Black Soldier Fly Larvae as a Novel Protein Feed Resource Promoting Circular Economy in Agriculture
Simple Summary
Abstract
1. Introduction
2. Waste Nutritional Profile and Comparative Advantages of BSFL
2.1. BSFL-Derived High-Value Feed: From Organic Substrates to Sustainable Nutrition
| Processing Matrix | KM | CM |
|---|---|---|
| Moisture | 78.41 ± 0.42 | \ |
| CP | 33.0 b ± 1.0 | 41.1 a ± 0.3 |
| EE | 34.3 b ± 0.4 | 30.1 a ± 0.4 |
| Ash | 9.6 ± 1.6 | 9.3 ± 1.8 |
| OM | \ | 59.8 a ± 0.4 |
| Ca | 2.0 b ± 1.41 | 3.2 a ± 2.32 |
| P | 4.1 ± 0.33 | 3.9 ± 0.31 |
| Ca:P | 8.3 | 5.2 |
| k | 5.7 b ± 0.04 | 4.9 a* ± 0.08 |
| Ma | 3.3 b ± 0.06 | 4.0 a ± 0.34 |
| Na | 2.0 ± 0.09 | 2.4 ± 0.12 |
| Fe | 2.2 b ± 0.00 | 0.6 a ± 0.43 |
| Cu | 0.2 a ± 0.00 | 0.4 a ± 0.00 |
| Isoleucine | 2.6 ± 4.5 | 1.6 ± 1.5 |
| Leucine | 2.9 ± 5.2 | 3.0 ± 5.2 |
| phenylalanine | 4.6 b ± 4.7 | 1.9 a ± 2.4 |
| Lysine | 4.7 ± 0.5 | 4.1 ± 0.6 |
| Methionine | 7.9 ± 0.8 | 6.1 ± 0.8 |
| Lauric acid | 7.1 a* ± 1.0 | 7.4 a ± 9.0 |
| Linoleic acid | 7.5 ± 0.1 | 5.8 ± 0.3 |
| Linolenic acid | 5.5 ab ± 0.5 | 5.6 a ± 0.1 |
| Arachidonic acid | 5.7 b ± 0.1 | 6.7 a ± 0.3 |
| References | [16,26] | [16] |
2.2. Comparative Analysis of Nutritional Characteristics and Composition in BSFL Under Different Substrate Conditions
2.3. Nutritional Composition Comparison Between BSFL and Other Insects
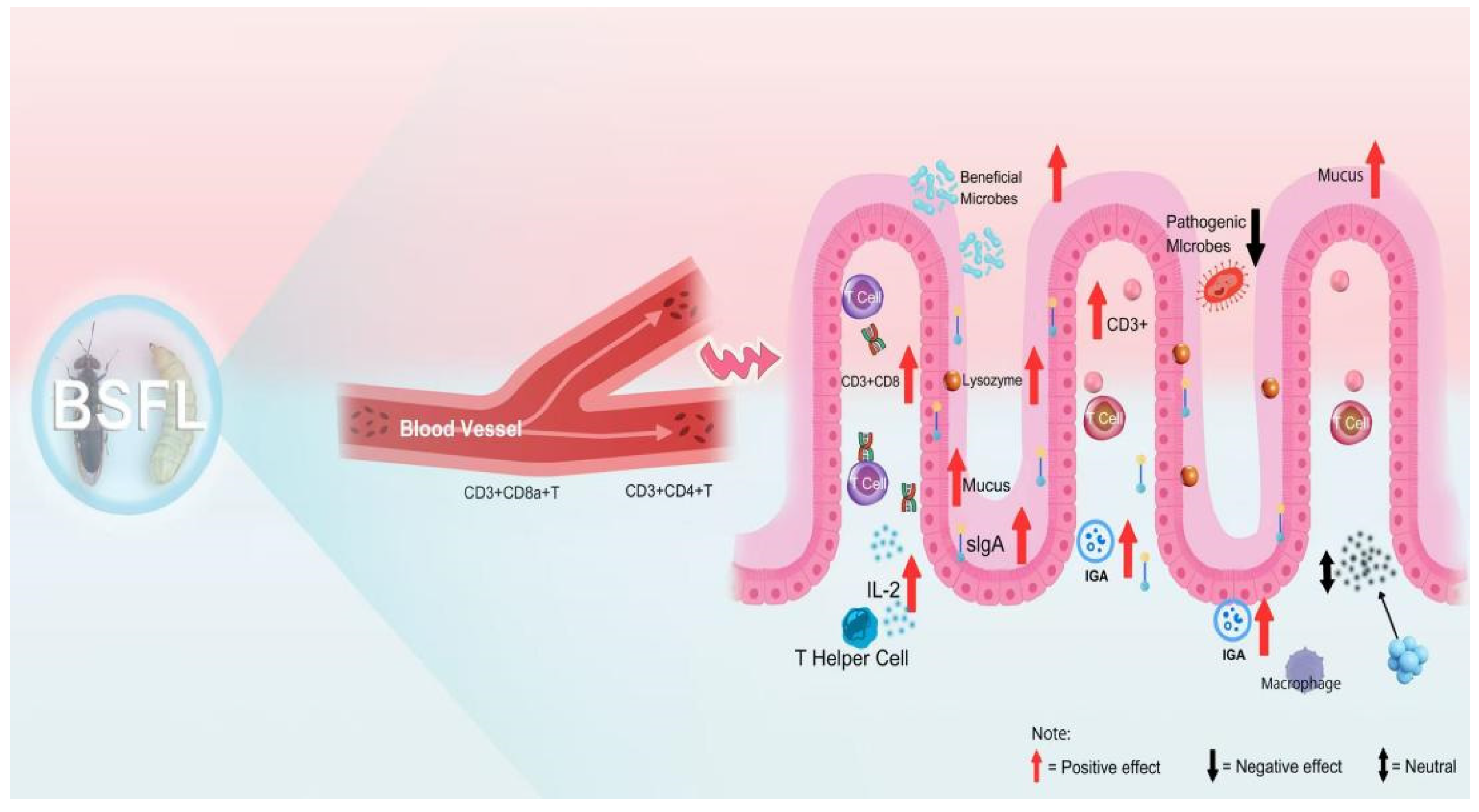
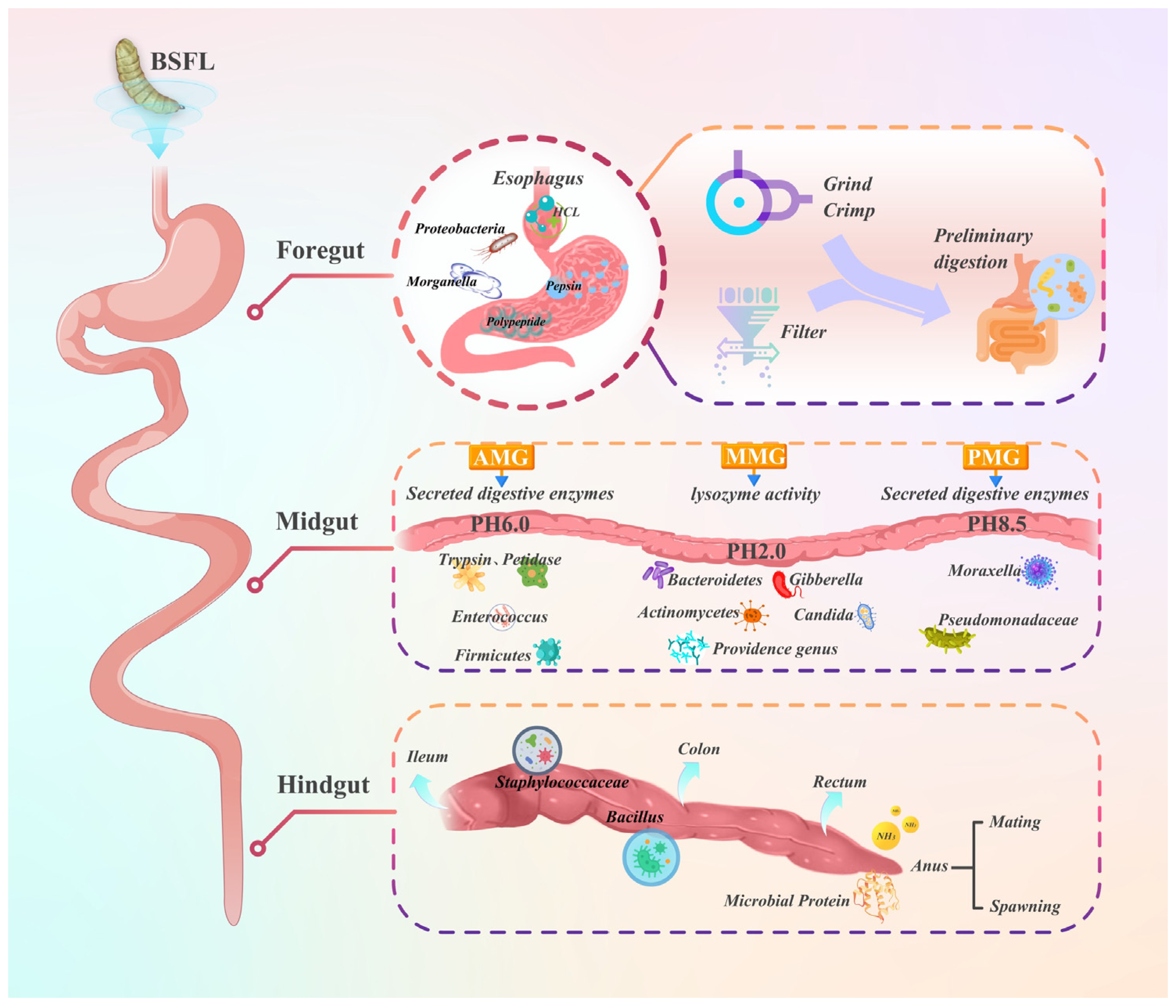
3. Intestinal Microbiota and Its Metabolic Pathways in BSFL
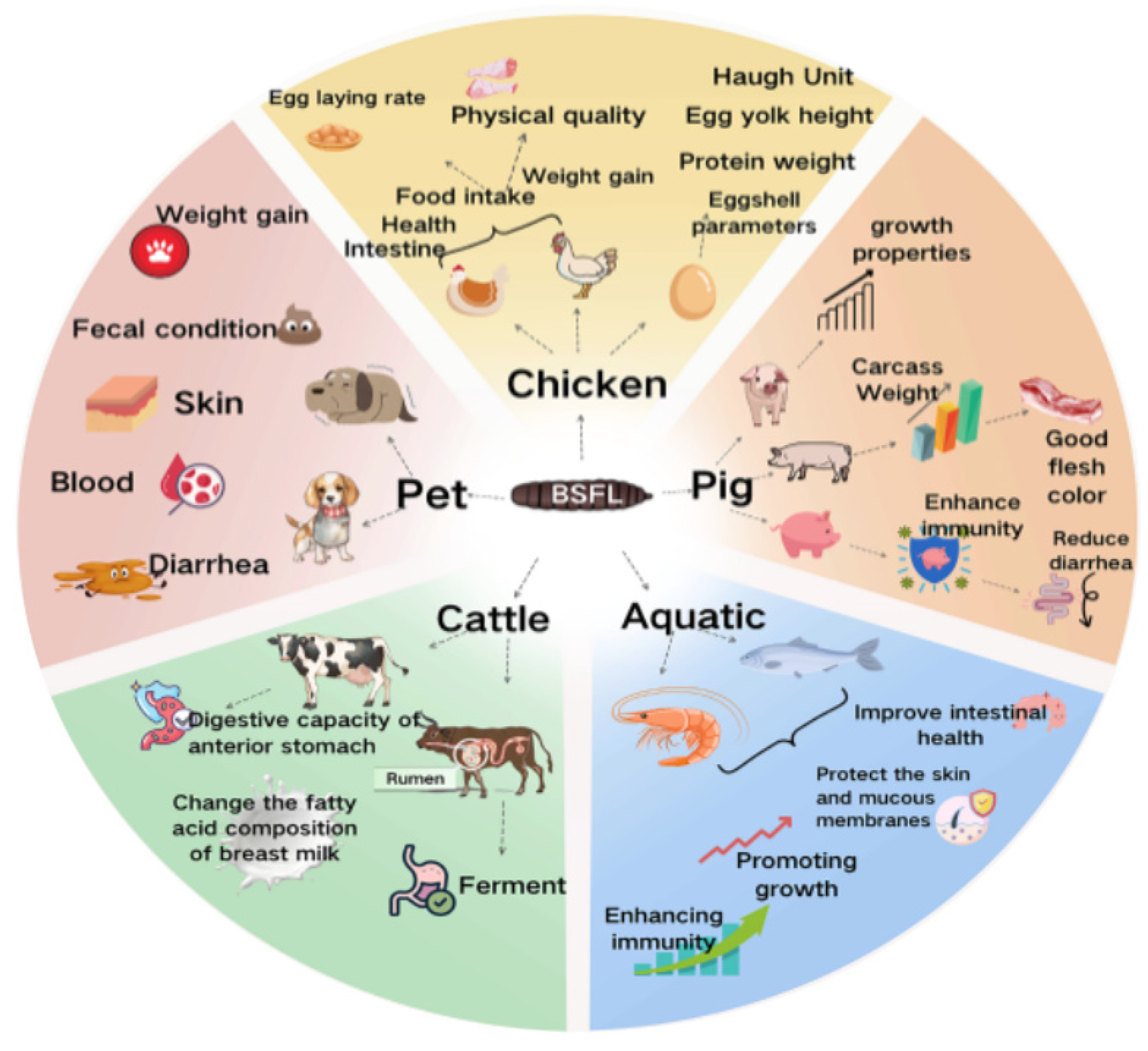
4. Applications of BSFL in Animal Production
4.1. Application in Poultry Production
| Poultry Species | Scientific Name | BSFL (%) | Impact | References |
|---|---|---|---|---|
| Broiler | \ | 4–25 | Improve growth performance, feeding rate, and nutrient conversion efficiency; optimize gut microbiota and immune function without affecting blood parameters or health. | [46,47,48,49,50] |
| 30 | Decreased intake of total DM and metabolizable energy, leading to a decline in protein utilization efficiency. Increased plasma uric acid and serum alkaline phosphatase concentrations. | [46] | ||
| 50–100 | Reduced growth performance, chicken flavor, and breast muscle ratio when replacing soybean meal. | [51,52] | ||
| Laying hens | \ | 5–25 | BSFL can effectively regulate the intestinal flora of laying hens, promote the production of short-chain fatty acids, improve gut health, and enhance the egg production rate and egg quality. At the same time, it does not affect egg weight, shell quality, or feed efficiency and has no negative impact on liver metabolism and overall health. | [53,54,55,56] |
| 50 | Decreased digestibility of dry matter, organic matter, and crude protein (possibly due to negative effects of chitin). Reduced serum cholesterol and triglycerides, increased serum globulins, and decreased albumin/globulin ratio. | [53] | ||
| Quail | Coturnix japonica | 20 | Significantly improve quail production performance, enhance liver and kidney function and metabolism–antioxidation–immune regulation, optimize egg quality, and promote the digestion and absorption of proteins and minerals. | [57,58] |
| Mandarin duck | Cairina moschata domestica | 9 | Defatted BSFL have no adverse effect on the slaughter characteristics and meat quality of Muscovy ducks. However, they changed the fatty acid profile. | [59] |
| Sichuan White Goose | \ | 1 | Significantly improve the daily weight gain of geese, enhance immunity (increase antibody titer and IgG/C3 levels, promote IL-6 and CD4 expression), and improve intestinal function (enhance barrier, regulate microbial balance). | [60] |
| Turkey | Meleagris gallopavo | 5 | No adverse effects on liver lipid status and histology. | [61] |
| 10–15 | Disturbs lipid metabolism and increases cholesterol, lipid oxidation, and liver fat deposition |
4.2. Application in Pig Production
4.3. Application in Aquaculture
| Test Animals | Scientific Name | BSFL (%) | Main Function | References |
|---|---|---|---|---|
| Atlantic salmon | Salmo salar | 6.25–12.5 | Had a lower immune response to the skin mucus protein profile of Atlantic salmon. Showed good intestinal health and normal metabolic response. | [75,76] |
| Largemouth bass | Micropterus salmoides | 1.0 | Improve growth performance and disease resistance, enhance antioxidant capacity, and promote intestinal health and microbiota. | [77] |
