First Test of a Potential Biological Control Agent of Argentine ants (Linepithema humile)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ant Collection
2.2. Aggression
2.3. Isolation and Identification of Fungal Strains
2.4. Inoculation Methods and Virulence Tests
2.5. Median Lethal Concentration Assays
2.6. Statistical Analyses
3. Results
3.1. Aggression
3.2. Isolation and Identification of Fungal Strains
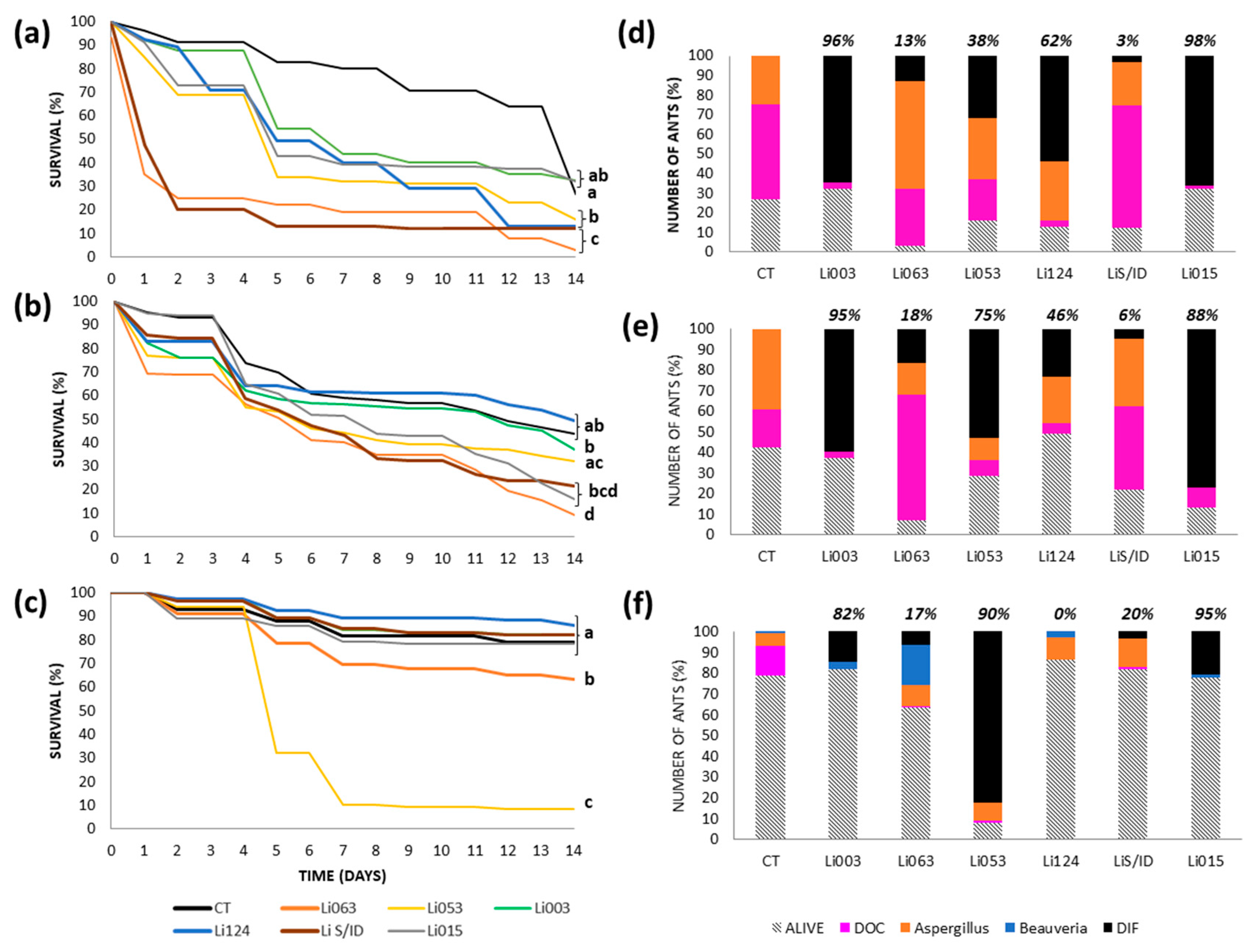
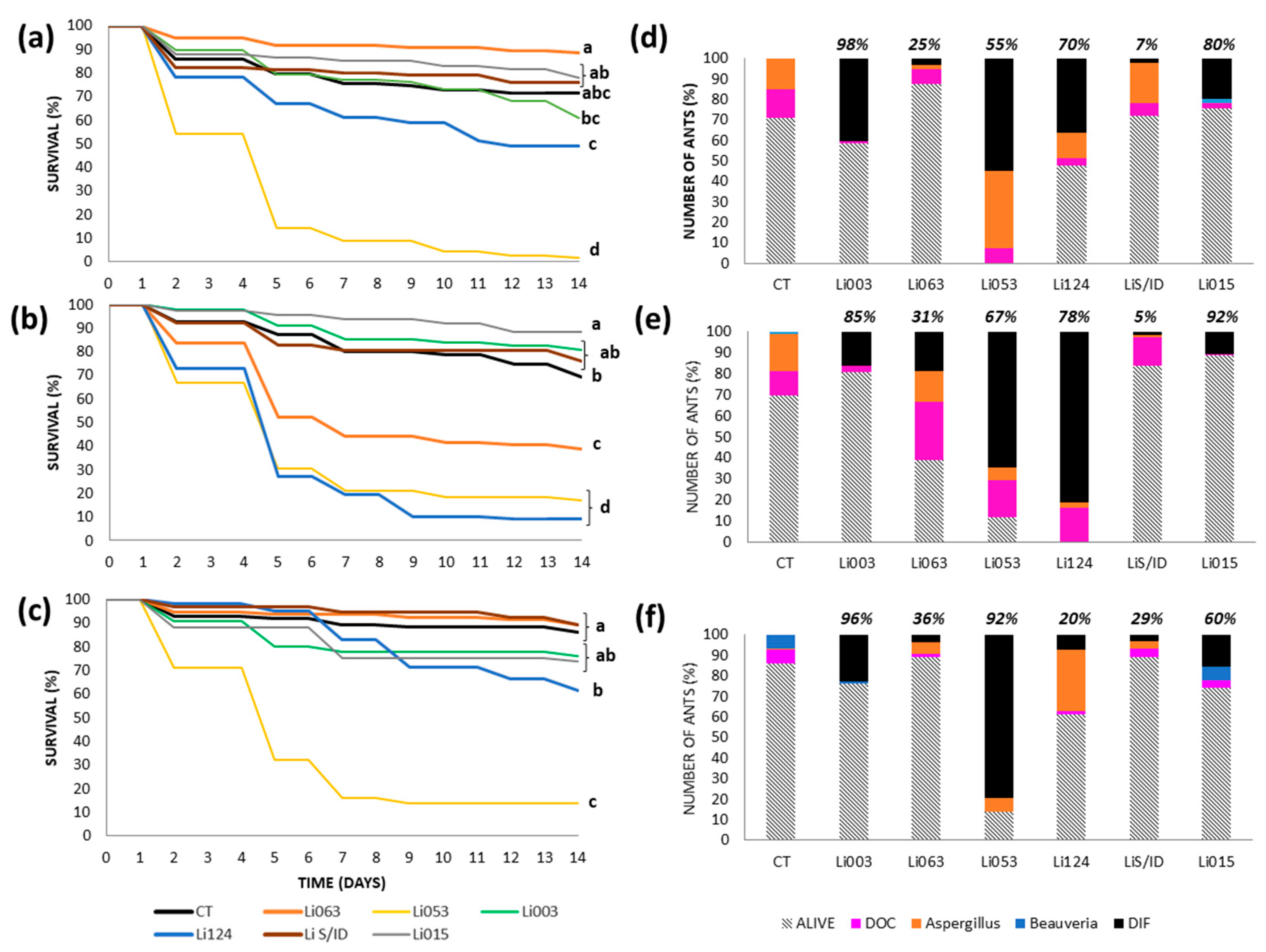
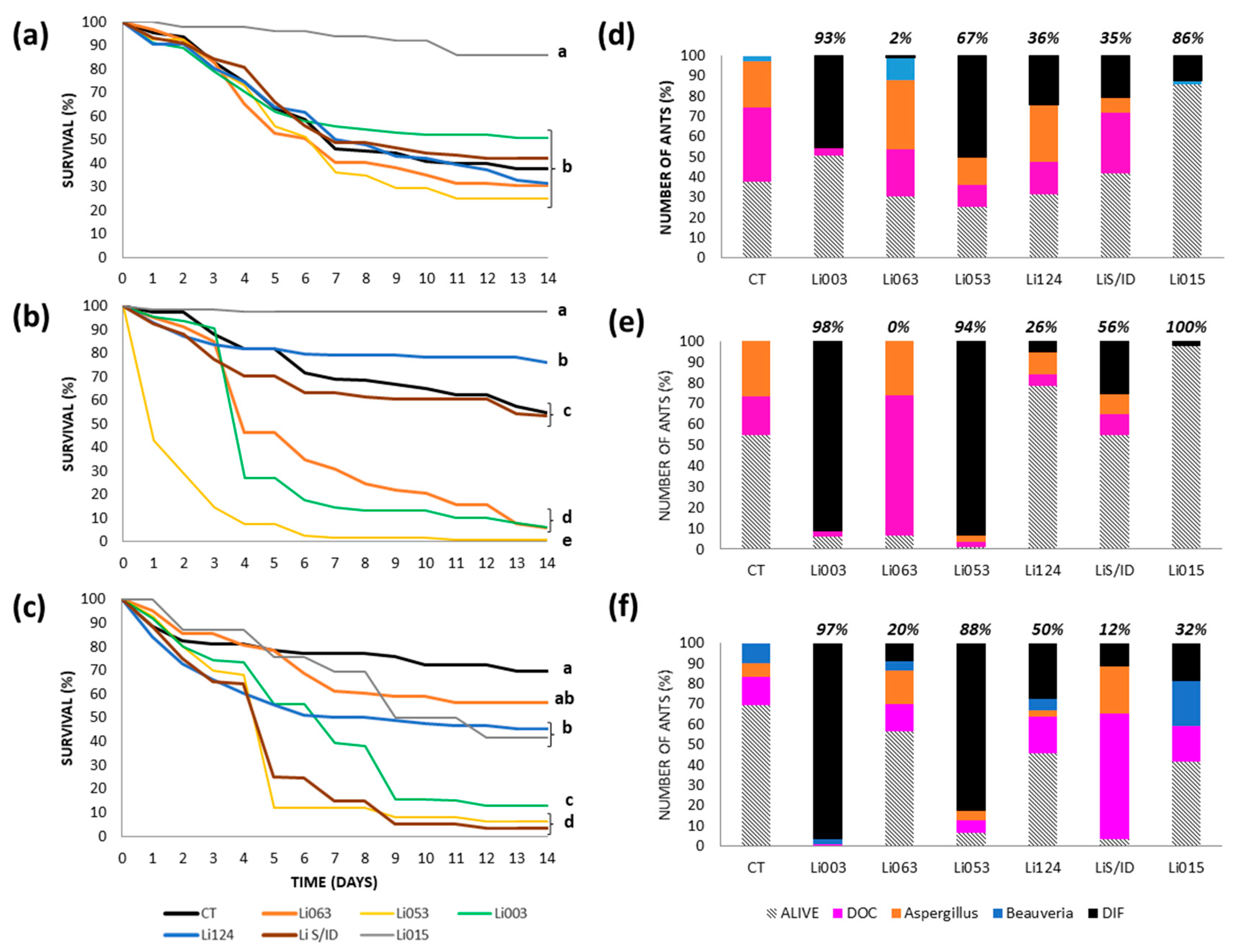
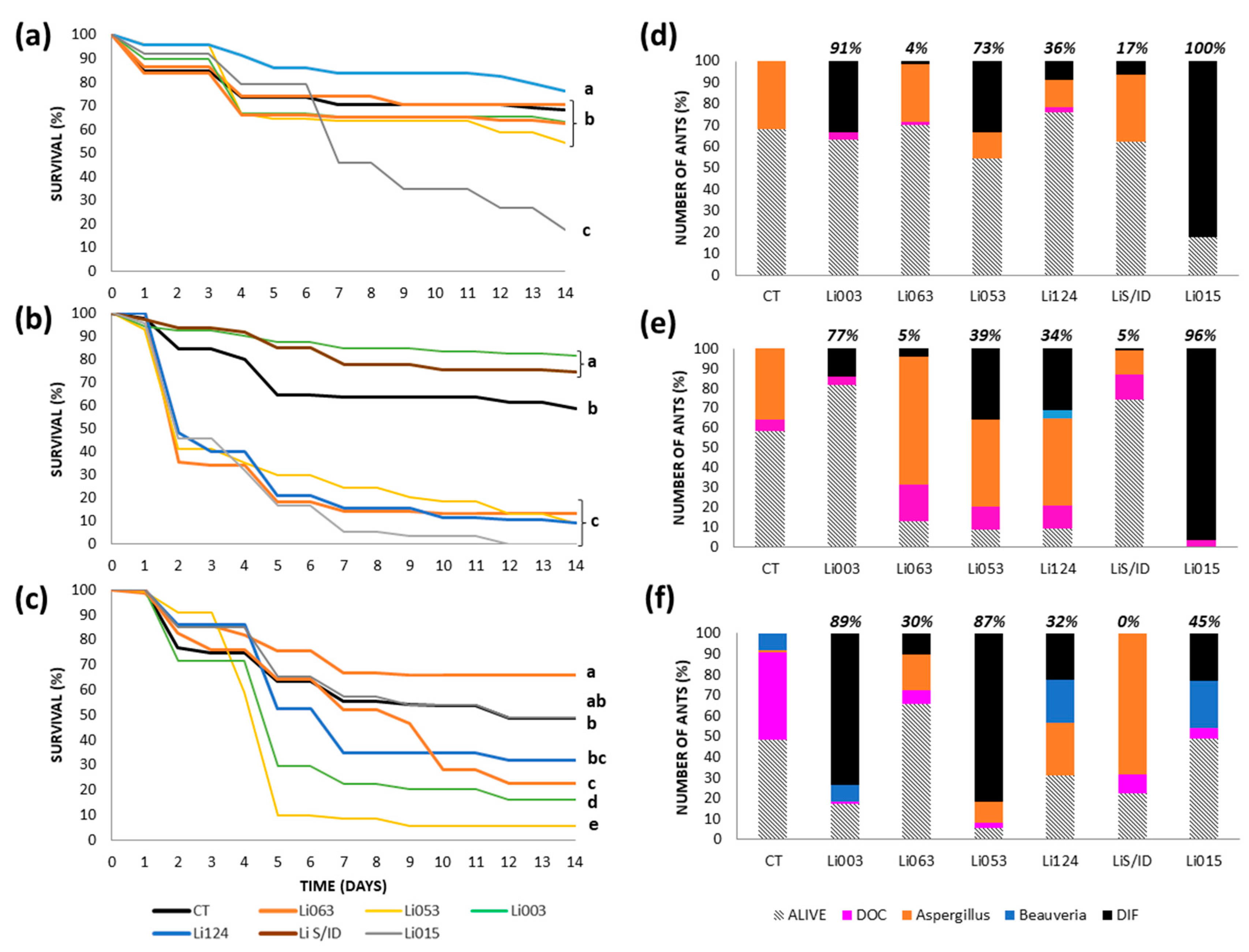
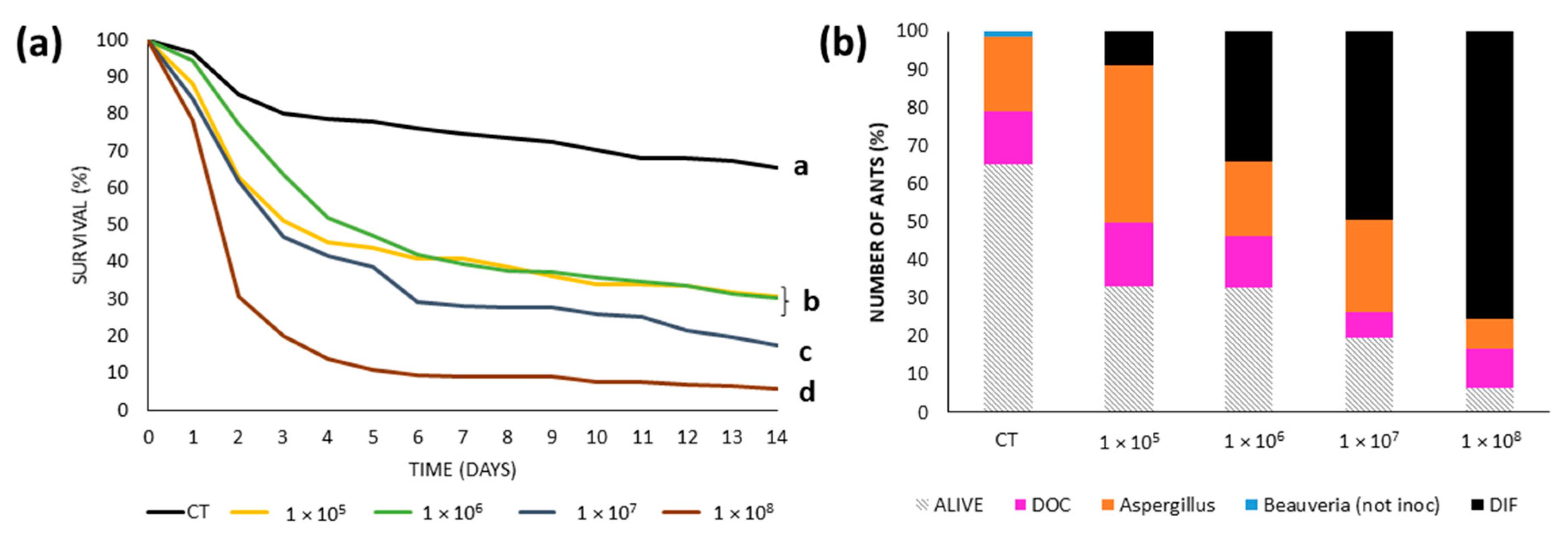
3.3. Virulence Tests: Survival and Cause of Death by Colony
3.3.1. Urban Site: Bernal
3.3.2. Urban Site: Gonnet
3.3.3. Reserves: RECS
3.3.4. Reserve: Rocha
3.4. Median Lethal Concentration
4. Discussion
4.1. Aggression
4.2. Survivorship
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holway, D.A.; Lach, L.; Suarez, A.V.; Tsutsui, N.D.; Case, T.J. The causes and consequences of ant invasions. Annu. Rev. Ecol. Evol. Syst. 2002, 33, 181–233. [Google Scholar] [CrossRef]
- Lach, L.; Parr, C.; Abbott, K. Ant Ecology; Universtity Press: Oxford, UK, 2010; pp. 231–310. [Google Scholar]
- Hoffmann, B.D.; Luque, G.M.; Bellard, C.; Holmes, N.D.; Donlan, C.J. Improving invasive ant eradication as a conservation tool: A review. Biol. Conserv. 2016, 198, 37–49. [Google Scholar] [CrossRef]
- Liu, H.K.; Lin, C.-C.; Huang, L.-H.; Huang, S.-A.; Huang, R.-N. Eradication and control strategies for red imported fire ants (Solenopsis invicta) in Taiwan. Sustainability 2020, 12, 3951. [Google Scholar] [CrossRef]
