Chitin Assessment in Insect-Based Products from Reference Methods to Near-Infrared Models
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chitin Estimation
2.1.1. Insect Samples
2.1.2. Reference Analysis
2.2. Near-Infrared Spectroscopy
2.2.1. Spectra Collection
2.2.2. Preprocessing and Multivariate Analysis
3. Results
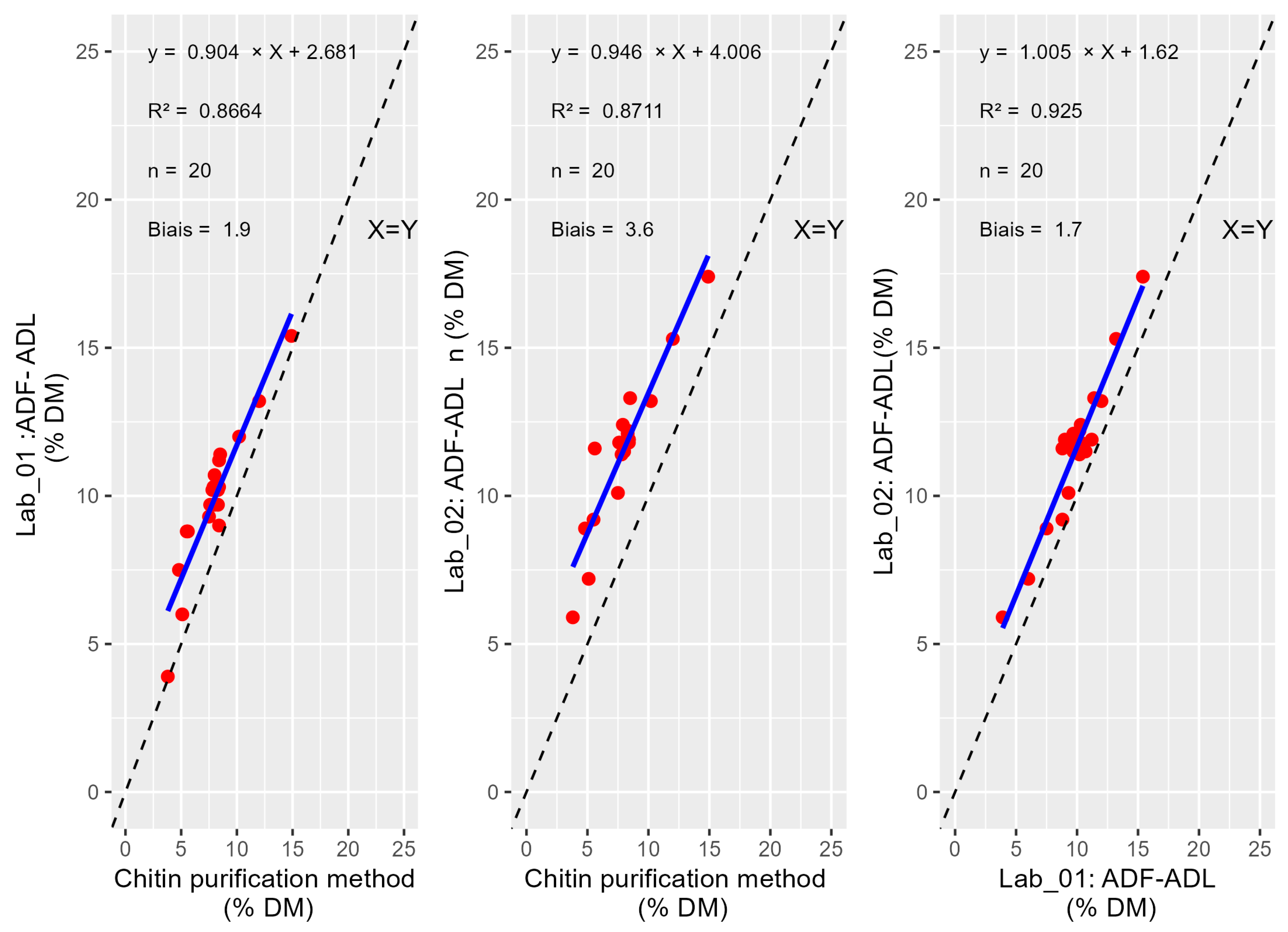
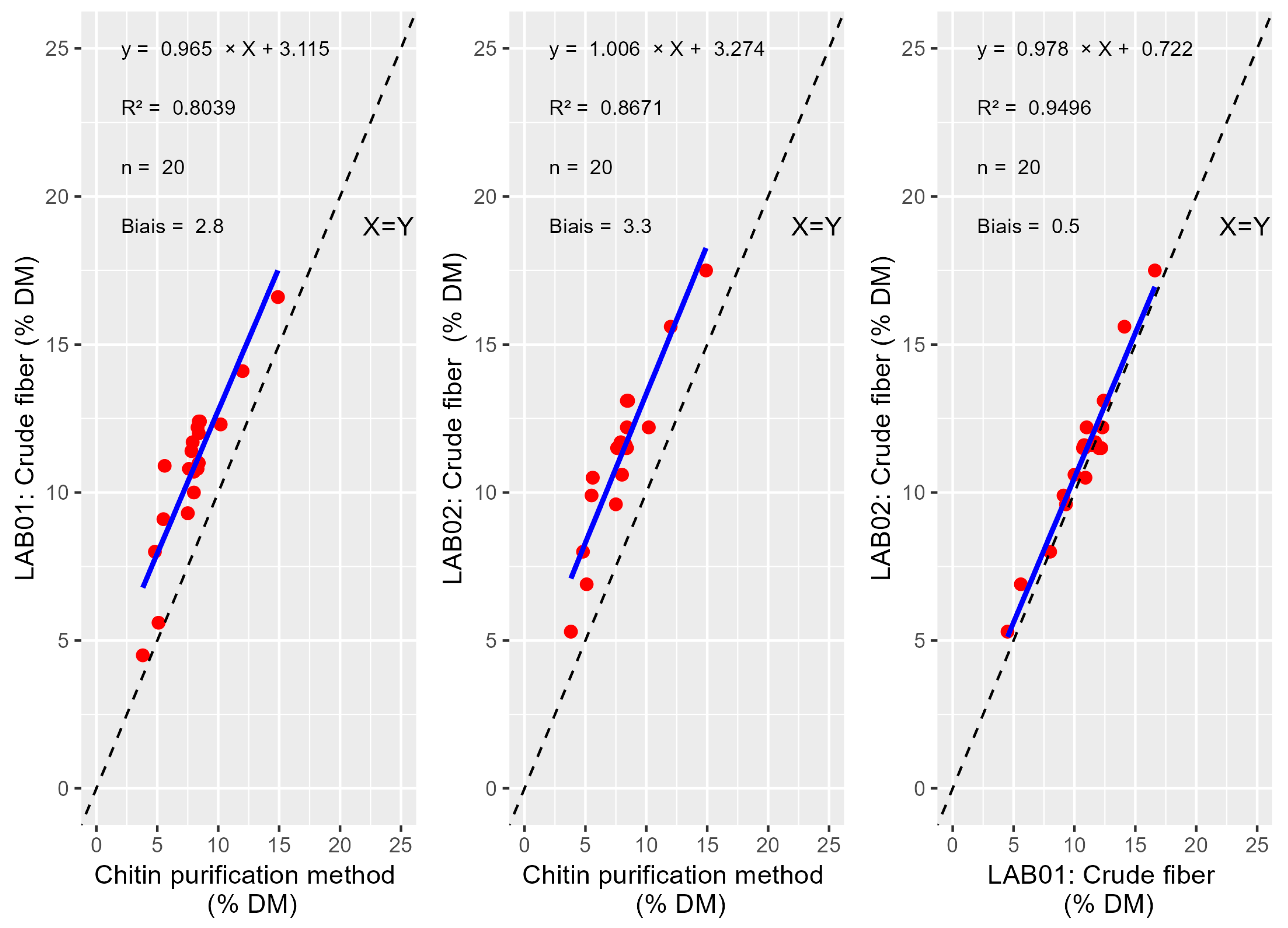
3.1. Chitin Estimation
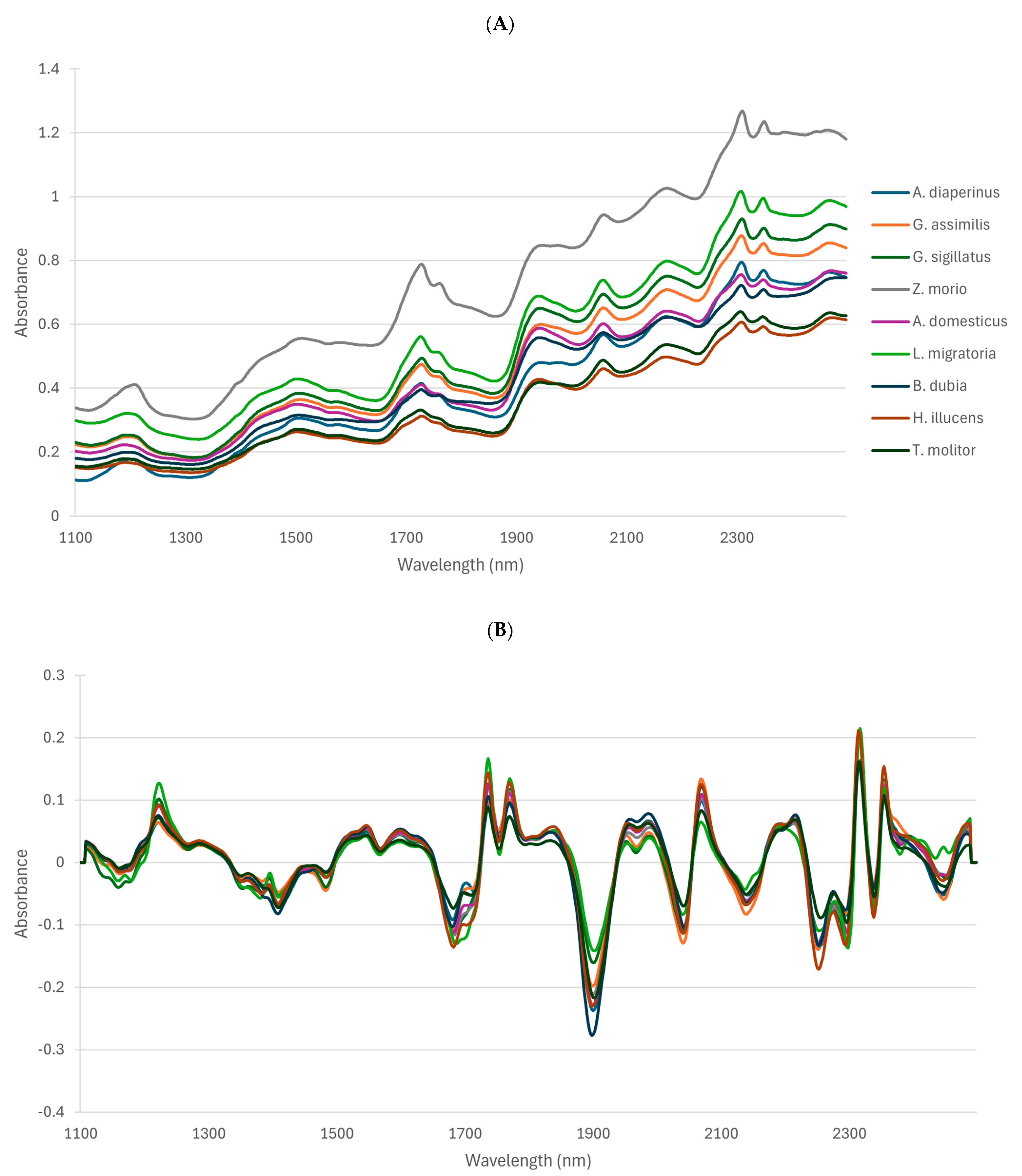
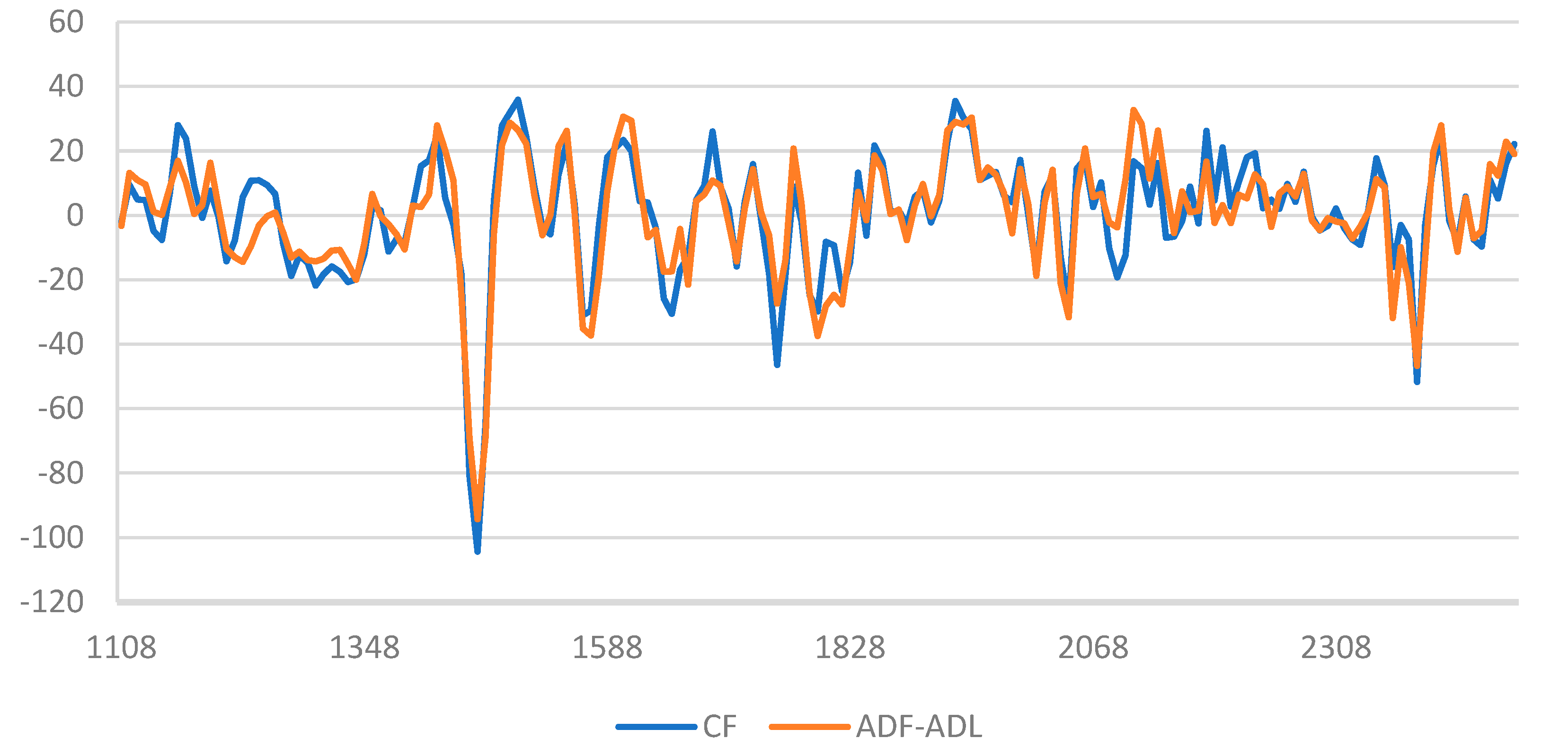
3.2. Near-Infrared Spectroscopy Analyses
3.2.1. NIRS Spectra
3.2.2. Calibration Results
4. Discussion
4.1. Chitin Estimation
4.2. Use of NIRS for Macronutrient Predictions
4.3. Implementation of NIRS in Industry
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hartmann, C.; Shi, J.; Giusto, A.; Siegrist, M. The psychology of eating insects: A cross-cultural comparison between Germany and China. Food Qual. Prefer. 2015, 44, 148–156. [Google Scholar] [CrossRef]
- Jensen, K.; Kristensen, T.N.; Heckmann, L.-H.L.; Sørensen, J.G. Breeding and maintaining high-quality insects. In Insects as Food and Feed: From Production to Consumption; Van Huis, A., Tomberlin, J.K., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2017; pp. 174–198. [Google Scholar] [CrossRef]
- Cadinu, L.A.; Barra, P.; Torre, F.; Delogu, F.; Madau, F.A. Insect Rearing: Potential, Challenges, and Circularity. Sustainability 2020, 12, 4567. [Google Scholar] [CrossRef]
- Guiné, R.P.F.; Correia, P.; Coelho, C.; Costa, C.A. The role of edible insects to mitigate challenges for sustainability. Open Agric. 2021, 6, 24–36. [Google Scholar] [CrossRef]
- Nogales-Mérida, S.; Gobbi, P.; Józefiak, D.; Mazurkiewicz, J.; Dudek, K.; Rawski, M.; Kierończyk, B.; Józefiak, A. Insect meals in fish nutrition. Rev. Aquac. 2019, 11, 1080–1103. [Google Scholar] [CrossRef]
- Kępińska-Pacelik, J.; Biel, W. Estimation of major nutrients in dry dog foods and their compliance with nutritional guidelines. Acta Sci. Pol. Zootech. 2021, 20, 35–46. [Google Scholar] [CrossRef]
