UV Radiation and Its Relation to DNA Methylation in Epidermal Cells: A Review
Abstract
1. Introduction
1.1. DNA Methylation
1.2. Ultraviolet Radiation (UV Radiation) and Epidermal Tissue
1.3. DNA Methylation in Epidermal Homeostasis
2. Results and Discussion
3. Materials and Methods
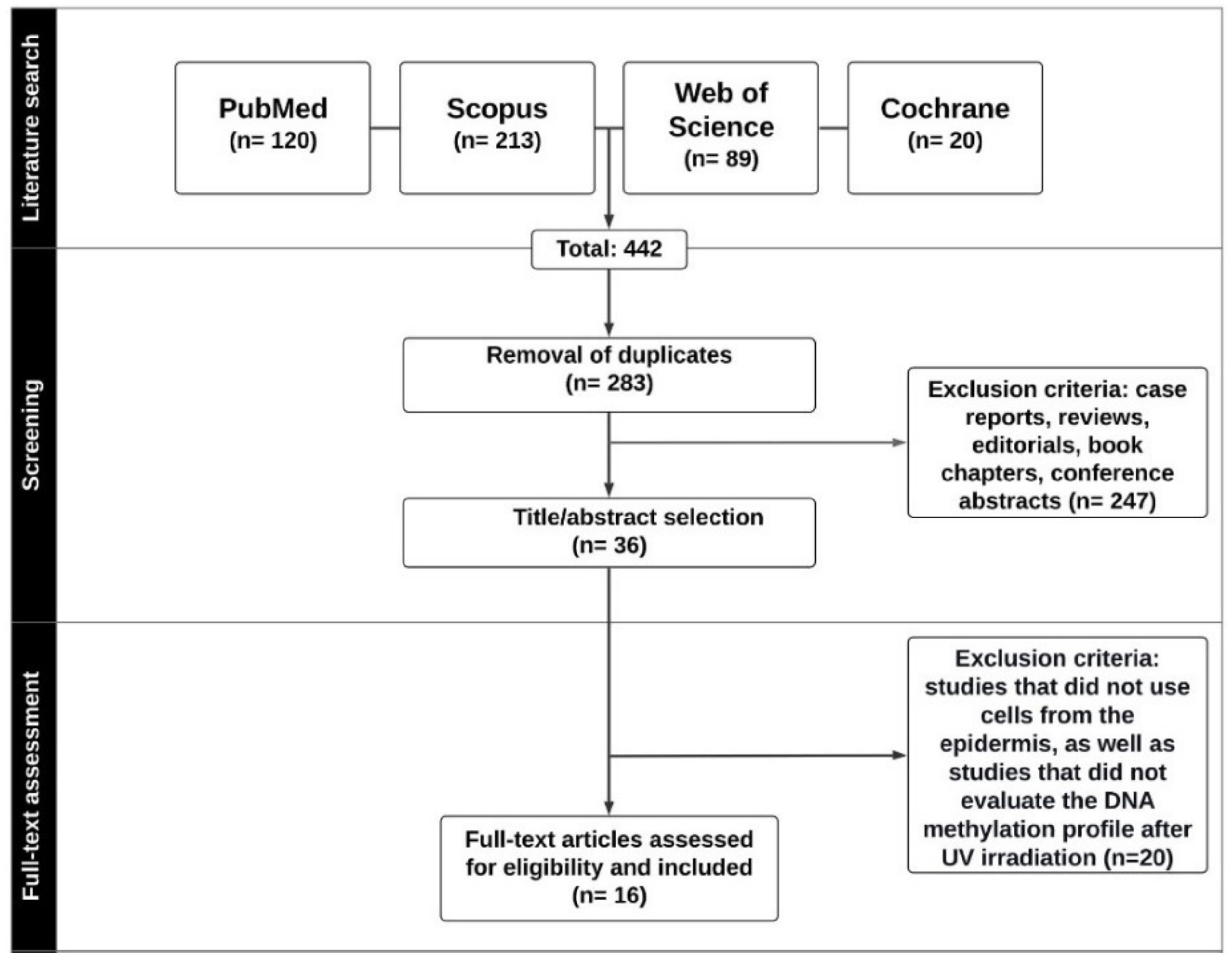
3.1. Literature Search Strategy
3.2. Inclusion and Exclusion Criteria
3.3. Data Extraction
4. Final Considerations and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Li, E.; Zhang, Y. DNA methylation in mammals. Cold Spring Harb. Perspect. Biol. 2014, 6, a019133. [Google Scholar] [CrossRef]
- Xiao, C.L.; Zhu, S.; He, M.; Chen, D.; Zhang, Q.; Chen, Y.; Yu, G.; Liu, J.; Xie, S.Q.; Luo, F.; et al. N6-Methyladenine DNA Modification in the Human Genome. Mol. Cell. 2018, 71, 306–318. [Google Scholar] [CrossRef]
- Lasman, L.; Hanna, J.H.; Novershtern, N. Role of m6A in Embryonic Stem Cell Differentiation and in Gametogenesis. Epigenomes 2020, 4, 5. [Google Scholar] [CrossRef]
- Nazki, F.H.; Sameer, A.S.; Ganaie, B.A. Folate: Metabolism, genes, polymorphisms and the associated diseases. Gene 2014, 533, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, K.D.; Helin, K. Role of TET enzymes in DNA methylation, development, and câncer. Gene Dev. 2016, 30, 733–750. [Google Scholar] [CrossRef] [PubMed]
- Field, A.E.; Robertson, N.A.; Wang, T.; Havas, A.; Ideker, T.; Adams, P.D. DNA Methylation Clocks in Aging: Categories, Causes, and Consequences. Mol. Cell. 2018, 71, 882–895. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, R.; Nwanaji-Enwerem, J.C.; Samet, M.; Ward-Caviness, C.K. DNA Methylation Age-Environmental Influences, Health Impacts, and Its Role in Environmental Epidemiology. Curr. Environ. Health Rep. 2018, 5, 317–327. [Google Scholar] [CrossRef]
- Meehan, R.R.; Thomson, J.P.; Lentini, A.; Nestor, C.E.; Pennings, S. DNA methylation as a genomic marker of exposure to chemical and environmental agents. Curr. Opin. Chem. Biol. 2018, 45, 48–56. [Google Scholar] [CrossRef]
- Herceg, Z. Epigenetics and cancer: Towards an evaluation of the impact of environmental and dietary factors. Mutagenesis 2007, 22, 91–103. [Google Scholar] [CrossRef]
- Stevens, A.J.; Rucklidge, J.J.; Kennedy, M.A. Epigenetics, nutrition and mental health.Is there a relationship? Nutr. Neurosci. 2018, 21, 602–613. [Google Scholar] [CrossRef]
- Halliday, G.M.; Agar, N.S.; Barnetson, R.S.C.; Ananthaswamy, H.N.; Jones, A.M. UV-A fingerprint mutations in human skin cancer. Photochem. Photobiol. 2005, 81, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Brash, D.E. UV-induced Melanin Chemiexcitation: A New Mode of Melanoma Pathogenesis. Toxicol. Pathol. 2016, 44, 552–554. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.I.; Christensen, L.; Baron, E. History of UV Lamps, Types, and Their Applications. In Ultraviolet Light in Human Health, Diseases and Environment, 1st ed.; Ahmad, S.I., Ed.; Springer International Publishing: New York, NY, USA, 2017; Volume 996, pp. 3–11. [Google Scholar]