- Plentovich, S.; Swenson, C.; Reimer, N.; Richardson, M.; Garon, N. The effects of hydramethylnon on the tropical fire ant, Solenopsis geminata (Hymenoptera: Formicidae), and non-target arthropods on Spit Island, Midway Atoll, Hawaii. J. Insect Conserv. 2010, 14, 459–465. [Google Scholar] [CrossRef]
- Davis, P.R.; Van Schagen, J.J.; Widmer, M.A.; Craven, T.J. A Review of Argentine Ant Research in Western Australia; Agricultural Protection Board of Western Australia: Perth, Australia, 1993; pp. 1–95. [Google Scholar]
- King, J.R. Pesticide-free management of invasive ants impacting ground-nesting wildlife populations. Wildl. Soc. Bull. 2024, 48, e1516. [Google Scholar] [CrossRef]
- Oi, D.; Porter, S.; Valles, S. A review of the biological control of fire ants. Myrmecol. News 2015, 21, 101–116. [Google Scholar]
- Chen, J.; Oi, D.H. Naturally occurring compounds/materials as alternatives to synthetic chemical insecticides for use in fire ant management. Insects 2020, 11, 758. [Google Scholar] [CrossRef]
- Vander Meer, R.K. Potential role of pheromones in fire ant control. In Pest Management in the Subtropics: Integrated Pest Management—A Florida Perspective; Rosen, D., Bennett, F.D., Capinera, J.L., Eds.; Intercept Limited: Andover, UK, 1996; pp. 223–232. [Google Scholar]
- Blust, W.E.; Wilson, B.H.; Koonce, K.L.; Nelson, B.D.; Sedberry, J.E. The Red Imported Fire Ant, Solenopsis Invicta Buren: Cultural Control and Effect on Hay Meadows; Bulletin-Louisiana Agricultural Experiment Station (No. 738): Franklinton, LA, USA, 1982; pp. 1–27. [Google Scholar]
