Distribution of Arsenic Inclusions in Rare Earth Steel Ingots
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
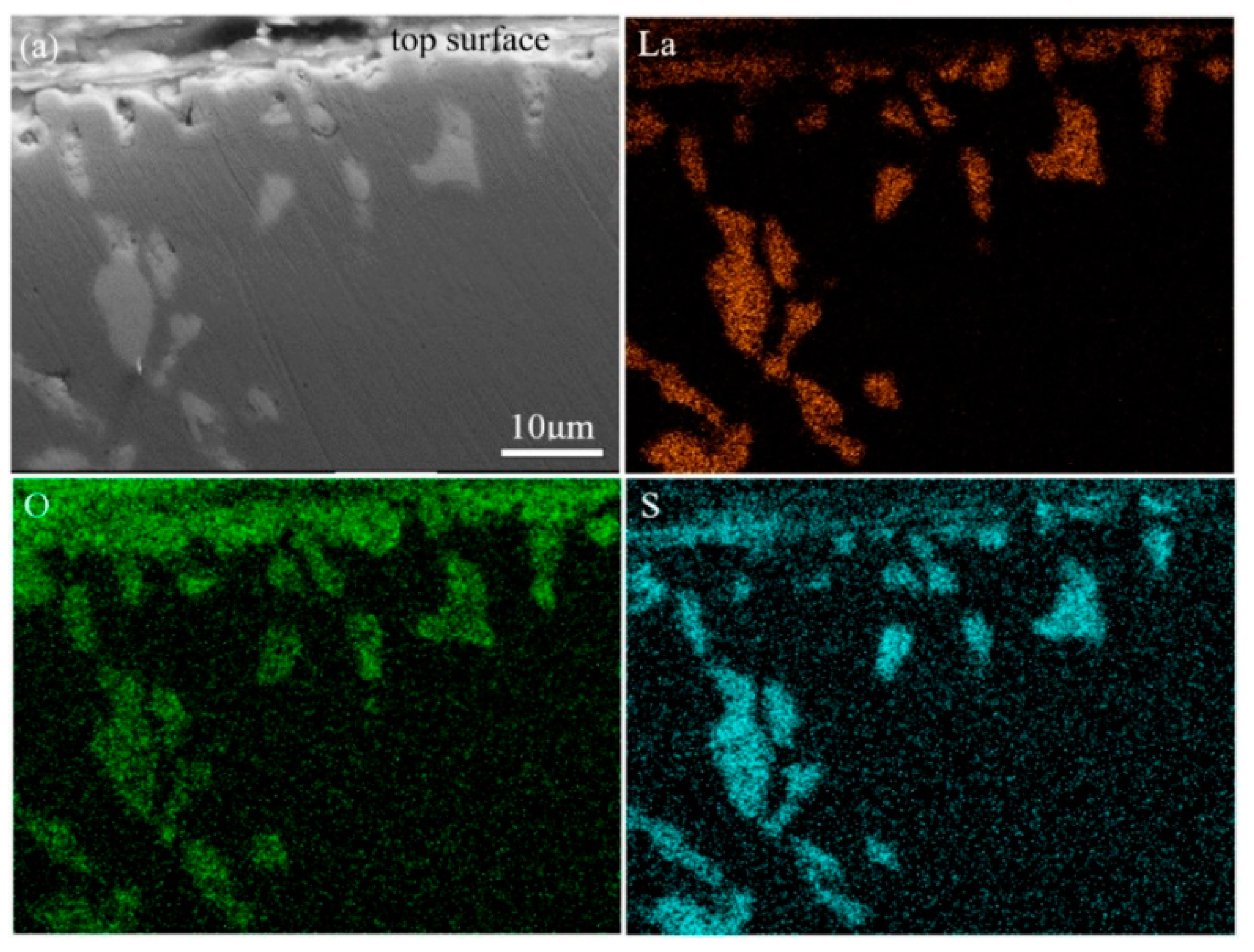
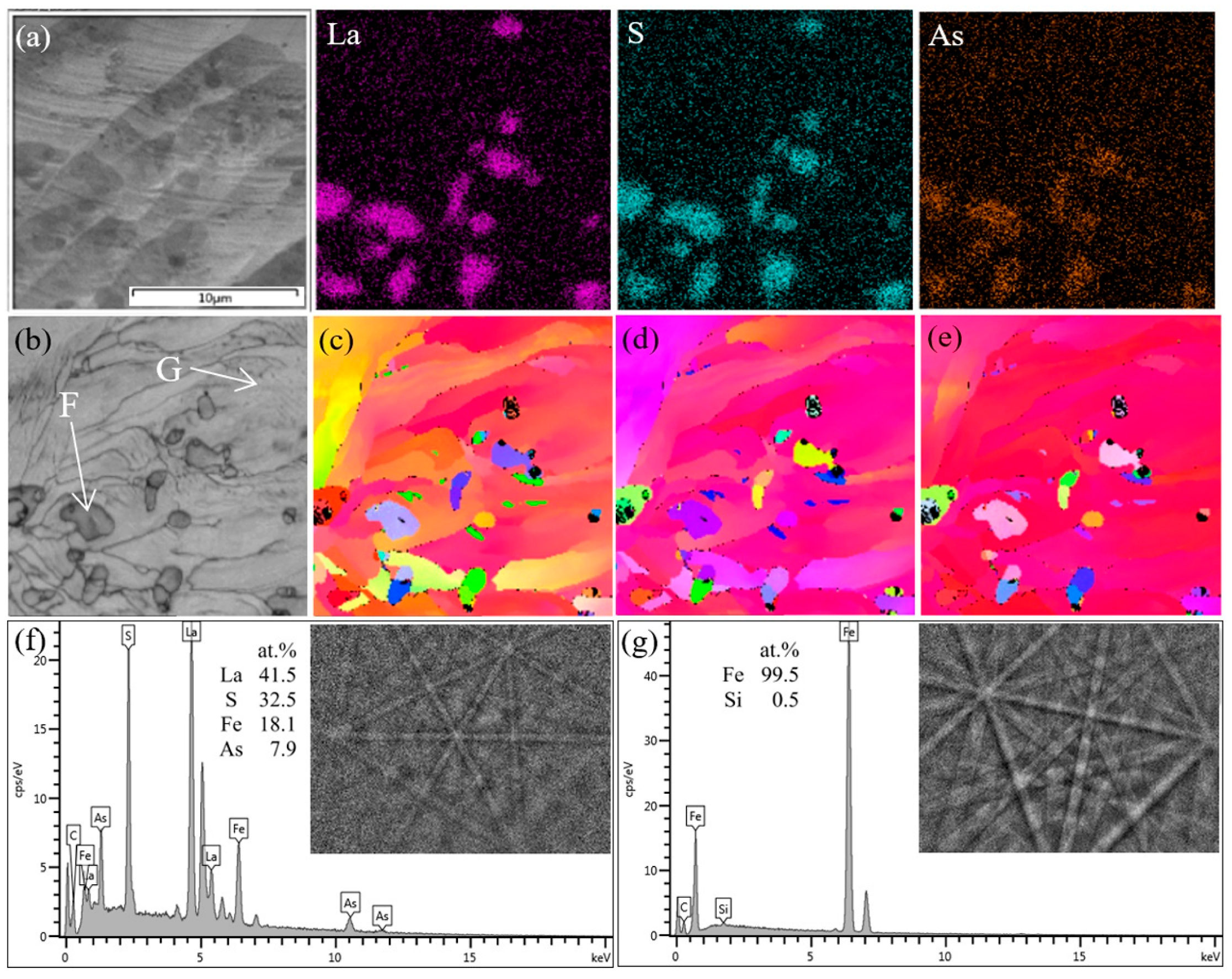
3.1. Types and Distribution of Inclusions
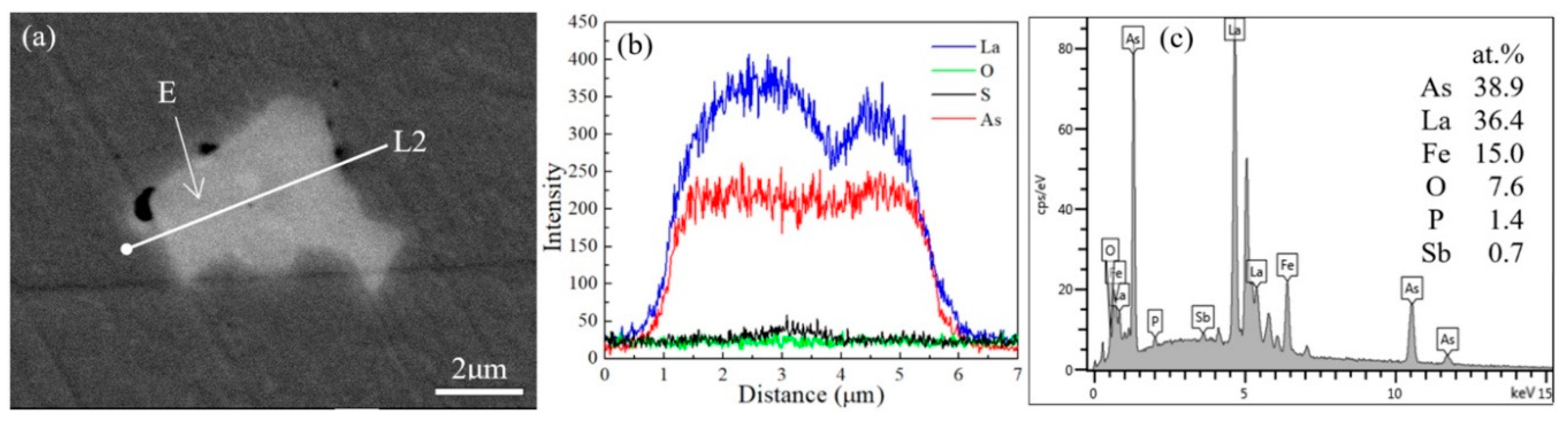
3.2. Chemical Composition Changes with La Addition
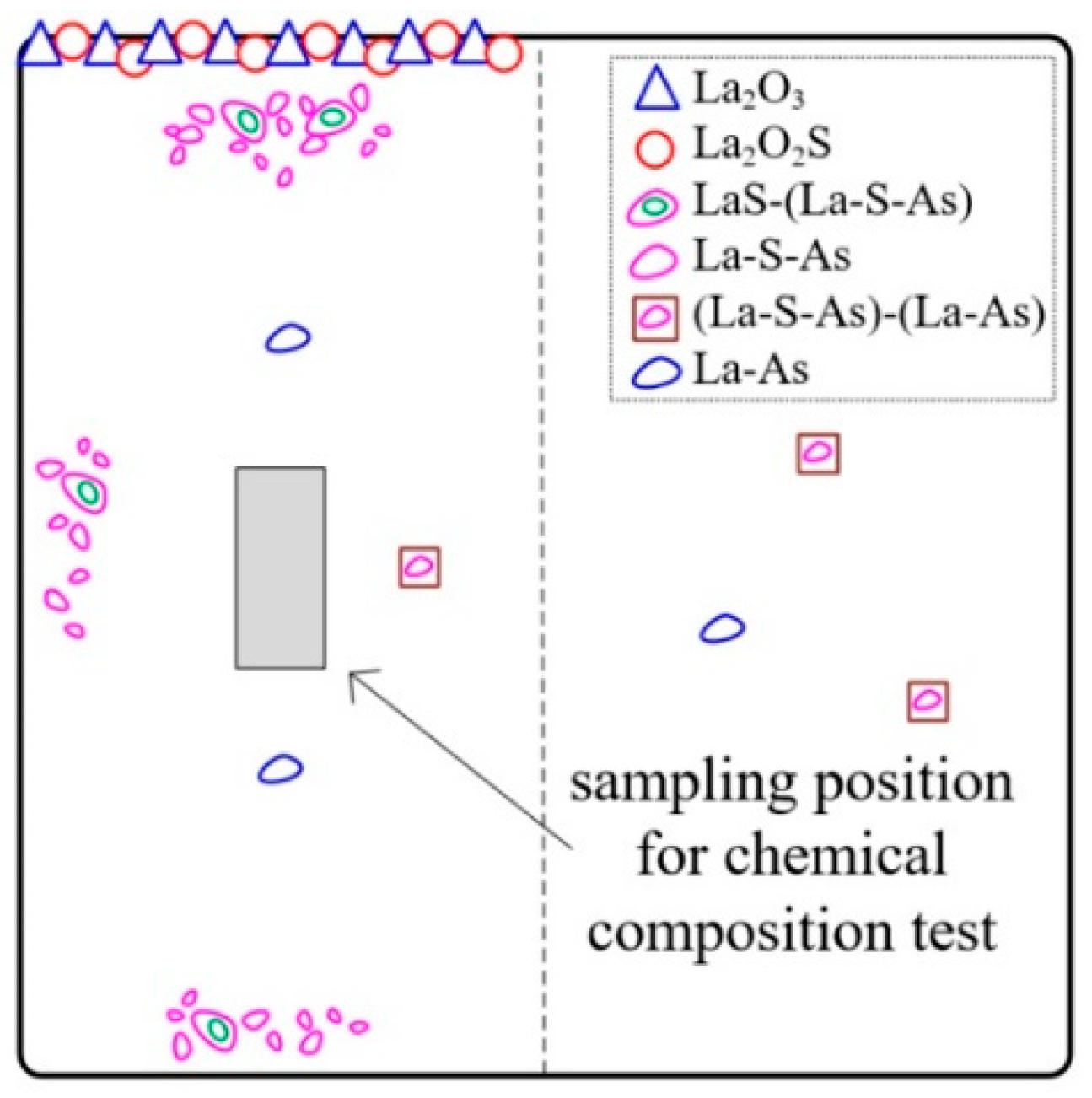
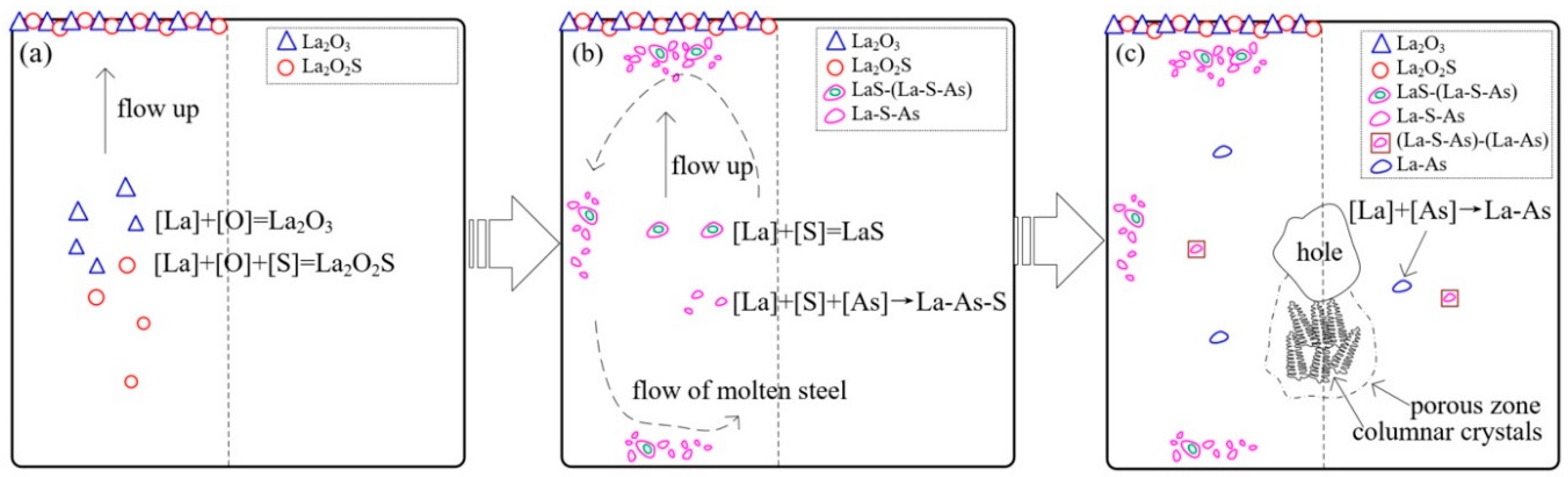
3.3. Formation Process and Distribution Mechanism of Inclusions
- (i)
- During the steelmaking process, La2O3 and La2O2S formed and floated up to the top surface of molten steel in the temperature range from 1600 °C to the liquidus, illustrated in Figure 9a. The formation of these inclusions took away most of the oxygen, including the part offered by alumina crucibles, as well as a part of the sulfur.
- (ii)
- As the consumption of soluble oxygen, lanthanum started to react mainly with sulfur and arsenic, and then LaS and La-S-As inclusions formed. As the temperature decreases, the first solidified part of the molten steel will be the surface, because the surface has the worst thermal insulation conditions. On the one hand, these inclusions floated up to the surface of molten steel and are easy to be captured. On the other hand, the unsolidified molten steel still flows, driven by the temperature gradient, as shown in Figure 9b. The overall solidification structures