- Anselmo, A.; Veys, P.; Stevens, F.; Fernández Pierna, J.A.; Michez, D.; Baeten, V. Insect meal as feed: Discrimination of particles issued from authorised and unauthorised species using Near Infrared Microscopy (NIRM). J. Insects Food Feed. 2024, 10, 1827–1839. [Google Scholar] [CrossRef]
- Sánchez-Muros, M.-J.; Barroso, F.G.; Manzano-Agugliaro, F. Insect meal as renewable source of food for animal feeding: A review. J. Clean. Prod. 2014, 65, 16–27. [Google Scholar] [CrossRef]
- Kröncke, N.; Benning, R. Determination of Moisture and Protein Content in Living Mealworm Larvae (Tenebrio molitor L.) Using Near-Infrared Reflectance Spectroscopy (NIRS). Insects 2022, 13, 560. [Google Scholar] [CrossRef]
- Canavoso, L.E.; Jouni, Z.E.; Karnas, K.J.; Pennington, J.E.; Wells, M.A. Fat metabolism in insects. Annu. Rev. Nutr. 2001, 21, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Kröncke, N.; Neumeister, M.; Benning, R. Near-infrared reflectance spectroscopy for quantitative analysis of fat and fatty acid content in living Tenebrio molitor larvae to detect the influence of substrate on larval composition. Insects 2023, 14, 114. [Google Scholar] [CrossRef] [PubMed]
- Finke, M.D. Estimate of Chitin in Raw Whole Insects. Zoo Biol. Publ. Affil. Am. Zoo Aquar. Assoc. 2007, 26, 105–115. [Google Scholar] [CrossRef]
- Eggink, K.M.; Dalsgaard, J. Chitin contents in different black soldier fly (Hermetia illucens) life stages. J. Insects Food Feed. 2023, 9, 855–864. [Google Scholar] [CrossRef]
- Henriques, B.S.; Garcia, E.S.; Azambuja, P.; Genta, F.A. Determination of chitin content in insects: An alternate method based on calcofluor staining. Front. Physiol. 2020, 11, 117. [Google Scholar] [CrossRef]
- Hahn, T.; Roth, A.; Febel, E.; Fijalkowska, M.; Schmitt, E.; Arsiwalla, T.; Zibek, S. New Methods for High-Accuracy Insect Chitin Measurement. J. Sci. Food Agric. 2018, 98, 5069–5073. [Google Scholar] [CrossRef] [PubMed]