- Wild, A.L. Taxonomy and distribution of the Argentine ant, Linepithema humile (Hymenoptera: Formicidae). Ann. Entomol. Soc. Am. 2004, 97, 1204–1215. [Google Scholar] [CrossRef]
- Schagen, J.J.; Davis, P.R.; Widmer, M.A. Ant pests of Western Australia, with particular reference to the Argentine ant (Linepithema humile). In Exotic Ants; CRC Press: Boca Raton, FL, USA, 1994; pp. 174–180. [Google Scholar]
- Holway, D.A. Distribution of the Argentine ant (Linepithema humile) in northern California. Conserv. Biol. 1995, 9, 1634–1637. [Google Scholar] [CrossRef]
- Suarez, A.V.; Holway, D.A.; Case, T.J. Patterns of spread in biological invasions dominated by long-distance jump dispersal: Insights from Argentine ants. Proc. Natl. Acad. Sci. USA 2001, 98, 1095–1100. [Google Scholar] [CrossRef]
- Giraud, T.; Pedersen, J.S.; Keller, L. Evolution of supercolonies: The Argentine ants of southern Europe. Proc. Natl. Acad. Sci. USA 2002, 99, 6075–6079. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.F.; Harris, R.J.; Stanley, M.C. Human-mediated range expansion of Argentine ants Linepithema humile (Hymenoptera: Formicidae) in New Zealand. Sociobiology 2005, 45, 1204–1215. [Google Scholar]
- Walters, A.C. Invasion of Argentine ants (Hymenoptera: Formicidae) in South Australia: Impacts on community composition and abundance of invertebrates in urban parklands. Austral Ecol. 2006, 31, 567–576. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, D.E.; Lyu, D.P. Discovery of the invasive Argentine ant, Linepithema humile (Mayr) (Hymenoptera: Formicidae: Dolichoderinae) in Korea. Korean J. Appl. Entomol. 2020, 59, 71–72. [Google Scholar]
