Effective Casting Technique of Nano-Particles Alloyed Austenitic Stainless Steel
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Casting Method
2.3. Thermodynamic Calculation with ThermoCalc Software
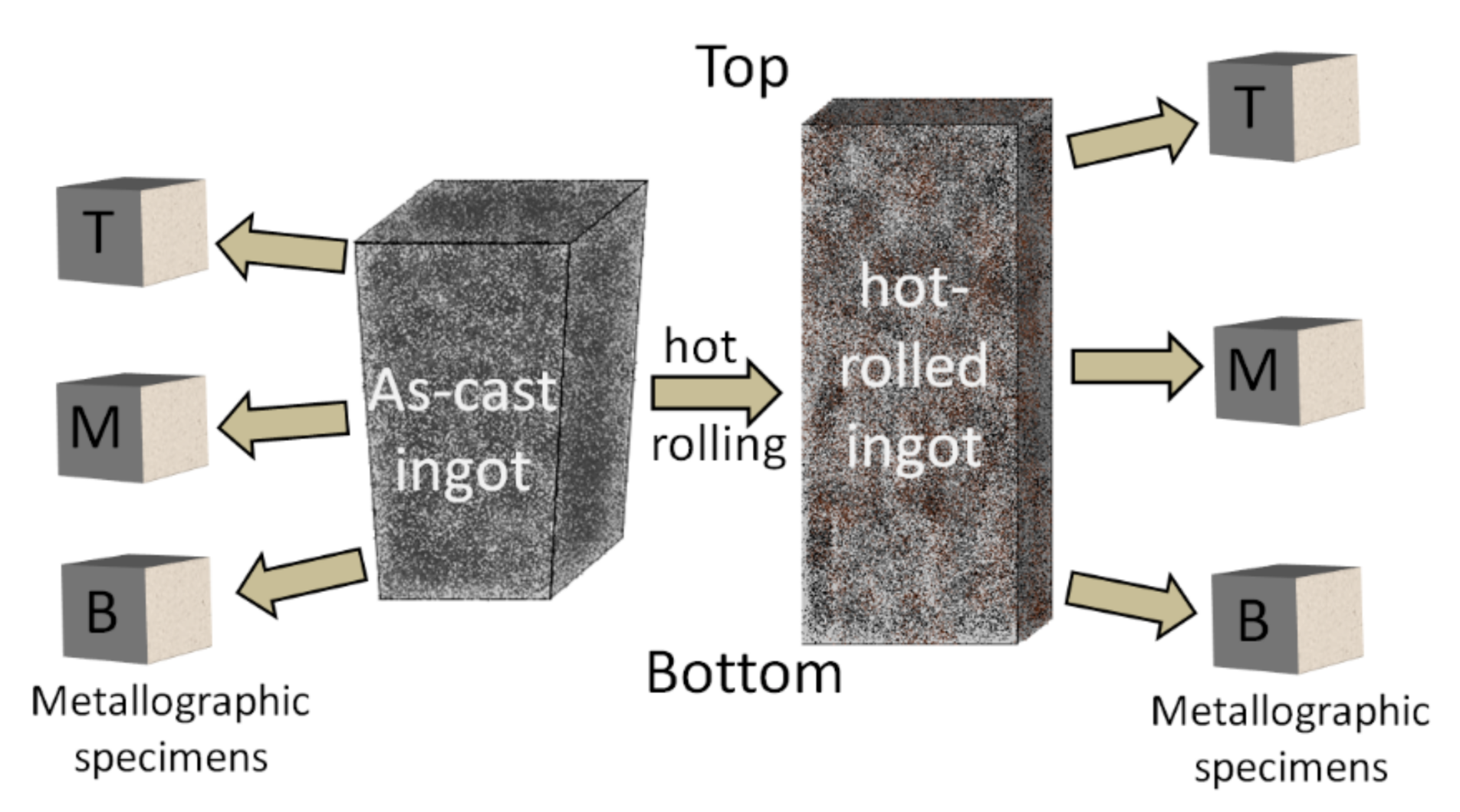
2.4. Microstructure Characterization
3. Results and Discussion
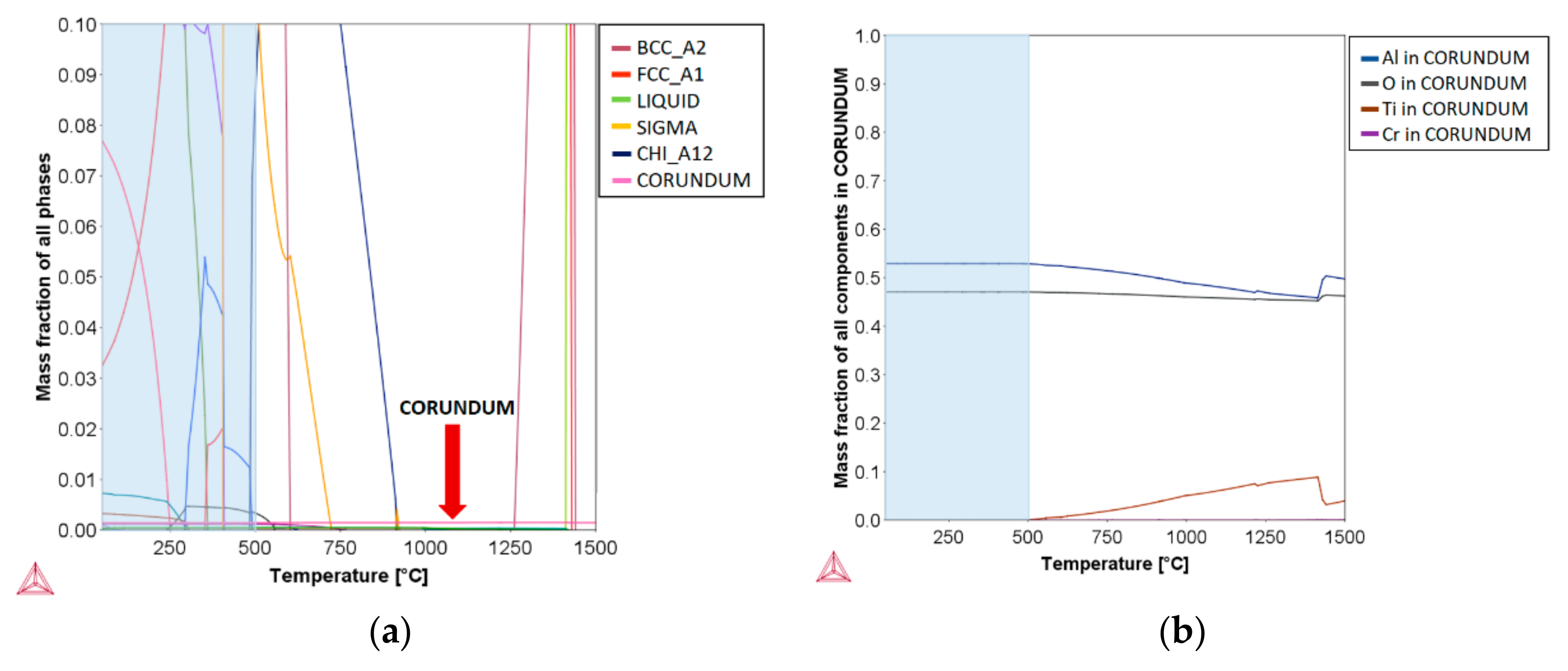
3.1. Thermodynamic Calculations
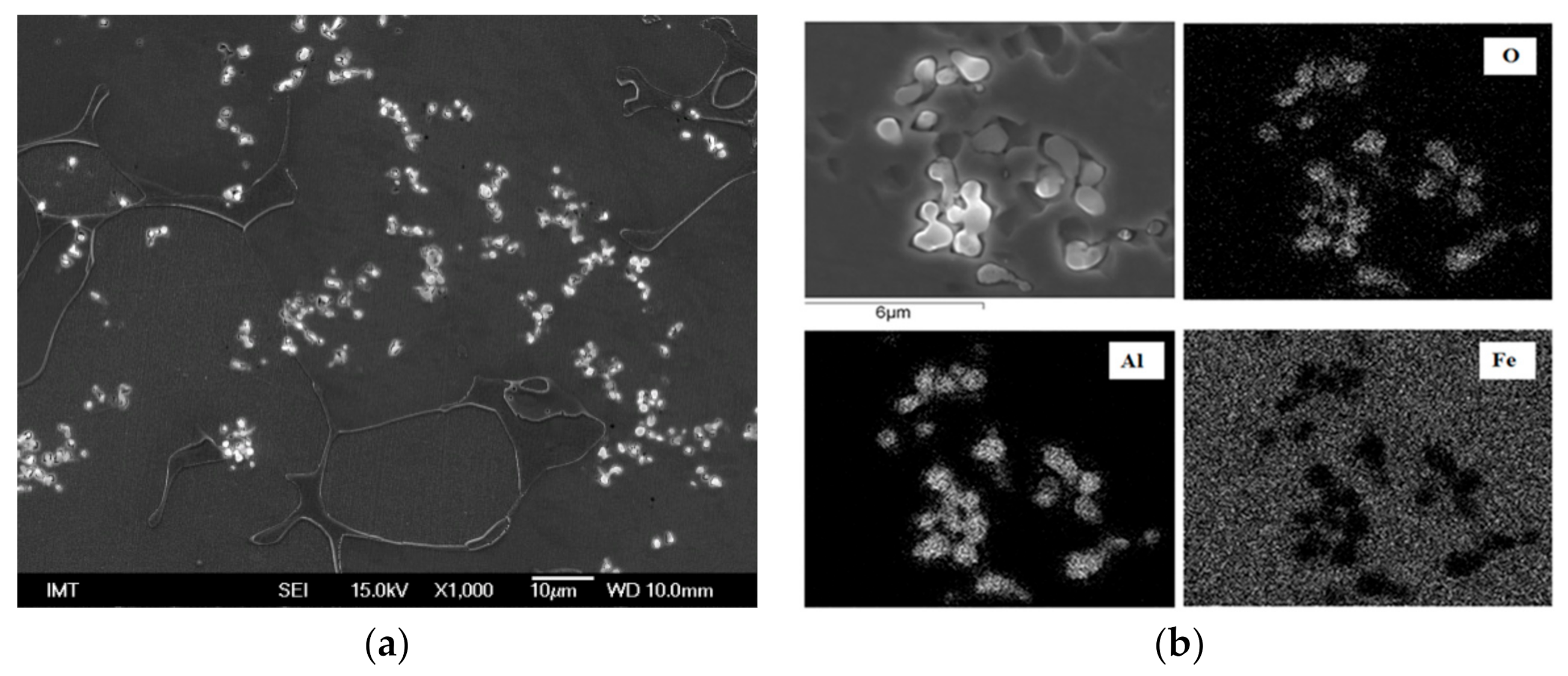
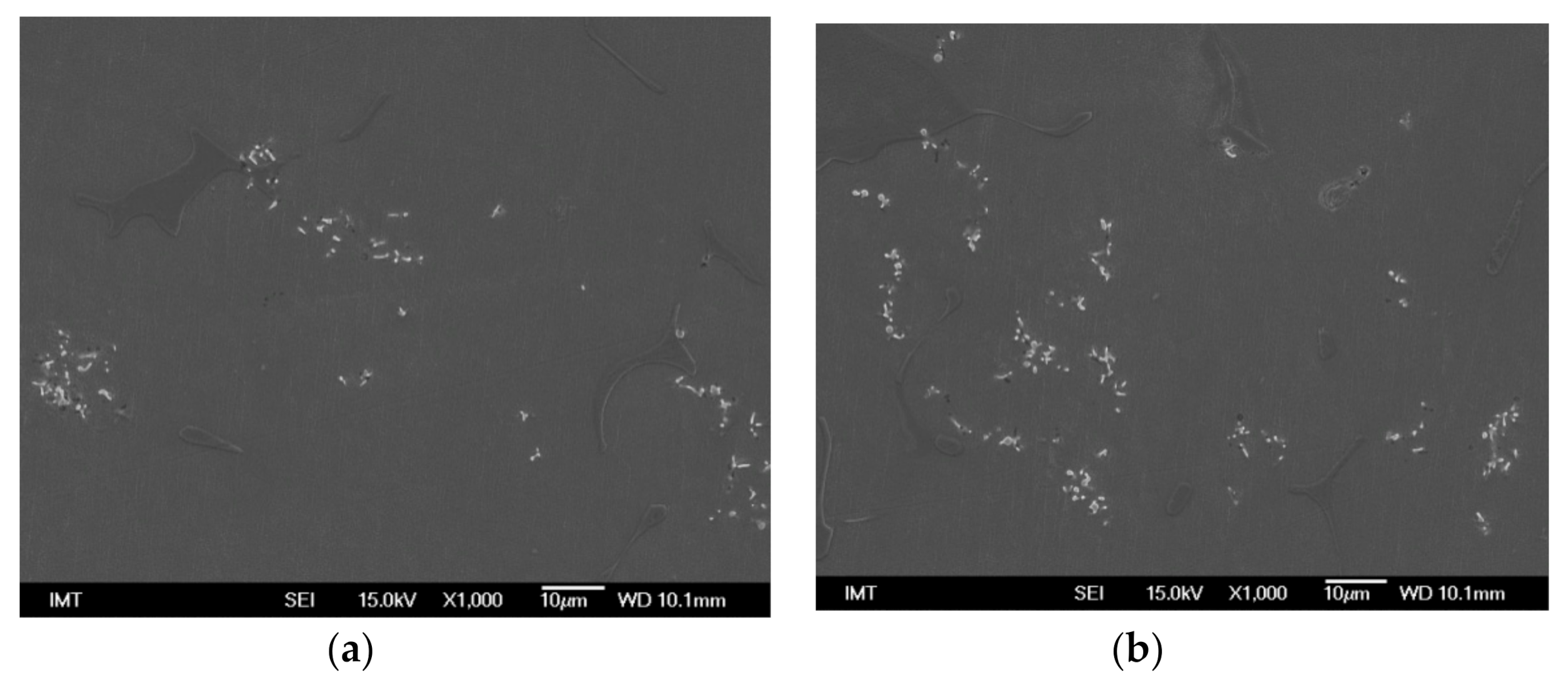
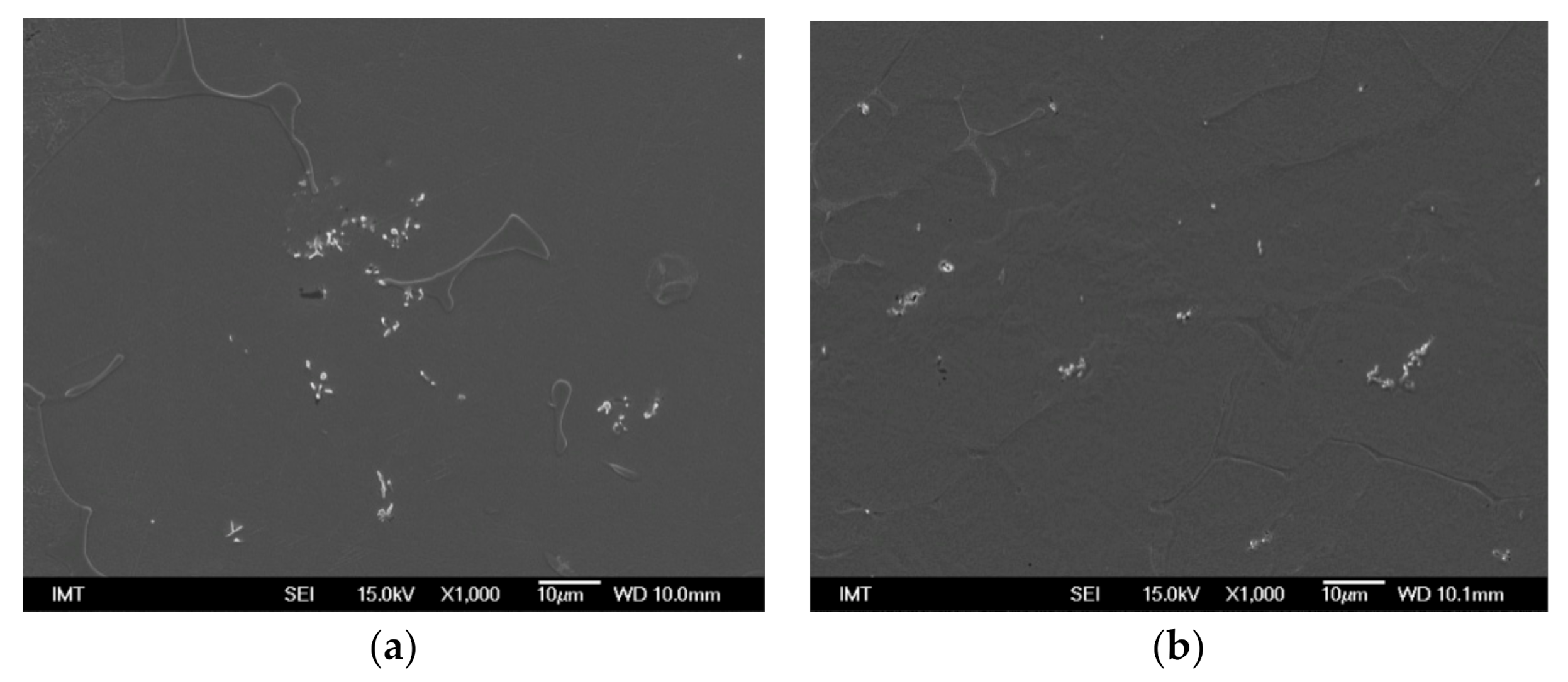
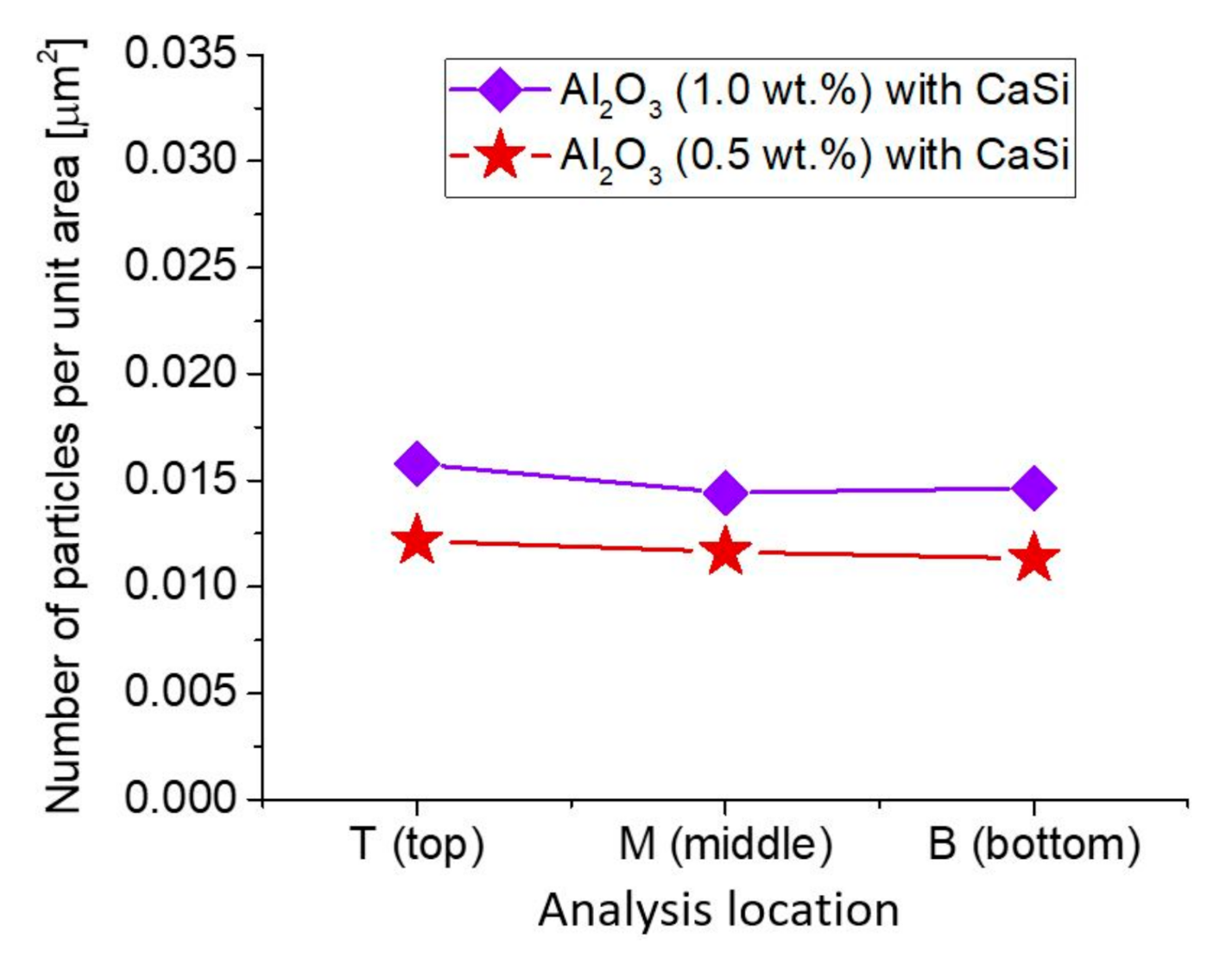
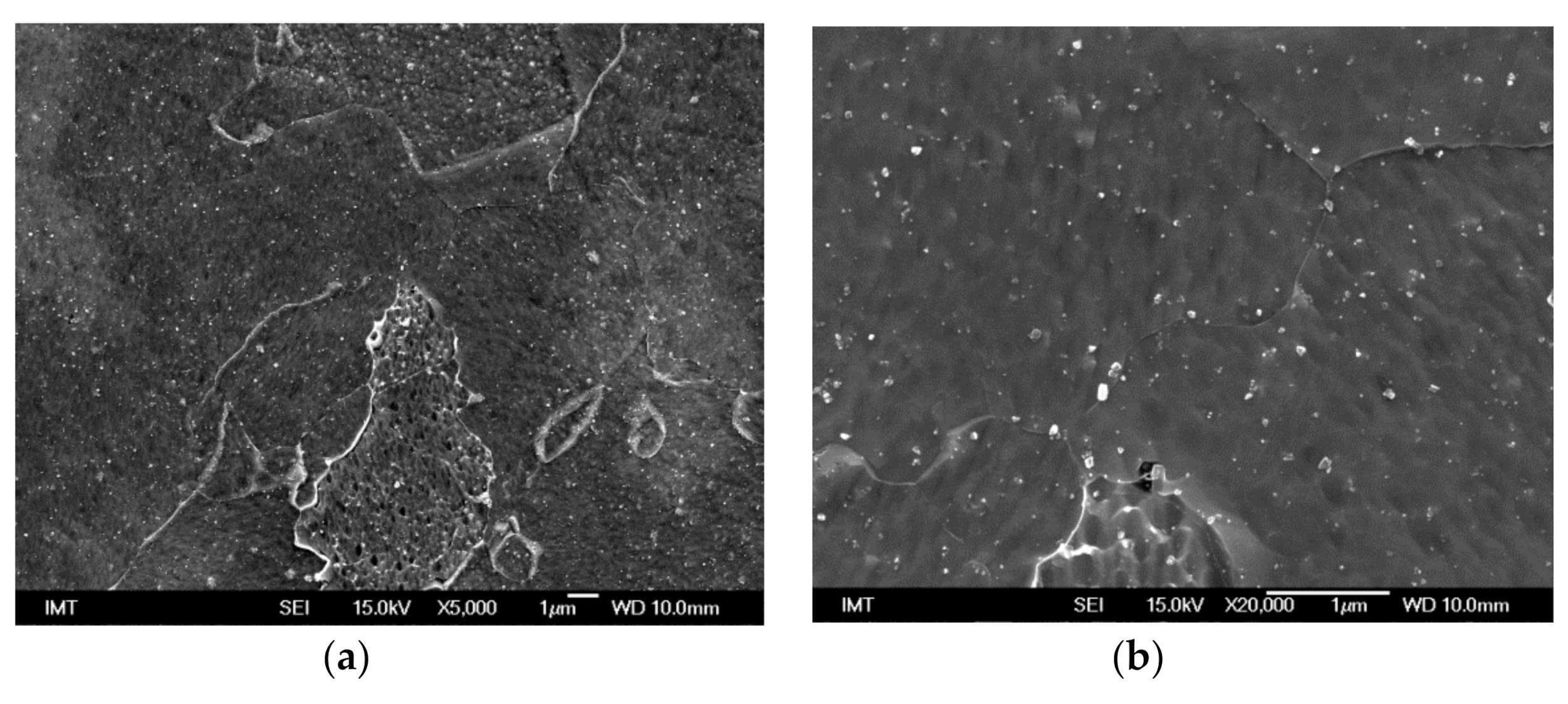
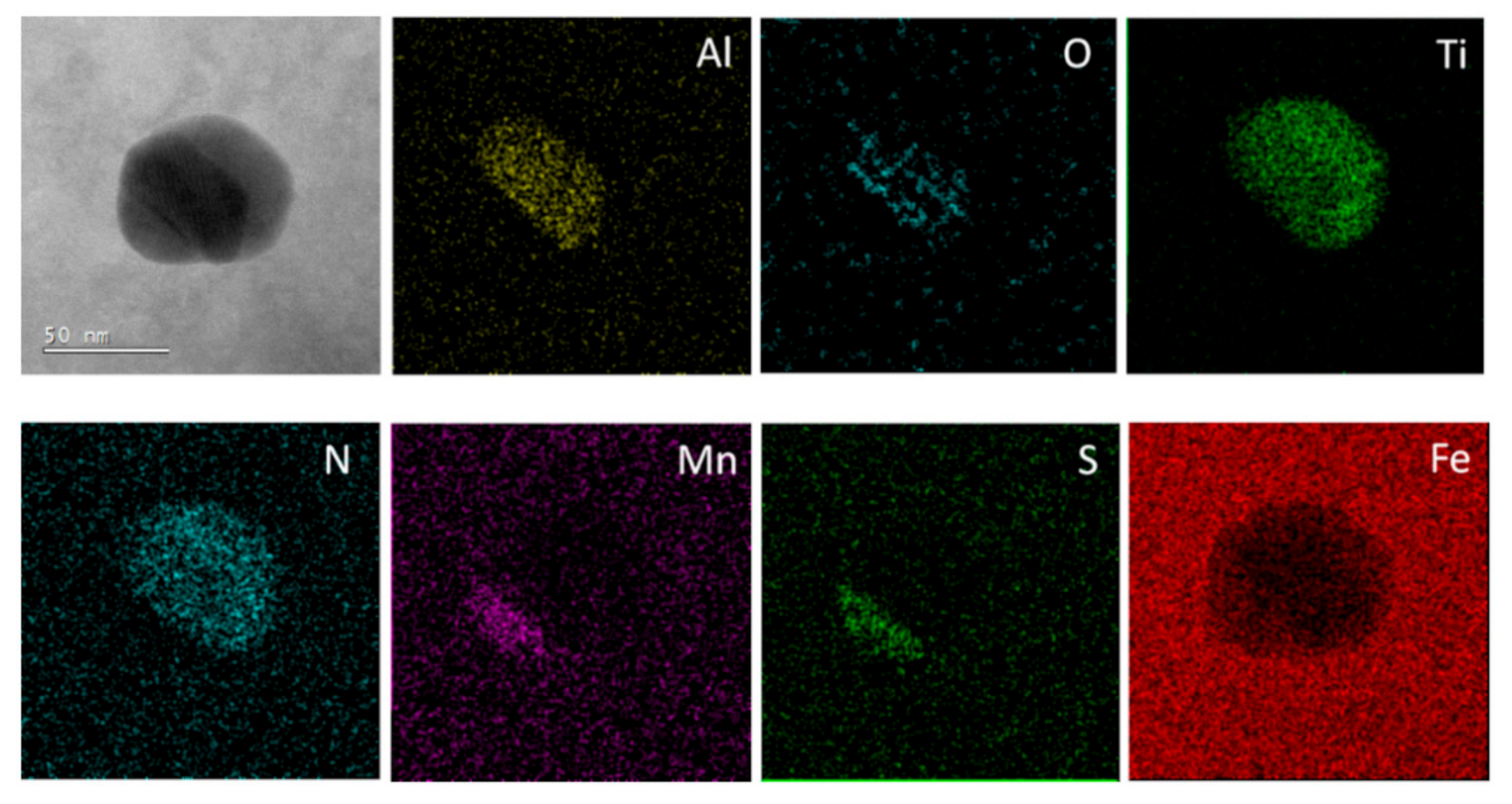
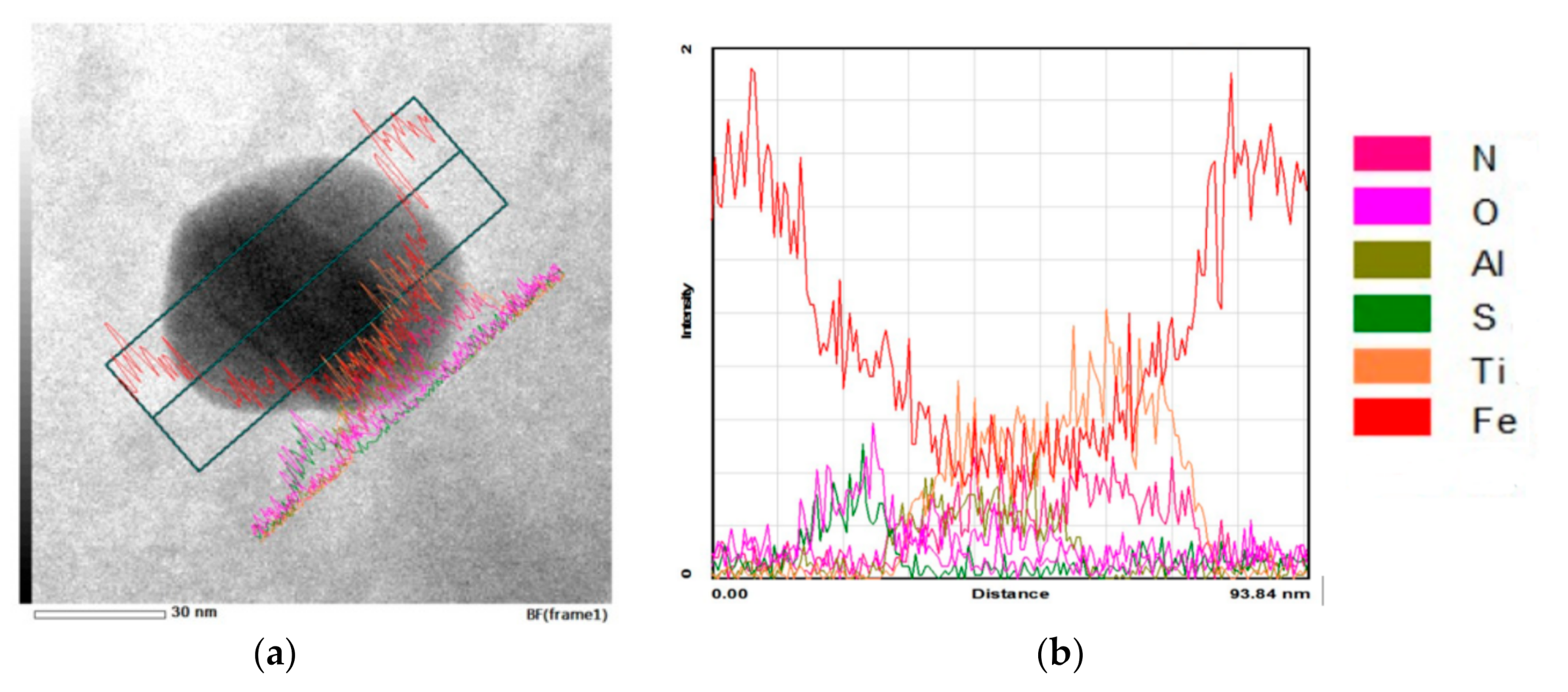
