Cytogenetic and Molecular Characterization of Sphaerophoria rueppellii (Diptera, Syrphidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Chromosome Preparation
2.2. C-Banding
2.3. DNA Extraction, Probes, and Fluorescence In Situ Hybridization (FISH)
2.4. Mitogenomic Sequencing and Assembly Strategies
2.5. Mitogenome Annotation and Sequence Analysis
2.6. Comparative Phylogenetics
3. Results and Discussion
3.1. Cytogenetic Analysis
3.2. Mitogenome Analysis, Gene Organization, and Sequence Analysis
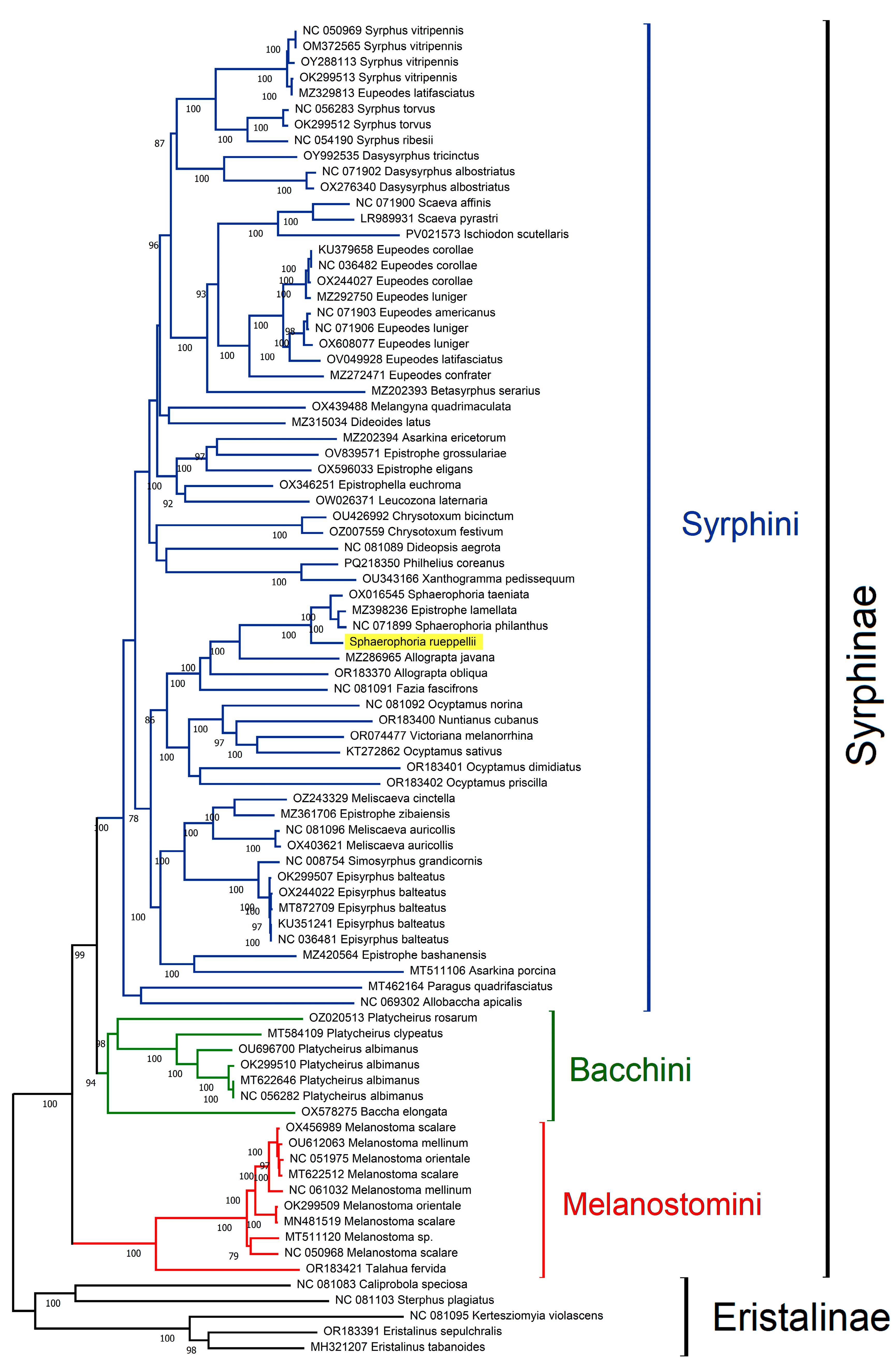
3.3. Phylogenetic Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dunn, L.; Lequerica, M.; Reid, C.R.; Latty, T. Dual ecosystem services of syrphid flies (Diptera: Syrphidae): Pollinators and biological control agents. Pest Manag. Sci. 2020, 76, 1973–1979. [Google Scholar] [CrossRef] [PubMed]
- Amorós-Jiménez, R.; Pineda, A.; Fereres, A.; Marcos-García, M.Á. Prey availability and abiotic requirements of immature stages of the aphid predator Sphaerophoria rueppellii. BioControl 2012, 63, 17–24. [Google Scholar] [CrossRef]
- Pérez-Bañón, C.; Petanidou, T.; Marcos-García, M.Á. Pollination in small islands by occasional visitors: The case of Daucus carota subsp. commutatus (Apiaceae) in the Columbretes archipelago. Spain. Plant Ecol. 2007, 192, 133–151. [Google Scholar] [CrossRef]
- Mengual, X.; Ståhls, G.; Rojo, S. Phylogenetic relationships and taxonomic ranking of pipizine flower flies (Diptera: Syrphidae) with implications for the evolution of aphidophagy. Cladistics 2015, 31, 491–508. [Google Scholar] [CrossRef]
- Wong, D.; Norman, H.; Creedy, T.J.; Jordaens, K.; Moran, K.M.; Young, A.; Mengual, X.; Skevington, J.H.; Vogler, A.P. The phylogeny and evolutionary ecology of hoverflies (Diptera: Syrphidae) inferred from mitochondrial genomes. Mol. Phylogenet. Evol. 2023, 184, 107759. [Google Scholar] [CrossRef]
- Rojo, S.; Gilbert, F.; Marcos-García, M.Á.; Nieto, J.M.; Mier, M.P. A World Review of Predatory Hoverflies (Diptera, Syrphidae: Syrphinae) and Their Prey; Ibero-American Center for Biodiversity (CIBIO): Murcia, Spain, 2003; 319p. [Google Scholar]
- Gómez-Polo, P.; Traugott, M.; Alomar, O.; Castañe, C.; Rojo, S.; Agustí, N. Identification of the most common predatory hoverflies of Mediterranean vegetable crops and their parasitism using multiplex PCR. J. Pest Sci. 2014, 87, 371–378. [Google Scholar] [CrossRef]
- Pekas, A.; de Craecker, I.; Boonen, S.; Wäckers, F.L.; Moerkens, R. One stone; two birds: Concurrent pest control and pollination services provided by aphidophagous hoverflies. BioControl 2020, 149, 104328. [Google Scholar] [CrossRef]
- Amorós-Jiménez, R.; Pineda, A.; Fereres, A.; Marcos-García, M.Á. Feeding preference of the aphidophagous hoverfly Sphaerophoria rueppellii affects the performance of its offspring. BioControl 2014, 54, 427–435. [Google Scholar] [CrossRef]
- Burgio, G.; Dindo, M.L.; Pape, T.; Whitmore, D.; Sommaggio, D. Diptera as predators in biological control: Applications and future perspectives. BioControl 2025, 70, 1–17. [Google Scholar] [CrossRef]
