Diverse Sublethal Effects of a Common Fungicide Impact the Behavior and Physiology of Honey Bees
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Acute Toxicity Tests
2.2.1. Acute Contact and Oral Toxicity Testing
2.2.2. Toxicity Evaluation
2.2.3. Risk Assessment
2.3. The Proboscis Extension Reflex Experiment
2.4. Enzyme Activity Testing
2.5. Analysis of Gut Microbiota
2.5.1. Sample Preparation
2.5.2. DNA Extraction and PCR Amplification
2.5.3. Illumina MiSeq Sequencing
2.5.4. Amplicon Sequence Processing and Analysis
2.6. Effects of Different Concentrations of Compound Fungicide on Intestinal Metabolites in the Hindgut of Bees
2.7. Data Analyses
3. Results
3.1. Toxicity Determination and Risk Assessment of Chunmanchun®
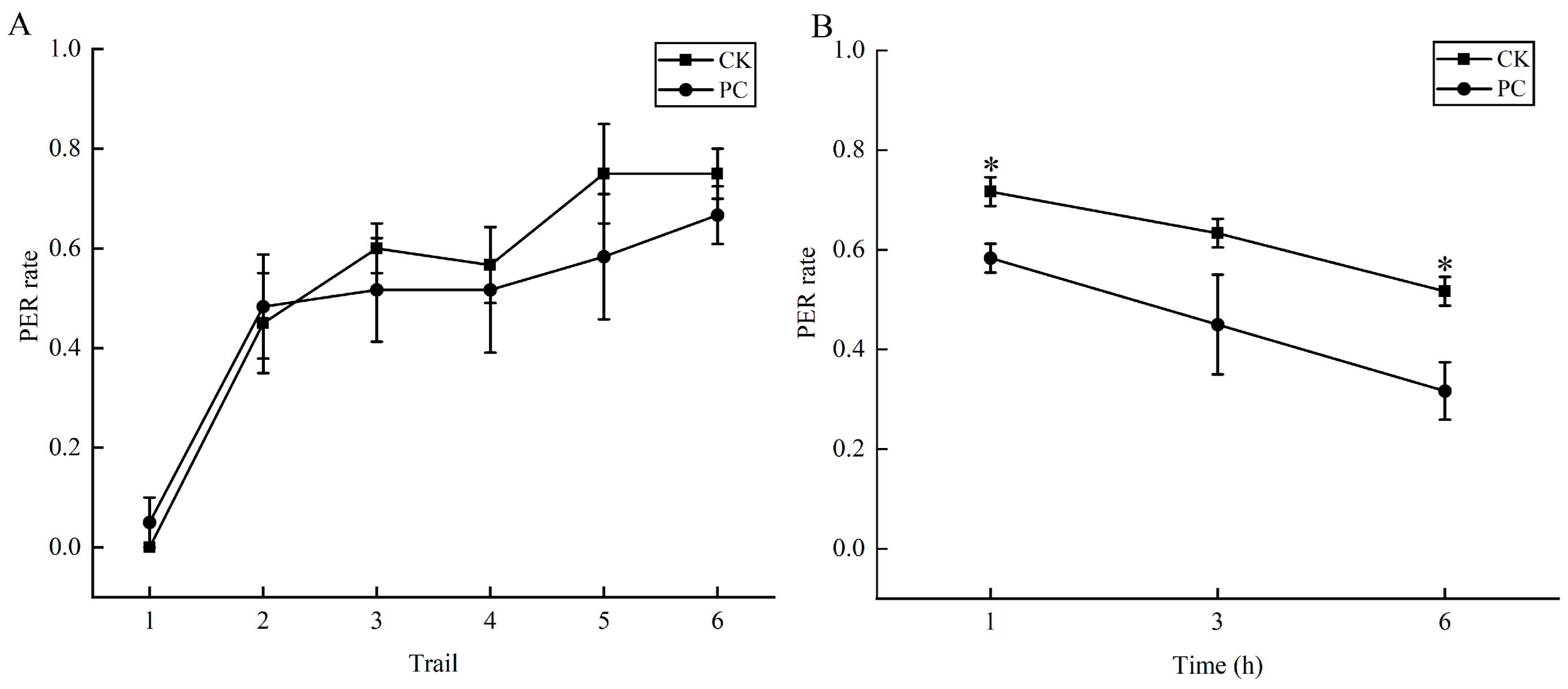
3.2. Effects of Chunmanchun® on Learning and Memory of Bees
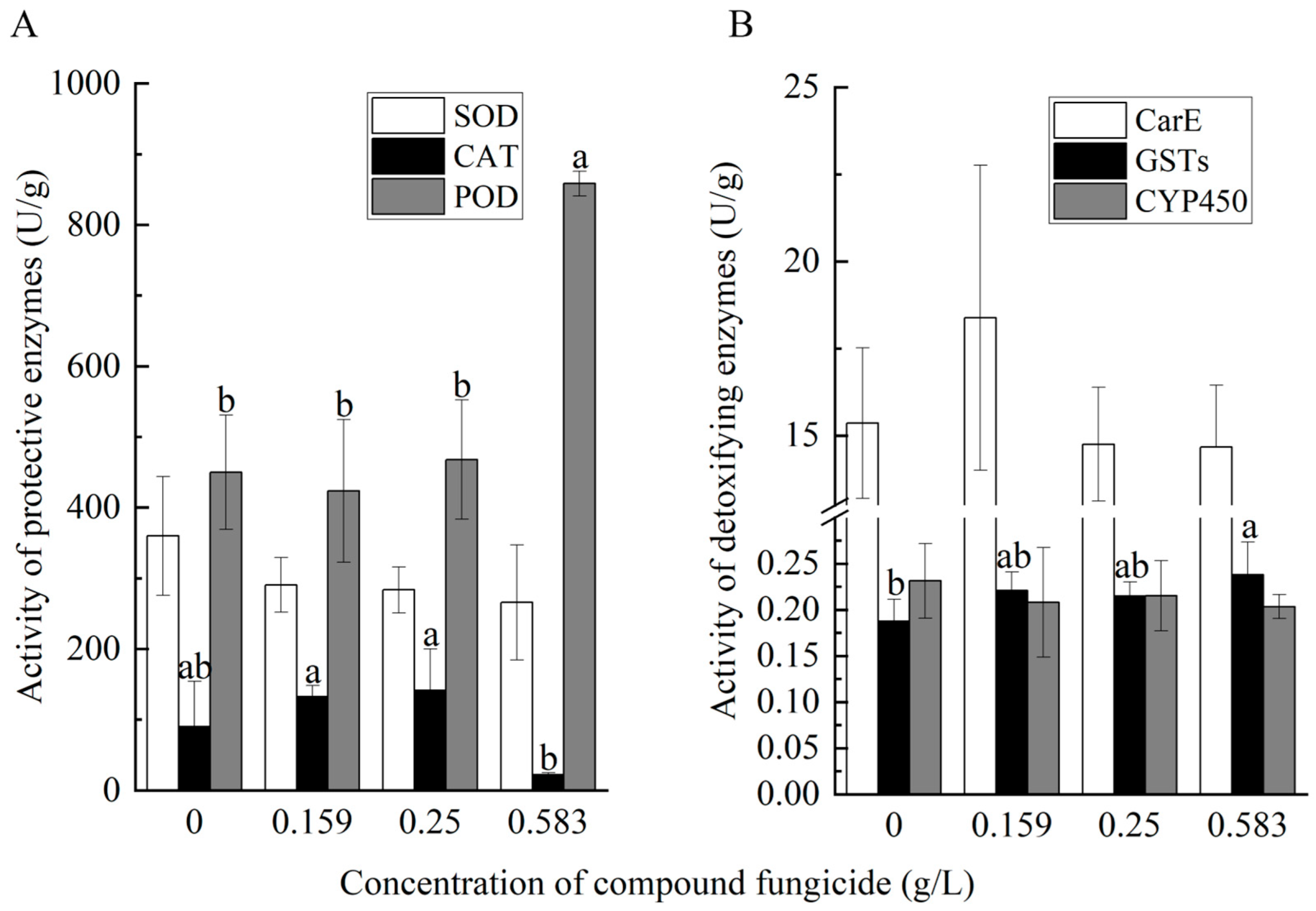
3.3. Effects of Chunmanchun® on Protective and Detoxifying Enzymes
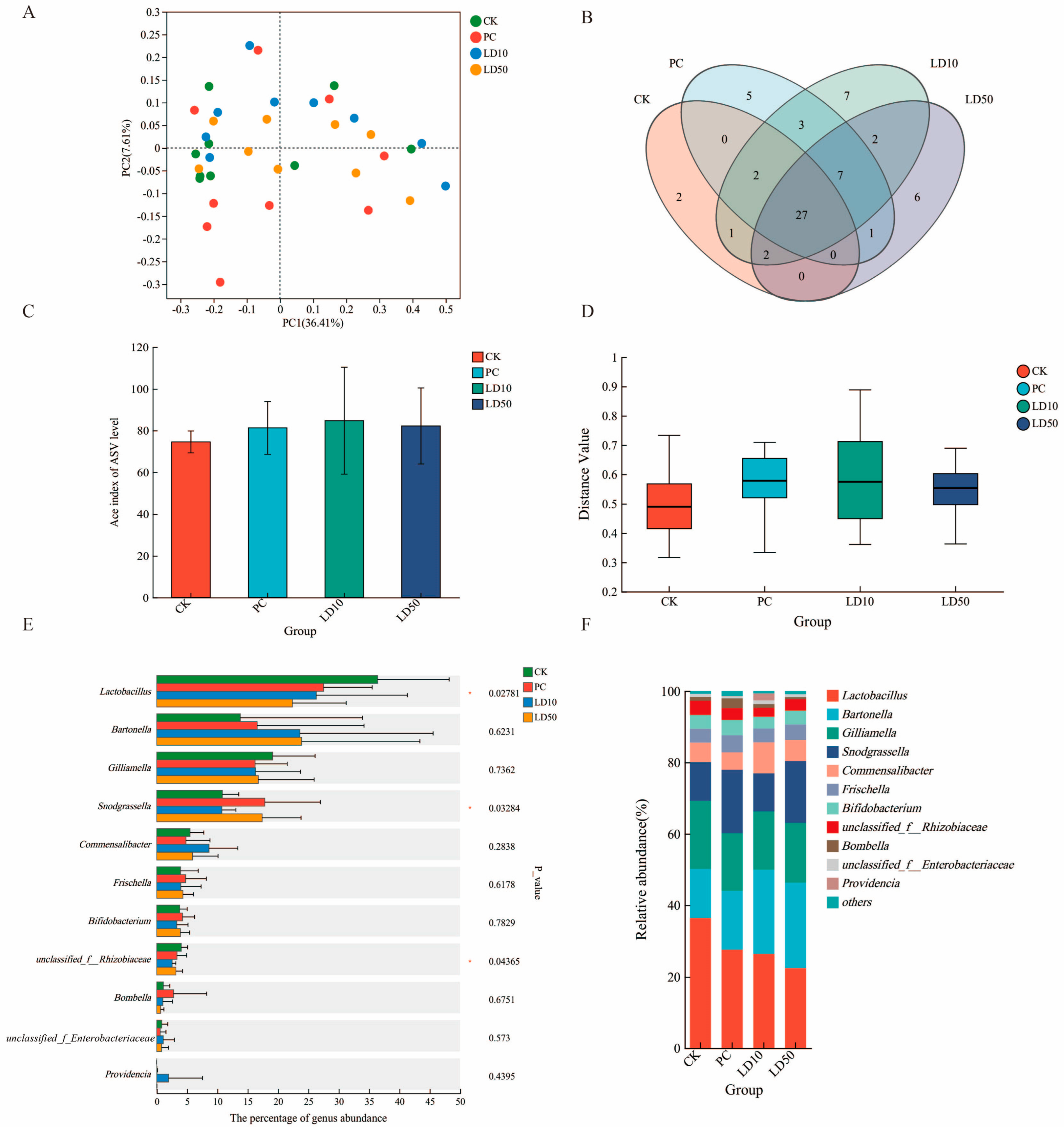
3.4. Gut Microbiome Effects of Compound Fungicide Exposure
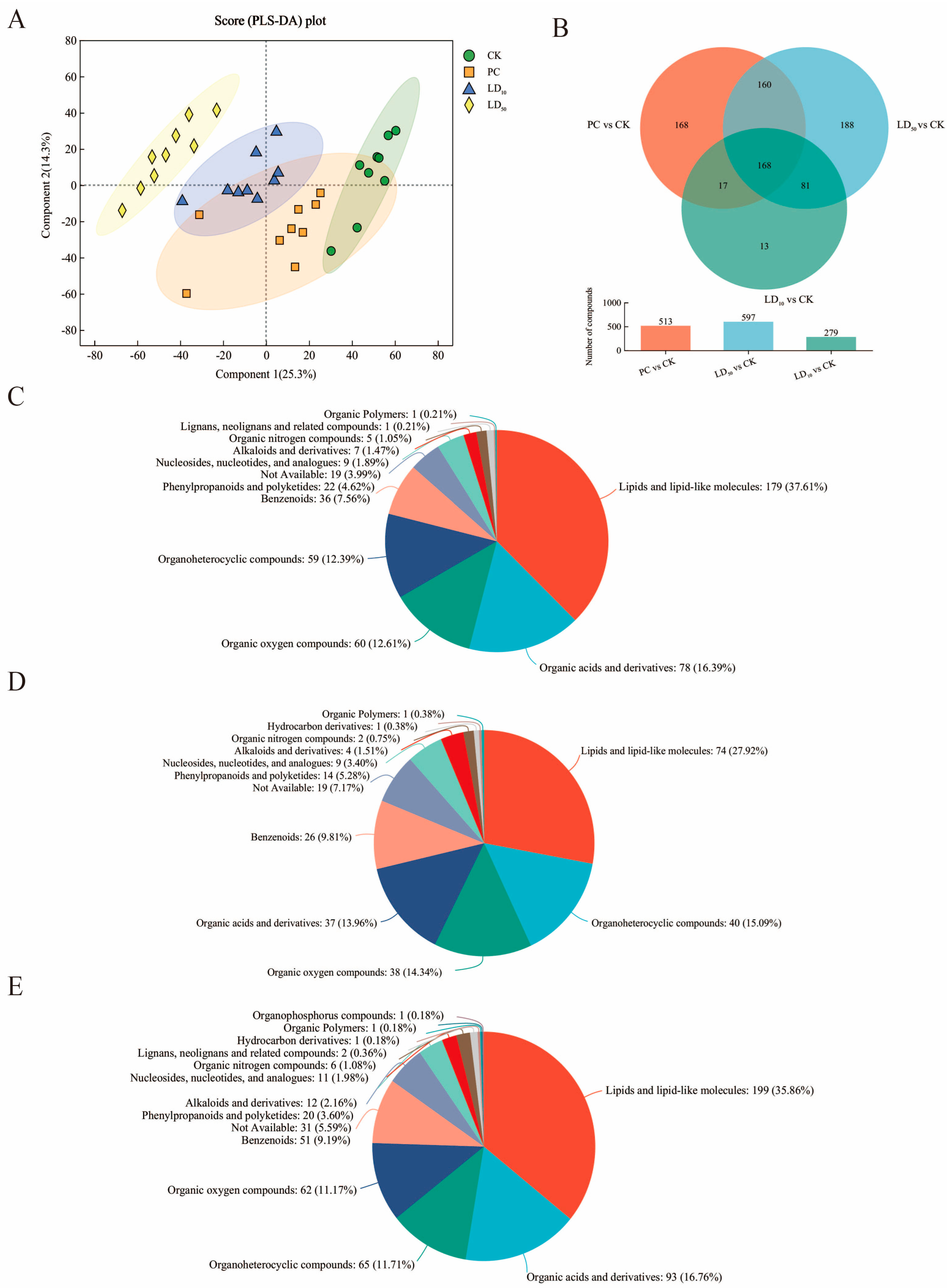
3.5. Hindgut Metabolome Effects of Compound Fungicide Exposure
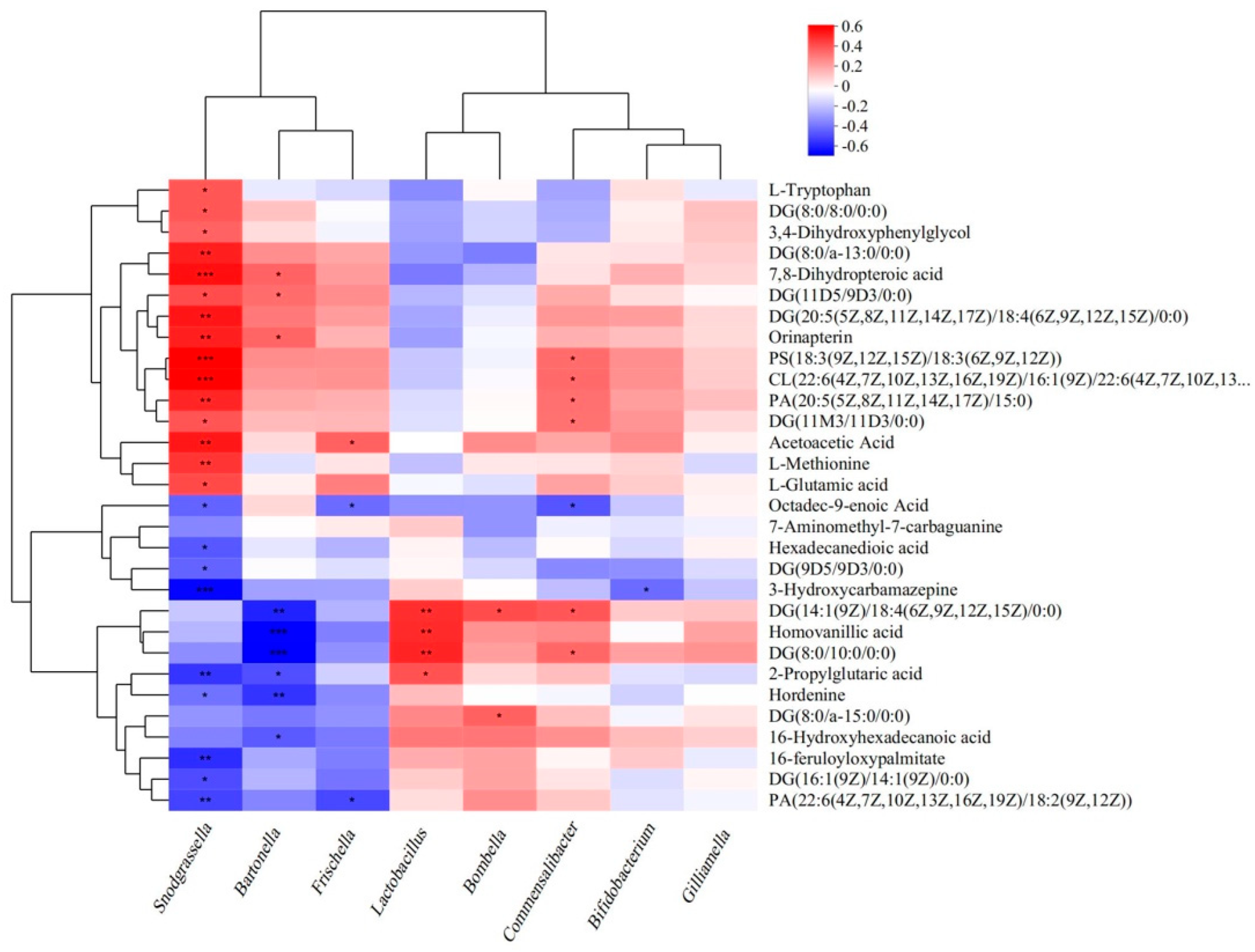
3.6. Relation Between Gut Microbiome and Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kevan, P.G.; Clark, E.A.; Thomas, V.G. Insect pollinators and sustainable agriculture. Am. J. Altern. Agric. 1990, 5, 13–22. