Simple Summary
Understanding how insects breathe is essential for both ecological research and pest control. However, accurately measuring the respiration rates of small-sized insects has been challenging due to a lack of suitable tools. This study evaluated the use of a portable photosynthesis system, originally developed for plants, to measure insect respiration. The results demonstrated that this method is effective across various insect species and developmental stages. Moreover, factors such as temperature, starvation, and specific chemicals significantly influenced respiration rates. Silencing a mitochondrial protein-coding gene through RNA interference reduced respiration and increased resistance to certain stresses. Overall, this study introduces a novel experimental method for measuring insect respiration, offering insights into their adaptation mechanisms and providing a potential tool for improved pest management. These findings also contribute valuable information to our understanding of how insects interact with their environment and respond to challenges such as climate change.
Abstract
Respiration rates in insects are critical for survival and environmental adaptation, being influenced by developmental stages, environmental conditions, and the regulation of mitochondrial protein-coding genes. However, methods for field-based measurements in small-sized insects remain limited. In this study, we established a portable photosynthesis system to quantify respiration rates in five small-sized insects (body length < 8 mm): Acyrthosiphon pisum, Aphis citricidus, Tuta absoluta, Tribolium castaneum, and Bactrocera dorsalis. We tested its effectiveness across life stages and under diverse treatments, including light/dark cycles, insecticides, temperature shifts, starvation, mitochondrial inhibitors, and RNA interference. The system exhibited high sensitivity and reproducibility rates, revealing stage-specific respiration patterns. Various treatments, as well as expression changes in mitochondrial protein-coding genes, significantly affected respiration rates. This study validates the portable system as a reliable tool for insect respiration studies and highlights regulatory networks associated with respiratory plasticity. These findings enhance experimental methodologies and advance our understanding of insect adaptation to environmental stressors and pest control strategies.
1. Introduction
The respiratory system in insects is essential for environmental adaptation, development, and survival [1,2]. To meet their high metabolic demands, insects depend on efficient gas exchange [3]. Respiration rates are influenced by multiple factors, including body size, activity level, food availability, temperature, and oxygen concentration [4,5]. Activities such as flight or mating can substantially increase respiration rates [6]. Temperature has a direct effect on insect respiration by influencing the metabolic rate, thereby affecting thermoregulation and respiratory efficiency under changing conditions [7]. These mechanisms underpin insects’ adaptability to environmental fluctuations, particularly in the context of global climate change [8,9]. Understanding insect respiration, therefore, holds ecological relevance and practical value in pest management [3,10]. For instance, strategies that target respiratory processes have led to the development of effective pesticides and control methods [11,12,13,14]. The respiration rate is thus a key parameter in studies of insect biology, ecology, and environmental interaction [4,15]. However, current techniques for measuring respiration in small-sized insects (body length < 8 mm) are limited by low sensitivity and poor field operability [16,17].
The portable photosynthesis system has become a widely used tool for quantifying photosynthetic parameters across diverse plant species and environmental conditions owing to its ease of use, standardized protocols, and high degree of experimental reproducibility [18,19,20,21,22]. Applications include studies in rapeseed [20], soybean [23], paper mulberry [24], cotton [25], mulberry [19], poplar [26], and switchgrass [27]. For example, it has been employed to measure the net photosynthesis, transpiration, intracellular CO2 concentration, and stomatal conductance in a Brassica napus variety resistant to Sclerotinia [20], as well as to assess the net photosynthetic rate in Pakchoi after seedling treatment with a polymeric hydrogel [28]. This system calculates the photosynthetic efficiency by simultaneously monitoring the CO2/H2O gas exchange, light intensity, and leaf temperature via integrated infrared sensors and environmental probes, enabling the precise quantification of photosynthesis and transpiration in field settings [29]. Based on these operational principles, the portable photosynthesis system offers untapped potential for measuring respiration in micro-insects by leveraging its high sensitivity to the CO2 flux, originally intended for detecting plant stomatal dynamics.
Mitochondria are essential organelles that regulate cellular respiration and energy production in eukaryotes [30]. In insects, mitochondrial genomes and associated genes play critical roles in respiratory function. Complete mitochondrial genome sequences have been reported for several species, including Leucinodes orbonalis [31], Rhyzopertha dominica [32], Choroterpes yixingensis [33], and Aphis citricidus [34], revealing 13 mitochondrial protein-coding genes. Although the expression patterns of these genes have been studied under various experimental conditions in some insect species [35,36,37], the relationship between their expression and respiration rates remains poorly understood in Acyrthosiphon pisum.
In this study, we developed a technique to measure respiration in small-sized insects, using a portable photosynthesis system. To validate its applicability, we assessed respiration rates across different life stages in multiple insect species and under various experimental conditions in Ac. pisum. This work presents a novel methodology for investigating insect respiration and contributes to a deeper understanding of insect adaptation mechanisms.
2. Materials and Methods
2.1. Insects
Ac. pisum was reared on Vicia faba seedlings, Aphis citricidus on Citrus sinensis, Tuta absoluta on Solanum lycopersicum, and Bactrocera dorsalis on an artificial diet, as previously described [38]. All the insects were maintained at 25 °C and 75% relative humidity and under a photoperiod of 14 h of light and 10 h of darkness. Tribolium castaneum was reared on a mixture of wheat flour and brewer’s yeast powder (in a 10:1 ratio) under conditions of 30 °C, 30% relative humidity, and a photoperiod cycle of 16 h of light and 8 h of darkness.
2.2. Sample Collection from Various Developmental Stages and Treatments
Developmental stages. Insects from different developmental stages of Ac. pisum and Ap. citricidus (first-instar nymphs (N1), second-instar nymphs (N2), third-instar nymphs, fourth-instar nymphs (N3), and three-day-old adults (AD)), Ap. citricidus (first-instar nymphs (N1), second-instar nymphs (N2), third-instar nymphs, fourth-instar nymphs (N3), three-day-old wingless adults (AD-WL), and three-day-old winged adults (AD-WL)), T. absoluta (eggs (E), larvae (L), pupae (P), and adults (AD)), B. dorsalis (eggs (E), first-instar larvae (L1), second-instar larvae (L2), third-instar larvae (L3), pupae (P), female adults (AD-F), and male adults (AD-M)), and T. castaneum (eggs (E), larvae (L), pupae (P), female adults (AD-F), and male adults (AD-M)) were collected for respiration rate measurements. For egg stages, 50 individuals were pooled per group; for larvae, pupae, and adults, 10 individuals were pooled. Each treatment included eight biological replicates.
