Inheritance of Stress Responses via Small Non-Coding RNAs in Invertebrates and Mammals
Abstract
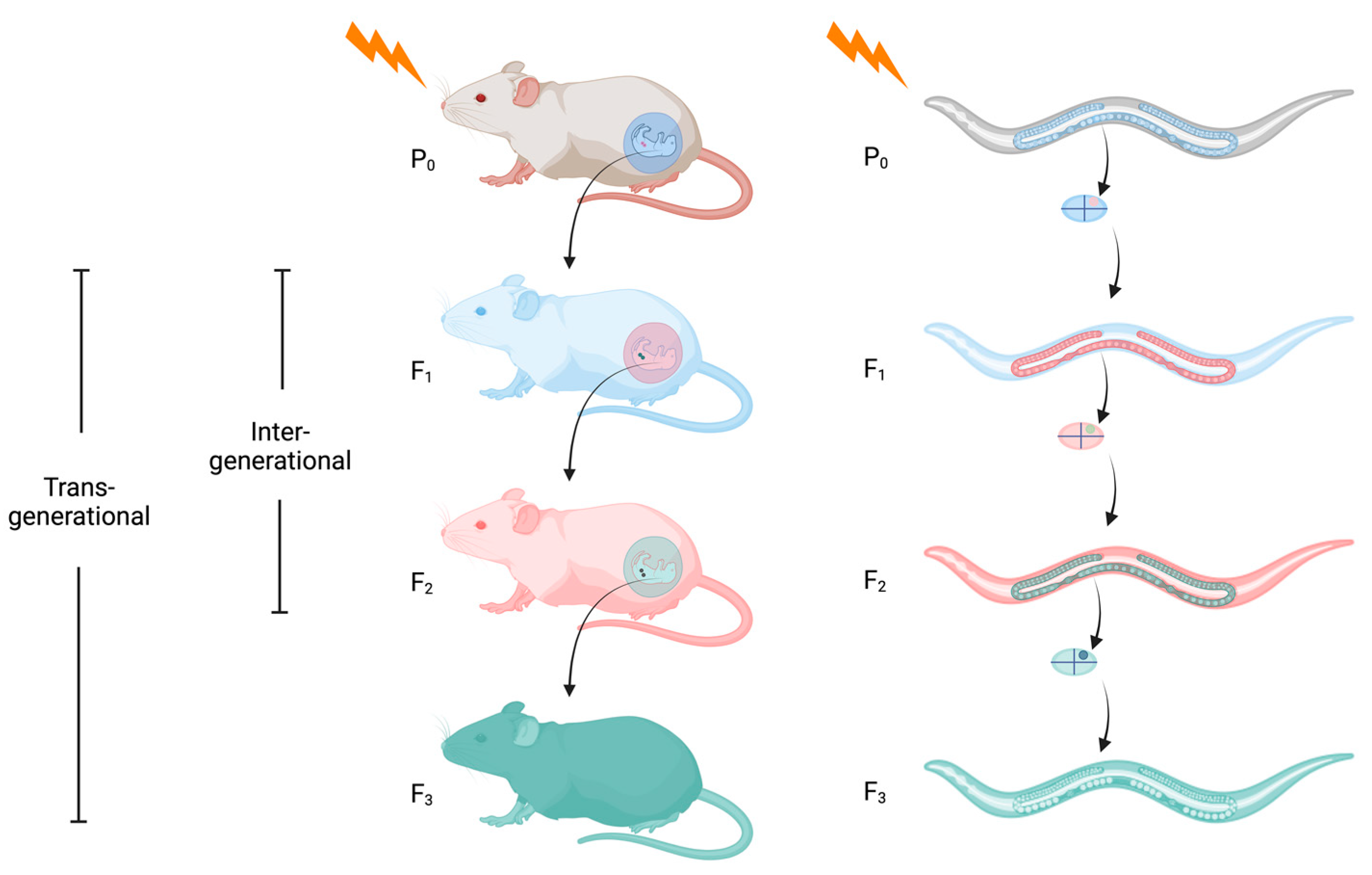
1. What Is Epigenetic Inheritance?
2. Biogenesis and Functions of sncRNAs
2.1. Small Interfering RNAs
2.2. Piwi-Interacting RNAs
2.3. MicroRNAs
2.4. Transfer RNA-Derived Fragments
2.5. Small Nucleolar RNAs
3. Germ Granules and Epigenetic Inheritance
4. Examples of Epigenetic Inheritance Induced by Biotic and Abiotic Stress
4.1. Heat Stress
4.2. Nutritional Stress
4.3. Pathogens
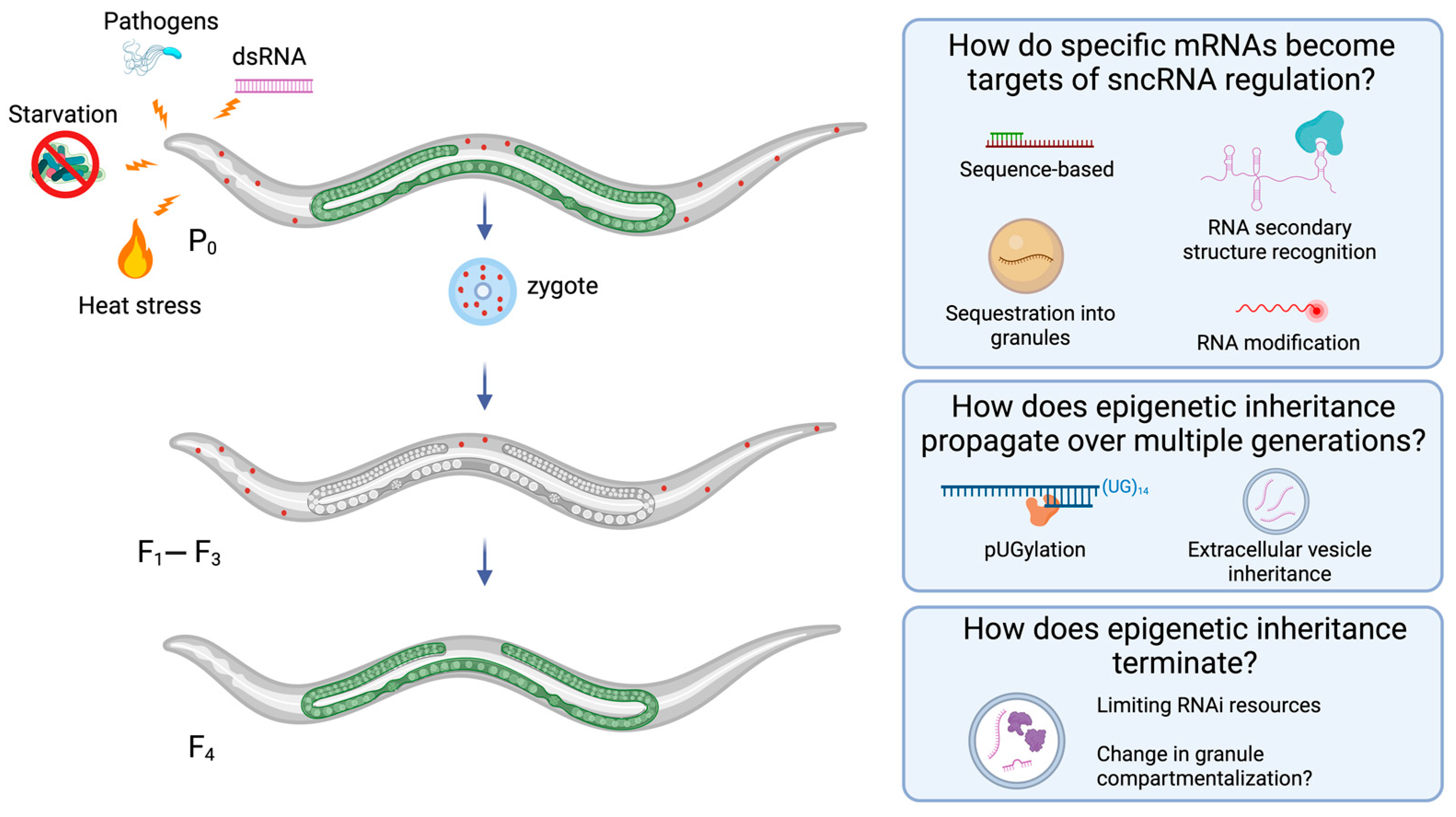
5. Progress on Open Questions in Epigenetic Inheritance Biology
5.1. How Do Specific mRNAs Become Targets of sncRNA Regulation?
5.2. How Does Epigenetic Inheritance Propagate over Generations?
5.3. How Does Epigenetic Inheritance Terminate?
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3′UTR | 3′ untranslated region |
| AGO | Argonaute |
| CM | conditioned media |
| dsRNA | double stranded RNA |
| endo-siRNA | endogenous siRNA |
| EV | extracellular vesicle |
| exo-siRNA | exogenous siRNA |
| FISH | fluorescent in situ hybridization |
| HFD | high fat diet |
| HMT | H3K9 methyltransferase |
| HPA | hypothalamic–pituitary–adrenal |
| LTR | long terminal repeat |
| LPD | low protein diet |
| MAPK | mitogen-activated protein kinase |
| miRNA | microRNA |
| Mrt | mortal germline phenotype |
| MSUS | maternal separation combined with unpredictable maternal stress |
| MZT | maternal-to-zygotic transition |
| NHG | nuclear RNAi-repressed heat-inducible gene |
| piRNA | Piwi interacting RNA |
| Piwi | p-element induced wimpy testis |
| RdRP | RNA dependent RNA polymerase |
| RNAe | RNA-induced epigenetic silencing |
| RNAi | RNA interference |
| SF1 | superfamily one |
| siRNA | small interfering RNA |
| sncRNA | small non-coding RNA |
| snoRNA | small nucleolar RNA |
| TE | transposable element |
| TEI | transgenerational epigenetic inheritance |
| TGF-β | transforming growth factor beta |
| tiRNA | stress-induced transfer RNA-derived small RNA |
| tRF | transfer RNA-derived fragment |
| tRNA | transfer RNA |
| tsRNA | tRNA-derived small RNA |
| VLP | viral-like particles |
| WAGO | worm specific Argonaute |
References
- Waddington, C.H. Canalization of Development and the Inheritance of Acquired Characters. Nature 1942, 150, 563–565. [Google Scholar] [CrossRef]
- Waddington, C.H. The Epigenotype. Int. J. Epidemiol. 2012, 41, 10–13. [Google Scholar] [CrossRef]
- MacDonald, J.L.; Tharin, S.; Hall, S.E. Epigenetic Regulation of Nervous System Development and Function. Neurochem. Int. 2022, 152, 105249. [Google Scholar] [CrossRef] [PubMed]
- Bird, A. Perceptions of Epigenetics. Nature 2007, 447, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, G.; Heard, E. Advances in Epigenetics Link Genetics to the Environment and Disease. Nature 2019, 571, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Heard, E.; Martienssen, R.A. Transgenerational Epigenetic Inheritance: Myths and Mechanisms. Cell 2014, 157, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, E.J.A.; Schedl, T. Biology of the Caenorhabditis elegans Germline Stem Cell System. Genetics 2019, 213, 1145–1188. [Google Scholar] [CrossRef]
- Skinner, M.K. What Is an Epigenetic Transgenerational Phenotype?: F3 or F2. Reprod. Toxicol. 2008, 25, 2–6. [Google Scholar] [CrossRef]
- Skinner, M.K.; Manikkam, M.; Guerrero-Bosagna, C. Epigenetic Transgenerational Actions of Environmental Factors in Disease Etiology. Trends Endocrinol. Metab. 2010, 21, 214–222. [Google Scholar] [CrossRef]
- Sun, D.; Maney, D.L.; Layman, T.S.; Chatterjee, P.; Yi, S.V. Regional Epigenetic Differentiation of the Z Chromosome between Sexes in a Female Heterogametic System. Genome Res. 2019, 29, 1673–1684. [Google Scholar] [CrossRef]
- Perez, M.F.; Lehner, B. Vitellogenins—Yolk Gene Function and Regulation in Caenorhabditis elegans. Front. Physiol. 2019, 10, 1067. [Google Scholar] [CrossRef] [PubMed]
- Smil, V. China’s Great Famine: 40 Years Later. BMJ 1999, 319, 1619–1621. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F. Epigenetics: Tales of Adversity. Nature 2010, 468, S20. [Google Scholar] [CrossRef] [PubMed]
- Lumey, L.H.; Stein, A.D.; Susser, E. Prenatal Famine and Adult Health. Annu. Rev. Public. Health 2011, 32, 237–262. [Google Scholar] [CrossRef] [PubMed]
- Tobi, E.W.; Slieker, R.C.; Luijk, R.; Dekkers, K.F.; Stein, A.D.; Xu, K.M.; Biobank-based Integrative Omics Studies Consortium; Slagboom, P.E.; van Zwet, E.W.; Lumey, L.H. DNA Methylation as a Mediator of the Association between Prenatal Adversity and Risk Factors for Metabolic Disease in Adulthood. Sci. Adv. 2018, 4, eaao4364. [Google Scholar] [CrossRef] [PubMed]
- Tobi, E.W.; van den Heuvel, J.; Zwaan, B.J.; Lumey, L.H.; Heijmans, B.T.; Uller, T. Selective Survival of Embryos Can Explain DNA Methylation Signatures of Adverse Prenatal Environments. Cell Rep. 2018, 25, 2660–2667. [Google Scholar] [CrossRef] [PubMed]
- Painter, R.C.; Osmond, C.; Gluckman, P.; Hanson, M.; Phillips, D.I.W.; Roseboom, T.J. Transgenerational Effects of Prenatal Exposure to the Dutch Famine on Neonatal Adiposity and Health in Later Life. BJOG 2008, 115, 1243–1249. [Google Scholar] [CrossRef]
- Cheng, Q.; Trangucci, R.; Nelson, K.N.; Fu, W.; Collender, P.A.; Head, J.R.; Hoover, C.M.; Skaff, N.K.; Li, T.; Li, X.; et al. Prenatal and Early-Life Exposure to the Great Chinese Famine Increased the Risk of Tuberculosis in Adulthood across Two Generations Proc. Natl. Acad. Sci. USA 2020, 117, 27549. [Google Scholar] [CrossRef]
- Bowers, M.E.; Yehuda, R. Intergenerational Transmission of Stress in Humans. Neuropsychopharmacology 2016, 41, 232–244. [Google Scholar] [CrossRef]
- Nomura, Y.; Rompala, G.; Pritchett, L.; Aushev, V.; Chen, J.; Hurd, Y.L. Natural Disaster Stress during Pregnancy Is Linked to Reprogramming of the Placenta Transcriptome in Relation to Anxiety and Stress Hormones in Young Offspring. Mol. Psychiatry 2021, 26, 6520–6530. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Lillycrop, K.A.; Burdge, G.C.; Gluckman, P.D.; Hanson, M.A. Epigenetic Mechanisms and the Mismatch Concept of the Developmental Origins of Health and Disease. Pediatr. Res. 2007, 61, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, B.; Goncalves, I.B.; Spence-Jones, H.C.; Bennett, N.C.; Heistermann, M.; Ganswindt, A.; Dubuc, C.; Gaynor, D.; Manser, M.B.; Clutton-Brock, T.H. The Influence of Stress Hormones and Aggression on Cooperative Behaviour in Subordinate Meerkats. Proc. R. Soc. B Biol. Sci. 2017, 284, 20171248. [Google Scholar] [CrossRef] [PubMed]
- Öst, A.; Lempradl, A.; Casas, E.; Weigert, M.; Tiko, T.; Deniz, M.; Pantano, L.; Boenisch, U.; Itskov, P.M.; Stoeckius, M.; et al. Paternal Diet Defines Offspring Chromatin State and Intergenerational Obesity. Cell 2014, 159, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Garbutt, J.S.; Little, T.J. Maternal Food Quantity Affects Offspring Feeding Rate in Daphnia Magna. Biol. Lett. 2014, 10, 20140356. [Google Scholar] [CrossRef] [PubMed]
- Zipple, M.N.; Archie, E.A.; Tung, J.; Altmann, J.; Alberts, S.C. Intergenerational Effects of Early Adversity on Survival in Wild Baboons. eLife 2019, 8, e47433. [Google Scholar] [CrossRef] [PubMed]
- Fallet, M.; Luquet, E.; David, P.; Cosseau, C. Epigenetic Inheritance and Intergenerational Effects in Mollusks. Gene 2020, 729, 144166. [Google Scholar] [CrossRef] [PubMed]
- Lamb, S.D.; Chia, J.H.Z.; Johnson, S.L. Paternal Exposure to a Common Herbicide Alters the Behavior and Serotonergic System of Zebrafish Offspring. PLoS ONE 2020, 15, e0228357. [Google Scholar] [CrossRef]
- Bringer, A.; Cachot, J.; Dubillot, E.; Prunier, G.; Huet, V.; Clérandeau, C.; Evin, L.; Thomas, H. Intergenerational Effects of Environmentally-Aged Microplastics on the Crassostrea Gigas. Environ. Pollut. 2022, 294, 118600. [Google Scholar] [CrossRef]
- Paul, S.C.; Singh, P.; Dennis, A.B.; Müller, C. Intergenerational Effects of Early-Life Starvation on Life History, Consumption, and Transcriptome of a Holometabolous Insect. Am. Nat. 2022, 199, E229–E243. [Google Scholar] [CrossRef]
- Bline, A.P.; Le Goff, A.; Allard, P. What Is Lost in the Weismann Barrier? J. Dev. Biol. 2020, 8, 35. [Google Scholar] [CrossRef]
- Cantone, I.; Fisher, A.G. Epigenetic Programming and Reprogramming during Development. Nat. Struct. Mol. Biol. 2013, 20, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Atlasi, Y.; Stunnenberg, H.G. The Interplay of Epigenetic Marks during Stem Cell Differentiation and Development. Nat. Rev. Genet. 2017, 18, 643–658. [Google Scholar] [CrossRef] [PubMed]
