Biosynthesis of Bioactive Human Neurotrophic Factor 3 in Silkworms and Its Biomedical Applications
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Silkworm Strain
2.2. Cell Line
2.3. Plasmid Construction
2.4. Transgenic Silkworm Generation
2.5. Genetic Analysis
2.6. Extraction of NT-3 from the Silkworm Cocoon
2.7. Quantitative Real-Time PCR Analysis
2.8. Western Blot Analysis
2.9. Quantification of NT-3
2.10. Immunofluorescence Staining
2.11. Scanning Electron Microscopy
2.12. Phenotypic Observation
2.13. Cell Viability
2.14. Cell Proliferation
2.15. Cell Differentiation
2.16. Wound Healing Assay
2.17. Neurite Outgrowth Assay
2.18. Statistical Analysis
3. Results
3.1. Generation of Human NT-3 Expression System in Silkworm
3.2. Expression of Human NT-3 Protein in Transgenic Silkworm
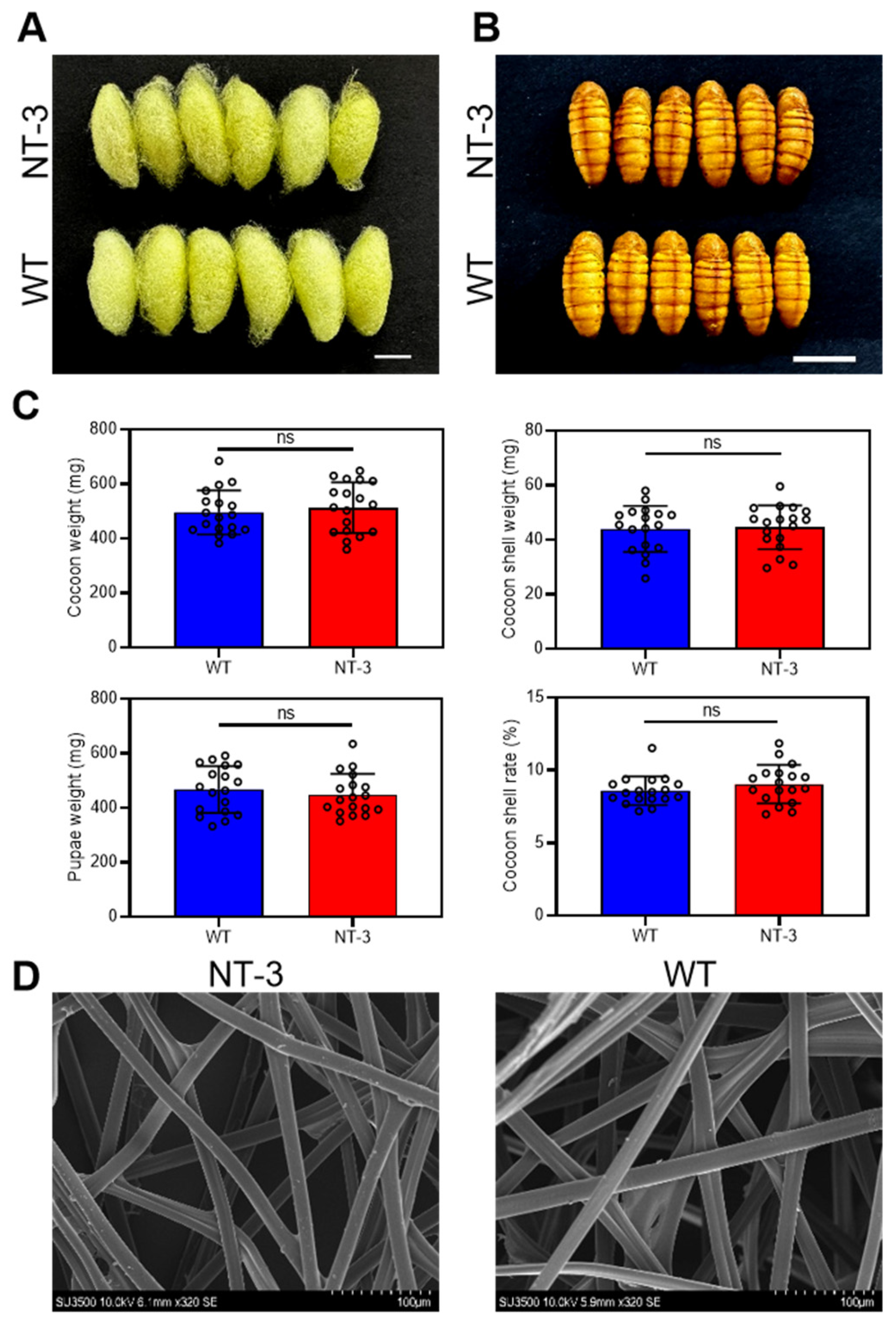
3.3. Effects of NT-3 Expression on Morphological Properties of Silkworm Cocoons
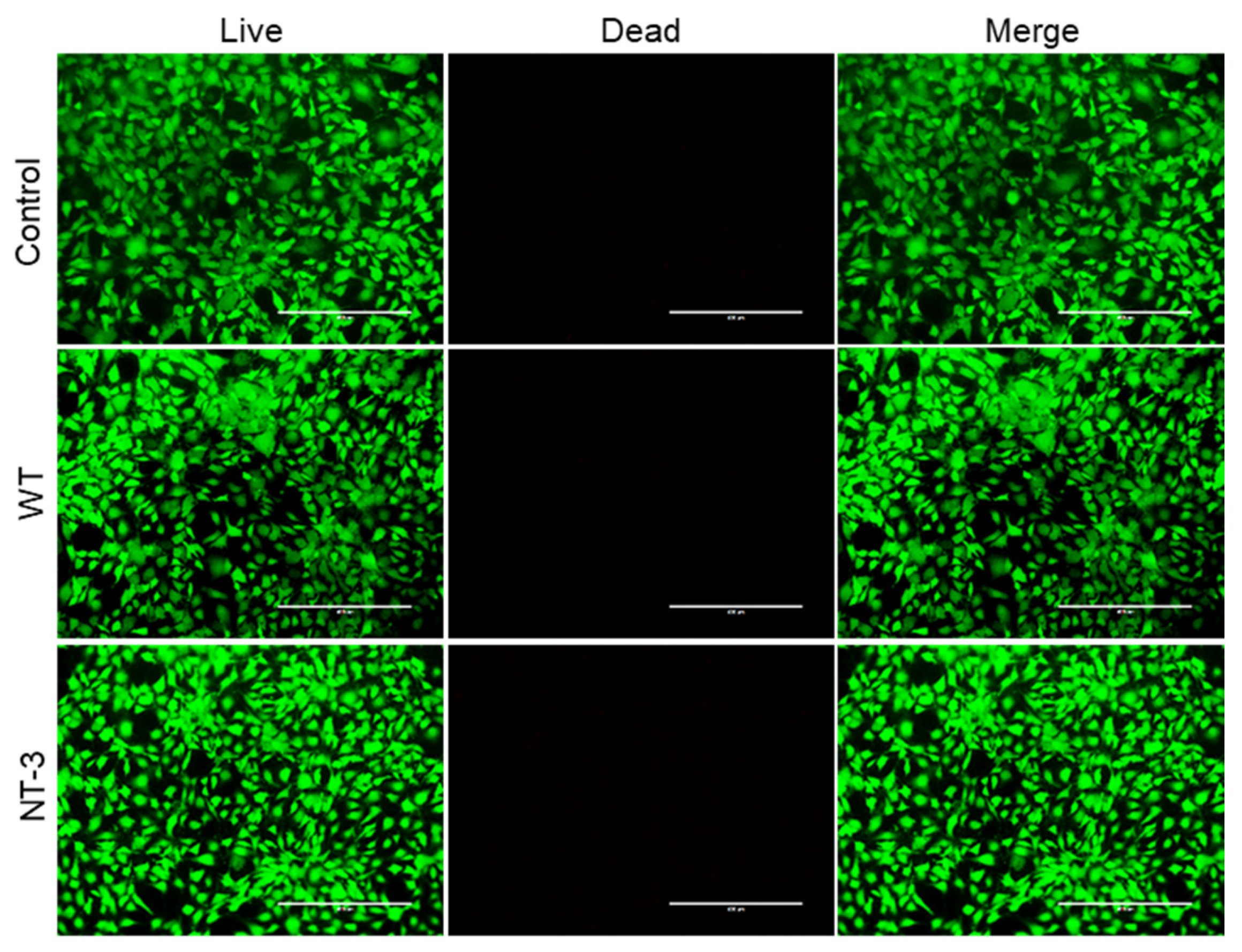
3.4. Cytotoxicity Analysis of Recombinant NT-3 Protein in HT-22 Cells
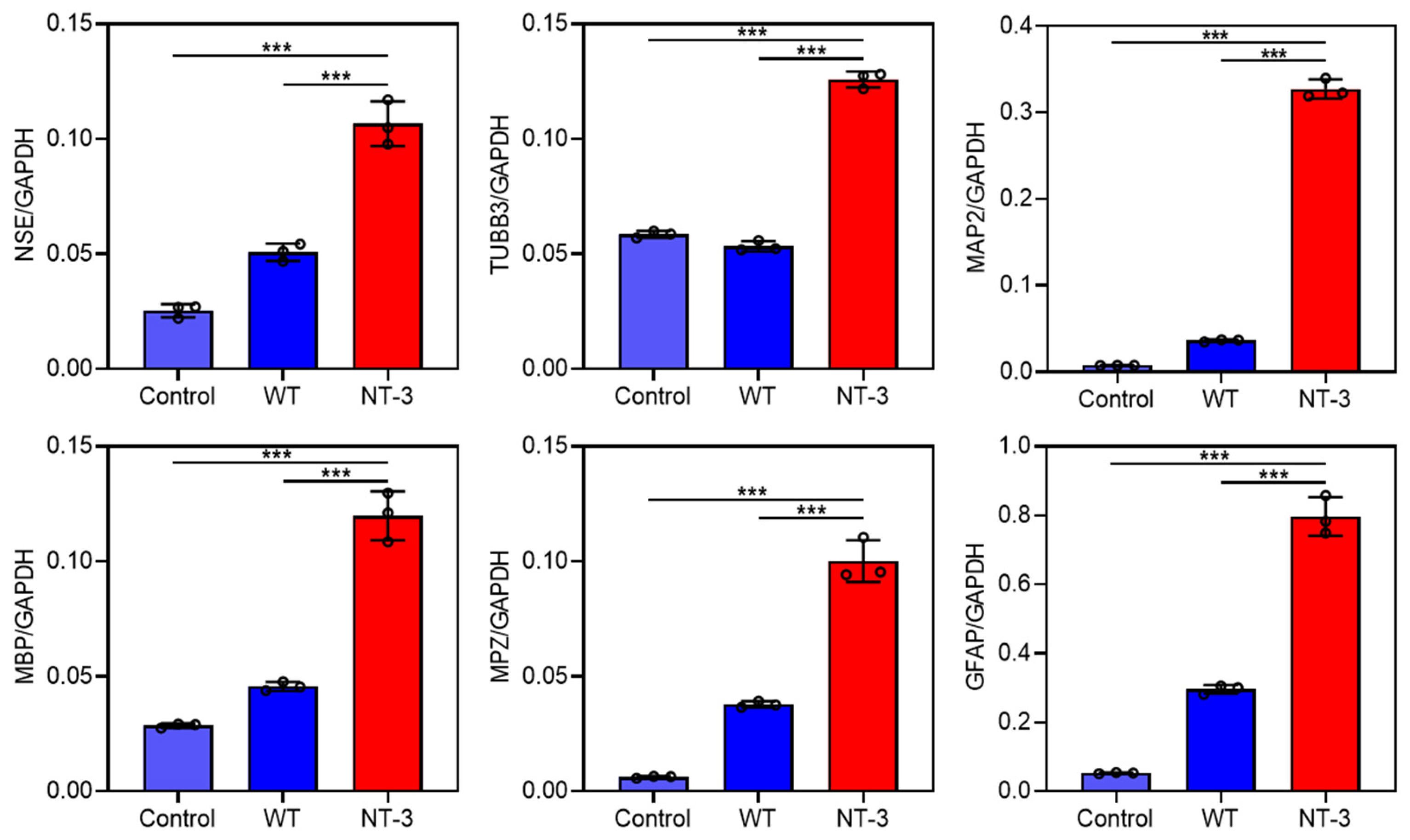
3.5. Differentiation Ability of Recombinant NT-3 Protein in HT-22 Cells
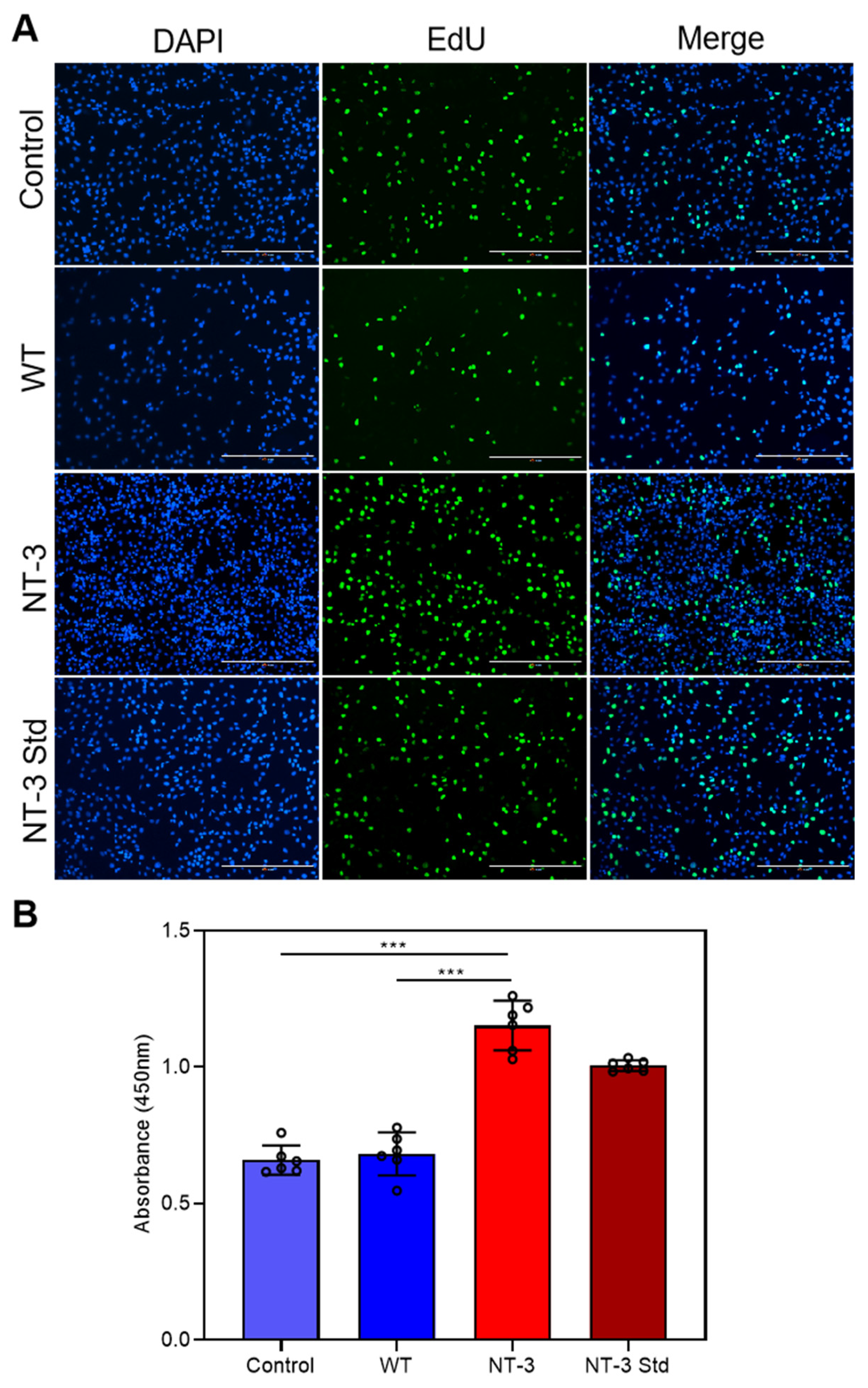
3.6. Proliferation Activity of Recombinant NT-3 Protein in HT-22 Cells
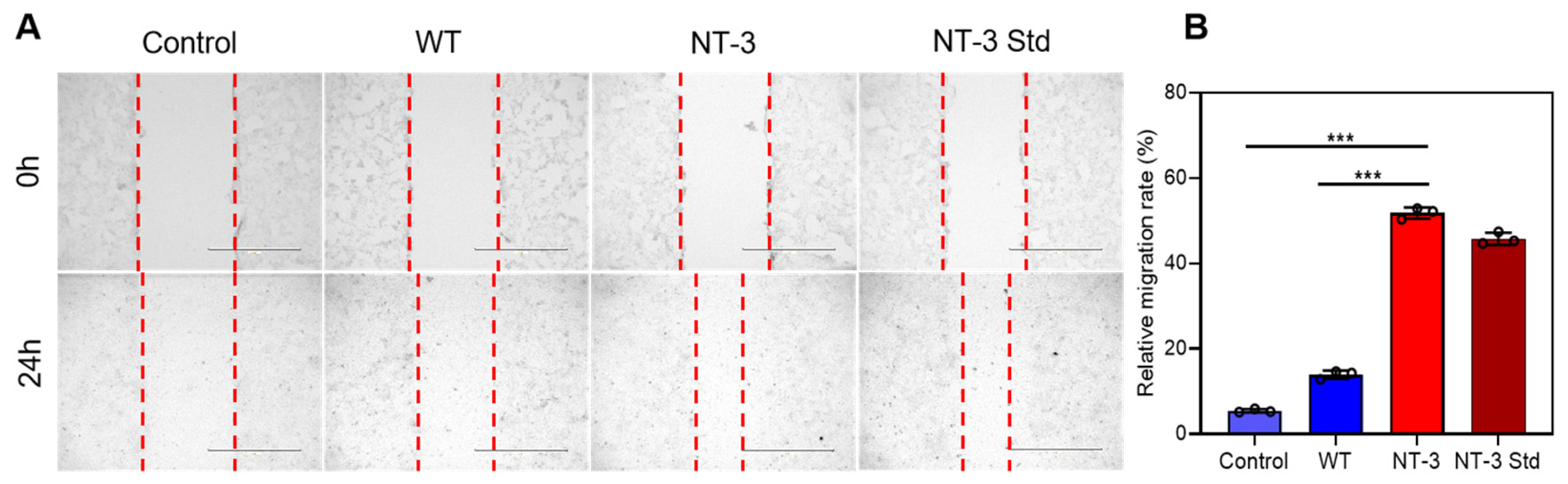
3.7. Migration Capacity of Recombinant NT-3 Protein in HT-22 Cells
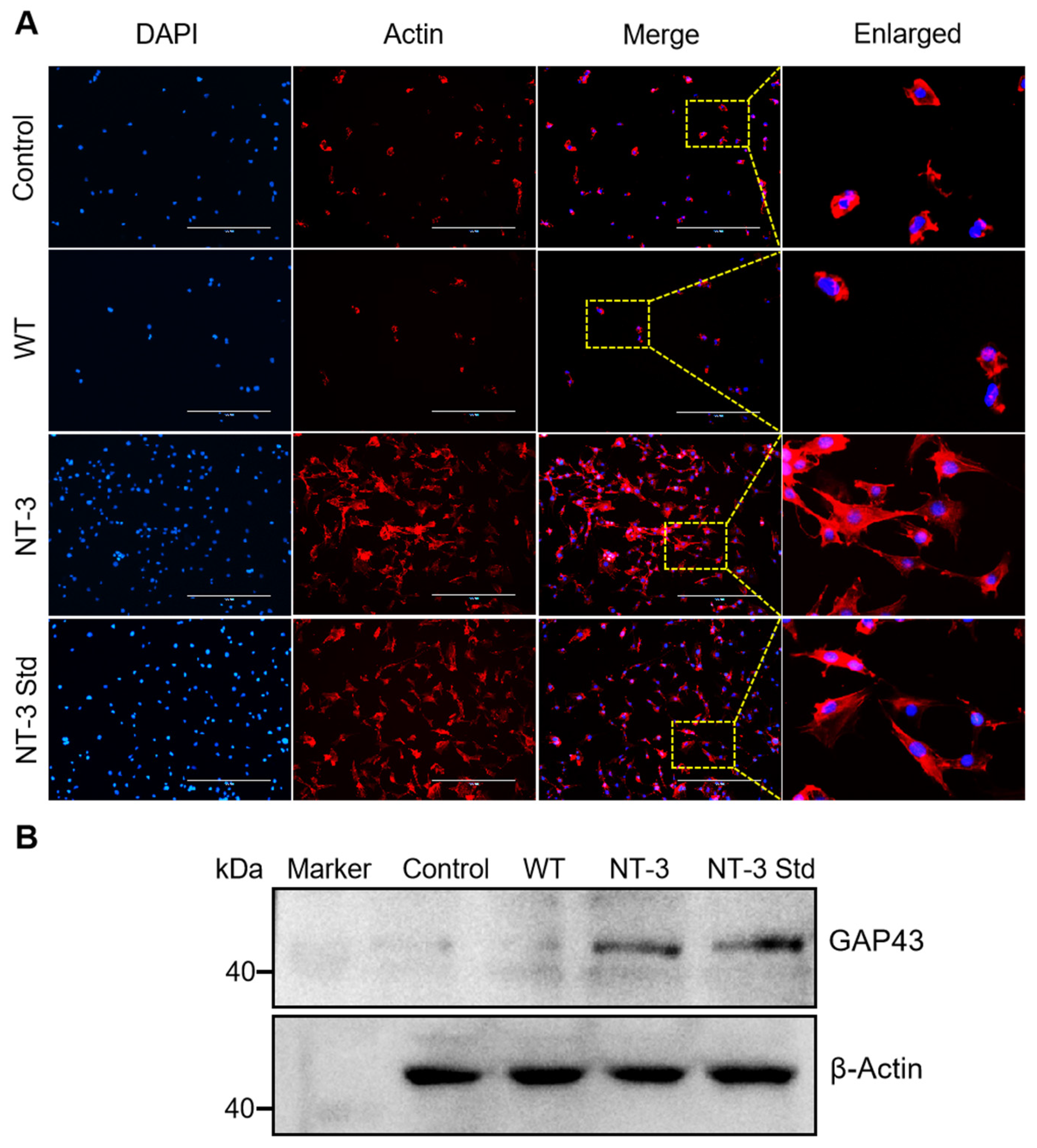