Photoperiod. Ac. pisum individuals, maintained under a 14:10 h light–dark cycle, were sampled at two-hour intervals to assess respiration rate variations throughout the photoperiod. Ten aphids were pooled per group, with eight biological replicates.
Temperatures. Ac. pisum was exposed to acute thermal stress at 35 °C and 4 °C for 4, 8, or 12 h. Respiratory measurements were taken immediately after treatment. Aphids reared at 25 °C served as controls. Ten individuals were pooled per group, with eight biological replicates.
Insecticides. Ac. pisum was treated with LC20 concentrations of avermectin, β-cypermethrin, and imidacloprid [39]. Aphids treated with acetone were used as controls. Ten individuals were pooled per group, with eight biological replicates.
Starvation. Ac. pisum was starved for 4, 8, or 12 h. After each starvation period, individuals were collected for respiration rate measurements. Normally fed aphids served as controls. Ten insects were pooled per group, with eight biological replicates.
Mitochondrial inhibitors. Ac. pisum was treated with mitochondrial inhibitors (rotenone and antimycin A). Aphids treated with acetone were used as controls. Ten insects were pooled per group, with eight biological replicates.
2.3. Measured Respiration Rates
Insect respiration rates were measured using a modified LI-6800 photosynthesis system (Ecotek Technology, Beijing, China), coupled with a custom insect chamber containing a ventilated lid and an artificial feeding device (Figure 1), according to the manufacturer’s instructions with slight modifications. Briefly, after assembling the system and performing preheating checks and zeroing calibration through the instrument’s startup protocol, we configured the operational parameters as follows: a 500 μmol s−1 airflow, a 25 °C temperature control, and 400 μmol mol−1 CO2 levels with active scrubbing. The humidity was maintained between 50 and 75% using the system’s water vapor subsystem. The insects were placed in the transparent chamber, which was sealed within the LI-6800 leaf chamber (Ecotek Technology, Beijing, China) and measurements were taken continuously for 10 min, with automatic data collection every 2 min. The insects were given time to reach a stable respiratory state (ΔCO2 remained within 0.01 μmol mol−1). Respiration rates were calculated as CO2 emissions per minute, normalized to the insect’s body mass (μmol g−1 min−1), reflecting gas-exchange dynamics under controlled environmental conditions.
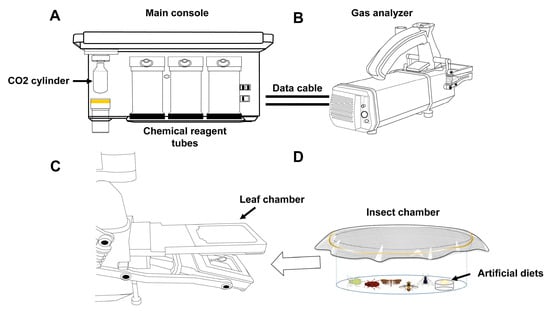
Figure 1.
Device for measuring respiration rates of small-sized insects, using the portable photosynthesis system. (A) Main console. (B) Gas analyzer. (C) Position of the leaf chamber on the gas analyzer. (D) Insect chamber, equipped with an artificial-diet provision system. The insect chamber is inserted into the leaf chamber for gas-exchange measurements when containing experimental insects.
2.4. RT-qPCR
The primers for 13 mitochondrial protein-coding genes are listed in Table S1. The total RNA was extracted from treated samples using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and cDNA was synthesized using the PrimeScript RT reagent kit (Takara, Dalian, China). Quantitative PCR (qPCR) analysis was performed with NovoStart SYBR qPCR SuperMix (Novoprotein, Shanghai, China) to quantify target gene expression levels. Standard curves were generated with serial cDNA dilutions to determine the amplification efficiency and CT values. Apactin and ApNADH were used as reference genes for normalization via qBASE+ [40,41].
2.5. RNA Interference Assay
Double-stranded RNA (dsRNA) was synthesized using the TranscriptAid T7 high-yield kit (Thermo Scientific, Wilmington, DE, USA). Aphids were topically treated with 500 ng/μL dsRNA, with dsGFP as a control. The treated aphids were fed artificial diets for 12 h before respiration rate measurements (Section 2.3). Each group contained ten individuals, with eight replicates.
2.6. Statistical Analyses
Statistical analyses were performed using GraphPad Prism 7.0 (GraphPad Software, San Diego, CA, USA). Multiple group comparisons, including respiration rates among different developmental stages and various treatments and transcriptional patterns of mitochondrial protein-coding genes during light/dark periods and across developmental stages, were analyzed using one-way ANOVA with LSD post hoc tests (p < 0.05). Pairwise comparisons, including transcriptional patterns of mitochondrial protein-coding genes in different treatments and the RNAi assay, were assessed using Student’s t-test (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
3. Results
3.1. Measurement of Respiration Rates in Different Insects, Using a Portable Photosynthesis-Monitoring System
To test the applicability of this method for various small-sized insects, we used a portable photosynthesis-monitoring system to measure the respiration rates of five insect species: Ac. pisum and Ap. citricidus (Hemiptera: Aphididae), Tu. absoluta (Lepidoptera: Gelechiidae), Tr. castaneum (Coleoptera: Tenebrionidae), and B. dorsalis (Diptera: Tephritidae) across different developmental stages or morphs. The results demonstrate that this method offers high sensitivity, ease of use, and excellent reproducibility. The respiration rate profiles revealed that second-instar nymphs exhibited the lowest respiration rates (1.16 μmol g−1 min−1), while first-instar nymphs showed the highest (1.93 μmol g−1 min−1) in Ac. pisum (Figure 2A). In Tu. absoluta, pupae had the lowest rates (0.03 μmol g−1 min−1), whereas larvae displayed the highest rates (2.58 μmol g−1 min−1) (Figure 2B). In Tr. Castaneum, eggs had the lowest respiration rates (0.07 μmol g−1 min−1), while larvae exhibited the highest (0.74 μmol g−1 min−1) (Figure 2C). In B. dorsalis, pupae displayed the lowest rates (0.06 μmol g−1 min−1), while first-instar larvae exhibited the highest (23.31 μmol g−1 min−1) (Figure 2D). In Ap. citricidus, winged adults (0.63 μmol g−1 min−1), wingless adults (0.64 μmol g−1 min−1), third-instar nymphs (0.72 μmol g−1 min−1), and fourth-instar nymphs (0.79 μmol g−1 min−1) had the lowest respiration rates, while first-instar nymphs showed the highest (1.71 μmol g−1 min−1) (Figure 2E).