| Spotted catfish | Ictalurus punctatus | \ | Altering the overall gene expression and activating both innate and adaptive immunity may support the species’ disease resistance. | [78] |
| Red hybrid tilapia | Oreochromis | 35–42 | Growth performance and increase the content of crude protein and fat in vivo. | [79] |
| Rainbow trout | Oncorhynchus mykiss | 4–10 | Improved the weight, feed intake, and growth performance and immune response of rainbow trout. | [80] |
| Juvenile Pacific white shrimp | Litopenaeus vannamei | 4.5–10.5 | The body weight gain, feed conversion ratio, and specific growth rate of Pacific white shrimp showed a linear improvement with increasing inclusion levels. | [81] |
| Juvenile mud crab | Scylla paramamosain | 25–50 | BSFO can safely replace fish oil in partial amounts, not only maintaining normal growth performance in mud crabs but also enhancing antioxidant capacity, improving immune response, optimizing lipid metabolism, and promoting mitochondrial function, making it a promising sustainable alternative feed ingredient. | [82] |
| Asian swamp eel | Monopterus albus | 24.1–32.8 | Damage the digestion and absorption of nutrients in Asian swamp eels. Excessive BSFL may induce liver lipid accumulation and affect intestinal morphology. | [83] |
| Hybrid grouper | Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂ | 30–50 | The replacement of fish meal with BSFL will weaken the intestinal wall, leading to vacuoles, a sparse striated border, and reduced villi. | [84] |
4.4. Application in Ruminants and Pets
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Protein Sources for the Animal Feed Industry. Available online: https://www.fao.org/4/y5019e/y5019e03.htm (accessed on 18 June 2025).
- Khalifah, A.; Abdalla, S.; Rageb, M.; Maruccio, L.; Ciani, F.; El-Sabrout, K. Could Insect Products Provide a Safe and Sustainable Feed Alternative for the Poultry Industry? A Comprehensive Review. Animals 2023, 13, 1534. [Google Scholar] [CrossRef] [PubMed]
- da-Silva, W.C.; da Silva, É.B.R.; da Silva, J.A.R.; Martorano, L.G.; Belo, T.S.; Sousa, C.E.L.; Camargo-Júnior, R.N.C.; Andrade, R.L.; de Santos, A.G.S.; de Carvalho, K.C.; et al. Nutritional Value of the Larvae of the Black Soldier Fly (Hermetia illucens) and the House Fly (Musca Domestica) as a Food Alternative for Farm Animals—A Systematic Review. Insects 2024, 15, 619. [Google Scholar] [CrossRef]
- Shelomi, M. Potential of Black Soldier Fly Production for Pacific Small Island Developing States. Animals 2020, 10, 1038. [Google Scholar] [CrossRef]
- Tomberlin, J.K.; van Huis, A.; Benbow, M.E.; Jordan, H.; Astuti, D.A.; Azzollini, D.; Banks, I.; Bava, V.; Borgemeister, C.; Cammack, J.A.; et al. Protecting the Environment through Insect Farming as a Means to Produce Protein for Use as Livestock, Poultry, and Aquaculture Feed. J. Insects Food Feed 2015, 1, 307–309. [Google Scholar] [CrossRef]
- Pimchan, T.; Hamzeh, A.; Siringan, P.; Thumanu, K.; Hanboonsong, Y.; Yongsawatdigul, J. Antibacterial Peptides from Black Soldier Fly (Hermetia illucens) Larvae: Mode of Action and Characterization. Sci. Rep. 2024, 14, 26469. [Google Scholar] [CrossRef]
- Xia, J.; Ge, C.; Yao, H. Antimicrobial Peptides from Black Soldier Fly (Hermetia illucens) as Potential Antimicrobial Factors Representing an Alternative to Antibiotics in Livestock Farming. Animals 2021, 11, 1937. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dong, Y.; Sun, Q.; Tan, X.; You, C.; Huang, Y.; Zhou, M. Growth and Fatty Acid Composition of Black Soldier Fly Hermetia illucens (Diptera: Stratiomyidae) Larvae Are Influenced by Dietary Fat Sources and Levels. Animals 2022, 12, 486. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Ristow, B.; Rahayu, T.; Putra, N.S.; Widya Yuwono, N.; Nisa’, K.; Mategeko, B.; Smetana, S.; Saki, M.; Nawaz, A.; et al. Black Soldier Fly Larvae (BSFL) and Their Affinity for Organic Waste Processing. Waste Manag. 2022, 140, 1–13. [Google Scholar] [CrossRef]
- Boakye-Yiadom, K.A.; Ilari, A.; Duca, D. Greenhouse Gas Emissions and Life Cycle Assessment on the Black Soldier Fly (Hermetia illucens L.). Sustainability 2022, 14, 10456. [Google Scholar] [CrossRef]
- Lisboa, H.M.; Nascimento, A.; Arruda, A.; Sarinho, A.; Lima, J.; Batista, L.; Dantas, M.F.; Andrade, R. Unlocking the Potential of Insect-Based Proteins: Sustainable Solutions for Global Food Security and Nutrition. Foods 2024, 13, 1846. [Google Scholar] [CrossRef] [PubMed]