- Gordon, R. Skin cancer: An overview of epidemiology and risk factors. Semin. Oncol. Nurs. 2013, 29, 160–169. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J. Nucleic. Acids 2010, 2010, 592980. [Google Scholar] [CrossRef]
- Yang, W. Surviving the sun: Repair and bypass of DNA UV lesions. Protein Sci. 2011, 20, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Geyer, S.; Weninger, W.; Guimberteau, J.C.; Wong, J.K. The dynamic anatomy and patterning of skin. Exp. Dermatol. 2016, 25, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Watt, F.M.; Jensen, K.B. Epidermal stem cell diversity and quiescence. EMBO Mol. Med. 2009, 1, 260–267. [Google Scholar] [CrossRef]
- Youssef, M.; Cuddihy, A.; Darido, C. Long-Lived Epidermal Cancer-Initiating Cells. Int. J. Mol. Sci. 2017, 18, 1369. [Google Scholar] [CrossRef]
- De Berker, D.; McGregor, J.; Mohd Mustapa, M.; Exton, L.; Hughes, B. British Association of Dermatologists’ guidelines for the care of patients with actinic keratosis 2017. Br. J. Dermatol. 2017, 176, 20–43. [Google Scholar] [CrossRef]
- INCA. Estimativa 2020: Incidência de Câncer no Brasil, 1st ed.; Instituto Nacional de Câncer José Alencar Gomes da Silva: Rio de Janeiro, Brazil, 2019; p. 120. [Google Scholar]
- Perera, E.; Gnaneswaran, N.; Staines, C.; Win, A.K.; Sinclair, R. Incidence and prevalence of non-melanoma skin cancer in Australia: A systematic review. Australas. J. Dermatol. 2015, 56, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.M.; Martin, B.; Ghafari, G.; Luong, J.; Nahar, V.K.; Pham, L.; Luo, J.; Savoy, M.; Sharma, M. Skin Cancer Knowledge, Attitudes, and Practices among Chinese Population: A Narrative Review. Dermatol. Res. Pract. 2018, 2018, 1965674. [Google Scholar] [CrossRef] [PubMed]
- Chipperfield, M.P.; Bekki, S.; Dhomse, S.; Harris, N.; Hassler, B.; Hossaini, R.; Steinbrecht, W.; Thiéblemont, R.; Weber, M. Detecting recovery of the stratospheric ozone layer. Nature 2017, 549, 211–218. [Google Scholar] [CrossRef]
- Marín, D.H.; Higgins, F.; Sanmartín, O.; López-Guerrero, J.A.; Carmen Bañó, M.; Carlos Igual, J.; Quilis, I.; Sandoval, J. Genome wide DNA methylation profiling identifies specific epigenetic features in high-risk cutaneous squamous cell carcinoma. PLoS ONE 2019, 14, e0223341. [Google Scholar]
- Sang, Y.; Deng, Y. Current insights into the epigenetic mechanisms of skin cancer. Dermatol. Ther. 2019, 32, e12964. [Google Scholar] [CrossRef] [PubMed]
- Miroshnikova, Y.A.; Cohen, I.; Ezhkova, E.; Wickström, S.A. Epigenetic gene regulation, chromatin structure, and force-induced chromatin remodelling in epidermal development and homeostasis. Curr. Opin. Genet. Dev. 2019, 55, 46–51. [Google Scholar] [CrossRef]
- Köhler, F.; Rodríguez-Paredes, M.J. DNA Methylation in Epidermal Differentiation, Aging, and Cancer. J. Investig. Dermatol. 2020, 140, 38–47. [Google Scholar] [CrossRef]
- Gifford, C.A.; Ziller, M.J.; Gu, H.; Trapnell, C.; Donaghey, J.; Tsankov, A.; Shalek, A.K.; Kelley, D.R.; Shishkin, A.A.; Issner, R.; et al. Transcriptional and epigenetic dynamics during specification of human embryonic stem cells. Cell 2013, 153, 1149–1163. [Google Scholar] [CrossRef]