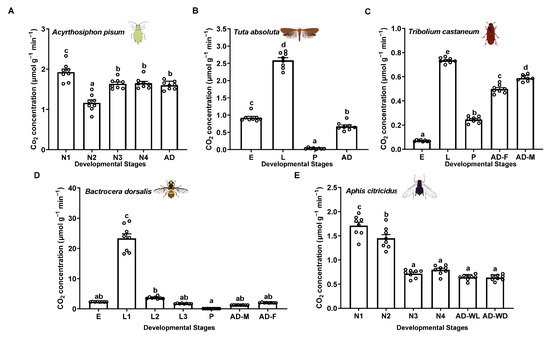
Figure 2.
Respiration rates of different insects, using the portable photosynthesis-monitoring system. (A) Acyrthosiphon pisum. (B) Tuta absoluta. (C) Tribolium castaneum. (D) Bactrocera dorsalis. (E) Aphis citricidus. Values represent the mean ± standard error (SE) of eight biological replicates. Lowercase letters denote statistically significant differences determined through one-way ANOVA with LSD post hoc testing (p < 0.05).
3.2. Measurement of Respiration Rates of Ac. pisum Under Various Treatments, Using a Portable Photosynthesis-Monitoring System
To assess the applicability of this method for measuring insect respiration under different conditions, we tested Ac. pisum exposed to light/dark cycles over a full photoperiod, insecticides, varying temperatures, starvation periods, and mitochondrial inhibitors at different concentrations. The respiration rates remained stable throughout the photoperiod, with only a slight initial increase followed by a subsequent decrease during light periods (Figure 3A). All three insecticides (imidacloprid, abamectin, and β-cypermethrin) reduced respiration rates (Figure 3B). High temperatures (32 °C) significantly increased the respiration rates, whereas low temperatures (4 °C) markedly decreased them (Figure 3C). Starvation also reduced respiration rates, with the reduction intensifying over time (Figure 3D). Both mitochondrial inhibitors (rotenone and antimycin A) suppressed respiration rates, with progressive decreases at higher concentrations (Figure 3E).
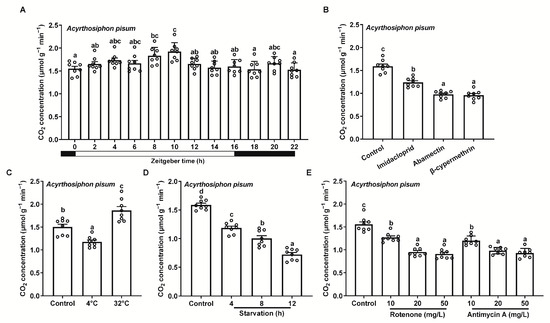
Figure 3.
Respiration rates of Acyrthosiphon pisum under different treatments, using the portable photosynthesis-monitoring system. (A) Light/dark cycles over a full photoperiod. (B) Insecticide exposure. (C) Varying temperatures. (D) Starvation at various time points. (E) Mitochondrial inhibitors. Values represent the mean ± standard error (SE) of eight biological replicates. Lowercase letters denote statistically significant differences determined through one-way ANOVA with LSD post hoc testing (p < 0.05).
3.3. Measurement of Respiration Rates of Ac. pisum Under the Silencing of a Key Mitochondrial Protein-Coding Genes, Using a Portable Photosynthesis-Monitoring System
To measure the respiration rates of Ac. pisum under the silencing of a key mitochondrial protein-coding gene, using the portable photosynthesis-monitoring system, we systematically quantified the expression profiles of 13 protein-coding genes. The expression profiles showed stable expression levels during light periods, with lower expression levels later in the light phase and dynamic changes during darkness (zeitgeber times ZT16–ZT22), peaking at ZT18 or ZT22 (Figure S1). Developmental-stage-specific profiles revealed higher gene expression levels in younger nymphs. For example, NAD1, NAD4, NAD5, Cytb, COX1, COX2, COX3, and ATP6 were highly expressed in second-instar nymphs, while NAD2, NAD4L, NAD6, and ATP8 peaked in third-instar nymphs (Figure S2). Larger insects (fourth-instar nymphs and adults) generally showed lower expression levels.
Treatment-induced changes included imidacloprid downregulating NAD2; abamectin reducing NAD5 and ATP8; and β-cypermethrin suppressing NAD2, NAD4, NAD4L, COX1, and ATP8 (Figure 4A). Low temperatures (4 °C) upregulated ATP8, while high temperatures decreased NAD4, NAD6, Cytb, COX3, and ATP6 (Figure 4B). Starvation specifically downregulated ATP8 (Figure 4C). Rotenone reduced NAD1, NAD2, NAD4, NAD4L, NAD5, NAD6, Cytb, and ATP8, whereas antimycin A suppressed NAD1, NAD2, NAD4, NAD4L, NAD6, and ATP8 (Figure 4D).
