3.1.1. Prothoracic Skeleton
The well-developed and highly sclerotized prothorax is the largest segment of the thorax. Anteriorly, the prothorax articulates with the head through the cervical membrane, where lateral cervical sclerites are absent [
28,
29]. All prothoracic sclerites fuse together to form a rigid ring-like structure. Dense short fine setae are present along the anterior and posterior margins of the prothorax, with additional scattered short thick setae on the prosternum.
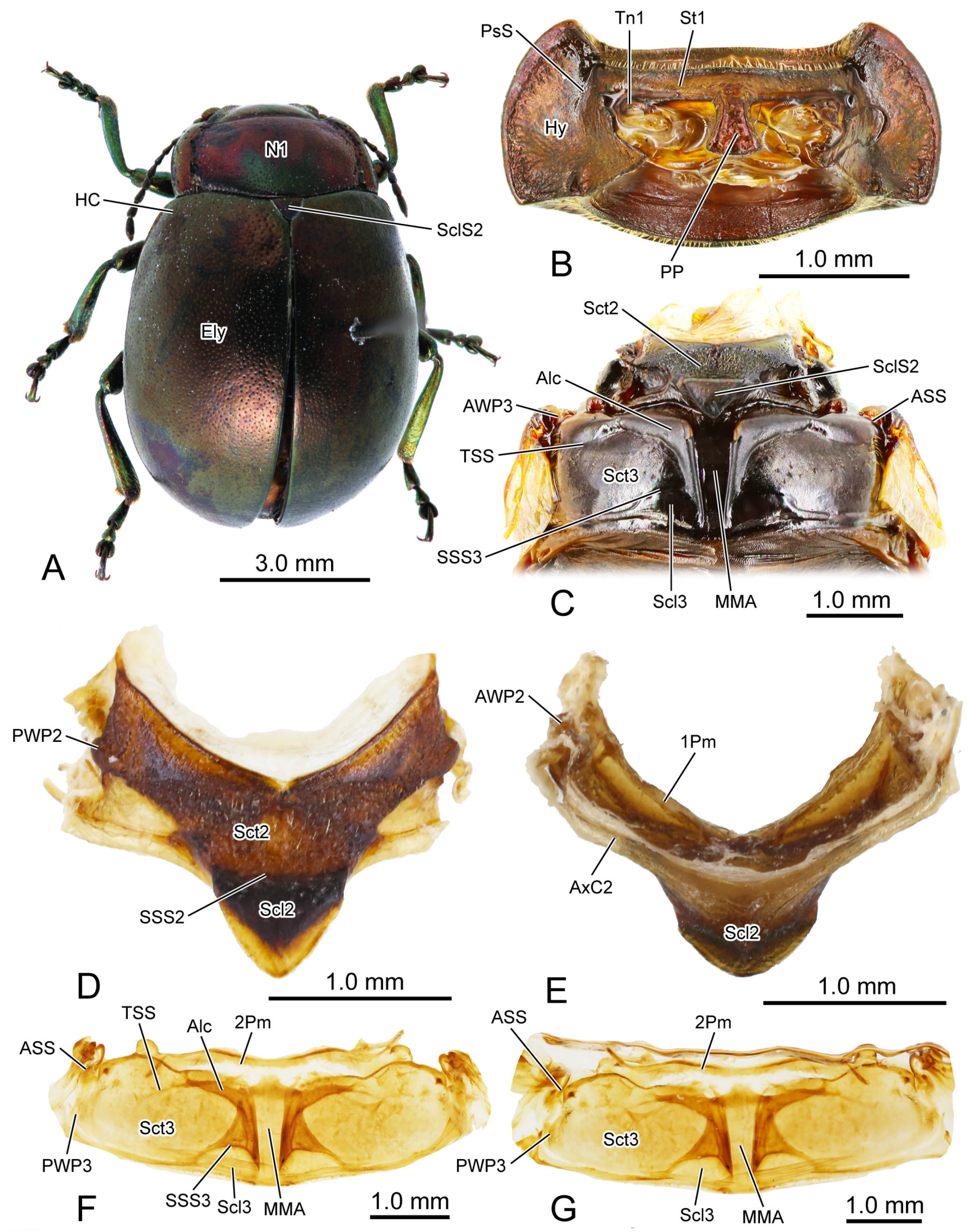
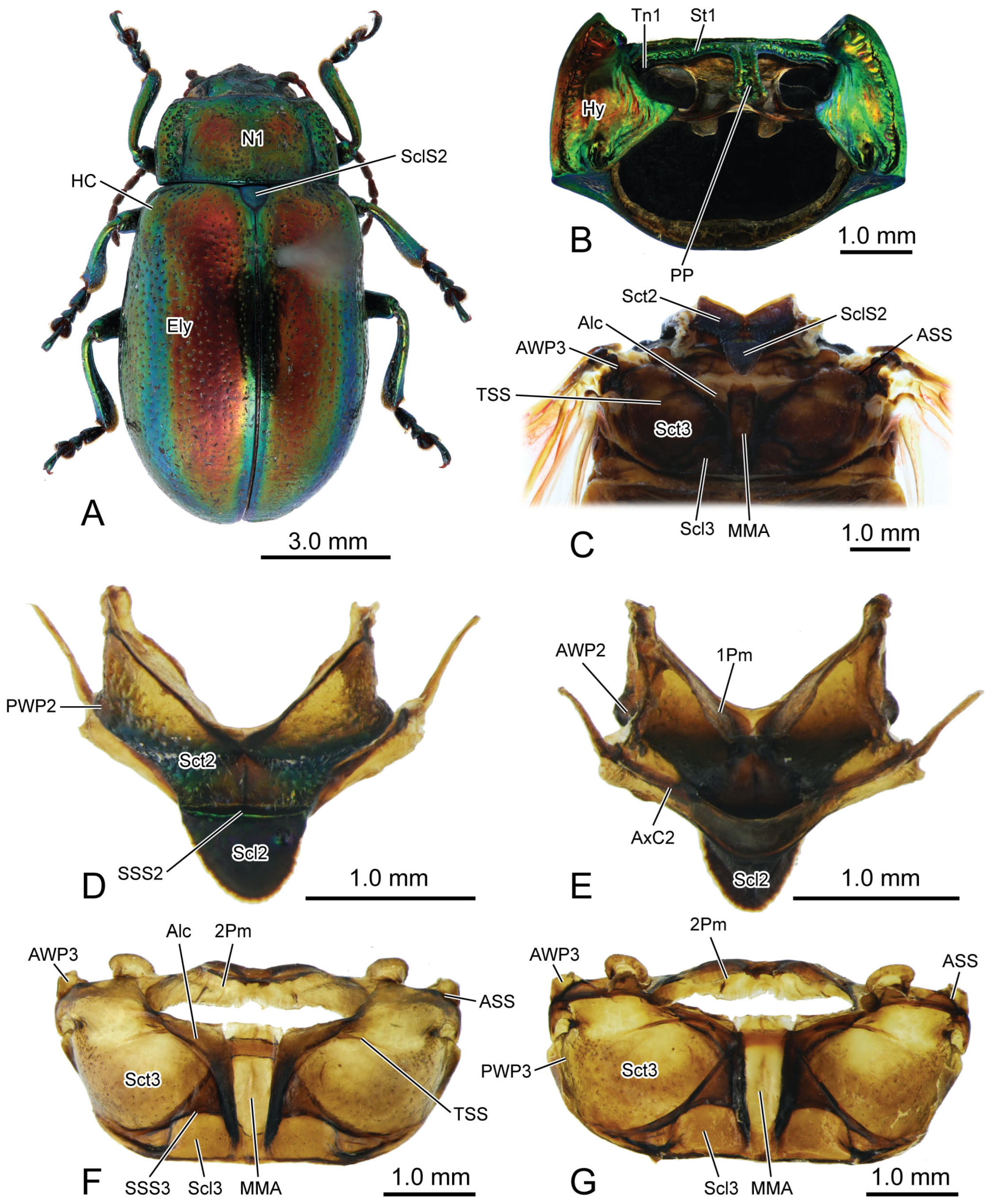
In the dorsal view, the width of the pronotum (N1:
Figure 1A,
Figure 3A,C and
Figure 6A) is 2.2 times its length. The punctures scattered across the median area of the pronotum are small and shallow, while those on the lateral side are larger and deeper. The anterior margin of the pronotum slightly concaves posterad, forming a shallow wide depression that accommodates the occiput. The antero-lateral corner of the pronotum forms an obtuse angle and projects antero-mediad. Laterally, a groove delimits the thickened margin from the central panel of the pronotum. The posterior margin of the central panel of the pronotum slightly bends posterad. Ventrally, it connects with the bent anterad prophragma (1Pm:
Figure 1E and
Figure 6A).