- Eckersley-Maslin, M.A.; Alda-Catalinas, C.; Reik, W. Dynamics of the Epigenetic Landscape during the Maternal-to-Zygotic Transition. Nat. Rev. Mol. Cell Biol. 2018, 19, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Greer, E.L.; Shi, Y. Histone Methylation: A Dynamic Mark in Health, Disease and Inheritance. Nat. Rev. Genet. 2012, 13, 343–357. [Google Scholar] [CrossRef] [PubMed]
- O’Kane, C.J.; Hyland, E.M. Yeast Epigenetics: The Inheritance of Histone Modification States. Biosci. Rep. 2019, 39, BSR20182006. [Google Scholar] [CrossRef] [PubMed]
- Saxton, D.S.; Rine, J. Epigenetic Memory Independent of Symmetric Histone Inheritance. eLife 2019, 8, e51421. [Google Scholar] [CrossRef] [PubMed]
- Tuscher, J.J.; Day, J.J. Multigenerational Epigenetic Inheritance: One Step Forward, Two Generations Back. Neurobiol. Dis. 2019, 132, 104591. [Google Scholar] [CrossRef] [PubMed]
- Fitz-James, M.H.; Cavalli, G. Molecular Mechanisms of Transgenerational Epigenetic Inheritance. Nat. Rev. Genet. 2022, 23, 325–341. [Google Scholar] [CrossRef]
- Shan, C.-M.; Fang, Y.; Jia, S. Leaving Histone Unturned for Epigenetic Inheritance. FEBS J. 2023, 290, 310–320. [Google Scholar] [CrossRef]
- Zion, E.; Chen, X. Studying Histone Inheritance in Different Systems Using Imaging-Based Methods and Perspectives. Biochem. Soc. Trans. 2023, 51, 1035–1046. [Google Scholar] [CrossRef]
- Kim, M.; Costello, J. DNA Methylation: An Epigenetic Mark of Cellular Memory. Exp. Mol. Med. 2017, 49, e322. [Google Scholar] [CrossRef] [PubMed]
- Illum, L.R.H.; Bak, S.T.; Lund, S.; Nielsen, A.L. DNA Methylation in Epigenetic Inheritance of Metabolic Diseases through the Male Germ Line. J. Mol. Endocrinol. 2018, 60, R39–R56. [Google Scholar] [CrossRef] [PubMed]
- Greeson, K.W.; Crow, K.M.S.; Edenfield, R.C.; Easley, C.A. Inheritance of Paternal Lifestyles and Exposures through Sperm DNA Methylation. Nat. Rev. Urol. 2023, 20, 356–370. [Google Scholar] [CrossRef] [PubMed]
- Kim, V.N.; Han, J.; Siomi, M.C. Biogenesis of Small RNAs in Animals. Nat. Rev. Mol. Cell Biol. 2009, 10, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Ahringer, J. (Ed.) Reverse genetics. In The C. elegans Research Community; WormBook: New York, NY, USA, 2006. [Google Scholar] [CrossRef]
- Billi, A.C. Endogenous RNAi pathways in C. elegans. In The C. elegans Research Community; WormBook: New York, NY, USA, 2014; pp. 1–49. [Google Scholar] [CrossRef]
- Napoli, C.; Lemieux, C.; Jorgensen, R. Introduction of a Chimeric Chalcone Synthase Gene into Petunia Results in Reversible Co-Suppression of Homologous Genes in Trans. Plant Cell 1990, 2, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Fire, A.; Albertson, D.; Harrison, S.W.; Moerman, D.G. Production of Antisense RNA Leads to Effective and Specific Inhibition of Gene Expression in C. elegans Muscle. Development 1991, 113, 503–514. [Google Scholar] [CrossRef]
- Izant, J.G.; Weintraub, H. Inhibition of Thymidine Kinase Gene Expression by Anti-Sense RNA: A Molecular Approach to Genetic Analysis. Cell 1984, 36, 1007–1015. [Google Scholar] [CrossRef]
- Romano, N.; Macino, G. Quelling: Transient Inactivation of Gene Expression in Neurospora crassa by Transformation with Homologous Sequences. Mol. Microbiol. 1992, 6, 3343–3353. [Google Scholar] [CrossRef]
- Guo, S.; Kemphues, K.J. Par-1, a Gene Required for Establishing Polarity in C. elegans Embryos, Encodes a Putative Ser/Thr Kinase That Is Asymmetrically Distributed. Cell 1995, 81, 611–620. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and Specific Genetic Interference by Double-Stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Grishok, A.; Tabara, H.; Mello, C.C. Genetic Requirements for Inheritance of RNAi in C. elegans. Science 2000, 287, 2494–2497. [Google Scholar] [CrossRef] [PubMed]
- Winston, W.M.; Molodowitch, C.; Hunter, C.P. Hunter Systemic RNAi in C. elegans Requires the Putative Transmembrane Protein SID-1. Science 2002, 295, 2456–2459. [Google Scholar] [CrossRef] [PubMed]
- Duxbury, M.S.; Ashley, S.W.; Whang, E.E. RNA Interference: A Mammalian SID-1 Homologue Enhances SiRNA Uptake and Gene Silencing Efficacy in Human Cells. Biochem. Biophys. Res. Commun. 2005, 331, 459–463. [Google Scholar] [CrossRef]
- Miska, E.A.; Ferguson-Smith, A.C. Transgenerational Inheritance: Models and Mechanisms of Non-DNA Sequence-Based Inheritance. Science 2016, 354, 59–63. [Google Scholar] [CrossRef]
- Rechavi, O.; Lev, I. Principles of Transgenerational Small RNA Inheritance in Caenorhabditis elegans. Curr. Biol. 2017, 27, R720–R730. [Google Scholar] [CrossRef] [PubMed]
- Seroussi, U.; Li, C.; Sundby, A.E.; Lee, T.L.; Claycomb, J.M.; Saltzman, A.L. Mechanisms of Epigenetic Regulation by C. elegans Nuclear RNA Interference Pathways. Semin. Cell Dev. Biol. 2022, 127, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Hutvágner, G.; McLachlan, J.; Pasquinelli, A.E.; Bálint, É.; Tuschl, T.; Zamore, P.D. A Cellular Function for the RNA-Interference Enzyme Dicer in the Maturation of the Let-7 Small Temporal RNA. Science 2001, 293, 834–838. [Google Scholar] [CrossRef]
- Ketting, R.F.; Fischer, S.E.J.; Bernstein, E.; Sijen, T.; Hannon, G.J.; Plasterk, R.H.A. Dicer Functions in RNA Interference and in Synthesis of Small RNA Involved in Developmental Timing in C. elegans. Genes. Dev. 2001, 15, 2654–2659. [Google Scholar] [CrossRef]
- Hutvagner, G.; Simard, M.J. Argonaute Proteins: Key Players in RNA Silencing. Nat. Rev. Mol. Cell Biol. 2008, 9, 22–32. [Google Scholar] [CrossRef]
- Castel, S.E.; Martienssen, R.A. RNA Interference in the Nucleus: Roles for Small RNAs in Transcription, Epigenetics and Beyond. Nat. Rev. Genet. 2013, 14, 100–112. [Google Scholar] [CrossRef]
- Seroussi, U.; Lugowski, A.; Wadi, L.; Lao, R.X.; Willis, A.R.; Zhao, W.; Sundby, A.E.; Charlesworth, A.G.; Reinke, A.W.; Claycomb, J.M. A Comprehensive Survey of C. elegans Argonaute Proteins Reveals Organism-Wide Gene Regulatory Networks and Functions. eLife 2023, 12, e83853. [Google Scholar] [CrossRef] [PubMed]
- Youngman, E.M.; Claycomb, J.M. From Early Lessons to New Frontiers: The Worm as a Treasure Trove of Small RNA Biology. Front. Genet. 2014, 5, 416. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.V.; de Jesus Domingues, A.M.; Ketting, R.F. Maternal and Zygotic Gene Regulatory Effects of Endogenous RNAi Pathways. PLoS Genet. 2019, 15, e1007784. [Google Scholar] [CrossRef] [PubMed]
- Ghildiyal, M.; Zamore, P.D. Small Silencing RNAs: An Expanding Universe. Nat. Rev. Genet. 2009, 10, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Claycomb, J.M.; Batista, P.J.; Pang, K.M.; Gu, W.; Vasale, J.J.; van Wolfswinkel, J.C.; Chaves, D.A.; Shirayama, M.; Mitani, S.; Ketting, R.F.; et al. The Argonaute CSR-1 and Its 22G-RNA Cofactors Are Required for Holocentric Chromosome Segregation. Cell 2009, 139, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Seth, M.; Shirayama, M.; Gu, W.; Ishidate, T.; Conte, D.; Mello, C.C. The C. elegans CSR-1 Argonaute Pathway Counteracts Epigenetic Silencing to Promote Germline Gene Expression. Dev. Cell 2013, 27, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Wedeles, C.J.; Wu, M.Z.; Claycomb, J.M. Protection of Germline Gene Expression by the C. elegans Argonaute CSR-1. Dev. Cell 2013, 27, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Cecere, G.; Hoersch, S.; O’Keeffe, S.; Sachidanandam, R.; Grishok, A. Global Effects of the CSR-1 RNA Interference Pathway on the Transcriptional Landscape. Nat. Struct. Mol. Biol. 2014, 21, 358–365. [Google Scholar] [CrossRef]
- Buckley, B.A.; Burkhart, K.B.; Gu, S.G.; Spracklin, G.; Kershner, A.; Fritz, H.; Kimble, J.; Fire, A.; Kennedy, S. A Nuclear Argonaute Promotes Multigenerational Epigenetic Inheritance and Germline Immortality. Nature 2012, 489, 447–451. [Google Scholar] [CrossRef]
- Gu, W.; Shirayama, M.; Conte, D.; Vasale, J.; Batista, P.J.; Claycomb, J.M.; Moresco, J.J.; Youngman, E.M.; Keys, J.; Stoltz, M.J.; et al. Distinct Argonaute-Mediated 22G-RNA Pathways Direct Genome Surveillance in the C. elegans Germline. Mol. Cell 2009, 36, 231–244. [Google Scholar] [CrossRef]
- Ashe, A.; Sapetschnig, A.; Weick, E.-M.; Mitchell, J.; Bagijn, M.P.; Cording, A.C.; Doebley, A.-L.; Goldstein, L.D.; Lehrbach, N.J.; Le Pen, J.; et al. PiRNAs Can Trigger a Multigenerational Epigenetic Memory in the Germline of C. elegans. Cell 2012, 150, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Vasale, J.J.; Gu, W.; Thivierge, C.; Batista, P.J.; Claycomb, J.M.; Youngman, E.M.; Duchaine, T.F.; Mello, C.C.; Conte, D. Sequential Rounds of RNA-Dependent RNA Transcription Drive Endogenous Small-RNA Biogenesis in the ERGO-1/Argonaute Pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 3582–3587. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.E.; Bennett, E.A.; Iskow, R.C.; Devine, S.E. Which Transposable Elements Are Active in the Human Genome? Trends Genet. 2007, 23, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, M.; VanKuren, N.W.; Zhao, R.; Zhang, X.; Kalsow, S.; Emerson, J.J. Hidden Genetic Variation Shapes the Structure of Functional Elements in Drosophila. Nat. Genet. 2018, 50, 20–25. [Google Scholar] [CrossRef]