- Xie, W.; Schultz, M.D.; Lister, R.; Hou, Z.; Rajagopal, N.; Ray, P.; Whitaker, J.W.; Tian, S.; Hawkins, R.D.; Leung, D.; et al. Epigenomic analysis of multilineage differentiation of human embryonic stem cells. Cell 2013, 153, 1134–1148. [Google Scholar] [CrossRef]
- Bock, C.; Beerman, I.; Lien, W.H.; Smith, Z.D.; Gu, H.; Boyle, P.; Gnirke, A.; Fuchs, E.; Rossi, D.J.; Meissner, A. DNA methylation dynamics during in vivo differentiation of blood and skin stem cells. Mol. Cell. 2012, 47, 633–647. [Google Scholar] [CrossRef]
- Rinaldi, L.; Datta, D.; Serrat, J.; Morey, L.; Solanas, G.; Avgustinova, A.; Blanco, E.; Pons, J.I.; Matallanas, D.; Von Kriegsheim, A.; et al. Dnmt3a and Dnmt3b Associate with Enhancers to Regulate Human Epidermal Stem Cell Homeostasis. Cell Stem Cell 2016, 19, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Sen, G.L.; Reuter, J.A.; Webster, D.E.; Zhu, L.; Khavari, P.A. DNMT1 maintains progenitor function in self-renewing somatic tissue. Nature 2010, 463, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Belokhvostova, D.; Berzanskyte, I.; Cujba, A.M.; Jowett, G.; Marshall, L.; Prueller, J.; Watt, F.M. Homeostasis, regeneration and tumor formation in the mammalian epidermis. Int. J. Dev. Biol. 2018, 62, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Chakrobarty, A.; Raman, G.; Banerjee, G. Cloning and identification of EDD gene from ultraviolet-irradiated HaCaT cells. Photodermatol. Photoimmunol. Photomed. 2006, 22, 278–284. [Google Scholar] [CrossRef]
- Chen, I.P.; Henning, S.; Faust, A.; Boukamp, P.; Volkmer, B.; Greinert, R. UVA-induced epigenetic regulation of P16(INK4a) in human epidermal keratinocytes and skin tumor derived cells. Photochem. Photobiol. Sci. 2012, 11, 180–190. [Google Scholar] [CrossRef]
- Lahtz, C.; Kim, S.I.; Bates, S.E.; Li, A.X.; Wu, X.; Pfeifer, G.P. UVB irradiation does not directly induce detectable changes of DNA methylation in human keratinocytes. F1000Research 2013, 2, 45. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, H.; Jiang, Q.; Gao, H.; Wang, C. Chemopreventive mechanism of polypeptides from Chlamy Farreri (PCF) against UVB-induced malignant transformation of HaCaT cells. Mutagenesis 2015, 30, 287–296. [Google Scholar] [CrossRef]
- Wang, D.; Huang, J.H.; Zeng, Q.H.; Gu, C.; Ding, S.; Lu, J.Y.; Chen, J.; Yang, S.B. Increased 5-hydroxymethylcytosine and Ten-eleven Translocation Protein Expression in Ultraviolet B-irradiated HaCaT Cells. Chin. Med. J. 2017, 130, 594–599. [Google Scholar] [CrossRef]
- Yamada, T.; Hasegawa, S.; Iwata, Y.; Arima, M.; Kobayashi, T.; Numata, S.; Nakata, S.; Sugiura, K.; Akamatsu, H. UV irradiation-induced DNA hypomethylation around WNT1 gene: Implications for solar lentigines. Exp. Dermatol. 2019, 28, 723–729. [Google Scholar] [CrossRef]
- Mittal, A.; Piyathilake, C.; Hara, Y.; Katiyar, S.K. Exceptionally high protection of photocarcinogenesis by topical application of (-)-epigallocatechin-3-gallate in hydrophilic cream in SKH-1 hairless mouse model: Relationship to inhibition of UVB-induced global DNA hypomethylation. Neoplasia 2003, 5, 555–565. [Google Scholar] [CrossRef]
