Simple Summary
Honeybees are essential for pollinating plants and maintaining ecosystems. This review focuses on two subspecies in Algeria, Apis mellifera intermissa and Apis mellifera sahariensis, examining how they adapt to environmental challenges like climate and disease. We highlight the role of Apis mellifera intermissa’s natural behaviors in resisting pests like the Varroa mite. Understanding the unique traits of these local bees is key for sustainable beekeeping and protecting the biodiversity they support, which is critical for food production and ecosystem health.
Abstract
Honeybees are vital for pollination and the overall health of ecosystems. Since the 18th century, the intricate biology of honeybees has been a subject of scientific inquiry. Understanding their biological and behavioral characteristics is essential for effective beekeeping, honey production, and ecosystem sustainability. This review examines the environmental impact and management practices on the health of local honeybees in Algeria, focusing on Apis mellifera intermissa and Apis mellifera sahariensis. We summarize research findings on genetic diversity, morphometric traits, behavioral characteristics, and adaptation of local honeybees. Additionally, we discuss the threats posed by abiotic and biotic stressors and highlight the importance of conservation and sustainable management. The reviewed studies indicate that environmental factors significantly influence the behavioral characteristics and adaptation of local honeybees. Notably, the hygienic behavior of A. m. intermissa contributes to their resistance against diseases and the Varroa destructor mite. Further research in these areas is important for enhancing our understanding of honeybee health and population dynamics in Algeria, thereby informing strategies for sustainable beekeeping practices.
1. Introduction
Honeybees play an essential role in pollination and maintaining ecosystems, contributing to biodiversity and agricultural productivity [1]. Understanding their behaviors is crucial for effective colony management, as these behaviors influence health, productivity, and resilience. Key behaviors such as foraging, thermoregulation, hygienic behavior, and grooming significantly affect colony stability and adaptability to environmental challenges. These behaviors are vital for managing pests and diseases, optimizing honey production, and ensuring sustainable beekeeping practices.
In Algeria, two subspecies of honeybees, Apis mellifera intermissa and Apis mellifera sahariensis hold particular significance due to their adaptations to the country’s diverse climatic zones. Algeria spans from a Mediterranean climate in the north [2] to arid and semi-arid regions with hyper-saline zones in the south [3]. A. m. intermissa is primarily found in North Africa, including Algeria and Tunisia (Figure 1), where it has adapted to the North African climate [4]. In contrast, A. m. sahariensis thrives in the harsh conditions of the Sahara Desert, with its range in Algeria including regions such as Béchar, Djebel Antar, and Beni Ounif [5].
Grooming behavior is known to influence honeybee resistance to pests like Varroa destructor and is shaped by genetic factors and natural selection [6]. However, grooming is just one aspect of their complex behavioral repertoire. Other key behaviors, including foraging efficiency, thermoregulation, and hygienic traits, are equally critical for colony survival and adaptation. Despite their ecological and economic importance, studies investigating the molecular and biochemical mechanisms underlying these behaviors in Algerian honeybees remain limited [7,8].
Conserving the genetic diversity of A. m. intermissa and A. m. sahariensis is vital due to their unique adaptations and increasing threats from habitat loss and climate change [9]. The recent availability of the western honeybee genome and transcriptome has provided valuable tools to explore the genetics of these subspecies, offering insights into their adaptation mechanisms [10,11].
This review synthesizes current knowledge about the behavioral characteristics and adaptations of Algerian honeybees, focusing on their role in sustaining local ecosystems and agriculture. By examining these subspecies’ behaviors, the study seeks to contribute to the development of sustainable beekeeping practices and conservation strategies that ensure the long-term survival of Algeria’s honeybee populations. Conserving local honeybee populations is critical for maintaining biodiversity and ecosystem services, which are essential for environmental stability and agricultural sustainability [8].
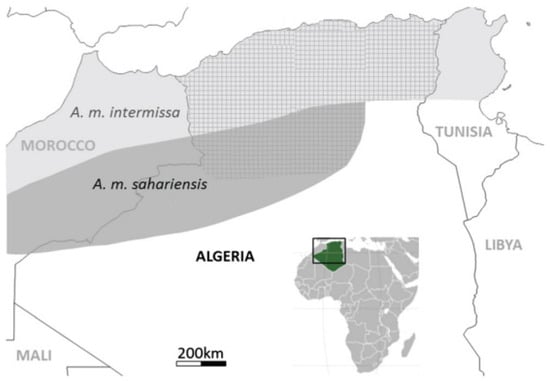

Figure 1.
Distribution Map of Local Honeybees in Algeria (A. m. intermissa and A. m. sahariensis). The square frame highlights the geographical focus on Algeria, and the green area on the inset map shows Algeria's location within the African continent. The distribution boundaries of the honeybee subspecies are based on Adjlane et al. [12] and previous studies [4,13,14].
2. Materials and Methods
2.1. Literature Review and Data Collection
A systematic literature review was conducted to gather publications on the behavior and adaptation of local honeybee subspecies in Algeria: Apis mellifera intermissa and Apis mellifera sahariensis. The search covered the period from 1916 to 2024, including studies in all languages. After removing duplicates, 85 articles were filtered by titles and abstracts, and 30 studies were selected after a full-text review.
2.2. Inclusion Criteria
Inclusion criteria included studies on the behavioral, physiological characteristics, and adaptation of these subspecies. Articles not meeting these criteria were excluded.
2.3. Data Synthesis and Analysis
A thematic analysis was used to organize the study results, categorizing behaviors, environmental adaptation, and disease resistance. A qualitative synthesis was preferred due to the diversity of methodologies used in the studies.
2.4. PRISMA Flowchart
A PRISMA flowchart was included to illustrate the study-selection process, from identification to the inclusion of studies.
3. Results
The literature search identified a total of 85 papers related to our study. From this search, we finally gathered 30 studies, a summary of variables studied, and methods used in studies on the behavior and adaptation of local Algerian honeybees according to the methodology used and subspecies of honeybees (Table 1), and from each one we harvested information on factors influencing adaptation processes of local honeybees in Algeria (abiotic stressors and biotic stressors), biological characteristics of local honeybees in Algeria; A. m. intermissa and A. m. sahariensis: general population structure, biometric analysis, morphological differences. As well, information about adaptation to specific climatic conditions, temperature, and to diseases, and natural selection were also included.

Table 1.
Summary of variables studied, methodologies applied, and subspecies investigated in research on the behavior and adaptation of local Algerian honeybees.