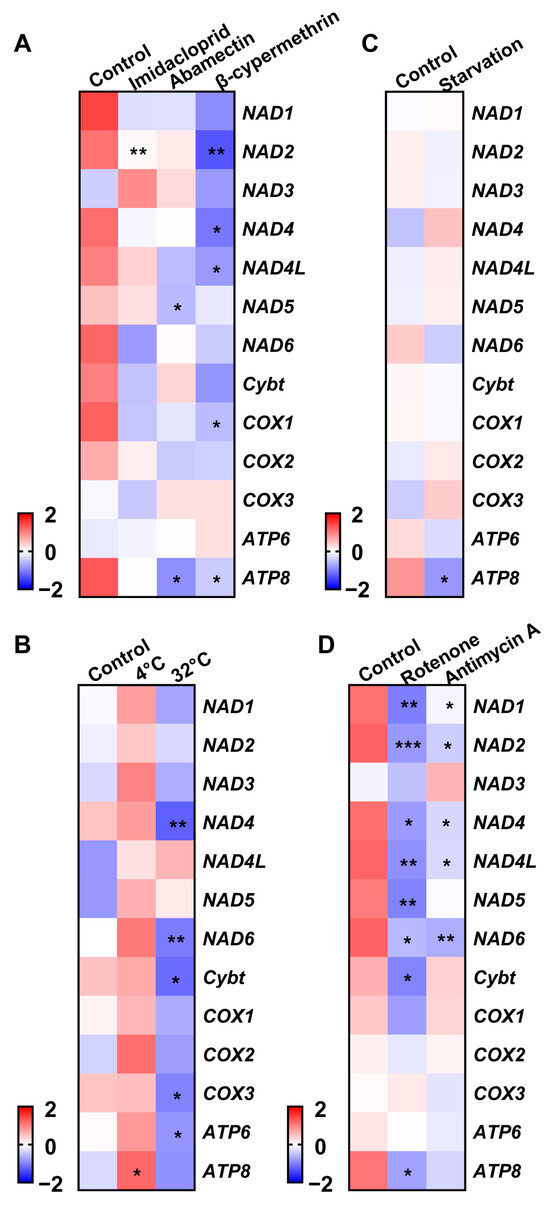
Figure 4.
Transcriptional patterns of mitochondrial protein-coding genes in Acyrthosiphon pisum under different treatments. (A) Insecticide exposure. (B) Varying temperatures. (C) Starvation. (D) Mitochondrial inhibitors. Values represent the mean ± standard error (SE) of three biological replicates. Asterisks denote statistically significant differences as determined by Student’s t-test (* p < 0.05, ** p < 0.01, *** p < 0.001).
Given the downregulation of ATP8 under multiple stresses (abamectin, β-cypermethrin, starvation, and rotenone), this gene was further used to measure the respiration rates of Ac. pisum under the silencing of a key mitochondrial protein-coding gene, using the portable photosynthesis-monitoring system. The successful silencing of ATP8 (44.1% efficiency) (Figure 5A) led to significant changes in other mitochondrial genes, with COX1 being significantly upregulated (Figure 5B). The silenced-gene insects exhibited significantly lower respiration rates compared to the dsGFP controls (0.91 vs. 1.34 μmol g−1 min−1, Figure 5C). Additionally, the dsATP8-treated groups demonstrated enhanced resistance to abamectin (a 15.2% lower mortality rate, Figure 5D) and β-cypermethrin (a 13.6% lower mortality rate, Figure 5E).
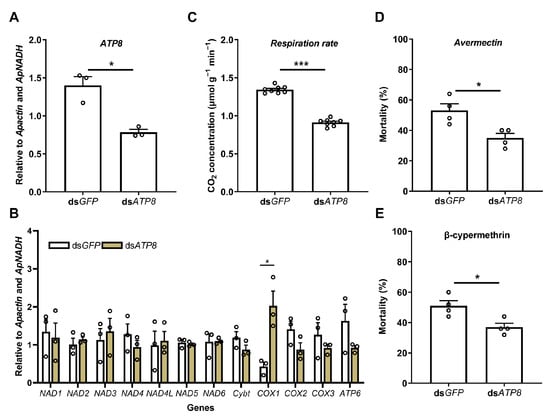
Figure 5.
Respiration rates of Acyrthosiphon pisum under the silencing of a key mitochondrial protein-coding gene (ATP8), using the portable photosynthesis-monitoring system. Effects of ATP8 silencing on the respiration rates and stress resistance of Acyrthosiphon pisum. (A) Relative expression level of ATP8 after ATP8 dsRNA treatment. (B) Relative expression levels of other mitochondrial protein-coding genes after ATP8 dsRNA treatment. (C) Respiration rates of Ac. pisum after ATP8 dsRNA treatment. (D) Mortality of Ac. pisum under avermectin treatment upon feeding dsRNA. (E) Mortality of Ac. pisum under β-cypermethrin treatment upon feeding dsRNA. Values represent the mean ± standard error (SE) of three biological replicates for gene expression levels, eight for respiration rate, and four for mortality. Asterisks denote statistically significant differences as determined by Student’s t-test (* p < 0.05, *** p < 0.001).
4. Discussion
In this study, we demonstrated the feasibility of using a portable photosynthesis system to measure respiration rates in small-sized insects, providing a practical and efficient approach for field-based studies. The results revealed significant variation in respiration rates across developmental stages and species, which aligns with previous reports showing that metabolic demands increase with higher energy requirements for activities such as flight, reproduction, stress resistance, and foraging [7,42]. A prior study indicated that geographic populations of three beetle species from different regions of Brazil exhibited significant differences in respiration rates [14]. Similarly, in Helicoverpa armigera, respiration rates fluctuated dynamically across developmental stages, peaking temporarily during interlarval molting and metamorphosis to pupae, sharply declining in the pupal phase, and rising again immediately before eclosion [15]. The high sensitivity and reproducibility rates of the portable photosynthesis system establish it as a valuable tool for future research, particularly in ecological and environmental studies requiring field measurements. Going forward, this system could be used to measure the respiration rates of extremely small insects and assess their stabilities under extreme environmental temperature and humidity conditions to test the detection limits of the system.