of the profile of the ingots can well explain this. As shown in Figure 9c, a hole exists at the center of the ingots, and some porous zones with strong columnar crystals exist below that can be seen even directly with the naked eyes. It is inferred that these porous zones are the last solidified part and have the highest temperature all the time. A temperature gradient, therefore, exists all the time also. In summary, the flow of molten steel, together with the buoyancy caused by the different densities between inclusions and molten steel, drives the inclusions to move. This leads to the enrichment of LaS and La-S-As inclusions at the surfaces of the ingots, especially near the top surface. It makes the inner part of the ingots almost free of LaS and La-S-As that dramatically decreases the concentrations of arsenic and sulfur.
- (iii)
- As the temperature went down, a few (La-S-As)-(La-As) and La-As inclusions formed during and after solidification, shown in Figure 9c. It is believed that the formation temperature of CeAs is at the end of solidification [3,29]. Therefore, the fact that La-As starts to form at the end of solidification is reasonable according to the similar chemical properties of La to Ce. However, these arsenic inclusions do not move away from the inner part of the ingots and do not affect the final arsenic concentration.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kondo, Y. Behaviour of copper during high temperature oxidation of steel containing copper. ISIJ Int. 2004, 44, 1576–1580. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, Z.; Xu, J. Grain boundary segregation of minor arsenic and nitrogen at elevated temperatures in a microalloyed steel. Int. J. Miner. Metall. Mater. 2012, 19, 399–403. [Google Scholar] [CrossRef]
- Xin, W.; Song, B.; Song, M.; Song, G. Effect of cerium on characteristic of inclusions and grain boundary segregation of arsenic in iron melts. Steel Res. Int. 2015, 86, 1430–1438. [Google Scholar] [CrossRef]
- Xin, W.; Zhang, J.; Jiang, Y.; Deng, Y.; Meng, Q.; Song, B. Effect of rare earth Ce on the isothermal oxidation behavior in air of arsenic bearing steels. Metall. Res. Technol. 2019, 116, 415. [Google Scholar] [CrossRef]
- Wang, H.; Bai, B.; Jiang, S.; Sun, L.; Wang, Y. An in situ study of the formation of rare earth inclusions in arsenic high carbon steels. ISIJ Int. 2019, 59, 1259–1265. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, F.; Li, C.; Tan, Y.; Wang, J. Effect of arsenic on microstructure and mechanical properties of 45 steel. Heat Treat. Met. 2010, 35, 33–38. [Google Scholar]
- Jiang, M.; Sun, T.; Qin, X.; Ji, E.; Wang, Z. Study on removing arsenic and tin from As-Sn bearing iron concentrates by direct reduction roasting of coal. Min. Metall. Eng. 2011, 31, 86–89. [Google Scholar]