- Angulo, E.; Guenard, B.; Balzani, P.; Bang, A.; Frizzi, F.; Masoni, A.; Abril, S.; Suarez, A.V.; Hoffmann, B.; Benelli, G.; et al. The Argentine ant, Linepithema humile: Natural history, ecology and impact of a successful invader. Entomol. Gen. 2024, 44, 41–61. [Google Scholar] [CrossRef]
- Holway, D.A. competitive mechanisms underlying the displacement of native ants by the invasive Argentine ant. Ecology 1999, 80, 238–251. [Google Scholar] [CrossRef]
- Cole, F.R.; Medeiros, A.C.; Loope, L.L.; Zuehlke, W.W. Effects of the Argentine ant on arthropod fauna of hawaiian high-elevation shrubland. Ecology 1992, 73, 1313–1322. [Google Scholar] [CrossRef]
- Holway, D.A. Effect of Argentine ant invasions on ground-dwelling arthropods in northern California riparian woodlands. Oecologia 1998, 116, 252–258. [Google Scholar] [CrossRef]
- Xu, Y.; Vargo, E.L.; Tsuji, K.; Wylie, R. Exotic ants of the Asia-Pacific: Invasion, national response, and ongoing needs. Annu. Rev. Entomol. 2022, 67, 27–42. [Google Scholar] [CrossRef]
- Silverman, J.; Brightwell, R.J. The Argentine ant: Challenges in managing an invasive unicolonial pest. Annu. Rev. Entomol. 2008, 53, 231–252. [Google Scholar] [CrossRef]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species a Selection from the Global Invasive Species Database; The Invasive Species Specialist Group (ISSG) a Specialist Group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN): Auckland, New Zealand, 2000; pp. 1–12. [Google Scholar]
- Tsutsui, N.D.; Case, T.J. Population genetics and colony structure of the Argentine ant (Linepithema humile) in its native and introduced ranges. Evolution 2001, 55, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Passera, L. Characteristics of Tramp Species. In Exotic Ants; CRC Press: Boca Raton, FL, USA, 1994; pp. 23–43. [Google Scholar]