- Orengo-Green, J.J.; Kanturski, M.; Ricarte, A.; Marcos-García, M.Á. A great little ally: Revealing the morphology of the immature stages of the aphid pest predator Sphaerophoria rueppellii (Wiedemann, 1830) (Diptera: Syrphidae). Eur. Zool. J. 2022, 89, 625–640. [Google Scholar] [CrossRef]
- Alba-Tercedor, J.; Marcos-García, M.Á. Revealing the larval anatomy of the hoverfly Sphaerophoria rueppellii (Wiedemann, 1820) (Diptera, Syrphidae) using micro-computed tomography. Sci. Rep. 2024, 14, 28848. [Google Scholar] [CrossRef] [PubMed]
- Orengo-Green, J.J.; Casas, J.L.; Marcos-García, M.Á. Effect of abiotic climatic factors on the gonadal maturation of the biocontrol agent Sphaerophoria rueppellii (Wiedemann, 1830) (Diptera: Syrphidae). Insects 2022, 13, 573. [Google Scholar] [CrossRef] [PubMed]
- Blackmon, H.; Ross, L.; Bachtrog, D. Sex determination, sex chromosomes, and karyotype evolution in insects. J. Hered. 2017, 108, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Fuller, Z.L.; Koury, S.A.; Phadnis, N.; Schaeffer, S.W. How chromosomal rearrangements shape adaptation and speciation: Case studies in Drosophila pseudoobscura and its sibling species Drosophila persimilis. Mol. Ecol. 2019, 28, 1283–1301. [Google Scholar] [CrossRef]
- Zacharopoulou, A.; Augustinos, A.A.; Drosopoulou, E.; Tsoumani, K.T.; Gariou-Papalexiou, A.; Franz, G.; Mathiopoulos, K.D.; Bourtzis, K.; Mavragani-Tsipidou, P. A review of more than 30 years of cytogenetic studies of Tephritidae in support of sterile insect technique and global trade. Entomol. Exp. Appl. 2017, 164, 204–225. [Google Scholar] [CrossRef]
- Sollazzo, G.; Gouvi, G.; Nikolouli, K.; Aumann, R.A.; Djambazian, H.; Whitehead, M.A.; Berube, P.; Chen, S.H.; Tsiamis, G.; Darby, A.C.; et al. Genomic and cytogenetic analysis of the Ceratitis capitata temperature-sensitive lethal region. G3 Genes Genomes Genet. 2023, 13, jkad074. [Google Scholar] [CrossRef]
- Kandul, N.P.; Lukhtanov, V.A.; Pierce, N.E. Karyotypic diversity and speciation in Agrodiaetus butterflies. Evolution 2007, 61, 546–559. [Google Scholar] [CrossRef]
- Cameron, S.L. How to sequence and annotate insect mitochondrial genomes for systematic and comparative genomics research. Syst. Entomol. 2014, 39, 400–411. [Google Scholar] [CrossRef]
- Cameron, S.L. Insect mitochondrial genomics: Implications for evolution and phylogeny. Annu. Rev. Entomol. 2014, 59, 95–117. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, H.; Zhu, X.; Wang, K.; Xue, H.; Ye, Z.; Zheng, C.; Bu, W. Mitochondrial introgression and mitonuclear discordance obscured the closely related species boundaries in Cletus Stål from China (Heteroptera: Coreidae). Mol. Phylogenet. Evol. 2023, 184, 107802. [Google Scholar] [CrossRef]
