Sardine Inclusion in a Food Waste-Based Substrate for Rearing Black Soldier Fly (Hermetia illucens) Larvae: Effects on Growth Performance, Body Composition, and Gut Microbiome
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Substrate
2.2. Feeding Trial and Experimental Conditions
2.3. Larval Growth Measurement and Sample Processing
2.4. Proximate Composition
2.5. Fatty Acid and Amino Acid Composition
2.6. Microbiome Analysis
2.7. Statistical Analyses
3. Results
3.1. Nutrient Composition of the Substrate
3.2. Growth Performance and Substrate Utilization
3.3. BSFL Proximate Composition
3.4. BSFL Fatty Acid Composition
3.5. BSFL Amino Acid Profile
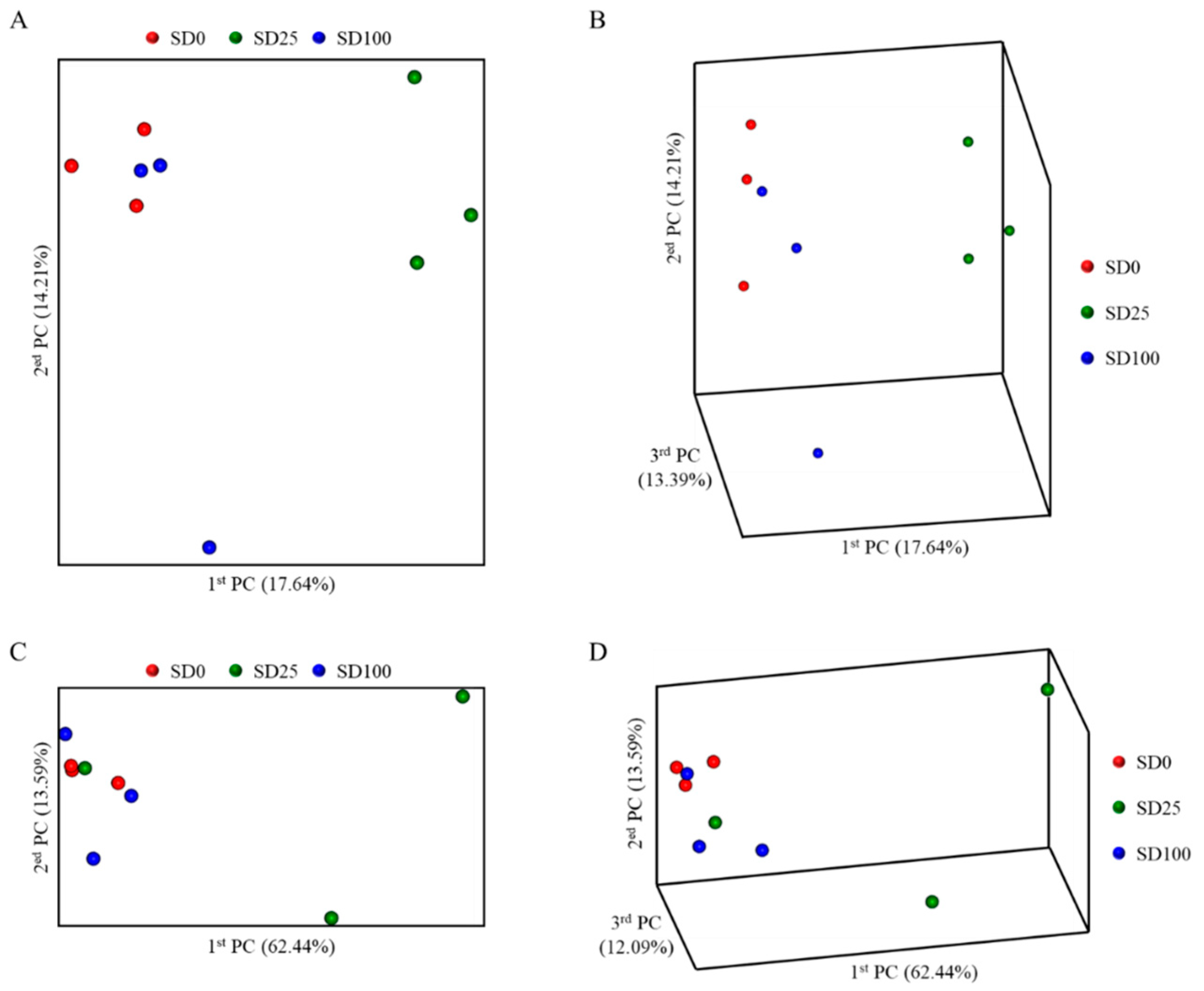
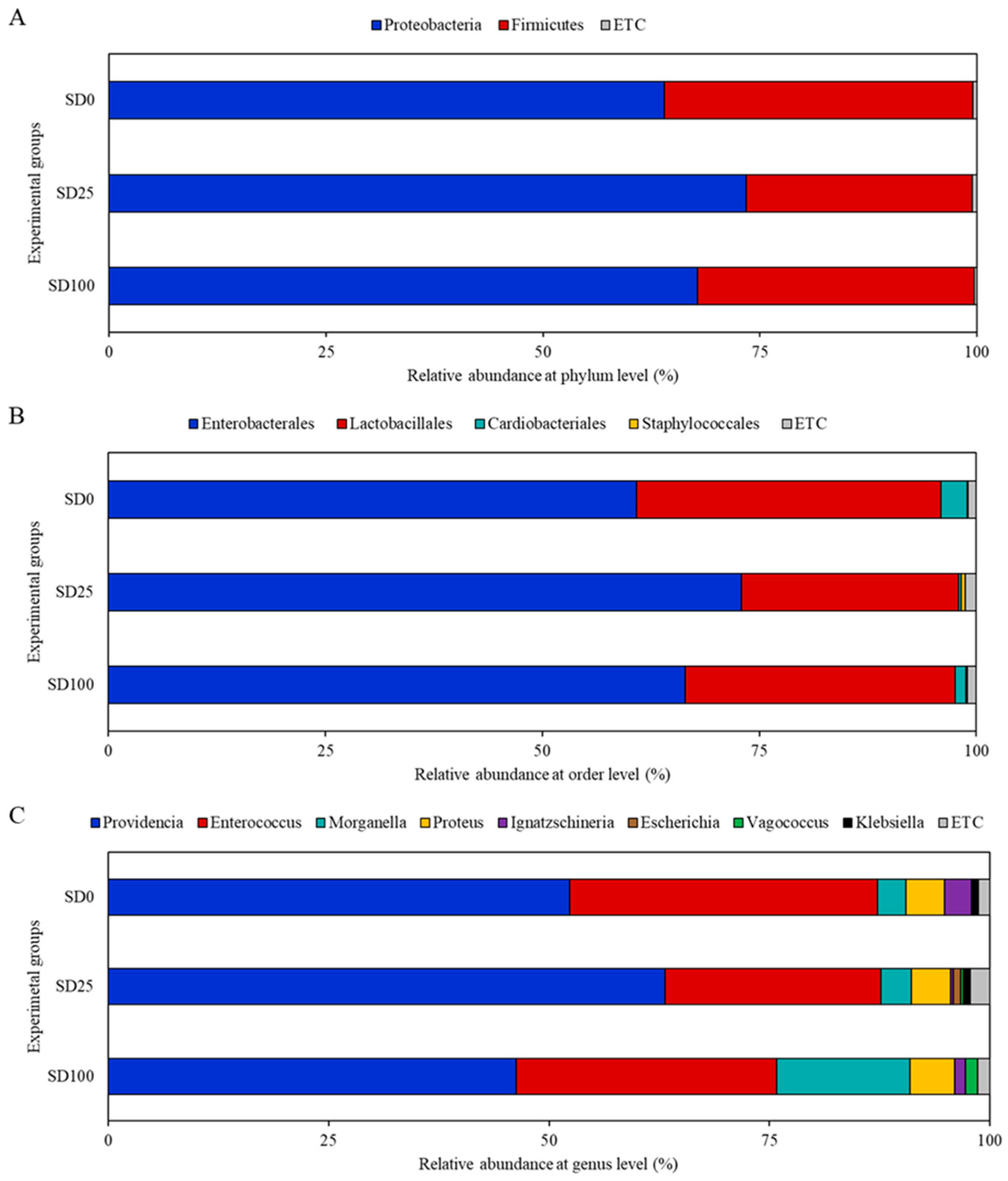
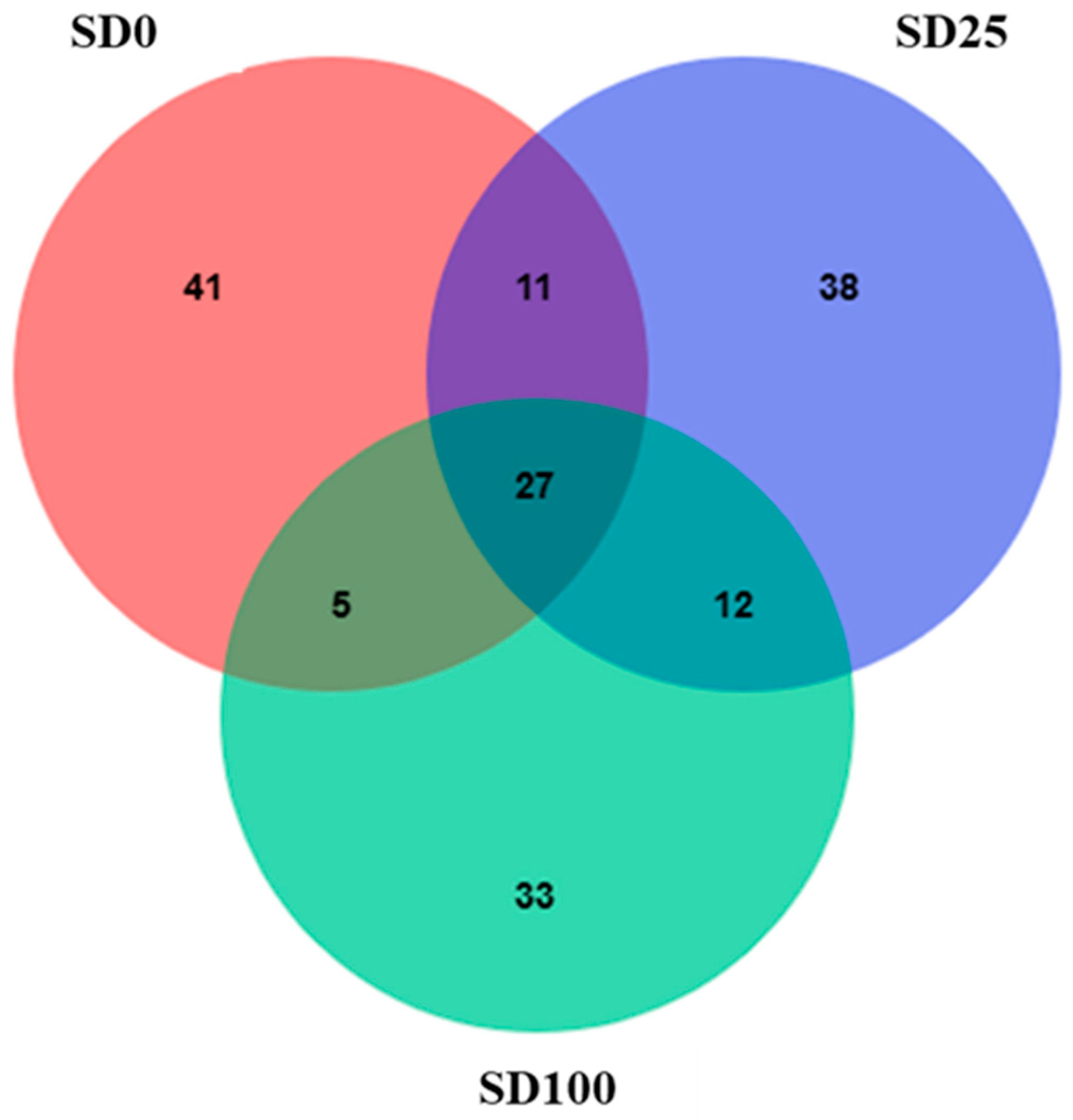
3.6. Microbiome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.L.; Low, P.J.; Ellis, J.R.; Reynolds, J.D. Climate change and distribution shifts in marine fishes. Science 2005, 308, 1912–1915. [Google Scholar] [CrossRef]
- Kim, J.G.; Kim, J.G. Changes in climate factors and catches of fisheries in the Republic of Korea over the three decades. Water 2023, 15, 1952. [Google Scholar] [CrossRef]
- KOSIS (Korean Statistical Information Service). Statistic Database for Fisheries Production. 2024. Available online: https://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1EW0004&vw_cd=MT_ZTITLE&list_id=K2_7&scrId=&seqNo=&lang_mode=ko&obj_var_id=&itm_id=&conn_path=MT_ZTITLE&path=%252FstatisticsList%252FstatisticsListIndex.do (accessed on 29 November 2024).
- Capanoglu, E.; Nemli, E.; Tomas-Barberan, F. Novel approaches in the valorization of agricultural wastes and their applications. J. Agric. Food Chem. 2022, 70, 6787–6804. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Towards the Future we Want: End Hunger and Make the Transition to Sustainable Agricultural and Food Systems; Policy paper for the Rio+ 20 Conference; Food and Agriculture Organization of the United Nations: Rome, Italy, 2012; pp. 13–22. [Google Scholar]
- Slorach, P.C.; Jeswani, H.K.; Cuéllar-Franca, R.; Azapagic, A. Environmental and economic implications of recovering resources from food waste in a circular economy. Sci. Total Environ. 2019, 693, 133516. [Google Scholar] [CrossRef]
- Guan, J.; Zhuang, H.; Lau, C.Y.; Leng, L.; Yeung, C.S.; Vuppaladadiyam, A.K.; Wang, H.; Tse, H.Y.; Leu, S.Y. Energy and carbon footprint analysis of municipal wastewater treatment process integrated with food waste disposer. J. Clean. Prod. 2022, 375, 134063. [Google Scholar] [CrossRef]
- Prepilková, V.; Poništ, J.; Schwarz, M.; Samešová, D. Challenges and opportunities for kitchen waste treatment—A review. Environ. Rev. 2023, 31, 632–642. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, C.; He, Z.; Li, Y.; Qin, Y.; Li, Y.Y. Stoichiometry and energy assessment comparison between thermophilic and mesophilic high-solid anaerobic co-digestion of lipids and food waste. Chem. Eng. J. 2023, 459, 141551. [Google Scholar] [CrossRef]