4. Discussion
The 30 selected papers provided valuable insights into the stressors influencing adaptive responses in local honeybees in Algeria, specifically focusing on abiotic and biotic stressors, biological characteristics, and the adaptation mechanisms of A. m. intermissa and A. m. sahariensis. However, it is important to emphasize that the relatively small number of studies on these North African subspecies clearly indicates that they are significantly understudied compared to Central European subspecies such as A. m. carnica and A. m. ligustica. A simple search on Google scholar “intermissa” OR “sahariensis” gives about 7870 studies, and for “ligustica” OR “carnica” about 33,600 studies. This lack of research highlights the need for further investigations into the unique adaptations and ecological roles of A. m. intermissa and A. m. sahariensis, particularly in response to specific climatic conditions, temperature variations, diseases, and natural selection in their local environments.
4.1. Factors Influencing Adaptation Processes on Local Honeybees in Algeria
Lin et al. [37] focus on the potential impacts of biotic and abiotic stressors on honeybee physiology and colony health. Several factors influencing adaptation processes of local honeybees in Algeria (Figure 2). Adjlane et al. [25] highlight the threats to local bee populations in Algeria, including bee diseases, insecticide poisoning, ecosystem degradation, and climate change.
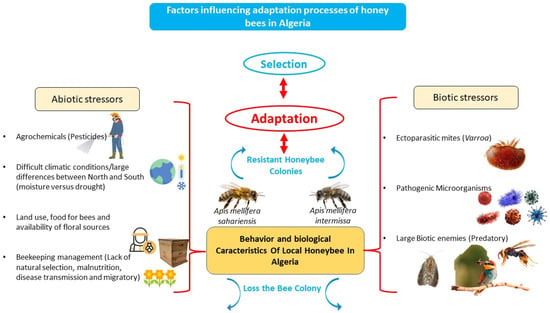
Figure 2.
Schematic description of factors influencing adaptation processes on local honeybees in Algeria.
4.1.1. Biotic Stressors
- a.
- Ectoparasitic mites (Varroa): V. destructor feeds on both the hemolymph and the fat body of its honeybee host, consuming nearly a microliter daily [38,39,40] and significantly interfering with honeybee physiology [41,42]. The life cycle of V. destructor consists of two stages: the phoretic dispersal phase, during which it parasitizes adult bees, and the reproductive phase, closely synchronized with the host’s development, during which it infests immature individuals [43]. Bee diseases, mainly represented by the V. destructor and bee poisoning by insecticides, threaten the survival of bee colonies in Algeria [25]. The impact of the parasitic mite V. destructor increases the risk of bee colony collapse and is influenced by local environmental factors such as temperature and humidity [44].
- b.
- Pathogenic microorganisms: The specific pathogenic microorganisms that cause biotic stress in honeybees include Mellisococcus plutonius, which is associated with European foulbrood (EFB) [45]. Other bacterial pathogens include the causative agents of American foulbrood (AFB), which are widely distributed and highly infectious [46]. Additionally, the fungal disease chalkbrood (CBD) affects honeybee broods [47]. Nosema ceranae is a microsporidian pathogen that has been identified as a cause of disease in honeybees [48]. These pathogens, along with other factors such as acaricide accumulation and unusual climatic conditions, contribute to the poor health status and vulnerability of honeybee colonies [49]. The development and progression of honeybee colonies are significantly influenced by various viruses, which pose a major threat to their health and well-being [50]. Viruses such as Deformed wing virus (DWV), Acute bee paralysis virus (ABPV), and Black Queen cell virus (BQCV) have been identified as having direct or indirect effects on individual bees and colony health [51]. Varroa exacerbates these effects by serving as a vector for these viruses, altering their transmission routes and potentially increasing their virulence [52]. Interestingly, while Varroa is associated with the spread of RNA viruses, it has been observed that the presence of certain viruses like SBV and BQCV can lead to a reduction in DWV viral titers, suggesting a complex interaction among viruses within honeybee colonies [52]. Moreover, the presence of Varroa mites and the associated viruses does not necessarily result in increased mortality of bee queens during the rearing process, indicating that the impact of viruses may vary depending on the context and the stage of bee development [53].
- c.
- Large biotic enemies: Honeybees face various biotic enemies during nesting and foraging, including predators such as the Asian hornet (Vespa velutina) and Asian giant hornet (Vespa mandarinia), as well as pests like wax moths (Galleria mellonella and Achroia grisella), small hive beetles (Aethina tumida), and opportunistic predators like praying mantises (Mantodea) and frogs (Batrachia). The Asian giant hornet (V. mandarinia), native to Asia, invades hives, causing forager homing failure and colony paralysis, significantly reducing A. mellifera survival rates [54,55,56,57]. Similarly, the Asian hornet (V. velutina) preys on A. mellifera near hive entrances, disrupting foraging activity [54]. Wax moths (G. mellonella and A. grisella) and small hive beetles (A. tumida) damage hive structures, consume brood and honey, and cause colony collapse, particularly in weakened hives [54,57]. These biotic threats underscore the importance of implementing effective pest and predator management strategies to protect honeybee colonies.
4.1.2. Abiotic Stressors
- a.
- Climate change and habitat degradation: Climate change in Algeria has resulted in rising temperatures, increasing by 1.5 °C over 3 decades, and a 20% decline in precipitation in northern regions [4,21,58,59,60,61,62]. These shifts exacerbate drought, reduce floral resources, and extend the foraging season, placing stress on bee populations. Despite this, A. m. intermissa demonstrates resilience to high temperatures, foraging even at 40 °C, which aids colony survival during heatwaves. However, erratic rainfall impacts floral diversity, posing nutritional challenges for bees [21]. Conservation of floral diversity and breeding climate-resilient strains are vital to address these challenges [58,59]. Deforestation and habitat loss can also affect bee populations and their behavior and may lead to a decline in pollination services [59]. The economic value of bee pollination for crop production in Algeria is influenced by the number of visits and the aggregate effects of various bee species, including honeybees, carpenter bees, stingless bees, bumblebees, and solitary bees [59], The absence of national legislation and standards for Algerian honey could hinder the development of beekeeping in Algeria. Research on honeybee infections and available treatment options in the country remains limited. There is also a lack of studies on the behavior, physiology, and evolution of honeybees in Algeria. Furthermore, the microbiota of Algerian honeybees and honey is still poorly understood [5,25,58,60].
- b.
