Neuromuscular Fatigue Profile of Prepubertal and Adult Female Handball Players
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Instrumentation
2.3. Testing Procedures
2.4. Data Analysis
2.5. Statistical Analysis
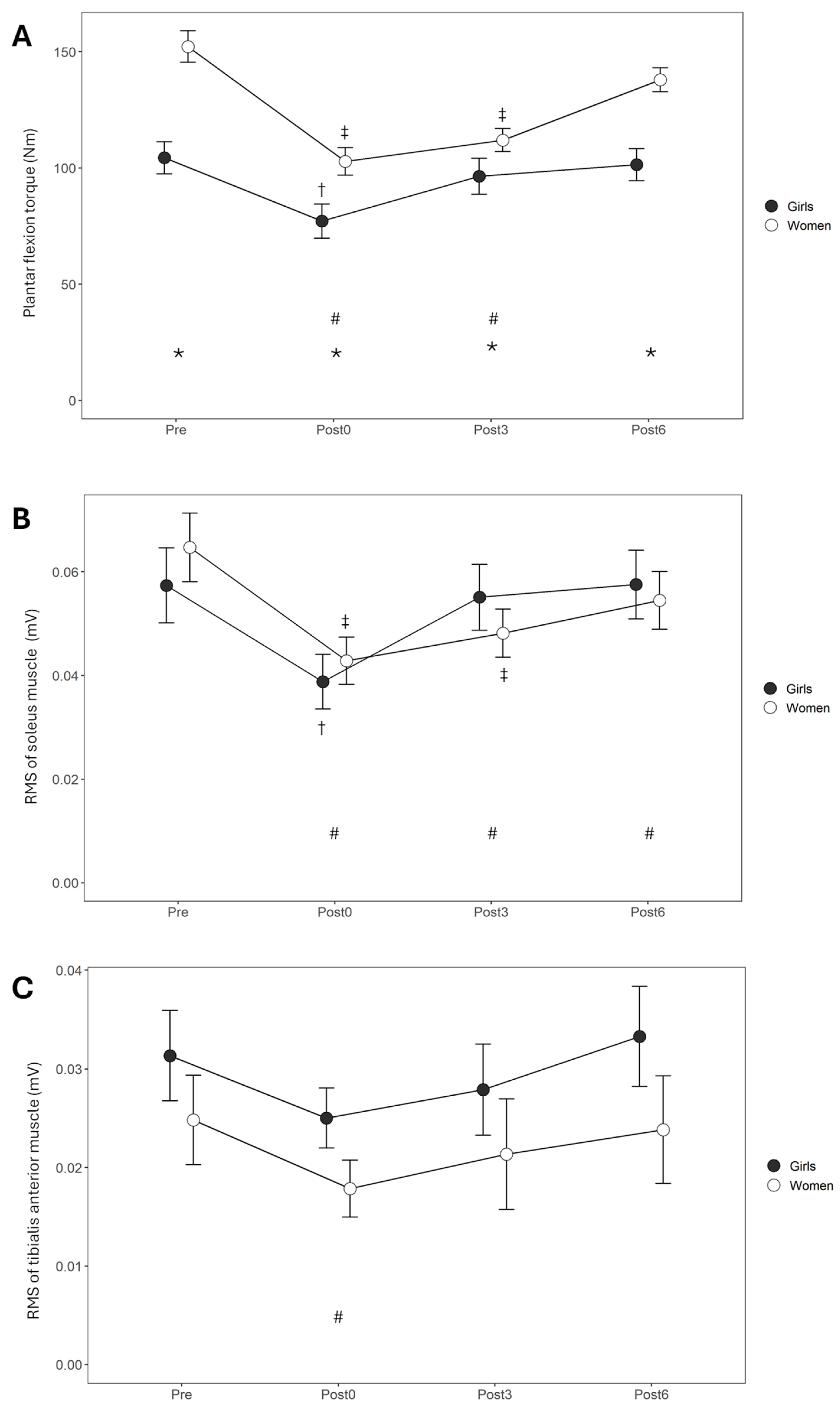
3. Results
3.1. During Sustained Isometric Contraction
3.2. Recovery
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karcher, C.; Buchheit, M. On-court demands of elite handball, with special reference to playing positions. Sports Med. 2014, 44, 797–814. [Google Scholar] [CrossRef] [PubMed]
- Laver, L.; Landreau, P.; Seil, R.; Popovic, N. (Eds.) Handball Sports Medicine; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Michalsik, L.B.; Madsen, K.; Aagaard, P. Match Performance and Physiological Capacity of Female Elite Team Handball Players. Int. J. Sports Med. 2013, 35, 595–607. [Google Scholar] [CrossRef]
- Póvoas, S.C.A.; Seabra, A.F.T.; Ascensão, A.A.M.R.; Magalhães, J.; Soares, J.M.C.; Rebelo, A.N.C. Physical and Physiological Demands of Elite Team Handball. J. Strength Cond. Res. 2012, 26, 3365–3375. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, C.; Navarro, R.M.; Karcher, C.; De La Rubia, A. Physical Demands during Official Competitions in Elite Handball: A Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 3353. [Google Scholar] [CrossRef] [PubMed]
- Michalsik, L.B.; Aagaard, P. Physical demands in elite team handball: Comparisons between male and female players. J. Sports Med. Phys. Fit. 2015, 55, 878–891. [Google Scholar]
- Edwards, R.H.T. Human Muscle Function and Fatigue; Ciba Foundation Symposium: Chichester, UK, 1981; Volume 82, pp. 1–18. [Google Scholar]
- Laurent, C.M.; Vervaecke, L.S.; Kutz, M.R.; Green, J.M. Sex-specific responses to self-paced, high-intensity interval training with variable recovery periods. J. Strength Cond. Res. 2014, 28, 920–927. [Google Scholar] [CrossRef]
- Laurent, C.M.; Green, J.M.; Bishop, P.A.; Sjökvist, J.; Schumacker, R.E.; Richardson, M.T.; Curtner-Smith, M. Effect of gender on fatigue and recovery following maximal intensity repeated sprint performance. J. Sports Med. Phys. Fit. 2010, 50, 243–253. [Google Scholar]
- Schmitz, B.; Niehues, H.; Thorwesten, L.; Klose, A.; Krüger, M.; Brand, S.-M. Sex Differences in High-Intensity Interval Training–Are HIIT Protocols Interchangeable Between Females and Males? Front. Physiol 2020, 11, 38. [Google Scholar] [CrossRef]
- Setuain, I.; Bikandi, E.; Amú Ruiz, F.A.; Urtasun, F.; Izquierdo, M. Horizontal jumping biomechanics among elite female handball players with and without anterior cruciate ligament reconstruction: An ISU based study. BMC Sports Sci. Med. Rehabil. 2019, 11, 30. [Google Scholar] [CrossRef]
- Albert, W.; Wrigley, A.; McLean, R.; Sleivert, G. Sex differences in the rate of fatigue development and recovery. Dyn. Med. 2006, 5, 2. [Google Scholar] [CrossRef]
- Besson, T.; Macchi, R.; Rossi, J.; Morio, C.Y.M.; Kunimasa, Y.; Nicol, C.; Vercruyssen, F.; Millet, G.Y. Sex Differences in Endurance Running. Sports Med. 2022, 52, 1235–1257. [Google Scholar] [CrossRef] [PubMed]