- Kvasnicka, F.; Kourimska, L.; Bleha, R.; Skvorovab, P.; Kulma, M.; Rajchl, A. Electrophoretic determination of chitin in insects. J. Chromatogr. A 2023, 26, 463952. [Google Scholar] [CrossRef] [PubMed]
- Abbas, O.; Pissard, A.; Baeten, V. Near-infrared, mid-infrared and Raman spectroscopy. In Chemical Analysis of Food—Techniques and Applications, 2nd ed.; Pico, Y., Ed.; Academic Press: Amsterdam, The Netherlands, 2020; Chapter 3; pp. 77–134. [Google Scholar] [CrossRef]
- Kröncke, N.; Wittke, S.; Steinmann, N.; Benning, R. Analysis of the Composition of Different Instars of Tenebrio molitor Larvae using Near-Infrared Reflectance Spectroscopy for Prediction of Amino and Fatty Acid Content. Insects 2023, 14, 310. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EC) No 152/2009 of 27 January 2009 laying down the methods of sampling and analysis for the official control of feed. Off. J. Eur. Union 2009, L 54, 1–130. Available online: https://data.europa.eu/eli/reg/2009/152/oj (accessed on 20 August 2025).
- Janssen, R.H.; Vincken, J.-P.; van den Broek, L.A.M.; Fogliano, V.; Lakemond, C.M.M. Nitrogen-to-protein conversion factors for three edible insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef]
- Boulos, S.; Tännler, A.; Nyström, L. Nitrogen-to-protein conversion factors for edible insects on the Swiss market: T. molitor, A. domesticus, and L. migratoria. Front. Nutr. 2020, 7, 89. [Google Scholar] [CrossRef]
- Ritvanen, T.; Pastell, H.; Welling, A.; Raatikainen, M. The nitrogen-to-protein conversion factor of two cricket species—Acheta domesticus and Gryllus bimaculatus. Agric. Food Sci. 2020, 29, 1–5. [Google Scholar] [CrossRef]
- AFNOR. NF V 18-122: Aliments des Animaux—Détermination Séquentielle des Constituants Pariétaux—Méthode par Traitement aux Détergents Neutre et Acide et à L’acide Sulfurique; AFNOR Éditions: Saint-Denis, France, 2013; 13p. [Google Scholar]
- Saeys, W.; Mouazen, A.; Ramon, H. Potential for Onsite and Online Analysis of Pig Manure using Visible and Near Infrared Reflectance Spectroscopy. Biosyst. Eng. 2005, 91, 393–402. [Google Scholar] [CrossRef]