- Laricchia, K.M.; Zdraljevic, S.; Cook, D.E.; Andersen, E.C. Natural Variation in the Distribution and Abundance of Transposable Elements across the Caenorhabditis elegans Species. Mol. Biol. Evol. 2017, 34, 2187–2202. [Google Scholar] [CrossRef] [PubMed]
- Barrón, M.G.; Fiston-Lavier, A.S.; Petrov, D.A.; González, J. Population Genomics of Transposable Elements in Drosophila. Annu. Rev. Genet. 2014, 48, 561–581. [Google Scholar] [CrossRef] [PubMed]
- Jonathan, N. Wells; Cedric Feschotte A Field Guide to Eukaryotic Transposable Elements. Annu. Rev. Genet. 2020, 54, 539–561. [Google Scholar] [CrossRef]
- Feschotte, C. Transposable Elements and the Evolution of Regulatory Networks. Nat. Rev. Genet. 2008, 9, 397–405. [Google Scholar] [CrossRef]
- Senft, A.D.; Macfarlan, T.S. Transposable Elements Shape the Evolution of Mammalian Development. Nat. Rev. Genet. 2021, 22, 691–711. [Google Scholar] [CrossRef]
- Chuong, E.B.; Elde, N.C.; Feschotte, C. Regulatory Activities of Transposable Elements: From Conflicts to Benefits. Nat. Rev. Genet. 2017, 18, 71–86. [Google Scholar] [CrossRef]
- Kathleen, H. Burns Our Conflict with Transposable Elements and Its Implications for Human Disease. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 51–70. [Google Scholar] [CrossRef]
- Wylie, A.; Jones, A.E.; D’Brot, A.; Lu, W.-J.; Kurtz, P.; Moran, J.V.; Rakheja, D.; Chen, K.S.; Hammer, R.E.; Comerford, S.A.; et al. P53 Genes Function to Restrain Mobile Elements. Genes. Dev. 2016, 30, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Wylie, A.; Jones, A.E.; Abrams, J.M. P53 in the Game of Transposons. BioEssays 2016, 38, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.; Jones, A.E.; Caillet, C.J.; Das, S.; Royer, S.K.; Abrams, J.M. P53 Directly Represses Human LINE1 Transposons. Genes. Dev. 2020, 34, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.W.; Siomi, M.C.; Siomi, H. PIWI-Interacting RNA: Its Biogenesis and Functions. Annu. Rev. Biochem. 2015, 84, 405–433. [Google Scholar] [CrossRef] [PubMed]
- Czech, B.; Munafò, M.; Ciabrelli, F.; Eastwood, E.L.; Fabry, M.H.; Kneuss, E.; Hannon, G.J. PiRNA-Guided Genome Defense: From Biogenesis to Silencing. Annu. Rev. Genet. 2018, 52, 131–157. [Google Scholar] [CrossRef] [PubMed]
- Ozata, D.M.; Gainetdinov, I.; Zoch, A.; O’Carroll, D.; Zamore, P.D. PIWI-Interacting RNAs: Small RNAs with Big Functions. Nat. Rev. Genet. 2019, 20, 89–108. [Google Scholar] [CrossRef]
- Picard, G. Non-Mendelian Female Sterility in Drosophila melanogaster: Hereditary Transmission of I Factor. Genetics 1976, 83, 107–123. [Google Scholar] [CrossRef]
- Kidwell, M.G.; Kidwell, J.F.; Sved, J.A. Hybrid Dysgenesis in Drosophila melanogaster: A Syndrome of Aberrant Traits Including Mutation, Sterility and Male Recombination. Genetics 1977, 86, 813–833. [Google Scholar] [CrossRef]
- Mérel, V.; Boulesteix, M.; Fablet, M.; Vieira, C. Transposable Elements in Drosophila. Mob. DNA 2020, 11, 23. [Google Scholar] [CrossRef]
- Luteijn, M.J.; van Bergeijk, P.; Kaaij, L.J.T.; Almeida, M.V.; Roovers, E.F.; Berezikov, E.; Ketting, R.F. Extremely Stable Piwi-Induced Gene Silencing in Caenorhabditis elegans. EMBO J. 2012, 31, 3422–3430. [Google Scholar] [CrossRef] [PubMed]
- Shirayama, M.; Seth, M.; Lee, H.-C.; Gu, W.; Ishidate, T.; Conte, D.; Mello, C.C. PiRNAs Initiate an Epigenetic Memory of Nonself RNA in the C. elegans Germline. Cell 2012, 150, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-C.; Gu, W.; Shirayama, M.; Youngman, E.; Conte, D.; Mello, C.C. C. elegans PiRNAs Mediate the Genome-Wide Surveillance of Germline Transcripts. Cell 2012, 150, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Belicard, T.; Jareosettasin, P.; Sarkies, P. The PiRNA Pathway Responds to Environmental Signals to Establish Intergenerational Adaptation to Stress. BMC Biol. 2018, 16, 103. [Google Scholar] [CrossRef] [PubMed]
- Brennecke, J.; Aravin, A.A.; Stark, A.; Dus, M.; Kellis, M.; Sachidanandam, R.; Hannon, G.J. Discrete Small RNA-Generating Loci as Master Regulators of Transposon Activity in Drosophila. Cell 2007, 128, 1089–1103. [Google Scholar] [CrossRef] [PubMed]
- Aravin, A.A.; Hannon, G.J.; Brennecke, J. The Piwi-PiRNA Pathway Provides an Adaptive Defense in the Transposon Arms Race. Science 2007, 318, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Klattenhoff, C.; Xi, H.; Li, C.; Lee, S.; Xu, J.; Khurana, J.S.; Zhang, F.; Schultz, N.; Koppetsch, B.S.; Nowosielska, A.; et al. The Drosophila HP1 Homolog Rhino Is Required for Transposon Silencing and PiRNA Production by Dual-Strand Clusters. Cell 2009, 138, 1137–1149. [Google Scholar] [CrossRef]
- Mohn, F.; Sienski, G.; Handler, D.; Brennecke, J. The Rhino-Deadlock-Cutoff Complex Licenses Noncanonical Transcription of Dual-Strand PiRNA Clusters in Drosophila. Cell 2014, 157, 1364–1379. [Google Scholar] [CrossRef]
- Andersen, P.R.; Tirian, L.; Vunjak, M.; Brennecke, J. A Heterochromatin-Dependent Transcription Machinery Drives PiRNA Expression. Nature 2017, 549, 54–59. [Google Scholar] [CrossRef]
- Siomi, M.C.; Miyoshi, T.; Siomi, H. PiRNA-Mediated Silencing in Drosophila Germlines. Semin. Cell Dev. Biol. 2010, 21, 754–759. [Google Scholar] [CrossRef]
- Gebert, D.; Neubert, L.K.; Lloyd, C.; Gui, J.; Lehmann, R.; Teixeira, F.K. Large Drosophila Germline PiRNA Clusters Are Evolutionarily Labile and Dispensable for Transposon Regulation. Mol. Cell 2021, 81, 3965–3978.e5. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans Heterochronic Gene Lin-4 Encodes Small RNAs with Antisense Complementarity to Lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Pasquinelli, A.E.; Reinhart, B.J.; Slack, F.; Martindale, M.Q.; Kuroda, M.I.; Maller, B.; Hayward, D.C.; Ball, E.E.; Degnan, B.; Müller, P.; et al. Conservation of the Sequence and Temporal Expression of Let-7 Heterochronic Regulatory RNA. Nature 2000, 408, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Ambros, V.; Ruvkun, G. Recent Molecular Genetic Explorations of Caenorhabditis elegans MicroRNAs. Genetics 2018, 209, 651–673. [Google Scholar] [CrossRef]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef]
- Gebert, L.F.R.; MacRae, I.J. Regulation of MicroRNA Function in Animals. Nat. Rev. Mol. Cell Biol. 2019, 20, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-H.; Chen, J.-A. Multifaceted Roles of MicroRNAs: From Motor Neuron Generation in Embryos to Degeneration in Spinal Muscular Atrophy. eLife 2019, 8, e50848. [Google Scholar] [CrossRef]
- Song, X.; Li, Y.; Cao, X.; Qi, Y. MicroRNAs and Their Regulatory Roles in Plant-Enviornment Interactions. Annu. Rev. Plant Biol. 2019, 70, 489–525. [Google Scholar] [CrossRef]
- Agbu, P.; Carthew, R.W. MicroRNA-Mediated Regulation of Glucose and Lipid Metabolism. Nat. Rev. Mol. Cell Biol. 2021, 22, 425–438. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. MiRBase: From MicroRNA Sequences to Function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V.N. Regulation of MicroRNA Biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Treiber, T.; Treiber, N.; Meister, G. Regulation of MicroRNA Biogenesis and Its Crosstalk with Other Cellular Pathways. Nat. Rev. Mol. Cell Biol. 2019, 20, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Dexheimer, P.J.; Cochella, L. MicroRNAs: From Mechanism to Organism. Front. Cell Dev. Biol. 2020, 8, 409. [Google Scholar] [CrossRef] [PubMed]
- Das, P.P.; Bagijn, M.P.; Goldstein, L.D.; Woolford, J.R.; Lehrbach, N.J.; Sapetschnig, A.; Buhecha, H.R.; Gilchrist, M.J.; Howe, K.L.; Stark, R.; et al. Piwi and PiRNAs Act Upstream of an Endogenous SiRNA Pathway to Suppress Tc3 Transposon Mobility in the Caenorhabditis elegans Germline. Mol. Cell 2008, 31, 79–90. [Google Scholar] [CrossRef]
- Gapp, K.; Jawaid, A.; Sarkies, P.; Bohacek, J.; Pelczar, P.; Prados, J.; Farinelli, L.; Miska, E.; Mansuy, I.M. Implication of Sperm RNAs in Transgenerational Inheritance of the Effects of Early Trauma in Mice. Nat. Neurosci. 2014, 17, 667–669. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, V.; Fourré, S.; De Abreu, D.A.F.; Derieppe, M.-A.; Remy, J.-J.; Rassoulzadegan, M. RNA-Mediated Paternal Heredity of Diet-Induced Obesity and Metabolic Disorders. Sci. Rep. 2015, 5, 18193. [Google Scholar] [CrossRef]
- Rodgers, A.B.; Morgan, C.P.; Leu, N.A.; Bale, T.L. Transgenerational Epigenetic Programming via Sperm MicroRNA Recapitulates Effects of Paternal Stress. Proc. Natl. Acad. Sci. USA 2015, 112, 13699–13704. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, A.B.; Morgan, C.P.; Bronson, S.L.; Revello, S.; Bale, T.L. Paternal Stress Exposure Alters Sperm MicroRNA Content and Reprograms Offspring HPA Stress Axis Regulation. J. Neurosci. 2013, 33, 9003–9012. [Google Scholar] [CrossRef]