- Wang, L.; Wang, H.; Xu, F.; Chen, F. Experimental study of microwave treating high arsenic ore. Metall. Eng. 2015, 2, 29–35. [Google Scholar] [CrossRef]
- Zhang, S.; Lv, Q.; Hu, X. Thermodynamics of arsenic removal from arsenic-bearing iron ores. Chin. J. Nonferrous Met. 2011, 21, 1705–1712. [Google Scholar]
- Dong, Y.; Shi, Z.; Zhang, L.; Peng, Y.; Hong, Y. Study on dearsenization of molten iron. Iron Steel 1984, 19, 1–7. [Google Scholar]
- Liu, S.; Sun, S. A study on dearsenication of molten iron and liquid steel with Ca-Si alloy. Specical Steel 2001, 22, 12–15. [Google Scholar]
- Fu, B.; Xue, Z.; Wu, G.; Wu, L.; Wu, Y.; Ke, C. Experimental study on the dearsenization of hot metal with CaC2-CaF2 slag. Chin. J. Proc. Eng. 2010, 10, 146–149. [Google Scholar]
- Wang, J.; Chang, L.; Zhou, L. Research on reduction dearsenication in Molten Iron (Steel). Adv. Mater. Res. 2012, 476, 273–280. [Google Scholar] [CrossRef]
- Luo, L.; Wang, J.; Wang, L.; Li, Z. Study on arsenic removal in molten steel. In Proceedings of the 6th International Symposium on High-Temperature Metallurgical Processing, TMS 2015, Orlando, FL, USA, 15–18 March 2015; pp. 669–705. [Google Scholar]
- Liu, S.; Sun, S. Dearsenication by vapourization during vacuum treatment of liquid steel. Specical Steel 2002, 23, 1–3. [Google Scholar]
