Taurine and Methylprednisolone Administration at Close Proximity to the Onset of Muscle Degeneration Is Ineffective at Attenuating Force Loss in the Hind-Limb of 28 Days Mdx Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Supplementation
2.2. Muscle Preparation for in Situ Contractile Protocol
2.3. Contractile Protocol
2.4. Statistics
3. Results
3.1. Effect of Taurine Supplementation on Body Mass, Muscle Mass, and in Situ Contractile Characteristics of Maximum and Specific Force Production
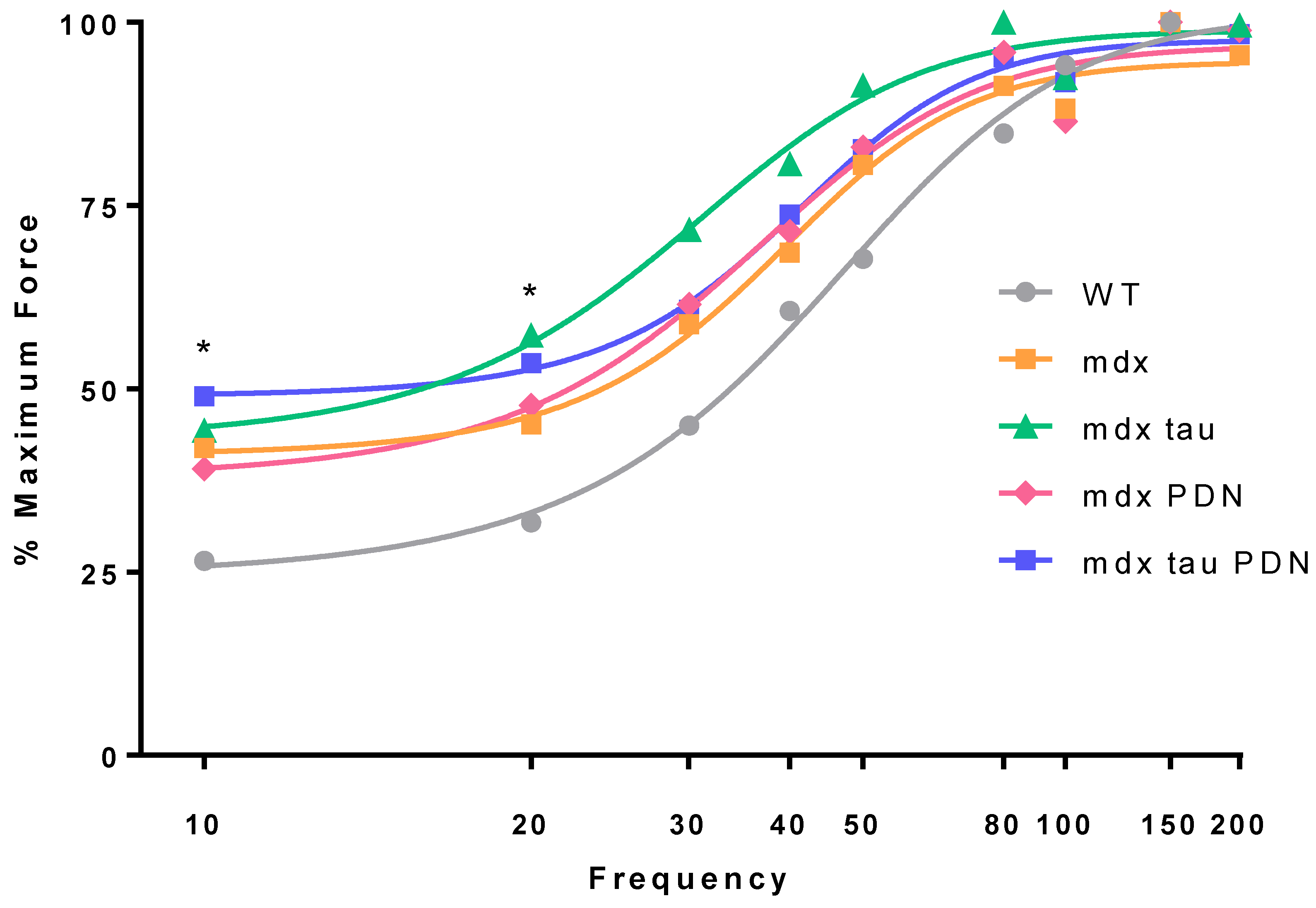
3.2. Force Frequency Relationship
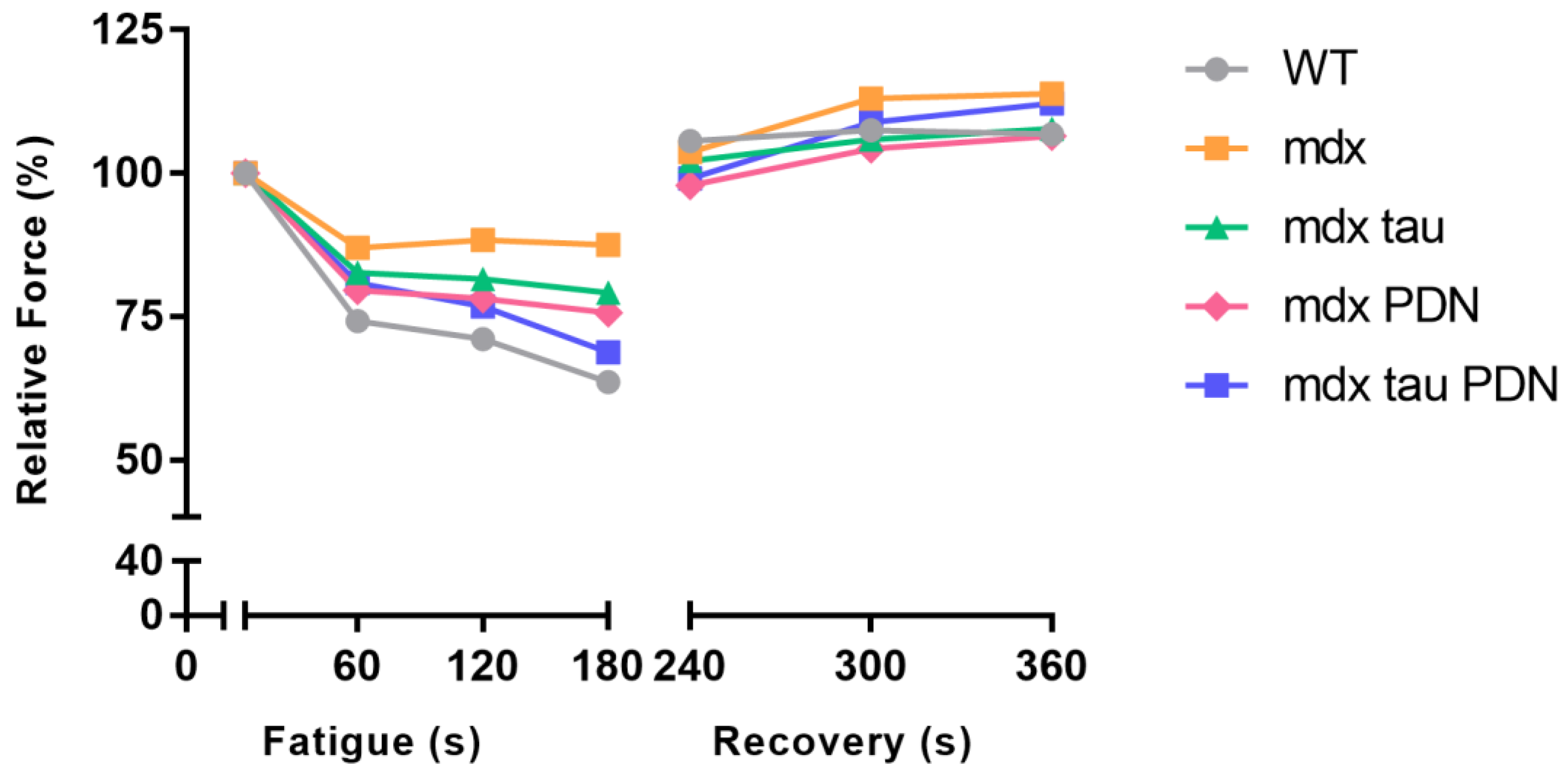
3.3. Fatigue Recovery
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bushby, K.; Finkel, R.; Birnkrant, D.J.; Case, L.E.; Clemens, P.R.; Cripe, L.; Kaul, A.; Kinnett, K.; McDonald, C.; Pandya, S.; et al. Diagnosis and management of duchenne muscular dystrophy, Part 1: Diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010, 9, 77–93. [Google Scholar] [CrossRef]
- Henricson, E.K.; Abresch, R.T.; Cnaan, A.; Hu, F.; Duong, T.; Arrieta, A.; Han, J.; Escolar, D.M.; Florence, J.M.; Clemens, P.R.; et al. The cooperative international neuromuscular research group duchenne natural history study: Glucocorticoid treatment preserves clinically meaningful functional milestones and reduces rate of disease progression as measured by manual muscle testing and other commonly used clinical trial outcome measures. Muscle Nerve 2013, 48, 55–67. [Google Scholar] [PubMed]
- Miyatake, S.; Shimizu-Motohashi, Y.; Takeda, S.; Aoki, Y. Anti-inflammatory drugs for duchenne muscular dystrophy: Focus on skeletal muscle-releasing factors. Drug Des. Dev. Ther. 2016, 10, 2745–2758. [Google Scholar]
- Yilmaz, O.; Karaduman, A.; Topaloglu, H. Prednisolone therapy in duchenne muscular dystrophy prolongs ambulation and prevents scoliosis. Eur. J. Neurol. 2004, 11, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Angelini, C.; Peterle, E. Old and new therapeutic developments in steroid treatment in duchenne muscular dystrophy. Acta Myol. 2012, 31, 9–15. [Google Scholar] [PubMed]
- De Luca, A.; Pierno, S.; Camerino, D.C. Taurine: The appeal of a safe amino acid for skeletal muscle disorders. J. Transl. Med. 2015, 13, 243. [Google Scholar] [CrossRef] [PubMed]