- Environmental Stressors: The environment plays a significant role in shaping the behavioral characteristics of A. m. intermissa in Algeria. Haddad et al. [60] highlights the adaptability of A. m. intermissa to varying climatic conditions and its cleaning behavior, which may be a response to pressures such as temperature fluctuations and parasite infestation. Additionally, Menail et al. [12] suggests that pathogen-host interactions in A. m. intermissa could be influenced by the bee’s ability to withstand higher temperatures, a trait that becomes increasingly relevant with global warming and the resultant habitat shifts. Contradictions or interesting facts emerge when considering the impact of environmental stressors, such as pathogens and insecticides, on A. m. intermissa. Menail et al. [21] reports the presence of various pathogens and a potential vector, Megaselia scalaris, which could influence bee behavior through disease pressure. Menail et al. [21] discusses the adverse effects of insecticides on bee health, including changes in hypopharyngeal gland development and survival, which could alter foraging behavior and colony maintenance activities. Certain types of pesticides have been found to affect the behavior and survival rates of honeybees. The combination of pesticides, such as imidacloprid, chlorpyrifos, and glyphosate, can produce synergistic changes in the flight ability and behavior of honeybees, resulting in a decrease in flying duration and distance [63]. Furthermore, worst-case environmental concentrations of pesticide mixtures have been shown to cause higher mortality rates and disturbances in biochemical markers in honeybees [64]. Pesticides, particularly those with neurotoxic properties, have been reported to impact the nervous systems of local bee subspecies in Algeria, such as A. m. intermissa and A. m. sahariensis [24]. In summary, environmental factors such as climate, pathogens, and anthropogenic stressors like insecticides, significantly influence the behavioral characteristics of A. m. intermissa in Algeria. Adaptations to high temperatures and cleaning behaviors are beneficial traits for coping with environmental challenges [21], while pathogen prevalence and insecticide exposure may induce stress responses that affect bee health and behavior [61,62]. Understanding these interactions is essential for the conservation and management of this important pollinator species.
- c.
- Beekeepers’ management: According to Aglagane et al. [7] A. m. sahariensis decreases with increasing human management (beekeepers) intensity and precipitation. This indicates that the level of human intervention and environmental conditions play a role in the genetic makeup of the honeybee populations. The study found that high rates of hybridization with A. m. intermissa jeopardize the genetic integrity of the Saharan honeybee. This hybridization is attributed to factors such as the modernization of the beekeeping sector, the importation of foreign queens, large-scale queen breeding, and the regular movement of colonies, which have heavily impacted the genetic pool of locally adapted subspecies and caused genetic pollution through introgression.
These studies collectively emphasize the importance of understanding and addressing the abiotic and biotic stressors faced by honeybees in Algeria, as they have significant implications for beekeeping, honey production, and overall agricultural health in the region.
4.2. Behaviour Characteristics of Local Honeybees in Algeria
4.2.1. General Population Structure Stressors
There are at least 26 recognized subspecies of Apis mellifera [35]. These subspecies are grouped at the level of five evolutionary branches: A (African), C (Carnica), M (Mellifera), O (Oriental), and Y (Yemenetica) (Figure 1). Several studies have been characterized for the subspecies of A. mellifera, which are based on their morphological and genetic differences [26,34,65]. In some regions, by mixing several races, other subspecies belong to one or more evolutionary branches. Achou [26] found that Algerian honeybee populations consist of three different lineages: African, North Mediterranean, and West Mediterranean. They also identified a low level of genetic introgression from non-local honeybees, possibly due to the import of foreign honeybees. Loucif-Ayad et al. [65] confirmed the African origin of Algerian honeybee populations and identified two subspecies, A. m. intermissa and A. m. sahariensis. Bouzeraa et al. [9]. further supported the presence of African lineages in northeastern Algeria and noted higher genetic diversity in northern populations compared to southern populations.
Research has found evidence of genetic diversity and hybridization between different subspecies of honeybees in Algeria, particularly between A. m. intermissa and A. m. sahariensis [12]. This genetic diversity and hybridization may contribute to the adaptability and evolution of honeybees in response to their specific environmental conditions. Achou et al. [19] assessed the genetic analysis of Algerian honeybee populations revealed three evolutionary lineages: African (A), North Mediterranean (C), and West Mediterranean (M). The study identified eight different mtDNA haplotypes, with A1, A2, A8, A9, A10, and A13 belonging to the African lineage, while M4 and C7 were imported haplotypes. The pairwise t-test showed no significant difference between the two non-local haplotypes, M4 vs. C7. The local Algerian honeybees maintained a significantly higher presence, 96.9%, compared to non-local honeybees. This suggests that honeybee subspecies in Algeria have the potential to adapt and evolve in response to their specific environmental conditions. Furthermore, the study found evidence of hybridization between different subspecies, particularly between A. m. intermissa and A. m. sahariensis [6].
4.2.2. Biometric Analysis
Bouzeraa et al. [20] conducted a morphometric study and found significant variations in various morphological traits among honeybees in the northeastern region of Algeria. Barour et al. [18,30] analysed the morphometric and forewing shape variations of A. m. intermissa in different regions of Algeria. They found distinct morpho clusters and shape differences between ecological regions, suggesting limited gene flow and possible anthropogenic introductions. A study by Bendjedid and Achou [27] discussed biometrics and performed statistical analyses on samples of bees from southern Algeria to define the position of this breed compared to others within A. mellifica from a morphological point of view. The description of the data by the univariate statistical method revealed that the bee of southern Algeria is small compared to that of Morocco, Tunisia, and northeastern Algeria for most morphological characters. The mean value of the yellow band width (coloring) of the two stations studied was 0.45 mm [66]. It was slightly higher than that given by Achou [66], which is of the order of 0.40 mm. This size differentiation was due to the low richness of the vegetation and the difficult climate of southern Algeria, which makes this bee have a lighter body to travel long distances in search of its food. We can attribute this differentiation to the existence of a north-south gradient for certain morphological characters [66]. Indeed, one of the first examples of the north-south gradient was provided for bees by a number of Russian authors, such as Chochlov [29], Michailov [67], Aplatov [68], and Ruttner and al [69], which found that the length of the tongue gradually decreased from north to south.
4.2.3. Morphological Differences
According to Bendjedid and Achou [27], the average value of the width of the yellow band (coloring) in the subspecie A. m. sahariensis shows a high dispersion, with a standard deviation ranging from 0.032 mm to 0.326 mm. Moreover, these results, concerning the coloring, confirm the descriptions left by the brother Adam [13] about the bee of southern Algeria. This author claims that a bee with yellow coat exists in very large numbers in this region. The same is true of the authors Garnery et al. [70], Franck et al. [14], and Loucif-Ayad [71], who confirm the existence of the yellow bee in southern Algeria (Figure 3).