- Duque-Gamboa, D.N.; Castillo-Cárdenas, M.F.; Hernández, L.M.; Guzmán, Y.C.; Manzano, M.R.; Toro-Perea, N. Mitochondrial DNA suggests cryptic speciation in Prodiplosis longifila Gagné (Diptera: Cecidomyiidae) associated with geographic distance and host specialization. Bull. Entomol. Res. 2018, 108, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Lea, V.; Uroš, S.; Jelena, J.; Sanja, B.; Biljana, S.; Mirko, Đ. Toward the development of the trojan female technique in pest insects: Male-specific influence of mitochondrial haplotype on reproductive output in the seed beetle Acanthoscelides obtectus. Evol. Appl. 2024, 17, e70065. [Google Scholar] [CrossRef] [PubMed]
- Breton, S.; Ghiselli, F.; Milani, L. Mitochondrial short-term plastic responses and long-term evolutionary dynamics in animal species. Genome Biol. Evol. 2021, 13, evab084. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.; Hu, H. Adaptive evidence of mitochondrial genes in Pteromalidae and Eulophidae (Hymenoptera: Chalcidoidea). PLoS ONE 2023, 18, e0294687. [Google Scholar] [CrossRef]
- Nanini, F.; Souza, P.G.C.; Soliman, E.P.; Zauza, E.A.V.; Domingues, M.M.; Santos, F.A.; Wilcken, C.F.; da Silva, R.S.; Corrêa, A.S. Genetic diversity, population structure and ecological niche modeling of Thyrinteina arnobia (Lepidoptera: Geometridae), a native Eucalyptus pest in Brazil. Sci. Rep. 2024, 14, 20963. [Google Scholar] [CrossRef]
- Levan, A.; Fredga, K.; Sandberg, A.A. Nomenclature for centromeric position on chromosomes. Hereditas 1964, 52, 201–220. [Google Scholar] [CrossRef]
- Sumner, A.T. A simple technique for demonstrating centromeric heterochromatin. Exp. Cell Res. 1972, 75, 304–306. [Google Scholar] [CrossRef]
- Mullin, P.G.; Harris, T.S.; Powers, T.O. Phylogenetic relationships of Nygolaimina and Dorylaimina (Nematoda: Dorylaimida) inferred from small subunit ribosomal DNA sequences. Nematology 2005, 7, 59–79. [Google Scholar] [CrossRef]
- Cabral-de-Mello, D.C.; Marec, F. Universal fluorescence in situ hybridization (FISH) protocol for mapping repetitive DNAs in insects and other arthropods. Mol. Genet. Genom. 2021, 296, 513–526. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Dierckxsens, N.; Mardulyn, P.; Smits, G. NOVOPlasty: De novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 2017, 45, e18. [Google Scholar] [CrossRef] [PubMed]
- Ojala, D.; Montoya, J.; Attardi, G. tRNA punctuation model of RNA processing in human mitochondria. Nature 1981, 290, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Sheffield, N.C.; Cameron, S.L.; Miller, K.B.; Whiting, M.F. What happens when the phylogenetic assumptions are violated?: The effect of base compositional heterogeneity and among-site rate heterogeneity in beetle mitochondrial phylogenomics. Syst. Entomol. 2010, 35, 429–448. [Google Scholar] [CrossRef]
- Mengual, X.; Mayer, C.; Burt, T.O.; Moran, K.M.; Dietz, L.; Nottebrock, G.; Pauli, T.; Young, A.D.; Brasseur, M.V.; Kukowka, S.; et al. Systematics and evolution of predatory flower flies (Diptera: Syrphidae) based on exon-capture sequencing. Syst. Entomol. 2023, 48, 250–277. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Talavera, G.; Castresana, J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 2007, 56, 564–577. [Google Scholar] [CrossRef]
- Chernomor, O.; von Haeseler, A.; Minh, B.Q. Terrace aware data structure for phylogenomic inference from supermatrices. Syst. Biol. 2016, 65, 997–1008. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef]