- Hopwood, H.J.; Bellinger, P.M.; Compton, H.R.; Bourne, M.N.; Minahan, C. The Relevance of Muscle Fiber Type to Physical Characteristics and Performance in Team-Sport Athletes. Int. J. Sports Physiol. Perform. 2023, 18, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Lamboley, C.; Rouffet, D.M.; Dutka, T.L.; McKenna, M.J.; Lamb, G.D. Effects of high-intensity intermittent exercise on the contractile properties of human type I and type II skeletal muscle fibers. J. Appl. Physiol. 2020, 128, 1207–1216. [Google Scholar] [CrossRef]
- Barič, A.; Hlebš, S.; Novak, S.; Brumat, P. Epidemiology of injuries in female and male senior Slovenian handball leagues. J. Sports Med. Phys. Fit. 2021, 61, 1644–1652. [Google Scholar] [CrossRef]
- Raya-González, J.; Clemente, F.M.; Beato, M.; Castillo, D. Injury Profile of Male and Female Senior and Youth Handball Players: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 3925. [Google Scholar] [CrossRef]
- Renstrom, P.; Ljungqvist, A.; A Arendt, E.; Beynnon, B.D.; Fukubayashi, T.; E Garrett, W.; Georgoulis, T.; E Hewett, T.; Johnson, R.P.; Krosshaug, T.; et al. Non-contact ACL injuries in female athletes: An International Olympic Committee current concepts statement. Br. J. Sports Med. 2008, 42, 394–412. [Google Scholar] [CrossRef]
- Langevoort, G.; Myklebust, G.; Dvorak, J.; Junge, A. Handball injuries during major international tournaments. Scand. J. Med. Sci. Sports 2007, 17, 400–407. [Google Scholar] [CrossRef]
- Kent-Braun, J.A.; Ng, A.V.; Doyle, J.W.; Towse, T.F. Human skeletal muscle responses vary with age and gender during fatigue due to incremental isometric exercise. J. Appl. Physiol. 2002, 93, 1813–1823. [Google Scholar] [CrossRef]
- Ratel, S.; Lazaar, N.; Williams, C.A.; Bedu, M.; Duché, P. Age differences in human skeletal muscle fatigue during high-intensity intermittent exercise. Acta Paediatr. 2003, 92, 1248–1254. [Google Scholar] [CrossRef]
- Patikas, D.; Kansizoglou, A.; Koutlianos, N.; Williams, C.A.; Hatzikotoulas, K.; Bassa, E.; Kotzamanidis, C. Fatigue and recovery in children and adults during sustained contractions at two different submaximal intensities. Appl. Physiol. Nutr. Metab. 2013, 38, 953–959. [Google Scholar] [CrossRef]
- Hatzikotoulas, K.; Patikas, D.A.; Ratel, S.; Bassa, E.I.; Kotzamanidis, C.M. Central and peripheral fatigability in boys and men during maximal contraction. Med. Sci. Sports Exerc. 2014, 46, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Halin, R.; Germain, P.; Bercier, S.; Kapitaniak, B.; Buttelli, O. Neuromuscular response of young boys versus men during sustained maximal contraction. Med. Sci. Sports Exerc. 2003, 35, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Falk, B.; Dotan, R. Child-adult differences in the recovery from high-intensity exercise. Exerc. Sport Sci. Rev. 2006, 34, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Grosset, J.-F.; Mora, I.; Lambertz, D.; Pérot, C. Voluntary activation of the triceps surae in prepubertal children. J. Electromyogr. Kinesiol. 2008, 18, 455–465. [Google Scholar] [CrossRef]
- De Ste Croix, M.B.A.; Priestley, A.M.; Lloyd, R.S.; Oliver, J.L. ACL injury risk in elite female youth soccer: Changes in neuromuscular control of the knee following soccer-specific fatigue. Scand. J. Med. Sci. Sports 2015, 25, e531–e538. [Google Scholar] [CrossRef]