Figure 3.
Original photos of the local honeybee in Algeria: (A) A. m. sahariensis (B) A. m. intermissa.
One study focusing on A. m. intermissa, the native honeybee subspecies in Algeria and North Africa, found that these bees are distinctly darker in color with light illumination on the tergites [21]. This study also noted that the bees are small in in size compared to Algerian honeybees, specifically the subspecies A. m. intermissa, which are smaller in size compared to other subspecies [16]. This smaller size may be due to genetic factors specific to the Algerian population, as well as adaptations to the local environmental conditions. As for the Saharan bee A. m. sahariensis, it is characterized by its small size, yellow color, non-aggressive nature, and remarkable resistance to conditions difficult to heat and dry in the middle [36]. The queen, very long and large, is of yellow-red color going to red-cauldron, with the tip of the abdomen often dark, sometimes even black. This queen, very prolific, settles her spawning with a lot of economy; in spring she arrives, thanks to the sweetness of time, to lay beyond the possibilities of incubators [4].
4.2.4. Nervousness and Aggressive Defense Behavior
Local honeybees in Algeria are known for their nervous and aggressive behavior when defending their hives [16]. Weller [36] discusses the existence of behavioral syndromes in individual honeybees, emphasizing the importance of considering both individual and colony-level personalities in understanding honeybee behavior. Lastly, Al-Etby [72] reviews the defense behavior of honeybee hives, highlighting factors such as colony strength, queen health, and the secretion of alarming pheromones that contribute to the aggressiveness of bees. The Tellian bee A. m. intermissa indicates a limited position by the races of Africa [32]. This bee is very aggressive, nervous, and characterized by a high tendency to swarm by several royal cells [73]. By comparing its genome with those of others under subspecies, the adaptive potential of A. m. intermissa is evolved for high temperatures and for resistance against pest infestations V. destructor [21]. This behavior is believed to be a result of their adaptation to the challenging environmental conditions of Algeria.
4.2.5. Abundant Use of Propolis
Propolis, a resinous substance collected by bees from tree buds, is used for hive construction and defense. Algerian honeybees have been observed to use propolis abundantly in their hives, which may be a result of their need for extra protection in their environment [16]. A. m. intermissa exhibits strong defensive capabilities, notably through its abundant use of propolis. This behavior is likely influenced by a combination of environmental pressures, pathogen prevalence, and resource availability, highlighting the subspecies’ remarkable adaptability to Algeria’s unique ecological conditions [34].
4.2.6. Foraging Behavior
One of the notable behavioral characteristics of local honeybees in Algeria is their foraging behavior. Studies have shown that Algerian honeybees exhibit specific foraging behaviors that are adapted to the local environment. They have been observed to have preferences for specific floral resources, such as certain plant species or nectar sources. Additionally, local honeybees in Algeria have been found to exhibit a high degree of resource efficiency during foraging, visiting multiple flowers within a short period to maximize their collection of nectar and pollen [16]. The Saharan bees forage very far away from their hive [4]. The foraging behavior of local honeybees in Algeria is influenced by several factors, including the availability of food sources, location, and the selected plant species in their foraging range. The morphological characteristics of honeybees may also play a role in their foraging behavior [23]. For example, a study by Abou-Shaara et al. found that the tongue length of Algerian honeybees is shorter compared to other subspecies. This may affect their ability to access nectar from deep floral structures and could explain their preference for certain plant species with more accessible nectar sources.
4.3. Adaptation to Specific Climatic Conditions, Temperature, and Diseases
4.3.1. Tolerance to Environmental Conditions
The subspecies A. m. sahariensis, which predominates in the south of Algeria, is known for its adaptation to drought conditions, indicating a high level of tolerance to the challenging environmental conditions of the region [12]. A recent study by Khedidji et al. [8] discusses the influence of various factors, such as diet, subspecies, and age, on the development, physiology, and behavior of local honeybees in Algeria (A. m. intermissa et A. m. sahariensis). The study showed that the amount of protein in the hemolymph was influenced by the subspecies, with the subspecies intermissa having more hemolymphatic proteins than sahariensis when the same amount of pollen was consumed. This suggests that intermissa optimizes its protein diet much better than sahariensis. The study also demonstrated that the subspecies have evolved under different floral environments, possibly making them more adapted to digest specific pollen diets, with the diet used in the study potentially being more adapted to intermissa.
4.3.2. Resistance Against V. destructor
In recent years, the study of honeybee behavior has shed light on two important traits that contribute to the resistance against V. destructor, a parasitic mite that poses a significant threat to honeybee populations [74]. This behavior, known as Varroa Sensitive Hygiene, is a heritable trait of Apis mellifera and can be incorporated into queen breeding programs as a sustainable strategy for controlling V. destructor [75]. Both Varroa Sensitive Hygiene and Suppression of Mite Reproduction are traits associated with the hygienic behavior of honeybees in response to V. destructor infestation [76].
The researchers observed that honeybees detected and removed diseased broods and V. destructor through uncapping and removal of infested cells. The timing of this hygienic behavior was found to be crucial in reducing the risk of disease transmission and improving colony fitness [22]. It was observed that honeybees displayed hygienic behavior promptly, removing diseased broods and V. destructor infested cells before further transmission of pathogens or pests could occur [77]. Another study focused on the behavioral characteristics of honeybees in Algeria and compared them to other social insects, such as ants and termites [78]. The study specifically investigated hygienic behavior in honeybees, which is an important form of social immunity for these insects [75]. The researchers aimed to understand the underlying behavioral mechanisms of hygienic behavior in honeybees and how it relates to diseases and parasites like V. destructor.
The first study by Adjlane and Haddad [76] on the behavioral characteristics of local honeybees in Algeria was conducted on 40 colonies of A. m. intermissa. The objective of the study was to evaluate the hygienic behavior of bees, which is an important factor in resistance to V. destructor, a parasitic mite that is a major threat to honeybees. The results of the study showed that local honeybees have a high hygienic behavior. The rate of removal of dead brood infested with V. destructor was 91.56% in spring and 83.55% in autumn. These results are higher than those reported for other honeybee races, suggesting that local honeybees are well adapted to the local environmental conditions and have a good resistance to V. destructor. The study on the grooming and removal behavior of A. m. intermissa in Tunisia against V. jacobsoni provides valuable insights into the resistance mechanisms of this subspecies. The results show that A. m. intermissa workers are highly effective at detecting and removing both artificially infested and freeze-killed brood, with removal rates of up to 75% and 97–99%, respectively. Additionally, the bees were observed to actively groom off V. destructor mites, with a large number of injured mites dropping from naturally infested colonies [33]. Overall, the studies from Tunisia and Algeria provide strong evidence that A. m. intermissa bees have evolved effective mechanisms to resist V. destructor mites.