- Yang, A.Y.; Lee, J.H.; Shu, L.; Zhang, C.; Su, Z.Y.; Lu, Y.; Huang, M.T.; Ramirez, C.; Pung, D.; Huang, Y.; et al. Genome-wide analysis of DNA methylation in UVB- and DMBA/TPA-induced mouse skin cancer models. Life Sci. 2014, 113, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Singh, T.; Katiyar, S.K. Honokiol inhibits ultraviolet radiation-induced immunosuppression through inhibition of ultraviolet-induced inflammation and DNA hypermethylation in mouse skin. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, R.; Sargsyan, D.; Yin, R.; Kuo, H.C.; Yang, I.; Wang, L.; Cheng, D.; Wang, C.; Li, S.; et al. UVB drives different stages of epigenome alterations during progression of skin cancer. Cancer Lett. 2019, 449, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yin, R.; Wu, R.; Ramirez, C.N.; Sargsyan, D.; Li, S.; Wang, L.; Cheng, D.; Wang, C.; Hudlikar, R.; et al. DNA methylome and transcriptome alterations and cancer prevention by triterpenoid ursolic acid in UVB-induced skin tumor in mice. Mol. Carcinog. 2019, 58, 1738–1753. [Google Scholar] [CrossRef]
- Grönniger, E.; Weber, B.; Heil, O.; Peters, N.; Stäb, F.; Wenck, H.; Korn, B.; Winnefeld, M.; Lyko, F. Aging and chronic sun exposure cause distinct epigenetic changes in human skin. PLoS Genet. 2010, 6, e1000971. [Google Scholar] [CrossRef]
- Gu, X.; Nylander, E.; Coates, P.J.; Fahraeus, R.; Nylander, K. Correlation between Reversal of DNA Methylation and Clinical Symptoms in Psoriatic Epidermis Following Narrow-Band UVB Phototherapy. J. Investig. Dermatol. 2015, 135, 2077–2083. [Google Scholar] [CrossRef]
- Vandiver, A.R.; Irizarry, R.A.; Hansen, K.D.; Garza, L.A.; Runarsson, A.; Li, X.; Chien, A.L.; Wang, T.S.; Leung, S.G.; Kang, S.; et al. Age and sun exposure-related widespread genomic blocks of hypomethylation in nonmalignant skin. Genome Biol. 2015, 16, 1–15. [Google Scholar] [CrossRef]
- Melo, A.R.D.S.; Barroso, H.; De Araújo, D.U.; Pereira, F.R.; De Oliveira, N.F.P. The influence of sun exposure on the DNA methylation status of MMP9, miR-137, KRT14 and KRT19 genes in human skin. Eur. J. Dermatol. 2015, 25, 436–443. [Google Scholar]
- Silva, M.B.D.; Melo, A.R.D.S.; Costa, L.D.A.; Barroso, H.; Oliveira, N.F.P.D. Global and gene-specific DNA methylation and hydroxymethylation in human skin exposed and not exposed to sun radiation. An. Bras. Dermatol. 2017, 92, 793–800. [Google Scholar] [CrossRef]
- Fitzpatrick, T.B. The validity and practicality of sun-reactive skin types I through VI. Arch. Dermatol. 1988, 124, 869–871. [Google Scholar] [CrossRef]
- Kader, F.; Ghai, M. DNA methylation-based variation between human populations. Mol. Genet. Genom. 2017, 292, 5–35. [Google Scholar] [CrossRef] [PubMed]
- Nwanaji-Enwerem, J.C.; Colicino, E. DNA Methylation-Based Biomarkers of Environmental Exposures for Human Population Studies. Curr. Environ. Health Rep. 2020, 7, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Wang, X.; Jin, P. Developing DNA methylation-based diagnostic biomarkers. Genet. Genom. 2018, 45, 87–97. [Google Scholar] [CrossRef]