- Sharma, U. Paternal Contributions to Offspring Health: Role of Sperm Small RNAs in Intergenerational Transmission of Epigenetic Information. Front. Cell Dev. Biol. 2019, 7, 215. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.-P.; Hu, H.; Lei, J.; Zhou, Z.; Yao, B.; Chen, L.; Liang, G.; Zhan, S.; Zhu, X.; et al. Sperm MicroRNAs Confer Depression Susceptibility to Offspring. Sci. Adv. 2021, 7, eabd7605. [Google Scholar] [CrossRef]
- Corrêa, R.L.; Steiner, F.A.; Berezikov, E.; Ketting, R.F. MicroRNA–Directed SiRNA Biogenesis in Caenorhabditis elegans. PLoS Genet. 2010, 6, e1000903. [Google Scholar] [CrossRef] [PubMed]
- Speer, J.; Gehrke, C.W.; Kuo, K.C.; Waalkes, T.P.; Borek, E. TRNA Breakdown Products as Markers for Cancer. Cancer 1979, 44, 2120–2123. [Google Scholar] [CrossRef] [PubMed]
- Levitz, R.; Chapman, D.; Amitsur, M.; Green, R.; Snyder, L.; Kaufmann, G.J. The Optional E. coli Prr Locus Encodes a Latent Form of Phage T4-induced Anticodon Nuclease. EMBO J. 1990, 9, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Shibata, Y.; Malhotra, A.; Dutta, A. A Novel Class of Small RNAs: TRNA-Derived RNA Fragments (TRFs). Genes. Dev. 2009, 23, 2639–2649. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yan, M.; Cao, Z.; Li, X.; Zhang, Y.; Shi, J.; Feng, G.; Peng, H.; Zhang, X.; Zhang, Y.; et al. Sperm TsRNAs Contribute to Intergenerational Inheritance of an Acquired Metabolic Disorder. Science 2016, 351, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Czech, A.; Wende, S.; Mörl, M.; Pan, T.; Ignatova, Z. Reversible and Rapid Transfer-RNA Deactivation as a Mechanism of Translational Repression in Stress. PLoS Genet. 2013, 9, e1003767. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.F.; Lehner, B. Intergenerational and Transgenerational Epigenetic Inheritance in Animals. Nat. Cell Biol. 2019, 21, 143–151. [Google Scholar] [CrossRef]
- Park, J.; Ahn, S.H.; Shin, M.G.; Kim, H.K.; Chang, S. TRNA-Derived Small RNAs: Novel Epigenetic Regulators. Cancers 2020, 12, 2773. [Google Scholar] [CrossRef]
- Kumar, P.; Anaya, J.; Mudunuri, S.B.; Dutta, A. Meta-Analysis of TRNA Derived RNA Fragments Reveals That They Are Evolutionarily Conserved and Associate with AGO Proteins to Recognize Specific RNA Targets. BMC Biol. 2014, 12, 78. [Google Scholar] [CrossRef]
- Kumar, P.; Mudunuri, S.B.; Anaya, J.; Dutta, A. TRFdb: A Database for Transfer RNA Fragments. Nucleic Acids Res. 2015, 43, D141–D145. [Google Scholar] [CrossRef]
- Weng, Q.; Wang, Y.; Xie, Y.; Yu, X.; Zhang, S.; Ge, J.; Li, Z.; Ye, G.; Guo, J. Extracellular Vesicles-Associated TRNA-Derived Fragments (TRFs): Biogenesis, Biological Functions, and Their Role as Potential Biomarkers in Human Diseases. J. Mol. Med. 2022, 100, 679–695. [Google Scholar] [CrossRef] [PubMed]
- Willis, I.M.; Moir, R.D. Moir Signaling to and from the RNA Polymerase III Transcription and Procesing Machinery. Annu. Rev. Biochem. 2018, 87, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Dubrovsky, E.B.; Dubrovskaya, V.A.; Levinger, L.; Schiffer, S.; Marchfelder, A. Drosophila RNase Z Processes Mitochondrial and Nuclear Pre-tRNA 3′ Ends in Vivo. Nucleic Acids Res. 2004, 32, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Jarrous, N.; Reiner, R. Human RNase P: A TRNA-Processing Enzyme and Transcription Factor. Nucleic Acids Res. 2007, 35, 3519–3524. [Google Scholar] [CrossRef] [PubMed]
- Maraia, R.J.; Lamichhane, T.N. 3′ Processing of Eukaryotic Precursor TRNAs. WIREs RNA 2011, 2, 362–375. [Google Scholar] [CrossRef]
- Hopper, A.K.; Nostramo, R.T. TRNA Processing and Subcellular Trafficking Proteins Multitask in Pathways for Other RNAs. Front. Genet. 2019, 10, 96. [Google Scholar] [CrossRef]
- Su, Z.; Wilson, B.; Kumar, P.; Dutta, A. Noncanonical Roles of TRNAs: TRNA Fragments and Beyond. Annu. Rev. Genet. 2020, 54, 47–69. [Google Scholar] [CrossRef]
- Tuck, A.C.; Tollervey, D. RNA in Pieces. Trends Genet. 2011, 27, 422–432. [Google Scholar] [CrossRef]
- Magee, R.; Rigoutsos, I. On the Expanding Roles of TRNA Fragments in Modulating Cell Behavior. Nucleic Acids Res. 2020, 48, 9433–9448. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, X.; Xie, Y.; Ye, G.; Guo, J. TRNA Derived Fragments:A Novel Player in Gene Regulation and Applications in Cancer. Front. Oncol. 2023, 13, 1063930. [Google Scholar] [CrossRef]
- Anderson, P.; Ivanov, P. TRNA Fragments in Human Health and Disease. FEBS Lett. 2014, 588, 4297–4304. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.P.; Hutvagner, G. TRNA-Derived Fragments (TRFs): Emerging New Roles for an Ancient RNA in the Regulation of Gene Expression. Life 2015, 5, 1638–1651. [Google Scholar] [CrossRef] [PubMed]
- Schorey, J.S.; Cheng, Y.; Singh, P.P.; Smith, V.L. Exosomes and Other Extracellular Vesicles in Host–Pathogen Interactions. EMBO Rep. 2015, 16, 24–43. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Chiou, N.-T.; Kageyama, R.; Ansel, K.M. Selective Export into Extracellular Vesicles and Function of TRNA Fragments during T Cell Activation. Cell Rep. 2018, 25, 3356–3370.e4. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Sun, F.; Conine, C.C.; Reichholf, B.; Kukreja, S.; Herzog, V.A.; Ameres, S.L.; Rando, O.J. Small RNAs Are Trafficked from the Epididymis to Developing Mammalian Sperm. Dev. Cell 2018, 46, 481–494.e6. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Yeom, J.-H.; Kay, M.A. Transfer RNA-Derived Small RNAs: Another Layer of Gene Regulation and Novel Targets for Disease Therapeutics. Mol. Ther. 2020, 28, 2340–2357. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Dutta, A. Function and Therapeutic Implications of TRNA Derived Small RNAs. Front. Mol. Biosci. 2022, 9, 888424. [Google Scholar] [CrossRef]
- Yu, M.; Lu, B.; Zhang, J.; Ding, J.; Liu, P.; Lu, Y. TRNA-Derived RNA Fragments in Cancer: Current Status and Future Perspectives. J. Hematol. Oncol. 2020, 13, 121. [Google Scholar] [CrossRef]
- Garcia-Silva, M.R.; Cabrera-Cabrera, F.; Cura das Neves, R.F.; Souto-Padrón, T.; de Souza, W.; Cayota, A. Gene Expression Changes Induced by Trypanosoma cruzi Shed Microvesicles in Mammalian Host Cells: Relevance of TRNA-Derived Halves. BioMed Res. Int. 2014, 2014, e305239. [Google Scholar] [CrossRef]
- Cooke, W.R.; Cribbs, A.; Zhang, W.; Kandzija, N.; Motta-Mejia, C.; Dombi, E.; Ri, R.; Cerdeira, A.S.; Redman, C.; Vatish, M. Maternal Circulating Syncytiotrophoblast-Derived Extracellular Vesicles Contain Biologically Active 5’-TRNA Halves. Biochem. Biophys. Res. Commun. 2019, 518, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Begley, T.J.; Dedon, P.C. TRNA Modifications Regulate Translation during Cellular Stress. FEBS Lett. 2014, 588, 4287–4296. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kuscu, C.; Dutta, A. Biogenesis and Function of Transfer RNA-Related Fragments (TRFs). Trends Biochem. Sci. 2016, 41, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Guzzi, N.; Bellodi, C. Stressin’ and Slicin’: Stress-Induced TRNA Fragmentation Codon-Adapts Translation to Repress Cell Growth. EMBO J. 2021, 40, e107097. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Conine, C.C.; Shea, J.M.; Boskovic, A.; Derr, A.G.; Bing, X.Y.; Belleannee, C.; Kucukural, A.; Serra, R.W.; Sun, F.; et al. Biogenesis and Function of TRNA Fragments during Sperm Maturation and Fertilization in Mammals. Science 2016, 351, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Gapp, K.; Miska, E.A. TRNA Fragments: Novel Players in Intergenerational Inheritance. Cell Res. 2016, 26, 395–396. [Google Scholar] [CrossRef] [PubMed]
- Ender, C.; Krek, A.; Friedländer, M.R.; Beitzinger, M.; Weinmann, L.; Chen, W.; Pfeffer, S.; Rajewsky, N.; Meister, G. A Human SnoRNA with MicroRNA-Like Functions. Mol. Cell 2008, 32, 519–528. [Google Scholar] [CrossRef]
- Brameier, M.; Herwig, A.; Reinhardt, R.; Walter, L.; Gruber, J. Human Box C/D SnoRNAs with MiRNA like Functions: Expanding the Range of Regulatory RNAs. Nucleic Acids Res. 2011, 39, 675–686. [Google Scholar] [CrossRef]
- He, X.; Chen, X.; Zhang, X.; Duan, X.; Pan, T.; Hu, Q.; Zhang, Y.; Zhong, F.; Liu, J.; Zhang, H.; et al. An Lnc RNA (GAS5)/SnoRNA-Derived PiRNA Induces Activation of TRAIL Gene by Site-Specifically Recruiting MLL/COMPASS-like Complexes. Nucleic Acids Res. 2015, 43, 3712–3725. [Google Scholar] [CrossRef]
- Zhong, F.; Zhou, N.; Wu, K.; Guo, Y.; Tan, W.; Zhang, H.; Zhang, X.; Geng, G.; Pan, T.; Luo, H.; et al. A SnoRNA-Derived PiRNA Interacts with Human Interleukin-4 Pre-MRNA and Induces Its Decay in Nuclear Exosomes. Nucleic Acids Res. 2015, 43, 10474–10491. [Google Scholar] [CrossRef]