- Boyes, J.W. Somatic chromosomes of some syrphid flies. Can. J. Genet. Cytol. 1959, 1, 39–48. [Google Scholar] [CrossRef]
- Boyes, J.W.; Van Brink, J.M. Chromosomes of Syrphidae. I. Variations in karyotype. Chromosoma 1964, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Boyes, J.W.; Van Brink, J.M. Chromosomes of Syrphidae. II. Karyotypes of five species in the tribe Sericomyiini. Chromosoma 1966, 19, 399–404. [Google Scholar] [CrossRef]
- Boyes, J.W.; Van Brink, J.M. Chromosomes of Syrphidae. III. Karyotypes of some species in the tribes Milesiini and Myoleptini. Chromosoma 1967, 22, 417–455. [Google Scholar] [CrossRef]
- Boyes, J.W.; Van Brink, J.M. Chromosomes of Syrphidae. V. Microchromosomes. Chromosoma 1970, 31, 207–216. [Google Scholar] [CrossRef]
- Boyes, J.W.; Van Brink, J.M. Chromosomes of Syrphidae. VI. The tribe Pipizini. Genetica 1972, 43, 321–333. [Google Scholar] [CrossRef]
- Boyes, J.W.; Van Brink, J.M.; Mehta, R.D. Chromosomes of Syrphidae. IV. Karyotypes of fourteen species in the tribe Chrysotoxini. Chromosoma 1968, 24, 233–242. [Google Scholar] [CrossRef]
- Boyes, J.W.; Van Brink, J.M.; Boyes, B.C. Chromosomes of Syrphinae (Diptera: Syrphidae); Genetics Society of Canada: Ottawa, ON, Canada, 1971; pp. 1–158. [Google Scholar]
- Boyes, J.W.; Boyes, B.C.; Van Brink, J.M.; Vockeroth, J.R. Cytotaxonomy of South American Syrphinae (Diptera: Syrphidae). Genetica 1973, 44, 368–415. [Google Scholar] [CrossRef]
- Boyes, J.W.; Van Brink, J.M.; Boyes, B.C.; Vockeroth, J.R. Chromosomes of Eristalinae and Microdontinae (Diptera: Syrphidae); Genetics Society of Canada: Ottawa, ON, Canada, 1980; pp. 1–137. [Google Scholar]
- Rozek, M.; Marcos-Garcia, M.Á.; Lachowska, D. C-banding patterns in chromosomes of four species of syrphid flies (Diptera, Syrphidae). Folia Biol. Krakow 1995, 43, 107–109. [Google Scholar]
- Rozek, M.; Lachowska, D.; Marcos-Garcia, M.Á. The C-banded karyotypes of Syrphis corolla (Fabr.) and Sphaerophoria scripta (L.) (Diptera, Syrphidae). Folia Biol. Krakow 1996, 44, 11–13. [Google Scholar]
- Khajuria, M.; Bhatti, A.A.; Tripathi, N.K. First report on the chromosomal analysis of three species of tribe Eristalini (Diptera: Syrphidae) from outer Himalayas, India. Biologia 2019, 74, 469–475. [Google Scholar] [CrossRef]
- Morelli, M.W.; Blackmon, H.; Hjelmen, C.E. Diptera and Drosophila Karyotype Databases: A useful dataset to guide evolutionary and genomic studies. Front. Ecol. Evol. 2022, 10, 832378. [Google Scholar] [CrossRef] [PubMed]
- Bailey, E.; Field, L.; Rawlings, C.; King, R.; Mohareb, F.; Pak, K.H.; Hughes, D.; Williamson, M.; Ganko, E.; Buer, B.; et al. A near-chromosome level genome assembly of the European hoverfly, Sphaerophoria rueppellii (Diptera: Syrphidae), provides comparative insights into insecticide resistance-related gene family evolution. BMC Genom. 2022, 23, 198. [Google Scholar] [CrossRef]