3.2. Microstructure and Particle Distribution
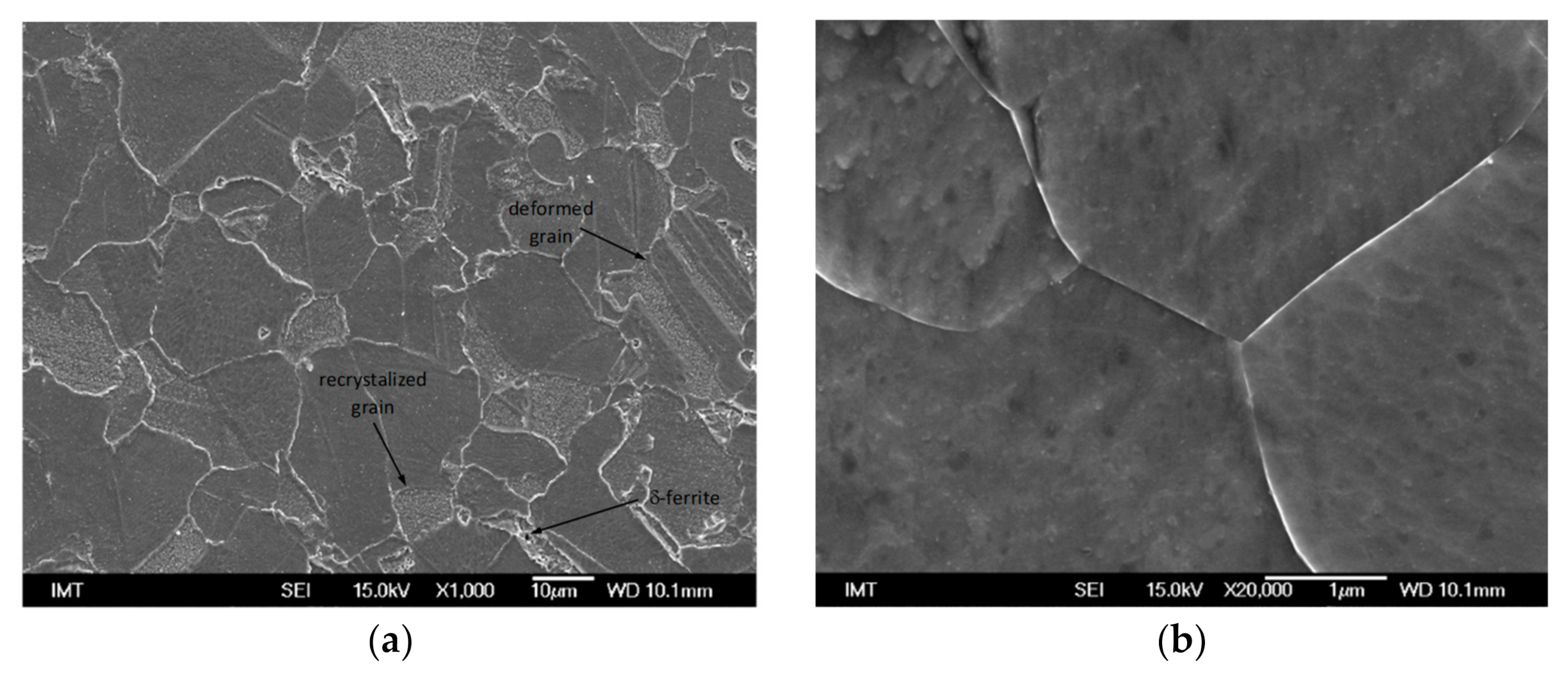
3.2.1. Reference Material
3.2.2. Submerging Method
3.2.3. Pouring over Method
3.2.4. Insertion of Sealed Tube into the Melt Flow
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaczmar, J.W.; Pietrzak, K.; Wlosinski, W. The production and application of metal matrix composite materials. J. Mater. Process. Technol. 2000, 106, 58–67. [Google Scholar]
- Casati, R.; Vedani, M. Metal Matrix Composites Reinforced by Nano-Particles—A Review. Metals 2014, 4, 65–83. [Google Scholar] [CrossRef]