- Anselmo, A.; Pissard, A.; Vincke, D.; Arnould, Q.; Lecler, B.; Gofflot, S.; Michez, D.; Baeten, V. Contribution of vibrational spectroscopy to the characterisation and detection of insect meal in compound feed. Ital. J. Anim. Sci. 2025, 24, 761–771. [Google Scholar] [CrossRef]
- Song, H.S.; Lee, K.T.; Park, S.M.; Kang, O.J.; Cheong, H.S. Measurement of Deproteinization and Deacetylation of Chitin and Chitosan by Near Infrared Spectroscopy. Korean J. Fish. Aquat. Sci. 2003, 36, 88–93. [Google Scholar] [CrossRef][Green Version]
- Johnson, J.B. An overview of near-infrared spectroscopy (NIRS) for the detection of insect pests in stored grains. J. Stored Prod. Res. 2020, 86, 101558. [Google Scholar] [CrossRef]
- Benes, E.; Biró, B.; Fodor, M.; Gere, A. Analysis of wheat flour-insect powder mixtures based on their near infrared spectra. Food Chem. X 2022, 13, 100266. [Google Scholar] [CrossRef]
- Kim, J.; Kurniawan, H.; Faqeerzada, M.A.; Kim, G.; Lee, H.; Kim, M.S.; Baek, I.; Cho, B.K. Proximate Content Monitoring of Black Soldier Fly Larval (Hermetia illucens) Dry Matter for Feed Material using Short-Wave Infrared Hyperspectral Imaging. Food Sci. Anim. Resour. 2023, 43, 1150–1169. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Tirado, J.P.; Amigo, J.; Barbin, D. Determination of protein content in single black fly soldier (Hermetia illucens L.) larvae by near infrared hyperspectral imaging (NIR-HSI) and chemometrics. Food Control 2022, 143, 109266. [Google Scholar] [CrossRef]
- Chen, S.; Tsai, C.-C.; Chen, R.; Yang, I.-C.; Hsu, S.H.-Y.; Chen, C.-T.; Yang, C.-W. Deacetylation of Chitinous Materials Using Near Infrared Spectroscopy. Eng. Agric. Environ. Food 2008, 1, 338–345. [Google Scholar] [CrossRef]
- Pinotti, L.; Ottoboni, M. Substrate as insect feed for bio-mass production. J. Insects Food Feed. 2021, 7, 585–596. [Google Scholar] [CrossRef]