- Deogharia, M.; Majumder, M. Guide SnoRNAs: Drivers or Passengers in Human Disease? Biology 2019, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Bratkovič, T.; Božič, J.; Rogelj, B. Functional Diversity of Small Nucleolar RNAs. Nucleic Acids Res. 2020, 48, 1627–1651. [Google Scholar] [CrossRef] [PubMed]
- Wajahat, M.; Bracken, C.P.; Orang, A. Emerging Functions for SnoRNAs and SnoRNA-Derived Fragments. Int. J. Mol. Sci. 2021, 22, 10193. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Du, Y.; Wen, J.; Lu, B.; Zhao, Y. SnoRNAs: Functions and Mechanisms in Biological Processes, and Roles in Tumor Pathophysiology. Cell Death Discov. 2022, 8, 1–10. [Google Scholar] [CrossRef]
- Voronina, E.; Seydoux, G.; Sassone-Corsi, P.; Nagamori, I. RNA Granules in Germ Cells. Cold Spring Harb. Perspect. Biol. 2011, 3, a002774. [Google Scholar] [CrossRef] [PubMed]
- Trcek, T.; Lehmann, R. All about the RNA after All. eLife 2017, 6, e24106. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.P.T.; Seydoux, G. Nuage Condensates: Accelerators or Circuit Breakers for SRNA Silencing Pathways? RNA 2022, 28, 58–66. [Google Scholar] [CrossRef]
- Wan, G.; Fields, B.D.; Spracklin, G.; Shukla, A.; Phillips, C.M.; Kennedy, S. Spatiotemporal Regulation of Liquid-like Condensates in Epigenetic Inheritance. Nature 2018, 557, 679–683. [Google Scholar] [CrossRef]
- Manage, K.I.; Rogers, A.K.; Wallis, D.C.; Uebel, C.J.; Anderson, D.C.; Nguyen, D.A.H.; Arca, K.; Brown, K.C.; Cordeiro Rodrigues, R.J.; de Albuquerque, B.F.; et al. A Tudor Domain Protein, SIMR-1, Promotes SiRNA Production at PiRNA-Targeted MRNAs in C. elegans. eLife 2020, 9, e56731. [Google Scholar] [CrossRef]
- Sundby, A.E.; Molnar, R.I.; Claycomb, J.M. Connecting the Dots: Linking Caenorhabditis elegans Small RNA Pathways and Germ Granules. Trends Cell Biol. 2021, 31, 387–401. [Google Scholar] [CrossRef]
- Batista, P.J.; Ruby, J.G.; Claycomb, J.M.; Chiang, R.; Fahlgren, N.; Kasschau, K.D.; Chaves, D.A.; Gu, W.; Vasale, J.J.; Duan, S.; et al. PRG-1 and 21U-RNAs Interact to Form the PiRNA Complex Required for Fertility in C. elegans. Mol. Cell 2008, 31, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Reinke, V. A C. elegans Piwi, PRG-1, Regulates 21U-RNAs during Spermatogenesis. Curr. Biol. 2008, 18, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Conine, C.C.; Batista, P.J.; Gu, W.; Claycomb, J.M.; Chaves, D.A.; Shirayama, M.; Mello, C.C. Argonautes ALG-3 and ALG-4 Are Required for Spermatogenesis-Specific 26G-RNAs and Thermotolerant Sperm in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2010, 107, 3588–3593. [Google Scholar] [CrossRef] [PubMed]
- Updike, D.; Strome, S. P Granule Assembly and Function in Caenorhabditis elegans Germ Cells. J. Androl. 2010, 31, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Updike, D.L.; Hachey, S.J.; Kreher, J.; Strome, S. P Granules Extend the Nuclear Pore Complex Environment in the C. elegans Germ Line. J. Cell Biol. 2011, 192, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.C.; Svendsen, J.M.; Tucci, R.M.; Montgomery, B.E.; Montgomery, T.A. ALG-5 Is a MiRNA-Associated Argonaute Required for Proper Developmental Timing in the Caenorhabditis elegans Germline. Nucleic Acids Res. 2017, 45, 9093–9107. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.T.; Lynch, T.R.; Crittenden, S.L.; Bingman, C.A.; Wickens, M.; Kimble, J. C. elegans Germ Granules Require Both Assembly and Localized Regulators for MRNA Repression. Nat. Commun. 2021, 12, 996. [Google Scholar] [CrossRef]
- Ishidate, T.; Ozturk, A.R.; Durning, D.J.; Sharma, R.; Shen, E.; Chen, H.; Seth, M.; Shirayama, M.; Mello, C.C. ZNFX-1 Functions within Perinuclear Nuage to Balance Epigenetic Signals. Mol. Cell 2018, 70, 639–649.e6. [Google Scholar] [CrossRef]
- Wan, Q.-L.; Meng, X.; Dai, W.; Luo, Z.; Wang, C.; Fu, X.; Yang, J.; Ye, Q.; Zhou, Q. N6-Methyldeoxyadenine and Histone Methylation Mediate Transgenerational Survival Advantages Induced by Hormetic Heat Stress. Sci. Adv. 2021, 7, eabc3026. [Google Scholar] [CrossRef]
- Xu, F.; Guang, S.; Feng, X. Distinct Nuclear and Cytoplasmic Machineries Cooperatively Promote the Inheritance of RNAi in Caenorhabditis elegans. Biol. Cell 2018, 110, 217–224. [Google Scholar] [CrossRef]
- Placentino, M.; de Jesus Domingues, A.M.; Schreier, J.; Dietz, S.; Hellmann, S.; de Albuquerque, B.F.; Butter, F.; Ketting, R.F. Intrinsically Disordered Protein PID-2 Modulates Z Granules and Is Required for Heritable PiRNA-Induced Silencing in the Caenorhabditis elegans Embryo. EMBO J. 2021, 40, e105280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Montgomery, T.A.; Gabel, H.W.; Fischer, S.E.J.; Phillips, C.M.; Fahlgren, N.; Sullivan, C.M.; Carrington, J.C.; Ruvkun, G. Mut-16 and Other Mutator Class Genes Modulate 22G and 26G SiRNA Pathways in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2011, 108, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.M.; Montgomery, T.A.; Breen, P.C.; Ruvkun, G. MUT-16 Promotes Formation of Perinuclear Mutator Foci Required for RNA Silencing in the C. elegans Germline. Genes. Dev. 2012, 26, 1433–1444. [Google Scholar] [CrossRef]
- Tsai, H.-Y.; Chen, C.-C.G.; Conte, D.; Moresco, J.J.; Chaves, D.A.; Mitani, S.; Yates, J.R.; Tsai, M.-D.; Mello, C.C. A Ribonuclease Coordinates SiRNA Amplification and MRNA Cleavage during RNAi. Cell 2015, 160, 407–419. [Google Scholar] [CrossRef]
- Uebel, C.J.; Anderson, D.C.; Mandarino, L.M.; Manage, K.I.; Aynaszyan, S.; Phillips, C.M. Distinct Regions of the Intrinsically Disordered Protein MUT-16 Mediate Assembly of a Small RNA Amplification Complex and Promote Phase Separation of Mutator Foci. PLoS Genet. 2018, 14, e1007542. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.P.T.; Zhang, W.L.; Seydoux, G. The Conserved Helicase ZNFX-1 Memorializes Silenced RNAs in Perinuclear Condensates. Nat. Cell Biol. 2022, 24, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Schott, D.; Yanai, I.; Hunter, C.P. Natural RNA Interference Directs a Heritable Response to the Environment. Sci. Rep. 2014, 4, 7387. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.S.; Kaletsky, R.; Murphy, C.T. Piwi/PRG-1 Argonaute and TGF-β Mediate Transgenerational Learned Pathogenic Avoidance. Cell 2019, 177, 1827–1841.e12. [Google Scholar] [CrossRef]
- Kaletsky, R.; Moore, R.S.; Vrla, G.D.; Parsons, L.R.; Gitai, Z.; Murphy, C.T. C. elegans Interprets Bacterial Non-Coding RNAs to Learn Pathogenic Avoidance. Nature 2020, 586, 445–451. [Google Scholar] [CrossRef]
- Ni, J.Z.; Kalinava, N.; Chen, E.; Huang, A.; Trinh, T.; Gu, S.G. A Transgenerational Role of the Germline Nuclear RNAi Pathway in Repressing Heat Stress-Induced Transcriptional Activation in C. elegans. Epigenet. Chromatin 2016, 9, 3. [Google Scholar] [CrossRef]
- Klosin, A.; Casas, E.; Hidalgo-Carcedo, C.; Vavouri, T.; Lehner, B. Transgenerational Transmission of Environmental Information in C. elegans. Science 2017, 356, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.K. Environmental Epigenetics and a Unified Theory of the Molecular Aspects of Evolution: A Neo-Lamarckian Concept That Facilitates Neo-Darwinian Evolution. Genome Biol. Evol. 2015, 7, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.K.; Nilsson, E.E. Role of Environmentally Induced Epigenetic Transgenerational Inheritance in Evolutionary Biology: Unified Evolution Theory. Environ. Epigenet. 2021, 7, dvab012. [Google Scholar] [CrossRef] [PubMed]
- Morran, L.T.; Cappy, B.J.; Anderson, J.L.; Phillips, P.C. Sexual Partners for the Stressed: Facultative Outcrossing in the Self-Fertilizing Nematode Caenorhabditis elegans. Evolution 2009, 63, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Ii, R.C.P.; Penley, M.J.; Morran, L.T. The Integral Role of Genetic Variation in the Evolution of Outcrossing in the Caenorhabditis elegans-Serratia marcescens Host-Parasite System. PLoS ONE 2016, 11, e0154463. [Google Scholar] [CrossRef]
- Leighton, D.H.W.; Choe, A.; Wu, S.Y.; Sternberg, P.W. Communication between Oocytes and Somatic Cells Regulates Volatile Pheromone Production in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2014, 111, 17905–17910. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Zhou, Y.; Chan, C.M.; Yang, H.; Yeung, C.; Chow, K.L. Chow SRD-1 in AWA Neurons Is the Receptor for Female Volatile Sex Pheromones in C. elegans Males. EMBO Rep. 2019, 20, e46288. [Google Scholar] [CrossRef]
- Toker, I.A.; Lev, I.; Mor, Y.; Gurevich, Y.; Fisher, D.; Houri-Zeevi, L.; Antonova, O.; Doron, H.; Anava, S.; Gingold, H.; et al. Transgenerational Inheritance of Sexual Attractiveness via Small RNAs Enhances Evolvability in C. elegans. Dev. Cell 2022, 57, 298–309.e9. [Google Scholar] [CrossRef]
- Seydoux, G. The P Granules of C. elegans: A Genetic Model for the Study of RNA–Protein Condensates. J. Mol. Biol. 2018, 430, 4702–4710. [Google Scholar] [CrossRef]