- Li, Y.; Zhang, T.; Liu, C.; Jiang, M. Thermodynamic and experimental studies on Al addition of 253MA steel. Metals 2019, 9, 433. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, C. Modification mechanism of spinel inclusions in medium manganese steel with rare earth treatment. Metals 2019, 9, 804. [Google Scholar] [CrossRef]
- Torkamani, H.; Raygan, S.; Mateo, C.G.; Rassizadehghani, J.; Vivas, J.; Palizdar, Y.; San-Martin, D. The influence of La and Ce addition on inclusion modification in cast niobium microalloyed steels. Metals 2017, 7, 377. [Google Scholar] [CrossRef]
- Xu, Y.; Song, S.; Wang, J. Effect of rare earth cerium on the creep properties of modified 9Cr-1Mo heat-resistant steel. Mater. Lett. 2015, 161, 616–619. [Google Scholar] [CrossRef]
- Wang, L.; Lin, Q.; Yue, L.; Liu, L.; Guo, F.; Wang, F. Study of application of rare earth elements in advanced low alloy steels. J. Alloys Compd. 2008, 451, 534–537. [Google Scholar] [CrossRef]
- Adabavazeh, Z.; Hwang, W.S.; Su, Y.H. Effect of adding cerium on microstructure and morphology of Ce-based inclusions formed in low-carbon steel. Sci. Rep. 2017, 7, 46503. [Google Scholar] [CrossRef]
- Liu, X.; Yang, J.; Yang, L.; Gao, X. Effect of Ce on inclusions and impact property of 2Cr13 stainless steel. J. Iron Steel Res. Int. 2010, 17, 59–64. [Google Scholar] [CrossRef]