- Marchi, A.; Pili, E. Ribosomal RNA genes in mosquitoes: Localization by fluorescence in situ hybridization (FISH). Heredity 1994, 72, 599–605. [Google Scholar] [CrossRef]
- Brianti, M.T.; Ananina, G.; Recco-Pimentel, S.M.; Klaczko, L.B. Comparative analysis of the chromosomal positions of rDNA genes in species of the tripunctata radiation of Drosophila. Cytogenet. Genome Res. 2009, 125, 149–157. [Google Scholar] [CrossRef]
- Madalena, C.R.; Díez, J.L.; Gorab, E. Chromatin structure of ribosomal RNA genes in dipterans and its relationship to the location of nucleolar organizers. PLoS ONE 2012, 7, e44006. [Google Scholar] [CrossRef]
- Falk, S.; Sudworth, J.; University of Oxford and Wytham Woods Genome Acquisition Lab; Darwin Tree of Life Barcoding Collective; Wellcome Sanger Institute Tree of Life Programme; Wellcome Sanger Institute Scientific Operations: DNA Pipelines Collective; Tree of Life Core Informatics Collective; Darwin Tree of Life Consortium. The genome sequence of a hoverfly, Sphaerophoria taeniata (Meigen, 1822). Wellcome Open Res. 2023, 8, 474. [Google Scholar] [CrossRef]
- Pu, D.Q.; Liu, H.L.; Gong, Y.Y.; Ji, P.C.; Li, Y.J.; Mou, F.S.; Wei, S.J. Mitochondrial genomes of the hoverflies Episyrphus balteatus and Eupeodes corollae (Diptera: Syrphidae), with a phylogenetic analysis of Muscomorpha. Sci. Rep. 2017, 7, 44300. [Google Scholar] [CrossRef]
- Hawkes, W.; Wotton, K.; Smith, M.; University of Oxford and Wytham Woods Genome Acquisition Lab; Natural History Museum Genome Acquisition Lab; Darwin Tree of Life Barcoding Collective; Wellcome Sanger Institute Tree of Life Programme; Wellcome Sanger Institute Scientific Operations: DNA Pipelines Collective; Tree of Life Core Informatics Collective; Darwin Tree of Life Consortium. The genome sequence of the two-banded wasp hoverfly, Chrysotoxum bicinctum (Linnaeus, 1758). Wellcome Open Res. 2021, 6, 321. [Google Scholar] [CrossRef]
- Boore, J.; Lavrov, D.; Brown, W. Gene translocation links insects and crustaceans. Nature 1998, 392, 667–668. [Google Scholar] [CrossRef]
- Sterling-Montealegre, R.A.; Prada, C.F. Variability and evolution of gene order rearrangement in mitochondrial genomes of arthropods (except Hexapoda). Gene 2023, 892, 147906. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yan, Y.; Li, J. Eighteen mitochondrial genomes of Syrphidae (Insecta: Diptera: Brachycera) with a phylogenetic analysis of Muscomorpha. PLoS ONE 2023, 18, e0278032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-X.; Hewitt, G.M. Insect mitochondrial control region: A review of its structure, evolution and usefulness in evolutionary studies. Biochem. Syst. Ecol. 1997, 25, 99–120. [Google Scholar] [CrossRef]
- Wolstenholme, D.R. Animal mitochondrial DNA: Structure and evolution. Int. Rev. Cytol. 1992, 141, 173–216. [Google Scholar] [CrossRef]
- Ruiz-Mena, A.; Mora, P.; Rico-Porras, J.M.; Kaufmann, B.; Seifert, B.; Palomeque, T.; Lorite, P. A comparative analysis of mitogenomes in species of the Tapinoma nigerrimum complex and other species of the genus Tapinoma (Formicidae, Dolichoderinae). Insects 2024, 15, 957. [Google Scholar] [CrossRef]
