphyry Copper Deposits. Metals 2020, 10, 1269. [Google Scholar] [CrossRef]
- Hirajima, T.; Miki, H.; Suyantara, G.P.W.; Matsuoka, H.; Elmahdy, A.M.; Sasaki, K.; Imaizumi, Y.; Kuroiwa, S. Selective flotation of chalcopyrite and molybdenite with H2O2 oxidation. Miner. Eng. 2017, 100, 83–92. [Google Scholar] [CrossRef]
- Miki, H.; Matsuoka, H.; Hirajima, T.; Suyantara, G.P.W.; Sasaki, K. Electrolysis Oxidation of Chalcopyrite and Molybdenite for Selective Flotation. Mater. Trans. 2017, 58, 761–767. [Google Scholar] [CrossRef]
- Yin, Z.; Sun, W.; Hu, Y.; Zhang, C.; Guan, Q.; Zhang, C. Separation of Molybdenite from Chalcopyrite in the Presence of Novel Depressant 4-Amino-3-thioxo-3,4-dihydro-1,2,4-triazin-5(2H)-one. Minerals 2017, 7, 146. [Google Scholar] [CrossRef]
- Wie, J.M.; Fuerstenau, D.W. The effect of dextrin on surface properties and the flotation of molybdenite. Int. J. Miner. Process. 1974, 1, 17–32. [Google Scholar] [CrossRef]
- Jorjani, E.; Barkhordari, H.R.; Tayebi Khorami, M.; Fazeli, A. Effects of aluminosilicate minerals on copper–molybdenum flotation from Sarcheshmeh porphyry ores. Miner. Eng. 2011, 24, 754–759. [Google Scholar] [CrossRef]
- Ansari, A.; Pawlik, M. Floatability of chalcopyrite and molybdenite in the presence of lignosulfonates. Part II. Hallimond tube flotation. Miner. Eng. 2007, 20, 609–616. [Google Scholar] [CrossRef]
- Yuan, D.; Cadien, K.; Liu, Q.; Zeng, H. Flotation separation of Cu-Mo sulfides by O-Carboxymethyl chitosan. Miner. Eng. 2019, 134, 202–205. [Google Scholar] [CrossRef]
- Yuan, D.; Cadien, K.; Liu, Q.; Zeng, H. Adsorption characteristics and mechanisms of O-Carboxymethyl chitosan on chalcopyrite and molybdenite. J. Colloid Interface Sci. 2019, 552, 659–670. [Google Scholar] [CrossRef]
- Kor, M.; Korczyk, P.M.; Addai-Mensah, J.; Krasowska, M.; Beattie, D.A. Carboxymethylcellulose Adsorption on Molybdenite: The Effect of Electrolyte Composition on Adsorption, Bubble–Surface Collisions, and Flotation. Langmuir 2014, 30, 11975–11984. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Cadien, K.; Liu, Q.; Zeng, H. Selective separation of copper-molybdenum sulfides using humic acids. Miner. Eng. 2019, 133, 43–46. [Google Scholar] [CrossRef]
- Chen, J.; Lan, L.; Liao, X. Depression effect of pseudo glycolythiourea acid in flotation separation of copper–molybdenum. Trans. Nonferrous Met. Soc. China 2013, 23, 824–831. [Google Scholar] [CrossRef]
- Li, M.; Wei, D.; Shen, Y.; Liu, W.; Gao, S.; Liang, G. Selective depression effect in flotation separation of copper–molybdenum sulfides using 2,3-disulfanylbutanedioic acid. Trans. Nonferrous Met. Soc. China 2015, 25, 3126–3132. [Google Scholar] [CrossRef]
- Yin, Z.; Sun, W.; Hu, Y.; Guan, Q.; Zhang, C.; Gao, Y.; Zhai, J. Depressing behaviors and mechanism of disodium bis (carboxymethyl) trithiocarbonate on separation of chalcopyrite and molybdenite. Trans. Nonferrous Met. Soc. China 2017, 27, 883–890. [Google Scholar] [CrossRef]