3.8. Neurite Outgrowth of Recombinant NT-3 Protein in HT-22 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, H.J.; Kaplan, D.L. Mechanism of silk processing in insects and spiders. Nature 2003, 424, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Zhou, Z.; Lu, C.; Cheng, D.; Dai, F.; Li, B.; Zhao, P.; Zha, X.; Cheng, T.; Chai, C.; et al. A draft sequence for the genome of the domesticated silkworm (Bombyx mori). Science 2004, 306, 1937–1940. [Google Scholar]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef]
- Kato, T.; Kajikawa, M.; Maenaka, K.; Park, E.Y. Silkworm expression system as a platform technology in life science. Appl. Microbiol. Biotechnol. 2010, 85, 459–470. [Google Scholar] [CrossRef]
- Tomita, M.; Munetsuna, H.; Sato, T.; Adachi, T.; Hino, R.; Hayashi, M.; Shimizu, K.; Nakamura, N.; Tamura, T.; Yoshizato, K. Transgenic silkworms produce recombinant human type III procollagen in cocoons. Nat. Biotechnol. 2003, 21, 52–56. [Google Scholar] [CrossRef]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef]
- Tatematsu, K.; Kobayashi, I.; Uchino, K.; Sezutsu, H.; Iizuka, T.; Yonemura, N.; Tamura, T. Construction of a binary transgenic gene expression system for recombinant protein production in the middle silk gland of the silkworm Bombyx mori. Transgenic Res. 2010, 19, 473–487. [Google Scholar] [CrossRef]
- Ramachandra, Y.L.; Bali, G.; Rai, S.P. Effect of temperature and relative humidity on spinning behaviour of silkworm (Bombyx mori.L). Indian J. Exp. Biol. 2001, 39, 87–89. [Google Scholar]