- Wang, Y.; Quan, J.; Cheng, X.; Li, C.; Yuan, Z. Relationship of black soldier fly larvae (BSFL) gut microbiota and bioconversion efficiency with properties of substrates. Waste Manag. 2024, 180, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Li, X.; Shimizu, N. Synthesis evaluation on thermophilic anaerobic co-digestion of tomato plant residue with cattle manure and food waste. Resour. Environ. Sustain. 2023, 13, 100119. [Google Scholar] [CrossRef]
- Zhang, S.; Liang, C.; Xiao, M.; Chui, C.; Wang, N.; Ji, Y.; Wang, Z.; Shi, J.; Liu, L. Metagenomic characterization of the enhanced performance of multicomponent synergistic thermophilic anaerobic co-digestion of food waste utilizing kitchen waste or garden waste as co-substrate. Water Res. 2023, 244, 120457. [Google Scholar] [CrossRef] [PubMed]
- Čičková, H.; Newton, G.L.; Lacy, R.C.; Kozánek, M. The use of fly larvae for organic waste treatment. Waste Manag. 2015, 35, 68–80. [Google Scholar] [CrossRef] [PubMed]
- James, M.T. The genus Hermetia in the United States (Diptera: Stratiomyidae). Bull. Brooklyn Entomol. Soc. 1935, 30, 165–170. [Google Scholar]
- Callan, E.M. Hermetia illucens (L.) (Diptera: Stratiomyidae), (a cosmopolitan American species long established in Australia and New Zealand). Entomol. S Mon. Mag. 1974, 109, 232–234. [Google Scholar]
- Sheppard, D.C.; Tomberlin, J.K.; Joyce, J.A.; Kiser, B.C.; Sumner, S.M. Rearing methods for the black soldier fly (Diptera: Stratiomyidae). J. Med. Entomol. 2002, 39, 695–698. [Google Scholar] [CrossRef]
- Wang, Y.S.; Shelomi, M. Review of black soldier fly (Hermetia illucens) as Animal Feed and Human Food. Foods 2017, 6, 91. [Google Scholar] [CrossRef]
- St-Hilaire, S.; Cranfill, K.; McGuire, M.A.; Mosley, E.E.; Tomberlin, J.K.; Newton, L.; Sealey, W.; Sheppard, C.; Irving, S. Fish offal recycling by the black soldier fly produces a foodstuff high in omega-3 fatty acids. J. World Aquacult. Soc. 2007, 38, 309–313. [Google Scholar] [CrossRef]
- Belghit, I.; Liland, N.S.; Gjesdal, P.; Biancarosa, I.; Menchetti, E.; Li, Y.; Waagbø, R.; Krogdahl, Å.; Lock, E.J. Black soldier fly larvae meal can replace fish meal in diets of sea-water phase Atlantic salmon (Salmo salar). Aquaculture 2019, 503, 609–619. [Google Scholar] [CrossRef]
- Magalhães, R.; Sánchez-López, A.; Leal, R.S.; Martínez-Llorens, S.; Oliva-Teles, A.; Peres, H. Black soldier fly (Hermetia illucens) pre-pupae meal as a fish meal replacement in diets for European seabass (Dicentrarchus labrax). Aquaculture 2017, 476, 79–85. [Google Scholar] [CrossRef]
- Oteri, M.; Di Rosa, A.R.; Lo Presti, V.; Giarratana, F.; Toscano, G.; Chiofalo, B. Black soldier fly larvae meal as alternative to fish meal for aquaculture feed. Sustainability 2021, 13, 5447. [Google Scholar] [CrossRef]
- Takakuwa, F.; Tanabe, R.; Nomura, S.; Inui, T.; Yamada, S.; Biswas, A.; Tanaka, H. Availability of black soldier fly meal as an alternative protein source to fish meal in red sea bream (Pagrus major, Temminck & Schlegel) fingerling diets. Aquacult. Res. 2022, 53, 36–49. [Google Scholar] [CrossRef]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef] [PubMed]
- Sealey, W.M.; Gaylord, T.G.; Barrows, F.T.; Tomberlin, J.K.; McGuire, M.A.; Ross, C.; St-Hilaire, S. Sensory analysis of rainbow trout, Oncorhynchus mykiss, fed enriched black soldier fly prepupae, Hermetia illucens. J. World Aquacult. Soc. 2011, 42, 34–45. [Google Scholar] [CrossRef]