The respiration rates of Ac. pisum were differentially influenced by environmental and chemical treatments. Light/dark cycles showed minimal effects on metabolic activity, suggesting stable respiratory regulation under variable light conditions. In contrast, significant changes were observed under insecticide exposure, with all the compounds reducing respiration rates, likely due to mitochondrial disruption. High temperatures increased respiration rates, while lower temperatures suppressed them. Starvation similarly reduced respiration, consistent with energy conservation strategies during nutrient deprivation. Mitochondrial inhibitors further confirmed respiratory suppression through targeted interference. These patterns align with observations in other insects. For example, starvation reduced respiration in H. armigera [15], and mitochondrial inhibitors (rotenone, malonate, and antimycin A) decreased respiratory activity in the flight muscles of Vespula vulgaris, Bombus impatiens, Apis mellifera, and Locusta migratoria [16]. Similar effects were reported for imidacloprid in Euschistus heros males [43], plant-derived compounds (lemongrass essential oil, geranyl acetate, cinnamon terpenoids, clove oils, and citral) in Sitophilus granarius and Ulomoides dermestoides [44,45,46], squamocin/tebufenozide in Anticarsia gemmatalis larvae [47,48], Cymbopogon citratus essential oil in Podisus nigrispinus [7], and chlorantraniliprole in Hypothenemus hampei [17], collectively demonstrating the broad conservation of metabolic responses across taxa.
Mitochondrial gene expression dynamics reveal key regulatory mechanisms underlying respiratory plasticity during development and in response to environmental stress. Under light–dark cycles, most mitochondrial genes exhibit stable expression levels, with small fluctuations observed at the end of light phases or during periods of darkness. Analyses across developmental stages revealed high expression levels of NAD1, NAD4, NAD5, Cytb, COX1-3, and ATP6 in early nymphal stages, reflecting the increased energy demands for growth. Lower expression levels in later nymphs and adults suggest a shift in energy use toward maintenance and reproduction, which aligns with observations of low ATG5 levels during early diapause, followed by peak expression after 12 weeks of diapause induction [49]. Cold stress was shown to increase ATP8 expression, likely as a response to maintain energy balance through adaptive processes, while starvation led to a decrease in ATP8 expression to conserve resources. Additionally, mitochondrial inhibitors generally reduced the expression levels of electron-transport-chain (ETC) genes, indicating the direct suppression of mitochondrial function. These results corroborate those of earlier studies. For example, lower expression levels of Cytb, NAD3, NAD5, NAD6, ATP6, and ATP8 were observed in Cryptolestes ferrugineus resistant to phosphine [37], contrasting with the higher expression levels of COX1-3, NAD1, NAD4, NAD5, ATP6, and Cytb in Monochamus alternatus during pinewood nematode infection [36]. Furthermore, imidacloprid exposure reduces the expression levels of COX3, NAD4, and NAD4L in Choroterpes yixingensis [9], while long-term cold stress lowered the expression levels of NAD1, NAD4, NAD4L, NAD5, COX1, COX3, ATP6, and ATP8 in C. ferrugineus [9]. These findings suggest both shared and distinct effects of chemical and cold stress on mitochondrial gene networks. Additionally, pyrethroid-resistant Anopheles sinensis populations exhibited higher NAD5 expression and lower ATP8 expression levels [50], further highlighting the evolutionary conservation of mitochondrial regulatory systems that enable adaptation to different stressors.
RNAi-targeting ATP8 provided functional evidence for its role in regulating respiration. The silencing of ATP8 significantly reduced respiration rates, indicating its critical role in maintaining aerobic energy production. Notably, the compensatory upregulation of COX1 was observed, suggesting the potential mitochondrial complementary mechanisms that help to stabilize respiration rates and energy homeostasis. Interestingly, the dsATP8-treated insects exhibited lower respiration rates and reduced mortality rates under exposure to abamectin and β-cypermethrin, implying that ATP8 downregulation may reduce metabolic rates, thereby decreasing toxin uptake and insecticide susceptibility. This protective effect could stem from optimized energy allocation to counteract chemical toxicity. Similar patterns have been observed in other mitochondrial genes. For instance, NAD6 knockdown resulted in increased mortality rates, whereas the suppression of COX1 reduced mortality rates following exposure to allyl isothiocyanate [51]. Similarly, NAD4 silencing led to the broad downregulation of mitochondrial genes (including NAD1, NAD2, NAD4L, COX1, COX3, ATP6, and ATP8) in a manner similar to that of the transcriptional cascade triggered by COX3 knockdown, which further reduced respiration rates but improved cold tolerance in C. ferrugineus [9]. Studies on B. dorsalis revealed that avermectin and malathion induced COX2 expression, and silencing COX2 increased pesticide-induced mortality rates [11]. These findings align with the idea that metabolic suppression may be linked to toxin tolerance. Reduced energy flux could limit toxin uptake or activate survival pathways, as seen in phosphine-resistant insects and nematodes with impaired mitochondrial function [52,53].
5. Conclusions
In conclusion, the use of a portable photosynthesis system for measuring respiration rates in small-sized insects opens up new possibilities for field-based studies, enabling a better understanding of how climate change and pest control measures affect insect population dynamics. Furthermore, the comprehensive analysis of mitochondrial gene expression, coupled with functional validation through RNAi, not only enhances our understanding of the molecular mechanisms governing respiration but also identifies potential targets for the development of more effective and environmentally friendly pest control strategies.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/insects16060616/s1: Table S1: Primers used in this study. Figure S1: Transcriptional patterns of mitochondrial protein-coding genes in Acyrthosiphon pisum during light/dark periods. Figure S2. Transcriptional patterns of mitochondrial protein-coding genes in Acyrthosiphon pisum during different developmental stages.