- Meijer, N.; Safitri, R.A.; Tao, W.; Hoek-Van den Hil, E.F. Review: European Union Legislation and Regulatory Framework for Edible Insect Production–Safety Issues. Animal 2025, 101468. [Google Scholar] [CrossRef] [PubMed]
- Astuti, D.A.; Wiryawan, K.G. Black Soldier Fly as Feed Ingredient for Ruminants. Anim. Biosci. 2022, 35, 356–363. [Google Scholar] [CrossRef]
- Penazzi, L.; Schiavone, A.; Russo, N.; Nery, J.; Valle, E.; Madrid, J.; Martinez, S.; Hernandez, F.; Pagani, E.; Ala, U.; et al. In Vivo and in Vitro Digestibility of an Extruded Complete Dog Food Containing Black Soldier Fly (Hermetia illucens) Larvae Meal as Protein Source. Front. Vet. Sci. 2021, 8, 653411. [Google Scholar] [CrossRef]
- Kim, W.; Bae, S.; Kim, A.; Park, K.; Lee, S.; Choi, Y.; Han, S.; Park, Y.; Koh, Y. Characterization of the Molecular Features and Expression Patterns of Two Serine Proteases in Hermetia illucens (Diptera: Stratiomyidae) Larvae. BMB Rep. 2011, 44, 387–392. [Google Scholar] [CrossRef]
- Shumo, M.; Osuga, I.M.; Khamis, F.M.; Tanga, C.M.; Fiaboe, K.K.M.; Subramanian, S.; Ekesi, S.; van Huis, A.; Borgemeister, C. The Nutritive Value of Black Soldier Fly Larvae Reared on Common Organic Waste Streams in Kenya. Sci. Rep. 2019, 9, 10110. [Google Scholar] [CrossRef]
- Shah, A.A.; Totakul, P.; Matra, M.; Cherdthong, A.; Hanboonsong, Y.; Wanapat, M. Nutritional Composition of Various Insects and Potential Uses as Alternative Protein Sources in Animal Diets. Anim. Biosci. 2022, 35, 317–331. [Google Scholar] [CrossRef]
- Liland, N.S.; Biancarosa, I.; Araujo, P.; Biemans, D.; Bruckner, C.G.; Waagbø, R.; Torstensen, B.E.; Lock, E.-J. Modulation of Nutrient Composition of Black Soldier Fly (Hermetia illucens) Larvae by Feeding Seaweed-Enriched Media. PLoS ONE 2017, 12, e0183188. [Google Scholar] [CrossRef]
- Erbland, P.; Alyokhin, A.; Perkins, L.B.; Peterson, M. Dose-Dependent Retention of Omega-3 Fatty Acids by Black Soldier Fly Larvae (Diptera: Stratiomyidae). J. Econ. Entomol. 2020, 113, 1221–1226. [Google Scholar] [CrossRef]
- Krupa, K.N.; Fritz, K.; Parmar, M. Omega-3 Fatty Acids. In Statpearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Wang, Y.-S.; Shelomi, M. Review of Black Soldier Fly (Hermetia illucens) as Animal Feed and Human Food. Foods 2017, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional Composition of Black Soldier Fly (Hermetia illucens) Prepupae Reared on Different Organic Waste Substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, E.; Belghit, I.; Johansen, J.; Leushuis, R.; Lock, E.-J.; Melsen, D.; Ramasamy Shanmugam, R.K.; Van Loon, J.; Paul, A. Growth and Safety Assessment of Feed Streams for Black Soldier Fly Larvae: A Case Study with Aquaculture Sludge. Animals 2019, 9, 189. [Google Scholar] [CrossRef]
- Proc, K.; Bulak, P.; Wiącek, D.; Bieganowski, A. Hermetia illucens Exhibits Bioaccumulative Potential for 15 Different Elements–Implications for Feed and Food Production. Sci. Total Environ. 2020, 723, 138125. [Google Scholar] [CrossRef]
- Kaczor, M.; Bulak, P.; Proc-Pietrycha, K.; Kirichenko-Babko, M.; Bieganowski, A. The Variety of Applications of Hermetia illucens in Industrial and Agricultural Areas—Review. Biology 2023, 12, 25. [Google Scholar] [CrossRef]
- Chang, C.-T.; Negi, S.; Rani, A.; Hu, A.H.; Pan, S.-Y.; Kumar, S. Food Waste and Soybean Curd Residue Composting by Black Soldier Fly. Environ. Res. 2022, 214, 113792. [Google Scholar] [CrossRef]
- Abdelmaksoud, E.M.; El-Sayed, W.; Rashwan, R.S.; Hegazy, S.A.; Abdelsalam, S.A. Optimize Using Black Soldier Fly Larvae Reared on Chicken Manure as an Alternative Feed in the Insect Farming Industry. Res. Sq. 2024. [Google Scholar] [CrossRef]
- China Agricultural Sciences Academy Beijing Veterinary Medicine Research Institute/National Key Laboratory of Animal Husbandry and Poultry Nutrition and Breeding; China Feed Database Information Network Center; National Agricultural Science Data Center (Animal Science). Explanation for the formulation of China Feed Composition and Nutritional Value Table (2024, 35th Edition). China Feed 2024, 1, 181. [Google Scholar] [CrossRef]
- Kim, Y.B.; Kim, D.-H.; Jeong, S.-B.; Lee, J.-W.; Kim, T.-H.; Lee, H.-G.; Lee, K.-W. Black Soldier Fly Larvae Oil as an Alternative Fat Source in Broiler Nutrition. Poult. Sci. 2020, 99, 3133–3143. [Google Scholar] [CrossRef]