- Altai, Z.; Hayford, C.F.; Phillips, A.T.M.; Moran, J.; Zhai, X.; Liew, B.X.W. Lower limb joint loading during high-impact activities: Implication for bone health. JBMR Plus 2024, 8, ziae119. [Google Scholar] [CrossRef]
- Bohm, S.; Mersmann, F.; Santuz, A.; Schroll, A.; Arampatzis, A. Muscle-specific economy of force generation and efficiency of work production during human running. eLife 2021, 10, e67182. [Google Scholar] [CrossRef]
- Stefanyshyn, D.J.; Nigg, B.M. Contribution of the lower extremity joints to mechanical energy in running vertical jumps and running long jumps. J. Sports Sci. 1998, 16, 177–186. [Google Scholar] [CrossRef]
- Steiner, M.; Baur, H.; Blasimann, A. Sex-specific differences in neuromuscular activation of the knee stabilizing muscles in adults —A systematic review. Arch. Physiother. 2023, 13, 4. [Google Scholar] [CrossRef]
- Souron, R.; Carayol, M.; Martin, V.; Piponnier, E.; Duché, P.; Gruet, M. Differences in time to task failure and fatigability between children and young adults: A systematic review and meta-analysis. Front. Physiol. 2022, 13, 1026012. [Google Scholar] [CrossRef]
- Bigland-Ritchie, B.; Woods, J.J. Changes in muscle contractile properties and neural control during human muscular fatigue. Muscle Nerve 1984, 7, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Place, N.; Bruton, J.D.; Westerblad, H. Mechanisms of fatigue induced by isometric contractions in exercising humans and in mouse isolated single muscle fibres. Clin. Exp. Pharmacol. Physiol. 2009, 36, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Bogdanis, G.C. Effects of Physical Activity and Inactivity on Muscle Fatigue. Front. Physiol. 2012, 3, 142. [Google Scholar] [CrossRef]
- Buchanan, P.A.; Vardaxis, V.G. Sex-Related and Age-Related Differences in Knee Strength of Basketball Players Ages 11–17 Years. J. Athl. Train. 2003, 38, 231–237. [Google Scholar]
- Dotan, R.; Falk, B. Task-Specific Sex Differences in Muscle Fatigue: Is There a Common Underlying Cause? Exerc. Sport Sci. Rev. 2010, 38, 36. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Tanner, J.M. Growth at Adolescence: With a General Consideration of the Effects of Hereditary and Environmental Factors Upon Growth and Maturation from Birth to Maturity, 2nd ed.; Blackwell Scientific Publications Ltd.: Oxford, UK, 1962. [Google Scholar]
- Hermens, H.J.; Freriks, B.; Merletti, R.; Stegeman, D.F.; Blok, J.H.; Rau, G.; Disselhorst-Klug, C.; Hägg, G. European Recommendations for Surface Electromyography; Roessingh Research and Development: Enshede, The Netherlands, 1999. [Google Scholar]
- Burnley, M.; Jones, A.M. Power–duration relationship: Physiology, fatigue, and the limits of human performance. Eur. J. Sport Sci. 2018, 18, 1–12. [Google Scholar] [CrossRef]
- Fuglevand, A.J.; Zackowski, K.M.; A Huey, K.; Enoka, R.M. Impairment of neuromuscular propagation during human fatiguing contractions at submaximal forces. J. Physiol. 1993, 460, 549–572. [Google Scholar] [CrossRef]
- Kent-Braun, J.A. Central and peripheral contributions to muscle fatigue in humans during sustained maximal effort. Eur. J. Appl. Physiol. 1999, 80, 57–63. [Google Scholar] [CrossRef]