- Wang, J.T.; Smith, J.; Chen, B.-C.; Schmidt, H.; Rasoloson, D.; Paix, A.; Lambrus, B.G.; Calidas, D.; Betzig, E.; Seydoux, G. Regulation of RNA Granule Dynamics by Phosphorylation of Serine-Rich, Intrinsically Disordered Proteins in C. elegans. eLife 2014, 3, e04591. [Google Scholar] [CrossRef]
- Putnam, A.; Cassani, M.; Smith, J.; Seydoux, G. A Gel Phase Promotes Condensation of Liquid P Granules in Caenorhabditis elegans Embryos. Nat. Struct. Mol. Biol. 2019, 26, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.C.; Updike, D.L. CSR-1 and P Granules Suppress Sperm-Specific Transcription in the C. elegans Germline. Development 2015, 142, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Rando, O.J. Metabolic Inputs into the Epigenome. Cell Metab. 2017, 25, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Aiken, C.E.; Tarry-Adkins, J.L.; Ozanne, S.E. Transgenerational Effects of Maternal Diet on Metabolic and Reproductive Ageing. Mamm. Genome 2016, 27, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Panera, N.; Mandato, C.; Crudele, A.; Bertrando, S.; Vajro, P.; Alisi, A. Genetics, Epigenetics and Transgenerational Transmission of Obesity in Children. Front. Endocrinol. 2022, 13, 1006008. [Google Scholar] [CrossRef] [PubMed]
- Massiera, F.; Barbry, P.; Guesnet, P.; Joly, A.; Luquet, S.; Moreilhon-Brest, C.; Mohsen-Kanson, T.; Amri, E.-Z.; Ailhaud, G. A Western-like Fat Diet Is Sufficient to Induce a Gradual Enhancement in Fat Mass over Generations. J. Lipid Res. 2010, 51, 2352–2361. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef]
- Lathigara, D.; Kaushal, D.; Wilson, R.B. Molecular Mechanisms of Western Diet-Induced Obesity and Obesity-Related Carcinogenesis—A Narrative Review. Metabolites 2023, 13, 675. [Google Scholar] [CrossRef]
- Tadros, W.; Lipshitz, H.D. The Maternal-to-Zygotic Transition: A Play in Two Acts. Development 2009, 136, 3033–3042. [Google Scholar] [CrossRef]
- Lee, M.T.; Bonneau, A.R.; Giraldez, A.J. Zygotic Genome Activation during the Maternal-to-Zygotic Transition. Annu. Rev. Cell Dev. Biol. 2014, 30, 581–613. [Google Scholar] [CrossRef]
- Vastenhouw, N.L.; Cao, W.X.; Lipshitz, H.D. The Maternal-to-Zygotic Transition Revisited. Development 2019, 146, dev161471. [Google Scholar] [CrossRef] [PubMed]
- Bazer, F.W.; Spencer, T.E.; Wu, G.; Cudd, T.A.; Meininger, C.J. Maternal Nutrition and Fetal Development. J. Nutr. 2004, 134, 2169–2172. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, B.; Harper, M.-E. In Utero Undernutrition Programs Skeletal and Cardiac Muscle Metabolism. Front. Physiol. 2016, 6, 401. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gallego, C.; García-Mantrana, I.; Martínez-Costa, C.; Salminen, S.; Isolauri, E.; Collado, M.C. The Microbiota and Malnutrition: Impact of Nutritional Status during Early Life. Annu. Rev. Nutr. 2019, 39, 267–290. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Xu, F.; Hirschfeld, M.; Brenig, B. Sperm Lipid Markers of Male Fertility in Mammals. Int. J. Mol. Sci. 2021, 22, 8767. [Google Scholar] [CrossRef]
- Mostafa, S.; Nader, N.; Machaca, K. Lipid Signaling During Gamete Maturation. Front. Cell Dev. Biol. 2022, 10, 814876. [Google Scholar] [CrossRef] [PubMed]
- L’Hernault, S.W. Spermatogenesis. In WormBook: The Online Review of C. elegans Biology [Internet]; WormBook: New York, NY, USA, 2006. [Google Scholar]
- Cheng, C.Y.; Mruk, D.D. The Biology of Spermatogenesis: The Past, Present and Future. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 1459–1463. [Google Scholar] [CrossRef]
- Reilly, J.N.; McLaughlin, E.A.; Stanger, S.J.; Anderson, A.L.; Hutcheon, K.; Church, K.; Mihalas, B.P.; Tyagi, S.; Holt, J.E.; Eamens, A.L.; et al. Characterisation of Mouse Epididymosomes Reveals a Complex Profile of MicroRNAs and a Potential Mechanism for Modification of the Sperm Epigenome. Sci. Rep. 2016, 6, 31794. [Google Scholar] [CrossRef]
- Kappil, M.; Wright, R.O.; Sanders, A.P. Sanders Developmental Origins of Common Disease: Epigenetic Contributions to Obesity. Annu. Rev. Genom. Hum. Genet. 2016, 17, 177–192. [Google Scholar] [CrossRef]
- Dupont, C.; Kappeler, L.; Saget, S.; Grandjean, V.; Lévy, R. Role of MiRNA in the Transmission of Metabolic Diseases Associated With Paternal Diet-Induced Obesity. Front. Genet. 2019, 10, 337. [Google Scholar] [CrossRef]
- Kaspar, D.; Hastreiter, S.; Irmler, M.; Hrabé de Angelis, M.; Beckers, J. Nutrition and Its Role in Epigenetic Inheritance of Obesity and Diabetes across Generations. Mamm. Genome 2020, 31, 119–133. [Google Scholar] [CrossRef] [PubMed]
- King, S.E.; Skinner, M.K. Epigenetic Transgenerational Inheritance of Obesity Susceptibility. Trends Endocrinol. Metab. 2020, 31, 478–494. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.-F.; Lin, R.C.Y.; Laybutt, D.R.; Barres, R.; Owens, J.A.; Morris, M.J. Chronic High-Fat Diet in Fathers Programs β 2-Cell Dysfunction in Female Rat Offspring. Nature 2010, 467, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Huypens, P.; Sass, S.; Wu, M.; Dyckhoff, D.; Tschöp, M.; Theis, F.; Marschall, S.; de Angelis, M.H.; Beckers, J. Epigenetic Germline Inheritance of Diet-Induced Obesity and Insulin Resistance. Nat. Genet. 2016, 48, 497–499. [Google Scholar] [CrossRef] [PubMed]
- de Castro Barbosa, T.; Ingerslev, L.R.; Alm, P.S.; Versteyhe, S.; Massart, J.; Rasmussen, M.; Donkin, I.; Sjögren, R.; Mudry, J.M.; Vetterli, L.; et al. High-Fat Diet Reprograms the Epigenome of Rat Spermatozoa and Transgenerationally Affects Metabolism of the Offspring. Mol. Metab. 2015, 5, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Shi, J.; Tuorto, F.; Li, X.; Liu, Y.; Liebers, R.; Zhang, L.; Qu, Y.; Qian, J.; et al. Dnmt2 Mediates Intergenerational Transmission of Paternally Acquired Metabolic Disorders through Sperm Small Non-Coding RNAs. Nat. Cell Biol. 2018, 20, 535–540. [Google Scholar] [CrossRef]
- Cropley, J.E.; Eaton, S.A.; Aiken, A.; Young, P.E.; Giannoulatou, E.; Ho, J.W.K.; Buckland, M.E.; Keam, S.P.; Hutvagner, G.; Humphreys, D.T.; et al. Male-Lineage Transmission of an Acquired Metabolic Phenotype Induced by Grand-Paternal Obesity. Mol. Metab. 2016, 5, 699–708. [Google Scholar] [CrossRef]
- Carone, B.R.; Fauquier, L.; Habib, N.; Shea, J.M.; Hart, C.E.; Li, R.; Bock, C.; Li, C.; Gu, H.; Zamore, P.D.; et al. Paternally Induced Transgenerational Environmental Reprogramming of Metabolic Gene Expression in Mammals. Cell 2010, 143, 1084–1096. [Google Scholar] [CrossRef]
- Billi, A.C.; Freeberg, M.A.; Kim, J.K. PiRNAs and SiRNAs Collaborate in Caenorhabditis elegans Genome Defense. Genome Biol. 2012, 13, 164. [Google Scholar] [CrossRef]
- Schlee, M.; Hartmann, G. Discriminating Self from Non-Self in Nucleic Acid Sensing. Nat. Rev. Immunol. 2016, 16, 566–580. [Google Scholar] [CrossRef]
- Cornec, A.; Poirier, E.Z. Interplay between RNA Interference and Transposable Elements in Mammals. Front. Immunol. 2023, 14, 1212086. [Google Scholar] [CrossRef] [PubMed]
- Palominos, M.F.; Verdugo, L.; Gabaldon, C.; Pollak, B.; Ortíz-Severín, J.; Varas, M.A.; Chávez, F.P.; Calixto, A. Transgenerational Diapause as an Avoidance Strategy against Bacterial Pathogens in Caenorhabditis elegans. mBio 2017, 8, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Burton, N.O.; Riccio, C.; Dallaire, A.; Price, J.; Jenkins, B.; Koulman, A.; Miska, E.A. Cysteine Synthases CYSL-1 and CYSL-2 Mediate C. elegans Heritable Adaptation to P. vranovensis Infection. Nat. Commun. 2020, 11, 1741. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.G.; Gracida, X.; Kagias, K.; Zhang, Y. C. elegans Aversive Olfactory Learning Generates Diverse Intergenerational Effects. J. Neurogenet. 2020, 34, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Houri-Ze’evi, L.; Korem, Y.; Sheftel, H.; Faigenbloom, L.; Toker, I.A.; Dagan, Y.; Awad, L.; Degani, L.; Alon, U.; Rechavi, O. A Tunable Mechanism Determines the Duration of the Transgenerational Small RNA Inheritance in C. elegans. Cell 2016, 165, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Houri-Zeevi, L.; Korem Kohanim, Y.; Antonova, O.; Rechavi, O. Three Rules Explain Transgenerational Small RNA Inheritance in C. elegans. Cell 2020, 182, 1186–1197.e12. [Google Scholar] [CrossRef] [PubMed]
- Houri-Zeevi, L.; Teichman, G.; Gingold, H.; Rechavi, O. Stress Resets Ancestral Heritable Small RNA Responses. eLife 2021, 10, e65797. [Google Scholar] [CrossRef]
- Meisel, J.D.; Panda, O.; Mahanti, P.; Schroeder, F.C.; Kim, D.H. Chemosensation of Bacterial Secondary Metabolites Modulates Neuroendocrine Signaling and Behavior of C. elegans. Cell 2014, 159, 267–280. [Google Scholar] [CrossRef]