- Daszkiewicz, T.; Murawska, D.; Kubiak, D.; Han, J. Chemical Composition and Fatty Acid Profile of the Pectoralis Major Muscle in Broiler Chickens Fed Diets with Full-Fat Black Soldier Fly (Hermetia illucens) Larvae Meal. Animals 2022, 12, 464. [Google Scholar] [CrossRef] [PubMed]
- Cullere, M.; Tasoniero, G.; Giaccone, V.; Miotti-Scapin, R.; Claeys, E.; De Smet, S.; Dalle Zotte, A. Black Soldier Fly as Dietary Protein Source for Broiler Quails: Apparent Digestibility, Excreta Microbial Load, Feed Choice, Performance, Carcass and Meat Traits. Animal 2016, 10, 1923–1930. [Google Scholar] [CrossRef]
- Khan, S.; Khan, R.U.; Alam, W.; Sultan, A. Evaluating the Nutritive Profile of Three Insect Meals and Their Effects to Replace Soya Bean in Broiler Diet. J. Anim. Physiol. Anim. Nutr. 2018, 102, e662–e668. [Google Scholar] [CrossRef]
- Jing, T.-Z.; Qi, F.-H.; Wang, Z.-Y. Most Dominant Roles of Insect Gut Bacteria: Digestion, Detoxification, or Essential Nutrient Provision? Microbiome 2020, 8, 38. [Google Scholar] [CrossRef]
- Benzertiha, A.; Kierończyk, B.; Kołodziejski, P.; Pruszyńska-Oszmałek, E.; Rawski, M.; Józefiak, D.; Józefiak, A. Tenebrio molitor and Zophobas morio Full-Fat Meals as Functional Feed Additives Affect Broiler Chickens’ Growth Performance and Immune System Traits. Poult. Sci. 2020, 99, 196–206. [Google Scholar] [CrossRef]
- Hong, J.; Han, T.; Kim, Y.Y. Mealworm (Tenebrio molitor Larvae) as an Alternative Protein Source for Monogastric Animal: A Review. Animals 2020, 10, 2068. [Google Scholar] [CrossRef] [PubMed]
- Magara, H.J.O.; Hugel, S.; Fisher, B.L. Nutritional Composition of Four Edible Grasshopper Species Frequently Consumed in Madagascar: Insights for Nutritional Contribution and Alternative Insect Farming. Foods 2025, 14, 1848. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Lozupone, C.A.; Hamady, M.; Knight, R.; Gordon, J.I. Worlds within Worlds: Evolution of the Vertebrate Gut Microbiota. Nat. Rev. Microbiol. 2008, 6, 776–788. [Google Scholar] [CrossRef]
- Tanga, C.M.; Waweru, J.W.; Tola, Y.H.; Onyoni, A.A.; Khamis, F.M.; Ekesi, S.; Paredes, J.C. Organic Waste Substrates Induce Important Shifts in Gut Microbiota of Black Soldier Fly (Hermetia illucens L.): Coexistence of Conserved, Variable, and Potential Pathogenic Microbes. Front. Microbiol. 2021, 12, 635881. [Google Scholar] [CrossRef]
- Ao, Y.; Yang, C.; Wang, S.; Hu, Q.; Yi, L.; Zhang, J.; Yu, Z.; Cai, M.; Yu, C. Characteristics and Nutrient Function of Intestinal Bacterial Communities in Black Soldier Fly (Hermetia illucens L.) Larvae in Livestock Manure Conversion. Microb. Biotechnol. 2020, 14, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, S.; Spampinato, G.; Macavei, L.I.; Lugli, L.; Candeliere, F.; Rossi, M.; Maistrello, L.; Amaretti, A. Effect of Rearing Temperature on Growth and Microbiota Composition of Hermetia illucens. Microorganisms 2020, 8, 902. [Google Scholar] [CrossRef]
- Querejeta, M.; Hervé, V.; Perdereau, E.; Marchal, L.; Herniou, E.A.; Boyer, S.; Giron, D. Changes in Bacterial Community Structure across the Different Life Stages of Black Soldier Fly (Hermetia illucens). Microb. Ecol. 2023, 86, 1254–1267. [Google Scholar] [CrossRef]
- IJdema, F.; De Smet, J.; Crauwels, S.; Lievens, B.; Van Campenhout, L. Meta-Analysis of Larvae of the Black Soldier Fly (Hermetia illucens) Microbiota Based on 16S rRNA Gene Amplicon Sequencing. FEMS Microbiol. Ecol. 2022, 98, fiac094. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, J.; Zhu, F.; Fan, M.; Zheng, J.; Cai, M.; Zheng, L.; Huang, F.; Yu, Z.; Zhang, J. Enhanced Protein Degradation by Black Soldier Fly Larvae (Hermetia illucens L.) and Its Gut Microbes. Front. Microbiol. 2023, 13, 1095025. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Fang, G.; Chen, K.; Song, Y.; Lu, T.; Tomberlin, J.K.; Zhan, S.; Huang, Y. A Gut Commensal Bacterium Promotes Black Soldier Fly Larval Growth and Development Partly via Modulation of Intestinal Protein Metabolism. mBio. 2023, 14, e0117423. [Google Scholar] [CrossRef]
- Chen, L.; Sun, H.; Song, H.; Wang, G.; Ma, X.; Tu, J.; Yang, L.; Li, J.; Wang, Y.; Meng, X.; et al. Dietary Inclusion of Defatted Black Soldier Fly Larvae Meal: Impacts on Laying Hen Performance, Egg Quality, Serum Biomarkers, and Intestinal Morphology. Front. Vet. Sci. 2025, 12, 1605077. [Google Scholar] [CrossRef]
- Seyedalmoosavi, M.M.; Mielenz, M.; Görs, S.; Wolf, P.; Daş, G.; Metges, C.C. Effects of Increasing Levels of Whole Black Soldier Fly (Hermetia illucens) Larvae in Broiler Rations on Acceptance, Nutrient and Energy Intakes and Utilization, and Growth Performance of Broilers. Poult. Sci. 2022, 101, 102202. [Google Scholar] [CrossRef] [PubMed]