- Li, M.; Wei, D.; Liu, Q.; Liu, W.; Zheng, J.; Sun, H. Flotation separation of copper–molybdenum sulfides using chitosan as a selective depressant. Miner. Eng. 2015, 83, 217–222. [Google Scholar] [CrossRef]
- Laskowski, J.S.; Castro, S.; Ramos, O. Effect of seawater main components on frothability in the flotation of Cu-Mo sulfide ore. Physicochem. Probl. Miner. Process. 2013, 17–29. [Google Scholar] [CrossRef]
- Park, I.; Tabelin, C.B.; Seno, K.; Jeon, S.; Inano, H.; Ito, M.; Hiroyoshi, N. Carrier-microencapsulation of arsenopyrite using Al-catecholate complex: Nature of oxidation products, effects on anodic and cathodic reactions, and coating stability under simulated weathering conditions. Heliyon 2020, 6, e03189. [Google Scholar] [CrossRef]
- Park, I.; Tabelin, C.B.; Seno, K.; Jeon, S.; Ito, M.; Hiroyoshi, N. Simultaneous suppression of acid mine drainage formation and arsenic release by Carrier-microencapsulation using aluminum-catecholate complexes. Chemosphere 2018, 205, 414–425. [Google Scholar] [CrossRef]
- McKibben, M.A.; Barnes, H.L. Oxidation of pyrite in low temperature acidic solutions: Rate laws and surface textures. Geochim. Cosmochim. Acta 1986, 50, 1509–1520. [Google Scholar] [CrossRef]
- Shirley, D.A. High-Resolution X-Ray Photoemission Spectrum of the Valence Bands of Gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Muir, I.J. X-ray photoelectron spectroscopic study of a pristine pyrite surface reacted with water vapour and air. Geochim. Cosmochim. Acta 1994, 58, 4667–4679. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters; Wiley-Interscience: New York, NY, USA, 1996. [Google Scholar]
- Morgan, B.; Lahav, O. The effect of pH on the kinetics of spontaneous Fe(II) oxidation by O2 in aqueous solution–basic principles and a simple heuristic description. Chemosphere 2007, 68, 2080–2084. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.Q.; Yang, C.R.; Wang, J.; Zhang, Y.; Jiao, F.; Zhao, H.B.; Zhu, S. Effect of Fe2+ and Cu2+ Ions on the Electrochemical Behavior of Massive Chalcopyrite in Bioleaching System. Adv. Mater. Res. 2013, 825, 472–476. [Google Scholar] [CrossRef]
- Holliday, R.I.; Richmond, W.R. An electrochemical study of the oxidation of chalcopyrite in acidic solution. J. Electroanal. Chem. Interfacial Electrochem. 1990, 288, 83–98. [Google Scholar] [CrossRef]
- Liang, C.-L.; Xia, J.-L.; Yang, Y.; Nie, Z.-Y.; Zhao, X.-J.; Zheng, L.; Ma, C.-Y.; Zhao, Y.-D. Characterization of the thermo-reduction process of chalcopyrite at 65 °C by cyclic voltammetry and XANES spectroscopy. Hydrometallurgy 2011, 107, 13–21. [Google Scholar] [CrossRef]
- Castro, S.; Lopez-Valdivieso, A.; Laskowski, J.S. Review of the flotation of molybdenite. Part I: Surface properties and floatability. Int. J. Miner. Process. 2016, 148, 48–58. [Google Scholar] [CrossRef]
- Park, I.; Higuchi, K.; Tabelin, C.B.; Jeon, S.; Ito, M.; Hiroyoshi, N. Suppression of arsenopyrite oxidation by microencapsulation using ferric-catecholate complexes and phosphate. Chemosphere. under review.