Figure 1.
Digital photography of Chrysolina sulcisollis. (A) habitus, dorsal view; (B) prothorax, ventral view; (C) pterothorax, dorsal view; (D) mesothoracic tergite, dorsal view. (E) mesothoracic tergite, ventral view. (F). metathoracic tergite, dorsal view. (G) metathoracic tergite, ventral view. Abbreviations: 1/2Pm—pro-/mesophragma; Alc—alacrista; ASS—antero-lateral scutal suture; AWP2/3—anterior notal wing process of meso-/metathorax; AxC2—axillary cord of mesothorax; Ely—elytron; HC—humeral callus; Hy—hypomeron; MMA—median membranous area; N1—pronotum; PP—prosternal process; PsS—notosternal suture; PWP2/3—posterior notal wing process of meso-/metathorax; Scl2/3—meso-/metascutellum; SclS2—mesoscutellar shield; Sct2/3—meso-/metascutum; SSS2/3—scutoscutellar suture of meso-/metathorax; St1—prosternum; Tn1—protrochantin; TSS—transverse scutal suture.
Figure 1.
Digital photography of Chrysolina sulcisollis. (A) habitus, dorsal view; (B) prothorax, ventral view; (C) pterothorax, dorsal view; (D) mesothoracic tergite, dorsal view. (E) mesothoracic tergite, ventral view. (F). metathoracic tergite, dorsal view. (G) metathoracic tergite, ventral view. Abbreviations: 1/2Pm—pro-/mesophragma; Alc—alacrista; ASS—antero-lateral scutal suture; AWP2/3—anterior notal wing process of meso-/metathorax; AxC2—axillary cord of mesothorax; Ely—elytron; HC—humeral callus; Hy—hypomeron; MMA—median membranous area; N1—pronotum; PP—prosternal process; PsS—notosternal suture; PWP2/3—posterior notal wing process of meso-/metathorax; Scl2/3—meso-/metascutellum; SclS2—mesoscutellar shield; Sct2/3—meso-/metascutum; SSS2/3—scutoscutellar suture of meso-/metathorax; St1—prosternum; Tn1—protrochantin; TSS—transverse scutal suture.
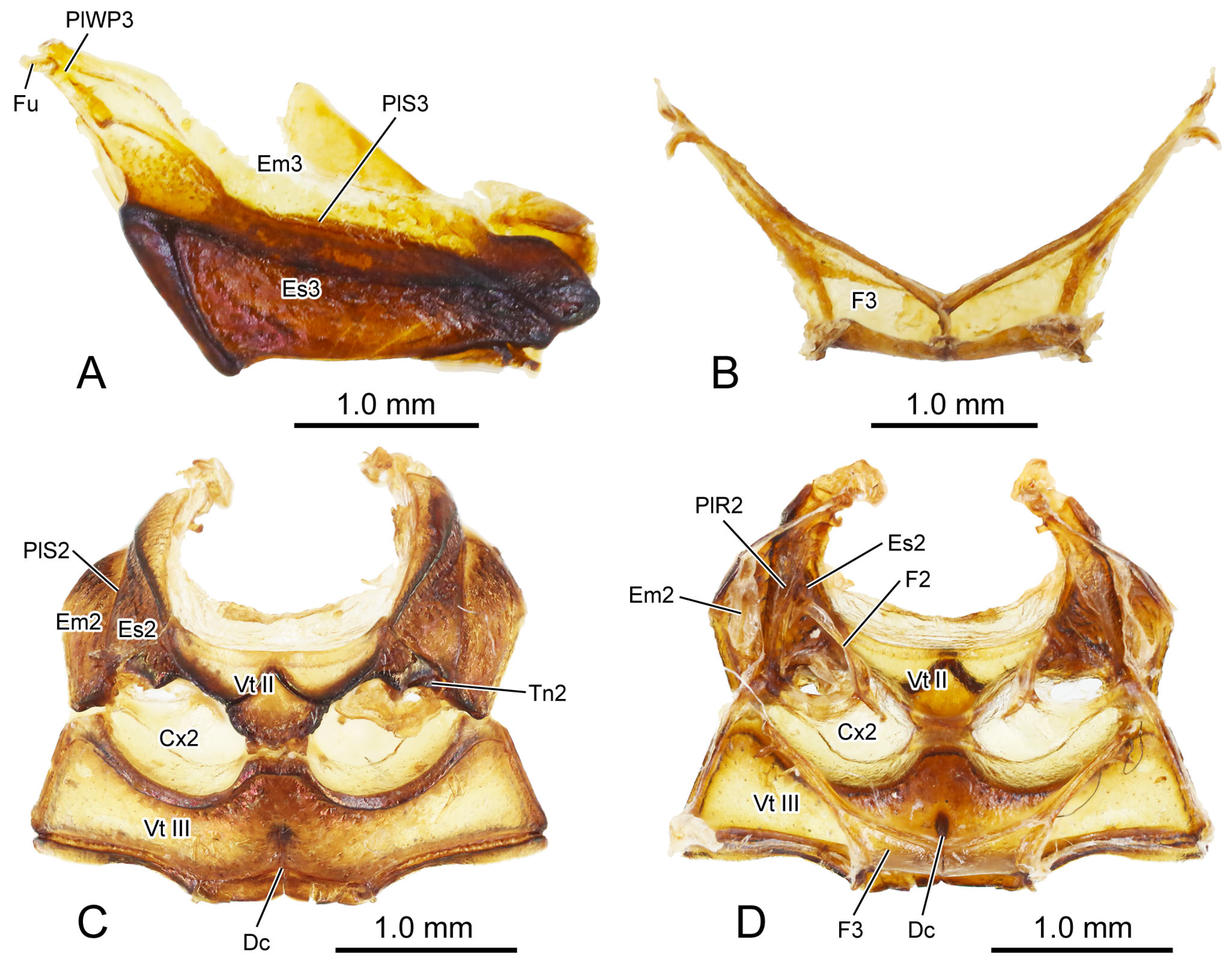
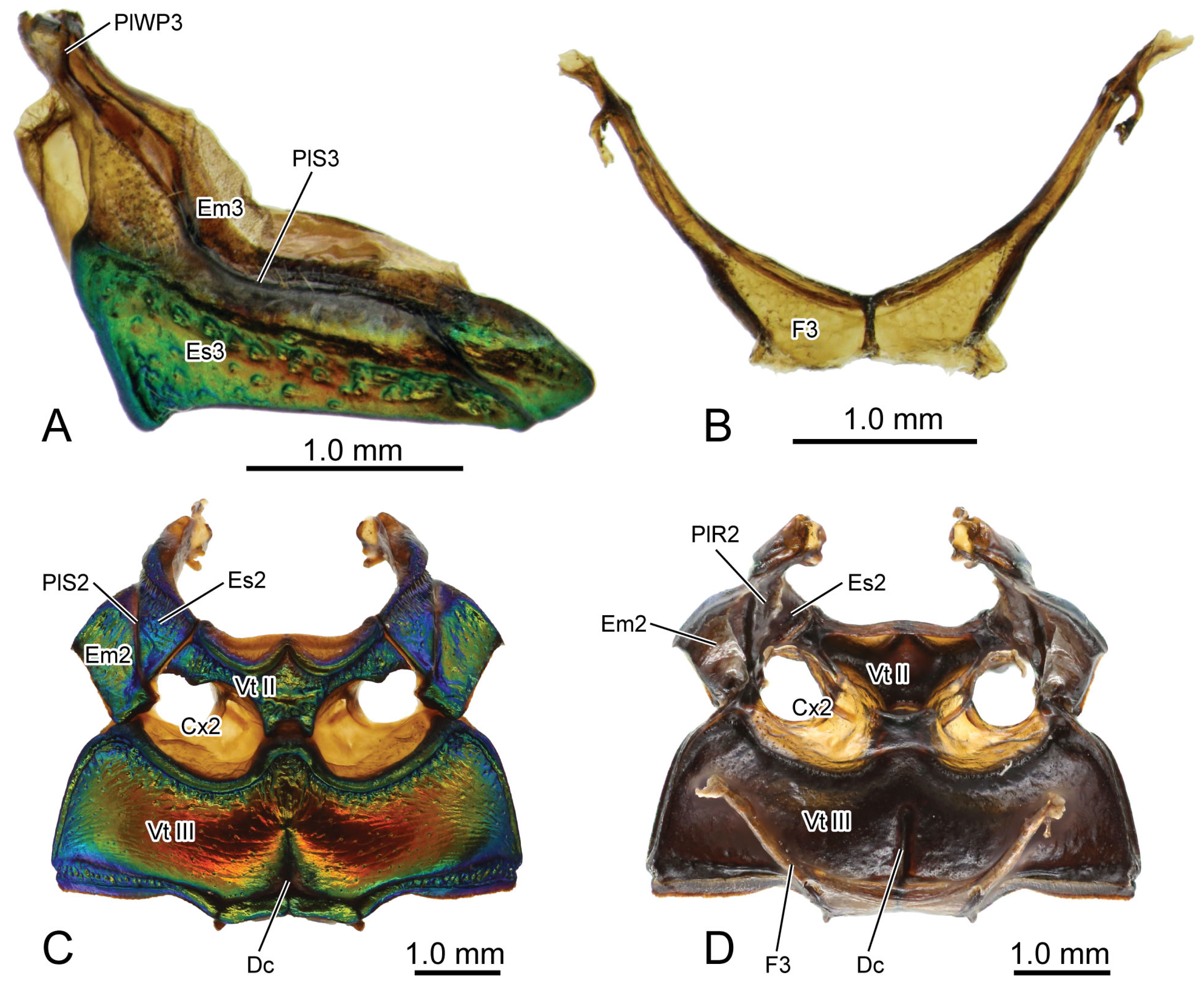
Figure 2.