- Mendez-Sanchez, C.; Güell, M.C.; Ferrando, M.; Rodriguez-Saona, L.; Jimenez-Flores, R.; Domingo, J.C.; de Lamo Castellvi, S. Prediction of fat content in edible insect powders using handheld FT-IR spectroscopic devices. LWT 2024, 207, 116652. [Google Scholar] [CrossRef]
- Riu, J.; Vega, A.; Boqué, R.; Giussani, B. Exploring the Analytical Complexities in Insect Powder Analysis Using Miniaturized NIR Spectroscopy. Foods 2022, 11, 3524. [Google Scholar] [CrossRef]
- Alagappan, S.; Hoffman, L.; Mikkelsen, D.; Mantilla, S.O.; James, P.; Yarger, O.; Cozzolino, D. Near-infrared spectroscopy (NIRS) for monitoring the nutritional composition of black soldier fly larvae (BSFL) and frass. J. Sci. Food Agric. 2024, 104, 1487–1496. [Google Scholar] [CrossRef]
- Brigode, C.; Hobbi, P.; Jafari, H.; Verwilghen, F.; Baeten, E.; Shavandi, A. Isolation and physicochemical properties of chitin polymer from insect farm side stream as a new source of renewable biopolymer. J. Clean. Prod. 2020, 275, 122924. [Google Scholar] [CrossRef]
- Son, Y.-J.; Hwang, I.-K.; Nho, C.W.; Kim, S.M.; Kim, S.H. Determination of Carbohydrate Composition in Mealworm (Tenebrio molitor L.) Larvae and Characterization of Mealworm Chitin and Chitosan. Foods 2021, 10, 640. [Google Scholar] [CrossRef]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Salvia, R.; Scieuzo, C.; Hahn, T.; Zibek, S.; Gagliardini, A.; Panariello, L.; Coltelli, M.B.; et al. Characterization of chitin and chitosan derived from Hermetia illucens, a further step in a circular economy process. Sci. Rep. 2022, 12, 6613. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Kuzhir, P.; Godeau, G. Update on chitin and chitosan from insects: Sources, production, characterization, and biomedical applications. Biomimetics 2024, 9, 297. [Google Scholar] [CrossRef] [PubMed]
- Fearn, T. Standardisation and calibration transfer for near infrared instruments: A review. J. Near Infrared Spectrosc. 2001, 9, 229–244. [Google Scholar] [CrossRef]
- Dardenne, P. Calibration transfer in near infrared spectroscopy. NIR News 2002, 13, 3–7. [Google Scholar] [CrossRef]