- Fonseca-Madrigal, J.; Karalazos, V.; Campbell, P.J.; Bell, J.G.; Tocher, D.R. Influence of dietary palm oil on growth, tissue fatty acid compositions, and fatty acid metabolism in liver and intestine in rainbow trout (Oncorhynchus mykiss). Aquacult. Nutr. 2005, 11, 241–250. [Google Scholar] [CrossRef]
- Kim, E.; Lee, S.M. Effect of different dietary composition of linoleic acid, eicosapentaenoic acid and docosahexaenoic acid on the growth and fatty acid profile of olive flounder Paralichthys olivaceus. Korean J. Fish. Aquat. Sci. 2019, 52, 49–58. [Google Scholar] [CrossRef]
- Kim, K.D.; Lee, S.M. Requirement of dietary n-3 highly unsaturated fatty acids for juvenile flounder (Paralichthys olivaceus). Aquaculture 2004, 229, 315–323. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, J.H.; Kim, K.D. Effect of dietary essential fatty acids on growth, body composition and blood chemistry of juvenile starry flounder (Platichthys stellatus). Aquaculture 2003, 225, 269–281. [Google Scholar] [CrossRef]
- Ruyter, B.M.S. Essential fatty acids in Atlantic salmon: Effects of increasing dietary doses of n-6 and n-3 fatty acids on growth, survival and fatty acid composition of liver, blood and carcass. Aquacult. Nutr. 2000, 6, 119–127. [Google Scholar] [CrossRef]
- Webster, C.D.; Lovell, R.T. Response of striped bass larvae fed brine shrimp from different sources containing different fatty acid compositions. Aquaculture 1990, 90, 49–61. [Google Scholar] [CrossRef]
- Arena, R.; Manuguerra, S.; Curcuraci, E.; Cusimano, M.; Lo Monaco, D.; Di Bella, C.; Santulli, A.; Messina, C.M. Fisheries and aquaculture by-products modulate growth, body composition, and omega-3 polyunsaturated fatty acid content in black soldier fly (Hermetia illucens) larvae. Front. Anim. Sci. 2023, 4, 1204767. [Google Scholar] [CrossRef]
- Scala, A.; Cammack, J.A.; Salvia, R.; Scieuzo, C.; Franco, A.; Bufo, S.A.; Tomberlin, J.K.; Falabella, P. Rearing substrate impacts growth and macronutrient composition of Hermetia illucens (L.) (Diptera: Stratiomyidae) larvae produced at an industrial scale. Sci. Rep. 2020, 10, 19448. [Google Scholar] [CrossRef]
- Rehman, K.U.; Ur Rehman, R.U.; Somroo, A.A.; Cai, M.; Zheng, L.; Xiao, X.; Ur Rehman, A.; Rehman, A.; Tomberlin, J.K.; Yu, Z.; et al. Enhanced bioconversion of dairy and chicken manure by the interaction of exogenous bacteria and black soldier fly larvae. J. Environ. Manag. 2019, 237, 75–83. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 17th ed.; Association of Official Analytical Chemists Incorporated: Washington, DC, USA, 2003. [Google Scholar]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Pamintuan, K.R.S.; Cajayon, J.A.B.; Dableo, G.B. Growth Characteristics and Lipid Content of Black Soldier Fly (Hermetia illucens) Larva Reared in milkfish Offal and Mixed Vegetable Wastes. In Proceedings of the 2019 6th International Conference on Biomedical and Bioinformatics Engineering, Shanghai, China, 13–15 November 2019; ACM: New York, NY, USA, 2019; pp. 163–168. [Google Scholar] [CrossRef]