- De Luca, C.J.; Erim, Z. Common Drive in Motor Units of a Synergistic Muscle Pair. J. Neurophysiol. 2002, 87, 2200–2204. [Google Scholar] [CrossRef]
- McNeil, C.J.; Giesebrecht, S.; Gandevia, S.C.; Taylor, J.L. Behaviour of the motoneurone pool in a fatiguing submaximal contraction. J. Physiol. 2011, 589, 3533–3544. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.V.B.; Edwards, S.C.; Van Tongeren, C.; Bawa, P. Properties of human motor units after prolonged activity at a constant firing rate. Exp. Brain Res. 2004, 154, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Adreani, C.M.; Hill, J.M.; Kaufman, M.P.; Christine, M.; Hill, J.M.; Marc, P. Responses of group III and IV muscle afferents to dynamic exercise. J. Appl. Physiol. 1997, 82, 1811–1817. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, S.K.; Weavil, J.C.; Mangum, T.S.; Jessop, J.E.; Richardson, R.S.; Morgan, D.E.; Amann, M. Group III/IV locomotor muscle afferents alter motor cortical and corticospinal excitability and promote central fatigue during cycling exercise. Clin. Neurophysiol. 2017, 128, 44–55. [Google Scholar] [CrossRef]
- Ratel, S.; Bedu, M.; Hennegrave, A.; Dore, E.; Duché, P. Effects of age and recovery duration on peak power output during repeated cycling sprints. Int. J. Sports Med. 2002, 23, 397–402. [Google Scholar] [CrossRef]
- Kanehisa, H.; Okuyama, H.; Ikegawa, S.; Fukunaga, T. Fatigability during repetitive maximal knee extensions in 14-year-old boys. Eur. J. Appl. Physiol. 1995, 72, 170–174. [Google Scholar] [CrossRef]
- Murphy, J.R.; Button, D.C.; Chaouachi, A.; Behm, D.G. Prepubescent males are less susceptible to neuromuscular fatigue following resistance exercise. Eur. J. Appl. Physiol. 2014, 114, 825–835. [Google Scholar] [CrossRef]
- Armatas, V.; Bassa, E.I.; Patikas, D.A.; Kitsas, I.; Zangelidis, G.; Kotzamanidis, C.M. Neuromuscular differences between men and prepubescent boys during a peak isometric knee extension intermittent fatigue test. Pediatr. Exerc. Sci. 2010, 22, 205–217. [Google Scholar] [CrossRef]
- Bontemps, B.; Piponnier, E.; Chalchat, E.; Blazevich, A.J.; Julian, V.; Bocock, O.; Duclos, M.; Martin, V.; Ratel, S. Children Exhibit a More Comparable Neuromuscular Fatigue Profile to Endurance Athletes Than Untrained Adults. Front. Physiol. 2019, 10, 119. [Google Scholar] [CrossRef]
- Inbar, O.; Bar-Or, O. Anaerobic characteristics in male children and adolescents. Med. Sci. Sports Exerc. 1986, 18, 264–269. [Google Scholar] [CrossRef]
- Kappenstein, J.; Ferrauti, A.; Runkel, B.; Fernandez-Fernandez, J.; Müller, K.; Zange, J. Changes in phosphocreatine concentration of skeletal muscle during high-intensity intermittent exercise in children and adults. Eur. J. Appl. Physiol. 2013, 113, 2769–2779. [Google Scholar] [CrossRef] [PubMed]
- Birat, A.; Bourdier, P.; Piponnier, E.; Blazevich, A.J.; Maciejewski, H.; Duché, P.; Ratel, S. Metabolic and fatigue profiles are comparable between prepubertal children and well-trained adult endurance athletes. Front. Physiol. 2018, 9, 387. [Google Scholar] [CrossRef] [PubMed]
- Henneman, E.; Somjen, G.; Carpenter, D.O. Functional significance of cell size in spinal motoneurones. J. Neurophysiol. 1965, 28, 560–580. [Google Scholar] [CrossRef]