- Mat, K.; Abdul Kari, Z.; Rusli, N.D.; Rahman, M.M.; Che Harun, H.; Al-Amsyar, S.M.; Mohd Nor, M.F.; Dawood, M.A.O.; Hassan, A.M. Effects of the Inclusion of Black Soldier Fly Larvae (Hermetia illucens) Meal on Growth Performance and Blood Plasma Constituents in Broiler Chicken (Gallus gallus Domesticus) Production. Saudi J. Biol. Sci. 2022, 29, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Ndotono, E.W.; Khamis, F.M.; Bargul, J.L.; Tanga, C.M. Insights into the Gut Microbial Communities of Broiler Chicken Fed Black Soldier Fly Larvae-Desmodium-Based Meal as a Dietary Protein Source. Microorganisms 2022, 10, 1351. [Google Scholar] [CrossRef]
- de Souza Vilela, J.; Andronicos, N.M.; Kolakshyapati, M.; Hilliar, M.; Sibanda, T.Z.; Andrew, N.R.; Swick, R.A.; Wilkinson, S.; Ruhnke, I. Black Soldier Fly Larvae in Broiler Diets Improve Broiler Performance and Modulate the Immune System. Anim. Nutr. 2021, 7, 695–706. [Google Scholar] [CrossRef]
- Lau, C.H.-F.; Capitani, S.; Tien, Y.-C.; Verellen, L.A.; Kithama, M.; Kang, H.; Kiarie, E.G.; Topp, E.; Diarra, M.S.; Fruci, M. Dynamic Effects of Black Soldier Fly Larvae Meal on the Cecal Bacterial Microbiota and Prevalence of Selected Antimicrobial Resistant Determinants in Broiler Chickens. Anim. Microbiome 2024, 6, 6. [Google Scholar] [CrossRef]
- Murawska, D.; Daszkiewicz, T.; Sobotka, W.; Gesek, M.; Witkowska, D.; Matusevičius, P.; Bakuła, T. Partial and Total Replacement of Soybean Meal with Full-Fat Black Soldier Fly (Hermetia illucens L.) Larvae Meal in Broiler Chicken Diets: Impact on Growth Performance, Carcass Quality and Meat Quality. Animals 2021, 11, 2715. [Google Scholar] [CrossRef]
- Dabbou, S.; Gai, F.; Biasato, I.; Capucchio, M.T.; Biasibetti, E.; Dezzutto, D.; Meneguz, M.; Plachà, I.; Gasco, L.; Schiavone, A. Black Soldier Fly Defatted Meal as a Dietary Protein Source for Broiler Chickens: Effects on Growth Performance, Blood Traits, Gut Morphology and Histological Features. J. Anim. Sci. Biotechnol. 2018, 9, 49. [Google Scholar] [CrossRef]
- Zhao, J.; Ban, T.; Miyawaki, H.; Hirayasu, H.; Izumo, A.; Iwase, S.; Kasai, K.; Kawasaki, K. Long-Term Dietary Fish Meal Substitution with the Black Soldier Fly Larval Meal Modifies the Caecal Microbiota and Microbial Pathway in Laying Hens. Animals 2023, 13, 2629. [Google Scholar] [CrossRef]
- Zhao, J.; Kawasaki, K.; Miyawaki, H.; Hirayasu, H.; Izumo, A.; Iwase, S.; Kasai, K. Egg Quality and Laying Performance of Julia Laying Hens Fed with Black Soldier Fly (Hermetia illucens) Larvae Meal as a Long-Term Substitute for Fish Meal. Poult. Sci. 2022, 101, 101986. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Shi, X.; Cai, R.; Shuai, Z.; Mao, W.; Khan, I.M.; Swelum, A.A.; Guo, J. Effect of Black Soldier Fly (Hermetia illucens) Larvae Meal and Oil on the Performance, Biochemical Profile, Intestinal Health and Gut Microbial Dynamics in Laying Hens. Poult. Sci. 2024, 103, 104460. [Google Scholar] [CrossRef] [PubMed]
- Bovera, F.; Loponte, R.; Pero, M.E.; Cutrignelli, M.I.; Calabrò, S.; Musco, N.; Vassalotti, G.; Panettieri, V.; Lombardi, P.; Piccolo, G.; et al. Laying Performance, Blood Profiles, Nutrient Digestibility and Inner Organs Traits of Hens Fed an Insect Meal from Hermetia illucens Larvae. Res. Vet. Sci. 2018, 120, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, G.; Li, Y.; Jiao, M.; Guo, J.; Shi, H.; Ji, X.; Zhang, W.; Quan, K.; Xia, W. Effects of Feeding Unprocessed Whole Black Soldier Fly (Hermetia illucens) Larvae on Performance, Biochemical Profile, Health Status, Egg Quality, Microbiome and Metabolome Patterns of Quails. Poult. Sci. 2025, 104, 105374. [Google Scholar] [CrossRef]
- Tahamtani, F.M.; Ivarsson, E.; Wiklicky, V.; Lalander, C.; Wall, H.; Rodenburg, T.B.; Tuyttens, F.A.M.; Hernandez, C.E. Feeding Live Black Soldier Fly Larvae (Hermetia illucens) to Laying Hens: Effects on Feed Consumption, Hen Health, Hen Behavior, and Egg Quality. Poult. Sci. 2021, 100, 101400. [Google Scholar] [CrossRef]
- Gariglio, M.; Dabbou, S.; Gai, F.; Trocino, A.; Xiccato, G.; Holodova, M.; Gresakova, L.; Nery, J.; Bellezza Oddon, S.; Biasato, I.; et al. Black Soldier Fly Larva in Muscovy Duck Diets: Effects on Duck Growth, Carcass Property, and Meat Quality. Poult. Sci. 2021, 100, 101303. [Google Scholar] [CrossRef]