- Eggink, K.M.; Lund, I.; Pedersen, P.B.; Hansen, B.W.; Dalsgaard, J. Biowaste and by-products as rearing substrates for black soldier fly (Hermetia illucens) larvae: Effects on larval body composition and performance. PLoS ONE 2022, 17, e0275213. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Danieli, P.P.; Lussiana, C.; Gasco, L.; Amici, A.; Ronchi, B. The effects of diet formulation on the yield, proximate composition, and fatty acid profile of the black soldier fly (Hermetia illucens L.) prepupae intended for animal feed. Animals 2019, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Cammack, J.A.; Tomberlin, J.K. The impact of diet protein and carbohydrate on select life-history traits of the black soldier fly Hermetia illucens (L.) (Diptera: Stratiomyidae). Insects 2017, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, J.C.; Pak, D. Feasibility of producing ethanol from food waste. Waste Manag. 2011, 31, 2121–2125. [Google Scholar] [CrossRef] [PubMed]
- Banks, I.J. To Assess the Impact of Black Soldier Fly (Hermetia Illucens) Larvae on Faecal Reduction in Pit Latrines. Ph.D. Thesis, London School of Hygiene & Tropical Medicine, London, UK, 2014. [Google Scholar] [CrossRef]
- Kim, W.T.; Bae, S.W.; Park, H.C.; Park, K.H.; Lee, S.B.; Choi, Y.C.; Han, S.M.; Koh, Y.H. The larval age and mouth morphology of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). Int. J. Ind. Entomol. Biomater. 2010, 21, 185–187. [Google Scholar]
- Meneguz, M.; Schiavone, A.; Gai, F.; Dama, A.; Lussiana, C.; Renna, M.; Gasco, L. Effect of rearing substrate on growth performance, waste reduction efficiency and chemical composition of black soldier fly (Hermetia illucens) larvae. J. Sci. Food Agric. 2018, 98, 5776–5784. [Google Scholar] [CrossRef]
- Purkayastha, D.; Sarkar, S.; Roy, P.; Kazmi, A.A. Isolation and morphological study of ecologically important insect “Hermetia illucens” collected from Roorkee compost plant. Pollution 2017, 3, 453–459. [Google Scholar] [CrossRef]
- Leroi, F.; Joffraud, J.J. Microbial degradation of seafood. In Aquaculture Microbiology and Biotechnology; Montet, D., Ray, R.C., Eds.; CRC Press: Boca Raton, FL, USA, 2011; Volume 2, pp. 47–72. [Google Scholar] [CrossRef]
- Hungerford, J.M. Scombroid poisoning: A review. Toxicon 2010, 56, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Lopes, I.G.; Lalander, C.; Vidotti, R.M.; Vinnerås, B. Using Hermetia illucens larvae to process biowaste from aquaculture production. J. Clean. Prod. 2020, 251, 119753. [Google Scholar] [CrossRef]
- Ewald, N.; Vidakovic, A.; Langeland, M.; Kiessling, A.; Sampels, S.; Lalander, C. Fatty acid composition of black soldier fly larvae (Hermetia illucens)—Possibilities and limitations for modification through diet. Waste Manag. 2020, 102, 40–47. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Wang, H.; Yang, Q.; Ur Rehman, K.; Li, W.; Cai, M.; Li, Q.; Mazza, L.; Zhang, J.; et al. Dynamic changes of nutrient composition throughout the entire life cycle of black soldier fly. PLoS ONE 2017, 12, e0182601. [Google Scholar] [CrossRef]