- Bakshi, S.R.; Lahiri, D.; Agarwal, A. Carbon nanotube reinforced metal matrix composites—A review. Int. Mater. Rev. 2010, 55, 41–64. [Google Scholar] [CrossRef]
- Hussainova, I. Effect of microstructure on the erosive wear of titanium carbide-based cermets. Wear 2003, 255, 121–128. [Google Scholar] [CrossRef]
- Sethi, G. Pressing to Full Density: Fundamental Limitations and Capabilities of High Density Powder Metallurgy. Ph.D. Thesis, Pennsylvania State University, University Park, PA, USA, December 2004. [Google Scholar]
- Swift, K.G.; Booker, J.D. Manufacturing Process Selection Handbook; Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar]
- Chawla, K.K. Metal matrix composites. In Composite Materials; Springer: Birmingham, AL, USA, 2012; pp. 197–248. [Google Scholar]
- Sarma, M.; Grants, I.; Kaldre, I.; Bojarevics, A.; Gerbeth, G. Casting technology for ODS steels - Dispersion of nanoparticles in liquid metals. IOP Conf. Ser. Mater. Sci. Eng. 2017, 228, 012020. [Google Scholar] [CrossRef]
- Chen, S.; Seda, P.; Krugla, M.; Rijkenberg, A. High-modulus steels reinforced with ceramic particles through ingot casting process. Mater. Sci. Technol. 2016, 32, 992–1003. [Google Scholar] [CrossRef]
- Kračun, A.; Jenko, D.; Godec, M.; Savilov, S.V.; Prieto, G.; Tuckart, W.; Podgornik, B. Nanoparticles Reinforcement for the Improved Strength and High-Temperature Wear Resistance of Mn-Cr Steel. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2018, 49, 5683–5694. [Google Scholar] [CrossRef]
- Thermo-Calc Software: Thermo-Calc. Available online: http://www.thermocalc.com/products-services/software/thermo-calc/ (accessed on 13 May 2020).
- Kračun, A.; Torkar, M.; Burja, J.; Podgornik, B. Microscopic characterization and particle distribution in a cast steel matrix composite. Mater. Tehnol. 2016, 50, 451–454. [Google Scholar] [CrossRef]
- Bazaka, K.; Baranov, O.; Cvelbar, U.; Podgornik, B.; Wang, Y.; Huang, S.; Xu, L.; Lim, J.W.M.; Levchenko, I.; Xu, S. Oxygen plasmas: A sharp chisel and handy trowel for nanofabrication. Nanoscale 2018, 10, 17494–17511. [Google Scholar] [PubMed]






| C | Si | Mn | P | Cu | S | Cr | Ni | Mo | Ti | N | Co | V |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.02 | 0.48 | 1.88 | 0.03 | 0.32 | 0.03 | 16.60 | 10.70 | 2.01 | 0.16 | 0.02 | 0.17 | 0.04 |
| Sample | Particle Size [nm] | Particle Concentration [%] | Method | Dispersant/Particle Activation |
|---|---|---|---|---|
| As-cast experiments | ||||
| R1 | - | - | Reference material | - |
| S1 | 500 | 1.0 | Submerging 1 | - |
| P1 | 500 | 0.5 | Pouring over method 2 | - |
| P2 | 1.0 | - | ||
| P3 | 2.5 | - | ||
| P4 | 50 | 0.5 | - | |
| P5 | 1.0 | - | ||
| P6 | 2.5 | - | ||
| F1 | 500 | 0.5 | Insertion into the melt flow during casting 3 | - |