- Feudale, R.N.; Woody, N.A.; Tan, H.; Myles, A.J.; Brown, S.D.; Ferré, J. Transfer of multivariate calibration models: A review. Chemometr. Intell. Lab. Syst. 2002, 64, 181–192. [Google Scholar] [CrossRef]
- Ni, D.; Nelis, J.; Dawson, A.; Bourne, N.; Juliano, P.; Colgrave, M.; Juhász, A.; Bose, U. Application of near-infrared spectroscopy and chemometrics for the rapid detection of insect protein adulteration from a simulated matrix. Food Control 2024, 159, 110268. [Google Scholar] [CrossRef]
- Pissard, A.; Marques, E.; Dardenne, P.; Lateur, M.; Pasquini, C.; Pimentel, M.; Fernández Pierna, J.; Baeten, V. Evaluation of a handheld ultra-compact NIR spectrometer for rapid and non-destructive determination of apple fruit quality. Postharvest Biol. Technol. 2021, 172, 111375. [Google Scholar] [CrossRef]
| Reference Method | Lab_01 | Lab_02 | |||
|---|---|---|---|---|---|
| Tenebrio molitor Sample | Chitin (% DM) | ADF-ADL (% DM) | CF (% DM) | ADF-ADL (% DM) | CF (% DM) |
| Sample_01 | 5.6 | 8.8 | 10.9 | 11.6 | 10.5 |
| Sample_02 | 8.5 | 11.4 | 12.4 | 13.3 | 13.1 |
| Sample_03 | 8.3 | 9.7 | 12.2 | 12.1 | 11.5 |
| Sample_04 | 8.4 | 10.3 | 12.0 | 11.8 | 11.5 |
| Sample_05 | 8.3 | 10.2 | 10.8 | 12.2 | 11.6 |
| Sample_06 | 7.9 | 10.3 | 11.7 | 12.4 | 11.7 |
| Sample_07 | 7.6 | 9.7 | 10.8 | 11.8 | 11.5 |
| Sample_08 | 7.8 | 10.2 | 11.4 | 11.4 | 11.6 |
| Sample_09 | 8.0 | 9.7 | 10.7 | 11.5 | 11.5 |
| Sample_10 | 8.4 | 9.0 | 11.0 | 11.9 | 12.2 |
| Sample_11 | 3.8 | 3.9 | 4.5 | 5.9 | 5.3 |
| Sample_12 | 5.1 | 6.0 | 5.6 | 7.2 | 6.9 |
| Sample_13 | 7.5 | 9.3 | 9.3 | 10.1 | 9.6 |
| Sample_14 | 10.2 | 12.0 | 12.3 | 13.2 | 12.2 |
| Sample_15 | 12.0 | 13.2 | 14.1 | 15.3 | 15.6 |
| Sample_16 | 14.9 | 15.4 | 16.6 | 17.4 | 17.5 |
| Sample_17 | 8.4 | 11.2 | 12.4 | 11.9 | 13.1 |
| Sample_18 | 4.8 | 7.5 | 8.0 | 8.9 | 8.0 |
| Sample_19 | 8.0 | 10.7 | 10.0 | 11.5 | 10.6 |
| Sample_20 | 5.5 | 8.8 | 9.1 | 9.2 | 9.9 |
| n | 20 | 20 | 20 | 20 | 20 |
| Mean | 8.0 | 9.9 | 10.8 | 11.5 | 11.3 |
| Min | 3.8 | 3.9 | 4.5 | 5.9 | 5.3 |