- Huxtable, R.J. Physiological actions of taurine. Physiol. Rev. 1992, 72, 101–163. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, L.; Granberg, K.E.; Briere, K.M.; Anderson, J.E. Nuclear magnetic resonance spectroscopy study of muscle growth, mdx dystrophy and glucocorticoid treatments: Correlation with repair. NMR Biomed. 1998, 11, 1–10. [Google Scholar] [CrossRef]
- Griffin, J.L.; Williams, H.J.; Sang, E.; Clarke, K.; Rae, C.; Nicholson, J.K. Metabolic profiling of genetic disorders: A multitissue 1h nuclear magnetic resonance spectroscopic and pattern recognition study into dystrophic tissue. Anal. Biochem. 2001, 293, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Barker, R.G.; Horvath, D.; van der Poel, C.; Murphy, R.M. Benefits of prenatal taurine supplementation in preventing the onset of acute damage in the mdx mouse. PLoS Curr. 2017, 9. [Google Scholar] [CrossRef]
- Terrill, J.R.; Grounds, M.D.; Arthur, P.G. Increased taurine in pre-weaned juvenile mdx mice greatly reduces the acute onset of myofibre necrosis and dystropathology and prevents inflammation. PLoS Curr. 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Grounds, M.D.; Radley, H.G.; Lynch, G.S.; Nagaraju, K.; De Luca, A. Towards developing standard operating procedures for pre-clinical testing in the mdx mouse model of duchenne muscular dystrophy. Neurobiol. Dis. 2008, 31, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Willmann, R.; Possekel, S.; Dubach-Powell, J.; Meier, T.; Ruegg, M.A. Mammalian animal models for duchenne muscular dystrophy. Neuromuscul. Disord. 2009, 19, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Cozzoli, A.; Rolland, J.F.; Capogrosso, R.F.; Sblendorio, V.T.; Longo, V.; Simonetti, S.; Nico, B.; De Luca, A. Evaluation of potential synergistic action of a combined treatment with alpha-methyl-prednisolone and taurine on the mdx mouse model of duchenne muscular dystrophy. Neuropathol. Appl. Neurobiol. 2011, 37, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Granchelli, J.A.; Pollina, C.; Hudecki, M.S. Pre-clinical screening of drugs using the mdx mouse. Neuromuscul. Disord. NMD 2000, 10, 235–239. [Google Scholar] [CrossRef]