- Tamura, T.; Thibert, C.; Royer, C.; Kanda, T.; Abraham, E.; Kamba, M.; Komoto, N.; Thomas, J.L.; Mauchamp, B.; Chavancy, G.; et al. Germline transformation of the silkworm Bombyx mori L. using a piggyBac transposon-derived vector. Nat. Biotechnol. 2000, 18, 81–84. [Google Scholar] [CrossRef]
- Wang, F.; Xu, H.; Yuan, L.; Ma, S.; Wang, Y.; Duan, X.; Duan, J.; Xiang, Z.; Xia, Q. An optimized sericin-1 expression system for mass-producing recombinant proteins in the middle silk glands of transgenic silkworms. Transgenic Res. 2013, 22, 925–938. [Google Scholar] [CrossRef]
- Wang, F.; Guo, C.; Yang, Q.; Li, C.; Zhao, P.; Xia, Q.; Kaplan, D.L. Protein composites from silkworm cocoons as versatile biomaterials. Acta Biomater. 2021, 121, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Wang, H.; Wei, K.; Yang, Y.; Zheng, R.Y.; Kim, I.S.; Zhang, K.Q. A Review of Structure Construction of Silk Fibroin Biomaterials from Single Structures to Multi-Level Structures. Int. J. Mol. Sci. 2017, 18, 237. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Lei, H.; Tian, C.; Ji, Y.; Wang, F.; Deng, H.; Zhou, H.; Chen, S.; Zhou, Y.; Meng, Z.; et al. An Efficient Biosynthetic System for Developing Functional Silk Fibroin-Based Biomaterials. Adv. Mater. 2025, 37, e2414878. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [PubMed]