- Inagaki, S.; Yamashita, O. Metabolic shift from lipogenesis to glycogenesis in the last instar larval fat body of the silkworm, Bombyx mori. Insect Biochem. 1986, 16, 327–331. [Google Scholar] [CrossRef]
- Li, X.; Dong, Y.; Sun, Q.; Tan, X.; You, C.; Huang, Y.; Zhou, M. Growth and fatty acid composition of black soldier fly Hermetia illucens (Diptera: Stratiomyidae) larvae are influenced by dietary fat sources and levels. Animals 2022, 12, 486. [Google Scholar] [CrossRef]
- Zhu, Z.; Rehman, K.U.; Yu, Y.; Liu, X.; Wang, H.; Tomberlin, J.K.; Sze, S.H.; Cai, M.; Zhang, J.; Yu, Z.; et al. De novo transcriptome sequencing and analysis revealed the molecular basis of rapid fat accumulation by black soldier fly (Hermetia illucens, L.) for development of insectival biodiesel. Biotechnol. Biofuels 2019, 12, 194. [Google Scholar] [CrossRef]
- Barragan-Fonseca, K.B.; Gort, G.; Dicke, M.; van Loon, J.J.A. Effects of dietary protein and carbohydrate on life-history traits and body protein and fat contents of the black soldier fly Hermetia illucens. Physiol. Entomol. 2019, 44, 148–159. [Google Scholar] [CrossRef]
- Matthäus, B.; Piofczyk, T.; Katz, H.; Pudel, F. Renewable resources from insects: Exploitation, properties, and refining of fat obtained by cold-pressing from Hermetia illucens (Black Soldier Fly) larvae. Eur. J. Lipid Sci. Technol. 2019, 121, 1800376. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Ramos-Bueno, R.P.; González-Fernández, M.J.; Fabrikov, D.; Sánchez-Muros, M.J.; Barroso, F.G. Insects as food: Fatty acid profiles, lipid classes, and sn-2 fatty acid distribution of lepidoptera larvae. Eur. J. Lipid Sci. Technol. 2018, 120, 1700391. [Google Scholar] [CrossRef]
- Dayrit, F.M. The properties of lauric acid and their significance in coconut oil. J. Am. Oil Chem. Soc. 2015, 92, 1–15. [Google Scholar] [CrossRef]
- Benito-Vicente, A.; Jebari-Benslaiman, S.; Galicia-Garcia, U.; Larrea-Sebal, A.; Uribe, K.B.; Martin, C. Molecular mechanisms of lipotoxicity-induced pancreatic β-cell dysfunction. Int. Rev. Cell Mol. Biol. 2021, 359, 357–402. [Google Scholar] [CrossRef]
- Keith, A.D. Fatty acid metabolism in Drosophila melanogaster: Interaction between dietary fatty acids and de novo synthesis. Comp. Biochem. Physiol. 1967, 21, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.L.; Dillwith, J.W.; Ryan, R.O.; Blomquist, G.J.; Reitz, R.C. Characterization of the acyl-CoA desaturase in the housefly, Musca domestica L. Insect Biochem. 1982, 12, 545–551. [Google Scholar] [CrossRef]
- Zeng, J.M.; Ye, W.F.; Noman, A.; Machado, R.A.R.; Lou, Y.G. The desaturase gene family is crucially required for fatty acid metabolism and survival of the brown planthopper, Nilaparvata lugens. Int. J. Mol. Sci. 2019, 20, 1369. [Google Scholar] [CrossRef]
- Hoc, B.; Genva, M.; Fauconnier, M.L.; Lognay, G.; Francis, F.; Caparros Megido, R. About lipid metabolism in Hermetia illucens (L. 1758): On the origin of fatty acids in prepupae. Sci. Rep. 2020, 10, 11916. [Google Scholar] [CrossRef]
- Renna, M.; Gasco, L.; Livorsi, L.; Mele, M.; Conte, G.; Meneguz, M.; Lussiana, C. Growth performance, proximate composition and fatty acid profile of black soldier fly larvae reared on two grape pomace varieties. Animal 2024, 18, 101240. [Google Scholar] [CrossRef] [PubMed]