- Beckenbach, A.T.; Stewart, J.B. Insect mitochondrial genomics 3: The complete mitochondrial genome sequences of representatives from two neuropteroid orders: A dobsonfly (order Megaloptera) and a giant lacewing and an owlfly (order Neuroptera). Genome 2009, 52, 31–38. [Google Scholar] [CrossRef]
- Ruiz-Mena, A.; Mora, P.; Montiel, E.E.; Palomeque, T.; Lorite, P. Complete nucleotide sequence of the mitogenome of Tapinoma ibericum (Hymenoptera: Formicidae: Dolichoderinae), gene organization and phylogenetic implications for the Dolichoderinae subfamily. Genes 2022, 13, 1325. [Google Scholar] [CrossRef]
- Taanman, J.W. The mitochondrial genome: Structure, transcription, translation and replication. Biochim. Biophys. Acta 1999, 1410, 103–123. [Google Scholar] [CrossRef]
- Dowton, M.; Cameron, S.L.; Dowavic, J.I.; Austin, A.D.; Whiting, M.F. Phylogenetic approaches for the analysis of mitochondrial genome sequence data in the Hymenoptera—A lineage with both rapidly and slowly evolving mitochondrial genomes. Mol. Phylogenet. Evol. 2009, 52, 512–519. [Google Scholar] [CrossRef]
- Pita, S.; Panzera, F.; Vela, J.; Mora, P.; Palomeque, T.; Lorite, P. Complete mitochondrial genome of Triatoma infestans (Hemiptera, Reduviidae, Triatominae), main vector of Chagas disease. Infect. Genet. Evol. 2017, 54, 158–163. [Google Scholar] [CrossRef]
- Morgan, B.; Wang, T.Y.; Chen, Y.Z.; Moctezuma, V.; Burgos, O.; Le, M.H.; Huang, J.P. Long-read sequencing data reveals dynamic evolution of mitochondrial genome size and the phylogenetic utility of mitochondrial DNA in Hercules beetles (Dynastes; Scarabaeidae). Genome Biol. Evol. 2022, 14, evac147. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, L.; Li, K.; Hong, Y.; Storey, K.B.; Zhang, J.; Yu, D. Nine mitochondrial genomes of Phasmatodea with two novel mitochondrial gene rearrangements and phylogeny. Insects 2023, 14, 485. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Xu, X.; Jin, X.; Yin, H.; Luo, J.; Liu, G.; Zhao, Q.; Chen, Z.; Bu, W.; Gao, S. Using high-resolution annotation of insect mitochondrial DNA to decipher tandem repeats in the control region. RNA Biol. 2019, 16, 830–837. [Google Scholar] [CrossRef]
- Anand, R.; Singh, S.P.; Sahu, N.; Singh, Y.T.; Mazumdar-Leighton, S.; Bentur, J.S.; Nair, S. Polymorphisms in the hypervariable control region of the mitochondrial DNA differentiate BPH populations. Front. Insect Sci. 2022, 2, 987718. [Google Scholar] [CrossRef]
- Arcaya, E.; Pérez-Bañón, C.; Mengual, X.; Zubcoff-Vallejo, J.J.; Rojo, S. Life table and predation rates of the syrphid fly Allograpta exotica, a control agent of the cowpea aphid Aphis craccivora. BioControl 2017, 115, 74–84. [Google Scholar] [CrossRef]
- Mengual, X.; Ståhls, G.; Rojo, S. First phylogeny of predatory flower flies (Diptera, Syrphidae, Syrphinae) using mitochondrial COI and nuclear 28S rRNA genes: Conflict and congruence with the current tribal classification. Cladistics 2008, 24, 543–562. [Google Scholar] [CrossRef]