- Bibel, M.; Barde, Y.A. Neurotrophins: Key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000, 14, 2919–2937. [Google Scholar] [CrossRef]
- Barres, B.A.; Raff, M.C.; Gaese, F.; Bartke, I.; Dechant, G.; Barde, Y.A. A crucial role for neurotrophin-3 in oligodendrocyte development. Nature 1994, 367, 371–375. [Google Scholar] [CrossRef]
- de la Rosa, E.J.; Arribas, A.; Frade, J.M.; Rodriguez-Tebar, A. Role of neurotrophins in the control of neural development: Neurotrophin-3 promotes both neuron differentiation and survival of cultured chick retinal cells. Neuroscience 1994, 58, 347–352. [Google Scholar] [CrossRef]
- Davis-Lopez de Carrizosa, M.A.; Morado-Diaz, C.J.; Tena, J.J.; Benitez-Temino, B.; Pecero, M.L.; Morcuende, S.R.; de la Cruz, R.R.; Pastor, A.M. Complementary actions of BDNF and neurotrophin-3 on the firing patterns and synaptic composition of motoneurons. J. Neurosci. 2009, 29, 575–587. [Google Scholar] [CrossRef]
- Lu, H.; Li, M.; Song, T.; Qian, Y.; Xiao, X.; Chen, X.; Zhang, P.; Feng, X.; Parker, T.; Liu, Y. Retrovirus delivered neurotrophin-3 promotes survival, proliferation and neuronal differentiation of human fetal neural stem cells in vitro. Brain Res. Bull. 2008, 77, 158–164. [Google Scholar] [CrossRef]
- Lin, S.; Wang, Y.; Zhang, C.; Xu, J. Modification of the neurotrophin-3 gene promotes cholinergic neuronal differentiation and survival of neural stem cells derived from rat embryonic spinal cord in vitro and in vivo. J. Int. Med. Res. 2012, 40, 1449–1458. [Google Scholar] [CrossRef]