The hygienic behavior of local honeybees in Algeria is influenced by environmental factors such as temperature, humidity, food availability, and exposure to pesticides [79,80,81,82]. Natural selection has led to the development of V. destructor resistance in honeybee populations in South Africa and in Africanized bees in South America. These populations showed a decrease in V. destructor numbers per hive and survived untreated after high initial V. destructor density and colony losses [83]. To develop programs for selecting honeybees resistant to V. destructor in Algeria, methods like natural selection, selective breeding, and artificial insemination can be employed [17,31,79]. Conservation and sustainable management of local honeybee can be achieved through protecting their habitat, educating beekeepers, and supporting them [84,85]. By implementing these measures, beekeepers can contribute to the preservation and sustainability of local honeybee populations in Algeria.
5. Conclusions
The study of behavioral characteristics and adaptations of local honeybees in Algeria provides critical insights into their response to diverse environmental conditions. These findings can inform actionable strategies for beekeepers and policymakers, focusing on improving management practices, enhancing colony health, and supporting sustainable beekeeping initiatives.
Protecting the genetic diversity of local honeybee populations is crucial for maintaining pollination services, which are fundamental to agricultural productivity, ecosystem stability, and food security. Targeted breeding programs that prioritize resilience traits, such as disease resistance and environmental adaptability, can help reduce colony losses and improve productivity. Policymakers can support these efforts by implementing national standards and legislation for honey production, ensuring the sustainability and quality of the industry.
Further research into the genetic and environmental factors influencing biological traits in Algerian honeybees will aid in refining breeding and conservation strategies. Additionally, understanding the specific hygienic behaviors linked to V. destructor infestation and viral infections will provide beekeepers with practical tools to improve colony fitness and reduce disease prevalence.
By emphasizing sustainable practices and reducing genetic introgression, Algeria can strengthen its agricultural sector and contribute to global honeybee conservation. Policymakers and beekeepers alike must collaborate to prioritize these efforts, ensuring the long-term resilience of ecosystems and the critical pollination services they provide.
Author Contributions
Conceptualization, Y.H., N.A. and N.H.; Methodology, N.A. and Y.H.; Validation, N.A. and N.H.; Writing—original draft preparation, Y.H.; Writing—review and editing, N.A. and N.H.; Supervision, N.A. and N.H.; Project administration, N.A.; Funding acquisition, N.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the MEDIBEES project Monitoring Mediterranean bee subspecies and their resilience to climate change for the sustainable improvement of agroecosystems. MEDIBEES is funded by the European Commission through the PRIMA program under Horizon 2020.
Acknowledgments
This research was conducted within the framework of the PRIMA MEDIBEES project, which provided invaluable support and resources. I express my sincere gratitude to the MEDIBEES project consortium for their collaborative spirit and facilitating this work. I am particularly indebted to Raquel Martin Hernandez, the project coordinator, for her generous guidance, insightful recommendations, and thorough review of this work. Her expertise and feedback significantly contributed to the quality and rigor of this research. I also gratefully acknowledge the Faculty of Agronomy Sciences, University of Boumerdes, Algeria, for providing the necessary facilities and support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nesrine, B.A.; Amel, S.A.; Noury, B. Recent evolution of climatic conditions in the Lower Tafna Watershed (North-West of Algeria). Plant Arch. 2022, 22, 162–167. [Google Scholar] [CrossRef]
- Bona, E.; Massa, N.; Toumatia, O.; Novello, G.; Cesaro, P.; Todeschini, V.; Boatti, L.; Mignone, F.; Titouah, H.; Zitouni, A.; et al. Climatic zone and soil properties determine the biodiversity of the soil bacterial communities associated to native plants from desert areas of north-central Algeria. Microorganisms 2021, 9, 1359. [Google Scholar] [CrossRef] [PubMed]
- Le Conte, Y.; Navajas, M. Climate change: Impact on honey bee populations and diseases. OIE Rev. Sci. Tech. 2008, 27, 485–510. [Google Scholar] [CrossRef]
- Haccour, P. Recherche sur la race d’abeille saharienne au Maroc. CR Soc. Sci. Nat. Phys. Extr. Belgique Apic. 1960, 25, 13–18. [Google Scholar]
- Dadoun, N.; Nait-Mouloud, M.; Mohammedi, A.; Sadeddine Zennouche, O. Differences in grooming behavior between susceptible and resistant honey bee colonies after 13 years of natural selection. Apidologie 2020, 51, 793–801. [Google Scholar] [CrossRef]
- Abed, F.; Bachir-Bouiadjra, B.; Dahloum, L.; Yakubu, A.; Haddad, A.; Homrani, A. Procruste analysis of forewing shape in two endemic honeybee subspecies Apis mellifera intermissa and A. m. sahariensis from the northwest of Algeria. Biodiversitas 2021, 22, 154–164. [Google Scholar] [CrossRef]
- Aglagane, A.; Oleksa, A.; Er-Rguibi, O.; Tofilski, A.; El Mouden, E.H.; Aamiri, A.; Aourir, M. Genetic diversity and population structure of the Saharan honey bee Apis mellifera sahariensis from southeastern Morocco: Introgression assessment and implications for conservation. Apidologie 2023, 54, 31. [Google Scholar] [CrossRef]