- Mervis, J.S.; McGee, J.S. DNA methylation and inflammatory skin diseases. Arch. Dermatol. Res. 2020, 312, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, E.S.; Chong, K.K.; Huynh, K.T.; Tanaka, R.; Hoon, D.S. Epigenetic biomarkers in skin cancer. Cancer Lett. 2014, 342, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Paredes, M.; Bormann, F.; Raddatz, G.; Gutekunst, J.; Lucena-Porcel, C.; Köhler, F.; Wurzer, E.; Schmidt, K.; Gallinat, S.; Wenck, H.; et al. Methylation profiling identifies two subclasses of squamous cell carcinoma related to distinct cells of origin. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.D.; Issa, J.P.J. The promise of epigenetic therapy: Reprogramming the cancer epigenome. Curr. Opin. Genet. Dev. 2017, 42, 68–77. [Google Scholar] [CrossRef]
- Singer, S.; Berneburg, M.J. Phototherapy. Dtsch. Dermatol. Ges. 2018, 16, 1120–1129. [Google Scholar] [CrossRef]
- Werner, R.J.; Kelly, A.D.; Issa, J.P.J. Epigenetics and Precision Oncology. Cancer J. 2017, 23, 262–269. [Google Scholar] [CrossRef]
| Country, Year [Reference] | Study Design | Cell Lines | Groups | UV Radiation Treatment | Methylation Type | Technique | Outcome and Conclusion | Other Outcomes |
|---|---|---|---|---|---|---|---|---|
| India, 2006 [36] | Comparison of UVA and UVB-irradiated and non-irradiated cells | HaCaT and A431 (epidermoid carcinoma) | Irradiated group x non-irradiated group | 6 cycles of exposure to UVA (150–200 mJ/cm2) and UVB (15–20 mJ/cm2). Cells were cultured for 1–2 days before re-exposure to the same radiation dosage | Site-specific (p16, MGMT, DAP Kinase, GSTP1) | COBRA | Hypermethylation of p16 was observed on the irradiated group | |
| Germany, 2011 [37] | Comparison of UVA-irradiated and non-irradiated cells | HaCaT | Irradiated group x non-irradiated group | UVA exposure (200 kJ/m2) once a week for 10 to 15 weeks, with a six-day interval between irradiation events | Site-specific (p16) | qMSP | Hypermethylation of p16 (up to 70%) as well as ↓ of its transcripts on the irradiated group | |
| USA, 2013 [38] | Comparison of cells submitted to different doses of radiation, as well as different periods of UV exposure and cell recovery | NHEK | Negative controls (non-irradiated cells); positive low and high-dose controls (one-time radiation); cells exposed to low or high doses of radiation with an 8 or 18-day growth period | 10 cycles of low (130 J/m2) or high-dose (260 J/m2) UVB irradiation with 2–3 days of “cell recovery” between each cycle. After the final irradiation, cells were grown for 8 or 18 days | Global and site-specific (CXXC5, PPP3CB, L17C, CCDC40, C21orf29) | MIRA combined with microarray analysis; COBRA | No differences were detected in the DNA methylation profile of irradiated cells. Further studies are encouraged. | |
| China, 2015 [39] | Evaluation of PCF effect upon DNA methylation in UVB-irradiated cells | HaCaT | Non-irradiated group (control); irradiated group, no treatment x irradiated group + PCF or vitamin C | 20 chronic UVB exposure cycles (10 mJ/cm2 for 15 min per cycle) with 24–48h intervals | Site-specific (p16, RASSF1A) | MS-HRM | Hypermethylation of p16 and RASSF1A, ↓ transcript levels of both genes, and ↑ in transcript and protein levels of DNMT3B in irradiated cells | PCF provoked demethylation on the studied tumor suppressor genes and generated better effects than vitamin C |