- Benowitz, L.I.; Routtenberg, A. GAP-43: An intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997, 20, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Chato-Astrain, J.; Roda, O.; Sanchez-Porras, D.; Miralles, E.; Alaminos, M.; Campos, F.; Garcia-Garcia, O.D.; Carriel, V. Peripheral nerve regeneration through nerve conduits evokes differential expression of growth-associated protein-43 in the spinal cord. Neural Regen. Res. 2023, 18, 1852–1856. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Z.; Lan, W.; Guo, H.; Chen, F.; Wang, F.; Shen, G.; Xia, Q.; Zhao, P. Bioengineered silkworm model for expressing human neurotrophin-4 with potential biomedical application. Front. Physiol. 2022, 13, 1104929. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Geng, W.; Jiang, X.; Chen, F.; Zhou, M.; Shen, G.; Lin, P.; Xia, Q.; Zhao, P.; Li, Z. Transgenic silkworm expressing bioactive human ciliary neurotrophic factor for biomedical application. Insect Sci. 2025, 32, 809–820. [Google Scholar] [CrossRef]
- Zhang, H.T.; Li, L.Y.; Zou, X.L.; Song, X.B.; Hu, Y.L.; Feng, Z.T.; Wang, T.T. Immunohistochemical distribution of NGF, BDNF, NT-3, and NT-4 in adult rhesus monkey brains. J. Histochem. Cytochem. 2007, 55, 1–19. [Google Scholar] [CrossRef]
- Ammendrup-Johnsen, I.; Naito, Y.; Craig, A.M.; Takahashi, H. Neurotrophin-3 Enhances the Synaptic Organizing Function of TrkC-Protein Tyrosine Phosphatase sigma in Rat Hippocampal Neurons. J. Neurosci. 2015, 35, 12425–12431. [Google Scholar] [CrossRef]