- Kudlow, B.A.; Zhang, L.; Han, M. Systematic Analysis of Tissue-Restricted MiRISCs Reveals a Broad Role for MicroRNAs in Suppressing Basal Activity of the C. elegans Pathogen Response. Mol. Cell 2012, 46, 530–541. [Google Scholar] [CrossRef]
- Liu, F.; He, C.-X.; Luo, L.-J.; Zou, Q.-L.; Zhao, Y.-X.; Saini, R.; Han, S.-F.; Knölker, H.-J.; Wang, L.-S.; Ge, B.-X. Nuclear Hormone Receptor Regulation of MicroRNAs Controls Innate Immune Responses in C. elegans. PLoS Pathog. 2013, 9, e1003545. [Google Scholar] [CrossRef]
- Dai, L.-L.; Gao, J.-X.; Zou, C.-G.; Ma, Y.-C.; Zhang, K.-Q. Mir-233 Modulates the Unfolded Protein Response in C. elegans during Pseudomonas Aeruginosa Infection. PLoS Pathog. 2015, 11, e1004606. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Ambros, V.R. Caenorhabditis elegans MicroRNAs of the Let-7 Family Act in Innate Immune Response Circuits and Confer Robust Developmental Timing against Pathogen Stress. Proc. Natl. Acad. Sci. USA 2015, 112, E2366–E2375. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-C.; Zhang, L.; Dai, L.-L.; Khan, R.U.; Zou, C.-G. Mir-67 Regulates P. Aeruginosa Avoidance Behavior in C. elegans. Biochem. Biophys. Res. Commun. 2017, 494, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Kuvbachieva, A.; Bestel, A.-M.; Tissir, F.; Maloum, I.; Guimiot, F.; Ramoz, N.; Bourgeois, F.; Moalic, J.-M.; Goffinet, A.M.; Simonneau, M. Identification of a Novel Brain-Specific and Reelin-Regulated Gene That Encodes a Protein Colocalized with Synapsin. Eur. J. Neurosci. 2004, 20, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Arellano-Carbajal, F.; Briseño-Roa, L.; Couto, A.; Cheung, B.H.H.; Labouesse, M.; Bono, M. de Macoilin, a Conserved Nervous System–Specific ER Membrane Protein That Regulates Neuronal Excitability. PLoS Genet. 2011, 7, e1001341. [Google Scholar] [CrossRef] [PubMed]
- Miyara, A.; Ohta, A.; Okochi, Y.; Tsukada, Y.; Kuhara, A.; Mori, I. Novel and Conserved Protein Macoilin Is Required for Diverse Neuronal Functions in Caenorhabditis elegans. PLoS Genet. 2011, 7, e1001384. [Google Scholar] [CrossRef]
- Neal, S.J.; Park, J.; DiTirro, D.; Yoon, J.; Shibuya, M.; Choi, W.; Schroeder, F.C.; Butcher, R.A.; Kim, K.; Sengupta, P. A Forward Genetic Screen for Molecules Involved in Pheromone-Induced Dauer Formation in Caenorhabditis elegans. G3 Genes Genomes Genet. 2016, 6, 1475–1487. [Google Scholar] [CrossRef]
- Moore, R.S.; Kaletsky, R.; Lesnik, C.; Cota, V.; Blackman, E.; Parsons, L.R.; Gitai, Z.; Murphy, C.T. The Role of the Cer1 Transposon in Horizontal Transfer of Transgenerational Memory. Cell 2021, 184, 4697–4712.e18. [Google Scholar] [CrossRef]
- Dennis, S.; Sheth, U.; Feldman, J.L.; English, K.A.; Priess, J.R. C. elegans Germ Cells Show Temperature and Age-Dependent Expression of Cer1, a Gypsy/Ty3-Related Retrotransposon. PLoS Pathog. 2012, 8, e1002591. [Google Scholar] [CrossRef]
- Feinberg, E.H.; Hunter, C.P. Transport of DsRNA into Cells by the Transmembrane Protein SID-1. Science 2003, 301, 1545–1547. [Google Scholar] [CrossRef]
- Shih, J.D.; Fitzgerald, M.C.; Sutherlin, M.; Hunter, C.P. The SID-1 Double-Stranded RNA Transporter Is Not Selective for DsRNA Length. RNA 2009, 15, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Minkina, O.; Hunter, C.P. Intergenerational Transmission of Gene Regulatory Information in Caenorhabditis elegans. Trends Genet. 2018, 34, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Marré, J.; Traver, E.C.; Jose, A.M. Extracellular RNA Is Transported from One Generation to the next in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2016, 113, 12496–12501. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Hunter, C.P. SID-1 Functions in Multiple Roles to Support Parental RNAi in Caenorhabditis elegans. Genetics 2017, 207, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Xu, X.; Lin, T.; Weng, S.; Liang, H.; Huang, M.; Dong, C.; Luo, Y.; He, J. Cloning, Characterization, and Biological Function Analysis of the SidT2 Gene from Siniperca Chuatsi. Dev. Comp. Immunol. 2011, 35, 692–701. [Google Scholar] [CrossRef]
- Elhassan, M.O.; Christie, J.; Duxbury, M.S. Homo Sapiens Systemic RNA Interference-Defective-1 Transmembrane Family Member 1 (SIDT1) Protein Mediates Contact-Dependent Small RNA Transfer and MicroRNA-21-Driven Chemoresistance. J. Biol. Chem. 2012, 287, 5267–5277. [Google Scholar] [CrossRef]
- Pratt, A.J.; Rambo, R.P.; Lau, P.-W.; MacRae, I.J. Preparation and Characterization of the Extracellular Domain of Human Sid-1. PLoS ONE 2012, 7, e33607. [Google Scholar] [CrossRef]
- Rouhana, L.; Weiss, J.A.; Forsthoefel, D.J.; Lee, H.; King, R.S.; Inoue, T.; Shibata, N.; Agata, K.; Newmark, P.A. RNA Interference by Feeding in Vitro–Synthesized Double-Stranded RNA to Planarians: Methodology and Dynamics. Dev. Dyn. 2013, 242, 718–730. [Google Scholar] [CrossRef]
- Li, W.; Koutmou, K.S.; Leahy, D.J.; Li, M. Systemic RNA Interference Deficiency-1 (SID-1) Extracellular Domain Selectively Binds Long Double-Stranded RNA and Is Required for RNA Transport by SID-1. J. Biol. Chem. 2015, 290, 18904–18913. [Google Scholar] [CrossRef]
- Kwak, J.E.; Wickens, M. A Family of Poly(U) Polymerases. RNA 2007, 13, 860–867. [Google Scholar] [CrossRef][Green Version]
- Spracklin, G.; Fields, B.; Wan, G.; Becker, D.; Wallig, A.; Shukla, A.; Kennedy, S. The RNAi Inheritance Machinery of Caenorhabditis elegans. Genetics 2017, 206, 1403–1416. [Google Scholar] [CrossRef] [PubMed]
- Sapetschnig, A.; Sarkies, P.; Lehrbach, N.J.; Miska, E.A. Tertiary SiRNAs Mediate Paramutation in C. elegans. PLoS Genet. 2015, 11, e1005078. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.G.; Pak, J.; Guang, S.; Maniar, J.M.; Kennedy, S.; Fire, A. A Transgenerational Impact of SiRNA on Chromatin: SiRNA Amplification in Caenorhabditis elegans Generates a Homology-Targeted Footprint of H3K9 Methylated Nucleosomes. Nat. Genet. 2012, 44, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Yan, J.; Pagano, D.J.; Dodson, A.E.; Fei, Y.; Gorham, J.; Seidman, J.G.; Wickens, M.; Kennedy, S. Poly(UG)-Tailed RNAs in Genome Protection and Epigenetic Inheritance. Nature 2020, 582, 283–288. [Google Scholar] [CrossRef]
- Preston, M.A.; Porter, D.F.; Chen, F.; Buter, N.; Lapointe, C.P.; Keles, S.; Kimble, J.; Wickens, M. Unbiased Screen of RNA Tailing Activities Reveals a Poly(UG) Polymerase. Nat. Methods 2019, 16, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.; Saari, B.; Anderson, P. Activation of a Transposable Element in the Germ Line but Not the Soma of Caenorhabditis elegans. Nature 1987, 328, 726–728. [Google Scholar] [CrossRef] [PubMed]
- Ketting, R.F.; Haverkamp, T.H.A.; van Luenen, H.G.A.M.; Plasterk, R.H.A. Mut-7 of C. elegans, Required for Transposon Silencing and RNA Interference, Is a Homolog of Werner Syndrome Helicase and RNaseD. Cell 1999, 99, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Tabara, H.; Sarkissian, M.; Kelly, W.G.; Fleenor, J.; Grishok, A.; Timmons, L.; Fire, A.; Mello, C.C. The Rde-1 Gene, RNA Interference, and Transposon Silencing in C. elegans. Cell 1999, 99, 123–132. [Google Scholar] [CrossRef]
- Chen, C.-C.G.; Simard, M.J.; Tabara, H.; Brownell, D.R.; McCollough, J.A.; Mello, C.C. A Member of the Polymerase β Nucleotidyltransferase Superfamily Is Required for RNA Interference in C. elegans. Curr. Biol. 2005, 15, 378–383. [Google Scholar] [CrossRef]
- Roschdi, S.; Yan, J.; Nomura, Y.; Escobar, C.A.; Petersen, R.J.; Bingman, C.A.; Tonelli, M.; Vivek, R.; Montemayor, E.J.; Wickens, M.; et al. An Atypical RNA Quadruplex Marks RNAs as Vectors for Gene Silencing. Nat. Struct. Mol. Biol. 2022, 29, 1113–1121. [Google Scholar] [CrossRef]
- Gent, J.I.; Lamm, A.T.; Pavelec, D.M.; Maniar, J.M.; Parameswaran, P.; Tao, L.; Kennedy, S.; Fire, A.Z. Distinct Phases of SiRNA Synthesis in an Endogenous RNAi Pathway in C. elegans Soma. Mol. Cell 2010, 37, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Varshney, D.; Spiegel, J.; Zyner, K.; Tannahill, D.; Balasubramanian, S. The Regulation and Functions of DNA and RNA G-Quadruplexes. Nat. Rev. Mol. Cell Biol. 2020, 21, 459–474. [Google Scholar] [CrossRef] [PubMed]
- Kharel, P.; Fay, M.; Manasova, E.V.; Anderson, P.J.; Kurkin, A.V.; Guo, J.U.; Ivanov, P. Stress Promotes RNA G-Quadruplex Folding in Human Cells. Nat. Commun. 2023, 14, 205. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.N.; Schisa, J.A.; Priess, J.R. P Granules in the Germ Cells of Caenorhabditis elegans Adults Are Associated with Clusters of Nuclear Pores and Contain RNA. Dev. Biol. 2000, 219, 315–333. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-Y.S.; Putnam, A.; Lu, T.; He, S.; Ouyang, J.P.T.; Seydoux, G. Recruitment of MRNAs to P Granules by Condensation with Intrinsically-Disordered Proteins. eLife 2020, 9, e52896. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Perales, R.; Kennedy, S. PiRNAs Coordinate Poly(UG) Tailing to Prevent Aberrant and Perpetual Gene Silencing. Curr. Biol. 2021, 31, 4473–4485.e3. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.M.; Updike, D.L. Germ Granules and Gene Regulation in the Caenorhabditis elegans Germline. Genetics 2022, 220, iyab195. [Google Scholar] [CrossRef]