| China, 2017 [40] | Comparison of UVB-irradiated and non-irradiated cells | HaCaT | Irradiated x non-irradiated cells | UVB exposure (40 mJ/cm2 or 80 mJ/cm2) for 24h | Global methylation and hydroxymethylation | IHC; IF | ↑ Global hydroxymethylation and ↑ transcript and protein levels of TETs 1, 2 and 3 in irradiated cells | |
| Japan, 2019 [41] | Comparison of UVB irradiated and non-irradiated cells, as well as between UV-exposed and non-exposed human facial regions | HDK1 and cells from human facial biopsies | Exposed group x non-exposed group | Exposure to different doses of UVB (10 or 100mJ/cm2) for two weeks (4× or less per week) for HDK1; regular sun exposure for facial samples | Global and site-specific (WNT1) | IHC; BS | Hypomethylation of WNT1 dose-dependent and ↑ transcript levels of WNT1 in irradiated cells. | Hypomethylation of WTN1; ↓ global methylation; ↓ DNMT1 levels in solar lentigines |
| Country, Year [Reference] | Study Design | Animal Model and Groups | Tissue or Cell Type | UV Radiation Treatment | Methylation Type | Technique | Outcome and Conclusion | Other Outcomes |
|---|---|---|---|---|---|---|---|---|
| USA, 2003 [42] | Effect of Epigallocatechin-3-gallate (EGCG) on the skin of mice submitted to a UV-induced carcinogenesis protocol | SKH-1 mice divided into 3 groups: non-irradiated (control), irradiated and irradiated + EGCG | Cells from dorsal skin biopsies (papillomas and carcinomas) and epidermal cells from the non-irradiated group | UVB (180 mJ/cm2); irradiation once a day for 10 days (tumor initiation); then 3× a week for 30 weeks (tumor promotion) | Global | IHC | Global hypomethylation and ↓ in DNMT1 activity on both irradiated groups | ECGC prevented global hypomethylation in the treated group |
| USA/South Coreia, 2014 [43] | Comparison of methylation profiles from skin tumors submitted to irradiation and DMBA/TPA-induced carcinogenesis | SKH-1 female mice from 7 to 8 weeks of age irradiated and non-irradiated (control) with UVB; CD-1 female mice of 6 weeks of age treated with DMBA/TPA | Cells from biopsies of dorsal skin tumors (papillomas and carcinomas); epidermal cells from the control group | UVB (280–320 nm; 70–80% of total energy); UVA (320–375 nm; 20–75% of the total energy). Mice were exposed to UV light (30 mJ/cm2) twice a week for 36 weeks | Whole-genome | MeDIP-Seq; IPA | Hypermethylation in 4140 genes and hypomethylation in 1863 from the UV group (sequences involved in the molecular mechanism of cancer were the most affected) | DMBA/TPA promoted an altered methylation profile when compared to controls. |