- Gilboa, S.; Klotz, J.H.; Nonacs, P. Urban infestation patterns of Argentine ants, Linepithema humile, in Los Angeles. Psyche J. Entomol. 2012, 2012, 925149. [Google Scholar] [CrossRef]
- Vega, S.J.; Rust, M.K. The Argentine ant-a significant invasive species in agricultural, urban and natural environment. Sociobiology 2001, 37, 3–26. [Google Scholar]
- Mgocheki, N.; Addison, P. Interference of ants (Hymenoptera: Formicidae) with biological control of the vine mealybug Planococcus Ficus (Signoret) (Hemiptera: Pseudococcidae). Biol. Control 2009, 49, 180–185. [Google Scholar] [CrossRef]
- Hooper-Bui, L.M.; Rust, M.K. Oral toxicity of abamectin, boric acid, fipronil, and hydramethylnon to laboratory colonies of Argentine ants (Hymenoptera: Formicidae). J. Econ. Entomol. 2000, 93, 858–864. [Google Scholar] [CrossRef]
- Klotz, J.H.; Rust, M.K.; Costa, H.S.; Reierson, D.A.; Kido, K. Strategies for controlling Argentine ants (Hymenoptera: Formicidae) with sprays and baits. J. Agric. Urban Entomol. 2002, 19, 85–94. [Google Scholar]
- Suiter, D.R.; Gochnour, B.M.; Holloway, J.B.; Vail, K.M. Alternative methods of ant (Hymenoptera: Formicidae) control with emphasis on the Argentine ant, Linepithema humile. Insects 2021, 12, 487. [Google Scholar] [CrossRef] [PubMed]
- Enzmann, B.L.; Kapheim, K.M.; Wang, T.B.; Nonacs, P. Giving them what they want: Manipulating argentine ant activity patterns with water. J. Appl. Entomol. 2012, 136, 588–595. [Google Scholar] [CrossRef]