- Mengual, X.; Ståhls, G.; Skevington, J.H. Life on an island: The phylogenetic placement of Loveridgeana and Afrotropical Sphaerophoria (Diptera: Syrphidae) inferred from molecular characters. Syst. Biodivers. 2020, 19, 22–53. [Google Scholar] [CrossRef]
- Zhao, R.; Li, H.; Wu, G.; Wang, Y.F. Codon usage bias analysis in the mitochondrial genomes of five Rhingia Scopoli (Diptera, Syrphidae, Eristalinae) species. Gene 2024, 917, 148466. [Google Scholar] [CrossRef]
- Guo, S.; Chen, J.; Song, N. Phylogenomic analysis of Syrphoidea (Diptera: Syrphidae, Pipunculidae) based on the expanded mitogenomic data. Arch. Insect Biochem. Physiol. 2023, 114, 1–13. [Google Scholar] [CrossRef]

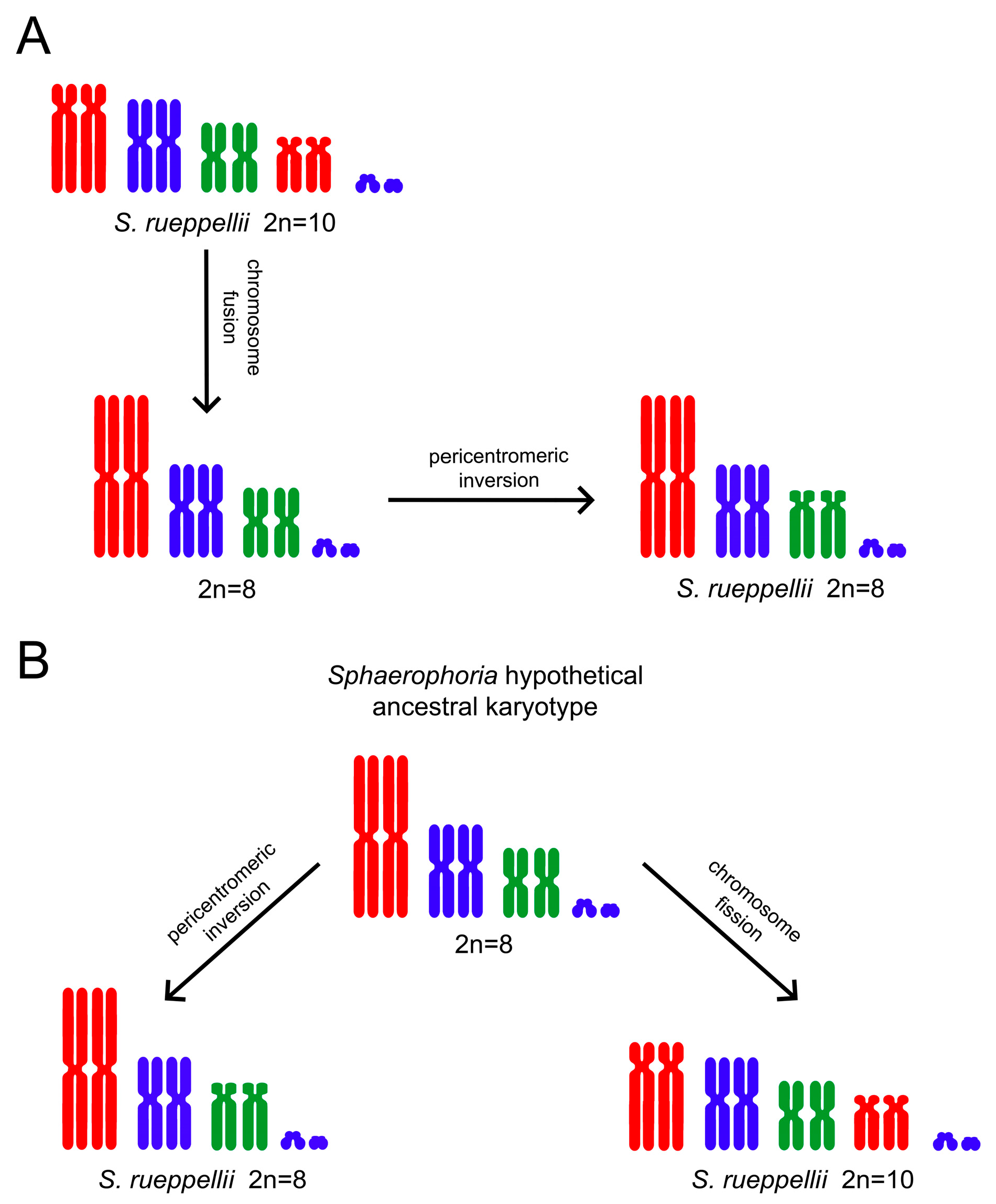
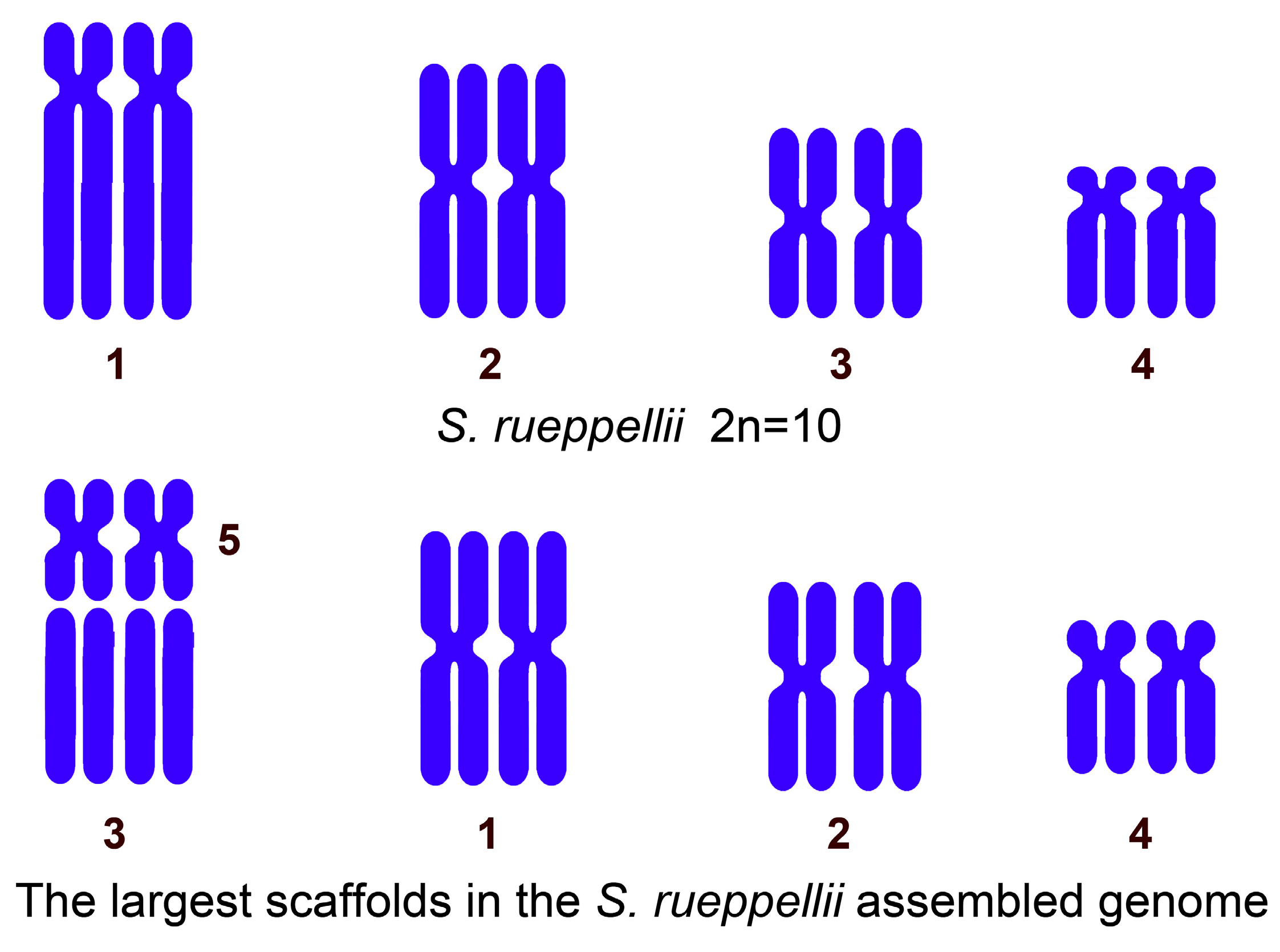
| Chromosome 1 | Chromosome 2 | Chromosome 3 | Chromosome 4 | |
|---|---|---|---|---|
| The relative length of each autosome in the 2n = 10 karyotype. | 27.9–28.8% | 25.5–27.8% | 22.0–24.5% | 15.9–18.0% |
| Scaffolds 3 + 5 | Scaffold 1 | Scaffold 2 | Scaffold 4 | |
| The percentage of each scaffold relative to the total assembled megabases. | 31.9% (19.14 + 12.73) | 27.6% | 22.3% | 16.5% |
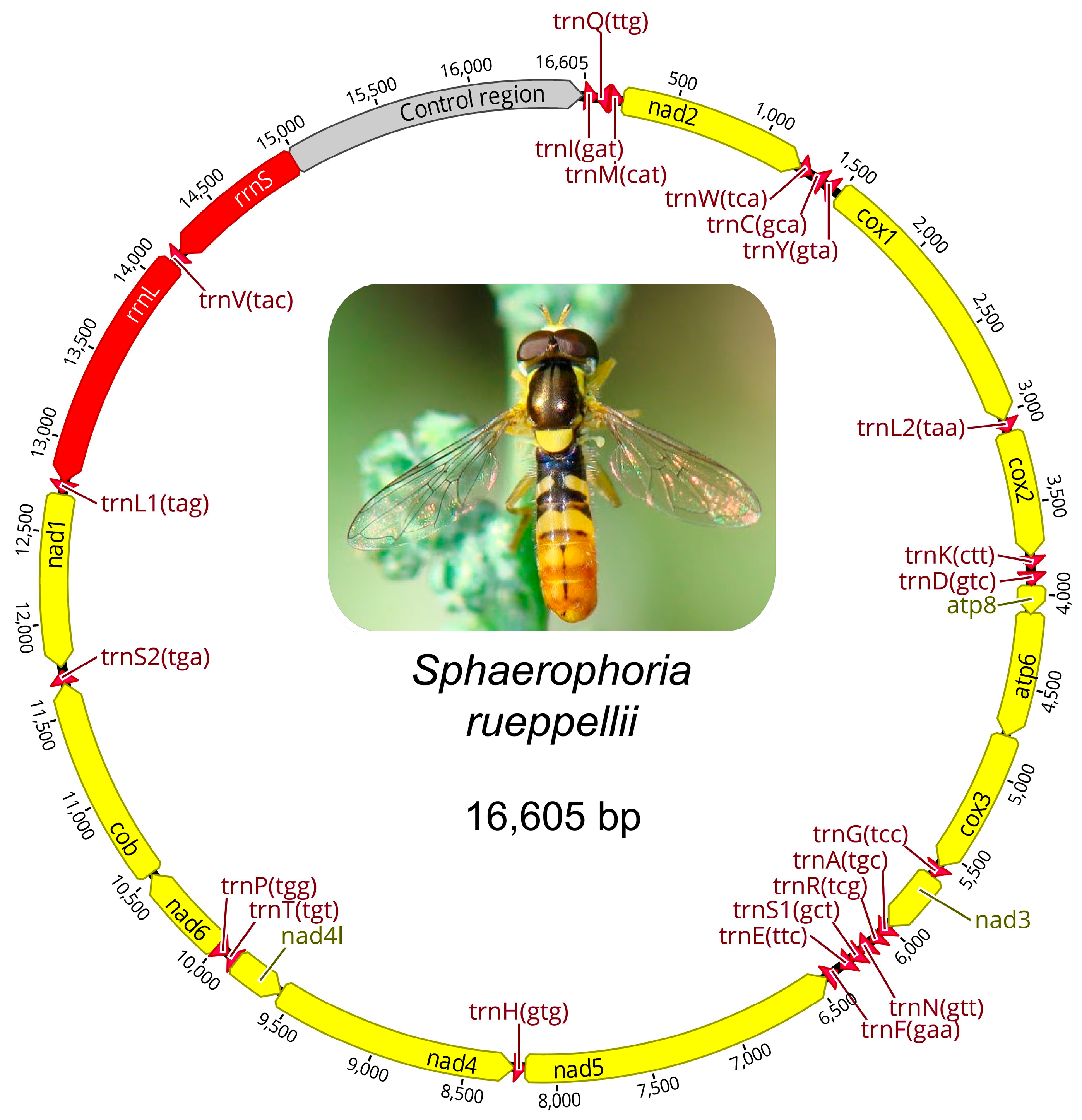
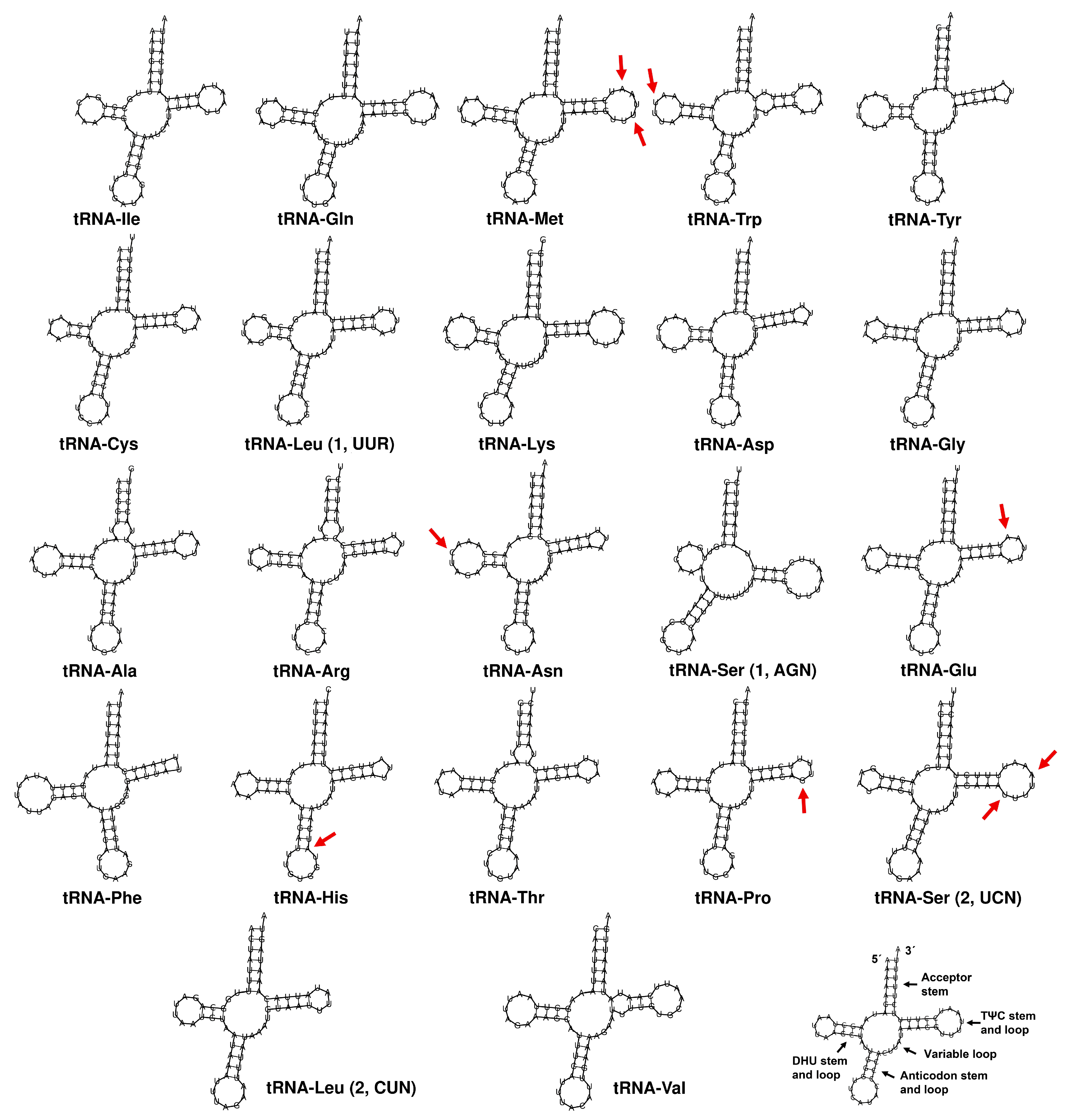
| Gene | Strand | Nucleotide Number | Length | IGN | Start Codon | Stop Codon | |
|---|---|---|---|---|---|---|---|
| (I) tRNA-Ile | H | 1 | 66 | 66 | −3 | ||
| (Q) tRNA-Gln | L | 64 | 132 | 69 | 3 | ||
| (M) tRNA-Met | H | 136 | 204 | 69 | 0 | ||
| nad2 | H | 205 | 1237 | 1033 | 0 | ATT | T– |
| (W) tRNA-Trp | H | 1238 | 1306 | 69 | 16 | ||
| (C) tRNA-Cys | L | 1323 | 1388 | 66 | 6 | ||
| (Y) tRNA-Tyr | L | 1395 | 1460 | 66 | 41 | ||
| cox1 | H | 1502 | 3035 | 1534 | 0 | TTG | T– |
| (L1) tRNA-Leu (UAA) | H | 3036 | 3101 | 66 | 4 | ||
| cox2 | H | 3106 | 3789 | 684 | 0 | ATG | TAA |
| (K) tRNA-Lys | H | 3790 | 3860 | 71 | 14 | ||
| (D) tRNA-Asp | H | 3875 | 3944 | 70 | 0 | ||
| atp8 | H | 3945 | 4106 | 162 | −7 | ATT | TAA |
| atp6 | H | 4100 | 4777 | 678 | 7 | ATG | TAA |
| cox3 | H | 4785 | 5573 | 789 | 3 | ATG | TAA |
| (G) tRNA-Gly | H | 5577 | 5642 | 66 | 0 | ||
| nad3 | H | 5643 | 5996 | 354 | 2 | ATT | TAA |
| (A) tRNA-Ala | H | 5999 | 6067 | 69 | −1 | ||
| (R) tRNA-Arg | H | 6067 | 6130 | 64 | 14 | ||
| (N) tRNA-Asn | H | 6145 | 6211 | 67 | 0 | ||
| (S1) tRNA-Ser (UCU) | H | 6212 | 6278 | 67 | 1 | ||
| (E) tRNA-Glu | H | 6280 | 6346 | 67 | 19 | ||
| (F) tRNA-Phe | L | 6366 | 6433 | 68 | 0 | ||
| nad5 | L | 6434 | 8169 | 1736 | 0 | ATG | TA– |
| (H) tRNA-His | L | 8170 | 8234 | 65 | 0 | ||
| nad4 | L | 8235 | 9575 | 1341 | −7 | ATG | TAA |
| nad4l | L | 9569 | 9865 | 297 | 2 | ATG | TAA |
| (T) tRNA-Thr | H | 9868 | 9933 | 66 | 0 | ||
| (P) tRNA-Pro | L | 9934 | 9999 | 66 | 2 | ||
| nad6 | H | 10,002 | 10,526 | 525 | 11 | ATT | TAA |
| cob | H | 10,538 | 11,674 | 1137 | 3 | ATG | TAA |
| (S2) tRNA-Ser (UGA) | H | 11,678 | 11,746 | 69 | 16 | ||
| nad1 | L | 11,763 | 12,710 | 948 | 1 | TTG | TAA |
| (L2) tRNA-Leu (UAG) | L | 12,712 | 12,776 | 65 | 0 | ||
| lrRNA | L | 12,777 | 14,111 | 1335 | 0 | ||
| (V) tRNA-Val | L | 14,112 | 14,182 | 71 | 0 | ||
| srRNA | L | 14,183 | 14,972 | 790 | 0 | ||