| USA, 2017 [44] | Effect of Honokiol (HK) on the skin’ methylation profile of both irradiated and non-irradiated | C3H/HeN female mice (5 to 6 weeks); 129 Ola/C57BL/6 mice, both COX-2 deficient and wild type. Groups were divided in non-irradiated (control), irradiated and irradiated + HK in two different concentrations (4% and 8%) | Skin biopsies from shaved dorsal region | Mice were irradiated with 150 mJ/cm2 of UVB radiation (280–320 nm; ≈ 80% of total energy) for 4 consecutive days and sacrificed 24 h after the last irradiation session | Global | IF; ELISA; Dot-blot analysis | Higher numbers of 5mC-positive cells; ↑ expression of Dnmt1, 3a and 3b, as well as Sp1 and 3; ↓ TET activity; ↑ global methylation levels in irradiated animals when compared to controls | Topical application of HK inhibited the UVB-induced formation of 5-mC-positive cells in a dose-dependent manner |
| USA, 2019 [45] | Analysis of methylation profile during different stages of radiation-induced carcinogenesis | SKH1 mice divided into two groups: non-irradiated (control) and submitted to UVB | Epidermal and tumor biopsies from different time points (2, 15 and 25 weeks); whole skin samples from the control group | UVB (60mJ/cm2), twice a week for 25 weeks. | Genome-wide, base resolution | MethylSeq; Pyrosequencing | Changes in 974 DMRs: 50% hypomethylated and 50% hypermethylated; methylation and expression are related in 60% of the DMRs (ex: Cdk4, Tgfbr2, Fgfr1, Bcl2l1, Pik3cb) in the epidermis of irradiated animals. | |
| USA, 2019 [46] | Analysis of effect of tripterpenoid ursolic acid (UA) upon the methylation profile of mice submitted to radiation-induced carcinogenesis | SKH-1 hairless mice divided into 3 groups: non-irradiated + acetone (control), irradiated + acetone and irradiated + UA | Epidermal cells from skin samples (control) and skin tumor cells from different time points (2, 15, and 25 weeks) | UVB (60mJ/cm2), twice a week for 25 weeks | Genome-wide, base resolution | MethylSeq; Pyrosequencing | Hypermethylation (Slco5a1, Ogfrl1, Bend6, Mgat4a, Creg2, Gm973, Slc4a3, Arl4c, Mlph, Twist2 Slco5a1, Ogfrl1, Bend6, Mgat4a, Creg2, Gm973, Slc4a3, Arl4c, Mlph, Twist2) and hypomethylation (Npbwr1, Plekhb2,Klf7, Mgat5, Ube2t, Phlda3, Kcnj9, Fbxo5, Plagl1,Myb); altered transcript levels in agreement with methylation analysis in irradiated animals; | Ursolic acid was able to reverse all observed methylation changes. |
| Country, Year [Reference] | Study Design | Groups | Tissue/Cell Lines | UV Radiation Exposition | Methylation Type | Technique | Outcome and Conclusion | Other Outcomes |
|---|---|---|---|---|---|---|---|---|
| Germany, 2010 [47] | Comparison of sun-exposed and non-exposed body areas of healthy individuals | 30 volunteers (10 suction blisters for male individuals; 20 punch biopsies for female individuals) | Dermal and epidermal cells from outer forearm and inner arm regions | Lifelong solar radiation | Genome-wide and site-specific | Human Methylation 27 BeadChip; BS (KRT75, SEC31L2, DDAH2, TET2) | Hypomethylation in chronically-exposed epidermis; hypomethylation of KRT5; ↑ expression levels of KRT5; hypermethylation of SEC31L2 | ≠ methylation profiles in male and female skin; age-related hypermethylation in 104 markers; hypermethylation of DDAH2 and TET2; ≠ methylation profile between dermis and epidermis |