- Burton, N.O.; Willis, A.; Fisher, K.; Braukmann, F.; Price, J.; Stevens, L.; Baugh, L.R.; Reinke, A.; Miska, E.A. Intergenerational Adaptations to Stress Are Evolutionarily Conserved, Stress-Specific, and Have Deleterious Trade-Offs. eLife 2021, 10, e73425. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, Y.; Hua, X.; Wang, D. Induction of Transgenerational Toxicity Is Associated with the Activated Germline Insulin Signals in Nematodes Exposed to Nanoplastic at Predicted Environmental Concentrations. Ecotoxicol. Environ. Saf. 2022, 243, 114022. [Google Scholar] [CrossRef]
- Jablonka, E.; Raz, G. Transgenerational Epigenetic Inheritance: Prevalence, Mechanisms, and Implications for the Study of Heredity and Evolution. Q. Rev. Biol. 2009, 84, 131–176. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Sun, Z. Lamarck Rises from His Grave: Parental Environment-Induced Epigenetic Inheritance in Model Organisms and Humans. Biol. Rev. 2017, 92, 2084–2111. [Google Scholar] [CrossRef] [PubMed]
- Horsthemke, B. A Critical View on Transgenerational Epigenetic Inheritance in Humans. Nat. Commun. 2018, 9, 2973. [Google Scholar] [CrossRef] [PubMed]
- Roth, O.; Beemelmanns, A.; Barribeau, S.M.; Sadd, B.M. Recent Advances in Vertebrate and Invertebrate Transgenerational Immunity in the Light of Ecology and Evolution. Heredity 2018, 121, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Skvortsova, K.; Iovino, N.; Bogdanović, O. Functions and Mechanisms of Epigenetic Inheritance in Animals. Nat. Rev. Mol. Cell Biol. 2018, 19, 774–790. [Google Scholar] [CrossRef] [PubMed]
- Senaldi, L.; Smith-Raska, M. Evidence for Germline Non-Genetic Inheritance of Human Phenotypes and Diseases. Clin. Epigenetics 2020, 12, 136. [Google Scholar] [CrossRef] [PubMed]
- Alcazar, R.M.; Lin, R.; Fire, A.Z. Transmission Dynamics of Heritable Silencing Induced by Double-Stranded RNA in Caenorhabditis elegans. Genetics 2008, 180, 1275–1288. [Google Scholar] [CrossRef]
- Vastenhouw, N.L.; Brunschwig, K.; Okihara, K.L.; Müller, F.; Tijsterman, M.; Plasterk, R.H.A. Long-Term Gene Silencing by RNAi. Nature 2006, 442, 882. [Google Scholar] [CrossRef]
- Kalinava, N.; Ni, J.Z.; Peterman, K.; Chen, E.; Gu, S.G. Decoupling the Downstream Effects of Germline Nuclear RNAi Reveals That H3K9me3 Is Dispensable for Heritable RNAi and the Maintenance of Endogenous SiRNA-Mediated Transcriptional Silencing in Caenorhabditis elegans. Epigenet. Chromatin 2017, 10, 6. [Google Scholar] [CrossRef]
- Kalinava, N.; Ni, J.Z.; Gajic, Z.; Kim, M.; Ushakov, H.; Gu, S.G. C. elegans Heterochromatin Factor SET-32 Plays an Essential Role in Transgenerational Establishment of Nuclear RNAi-Mediated Epigenetic Silencing. Cell Rep. 2018, 25, 2273–2284.e3. [Google Scholar] [CrossRef]
- Woodhouse, R.M.; Buchmann, G.; Hoe, M.; Harney, D.J.; Low, J.K.K.; Larance, M.; Boag, P.R.; Ashe, A. Chromatin Modifiers SET-25 and SET-32 Are Required for Establishment but Not Long-Term Maintenance of Transgenerational Epigenetic Inheritance. Cell Rep. 2018, 25, 2259–2272.e5. [Google Scholar] [CrossRef]
- Andersen, E.C.; Horvitz, H.R. Two C. elegans Histone Methyltransferases Repress Lin-3EGF Transcription to Inhibit Vulval Development. Development 2007, 134, 2991–2999. [Google Scholar] [CrossRef] [PubMed]
- Greer, E.L.; Beese-Sims, S.E.; Brookes, E.; Spadafora, R.; Zhu, Y.; Rothbart, S.B.; Aristizábal-Corrales, D.; Chen, S.; Badeaux, A.I.; Jin, Q.; et al. A Histone Methylation Network Regulates Transgenerational Epigenetic Memory in C. elegans. Cell Rep. 2014, 7, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Lev, I.; Seroussi, U.; Gingold, H.; Bril, R.; Anava, S.; Rechavi, O. MET-2-Dependent H3K9 Methylation Suppresses Transgenerational Small RNA Inheritance. Curr. Biol. 2017, 27, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Kerr, S.C.; Ruppersburg, C.C.; Francis, J.W.; Katz, D.J. SPR-5 and MET-2 Function Cooperatively to Reestablish an Epigenetic Ground State during Passage through the Germ Line. Proc. Natl. Acad. Sci. USA 2014, 111, 9509–9514. [Google Scholar] [CrossRef] [PubMed]
- Perales, R.; Pagano, D.; Wan, G.; Fields, B.D.; Saltzman, A.L.; Kennedy, S.G. Transgenerational Epigenetic Inheritance Is Negatively Regulated by the HERI-1 Chromodomain Protein. Genetics 2018, 210, 1287–1299. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.J.; Hunter, C.P. The Influence of Competition Among C. elegans Small RNA Pathways on Development. Genes 2012, 3, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Brunquell, J.; Morris, S.; Lu, Y.; Cheng, F.; Westerheide, S.D. The Genome-Wide Role of HSF-1 in the Regulation of Gene Expression in Caenorhabditis elegans. BMC Genom. 2016, 17, 559. [Google Scholar] [CrossRef] [PubMed]
- Joutsen, J.; Sistonen, L. Tailoring of Proteostasis Networks with Heat Shock Factors. Cold Spring Harb. Perspect. Biol. 2019, 11, a034066. [Google Scholar] [CrossRef]
- Brunquell, J.; Snyder, A.; Cheng, F.; Westerheide, S.D. HSF-1 Is a Regulator of MiRNA Expression in Caenorhabditis elegans. PLoS ONE 2017, 12, e0183445. [Google Scholar] [CrossRef]
- Schreiner, W.P.; Pagliuso, D.C.; Garrigues, J.M.; Chen, J.S.; Aalto, A.P.; Pasquinelli, A.E. Remodeling of the Caenorhabditis elegans Non-Coding RNA Transcriptome by Heat Shock. Nucleic Acids Res. 2019, 47, 9829–9841. [Google Scholar] [CrossRef]
- Zheng, X.; Beyzavi, A.; Krakowiak, J.; Patel, N.; Khalil, A.S.; Pincus, D. Hsf1 Phosphorylation Generates Cell-to-Cell Variation in Hsp90 Levels and Promotes Phenotypic Plasticity. Cell Rep. 2018, 22, 3099–3106. [Google Scholar] [CrossRef] [PubMed]
- Andrusiak, M.G.; Jin, Y. Context Specificity of Stress-Activated Mitogen-Activated Protein (MAP) Kinase Signaling: The Story as Told by Caenorhabditis elegans. J. Biol. Chem. 2016, 291, 7796–7804. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, T.K.; Steinbaugh, M.J.; Hourihan, J.M.; Ewald, C.Y.; Isik, M. SKN-1/Nrf, Stress Responses, and Aging in Caenorhabditis elegans. Free Radic. Biol. Med. 2015, 88, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Ow, M.C.; Nichitean, A.M.; Hall, S.E. Somatic Aging Pathways Regulate Reproductive Plasticity in Caenorhabditis elegans. eLife 2021, 10, e61459. [Google Scholar] [CrossRef] [PubMed]
- Updike, D.L.; Knutson, A.K.; Egelhofer, T.A.; Campbell, A.C.; Strome, S. Germ-Granule Components Prevent Somatic Development in the C. elegans Germline. Curr. Biol. 2014, 24, 970–975. [Google Scholar] [CrossRef]
- Rochester, J.D.; Min, H.; Gajjar, G.A.; Sharp, C.S.; Maki, N.J.; Rollins, J.A.; Keiper, B.D.; Graber, J.H.; Updike, D.L. GLH-1/Vasa Represses Neuropeptide Expression and Drives Spermiogenesis in the C. elegans Germline. Dev. Biol. 2022, 492, 200–211. [Google Scholar] [CrossRef]
- Chen, W.; Brown, J.S.; He, T.; Wu, W.-S.; Tu, S.; Weng, Z.; Zhang, D.; Lee, H.-C. GLH/VASA Helicases Promote Germ Granule Formation to Ensure the Fidelity of PiRNA-Mediated Transcriptome Surveillance. Nat. Commun. 2022, 13, 5306. [Google Scholar] [CrossRef]
- Spike, C.A.; Bader, J.; Reinke, V.; Strome, S. DEPS-1 Promotes P-Granule Assembly and RNA Interference in C. elegans Germ Cells. Development 2008, 135, 983–993. [Google Scholar] [CrossRef]
- Suen, K.M.; Braukmann, F.; Butler, R.; Bensaddek, D.; Akay, A.; Lin, C.-C.; Milonaitytė, D.; Doshi, N.; Sapetschnig, A.; Lamond, A.; et al. DEPS-1 Is Required for PiRNA-Dependent Silencing and PIWI Condensate Organisation in Caenorhabditis elegans. Nat. Commun. 2020, 11, 4242. [Google Scholar] [CrossRef]
- Nguyen, D.A.H.; Phillips, C.M. Arginine Methylation Promotes SiRNA-Binding Specificity for a Spermatogenesis-Specific Isoform of the Argonaute Protein CSR-1. Nat. Commun. 2021, 12, 4212. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ow, M.C.; Hall, S.E. Inheritance of Stress Responses via Small Non-Coding RNAs in Invertebrates and Mammals. Epigenomes 2024, 8, 1. https://doi.org/10.3390/epigenomes8010001
Ow MC, Hall SE. Inheritance of Stress Responses via Small Non-Coding RNAs in Invertebrates and Mammals. Epigenomes. 2024; 8(1):1. https://doi.org/10.3390/epigenomes8010001
Chicago/Turabian StyleOw, Maria C., and Sarah E. Hall. 2024. "Inheritance of Stress Responses via Small Non-Coding RNAs in Invertebrates and Mammals" Epigenomes 8, no. 1: 1. https://doi.org/10.3390/epigenomes8010001
APA StyleOw, M. C., & Hall, S. E. (2024). Inheritance of Stress Responses via Small Non-Coding RNAs in Invertebrates and Mammals. Epigenomes, 8(1), 1. https://doi.org/10.3390/epigenomes8010001