- Zeng, L.; Li, X.; Shi, Y.; Qi, Y.; Huang, D.; Tadé, M.; Wang, S.; Liu, S. FePO4 based single chamber air-cathode microbial fuel cell for online monitoring levofloxacin. Biosens. Bioelectron. 2017, 91, 367–373. [Google Scholar] [CrossRef]
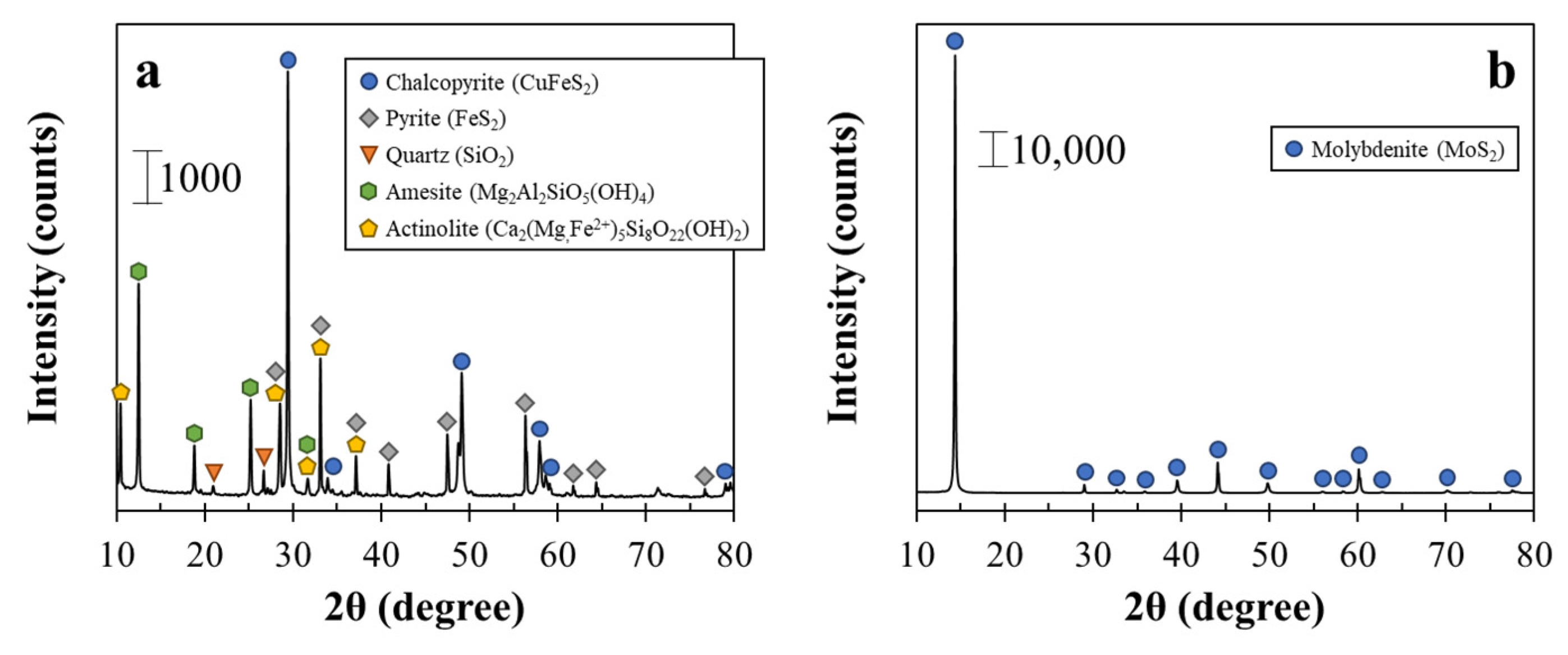
| Chalcopyrite | Molybdenite | ||
|---|---|---|---|
| Elements | wt.% | Elements | wt.% |
| Cu | 23.2 | Mo | 56.8 |
| Fe | 32.6 | S | 43.0 |
| S | 29.4 | Others | 0.2 |
| Si | 9.5 | - | - |
| Others | 5.3 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, I.; Hong, S.; Jeon, S.; Ito, M.; Hiroyoshi, N. Flotation Separation of Chalcopyrite and Molybdenite Assisted by Microencapsulation Using Ferrous and Phosphate Ions: Part I. Selective Coating Formation. Metals 2020, 10, 1667. https://doi.org/10.3390/met10121667
Park I, Hong S, Jeon S, Ito M, Hiroyoshi N. Flotation Separation of Chalcopyrite and Molybdenite Assisted by Microencapsulation Using Ferrous and Phosphate Ions: Part I. Selective Coating Formation. Metals. 2020; 10(12):1667. https://doi.org/10.3390/met10121667
Chicago/Turabian StylePark, Ilhwan, Seunggwan Hong, Sanghee Jeon, Mayumi Ito, and Naoki Hiroyoshi. 2020. "Flotation Separation of Chalcopyrite and Molybdenite Assisted by Microencapsulation Using Ferrous and Phosphate Ions: Part I. Selective Coating Formation" Metals 10, no. 12: 1667. https://doi.org/10.3390/met10121667
APA StylePark, I., Hong, S., Jeon, S., Ito, M., & Hiroyoshi, N. (2020). Flotation Separation of Chalcopyrite and Molybdenite Assisted by Microencapsulation Using Ferrous and Phosphate Ions: Part I. Selective Coating Formation. Metals, 10(12), 1667. https://doi.org/10.3390/met10121667