Digital photography of Chrysolina sulcisollis. (A) metathorax, lateral view; (B) metafurca, rear view; (C) meso- and metaventrites, ventral view; (D) meso- and metaventrite, dorsal view. Abbreviations: Cx2—mesocoxa; Dc—metathoracic discrimen; Em2/3—mes-/metepimeron; Es2/3—mes-/metanepisternum; F2/3—meso-/metafurca; Fu—fulcrum; PlWP3—pleural wing process of metathorax; PlR2—mesopleural ridge; PlS2/3—meso-/metapleural suture; Tn2—mesotrochantin; Vt II/III—meso-/metaventrite.
Figure 2.
Digital photography of Chrysolina sulcisollis. (A) metathorax, lateral view; (B) metafurca, rear view; (C) meso- and metaventrites, ventral view; (D) meso- and metaventrite, dorsal view. Abbreviations: Cx2—mesocoxa; Dc—metathoracic discrimen; Em2/3—mes-/metepimeron; Es2/3—mes-/metanepisternum; F2/3—meso-/metafurca; Fu—fulcrum; PlWP3—pleural wing process of metathorax; PlR2—mesopleural ridge; PlS2/3—meso-/metapleural suture; Tn2—mesotrochantin; Vt II/III—meso-/metaventrite.
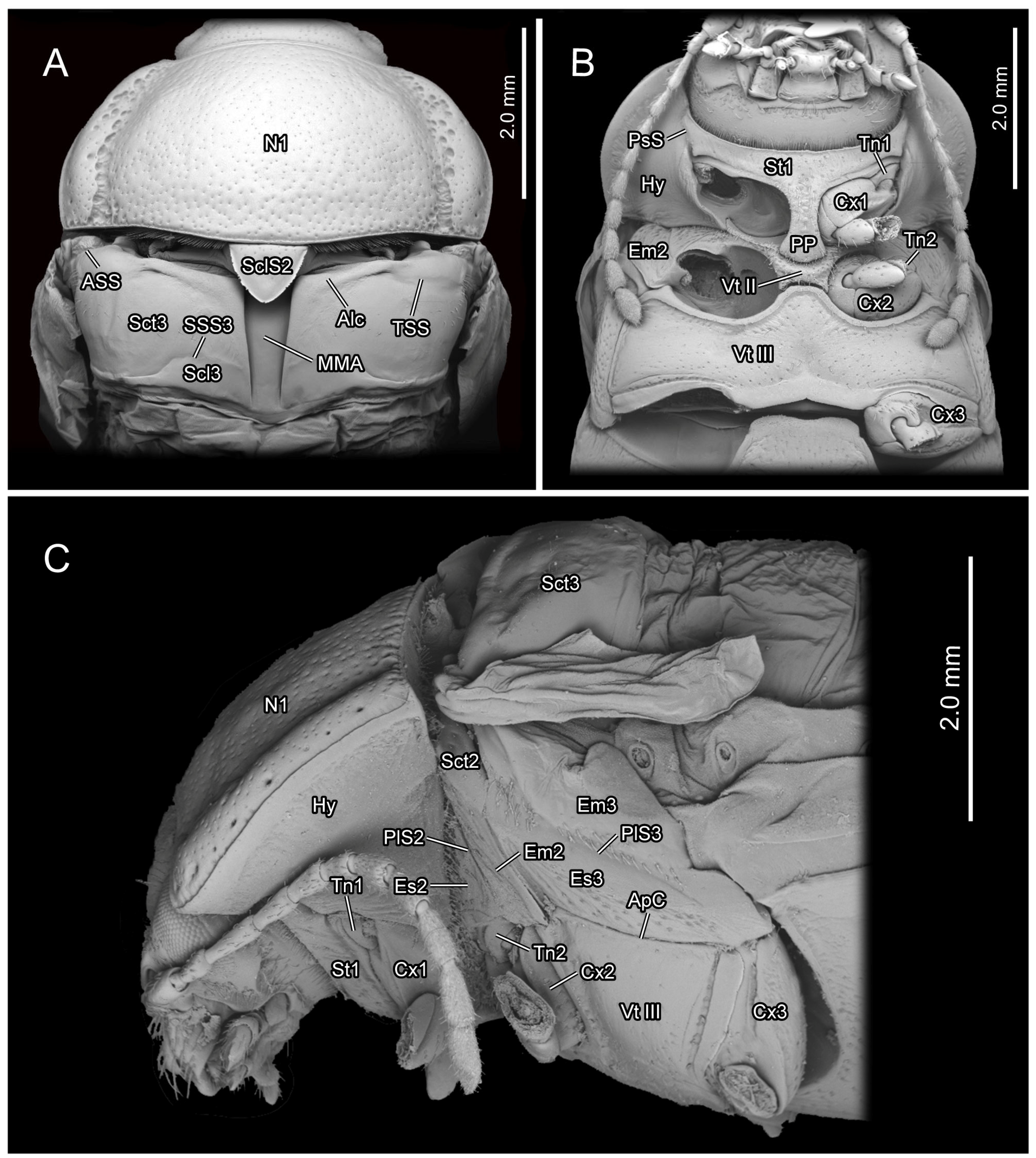
In the ventral view of the prothorax, the bulged hypomeron (Hy:
Figure 1B and
Figure 3B,C) connects laterally with the thickened pronotal margin and occupies the lateral part of the sclerotic structure on the ventral side. The notosternal suture (PsS:
Figure 1B) extends postero-laterad from the antero-lateral corner of the prosternum to the anterior 1/3 of the prothoracic ventral side, delimiting the T-shaped prosternum (St1:
Figure 1B,C) from the lateral hypomera. The anterior portion of the prosternum is transversely slender, while the postero-median portion bears a long, trapezoid prosternal process (PP:
Figure 1B and
Figure 3B) that extends posterad between the paired procoxae. The prosternal process is narrow anteriorly, wide posteriorly and curved proximad laterally, with its posterior margin slightly bending posteriorly. The anterad bent protrochantin (Tn1:
Figure 1B and
Figure 3B) is nearly a flat trapezoid, with both the long anterior and short posterior margins bent anterad. Anteriorly, the protrochantin attaches to the postero-lateral margin of the anterior portion of the prosternum, and posteriorly it connects to the procoxal base. The slender crytopleuron (Crpl:
Figure 6A) is situated near the lateral margin of the procoxal rim and extends dorsad along the hypomeron. Its dorsal portion broadens into a fan shape near the dorso-lateral margin of the pronotum. The short paired arms of the profurca (F1:
Figure 6A) arise dorso-laterad separately from posterior margin of the prosternum, with each tip expanding into a posterad bent tray-like structure.