- Khedidji, H.; Abderrahmani, K.; Oulebsir-Mohandkaci, H.; Ladjali-Mohammedi, K.; Mohammedi, A. Effects of Pollen Deprivation in Groups of Tellian (Apis mellifera intermissa) and Saharan (Apis mellifera sahariensis) Honey Bees under Controlled Conditions. Insects 2022, 13, 727. [Google Scholar] [CrossRef]
- Bouzeraa, H.; Sellami, H.; Gdoura, R.; Achou, M.; Soltani, N. Genetic diversity of the Honeybee Apis mellifera Linnaeus, 1758 (Hymenoptera Apidae) from Jijel (Northeast Algeria). Biodivers J. 2020, 11, 7–14. [Google Scholar] [CrossRef]
- Altaye, S.Z.; Meng, L.; Lu, Y.; Li, J. The emerging proteomic research facilitates in-depth understanding of the biology of honeybees. Int. J. Mol. Sci. 2019, 20, 4252. [Google Scholar] [CrossRef]
- Diao, Q.; Sun, L.; Zheng, H.; Zeng, Z.; Wang, S.; Xu, S.; Zheng, H.; Chen, Y.P.; Shi, Y.; Wang, Y.; et al. Genomic and transcriptomic analysis of the Asian honeybee Apis cerana provides novel insights into honeybee biology. Sci. Rep. 2018, 8, 822. [Google Scholar] [CrossRef]
- Menail, A.H.; Piot, N.; Meeus, I.; Smagghe, G.; Loucif-Ayad, W. Large pathogen screening reveals first report of Megaselia scalaris (Diptera: Phoridae) parasitizing Apis mellifera intermissa (Hymenoptera: Apidae). J. Invertebr. Pathol. 2016, 137, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Adam, F.A. La Recherche des Meilleures Lignées D’abeilles (Second Voyage). Bee World 1953, 35, 193–203. [Google Scholar] [CrossRef]
- Franck, P.; Garnery, L.; Loiseau, A.; Oldroyd, B.P.; Hepburn, H.R.; Solignac, M.; Cornuet, J.M. Genetic diversity of the honeybee in Africa: Microsatellite and mitochondrial data. Heredity 2001, 86, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Peyvel, C. L’espèce Apis mellifera: Les grandes races géographiques. Bull Tech Apic. 1994, 21, 129–138. [Google Scholar]
- Baldensperger, P.J. North African bees. Bee World 1923, 5, 175–176. [Google Scholar] [CrossRef]
- Chahbar, N.; Hamadi, K. Les abeilles domestiques locales et l’environnement. Un modèle parfait pour la sensibilisation environnementale. L’éducateur 2020, 23, 136–143. [Google Scholar]
- Barour, C.; Tahar, A.; Baylac, M. Forewing shape variation in Algerian honey bee populations of Apis mellifera intermissa (Buttel-Reepen, 1906) (Hymenoptera: Apidae): A landmark-based geometric morphometrics analysis. African Entomol. 2011, 19, 11–22. [Google Scholar] [CrossRef]
- Achou, M.; Wahida, L.; Legout, H.; Hayan, H.; Alburaki, M.; Garnery, L. An Insightful Molecular Analysis Reveals Foreign Honeybees Among Algerian Honeybee Populations (Apis mellifera L.). J. Data Min. Genom. Proteomics. 2015, 6, 166. [Google Scholar]
- Bouzeraa, H.; Achou, M.; Sellami, H.; Slotani, N. Study of the morphometric diversity of the population of honeybees (Apis Mellifera) in the North-East Algeria. Eur. J. Exp. Biol. 2016, 6, 6–12. [Google Scholar]
- Menail, A.H.; Boutefnouchet-Bouchema, W.F.; Haddad, N.; Taning, C.N.T.; Smagghe, G.; Loucif-Ayad, W. Effects of thiamethoxam and spinosad on the survival and hypopharyngeal glands of the African honey bee (Apis mellifera intermissa). Entomol. Gen. 2020, 40, 207. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Wahed, A.A.A.; Zhao, C.; Saeed, A.; Zou, X.; Guo, Z.; Hegazi, A.G.; Shehata, A.A.; El-Seedi, H.H.R.; Algethami, A.F.; et al. A Spotlight on the Egyptian Honeybee (Apis mellifera lamarckii). Animals 2022, 12, 2749. [Google Scholar] [CrossRef] [PubMed]
- Buchegger, M.; Buechler, R.; Fuerst-Waltl, B.; Kovacic, M.; Willam, A. Relationships between resistance characteristics of honey bees (Apis mellifera) against Varroa mites (Varroa destructor). J. Cent. Eur. Agric. 2018, 19, 954–958. [Google Scholar] [CrossRef]
- Miguel, I.; Iriondo, M.; Garnery, L.; Sheppard, W.; Estonba, A. Gene flow within the M evolutionary lineage of Apis mellifera: Role of the Pyrenees, isolation by distance and post-glacial re-colonization routes in the western Europe. Apidologie 2007, 38, 141–155. [Google Scholar] [CrossRef]
- Adjlane, N.; Doumandji, S.E.; Haddad, N. Situation de l’apiculture en Algérie: Facteurs menaçant la survie des colonies d’abeilles locales Apis mellifera intermissa. Cah. Agric. 2012, 21, 235–241. [Google Scholar]
- Loucif-Ayad, W.; Achou, M.; Legout, H.; Alburaki, M.; Garnery, L. Genetic assessment of Algerian honeybee populations by microsatellite markers. Apidologie 2015, 46, 392–402. [Google Scholar] [CrossRef]
- Bendjedid, H.; Achou, M. Etude de la Diversité Morphométrique de Deux Populations d’Abeilles Domestiques (Apis Mellifera Intermissa et Apis Mellifera Sahariensis) du Sud Algérien. Synthèse Rev. des Sci. et Technol. 2014, 95, 84–95. [Google Scholar] [CrossRef]
- Khedim, R.; Halfaoui, Y.F.; Mediouni, R.M.; Gaouar, S.; Bechir, S. Characterization of isolated colonies of honey bees (Apis mellifera intermissa), in Northern Algeria by classical morphometry approach. Genet Biodivers J. 2023, 7, 163–176. [Google Scholar]
- Chochlov, B.A. Investigations on the Length of the Bee Tongue; Ministry of Agriculture: Petrograd, Russia, 1916; pp. 17–41. (In Russian) [Google Scholar]
- Barour, C.; Tahar, A.; Radloff, S.E.; Hepburn, H.R. Multivariate analysis of honeybees, Apis mellifera Linnaeus (Hymenoptera: Apidae) of the northeastern and southern regions of Algeria. African Entomol. 2005, 13, 17–23. [Google Scholar]