- De Luca, A.; Pierno, S.; Liantonio, A.; Cetrone, M.; Camerino, C.; Fraysse, B.; Mirabella, M.; Servidei, S.; Ruegg, U.T.; Conte Camerino, D. Enhanced dystrophic progression in mdx mice by exercise and beneficial effects of taurine and insulin-like growth factor-1. J. Pharmacol. Exp. Ther. 2003, 304, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Burkholder, T.J.; Fingado, B.; Baron, S.; Lieber, R.L. Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb. J. Morphol. 1994, 221, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Terrill, J.R.; Pinniger, G.J.; Graves, J.A.; Grounds, M.D.; Arthur, P.G. Increasing taurine intake and taurine Synthesis improves skeletal muscle function in the mdx mouse model for duchenne muscular dystrophy. J. Physiol. 2016, 594, 3095–3110. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Pierno, S.; Camerino, C.; Huxtable, R.J.; Camerino, D.C. Effect of taurine on excitation-contraction coupling of extensor digitorum longus muscle of dystrophic mdx mouse. Adv. Exp. Med. Biol. 1998, 442, 115–119. [Google Scholar] [PubMed]
- Horvath, D.M.; Murphy, R.M.; Mollica, J.P.; Hayes, A.; Goodman, C.A. The effect of taurine and beta-alanine supplementation on taurine transporter protein and fatigue resistance in skeletal muscle from mdx mice. Amino Acids 2016, 48, 2635–2645. [Google Scholar] [CrossRef] [PubMed]
- Spaeth, D.G.; Schneider, D.L. Turnover of taurine in rat tissues. J. Nutr. 1974, 104, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Oja, S.; Lehtinen, I.; Lähdesmäki, P. Taurine transport rates between plasma and tissues in adult and 7-day-old mice. Q. J. Exp. Physiol. Cogn. Med. Sci. 1976, 61, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.G.; Whitehead, N.P.; Froehner, S.C. Absence of dystrophin disrupts skeletal muscle signaling: Roles of Ca2+, reactive oxygen species, and nitric oxide in the development of muscular dystrophy. Physiol. Rev. 2016, 96, 253–305. [Google Scholar] [CrossRef] [PubMed]
- Terrill, J.R.; Grounds, M.D.; Arthur, P.G. Taurine deficiency, synthesis and transport in the mdx mouse model for duchenne muscular dystrophy. Int. J. Biochem. Cell Boil. 2015, 66, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, E.P.; Reeves, E.; Damsker, J.; Nagaraju, K.; McCall, J.M.; Connor, E.M.; Bushby, K. Novel approaches to corticosteroid treatment in duchenne muscular dystrophy. Phys. Med. Rehabilit. Clin. N. Am. 2012, 23, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Ghosh, D.; Srivastava, N.K.; Kumar, A.; Mittal, B.; Pandey, C.M.; Singh, U. Prednisolone in duchenne muscular dystrophy with imminent loss of ambulation. J. Neurol. 