- Gruber, M.A.M.; Cooling, M.; Baty, J.W.; Buckley, K.; Friedlander, A.; Quinn, O.; Russell, J.F.E.J.; Sébastien, A.; Lester, P.J. Single-stranded RNA viruses infecting the invasive Argentine ant, Linepithema humile. Sci. Rep. 2017, 7, 3304. [Google Scholar] [CrossRef]
- Orr, M.R.; Seike, S.H.; Benson, W.W.; Dahlsten, D.L. Host specificity of Pseudacteon (Diptera: Phoridae) parasitoids that attack Linepithema (Hymenoptera: Formicidae) in South America. Environm. Entomol. 2001, 30, 742–747. [Google Scholar] [CrossRef]
- Plowes, R.M.; Folgarait, P.J.; Gilbert, L.E. The introduction of the fire ant parasitoid Pseudacteon nocens in North America: Challenges when establishing small populations. BioControl 2012, 57, 503–514. [Google Scholar] [CrossRef]
- Folgarait, P.J.; Guillade, A.C. Parasitoides de Hormigas. In Hormigas de Colombia, 1st ed.; Fernández, F., Guerrero, R.J., Delsinne, T., Eds.; Universidad Nacional de Colombia: Bogotá, Colombia, 2019; pp. 255–318. [Google Scholar]
- Kesäniemi, J.; Koskimäki, J.J.; Jurvansuu, J. Corpse management of the invasive Argentine ant inhibits growth of pathogenic fungi. Sci. Rep. 2019, 9, 7593. [Google Scholar] [CrossRef]
- Pavan, M.; Ronchetti, G. Studi sulla morfologia esterna e anatomia interna dell’operaia di Iridomyrmex humilis Mayr e ricerche chimiche e biologiche sulla iridomirmecina. In Atti Della Societa Italiana di Scienze Naturali Milano; Società Italiana di Scienze Naturali: Milano, Italy, 1955; pp. 379–477. [Google Scholar]
- Harris, R.J. Potential Impact of the Argentine Ant (Linepithema Humile) in New Zealand and Options for its Control; Department of Conservation: Wellington, New Zealand, 2002; pp. 1–36.