- Page, E.J.R.; Fondrk, M.K. The effects of colony-level selection on the social organization of honey bee (Apis mellifera L.) colonies: Colony-level components of pollen hoarding. Behav. Ecol. Sociobiol. 1995, 36, 135–144. [Google Scholar] [CrossRef]
- Clément, H. Le traité Rustica de l’apiculture; Traité Rustica: Paris, France, 2002; p. 528. [Google Scholar]
- Boecking, O.; Spivak, M. Behavioral defenses of honey bees against Varroa jacobsoni Oud. Apidologie 1999, 30, 141–158. [Google Scholar] [CrossRef]
- Ruttner, F. Biogeography and Taxonomy of Honey Bees; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1988. [Google Scholar]
- Ruttner, F.; Tassencourt, L.; Louveaux, J. Biometrical-statistical analysis of the geographic variability of Apis mellifera. Apidologie 1978, 9, 363–381. [Google Scholar] [CrossRef]
- Weller, A. Behavioral Syndromes in Individual Honeybees. Ph.D. Thesis, University of Colorado, Boulder, CO, USA, 2015. [Google Scholar]
- Hillayová, M.K.; Korený, Ľ.; Škvarenina, J. The local environmental factors impact the infestation of bee colonies by mite Varroa destructor. Ecol. Indic. 2022, 141, 109104. [Google Scholar] [CrossRef]
- Ramsey, S.; Gulbronson, C.; Mowery, J.; Ochoa, R.; Vanengelsdorp, D.; Bauchan, G. A multi- microscopy approach to discover the feeding site and host tissue consumed by Varroa destructor on host honey bees. Microsc. Microanal. 2018, 24, 1258–1269. [Google Scholar] [CrossRef]
- Ramsey, S.; Ochoa, R.; Bauchan, G. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci. USA 2019, 116, 1792–1801. [Google Scholar] [CrossRef]
- Posada-Florez, F.; Sonenshine, D.; Egekwu, N.; Rice, C.; Lupitskyy, R.; Cook, S. Insights into the metabolism and behaviour of Varroa destructor mites from analysis of their waste excretions. Parasitology 2019, 146, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Amdam, G.; Norberg, K.; Hagen, A.; Omholt, S. Social exploitation of vitellogenin. Proc. Natl. Acad. Sci. USA 2003, 100, 1799–1802. [Google Scholar] [CrossRef]
- Arrese, E.; Soulages, J. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Traynor, K.; Mondet, F.; de Miranda, J. Varroa destructor: A complex parasite, crippling honey bees worldwide. Trends Parasitol. 2020, 36, 592–606. [Google Scholar] [CrossRef]
- Floyd, A.S.; Mott, B.M.; Maes, P.; Copeland, D.C.; McFrederick, Q.S.; Anderson, K.E. Microbial ecology of european foul brood disease in the honey bee (Apis mellifera): Towards a microbiome understanding of disease susceptibility. Insects 2020, 11, 555. [Google Scholar] [CrossRef]
- Ye, M.H.; Fan, S.H.; Li, X.Y.; Tarequl, I.M.; Yan, C.X.; Wei, W.H.; Yang, S.M.; Zhou, B. Microbiota dysbiosis in honeybee (Apis mellifera L.) larvae infected with brood diseases and foraging bees exposed to agrochemicals. R. Soc. Open Sci. 2021, 8, 201805. [Google Scholar] [CrossRef]
- Forsgren, E.; Locke, B.; Sircoulomb, F.; Schäfer, M.O. Bacterial Diseases in Honeybees. Curr. Clin. Microbiol. Rep. 2018, 5, 18–25. [Google Scholar] [CrossRef]
- Alonso-Prados, E.; González-Porto, A.V.; Bernal, J.L.; Bernal, J.; Martín-Hernández, R.; Higes, M. A case report of chronic stress in honey bee colonies induced by pathogens and acaricide residues. Pathogens 2021, 10, 955. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Maeng, S.; Cho, S.J.; Park, H.J.; Kim, K.; Lee, J.K.; Srinivasan, S. The Ascosphaera apis Infection (Chalkbrood Disease) Alters the Gut Bacteriome Composition of the Honeybee. Pathogens 2023, 12, 734. [Google Scholar] [CrossRef]
- Tantillo, G.; Bottaro, M.; Di Pinto, A.; Martella, V.; Di Pinto, P.; Terio, V. Virus infections of honeybees Apis mellifera. Ital. J. Food Saf. 2015, 4, 157–168. [Google Scholar] [CrossRef]
- Petrović, T.; Vidanović, D.; Lupulović, D.; Lazić, G.; Lazić, S. Honeybee Viruses Presence in Serbian Apiaries: A Review. Arch Vet. Med. 2021, 14, 97–117. [Google Scholar] [CrossRef]
- Remnant, E.; Mather, N.; Gillard, T.; Yagound, B.; Beekman, M. Vector-mediated viral transmission favours less virulent viruses. bioRxiv 2018. [Google Scholar] [CrossRef]
- Beims, H.; Janke, M.; von der Ohe, W.; Steinert, M. Influence of virus abundances in donor colonies and nurse hives on queens of Apis mellifera during the rearing process. Open Vet. J. 2023, 13, 879–893. [Google Scholar] [CrossRef] [PubMed]
- Requier, F.; Rome, Q.; Chiron, G. Predation of the invasive Asian hornet affects foraging activity and survival probability of honey bees in Western Europe. J. Pest Sci. 2019, 92, 567–578. [Google Scholar] [CrossRef]
- Zhu, G.; Illan, G.J.; Looney, C.; Crowder, D. Assessing the ecological niche and invasion potential of the Asian giant hornet. Proc. Natl. Acad. Sci. USA 2020, 117, 246–258. [Google Scholar] [CrossRef]
- Werenkraut, V.; Arbetman, M.; Fergnani, P. The Oriental hornet (Vespa orientalis L.): A threat to the Americas? Neotrop. Entomol. 2022, 51, 330–358. [Google Scholar] [CrossRef]
- Tan, K.; Radloff, S.; Li, J. Bee-hawking by the wasp, Vespa velutina, on the honeybees Apis cerana and A. mellifera. Naturwissen schaften. 2007, 94, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.L.J.; Kingston, J.M.; Albrecht, M.; Holway, D.A.; Kohn, J.R. The worldwide importance of honey bees as pollinators in natural habitats. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172140. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elshafiey, E.H.; Shetaia, A.A.; El-Wahed, A.A.A.; Algethami, A.F.; Musharraf, S.G.; AlAjmi, M.F.; Zhao, C.; Masry, S.H.D.; Abdel-Daim, M.M.; et al. Overview of bee pollination and its economic value for crop production. Insects 2021, 12, 688. [Google Scholar] [CrossRef]