3.1.2. Prothoracic Musculature
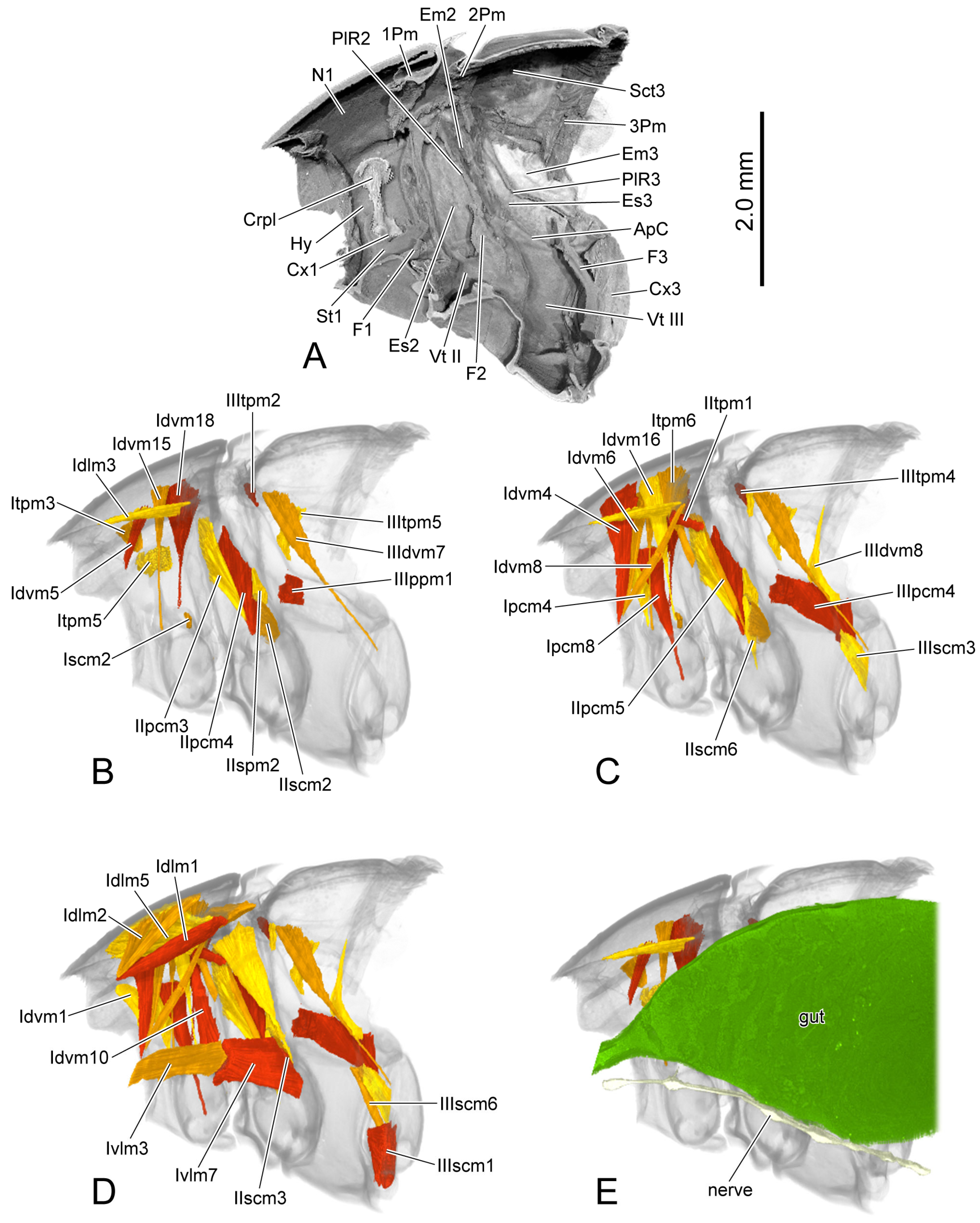
Idlm1 M. prophragma-occipitalis. O (=origin): antero-lateral area of ventral face of prophragma. I (=insertion): dorso-lateral area of occiput. Long triangular, narrowing towards occiput, straight.
Idlm2 M. pronoto-occipitalis. O: postero-median area of pronotum. I: dorso-lateral area of occiput. Long triangular, narrowing towards occiput, straight, broad, flat.
Idlm3 M. prophragma-cervicalis. O: lateral part of anterior margin of prophragma. I: dorso-lateral area of cervical membrane. Long triangular, narrowing towards cervical membrane, straight, slender.
Idlm5 M. pronoto-phragmalis anterior. O: antero-median area of pronotum. I: antero-lateral area of ventral face of prophragma. Irregular quadrilateral, original end broader than insertional end, straight, broad, flat.
Idvm1 M. cervico-occipitalis anterior. O: ventro-lateral area of ventral cervical membrane. I: dorso-lateral area of occipitale. Long triangular, narrowing towards occipitale, bent dorso-posterad.
Idvm4 M. pronoto-cervicalis lateralis. O: meso-lateral area of pronotum. I: postero-ventral area of occiput. Long triangular, narrowing towards occiput, straight.
Idvm5 M. pronoto-cervicalis anterior. O: meso-lateral area of pronotum. I: dorso-lateral area of cervical membrane. Approximate parallelogram, original end broader than insertional end, straight, slender.
Idvm6 M. pronoto-cervicalis medialis. O: meso-lateral area of pronotum. I: ventro-lateral area of ventral cervical membrane. Long triangular, narrowing towards cervical membrane, slightly bent postero-proximad, long.
Idvm8 M. prophragma-tentoralis. O: antero-lateral area of ventral face of prophragma. I: occipitale. Broad medially and narrowing towards both ends, slightly bent postero-laterad, long, slender.
Idvm10 M. profurca-phragmalis. O: dorso-lateral part of profurcal arm. I: antero-lateral area of ventral face of prophragma. Long triangular, narrowing towards prophragma, straight.
Idvm15 M. pronoto-trochantinocoxalis. O: postero-lateral area of pronotum. I: antero-proximal margin of procoxal rim. Long triangular, narrowing towards procoxal rim, straight, long, slender.
Idvm16 M. pronoto-coxalis anterior. O: postero-lateral area of pronotum. I: postero-lateral margin of procoxal rim. Long triangular, narrowing towards procoxal rim, straight, long.
Idvm18 M. pronoto-coxalis lateralis. O: postero-lateral area of pronotum. I: postero-lateral margin of procoxal rim. Long triangular, narrowing towards procoxal rim, straight, long.
Itpm3 M. pronoto-pleuralis anterior. O: antero-lateral area of pronotum. I: antero-dorsal area of lateral face of fan-shaped crytopleural dorsal portion. Triangular, narrowing towards crytopleuron, straight, short.
Itpm5 M. pronoto-apodemalis posterior. O: postero-lateral area of pronotum. I: postero-ventral area of lateral face of fan-shaped cryptopleural dorsal portion. Irregular quadrilateral, original end broader than insertional end, straight, short.
Itpm6 M. pronoto-intersegmentalis. O: postero-lateral area of pronotum. I: intersegmental membrane between pro- and mesothorax. Long triangular, narrowing towards intersegmental membrane, bent antero-laterad, long.
Ipcm4 M. propleuro-coxalis superior. O: antero-ventral area of proximal face of cryptopleural dorsal portion. I: antero-lateral margin of procoxal rim. Long triangular, narrowing towards procoxal rim, straight.
Ipcm8 M. propleuro-trochanteralis. O: postero-dorsal area of proximal face of cryptopleural dorsal portion. I: protrochanter. Long triangular, narrowing towards protrochanter, straight, long.
Ivlm3 M. profurca-tentorialis. O: dorsal area of anterior face of profurcal arm. I: ventro-median area of occipitale. Parallelogram, straight, long, broad.