- Hernandez-Echeagaray, E. Neurotrophin-3 modulates synaptic transmission. Vitam. Horm. 2020, 114, 71–89. [Google Scholar]
- Guo, Y.; Su, Z.J.; Chen, Y.K.; Chai, Z. Brain-derived neurotrophic factor/neurotrophin 3 regulate axon initial segment location and affect neuronal excitability in cultured hippocampal neurons. J. Neurochem. 2017, 142, 260–271. [Google Scholar] [CrossRef]
- Safina, D.R.; Rafieva, L.M.; Koval, A.V.; Shkurina, E.E.; Dmitrieva, V.G.; Raevskaia, N.M.; Gasanov, E.V.; Demidiuk, I.V.; Kostrov, S.V. Oligomeric organization of recombinant human neurotrophins expressed in Escherichia coli cells. Bioorg. Chem. 2008, 34, 327–332. [Google Scholar] [CrossRef]
- Chen, X.; Frisina, R.D.; Bowers, W.J.; Frisina, D.R.; Federoff, H.J. HSV amplicon-mediated neurotrophin-3 expression protects murine spiral ganglion neurons from cisplatin-induced damage. Mol. Ther. 2001, 3, 958–963. [Google Scholar] [CrossRef]
- Wang, F.; Wang, R.; Wang, Y.; Zhao, P.; Xia, Q. Large-scale production of bioactive recombinant human acidic fibroblast growth factor in transgenic silkworm cocoons. Sci. Rep. 2015, 5, 16323. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, Y.; Li, Y.; Pan, W.; Tan, G. Silk Fibroin Combined with Electrospinning as a Promising Strategy for Tissue Regeneration. Macromol. Biosci. 2023, 23, e2200380. [Google Scholar] [CrossRef]