- Le Conte, Y.; Meixner, M.D.; Brandt, A.; Carreck, N.L.; Costa, C.; Mondet, F.; Büchler, R. Geographical distribution and selection of european honey bees resistant to Varroa destructor. Insects 2020, 11, 873. [Google Scholar] [CrossRef] [PubMed]
- Haddad, N.J.; Loucif-Ayad, W.; Adjlane, N.; Saini, D.; Manchiganti, R.; Krishnamurthy, V.; AlShagoor, B.; Batainh, A.M.; Mugasimangalam, R. Draft genome sequence of the Algerian bee Apis mellifera intermissa. Genomics Data 2015, 4, 24–25. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Li, Z.; Huang, Q.; Zeng, Z.J. The effects of sublethal doses of imidacloprid and deltamethrin on honeybee foraging time and the brain transcriptome. Int. J. Mol. Sci. 2022, 23, 14278. [Google Scholar] [CrossRef]
- Christen, V. Different effects of pesticides on transcripts of the endocrine regulation and energy metabolism in honeybee foragers from different colonies. Sci. Rep. 2023, 13, 1985. [Google Scholar] [CrossRef] [PubMed]
- Chahbar, N.; Chahbar, M.; Doumandji, S. Evaluation of acute toxicity of thiamethoxam in algerian honeybee Apis mellifera intermissa and Apis mellifera sahariensis. Int. J. Zool. 2014, 4, 29–40. [Google Scholar]
- Miguel, I.; Baylac, M.; Iriondo, M.; Manzano, C.; Garnery, L.; Estonba, A. Both geometric morphometric and microsatellite data consistently support the differentiation of the Apis mellifera M evolutionary branch. Apidologie 2011, 42, 150–161. [Google Scholar] [CrossRef]
- Adjlane, N.; Dainat, B.; Gauthier, L.; Dietemann, V. Atypical viral and parasitic pattern in Algerian honey bee subspecies Apis mellifera intermissa and A. m. sahariensis. Apidologie 2016, 47, 631–641. [Google Scholar] [CrossRef]
- Achou, M. Caractérisation Morphomètrique, Biochimique Et Moléculaire Des Populations D’abeilles Domestiques De l’Est Algérien. Effets Physiopathologiques De Son Parasite Majeur Varroa destructor. Ph.D. Thesis, Université Badji Mokhtar, Annaba, Algérie, 2007. [Google Scholar]
- Michailov, A.S. On the increasing variability of the honey bee from north to south. Opytn Paseca. 1926, 12, 13–14. (In Russian) [Google Scholar]
- Alpatov, W.W. Über die Verkleinerung der Rüssellänge der Honigbiene vom Süden nach Norden hin. Zool Anzeiger. 1925, 65, 103–111. [Google Scholar]
- Ruttner, F.; Pourasghar, D.; Kauhausen, D. Die honigbienen des Iran. 2. Apis mellifera meda Skorikow, die Persische Biene. Apidologie 1985, 16, 241–264. [Google Scholar]
- Garnery, L.; Mosshine, E.H.; Oldroyd, B.P.; Cornuet, J.M. Mitochondrial DNA variation in Moroccan and Spanish honey bee populations. Mol. Ecol. 1995, 4, 465–471. [Google Scholar] [CrossRef]
- Loucif-Ayad, W. Etude de la Diversité Génétique Des Abeilles Domestiques Algériennes (Apis mellifera L.) Et Évaluation De l’Effet De Divers Acaricides Sur Les Abeilles Et Leur Parasite Varroa destructor. Ph.D. Thesis, Université de Annaba, Annaba, Algérie, 2009. [Google Scholar]
- Al-Etby, M.A. Defense Behavior of Honeybee Apis mellifera L. Hives: A Review. Arab. J. Plant Prot. 2023, 41, 85–92. [Google Scholar] [CrossRef]
- Abou-Shaara, H.F. The foraging behaviour of honey bees, Apis mellifera: A review. Vet. Med. 2014, 59, 1–10. [Google Scholar] [CrossRef]
- Spivak, M.; Danka, R.G. Perspectives on hygienic behavior in Apis mellifera and other social insects. Apidologie 2021, 52, 1–16. [Google Scholar] [CrossRef]
- Sprau, L.; Hasselmann, M.; Rosenkranz, P. Reproduction of Varroa destructor does not elicit Varroa Sensitive Hygiene (VSH) or recapping behaviour in honey bee colonies (Apis mellifera). Apidologie 2021, 52, 1048–1059. [Google Scholar] [CrossRef]
- Adjlane, N.; Haddad, N. The first data on hygienic behavior of Apis mellifera intermissa in Algeria. J. Biol. Earth Sci. 2014, 4, B1–B5. [Google Scholar]
- Brothers, D.; Tschuch, G.; Burger, F. Associations of mutillid wasps (Hymenoptera, Mutillidae) with eusocial insects. Insectes Soc. 2000, 47, 201–211. [Google Scholar] [CrossRef]
- Boecking, O.; Ritter, W. Grooming and removal behaviour of Apis mellifera intermissa in Tunisia against Varroa jacobsoni. J. Apic. Res. 1993, 32, 127–134. [Google Scholar] [CrossRef]
- Fries, I.; Raina, S. American Foulbrood and African Honey Bees (Hymenoptera: Apidae). J. Econ. Entomol. 2003, 96, 1641–1646. [Google Scholar] [CrossRef]
- Evans, J.D.; Spivak, M. Socialized medicine: Individual and communal disease barriers in honey bees. J. Invertebr. Pathol. 2010, 103, S62–S72. [Google Scholar] [CrossRef]
- Johnson, B.R. Division of labor in honeybees: Form, function, and proximate mechanisms. Behav. Ecol. Sociobiol. 2010, 64, 305–316. [Google Scholar] [CrossRef]
- van Alphen, J.J.M.; van Alphen, J.J.M.; Fernhout, B.J. Erratum: Natural selection, selective breeding, and the evolution of resistance of honeybees (Apis mellifera) against Varroa. Zool Lett. 2020, 6, 6. [Google Scholar] [CrossRef]
- Dietemann, V.; Nazzi, F.; Martin, S.J.; Anderson, D.L.; Locke, B.; Delaplane, K.S.; Wauquiez, Q.; Tannahill, C.; Frey, E.; Ziegelmann, B.; et al. Standard methods for varroa research Métodos estándar de la investigación en varroa étodos. J. Apic. Res. 2013, 52, 1–54. [Google Scholar]
- Haider, Y.; Adjlane, N.; Haddad, N. Results of the national survey on Algerian beekeeping (breeding practices, health situation). South Florida J. Dev. 2024, 5, e4618. [Google Scholar] [CrossRef]
- Haider, Y.; Adjlane, N.; Martin-hernande, R.; Haddad, N.; Khemmouli, A. Sustainable beekeeping in Algeria: Exploring practices, challenges, and local honeybee traits for natural resource management. Nat. Resour. Sustain Dev. 2024, 14, 257–278. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).