- Xie, Y.; Hao, Y.; Gui, F.; Li, X.; Huang, H.; Yang, P.; Zhong, C.; Cao, L. Hermetia illucens Larvae Meal Enhances Immune Response by Improving Serum Immunoglobulin, Intestinal Barrier and Gut Microbiota of Sichuan White Geese after Avian Influenza Vaccination. Vet. Sci. 2024, 11, 615. [Google Scholar] [CrossRef]
- Ognik, K.; Kozłowski, K.; Stępniowska, A.; Listos, P.; Józefiak, D.; Zduńczyk, Z.; Jankowski, J. Antioxidant Status and Liver Function of Young Turkeys Receiving a Diet with Full-Fat Insect Meal from Hermetia illucens. Animals 2020, 10, 1339. [Google Scholar] [CrossRef]
- Salahuddin, M.; Abdel-Wareth, A.A.A.; Hiramatsu, K.; Tomberlin, J.K.; Luza, D.; Lohakare, J. Flight toward Sustainability in Poultry Nutrition with Black Soldier Fly Larvae. Animals 2024, 14, 510. [Google Scholar] [CrossRef]
- Yu, M.; Li, Z.; Chen, W.; Wang, G.; Rong, T.; Liu, Z.; Wang, F.; Ma, X. Hermetia illucens Larvae as a Fishmeal Replacement Alters Intestinal Specific Bacterial Populations and Immune Homeostasis in Weanling Piglets. J. Anim. Sci. 2020, 98, skz395. [Google Scholar] [CrossRef] [PubMed]
- Biasato, I.; Ferrocino, I.; Colombino, E.; Gai, F.; Schiavone, A.; Cocolin, L.; Vincenti, V.; Capucchio, M.T.; Gasco, L. Effects of Dietary Hermetia illucens Meal Inclusion on Cecal Microbiota and Small Intestinal Mucin Dynamics and Infiltration with Immune Cells of Weaned Piglets. J. Anim. Sci. Biotechnol. 2020, 11, 64. [Google Scholar] [CrossRef]
- Boontiam, W.; Phaengphairee, P.; Hong, J.; Kim, Y.Y. Full-Fatted Hermetia illucens Larva as a Protein Alternative: Effects on Weaning Pig Growth Performance, Gut Health, and Antioxidant Status under Poor Sanitary Conditions. J. Appl. Anim. Res. 2022, 50, 732–739. [Google Scholar] [CrossRef]
- Spranghers, T.; Michiels, J.; Vrancx, J.; Ovyn, A.; Eeckhout, M.; De Clercq, P.; De Smet, S. Gut Antimicrobial Effects and Nutritional Value of Black Soldier Fly (Hermetia illucens L.) Prepupae for Weaned Piglets. Anim. Feed Sci. Technol. 2018, 235, 33–42. [Google Scholar] [CrossRef]
- Driemeyer, H. Evaluation of Black Soldier Fly (Hermetia illucens) Larvae as an Alternative Protein Source in Pig Creep Diets in Relation to Production, Blood and Manure Microbiology Parameters. Master’s Thesis, Stellenbosch University, Stellenbosch, South Africa, 2016. [Google Scholar]
- Maurer, V.; Holinger, M.; Amsler, Z.; Früh, B.; Wohlfahrt, J.; Stamer, A.; Leiber, F. Replacement of Soybean Cake by Hermetia illucens Meal in Diets for Layers. J. Insects Food Feed 2016, 2, 83–90. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, M.; Yuan, B.; Jin, X.; Zhang, X.; Xie, G.; Wang, Z.; Lv, Y.; Wang, W.; Huang, Y. Growth Performance and Meat Quality of Growing Pigs Fed with Black Soldier Fly (Hermetia illucens) Larvae as Alternative Protein Source. Processes 2022, 10, 1498. [Google Scholar] [CrossRef]
- Yu, M.; Li, Z.; Chen, W.; Rong, T.; Wang, G.; Li, J.; Ma, X. Use of Hermetia illucens Larvae as a Dietary Protein Source: Effects on Growth Performance, Carcass Traits, and Meat Quality in Finishing Pigs. Meat Sci. 2019, 158, 107837. [Google Scholar] [CrossRef]
- Kim, C.-H.; Ryu, J.; Lee, J.; Ko, K.; Lee, J.; Park, K.Y.; Chung, H. Use of Black Soldier Fly Larvae for Food Waste Treatment and Energy Production in Asian Countries: A Review. Processes 2021, 9, 161. [Google Scholar] [CrossRef]
- Yao, W.; Zhang, C.; Mao, H.; Hua, G.; Liu, Q.; Zhao, S.; Shuang, H.; Poolsawat, L.; Yuan, S.; Wang, J.; et al. Effects of Dietary Defatted Black Soldier Fly (Hermetia illucens) Larvae Meal Substituting Fish Meal on Growth, Antioxidative Capacity, Immunity, Intestinal Histology and Microbiota of Juvenile Chinese Mitten Crab (Eriocheir sinensis). Aquac. Rep. 2024, 38, 102302. [Google Scholar] [CrossRef]
- Soetemans, L.; Uyttebroek, M.; Bastiaens, L. Characteristics of Chitin Extracted from Black Soldier Fly in Different Life Stages. Int. J. Biol. Macromol. 2020, 165, 3206–3214. [Google Scholar] [CrossRef]
- Fisher, H.J.; Collins, S.A.; Hanson, C.; Mason, B.; Colombo, S.M.; Anderson, D.M. Black Soldier Fly Larvae Meal as a Protein Source in Low Fish Meal Diets for Atlantic Salmon (Salmo salar). Aquaculture 2020, 521, 734978. [Google Scholar] [CrossRef]
- Kousoulaki, K.; Sveen, L.; Norén, F.; Espmark, Å. Atlantic Salmon (Salmo salar) Performance Fed Low Trophic Ingredients in a Fish Meal and Fish Oil Free Diet. Front. Physiol. 2022, 13, 884740. [Google Scholar] [CrossRef] [PubMed]