| F2 | CaSi (1:1) | |||
| F3 | 50 | - | ||
| F4 | CaSi (1:1) | |||
| F5 | 50 | 0.5 | Insertion into the melt flow during casting 4 | CaSi (1:1) |
| F6 | 1.0 | CaSi (1:1) | ||
| Cast and hot rolled experiments | ||||
| R2 | - | - | Reference material | - |
| A1 | 50 | 0.5 | Insertion into the melt flow during casting 4 | - |
| A2 | CaSi (1:1) | |||
| A3 | activated | |||
| A4 | CaSi (1:1)/activated | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kračun, A.; Kafexhiu, F.; Tehovnik, F.; Podgornik, B. Effective Casting Technique of Nano-Particles Alloyed Austenitic Stainless Steel. Metals 2020, 10, 1287. https://doi.org/10.3390/met10101287
Kračun A, Kafexhiu F, Tehovnik F, Podgornik B. Effective Casting Technique of Nano-Particles Alloyed Austenitic Stainless Steel. Metals. 2020; 10(10):1287. https://doi.org/10.3390/met10101287
Chicago/Turabian StyleKračun, Ana, Fevzi Kafexhiu, Franc Tehovnik, and Bojan Podgornik. 2020. "Effective Casting Technique of Nano-Particles Alloyed Austenitic Stainless Steel" Metals 10, no. 10: 1287. https://doi.org/10.3390/met10101287
APA StyleKračun, A., Kafexhiu, F., Tehovnik, F., & Podgornik, B. (2020). Effective Casting Technique of Nano-Particles Alloyed Austenitic Stainless Steel. Metals, 10(10), 1287. https://doi.org/10.3390/met10101287