Author Contributions
Conceptualization, B.-Y.D.; methodology, B.-Y.D.; investigation, B.-Y.D., Q.-Q.X., Y.-H.Z., Y.-J.L. and Y.Z.; resources, B.-Y.D.; data curation, B.-Y.D. and Q.-Q.X.; writing—original draft preparation, B.-Y.D.; writing—review and editing, B.-Y.D. and Q.-Q.X.; visualization, B.-Y.D. and Q.-Q.X.; supervision, B.-Y.D.; project administration, B.-Y.D.; funding acquisition, B.-Y.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 32302338, and the Natural Science Foundation of Chongqing, grant number CSTB2024NSCQ-MSX0794.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank the anonymous reviewers for their invaluable comments and suggestions about this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Westneat, M.W.; Betz, O.; Blob, R.W.; Fezzaa, K.; Cooper, W.J.; Lee, W.K. Tracheal respiration in insects visualized with synchrotron X-ray imaging. Science 2003, 299, 558–560. [Google Scholar] [CrossRef] [PubMed]
- Contreras, H.L.; Bradley, T.J. Metabolic rate controls respiratory pattern in insects. J. Exp. Biol. 2009, 212, 424–428. [Google Scholar] [CrossRef]
- Harrison, J.F.; Greenlee, K.J.; Verberk, W.C.E.P. Functional hypoxia in insects: Definition, assessment, and consequences for physiology, ecology, and evolution. Annu. Rev. Entomol. 2018, 63, 303–325. [Google Scholar] [CrossRef] [PubMed]
- Hetz, S.K.; Bradley, T.J. Insects breathe discontinuously to avoid oxygen toxicity. Nature 2005, 433, 516–519. [Google Scholar] [CrossRef]
- Lalouette, L.; Williams, C.M.; Hervant, F.; Sinclair, B.J.; Renault, D. Metabolic rate and oxidative stress in insects exposed to low temperature thermal fluctuations. Comp. Biochem. Physiol. A-Mol. Integr. Physiol. 2011, 158, 229–234. [Google Scholar] [CrossRef]
- Contreras, H.L.; Bradley, T.J. Transitions in insect respiratory patterns are controlled by changes in metabolic rate. J. Insect Physiol. 2010, 56, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Clissold, F.J.; Simpson, S.J. Temperature, food quality and life history traits of herbivorous insects. Curr. Opin. Insect Sci. 2015, 11, 63–70. [Google Scholar] [CrossRef]
- Verberk, W.C.E.P.; Bilton, D.T. Respiratory control in aquatic insects dictates their vulnerability to global warming. Biol. Lett. 2013, 9, 20130473. [Google Scholar] [CrossRef]
- Yuan, G.-Q.; Chen, M.-Q.; Hou, Q.-L.; Tang, P.-A.; Chen, E.-H. Identification and functional analysis of mitochondrial protein-coding genes associated with the adaptability to cold stress of the rusty grain beetle Cryptolestes ferrugineus. J. Stored Prod. Res. 2025, 112, 102626. [Google Scholar] [CrossRef]
- Nayak, M.K.; Daglish, G.J.; Phillips, T.W.; Ebert, P.R. Resistance to the fumigant phosphine and its management in insect pests of stored products: A global perspective. Annu. Rev. Entomol. 2020, 65, 333–350. [Google Scholar] [CrossRef]
- Jiang, S.-D.; Wang, L.; Wang, L.; Sun, J.; Wang, J.J.; Wei, D.D. Mitochondrial coding genes mediate insecticide tolerance in the oriental fruit fly, Bactrocera dorsalis (Hendel). Pest. Biochem. Physiol. 2024, 199, 105763. [Google Scholar] [CrossRef] [PubMed]
- Brügger, B.P.; Martínez, L.C.; Plata-Rueda, A.; Castro, B.M.d.C.e.; Soares, M.A.; Wilcken, C.F.; Carvalho, A.G.; Serrão, J.E.; Zanuncio, J.C. Bioactivity of the Cymbopogon citratus (Poaceae) essential oil and its terpenoid constituents on the predatory bug, Podisus nigrispinus (Heteroptera: Pentatomidae). Sci. Rep. 2019, 9, 8358. [Google Scholar] [CrossRef]
- Plata-Rueda, A.; Martínez, L.C.; Santos, M.H.D.; Fernandes, F.L.; Wilcken, C.F.; Soares, M.A.; Serrão, J.E.; Zanuncio, J.C. Insecticidal activity of garlic essential oil and their constituents against the mealworm beetle, Tenebrio molitor Linnaeus (Coleoptera: Tenebrionidae). Sci. Rep. 2017, 7, 46406. [Google Scholar] [CrossRef]
- Sousa, A.H.; Faroni, L.R.D.A.; Guedes, R.N.C.; Tótola, M.R.; Urruchi, W.I. Ozone as a management alternative against phosphine-resistant insect pests of stored products. J. Stored Prod. Res. 2008, 44, 379–385. [Google Scholar] [CrossRef]
- Jiang, T.; Ma, L.; Liu, X.-Y.; Xiao, H.-J.; Zhang, W.-N. Effects of starvation on respiratory metabolism and energy metabolism in the cotton bollworm Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). J. Insect Physiol. 2019, 119, 103951. [Google Scholar] [CrossRef] [PubMed]
- Teulier, L.; Weber, J.M.; Crevier, J.; Darveau, C.A. Proline as a fuel for insect flight: Enhancing carbohydrate oxidation in hymenopterans. Proc. R. Soc. B 2016, 283, 20160333. [Google Scholar] [CrossRef]
- Plata-Rueda, A.; Martínez, L.C.; Costa, N.C.R.; Zanuncio, J.C.; de Sena Fernandes, M.E.; Serrão, J.E.; Guedes, R.N.C.; Fernandes, F.L. Chlorantraniliprole–mediated effects on survival, walking abilities, and respiration in the coffee berry borer, Hypothenemus hampei. Ecotoxicol. Environ. Saf. 2019, 172, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Liu, Y.; Liu, Y.; Dai, C.; Zhang, Y.; Zhou, F.; Zhu, Y. Salicylic acid modulates the osmotic system and photosynthesis rate to enhance the drought tolerance of Toona ciliata. Plants 2023, 12, 4187. [Google Scholar] [CrossRef]