- Lopez, E.; Orduz, S. Metarhizium anisopliae and Trichoderma viride for control of nests of the fungus-growing ant, Atta cephalotes. Biol. Control 2003, 27, 194–200. [Google Scholar] [CrossRef]
- Folgarait, P.J.; Goffré, D. Biological control of leaf-cutter ants using pathogenic fungi: Experimental laboratory and field studies. Entomol. Exp. Appl. 2021, 169, 813–824. [Google Scholar] [CrossRef]
- Folgarait, P.J.; Goffré, D. Control of pest ants by pathogenic fungi: State of the art. Front. Fungal Biol. 2023, 4, 1199110. [Google Scholar] [CrossRef]
- Suarez, A.V.; Tsutsui, N.D.; Holway, D.A.; Case, T.J. Behavioral and genetic differentiation between native and introduced populations of the Argentine ant. Biol. Invasions 1999, 1, 43–53. [Google Scholar] [CrossRef]
- Möller, E.M.; Bahnweg, G.; Sandermann, H.; Geiger, H.H. A Simple and efficient protocol for isolation of high molecular weight DNA from filamentous fungi, fruit bodies, and infected plant tissues. Nucleic Acids Res. 1992, 20, 6115–6116. [Google Scholar] [CrossRef]
- Rehner, S.A.; Samuels, G.J. Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycol. Res. 1994, 98, 625–634. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Goffré, D.; Jensen, A.B.; Lopez Lastra, C.C.; Humber, R.A.; Folgarait, P.J. Conidiobolus lunulus, a new entomophthoralean species isolated from leafcutter ants. Mycologia 2021, 113, 56–64. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Finney, D.J. Probit Analysis: A Statistical Treatment of the Sigmoid Response Curve; Macmillan: Oxford, UK, 1947; pp. 1–256. [Google Scholar]
- Abbott, W.S. Abbott’s Formula. J. Econ. Entomol. 1925, 18, 267–268. [Google Scholar]
- SYSTAT, Version 13; Systat Software Inc.: Chicago, IL, USA, 2009.
- Rehner, S.A.; Kepler, R.M. Species limits, phylogeography and reproductive mode in the Metarhizium anisopliae complex. J. Invertebr. Pathol. 2017, 148, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, H.; Cano, J.; Gené, J.; García, D.; Hernández, M.; Guarro, J. Polyphasic analysis of Purpureocillium lilacinum isolates from different origins and proposal of the new species Purpureocillium lavendulum. Mycologia 2013, 105, 151–161. [Google Scholar] [CrossRef]
- Rombach, M.C.; Humber, R.A.; Evans, H.C. Metarhizium album, a fungal pathogen of leaf-and planthoppers of rice. Trans. Br. Mycol. Soc. 1987, 88, 451–459. [Google Scholar] [CrossRef]
- Lombard, L.; Sandoval-Denis, M.; Lamprecht, S.C.; Crous, P.W. Epitypification of Fusarium oxysporum—Clearing the taxonomic chaos. Persoonia Mol. Phylogeny Evol. Fungi 2019, 43, 1–47. [Google Scholar] [CrossRef]
- Horn, B.W. Aspergillus caelatus, a new species in section Flavi. Mycotaxon 1997, 61, 185–191. [Google Scholar]
- Rehner, S.A.; Minnis, A.M.; Sung, G.; Luangsa-ard, J.J.; Devotto, L.; Humber, R.A. Phylogeny and systematics of the anamorphic, entomopathogenic genus Beauveria. Mycologia 2011, 103, 1055–1073. [Google Scholar] [CrossRef]
- Pedersen, J.S.; Krieger, M.J.B.; Vogel, V.; Giraud, T.; Keller, L. Native supercolonies of unrelated individuals in the invasive Argentine ant. Evolution 2006, 60, 782–791. [Google Scholar]
- Vogel, V.; Pedersen, J.S.; D’Ettorre, P.; Lehmann, L.; Keller, L. Dynamics and genetic structure of Argentine ant supercolonies in their native range. Evolution 2009, 63, 1627–1639. [Google Scholar] [CrossRef]
- Thomas, M.L.; Payne-Makrisâ, C.M.; Suarez, A.V.; Tsutsui, N.D.; Holway, D.A. When supercolonies collide: Territorial aggression in an invasive and unicolonial social insect. Mol. Ecol. 2006, 15, 4303–4315. [Google Scholar] [CrossRef] [PubMed]
- Sunamura, E.; Hatsumi, S.; Karino, S.; Nishisue, K.; Terayama, M.; Kitade, O.; Tatsuki, S. Four mutually incompatible Argentine ant supercolonies in Japan: Inferring invasion history of introduced Argentine ants from their social structure. Biol. Invasions 2009, 11, 2329–2339. [Google Scholar] [CrossRef]
- Roulston, T.H.; Buczkowski, G.; Silverman, J. Nestmate discrimination in ants: Effect of bioassay on aggressive behavior. Insectes Soc. 2003, 50, 151–159. [Google Scholar] [CrossRef]