2006, 253, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Pierno, S.; Liantonio, A.; Cetrone, M.; Camerino, C.; Simonetti, S.; Papadia, F.; Camerino, D.C. Alteration of excitation-contraction coupling mechanism in extensor digitorum longus muscle fibres of dystrophic mdx mouse and potential efficacy of taurine. Br. J. Pharmacol. 2001, 132, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Schuller-Levis, G.B.; Park, E. Taurine: New implications for an old amino acid. FEMS Microbiol. Lett. 2003, 226, 195–202. [Google Scholar] [CrossRef]
- Krahn, M.J.; Anderson, J.E. Anabolic steroid treatment increases myofiber damage in mdx mouse muscular dystrophy. J. Neurol. Sci. 1994, 125, 138–146. [Google Scholar] [CrossRef]
- Lynch, G.S.; Hinkle, R.T.; Chamberlain, J.S.; Brooks, S.V.; Faulkner, J.A. Force and power output of fast and slow skeletal muscles from mdx mice 6–28 months old. J. Physiol. 2001, 535, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.; Williams, D.A. Contractile function and low-intensity exercise effects of old dystrophic (mdx) mice. Am. J. Physiol. 1998, 274, C1138–C1144. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.M.; Moore, R.L. Myosin light chain phosphorylation and tension potentiation in mouse skeletal muscle. Am. J. Physiol. 1989, 257, C1012–C1019. [Google Scholar] [CrossRef] [PubMed]
- Abbate, F.; Sargeant, A.J.; Verdijk, P.W.L.; de Haan, A. Effects of high-frequency initial pulses and posttetanic potentiation on power output of skeletal muscle. J. Appl. Physiol. 2000, 88, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.C.; Ong, S.G.; LaGory, E.L.; Kraft, P.E.; Giaccia, A.J.; Wu, J.C.; Blau, H.M. Telomere shortening and metabolic compromise underlie dystrophic cardiomyopathy. Proc. Natl. Acad. Sci. USA 2016, 113, 13120–13125. [Google Scholar] [CrossRef] [PubMed]

| - | Body Mass (g) | TA Mass (mg) | Lo (mm) | CSA (mm) | Pt (mN) | TTP (ms) | ½RT (ms) |
|---|---|---|---|---|---|---|---|