| Max | 14.9 | 15.4 | 16.6 | 17.4 | 17.5 |
| Range | 11.1 | 11.5 | 12.1 | 11.5 | 12.2 |
| Reference Method | ADF-ADL_Lab_01 | CF_Lab_01 | ADF-ADL_Lab_02 | CF-Lab_02 | |
| Reference method | x | 0.5985 | 0.6276 | 0.2778 | 0.6059 |
| ADF-ADL_lab_01 | x | 0.9781 | 0.5554 | 0.6191 | |
| CF_Lab_01 | x | 0.0927 | 0.3613 | ||
| ADF-ADL_Lab_02 | x | 0.3665 | |||
| CF-Lab_02 | x |
| Reference Method | ADF-ADL_Lab_01 | CF_Lab_01 | ADF-ADL_Lab_02 | CF-Lab_02 | |
| Reference method | x | 1.556 × 10−8 | 1.871 × 10−9 | 3.737 × 10−13 | 4.762 × 10−12 |
| ADF-ADL_lab_01 | x | 6.469 × 10−5 | 1.607 × 10−9 | 1.685 × 10−7 | |
| CF_Lab_01 | x | 2.183 × 10−5 | 2.220 × 10−3 | ||
| ADF-ADL_Lab_02 | x | 6.632 × 10−2 | |||
| CF-Lab_02 | x |
| Reference Method | Lab_01 | Lab_02 | |||
|---|---|---|---|---|---|
| Species | Chitin (% DM) | ADF-ADL (%DM) | CF (%DM) | ADF-ADL (%DM) | CF (%DM) |
| Hermetia illucens | 5.4 | 8.6 | 7.4 | 8.3 | 6.9 |
| Gryllus assimilis | 5.8 | 7.9 | 10.1 | 10.8 | 10.1 |
| Calibration Set | Validation Set | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Moist (%) | Prot (%) | Fat (%) | Cell (%) | ADF-ADL (%) | Moist (%) | Prot (%) | Fat (%) | Cell (%) | ADF-ADL (%) | |
| N | 100 | 128 | 88 | 100 | 100 | 26 | 26 | 20 | 26 | 26 |
| Mean | 4.75 | 59.90 | 16.05 | 8.25 | 8.20 | 4.70 | 59.21 | 18.22 | 9.39 | 9.24 |
| Minimum | 1.50 | 16.59 | 1.56 | 0.1 | 0.09 | 1.70 | 47.38 | 4.56 | 5.98 | 5.2 |
| Maximum | 10.31 | 81.90 | 40.96 | 17.05 | 16.96 | 8.28 | 74.07 | 33.99 | 14.06 | 14.97 |
| SD | 1.93 | 12.10 | 9.77 | 3.72 | 3.82 | 1.74 | 8.63 | 8.08 | 2.52 | 2.79 |
| Constituent | Pretreatment | N | SD | LVs | SEC | R2c | SECV | R2cv | RPDcv |
|---|---|---|---|---|---|---|---|---|---|
| Moist (%) | None | 89 | 1.81 | 7 | 0.41 | 0.95 | 0.48 | 0.93 | 3.8 |
| D1 | 87 | 1.85 | 8 | 0.34 | 0.96 | 0.48 | 0.93 | 3.9 | |
| SNVD-D1 | 87 | 1.86 | 8 | 0.18 | 0.99 | 0.39 | 0.95 | 4.8 | |
| Prot (%) | None | 115 | 8.98 | 6 | 2.17 | 0.94 | 2.23 | 0.94 | 4.0 |
| D1 | 116 | 9.65 | 6 | 1.78 | 0.96 | 2.39 | 0.94 | 4.0 | |