- Wen, B.; Song, B.; Pan, N.; Shu, Q.; Hu, X.; Mao, J. Influence of Ce on characteristics of inclusions and microstructure of pure iron. J. Iron Steel Res. Int. 2011, 18, 38–44. [Google Scholar] [CrossRef]
- Xin, W.; Zhang, J.; Luo, G.; Wang, R.; Meng, Q.; Song, B. Improvement of hot ductility of C-Mn steel containing arsenic by rare earth Ce. Metall. Res. Technol. 2018, 115, 419. [Google Scholar] [CrossRef]
- Wang, H.; Xiong, L.; Zhang, L.; Wang, Y.; Shu, Y.; Zhou, Y. Investigation of RE-O-S-As inclusions in high carbon steels. Metall. Mater. Trans. B 2017, 48, 2849–2858. [Google Scholar] [CrossRef]
- Vahed, A.; Kay, D.A.R. Thermodynamics of rare earths in steelmaking. Metall. Trans. B 1976, 7, 375–383. [Google Scholar] [CrossRef]
- Lu, W.K.; McLean, A. Thermodynamic behaviour of rare-earth elements in molten steel. Ironmak. Steelmak. 1974, 1, 228–233. [Google Scholar]
- Xin, W.; Song, B.; Huang, C.; Song, M.; Song, G. Effect of arsenic content and quenching temperature on solidification microstructure and arsenic distribution in iron-arsenic alloys. Int. J. Miner. Metall. Mater. 2015, 22, 704–713. [Google Scholar] [CrossRef]
- Li, Y.; Sun, M.; Jiang, Z.; Chen, C.; Chen, K.; Huang, X.; Sun, S.; Li, H. Inclusion modification in C104Cr saw wire steel with different cerium content. Metals 2019, 9, 54. [Google Scholar] [CrossRef]
- Villars, P. AULING FILE in: Inorganic Solid Phases, SpringerMaterials (Online Database); Springer: Heidelberg, Germany, 2012. [Google Scholar]