- Shi, S.; Li, H.; Wang, X.; Wang, Z.; Xu, J.; He, X.; Yang, Z.a. Greater biomass production under elevated CO2 is attributed to physiological optimality, trade-offs in nutrient allocation, and oxidative defense in drought-stressed mulberry. Antioxidants 2025, 14, 383. [Google Scholar] [CrossRef]
- Yu, M.; Fan, Y.; Li, X.; Chen, X.; Yu, S.; Wei, S.; Li, S.; Chang, W.; Qu, C.; Li, J.; et al. LESION MIMIC MUTANT 1 confers basal resistance to Sclerotinia sclerotiorum in rapeseed via a salicylic aciddependent pathway. J. Exp. Bot. 2023, 74, 5620–5634. [Google Scholar] [CrossRef]
- Zveushe, O.K.; Sajid, S.; Dong, F.; Han, Y.; Zeng, F.; Geng, Y.; Shen, S.; Xiang, Y.; Kang, Q.; Zhang, Y.; et al. Different sex combinations of Populus cathayana affect soil respiration and tea litter decomposition by influencing plant growth and soil functional microbial diversity. Plant Soil 2023, 490, 631–650. [Google Scholar] [CrossRef]
- Wang, S.; Wang, T.; Gao, L.; Du, H.; Wang, D.; Ma, M.; Rennenberg, H. Iron addition promotes mercury removal from soil by Robinia pseudoacacia-rhizobia symbiosis. Tree Physiol. 2025, 45, tpae166. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Li, X.; Chen, M.; Xu, X.; Zhang, W.; Chi, H.; Shao, P.; Tang, F.; Gong, T.; Guo, M.; et al. Excellent canopy structure in soybeans can improve their photosynthetic performance and increase yield. Agriculture 2024, 14, 1783. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, F.; Li, H.; Su, Y.; Wu, Y. Stable nitrogen isotopes as an effective tool for estimating the nitrogen demand of Broussonetia papyrifera (L.) vent seedlings under variable nitrate concentrations. Agronomy 2023, 13, 1663. [Google Scholar] [CrossRef]
- Kayoumu, M.; Iqbal, A.; Muhammad, N.; Li, X.; Li, L.; Wang, X.; Gui, H.; Qi, Q.; Ruan, S.; Guo, R.; et al. Phosphorus availability affects the photosynthesis and antioxidant system of contrasting Low-P-Tolerant cotton genotypes. Antioxidants 2023, 12, 466. [Google Scholar] [CrossRef]
- Li, Y.; Ruan, S.; Li, D.; Liu, J.; Hu, Q.; Dian, Y.; Yu, Z.; Zhou, J. Photosynthetic difference of six poplar genotypes and estimation of photosynthetic capacities based on leaf hyperspectral reflectance. For. Res. 2024, 4, e037. [Google Scholar] [CrossRef]
- Chieppa, J.; Brown, T.; Giresi, P.; Juenger, T.E.; Resco de Dios, V.; Tissue, D.T.; Aspinwall, M.J. Climate and stomatal traits drive covariation in nighttime stomatal conductance and daytime gas exchange rates in a widespread C4 grass. New Phytol. 2021, 229, 2020–2034. [Google Scholar] [CrossRef]
- Feng, Q.; Luo, Y.; Liang, M.; Cao, Y.; Wang, L.; Liu, C.; Zhang, X.; Ren, L.; Wang, Y.; Wang, D.; et al. Rhizobacteria protective hydrogel to promote plant growth and adaption to acidic soil. Nat. Commun. 2025, 16, 1684. [Google Scholar] [CrossRef]
- Riches, M.; Lee, D.; Farmer, D.K. Simultaneous leaf-level measurement of trace gas emissions and photosynthesis with a portable photosynthesis system. Atmos. Meas. Tech. 2020, 13, 4123–4139. [Google Scholar] [CrossRef]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling. Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Despabiladeras, J.B.; Bautista, M.A.M. Complete mitochondrial genome of the eggplant fruit and Shoot Borer, Leucinodes orbonalis Guenée (Lepidoptera: Crambidae), and comparison with other pyraloid moths. Insects 2024, 15, 220. [Google Scholar] [CrossRef] [PubMed]
- Perkin, L.C.; Smith, T.P.L.; Oppert, B. Variants in the mitochondrial genome sequence of Rhyzopertha dominica (Fabricius) (Coleoptera: Bostrycidae). Insects 2021, 12, 387. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, S.; Wu, X.; Wei, Q.; Shang, Y.; Sun, G.; Mei, X.; Dong, Y.; Sha, W.; Zhang, H. High-altitude adaptation in vertebrates as revealed by mitochondrial genome analyses. Ecol. Evol. 2021, 11, 15077–15084. [Google Scholar] [CrossRef] [PubMed]
- Francoso, E.; Zuntini, A.R.; Ricardo, P.C.; Santos, P.K.F.; Araujo, N.d.S.; Silva, J.P.N.; Goncalves, L.T.; Brito, R.; Gloag, R.; Taylor, B.A.; et al. Rapid evolution, rearrangements and whole mitogenome duplication in the Australian stingless bees Tetragonula (Hymenoptera: Apidae): A steppingstone towards understanding mitochondrial function and evolution. Int. J. Biol. Macromol. 2023, 242, 124568. [Google Scholar] [CrossRef]
- Guan, J.-Y.; Zhang, Z.-Y.; Cao, Y.-R.; Xu, X.-D.; Storey, K.B.; Yu, D.-N.; Zhang, J.-Y. The complete mitochondrial genome of Choroterpes (Euthralus) yixingensis (Ephemeroptera: Leptophlebiidae) and its mitochondrial protein-coding gene expression under imidacloprid stress. Gene 2021, 800, 145833. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Guan, J.-Y.; Cao, Y.-R.; Dai, X.-Y.; Storey, K.B.; Yu, D.-N.; Zhang, J.-Y. Mitogenome analysis of four Lamiinae species (Coleoptera: Cerambycidae) and gene expression responses by Monochamus alternatus when infected with the parasitic nematode, Bursaphelenchus mucronatus. Insects 2021, 12, 453. [Google Scholar] [CrossRef]