Ivlm7 M. profurca-mesofurcalis. O: posterior face of profurcal arm. I: anterior face of mesofurcal arm. Irregular quadrilateral, original end narrower than insertional end, slightly bent dorso-proximad, large, flat.
Iscm2 M. profurca-coxalis posterior. O: ventro-lateral margin of profurcal arm. I: postero-lateral margin of procoxal rim. Long triangular, narrowing towards procoxal rim, straight, short.
Figure 3.
SEM photography of Chrysolina sulcisollis. (A) thorax without elytra, dorsal view; (B) thorax, ventral view; (C) thorax without elytra, lateral view. Abbreviations: Alc—alacrista; ApC—anapleural cleft; ASS—antero-lateral scutal suture; Cx1/2/3—pro-/meso-/metacoxal; Em2/3—mes-/metepimeron; Es2/3—mes-/metanepisternum; Hy—hypomeron; MMA—median membranous area; N1—pronotum; PlS2/3—meso-/metapleural suture; PP—prosternal process; PsP—notosternal suture; Scl3—metascutellum; SclS2—mesoscutellar shield; Sct2/3—meso-/metascutum; SSS3—scutoscutellar suture of metathorax; St1—prosternum; Tn1/2—pro-/mesotrochantin; TSS—transverse scutal suture; Vt II/III—meso-/metaventrite.
Figure 3.
SEM photography of Chrysolina sulcisollis. (A) thorax without elytra, dorsal view; (B) thorax, ventral view; (C) thorax without elytra, lateral view. Abbreviations: Alc—alacrista; ApC—anapleural cleft; ASS—antero-lateral scutal suture; Cx1/2/3—pro-/meso-/metacoxal; Em2/3—mes-/metepimeron; Es2/3—mes-/metanepisternum; Hy—hypomeron; MMA—median membranous area; N1—pronotum; PlS2/3—meso-/metapleural suture; PP—prosternal process; PsP—notosternal suture; Scl3—metascutellum; SclS2—mesoscutellar shield; Sct2/3—meso-/metascutum; SSS3—scutoscutellar suture of metathorax; St1—prosternum; Tn1/2—pro-/mesotrochantin; TSS—transverse scutal suture; Vt II/III—meso-/metaventrite.
3.1.3. Mesothoracic Skeleton
The exposed part of the mesothoracic tergite from the dorsal view is triadius-shaped. The dense short white setae cover the mesoscutum, whereas the mesoscutellum (Scl2:
Figure 1D,E) has no setae. The mesoscutum (Sct2:
Figure 2C,D) occupies the anterior two branches and the central area of the triadius-shape. The small median process on the antero-lateral margin of the mesoscutum is the anterior notal wing process (AWP2:
Figure 1E), and the inflated obtuse postero-lateral corner is the posterior notal wing process (PWP2:
Figure 1D). The nearly equilaterally triangular posterior branch of the mesothorax in the dorsal view is the mesoscutellum, which is delimited from the anterior mesoscutum by the transverse scutoscutellar suture (SSS2:
Figure 2D). The prominently raised triangular mesoscutellar shield (SclS2:
Figure 1C and
Figure 3A) aligns with the antero-proximal edge of the elytron when the elytra are closed. In the ventral view of the mesothoracic tergite, the axillary cord (AxC2:
Figure 1E) runs along the scutoscutellar suture and the posterior margin of the mesoscutum, extending to a position behind the posterior notal process. The slender transverse mesophragma (2Pm:
Figure 1F,G and
Figure 6A) medially bends posterad and laterally links to the antero-proximal corners of the paired yoke plates.
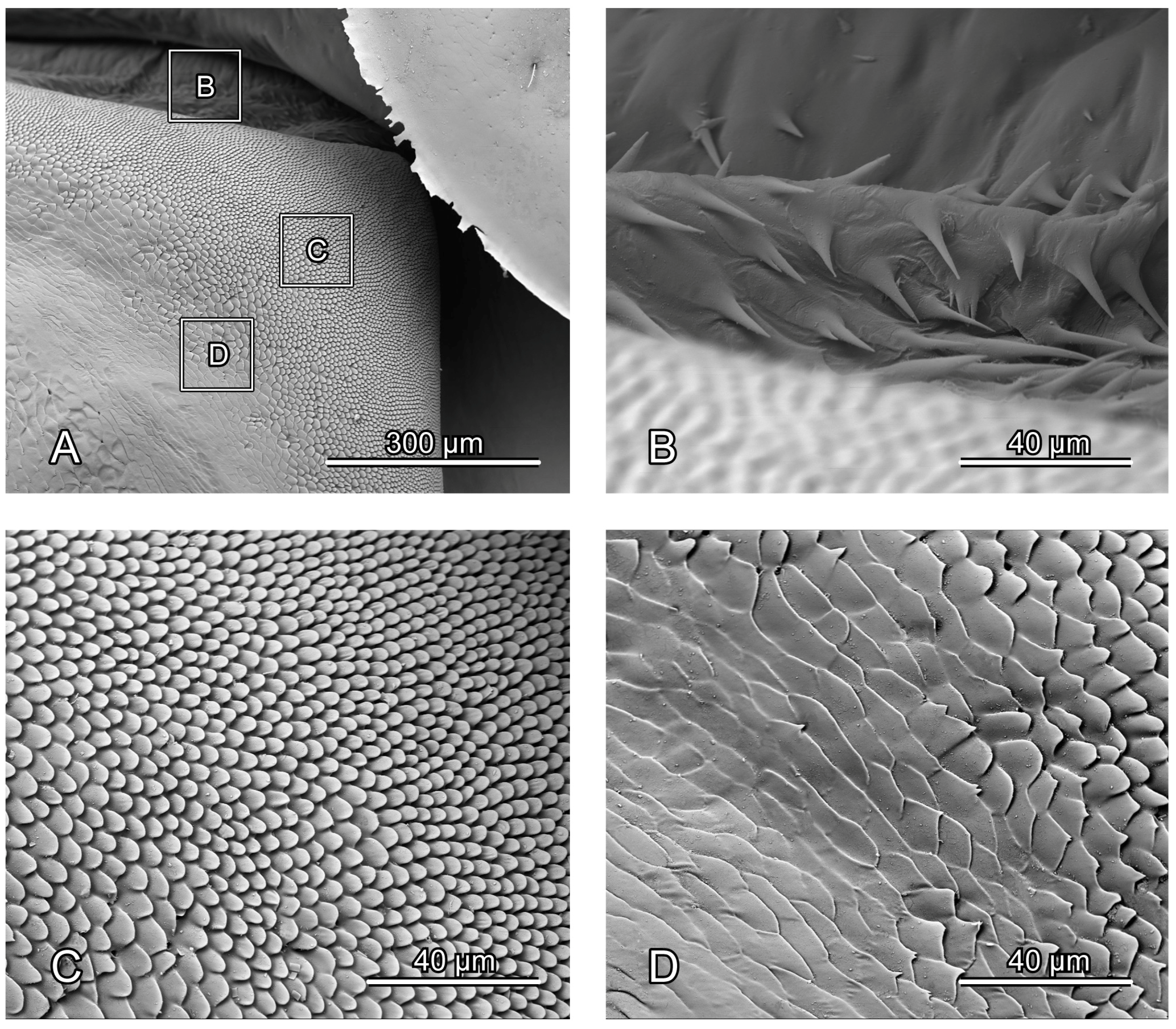
Figure 4.
SEM photography of alacrista of Chrysolina sulcisollis. (A) alacrista; (B,C) setae on different parts of alacrista; (D) scales on lateral margin of alacrista.
Figure 4.
SEM photography of alacrista of Chrysolina sulcisollis. (A) alacrista; (B,C) setae on different parts of alacrista; (D) scales on lateral margin of alacrista.
The plicated mesopleuron is a nearly long triangle. The mesopleural suture (PlS2:
Figure 2C and
Figure 3C) extends from the antero-dorsal corner to the meso-ventral area of the mesopleuron, dividing it into the anterior mesanepisternum (Es2:
Figure 2C,D,
Figure 3C and
Figure 6A) and the posterior mesepimeron (Em2:
Figure 2C,D,
Figure 3B,C and
Figure 6A). The mesopleural suture invaginates inward, forming a mesopleural ridge (PlR2:
Figure 2D and
Figure 6A).