- Weththasinghe, P.; Lagos, L.; Cortés, M.; Hansen, J.Ø.; Øverland, M. Dietary Inclusion of Black Soldier Fly (Hermetia illucens) Larvae Meal and Paste Improved Gut Health but Had Minor Effects on Skin Mucus Proteome and Immune Response in Atlantic Salmon (Salmo Salar). Front. Immunol. 2021, 12, 599530. [Google Scholar] [CrossRef]
- Xu, F.-M.; Hou, S.-W.; Wang, G.-X.; Gong, J.-Y.; Zhou, L.; Huang, Y.-H.; Huang, X.-D.; Liu, L. Effects of Zymolytic Black Soldier Fly (Hermetia illucens) Pulp as Dietary Supplementation in Largemouth Bass (Micropterus salmoides). Aquacult. Rep. 2021, 21, 100823. [Google Scholar] [CrossRef]
- Sankappa, N.M.; Lange, M.D.; Yildirim-Aksoy, M.; Eljack, R.; Kucuktas, H.; Beck, B.H.; Abernathy, J.W. Transcriptome Analysis and Immune Gene Expression of Channel Catfish (Ictalurus punctatus) Fed Diets with Inclusion of Frass from Black Soldier Fly Larvae. Front. Physiol. 2024, 14, 1330368. [Google Scholar] [CrossRef]
- Aisyah, H.N.; Athirah, Z.a.R.; Hanani, W.R.; Arshad, S.S.; Hassim, H.A.; Nazarudin, M.F.; Ina-Salwany, M.Y. The Effect of Feeding Black Soldier Fly Larvae on Growth Performance, Protein, and Fat Content of Red Hybrid Tilapia (Oreochromis Spp.). Vet. World 2022, 15, 2453–2457. [Google Scholar] [CrossRef] [PubMed]
- Borland, M.; Riesenbach, C.; Shandilya, U.; Chiasson, M.A.; Karrow, N.A.; Huyben, D. Growth Performance, Hepatic Gene Expression, and Plasma Biochemistry of Rainbow Trout Fed Full-Fat Meal, Defatted Meal, Oil and Chitin from Black Soldier Flies. Comp. Immunol. Rep. 2024, 6, 200149. [Google Scholar] [CrossRef]
- Richardson, A.; Dantas-Lima, J.; Lefranc, M.; Walraven, M. Effect of a Black Soldier Fly Ingredient on the Growth Performance and Disease Resistance of Juvenile Pacific White Shrimp (Litopenaeus vannamei). Animals 2021, 11, 1450. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, T.; Jin, M.; Li, X.; Xie, S.; Cui, Y.; Zhou, Q. Black Soldier Fly Larvae Oil Can Partially Replace Fish Oil in the Diet of the Juvenile Mud Crab (Scylla paramamosain). Anim. Nutr. 2025, 20, 469–486. [Google Scholar] [CrossRef]
- Xiang, Y.; Gao, S.; Luo, Y.; Tang, G.; Zou, X.; Xie, K.; Niu, W.; Li, X.; Xiang, J.; Zhang, L.; et al. Fermented Black Soldier Fly Larvae as a Sustainable Replacement for Marine Fish in Asian Swamp Eel Diets. Vet. World 2025, 18, 1002–1013. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, J.; Yong, Y.-S.; Chen, Y.; Chen, B.; Cao, J.; Peng, K.; Wang, G.; Huang, H.; Loh, J.-Y. Impacts of Black Soldier Fly (Hermetia illucens) Larval Meal on Intestinal Histopathology and Microbiome Responses in Hybrid Grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂): A Comprehensive Analysis. Animals 2024, 14, 3596. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Chen, S.; Paengkoum, S.; Taethaisong, N.; Meethip, W.; Surakhunthod, J.; Wang, Q.; Thongpea, S.; Paengkoum, P. Effects of Black Soldier Fly (Hermetia illucens L., BSF) Larvae Addition on in Vitro Fermentation Parameters of Goat Diets. Insects 2024, 15, 343. [Google Scholar] [CrossRef] [PubMed]
- Prachumchai, R.; Cherdthong, A. Black Soldier Fly Larva Oil in Diets with Roughage to Concentrate Ratios on Fermentation Characteristics, Degradability, and Methane Generation. Animals 2023, 13, 2416. [Google Scholar] [CrossRef]
- Shah, A.M.; Qazi, I.H.; Matra, M.; Wanapat, M. Role of Chitin and Chitosan in Ruminant Diets and Their Impact on Digestibility, Microbiota and Performance of Ruminants. Fermentation 2022, 8, 549. [Google Scholar] [CrossRef]
- Nekrasov, R.V.; Ivanov, G.A.; Chabaev, M.G.; Zelenchenkova, A.A.; Bogolyubova, N.V.; Nikanova, D.A.; Sermyagin, A.A.; Bibikov, S.O.; Shapovalov, S.O. Effect of Black Soldier Fly (Hermetia illucens L.) Fat on Health and Productivity Performance of Dairy Cows. Animals 2022, 12, 2118. [Google Scholar] [CrossRef]
- Braamhaar, D.J.M.; Pellikaan, W.F.; List, D.; Korir, D.; Tanga, C.M.; Oosting, S.J. Defatted Black Soldier Fly Larvae Meal as a Substitute of Soybean Meal in Dairy Cow Diets. Animal 2025, 19, 101476. [Google Scholar] [CrossRef]
- Carrasco, M.N.; Drewery, M.L. Mealworm Larvae and Black Soldier Fly Larvae as Novel Protein Supplements for Cattle Consuming Low-Quality Forage. Transl. Anim. Sci. 2024, 8, txae122. [Google Scholar] [CrossRef]
- Sudwischer, P.; Krüger, B.; Sitzmann, W.; Hellwig, M. Chitin Analysis in Insect-based Feed Ingredients and Mixed Feed: Development of a Cost-effective and Practical Method. J. Anim. Physiol. Anim. Nutr. 2025, 109, 854–866. [Google Scholar] [CrossRef]