- Chen, E.-H.; Duan, J.-Y.; Song, W.; Wang, D.-X.; Tang, P.-A. RNA-seq analysis reveals mitochondrial and cuticular protein genes are associated with phosphine resistance in the rusty grain beetle (Coleoptera: Laemophloeidae). J. Econ. Entomol. 2021, 114, 440–453. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.-H.; Wei, D.; Wei, D.-D.; Yuan, G.-R.; Wang, J.-J. The effect of dietary restriction on longevity, fecundity, and antioxidant responses in the oriental fruit fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). J. Insect Physiol. 2013, 59, 1008–1016. [Google Scholar] [CrossRef]
- Qiang, W.X.; Zhong, L.C.; Tian, X.Y.; Zhou, S. Effects of sublethal dosage of imidacloprid, abamectin and beta-cypermethrin on the development and reproduction of green of the morph of pea aphid (Acyrthosiphon pisum). Acta Prataculturae Sin. 2014, 23, 279–286. [Google Scholar]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007, 8, R19. [Google Scholar] [CrossRef]
- Yang, C.; Pan, H.; Liu, Y.; Zhou, X. Selection of reference genes for expression analysis using quantitative real-time PCR in the pea aphid, Acyrthosiphon pisum (Harris) (Hemiptera, Aphidiae). PLoS ONE 2014, 9, e110454. [Google Scholar] [CrossRef] [PubMed]
- Lebenzon, J.E.; Overgaard, J.; Jørgensen, L.B. Chilled, starved or frozen: Insect mitochondrial adaptations to overcome the cold. Curr. Opin. Insect Sci. 2023, 58, 101076. [Google Scholar] [CrossRef] [PubMed]
- Haddi, K.; Mendes, M.V.; Barcellos, M.S.; Lino-Neto, J.; Freitas, H.L.; Guedes, R.N.C.; Oliveira, E.E. Sexual success after stress? Imidacloprid-induced hormesis in males of the neotropical stink bug Euschistus heros. PLoS ONE 2016, 11, e0156616. [Google Scholar] [CrossRef] [PubMed]
- Plata-Rueda, A.; Rolim, G.D.S.; Wilcken, C.F.; Zanuncio, J.C.; Serrão, J.E.; Martínez, L.C. Acute toxicity and sublethal effects of lemongrass essential oil and their components against the Granary weevil, Sitophilus granarius. Insects 2020, 11, 379. [Google Scholar] [CrossRef]
- Plata-Rueda, A.; Martínez, L.C.; Rolim, G.d.S.; Coelho, R.P.; Santos, M.H.; Tavares, W.d.S.; Zanuncio, J.C.; Serrão, J.E. Insecticidal and repellent activities of Cymbopogon citratus (Poaceae) essential oil and its terpenoids (citral and geranyl acetate) against Ulomoides dermestoides. Crop Prot. 2020, 137, 105299. [Google Scholar] [CrossRef]
- Plata-Rueda, A.; Campos, J.M.; da Silva Rolim, G.; Martínez, L.C.; Dos Santos, M.H.; Fernandes, F.L.; Serrão, J.E.; Zanuncio, J.C. Terpenoid constituents of cinnamon and clove essential oils cause toxic effects and behavior repellency response on granary weevil, Sitophilus granarius. Ecotoxicol. Environ. Saf. 2018, 156, 263–270. [Google Scholar] [CrossRef]
- Fiaz, M.; Martínez, L.C.; Costa, M.d.S.; Cossolin, J.F.S.; Plata-Rueda, A.; Gonçalves, W.G.; Sant’Ana, A.E.G.; Zanuncio, J.C.; Serrão, J.E. Squamocin induce histological and ultrastructural changes in the midgut cells of Anticarsia gemmatalis (Lepidoptera: Noctuidae). Ecotoxicol. Environ. Saf. 2018, 156, 1–8. [Google Scholar] [CrossRef]
- Fiaz, M.; Martínez, L.C.; Plata-Rueda, A.; Gonçalves, W.G.; Shareef, M.; Zanuncio, J.C.; Serrão, J.E. Toxicological and morphological effects of tebufenozide on Anticarsia gemmatalis (Lepidoptera: Noctuidae) larvae. Chemosphere 2018, 212, 337–345. [Google Scholar] [CrossRef]
- Lebenzon, J.E.; Denezis, P.W.; Mohammad, L.; Mathers, K.E.; Turnbull, K.F.; Staples, J.F.; Sinclair, B.J. Reversible mitophagy drives metabolic suppression in diapausing beetles. Proc. Natl. Acad. Sci. USA 2022, 119, e2201089119. [Google Scholar] [CrossRef]
- Ding, Y.-R.; Van, Z.-T.; Si, F.-L.; Li, X.-D.; Mao, Q.-M.; Asghar, S.; Chen, B. Mitochondrial genes associated with pyrethroid resistance revealed by mitochondrial genome and transcriptome analyses in the malaria vector Anopheles sinensis (Diptera: Culicidae). Pest Manag. Sci. 2020, 76, 769–778. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, Y.; Wu, H.; Xu, N.; Ma, Z.; Zhang, C. Function of four mitochondrial genes in fumigation lethal mechanisms of Allyl Isothiocyanate against Sitophilus zeamais adults. Pest Biochem. Physiol. 2021, 179, 104947. [Google Scholar] [CrossRef] [PubMed]
- Steven, Z.; Jujiao, K.; Paul, E. Mitochondrial modulation of phosphine toxicity and resistance in Caenorhabditis elegans. Toxicol. Sci. 2008, 102, 179–186. [Google Scholar]
- Pimentel, M.A.G.; Faroni, L.R.D.A.; Tótola, M.R.; Guedes, R.N.C. Phosphine resistance, respiration rate and fitness consequences in stored-product insects. Pest Manag. Sci. 2007, 63, 876–881. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).