| WT (n = 10) | 14 ± 2 | 20.6 ± 3 | 10 ± 0.6 | 3.3 ± 0.4 | 118 ± 20 | 26 ± 5 | 21 ± 5 |
| mdx (n = 8) | 15 ± 1 | 23.5 ± 3 | 11 ± 0.5 * | 3.4 ± 0.3 | 109 ± 33 ^ | 29 ± 3 * | 32 ± 10 * |
| mdx tau (n = 6) | 13 ± 2 | 19.3 ± 5 | 10 ± 0.8 | 3.0 ± 0.8 | 91 ± 18 ^ | 27 ± 3 | 28 ± 10 |
| mdx PDN (n = 6) | 15 ± 2 | 25.3 ± 4 | 11 ± 0.5 | 3.7 ± 0.4 | 150 ± 20 | 29 ± 2 | 30 ± 5 |
| mdx tau + PDN (n = 6) | 13 ± 2 | 19.0 ± 6 | 10 ± 0.5 | 3.0 ± 0.8 | 109 ± 14 ^ | 29 ± 3 | 32 ± 8 |
| - | Fatigue (mN) | Recovery (mN) | |||||
|---|---|---|---|---|---|---|---|
| - | 0 (s) | 60 (s) | 120 (s) | 180 (s) | 240 (s) | 300 (s) | 360 (s) |
| WT | 1 | 74 ± 12 | 71 ± 15 | 64 ± 9 | 106 ± 19 | 108 ±20 | 107 ± 17 |
| mdx | 1 | 87 ± 14 | 88 ± 19 | 87 ± 24 | 112 ± 20 | 131 ± 40 | 134 ± 37 |
| mdx tau | 1 | 83 ± 7 | 82 ± 8 | 79 ± 10 | 102 ± 12 | 106 ± 10 | 108 ± 10 |
| mdx PDN | 1 | 80 ± 4 | 78 ± 5 | 76 ± 7 | 98 ± 20 | 104 ± 18 | 106 ± 15 |
| mdx tau + PDN | 1 | 80 ± 13 | 77 ± 19 | 69 ± 26 | 99 ± 32 | 108 ± 30 | 112 ± 29 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barker, R.G.; Van der Poel, C.; Horvath, D.; Murphy, R.M. Taurine and Methylprednisolone Administration at Close Proximity to the Onset of Muscle Degeneration Is Ineffective at Attenuating Force Loss in the Hind-Limb of 28 Days Mdx Mice. Sports 2018, 6, 109. https://doi.org/10.3390/sports6040109
Barker RG, Van der Poel C, Horvath D, Murphy RM. Taurine and Methylprednisolone Administration at Close Proximity to the Onset of Muscle Degeneration Is Ineffective at Attenuating Force Loss in the Hind-Limb of 28 Days Mdx Mice. Sports. 2018; 6(4):109. https://doi.org/10.3390/sports6040109
Chicago/Turabian StyleBarker, Robert G., Chris Van der Poel, Deanna Horvath, and Robyn M. Murphy. 2018. "Taurine and Methylprednisolone Administration at Close Proximity to the Onset of Muscle Degeneration Is Ineffective at Attenuating Force Loss in the Hind-Limb of 28 Days Mdx Mice" Sports 6, no. 4: 109. https://doi.org/10.3390/sports6040109
APA StyleBarker, R. G., Van der Poel, C., Horvath, D., & Murphy, R. M. (2018). Taurine and Methylprednisolone Administration at Close Proximity to the Onset of Muscle Degeneration Is Ineffective at Attenuating Force Loss in the Hind-Limb of 28 Days Mdx Mice. Sports, 6(4), 109. https://doi.org/10.3390/sports6040109