- Barroso, F.G.; Sánchez-Muros, M.J.; Rincón, M.Á.; Rodriguez-Rodriguez, M.; Fabrikov, D.; Morote, E.; Guil-Guerrero, J.L. Production of n-3-rich insects by bioaccumulation of fishery waste. J. Food Compos. Anal. 2019, 82, 103237. [Google Scholar] [CrossRef]
- Rodrigues, D.P.; Calado, R.; Pinho, M.; Rosário Domingues, M.; Antonio Vázquez, J.; Ameixa, O.M.C.C. Bioconversion and performance of Black Soldier Fly (Hermetia illucens) in the recovery of nutrients from expired fish feeds. Waste Manag. 2022, 141, 183–193. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; Finke, M.D. Nutritional value of insects and ways to manipulate their composition. J. Insects Food Feed 2021, 7, 639–659. [Google Scholar] [CrossRef]
- Huis, A.V.; Itterbeeck, J.V.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Verkerk, M.C.; Tramper, J.; Van Trijp, J.C.M.; Martens, D.E. Insect cells for human food. Biotechnol. Adv. 2007, 25, 198–202. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Gold, M.; Von Allmen, F.; Zurbrügg, C.; Zhang, J.; Mathys, A. Identification of bacteria in two food waste black soldier fly larvae rearing residues. Front. Microbiol. 2020, 11, 582867. [Google Scholar] [CrossRef]
- Ao, Y.; Yang, C.; Wang, S.; Hu, Q.; Yi, L.; Zhang, J.; Yu, Z.; Cai, M.; Yu, C. Characteristics and nutrient function of intestinal bacterial communities in black soldier fly (Hermetia illucens L.) larvae in livestock manure conversion. J. Microbiol. Biotechnol. 2021, 14, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shen, J.; Wang, H.; Liu, M.; Wu, L.; Ping, F.; He, Q.; Li, H.; Zheng, C.; Xu, X. Attenuation of veterinary antibiotics in full-scale vermicomposting of swine manure via the housefly larvae (Musca domestica). Sci. Rep. 2014, 4, 6844. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Hu, R.; Zhang, K.; Ma, S.; Zheng, L.; Yu, Z.; Zhang, J. Resistance of black soldier fly (Diptera: Stratiomyidae) larvae to combined heavy metals and potential application in municipal sewage sludge treatment. Environ. Sci. Pollut. Res. Int. 2018, 25, 1559–1567. [Google Scholar] [CrossRef]
- Cai, M.; Ma, S.; Hu, R.; Tomberlin, J.K.; Thomashow, L.S.; Zheng, L.; Li, W.; Yu, Z.; Zhang, J. Rapidly mitigating antibiotic resistant risks in chicken manure by Hermetia illucens bioconversion with intestinal microflora. Environ. Microbiol. 2018, 20, 4051–4062. [Google Scholar] [CrossRef]
- Cai, M.; Ma, S.; Hu, R.; Tomberlin, J.K.; Yu, C.; Huang, Y.; Zhan, S.; Li, W.; Zheng, L.; Yu, Z.; et al. Systematic characterization and proposed pathway of tetracycline degradation in solid waste treatment by Hermetia illucens with intestinal microbiota. Environ. Pollut. 2018, 242, 634–642. [Google Scholar] [CrossRef]
- Li, X.Y.; Mei, C.; Luo, X.Y.; Wulamu, D.; Zhan, S.; Huang, Y.P.; Yang, H. Dynamics of the intestinal bacterial community in black soldier fly larval guts and its influence on insect growth and development. Insect Sci. 2023, 30, 947–963. [Google Scholar] [CrossRef]