- Lexell, J.; Sjöström, M.; Nordlund, A.S.; Taylor, C.C. Growth and development of human muscle: A quantitative morphological study of whole vastus lateralis from childhood to adult age. Muscle Nerve 1992, 15, 404–409. [Google Scholar] [CrossRef]
- Sjöström, M.; Lexell, J.; Downham, D.Y. Differences in fiber number and fiber type proportion within fascicles. A quantitative morphological study of whole vastus lateralis muscle from childhood to old age. Anat. Rec. 1992, 234, 183–189. [Google Scholar] [CrossRef]
- Hamada, T.; Sale, D.G.; MacDougall, J.D.; Tarnopolsky, M.A. Interaction of fibre type, potentiation and fatigue in human knee extensor muscles. Acta Physiol. Scand. 2003, 178, 165–173. [Google Scholar] [CrossRef]
- Behm, D.G.; Power, K. Comparison of interpolation and central activation ratios as measures of muscle inactivation. Muscle Nerve 2001, 24, 925–934. [Google Scholar] [CrossRef]
- Hatzikotoulas, K.; Patikas, D.A.; Bassa, E.I.; Hadjileontiadis, L.J.; Koutedakis, Y.; Kotzamanidis, C.M. Submaximal fatigue and recovery in boys and men. Int. J. Sports Med. 2009, 30, 741–746. [Google Scholar] [CrossRef]
- Hunter, S.K.; Enoka, R.M. Sex differences in the fatigability of arm muscles depends on absolute force during isometric contractions. J. Appl. Physiol. 2001, 91, 2686–2694. [Google Scholar] [CrossRef]
- McNulty, K.L.; Elliott-Sale, K.J.; Dolan, E.; Swinton, P.A.; Ansdell, P.; Goodall, S.; Thomas, K.; Hicks, K.M. The Effects of Menstrual Cycle Phase on Exercise Performance in Eumenorrheic Women: A Systematic Review and Meta-Analysis. Sports Med. 2020, 50, 1813–1827. [Google Scholar] [CrossRef]
- Cheng, G.; Zhang, Z.; Shi, Z.; Qiu, Y. An investigation into how the timing of nutritional supplements affects the recovery from post-exercise fatigue: A systematic review and meta-analysis. Front. Nutr. 2025, 12, 1567438. [Google Scholar] [CrossRef]
- Ruscello, B.; Esposito, M.; Partipilo, F.; Di Cicco, D.; Filetti, C.; Pantanella, L.; D’OTtavio, S. Exercise-to-rest ratios in repeated sprint ability training in women’s soccer. J. Sports Med. Phys. Fit. 2018, 58, 1790–1799. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papavasileiou, A.; Bassa, E.; Xenofondos, A.; Meletakos, P.; Noutsos, K.; Patikas, D.A. Neuromuscular Fatigue Profile of Prepubertal and Adult Female Handball Players. Sports 2025, 13, 230. https://doi.org/10.3390/sports13070230
Papavasileiou A, Bassa E, Xenofondos A, Meletakos P, Noutsos K, Patikas DA. Neuromuscular Fatigue Profile of Prepubertal and Adult Female Handball Players. Sports. 2025; 13(7):230. https://doi.org/10.3390/sports13070230
Chicago/Turabian StylePapavasileiou, Anastasia, Eleni Bassa, Anthi Xenofondos, Panagiotis Meletakos, Konstantinos Noutsos, and Dimitrios A. Patikas. 2025. "Neuromuscular Fatigue Profile of Prepubertal and Adult Female Handball Players" Sports 13, no. 7: 230. https://doi.org/10.3390/sports13070230
APA StylePapavasileiou, A., Bassa, E., Xenofondos, A., Meletakos, P., Noutsos, K., & Patikas, D. A. (2025). Neuromuscular Fatigue Profile of Prepubertal and Adult Female Handball Players. Sports, 13(7), 230. https://doi.org/10.3390/sports13070230