- Ma, L.; Sun, Y.; Cheng, Q.; Yang, Z.; Wang, J.; Xu, Z.; Yang, M.; Shuai, Y. Silk Protein-Mediated Biomineralization: From Bioinspired Strategies and Advanced Functions to Biomedical Applications. ACS Appl. Mater. Interfaces 2023, 15, 33191–33206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yin, M.; Dai, J.; Lin, Q.; Yu, H.; Liu, Z. Gene Cloning and Expression of Human Neurotrophin-3 in E. Coli. J. Navy Med. 1999, 20, 125–127. [Google Scholar]
- Park, H.; Poo, M.M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013, 14, 7–23. [Google Scholar] [CrossRef]
- Nieto-Estevez, V.; Defterali, C.; Vicario, C. Distinct Effects of BDNF and NT-3 on the Dendrites and Presynaptic Boutons of Developing Olfactory Bulb GABAergic Interneurons In Vitro. Cell. Mol. Neurobiol. 2022, 42, 1399–1417. [Google Scholar] [CrossRef]
- Sterne, G.D.; Brown, R.A.; Green, C.J.; Terenghi, G. NT-3 modulates NPY expression in primary sensory neurons following peripheral nerve injury. J. Anat. 1998, 193, 273–281. [Google Scholar] [CrossRef]
- Sun, X.; Ni, S.; Zhou, Q.; Zou, D. Exogenous NT-3 Promotes Phenotype Switch of Resident Macrophages and Improves Sciatic Nerve Injury through AMPK/NF-kappaB Signaling Pathway. Neurochem. Res. 2024, 49, 2600–2614. [Google Scholar] [CrossRef]
- Chang, K.; Wu, J.G.; Ma, T.L.; Hsu, S.H.; Cho, K.S.; Yu, Z.; Lennikov, A.; Ashok, A.; Rajagopalan, A.; Chen, M.H.; et al. Bioengineering strategy to promote CNS nerve growth and regeneration via chronic glutamate signaling. Acta Biomater. 2024, 190, 165–177. [Google Scholar] [CrossRef]