- Silvaraju, S.; Zhang, Q.H.; Kittelmann, S.; Puniamoorthy, N. Genetics, age, and diet influence gut bacterial communities and performance of black soldier fly larvae (Hermetia illucens). Anim. Microbiome 2024, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yao, H.; Wang, C. Black soldier fly larvae can effectively degrade oxytetracycline bacterial residue by means of the gut bacterial community. Front. Microbiol. 2021, 12, 663972. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Sun, M.; Zhang, J.; Lei, A.; Chen, H.; Kang, X.; Ni, H.; Yang, S. Comparative metagenomic and metatranscriptomic analyses reveal the response of black soldier fly (Hermetia illucens) larvae intestinal microbes and reduction mechanisms to high concentrations of tetracycline. Toxics 2023, 11, 611. [Google Scholar] [CrossRef] [PubMed]
| FW | SD | |
|---|---|---|
| Proximate composition | ||
| Moisture | 84.81 | 69.72 |
| Crude protein | 4.57 | 15.90 |
| Crude lipid | 5.09 | 15.43 |
| Ash | 1.66 | 3.54 |
| Crude fiber | 3.49 | 0.15 |
| Major fatty acid composition | ||
| C14:0 | 1.56 | 8.92 |
| C16:0 | 20.98 | 20.89 |
| C18:0 | 8.43 | 4.46 |
| C16:1 | 2.21 | 11.21 |
| C18:1n-9 | 32.81 | 11.81 |
| C18:2n-6 | 24.83 | 3.12 |
| C18:3n-3 | 3.83 | 1.48 |
| C20:5n-3 (EPA) | 0.99 | 15.08 |
| C22:6n-3 (DHA) | 1.68 | 8.35 |
| Amino acid composition | ||
| Arginine | 3.79 | 3.38 |
| Histidine | 1.78 | 1.86 |
| Isoleucine | 3.58 | 5.22 |
| Leucine | 7.12 | 7.30 |
| Lysine | 6.86 | 8.81 |
| Methionine + Cystine | 3.79 | 4.89 |
| Phenylalanine | 3.03 | 2.50 |
| Threonine | 3.64 | 3.00 |
| Valine | 6.59 | 6.55 |
| SR 1 | DR 2 | IGT 3 | LTB 4 | BR 5 | LL 6 | LH 7 | |
|---|---|---|---|---|---|---|---|
| SD0 | 89.6 ± 0.9 | 86.0 ± 0.4 a | 93.3 ± 1.0 a | 1.12 ± 0.01 a | 13.2 ± 0.1 a | 28.0 ± 0.5 a | 5.88 ± 0.02 a |
| SD25 | 85.3 ± 1.1 | 79.4 ± 0.5 b | 88.9 ± 1.1 ab | 1.07 ± 0.01 ab | 12.5 ± 0.2 ab | 26.7 ± 0.3 a | 5.82 ± 0.04 a |
| SD50 | 82.7 ± 1.1 | 73.1 ± 1.0 c | 86.1 ± 1.1 b | 1.03 ± 0.01 b | 12.2 ± 0.2 b | 27.4 ± 0.2 a | 5.92 ± 0.02 a |
| SD75 | 80.0 ± 3.1 | 69.7 ± 1.0 d | 66.7 ± 2.5 c | 0.80 ± 0.03 c | 9.4 ± 0.4 c | 23.6 ± 0.2 b | 4.88 ± 0.02 b |
| SD100 | 84.4 ± 2.9 | 63.3 ± 0.2 e | 42.2 ± 0.6 d | 0.51 ± 0.01 d | 6.0 ± 0.1 d | 20.3 ± 0.2 c | 3.77 ± 0.07 c |
| Moisture | Crude Protein | Crude Lipid | Ash | |
|---|---|---|---|---|
| SD0 | 4.16 ± 0.11 | 39.6 ± 0.2 c | 34.8 ± 0.1 ab | 10.22 ± 0.34 a |
| SD25 | 4.68 ± 0.20 | 40.8 ± 0.4 bc | 33.1 ± 0.5 ab | 9.59 ± 0.11 a |
| SD50 | 4.97 ± 0.44 | 40.6 ± 0.4 bc | 31.0 ± 0.3 b | 9.79 ± 0.06 a |
| SD75 | 5.79 ± 0.06 | 43.2 ± 0.6 a | 32.6 ± 0.6 ab | 8.32 ± 0.20 b |
| SD100 | 5.63 ± 0.08 | 41.8 ± 0.2 ab | 36.4 ± 0.1 a | 5.85 ± 0.03 c |
| SD0 | SD25 | SD50 | SD75 | SD100 | |
|---|---|---|---|---|---|
| C10:0 | 2.49 ± 0.04 a | 2.52 ± 0.16 a | 2.59 ± 0.13 a | 2.12 ± 0.03 ab | 1.93 ± 0.10 b |
| C12:0 | 51.2 ± 0.2 a | 46.3 ± 2.1 ab | 44.2 ± 0.2 ab | 41.9 ± 1.5 ab | 34.8 ± 0.4 b |
| C14:0 | 6.16 ± 0.08 c | 7.05 ± 0.16 b | 7.19 ± 0.09 b | 8.06 ± 0.04 a | 8.30 ± 0.13 a |
| C16:0 | 11.9 ± 0.2 b | 13.1 ± 0.4 ab | 12.6 ± 0.1 ab | 13.7 ± 0.4 b | 15.2 ± 0.2 a |
| C18:0 | 1.92 ± 0.03 b | 2.11 ± 0.04 ab | 2.02 ± 0.04 ab | 2.24 ± 0.18 ab | 2.95 ± 0.12 a |