- Hughes, W.O.H.; Boomsma, J.J. Let your enemy do the work: Within-host interactions between two fungal parasites of leaf-cutting ants. Proc. R. Soc. B Biol. Sci. 2004, 271, S104–S106. [Google Scholar] [CrossRef] [PubMed]
- Goffré, D.; Folgarait, P.J. Purpureocillium lilacinum, potential agent for biological control of the leaf-cutting ant Acromyrmex lundii. J. Invertebr. Pathol. 2015, 130, 107–115. [Google Scholar] [CrossRef]
- Folgarait, P.J.; Goffré, D.; Giraldo Osorio, A. Beauveria bassiana for the control of leafcutter ants: Strain and host differences. Biocontrol Sci. Technol. 2020, 30, 996–1005. [Google Scholar] [CrossRef]
- Uma Devi, K.; Padmavathi, J.; Uma Maheswara Rao, C.; Khan, A.A.P.; Mohan, M.C. A study of host specificity in the entomopathogenic fungus Beauveria bassiana (Hypocreales, Clavicipitaceae). Biocontrol Sci. Technol. 2008, 18, 975–989. [Google Scholar] [CrossRef]
- Vestergaard, S.; Cherry, A.; Keller, S.; Goettel, M. Safety of hyphomycete fungi as microbial control agents. In Environmental Impacts of Microbial Insecticides: Need and Methods for Risk Assessment; Hokkanen, H.M.T., Hajek, A.E., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 35–62. [Google Scholar]
- Zimmermann, G. Review on safety of the entomopathogenic fungi Beauveria bassiana and Beauveria brongniartii. Biocontrol Sci. Technol. 2007, 17, 553–596. [Google Scholar] [CrossRef]
- Rajput, M.; Sajid, M.S.; Rajput, N.A.; George, D.R.; Usman, M.; Zeeshan, M.; Iqbal, O.; Bhutto, B.; Atiq, M.; Rizwan, H.M.; et al. Entomopathogenic fungi as alternatives to chemical acaricides: Challenges, opportunities and prospects for sustainable tick control. Insects 2024, 15, 1017. [Google Scholar] [CrossRef]
| Sites/Time | Morning (9:00 to 12:00) | Midday to Early Afternoon (12:00 to 17:00) | Late Afternoon (17:00 Onwards) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Score | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 |
| Rocha CT | 3 | 2 | 14 | 1 | 7 | ||||||||||
| RECS CT | 3 | 7 | 5 | ||||||||||||
| GONNET CT | 6 | 7 | 6 | 1 | |||||||||||
| BERNAL CT | 6 | 7 | 7 | ||||||||||||
| RECS vs. ROCHA | 2 | 1 | 3 | 1 | 1 | 4 | 2 | 4 | |||||||
| GONNET vs. BERNAL | 1 | 5 | 1 | 1 | 4 | 1 | 2 | 2 | 1 | ||||||
| ROCHA vs. BERNAL | 1 | 10 | 7 | 13 | |||||||||||
| RECS vs. BERNAL | 6 | 1 | 5 | 2 | 1 | 3 | |||||||||
| RECS vs. GONNET | 1 | 1 | 4 | 2 | 2 | 1 | 3 | 2 | 4 | ||||||
| GONNET vs. ROCHA | 2 | 4 | 6 | 2 | 7 | ||||||||||
| Strain | Sampling Site | 28S rDNA BLAST Result—Species and % Identity | Final Species Identification |
|---|---|---|---|
| Li053 | ROCHA | Beauveria bassiana ATCC 26854 and B. bassiana CBS:212.61 (99.68%) | Beauveria bassiana |
| LiS/ID | ROCHA | Metarhizium anisopliae CBS:662.67 (97.96%) and M. album ARSEF 2082 (92%) | Metarhizium album |
| Li124 | ROCHA | Purpureocillium lilacinum CBS:129410 and P. lavendulum CBS:128678 (100%), and P. lilacinum ATCC 10114 (99.29%) | Purpureocillium lilacinum |
| Li063 | ROCHA | Fusarium incarnatum CBS:173.30, F. oxysporum CBS:130308, F. oxysporum f. cubense strain ATCC 96285 and F. redolens CBS:128.73 (100%) | Fusarium oxysporum |
| Li003 | RECS | Aspergillus caelatus CBS:763.97 (99.75%) | Aspergillus caelatus |
| Li015 | ROCHA | Aspergillus caelatus CBS:763.97 (94.84%) | Aspergillus caelatus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Folgarait, P.J.; Goffré, D. First Test of a Potential Biological Control Agent of Argentine ants (Linepithema humile). Insects 2025, 16, 677. https://doi.org/10.3390/insects16070677
Folgarait PJ, Goffré D. First Test of a Potential Biological Control Agent of Argentine ants (Linepithema humile). Insects. 2025; 16(7):677. https://doi.org/10.3390/insects16070677
Chicago/Turabian StyleFolgarait, Patricia J., and Daniela Goffré. 2025. "First Test of a Potential Biological Control Agent of Argentine ants (Linepithema humile)" Insects 16, no. 7: 677. https://doi.org/10.3390/insects16070677
APA StyleFolgarait, P. J., & Goffré, D. (2025). First Test of a Potential Biological Control Agent of Argentine ants (Linepithema humile). Insects, 16(7), 677. https://doi.org/10.3390/insects16070677