- Verlinden, A.; Hesta, M.; Millet, S.; Janssens, G.P.J. Food Allergy in Dogs and Cats: A Review. Crit. Rev. Food Sci. Nutr. 2006, 46, 259–273. [Google Scholar] [CrossRef]
- Valdés, F.; Villanueva, V.; Durán, E.; Campos, F.; Avendaño, C.; Sánchez, M.; Domingoz-Araujo, C.; Valenzuela, C. Insects as Feed for Companion and Exotic Pets: A Current Trend. Animals 2022, 12, 1450. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, O.; İnal, F.; Alataş, M.S.; İnanç, Z.S.; Damar, S.; Ahmed, I.; Uludağ, M.; Çalıkoğlu, T. Substitution of Poultry Fat with Black Soldier Fly (Hermetia illucens) Larvae Fat in Dog Diets: Effects on Digestibility, Palatability, Peroxidation of Dry Food, Immunity, Blood Biochemistry, and Faecal Characteristics of Adult Dogs. Vet. Sci. 2025, 12, 311. [Google Scholar] [CrossRef] [PubMed]
- Kerr, K.R.; Gajda, A.M.; Daristotle, L.; Frantz, N. PSIX-5 Performance of Black Soldier Fly Larvae Meal as Secondary Protein Sources in Extruded Dog Diets. J. Anim. Sci. 2024, 102, 577–578. [Google Scholar] [CrossRef]
- Wei, Y.; Xue, L.; Ma, D.; Weng, Y.; Liu, M.; Li, L.; Dai, Z.; Zhao, Z.; Wang, H.; Xu, X. The Effect of Dietary Protein Hydrolysate from Black Soldier Fly Larvae and Schizochytrium on Palatability, Nutrient Metabolites and Health Status in Beagle Dogs. Metabolites 2024, 14, 165. [Google Scholar] [CrossRef]
- Seo, K.; Cho, H.-W.; Chun, J.; Jeon, J.; Kim, C.; Kim, M.; Park, K.; Kim, K. Evaluation of Fermented Oat and Black Soldier Fly Larva as Food Ingredients in Senior Dog Diets. Animals 2021, 11, 3509. [Google Scholar] [CrossRef]
- Abd El-Wahab, A.; Meyer, L.; Kölln, M.; Chuppava, B.; Wilke, V.; Visscher, C.; Kamphues, J. Insect Larvae Meal (Hermetia illucens) as a Sustainable Protein Source of Canine Food and Its Impacts on Nutrient Digestibility and Fecal Quality. Animals 2021, 11, 2525. [Google Scholar] [CrossRef]
- Jian, S.; Zhang, L.; Ding, N.; Yang, K.; Xin, Z.; Hu, M.; Zhou, Z.; Zhao, Z.; Deng, B.; Deng, J. Effects of Black Soldier Fly Larvae as Protein or Fat Sources on Apparent Nutrient Digestibility, Fecal Microbiota, and Metabolic Profiles in Beagle Dogs. Front. Microbiol. 2022, 13, 1044986. [Google Scholar] [CrossRef] [PubMed]
| Raw Material | Ash | CP | CF | EE | References |
|---|---|---|---|---|---|
| Kitchen waste BSFL | 4.33 | 23.24 | 22.18 | 9.19 | [26] |
| Chicken manure BSFL | 43.33 | 25.2 | 5.7 | 12.77 | [27] |
| SM | 4.8 | 46.8 | 3.9 | 1.0 | [28] |
| FM | 20.8 | 53.5 | 0.8 | 10.0 |
| Nutritional Composition | Insect Species | |||||
|---|---|---|---|---|---|---|
| FF BSFL | DF BSFL | Musca domestica | Tenebrio molitor | Acrida cinerea | Bombyx mori | |
| CP | 43.10 | 51.83 | 50.0 | 53.0 | 58.09 ± 0.09 | 54.0 |
| CF | \ | \ | 18.9 | 3.1 | 4.46 ± 0.04 | 3.9 |
| EE | 38.6 | 14.71 | 2.7 | 3.6 | 8.88 ± 0.11 | 2.5 |
| Ash | 2.7 | 7.27 | 10.1 | 26.8 | 7.14 ± 0.16 | 5.8 |
| SFA | 70.72 | 65.01 | \ | 20.99 | 25.0 ± 0.71 | \ |
| Isoleucine | 1.91 | 2.24 | 3.2 | 4.6 | 43.7 ± 0.09 | 3.9 |
| Threonine | 1.62 | 1.93 | 3.78 | 4.0 | \ | 4.8 |
| Methionine | 0.71 | 0.62 | 2.2 | 1.5 | 20.6 ± 0.06 | 3.0 |
| Phenylalanine | 1.64 | 1.69 | 4.6 | 4.0 | 32.6 ± 0.19 | 4.4 |
| Lysine | 2.3 | 1.96 | 6.1 | 5.4 | 15.5 ± 0.13 | 6.1 |
| Chitin | 6.7 | \ | \ | 8.91 | \ | \ |
| References | [22,29] | [30,31] | [32] | [32,33,34,35] | [36] | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, H.; Zhang, B.; Shi, J.; He, S.; Dai, S.; Zhao, Z.; Wu, D.; Li, J. Black Soldier Fly Larvae as a Novel Protein Feed Resource Promoting Circular Economy in Agriculture. Insects 2025, 16, 830. https://doi.org/10.3390/insects16080830
Su H, Zhang B, Shi J, He S, Dai S, Zhao Z, Wu D, Li J. Black Soldier Fly Larvae as a Novel Protein Feed Resource Promoting Circular Economy in Agriculture. Insects. 2025; 16(8):830. https://doi.org/10.3390/insects16080830
Chicago/Turabian StyleSu, Hongren, Bin Zhang, Jingyi Shi, Shichun He, Sifan Dai, Zhiyong Zhao, Dongwang Wu, and Jun Li. 2025. "Black Soldier Fly Larvae as a Novel Protein Feed Resource Promoting Circular Economy in Agriculture" Insects 16, no. 8: 830. https://doi.org/10.3390/insects16080830
APA StyleSu, H., Zhang, B., Shi, J., He, S., Dai, S., Zhao, Z., Wu, D., & Li, J. (2025). Black Soldier Fly Larvae as a Novel Protein Feed Resource Promoting Circular Economy in Agriculture. Insects, 16(8), 830. https://doi.org/10.3390/insects16080830