| C16:1 | 1.80 ± 0.15 e | 3.82 ± 0.32 d | 5.50 ± 0.10 c | 7.59 ± 0.22 b | 9.81 ± 0.04 a |
| C18:1n-9 | 11.9 ± 0.1 | 11.7 ± 0.4 | 10.5 ± 0.1 | 10.7 ± 0.6 | 11.8 ± 0.4 |
| C18:2n-6 | 10.4 ± 0.02 a | 8.51 ± 0.29 ab | 6.99 ± 0.05 ab | 5.46 ± 0.18 ab | 3.98 ± 0.06 b |
| C18:3n-3 | 1.34 ± 0.02 | 1.01 ± 0.09 | 1.05 ± 0.01 | 0.97 ± 0.03 | 1.01 ± 0.02 |
| C20:5n-3 | 0.73 ± 0.01 d | 2.27 ± 0.15 c | 3.32 ± 0.08 b | 3.54 ± 0.15 b | 4.87 ± 0.16 a |
| C22:6n-3 | n.d. b | 0.21 ± 0.02 ab | 0.61 ± 0.09 ab | 0.39 ± 0.02 ab | 0.97 ± 0.11 a |
| EPA + DHA | 0.72 ± 0.01 d | 2.48 ± 0.14 c | 3.93 ± 0.17 b | 3.93 ± 0.16 b | 5.84 ± 0.26 a |
| ∑SFA | 73.6 ± 0.0 a | 71.9 ± 1.5 ab | 70.2 ± 0.1 ab | 69.9 ± 1.0 ab | 65.9 ± 0.6 b |
| ∑MUFA | 13.7 ± 0.0 b | 15.7 ± 0.8 ab | 16.7 ± 0.0 ab | 18.5 ± 0.8 ab | 22.4 ± 0.4 a |
| ∑n-3 PUFA | 2.07 ± 0.03 d | 3.50 ± 0.23 c | 4.98 ± 0.16 b | 4.90 ± 0.19 b | 6.85 ± 0.24 a |
| ∑n-6 PUFA | 10.39 ± 0.02 a | 8.57 ± 0.32 ab | 7.24 ± 0.06 ab | 5.97 ± 0.11 ab | 4.67 ± 0.09 b |
| SD0 | SD25 | SD50 | SD75 | SD100 | |
|---|---|---|---|---|---|
| Arginine | 5.23 ± 0.10 | 5.21 ± 0.11 | 5.23 ± 0.12 | 5.26 ± 0.31 | 5.01 ± 0.03 |
| Histidine | 3.61 ± 0.33 | 3.72 ± 0.08 | 3.47 ± 0.04 | 3.97 ± 0.14 | 3.38 ± 0.10 |
| Isoleucine | 4.70 ± 0.04 | 4.47 ± 0.08 | 4.69 ± 0.01 | 4.62 ± 0.08 | 4.63 ± 0.01 |
| Leucine | 7.12 ± 0.11 | 7.17 ± 0.05 | 7.26 ± 0.14 | 7.33 ± 0.10 | 6.97 ± 0.04 |
| Lysine | 6.19 ± 0.03 ab | 6.33 ± 0.02 a | 6.19 ± 0.09 ab | 6.37 ± 0.12 a | 5.86 ± 0.06 b |
| Methionine + Cystine | 2.17 ± 0.07 | 2.06 ± 0.10 | 2.05 ± 0.24 | 2.04 ± 0.22 | 1.73 ± 0.26 |
| Phenylalanine | 4.68 ± 0.15 | 4.29 ± 0.09 | 4.34 ± 0.08 | 4.15 ± 0.28 | 3.92 ± 0.04 |
| Threonine | 3.80 ± 0.06 ab | 3.92 ± 0.02 a | 3.85 ± 0.10 a | 3.68 ± 0.09 ab | 3.51 ± 0.02 b |
| Valine | 6.65 ± 0.04 | 6.37 ± 0.03 | 6.76 ± 0.13 | 6.67 ± 0.16 | 6.48 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.-M.; Min, B.H.; Hur, S.W.; Bae, J.; Park, K.H.; Kim, K.W. Sardine Inclusion in a Food Waste-Based Substrate for Rearing Black Soldier Fly (Hermetia illucens) Larvae: Effects on Growth Performance, Body Composition, and Gut Microbiome. Insects 2025, 16, 977. https://doi.org/10.3390/insects16090977
Jeong S-M, Min BH, Hur SW, Bae J, Park KH, Kim KW. Sardine Inclusion in a Food Waste-Based Substrate for Rearing Black Soldier Fly (Hermetia illucens) Larvae: Effects on Growth Performance, Body Composition, and Gut Microbiome. Insects. 2025; 16(9):977. https://doi.org/10.3390/insects16090977
Chicago/Turabian StyleJeong, Seong-Mok, Byung Hwa Min, Sang Woo Hur, Jinho Bae, Ki Hwan Park, and Kang Woong Kim. 2025. "Sardine Inclusion in a Food Waste-Based Substrate for Rearing Black Soldier Fly (Hermetia illucens) Larvae: Effects on Growth Performance, Body Composition, and Gut Microbiome" Insects 16, no. 9: 977. https://doi.org/10.3390/insects16090977
APA StyleJeong, S.-M., Min, B. H., Hur, S. W., Bae, J., Park, K. H., & Kim, K. W. (2025). Sardine Inclusion in a Food Waste-Based Substrate for Rearing Black Soldier Fly (Hermetia illucens) Larvae: Effects on Growth Performance, Body Composition, and Gut Microbiome. Insects, 16(9), 977. https://doi.org/10.3390/insects16090977