| Sweden, 2015 [48] | Effect of phototherapy upon the skin methylation profile of patients with psoriasis | 24 individuals divided into 2 groups: psoriasis patients (n = 12) and healthy subjects (n = 12, control group) | Epidermis cells obtained from punch biopsies | Whole body narrow band (311–312 nm) UVB light irradiation in a cabinet equipped with fluorescent lamps (UVB TL100W/01, Philips); 24 sessions in for 2–3 months | Genome-scale | Human Methylation 450 | Hypomethylation on psoriasis group; after phototherapy, the methylation profile was similar to that of the healthy group. | Altering in the methylation profile was associated with a better response to treatment. |
| USA, 2015 [49] | Comparison of sun-exposed and non-exposed areas of the body in healthy and skin cancer patients | 36 individuals <35 and >60 years old divided into 2 groups: healthy subjects (n = 26) and squamous cell carcinoma (SCC) patients (n = 10) | Dermis and epidermis cells obtained by punch biopsies of the arm (upper inner arm) and face (outer forearm or lateral epicanthus) | Lifelong solar radiation | Genome-scale | Human Methylation 450; WGBS | Hypomethylation blocks throughout the genome in sun-exposed regions, mainly in older individuals. | Hypomethylation in sun-exposed regions of healthy volunteers was similar to the profile of individuals with SCC. |
| Brazil, 2015 [50] | Comparison of sun-exposed and non-exposed areas of the body | Corpses of both genders, over 30 years old and with healthy skin (n = 28) | Skin punch biopsies (dermis + epidermis) from the inner arm or outer forearm | Lifelong solar radiation | Site-specific | MSP (MMP9, miR-137, KRT14); MSRE (KRT19) | No difference was detected between exposed and non-exposed regions | Methylated profile of miR-137 seemingly more frequent in women |
| Brazil, 2017 [51] | Comparison of sun-exposed and non-exposed areas of the body | Corpses from both genders, ranging from 18–89 years old and with healthy skin (n = 24) | Skin punch biopsies (dermis + epidermis) from the inner arm or outer forearm | Lifelong solar radiation | Global and site-specific. | ELISA; MSP (miR-9-1, miR-9-3, MTHFR) | No difference was detected between exposed and non-exposed regions | global methylation levels > global hydroxymethylation levels |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, N.F.P.; de Souza, B.F.; de Castro Coêlho, M. UV Radiation and Its Relation to DNA Methylation in Epidermal Cells: A Review. Epigenomes 2020, 4, 23. https://doi.org/10.3390/epigenomes4040023
de Oliveira NFP, de Souza BF, de Castro Coêlho M. UV Radiation and Its Relation to DNA Methylation in Epidermal Cells: A Review. Epigenomes. 2020; 4(4):23. https://doi.org/10.3390/epigenomes4040023
Chicago/Turabian Stylede Oliveira, Naila Francis Paulo, Beatriz Fernandes de Souza, and Marina de Castro Coêlho. 2020. "UV Radiation and Its Relation to DNA Methylation in Epidermal Cells: A Review" Epigenomes 4, no. 4: 23. https://doi.org/10.3390/epigenomes4040023
APA Stylede Oliveira, N. F. P., de Souza, B. F., & de Castro Coêlho, M. (2020). UV Radiation and Its Relation to DNA Methylation in Epidermal Cells: A Review. Epigenomes, 4(4), 23. https://doi.org/10.3390/epigenomes4040023