- Korshunova, I.; Mosevitsky, M. Role of the growth-associated protein GAP-43 in NCAM-mediated neurite outgrowth. Adv. Exp. Med. Biol. 2010, 663, 169–182. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, W.; Lu, L.; Li, T.; Zhou, M.; Chen, W.; Tan, H.; Zhong, D.; Shen, G.; Lin, P.; Xia, Q.; et al. Biosynthesis of Bioactive Human Neurotrophic Factor 3 in Silkworms and Its Biomedical Applications. Insects 2025, 16, 676. https://doi.org/10.3390/insects16070676
Geng W, Lu L, Li T, Zhou M, Chen W, Tan H, Zhong D, Shen G, Lin P, Xia Q, et al. Biosynthesis of Bioactive Human Neurotrophic Factor 3 in Silkworms and Its Biomedical Applications. Insects. 2025; 16(7):676. https://doi.org/10.3390/insects16070676
Chicago/Turabian StyleGeng, Wenjing, Liang Lu, Tangmin Li, Mingyi Zhou, Wei Chen, Hao Tan, Debin Zhong, Guanwang Shen, Ping Lin, Qingyou Xia, and et al. 2025. "Biosynthesis of Bioactive Human Neurotrophic Factor 3 in Silkworms and Its Biomedical Applications" Insects 16, no. 7: 676. https://doi.org/10.3390/insects16070676
APA StyleGeng, W., Lu, L., Li, T., Zhou, M., Chen, W., Tan, H., Zhong, D., Shen, G., Lin, P., Xia, Q., Zhao, P., & Li, Z. (2025). Biosynthesis of Bioactive Human Neurotrophic Factor 3 in Silkworms and Its Biomedical Applications. Insects, 16(7), 676. https://doi.org/10.3390/insects16070676