| SNVD-D1 | 120 | 10.96 | 8 | 1.20 | 0.99 | 1.59 | 0.98 | 6.9 | |
| Fat (%) | None | 83 | 8.86 | 8 | 1.07 | 0.98 | 1.26 | 0.98 | 7.0 |
| D1 | 81 | 8.68 | 5 | 0.82 | 0.99 | 1.00 | 0.99 | 8.7 | |
| SNVD-D1 | 80 | 8.68 | 6 | 0.78 | 0.99 | 1.00 | 0.99 | 8.7 | |
| Cell (%) | None | 95 | 3.46 | 8 | 1.67 | 0.77 | 1.89 | 0.70 | 1.8 |
| D1 | 97 | 3.57 | 8 | 0.92 | 0.93 | 1.23 | 0.88 | 2.9 | |
| SNVD-D1 | 97 | 3.63 | 8 | 0.82 | 0.95 | 1.22 | 0.88 | 3.0 | |
| ADF-ADL (%) | None | 92 | 3.50 | 8 | 1.55 | 0.80 | 2.10 | 0.66 | 1.7 |
| D1 | 92 | 3.55 | 8 | 0.91 | 0.93 | 1.38 | 0.85 | 2.6 | |
| SNVD-D1 | 93 | 3.64 | 8 | 0.80 | 0.95 | 1.13 | 0.90 | 3.2 |
| N Val | SD | SEP | R2p | RPDp | |
|---|---|---|---|---|---|
| Moist (%) | 26 | 1.74 | 0.19 | 0.99 | 9.2 |
| Prot (%) | 26 | 8.63 | 1.53 | 0.97 | 5.6 |
| Fat (%) | 20 | 8.08 | 1.44 | 0.97 | 5.6 |
| Cell (%) | 26 | 2.52 | 1.03 | 0.83 | 2.4 |
| ADF-ADL (%) | 26 | 2.79 | 1.32 | 0.77 | 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pissard, A.; Gofflot, S.; Baeten, V.; Lecler, B.; Lorrette, B.; Morin, J.-F.; Debode, F. Chitin Assessment in Insect-Based Products from Reference Methods to Near-Infrared Models. Insects 2025, 16, 924. https://doi.org/10.3390/insects16090924
Pissard A, Gofflot S, Baeten V, Lecler B, Lorrette B, Morin J-F, Debode F. Chitin Assessment in Insect-Based Products from Reference Methods to Near-Infrared Models. Insects. 2025; 16(9):924. https://doi.org/10.3390/insects16090924
Chicago/Turabian StylePissard, Audrey, Sébastien Gofflot, Vincent Baeten, Bernard Lecler, Bénédicte Lorrette, Jean-François Morin, and Frederic Debode. 2025. "Chitin Assessment in Insect-Based Products from Reference Methods to Near-Infrared Models" Insects 16, no. 9: 924. https://doi.org/10.3390/insects16090924
APA StylePissard, A., Gofflot, S., Baeten, V., Lecler, B., Lorrette, B., Morin, J.-F., & Debode, F. (2025). Chitin Assessment in Insect-Based Products from Reference Methods to Near-Infrared Models. Insects, 16(9), 924. https://doi.org/10.3390/insects16090924