| Control Region | 14,973 | 16,605 | 1633 | ||||
| Codon | n | RSCU | % | Codon | n | % | RSCU | Codon | n | % | RSCU | Codon | n | % | RSCU |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UUU(F) | 324 | 1.91 | 8.66 | UCU(S) | 124 | 3.31 | 3.06 | UAU(Y) | 184 | 4.92 | 1.96 | UGU(C) | 38 | 1.02 | 2 |
| UUC(F) | 16 | 0.09 | 0.43 | UCC(S) | 5 | 0.13 | 0.12 | UAC(Y) | 4 | 0.11 | 0.04 | UGC(C) | 0 | - | 0 |
| UUA(L) | 536 | 5.54 | 14.33 | UCA(S) | 96 | 2.57 | 2.37 | UAA(*) | 13 | 0.35 | 2 | UGA(W) | 96 | 2.57 | 1.96 |
| UUG(L) | 10 | 0.1 | 0.27 | UCG(S) | 0 | - | 0 | UAG(*) | 0 | - | 0 | UGG(W) | 2 | 0.05 | 0.04 |
| CUU(L) | 18 | 0.19 | 0.48 | CCU(P) | 81 | 2.17 | 2.53 | CAU(H) | 69 | 1.84 | 1.92 | CGU(R) | 20 | 0.53 | 1.43 |
| CUC(L) | 0 | 0 | - | CCC(P) | 6 | 0.16 | 0.19 | CAC(H) | 3 | 0.08 | 0.08 | CGC(R) | 0 | - | 0 |
| CUA(L) | 16 | 0.17 | 0.43 | CCA(P) | 40 | 1.07 | 1.25 | CAA(Q) | 70 | 1.87 | 1.97 | CGA(R) | 35 | 0.94 | 2.5 |
| CUG(L) | 0 | 0 | - | CCG(P) | 1 | 0.03 | 0.03 | CAG(Q) | 1 | 0.03 | 0.03 | CGG(R) | 1 | 0.03 | 0.07 |
| AUU(I) | 374 | 1.98 | 10.00 | ACU(T) | 85 | 2.27 | 1.85 | AAU(N) | 210 | 5.61 | 1.92 | AGU(S) | 42 | 1.12 | 1.04 |
| AUC(I) | 3 | 0.02 | 0.08 | ACC(T) | 2 | 0.05 | 0.04 | AAC(N) | 9 | 0.24 | 0.08 | AGC(S) | 2 | 0.05 | 0.05 |
| AUA(M) | 285 | 1.9 | 7.62 | ACA(T) | 97 | 2.59 | 2.11 | AAA(K) | 83 | 2.22 | 1.8 | AGA(S) | 55 | 1.47 | 1.36 |
| AUG(M) | 15 | 0.1 | 0.40 | ACG(T) | 0 | - | 0 | AAG(K) | 9 | 0.24 | 0.2 | AGG(S) | 0 | - | 0 |
| GUU(V) | 71 | 1.75 | 1.90 | GCU(A) | 88 | 2.35 | 2.32 | GAU(D) | 63 | 1.68 | 1.88 | GGU(G) | 41 | 1.10 | 0.79 |
| GUC(V) | 0 | 0 | - | GCC(A) | 3 | 0.08 | 0.08 | GAC(D) | 4 | 0.11 | 0.12 | GGC(G) | 0 | - | 0 |
| GUA(V) | 89 | 2.2 | 2.38 | GCA(A) | 60 | 1.60 | 1.58 | GAA(E) | 70 | 1.87 | 1.92 | GGA(G) | 159 | 4.25 | 3.07 |
| GUG(V) | 2 | 0.05 | 0.05 | GCG(A) | 1 | 0.03 | 0.03 | GAG(E) | 3 | 0.08 | 0.08 | GGG(G) | 7 | 0.19 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorite, P.; Rico-Porras, J.M.; Palomeque, T.; Marcos-García, M.Á.; Cabral-de-Mello, D.C.; Mora, P. Cytogenetic and Molecular Characterization of Sphaerophoria rueppellii (Diptera, Syrphidae). Insects 2025, 16, 604. https://doi.org/10.3390/insects16060604
Lorite P, Rico-Porras JM, Palomeque T, Marcos-García MÁ, Cabral-de-Mello DC, Mora P. Cytogenetic and Molecular Characterization of Sphaerophoria rueppellii (Diptera, Syrphidae). Insects. 2025; 16(6):604. https://doi.org/10.3390/insects16060604
Chicago/Turabian StyleLorite, Pedro, José M. Rico-Porras, Teresa Palomeque, Mª Ángeles Marcos-García, Diogo C. Cabral-de-Mello, and Pablo Mora. 2025. "Cytogenetic and Molecular Characterization of Sphaerophoria rueppellii (Diptera, Syrphidae)" Insects 16, no. 6: 604. https://doi.org/10.3390/insects16060604
APA StyleLorite, P., Rico-Porras, J. M., Palomeque, T., Marcos-García, M. Á., Cabral-de-Mello, D. C., & Mora, P. (2025). Cytogenetic and Molecular Characterization of Sphaerophoria rueppellii (Diptera, Syrphidae). Insects, 16(6), 604. https://doi.org/10.3390/insects16060604