The flat T-shaped mesoventrite (Vt II:
Figure 2C,D,
Figure 3B and
Figure 6A) is covered with thick setae. It is positioned anteriorly attaching to the posterior margin of the prothorax, with its antero-median part overlapped by the terminal portion of the prosternal process and its lateral margin connecting to the antero-ventral margin of the mesanepisternum. The bulged meso-posterior region of the mesoventrite is delineated by a medially anterad concaved margin. The small and anterad bent mesotrochantin (Tn2:
Figure 2C and
Figure 3B) antero-laterally attaches to the postero-lateral margin of the mesoventrite. The slender arm of the mesofurca (F2:
Figure 2D and
Figure 6A) extends dorso-laterad from the posterior margin of the mesoventrite, with its slightly expanded dorsal part positioned near the ventral part of the mesopleural ridge and bent antero-dorsad.
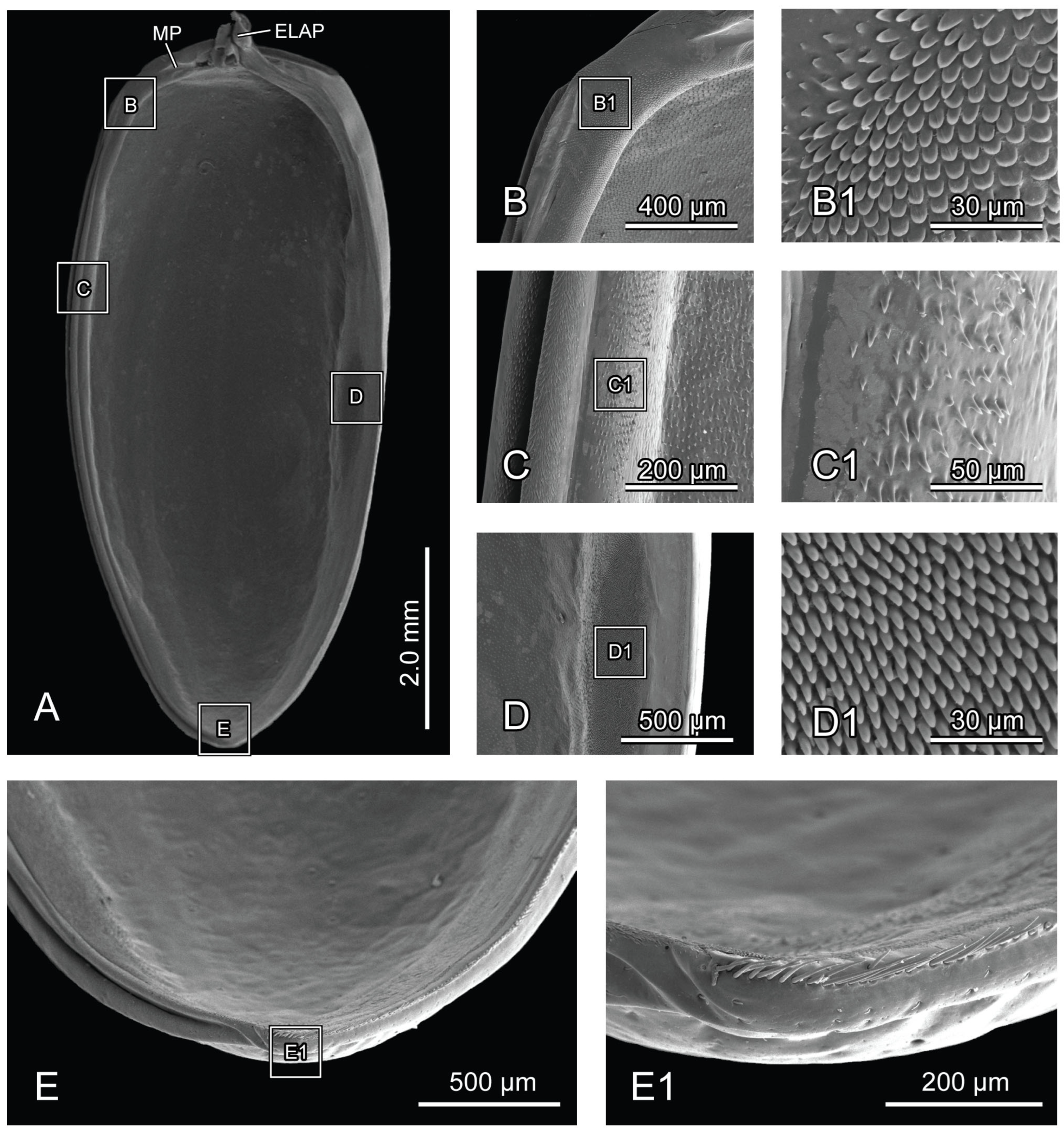
Figure 5.
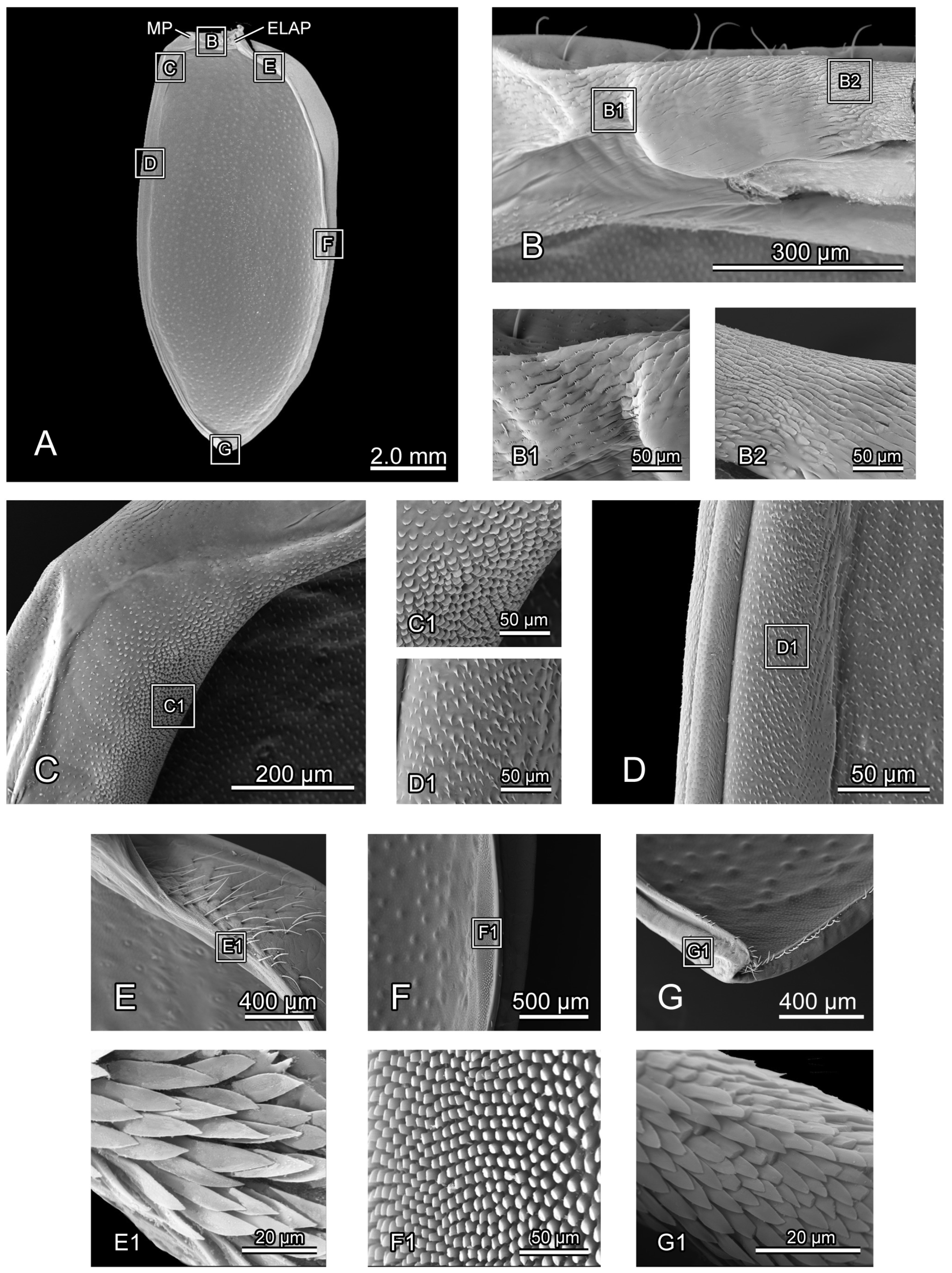
SEM photography of elytron of Chrysolina sulcisollis. (A) elytron, ventral view; (B) elytral base; (B1) michrotrichia on fold ridges in latero-anterior area of elytral base; (B2) wrinkles of elytral base; (C) anterior area of mesal suture; (C1) scales on antero-inner margin of mesal suture; (D) median area of mesal suture; (D1) setae on meso-inner region of mesal suture; (E) anterior area of epipleuron; (E1) setae on antero-inner region of the ridge; (F) median area of epipleuron. (F1) setae on meso-inner region of the ridge. (G) posterior area of elytron. (G1) setae on postero-lateral region of mesal suture. Abbreviations: ELAP—articulating process of elytron; MP—mesal process.