- Kwon, S.K.; Park, J.S.; Park, J.H. Influence of refractory-steel interfacial reaction on the formation behavior of inclusions in Ce-containing stainless steel. ISIJ Int. 2015, 55, 2589–2596. [Google Scholar] [CrossRef]
- Kwon, S.K.; Kong, Y.M.; Park, J.H. Effect of Al deoxidation on the formation behavior of inclusions in Ce-added stainless steel melts. Met. Mater. Int. 2014, 20, 959–966. [Google Scholar] [CrossRef]




| Elements | C | Si | Mn | P | S | Al | O | As |
|---|---|---|---|---|---|---|---|---|
| Concentrations | 0.79 | 0.21 | 0.61 | 0.011 | 0.015 | 0.001 | 0.0024 | 0.010 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Jiang, S.; Yu, P.; Bai, B.; Sun, L.; Wang, Y. Distribution of Arsenic Inclusions in Rare Earth Steel Ingots. Metals 2020, 10, 146. https://doi.org/10.3390/met10010146
Wang H, Jiang S, Yu P, Bai B, Sun L, Wang Y. Distribution of Arsenic Inclusions in Rare Earth Steel Ingots. Metals. 2020; 10(1):146. https://doi.org/10.3390/met10010146
Chicago/Turabian StyleWang, Hongpo, Silu Jiang, Peng Yu, Bin Bai, Lifeng Sun, and Yu Wang. 2020. "Distribution of Arsenic Inclusions in Rare Earth Steel Ingots" Metals 10, no. 1: 146. https://doi.org/10.3390/met10010146
APA StyleWang, H., Jiang, S., Yu, P., Bai, B., Sun, L., & Wang, Y. (2020). Distribution of Arsenic Inclusions in Rare Earth Steel Ingots. Metals, 10(1), 146. https://doi.org/10.3390/met10010146