Figure 5.
SEM photography of elytron of Chrysolina sulcisollis. (A) elytron, ventral view; (B) elytral base; (B1) michrotrichia on fold ridges in latero-anterior area of elytral base; (B2) wrinkles of elytral base; (C) anterior area of mesal suture; (C1) scales on antero-inner margin of mesal suture; (D) median area of mesal suture; (D1) setae on meso-inner region of mesal suture; (E) anterior area of epipleuron; (E1) setae on antero-inner region of the ridge; (F) median area of epipleuron. (F1) setae on meso-inner region of the ridge. (G) posterior area of elytron. (G1) setae on postero-lateral region of mesal suture. Abbreviations: ELAP—articulating process of elytron; MP—mesal process.
The elytron (Ely:
Figure 1A) is long and nearly oval in shape (
Figure 5A), with a length 2.17 times its width. From the dorsal view, circualar punctures are irregularly distributed across the discal region (
Figure 1A), and the anterior margin of the elytra bears a slightly protruding humeral callus (HC:
Figure 1A). From the ventral view, a sharp angulate lateral articulating process of elytron (ELAP:
Figure 5A) extends antero-laterad, with its broad base positioned along the elytral base. The edge surrounding the discal region of the elytron is densely covered with setae or scales. The elytral base (
Figure 5B) has dense wrinkles (
Figure 5B2), with the fold ridges in the latero-anterior area bearing several michrotrichia (
Figure 5B1). A prominent mesal process (MP:
Figure 5A) is situated antero-proximally at the elytral base. The mesal suture is situated along the inner edge, forming the interlocking mechanism with the mesoscutellum and the elytron on the opposite side. The antero-proximal corner of the mesal suture protrudes antero-proximad and fits into the postero-lateral outline of the mesoscutellar shield. The mesal suture has obtuse scales along its antero-inner margin (
Figure 5C,C1), spinous setae in the meso-inner region (
Figure 5D,D1), and lanceolate setae along its postero-lateral margin (
Figure 5G1). The epipleuron, located along the lateral edge, has a slender raised inner ridge. Bent setae are present on the anterior area of the epipeluron and along the lateral margin of the inner ridge (
Figure 5E,G). The inner margin of the ridge has lanceolate setae in the anterior region (
Figure 5E1) and nearly cylinder-shaped setae in the median region (
Figure 5F,F1).
3.1.4. Mesothoracic Musculature
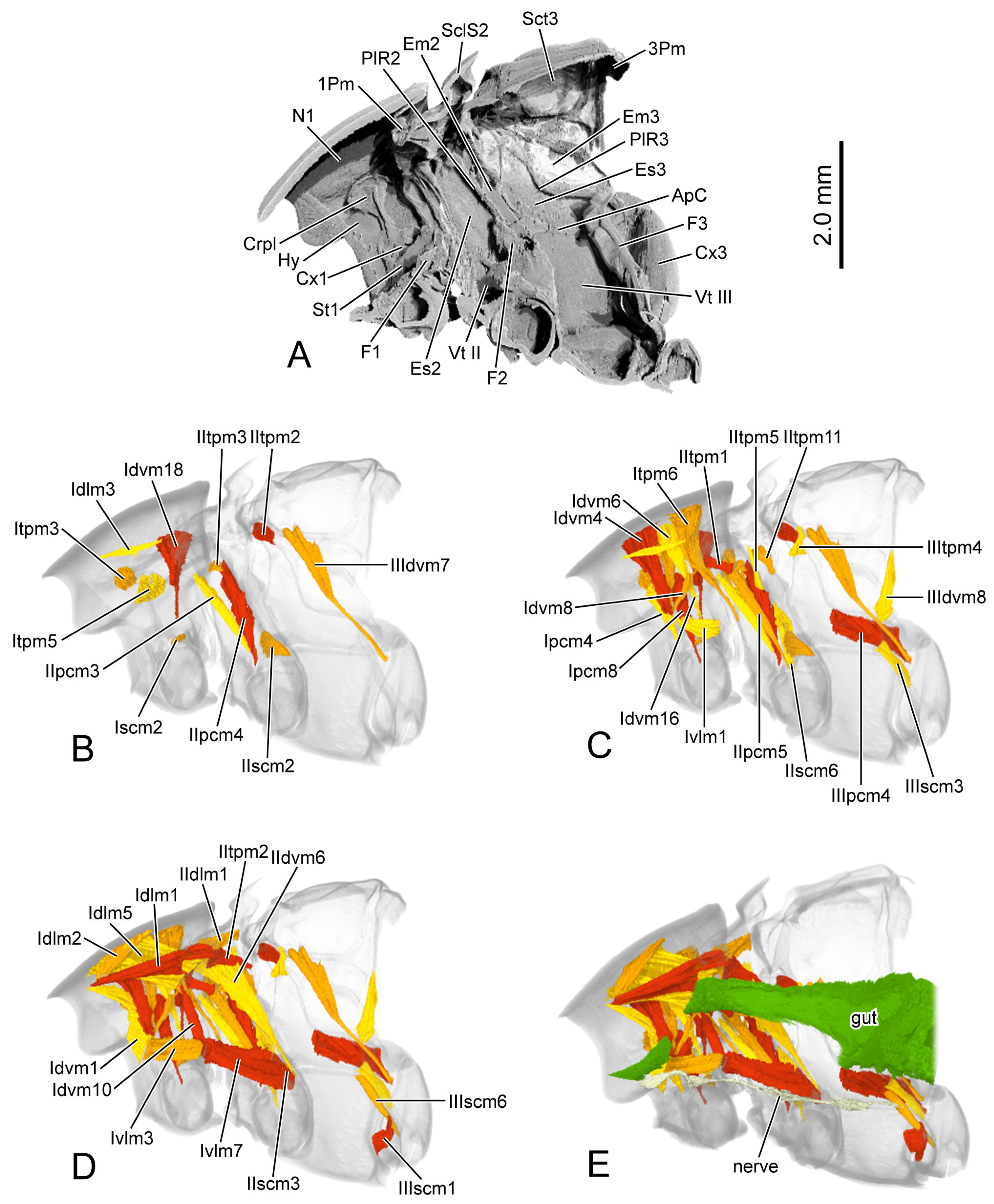
IIdlm1 M. prophragma-mesopragmalis. O: meso-lateral region of ventral face of prophragma. I: meso-lateral region of mesophragma. Irregular quadrilateral, original end broader than insertional end, straight, large, flat.
IIdvm6 M. mesocoxa-subalaris. O: postero-lateral margin of mesocoxal rim. I: postero-lateral margin of mesonotum. Long triangular, narrowing towards mesocoxal rim, slightly bent postero-proximad, long, thick.
IItpm1 M. prophragma-mesanepisternalis. O: antero-lateral corner of ventral face of prophragma. I: antero-dorsal margin of mesanepisternum. Parallelogram, short, straight.
IIspm2 M. mesofurca-pleuralis. O: lateral margin of dorsal part of mesofurcal arm. I: ventral part of mesopleural ridge. Irregular quadrilateral, original end narrower than insertional end, slightly bent postero-laterad, flat, short.
IIpcm3 M. mesanepisterno-coxalis anterior. O: antero-dorsal area of mesanepisternum. I: antero-lateral margin of mesocoxal rim. Long triangular, narrowing towards mesocoxal rim, straight, long.
IIpcm4 M. mesanepisterno-coxalis posterior. O: posterior area of mesanepisternum. I: antero-lateral margin of mesocoxal rim. Approximate long triangular, narrowing towards mesocoxal rim, straight, long.
IIpcm5 M. mesanepisterno-trochanteralis. O: dorsal area of mesanepisternum. I: mesotrochanter. Long triangular, narrowing towards mesotrochanter, straight, long.
IIscm2 M. mesofurca-coxalis posterior. O: postero-lateral part of mesofurcal arm. I: postero-lateral margin of mesocoxal rim. Triangular, narrowing towards mesocoxal rim, straight, short.
IIscm3 M. mesofurca-coxalis posterior. O: postero-median part of mesofurcal arm. I: proximal margin of mesocoxal rim. Triangular, narrowing towards mesocoxal rim, slightly bent proximad, short.
IIscm6 M. mesofurca-trochanteralis. O: ventral face of dorsal part of mesofurcal arm. I: mesotrochanter. Long triangular, narrowing towards mesotrochanter, slightly bent antero-proximad, long.
Figure 6.
3D reconstruction of thoracic skeleton, musculature and other organs of Chrysolina sulcisollis in sagittal section. (A–E) indicate a gradual movement from the lateral position of the thorax to the proximal position. Abbreviations: 1/2/3Pm—pro-/meso-/metaphragma; ApC—anapleural cleft; Crpl—crytopleuron; Cx1/3—pro-/metacoxal; Em2/3—mes-/metepimeron; Es2/3—mes-/metanepisternum; F1/2/3—pro-/meso-/metafurca; Hy—hypomeron; N1—pronotum; PlR2/3—meso-/metapleural suture; Sct3—metascutum; St1—prosternum; Vt II/III—meso-/metaventrite.
Figure 6.
3D reconstruction of thoracic skeleton, musculature and other organs of Chrysolina sulcisollis in sagittal section. (A–E) indicate a gradual movement from the lateral position of the thorax to the proximal position. Abbreviations: 1/2/3Pm—pro-/meso-/metaphragma; ApC—anapleural cleft; Crpl—crytopleuron; Cx1/3—pro-/metacoxal; Em2/3—mes-/metepimeron; Es2/3—mes-/metanepisternum; F1/2/3—pro-/meso-/metafurca; Hy—hypomeron; N1—pronotum; PlR2/3—meso-/metapleural suture; Sct3—metascutum; St1—prosternum; Vt II/III—meso-/metaventrite.
3.1.5. Metathoracic Skeleton
The metathorax is 4.31 times as long as its width. The metanotum is less sclerotized than the pro- and mesonotum. The intersegmental membrane between meso- and metatergite has sharp spines (
Figure 4A,B). The metascutum (Sct3:
Figure 1C,F,G,
Figure 3A,C and
Figure 6A) has a stripe-like median membranous area (MMA:
Figure 1F,G and
Figure 3A) that gradually narrows from anterior to posterior. The bulged alacrista (Alc:
Figure 1C,F and
Figure 3A) extends along the latero-anterior margin of the median depression to the antero-proximal margin of the metascutum. Dense oval-shaped setae are distributed on the alacrista (
Figure 4A,C), while its lateral margin is covered by overlapping irregular scales (
Figure 4A,D). The transverse scutal suture (TSS:
Figure 1C,F,G and
Figure 3A) extends latero-posterad from the distal end of the alacrista to the antero-lateral area of the metascutum. The antero-lateral scutal suture (ASS:
Figure 1C,F,G and
Figure 3A) extends along the antero-lateral margin of the metascutum, originating from the junction between the yoke plate and the metascutum, and delineates the long triangular anterior notal wing process (AWP3:
Figure 1C) from the rest of the metascutum. Posteriorly, the obtuse posterior notal wing process (PWP3:
Figure 1F,G) is positioned near the latero-posteral margin of the metascutum. The metascutellum (Scl3:
Figure 1C,F,G and
Figure 3A) is divided medially into a pair of semicircles by the median depression. It is separated from the anterior metascutum by scuto-scutellar suture (SSS3:
Figure 1C,F,G and
Figure 3A), which bends anterad. The narrow metaphragma (3Pm:
Figure 6A) is located along the posterior margin of the metanotum and expands antero-ventrad.
The curved metapleural suture (PlS3:
Figure 2A and
Figure 3C) divides the irregularly quadrangular metapleuron into an antero-ventral metanepisternum (Es3:
Figure 2A and
Figure 3C) and a postero-dorsal metepimeron (Em3:
Figure 2A and
Figure 3C), and evaginates inward to form the metapleural ridge (PlR3:
Figure 6A). The pleural wing process of the metathorax (PlWP3:
Figure 2A) extends antero-dorsad from the antero-dorsal corner of the metanepisternum to articulate with the wing base. At its apex, it bifurcates, with the anterior branch forming the fulcrum (Fu:
Figure 2A). The anterior margin of the metanepisternum has a bulged, highly sclerotized and narrow region. Ventrally the metapleuron is separated from the metaventrite by the anapleural cleft (ApC:
Figure 3C).
The transverse metaventrite (Vt III:
Figure 2C,D,
Figure 3B,C and
Figure 6A) is covered by scattered short setae. Both its anterior and posterior margins have a pair of depressions that, respectively, accommodate the meso- and metacoxae. The antero-median process and antero-lateral corner, respectively, connect to the posterior margin of the mesoventrite and the ventro-posterior corners of the mesepimeron. Additionally, its postero-median margin and postero-lateral corners connect to the first abdominal sternite. The arm of the metafurca (F3:
Figure 2B and
Figure 6A) extends dorso-laterad from the posterior margin of the metaventrite, gradually tapering distally, and terminates a dorsoventrally forked apex. The metathoracic discrimen (Dc:
Figure 2C,D) lies along the midline, extending from the center to the posterior margin of the metaventrite, where it supports the posterior metafurca.
3.1.6. Metathoracic Musculature
IIIdvm7 M. metanoto-trochanteralis. O: antero-lateral area of metanotum. I: metatrochanter. Long triangular, narrowing towards metatrochanter, slightly bent antero-proximad, long.
IIIdvm8 M. metafurca-phragmalis. O: apex of dorsal branch of metafurcal arm. I: latero-ventral area of anterior face of metaphragma. Long triangular, narrowing towards metascutum, slender, bent ventro-laterad.
IIItpm2 M. metapleural-prealaris. O: apex of metapleural ridge. I: lateral area of mesophragma. Long triangular, narrowing towards metapleural ridge, straight, short.
IIItpm4 M. mesonoto-pleuralis anterior. O: dorsal part of metapleual ridge. I: antero-lateral area of metanotum. Rectangular, slightly bent proximad, short, slender.
IIItpm5 M. metanoto-pleuralis medialis. O: dorso-median part of metapleural ridge. I: latero-median margin of metascutum. Irregular quadrilateral, original end broader than insertional end, bent anterad.
IIIppm1 M. metatransanapleuralis. O: median part of metapleural ridge. I: antero-lateral margin of metaventrite. Irregular quadrilateral, original end narrower than insertional end, straight, thick, flat.
IIIpcm4 M. metanepisterno-coxalis posterior. O: postero-ventral area of metanepisternum, beneath metapleural ridge. I: antero-lateral margin of metacoxal rim. Approximately triangular, narrowing towards metacoxal rim, straight, large.
IIIscm1 M. metafurca-coxalis anterior. O: proximo-ventral part of metafurcal arm. I: antero-proximal margin of metacoxal rim. Flat triangular, narrowing towards metacoxal rim, straight, large, flat.
IIIscm3 M. metafurca-coxalis medialis. O: postero-median part of metafurcal arm. I: meso-proximal margin of metacoxal rim. Irregular quadrilateral, original end narrower than insertional end, straight.
IIIscm6 M. metafurca-trochanteralis. O: latero-ventral part of metafurcal arm. I: metatrochanter. Long triangular, narrowing towards metatrochanter, straight, long, slender.