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elshafiey, E.H.; Shetaia, A.A.; El-Wahed, A.A.A.; Algethami, A.F.; Musharraf, S.G.; AlAjmi, M.F.; Zhao, C.; Masry, S.H.D.; Abdel-Daim, M.M.; et al. Overview of bee pollination and its economic value for crop production. Insects 2021, 12, 688. [Google Scholar] [CrossRef]
- Nabhan, G.P.; Buchmann, S.L. Nature’s Services: Societal Dependence on Natural Ecosystems; Island Press: Washington, DC, USA, 1997; pp. 133–150. [Google Scholar]
- Michener, C.D. The Bees of the World; The Johns Hopkins University Press: Baltimore, MD, USA, 2007. [Google Scholar]
- Klein, A.M.; Vaissiere, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef]
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J. Safeguarding pollinators and their values to human well-being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef]
- Goulson, D.; Nicholls, E.; Botias, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef] [PubMed]
- Zattara, E.E.; Aizen, M.A. Worldwide occurrence records suggest a global decline in bee species richness. One Earth 2021, 4, 114–123. [Google Scholar] [CrossRef]
- Krupke, C.H.; Hunt, G.J.; Eitzer, B.D.; Andino, G.; Given, K. Multiple routes of pesticide exposure for honey bees living near agricultural fields. PLoS ONE 2012, 7, e29268. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Blacquiere, T.; Field, L.M.; Hails, R.S.; Potts, S.G.; Raine, N.E.; Vanbergen, A.J.; McLean, A.R. A restatement of recent advances in the natural science evidence base concerning neonicotinoid insecticides and insect pollinators. Proc. R. Soc. B Biol. Sci. 2015, 282, 20151821. [Google Scholar] [CrossRef]
- Sanchez-Bayo, F.; Goka, K. Impacts of Pesticides on Honey Bees. In Beekeeping and Bee Conservation: Advances in Research; Chambó, E., Ed.; IntechOpen: London, UK, 2016; pp. 77–97. [Google Scholar]
- Moffat, C.; Pacheco, J.G.; Sharp, S.; Samson, A.J.; Bollan, K.A.; Huang, J.; Buckland, S.T.; Connolly, C.N. Chronic exposure to neonicotinoids increases neuronal vulnerability to mitochondrial dysfunction in the bumblebee (Bombus terrestris). FASEB J. 2015, 29, 2112–2119. [Google Scholar] [CrossRef]
- Pineaux, M.; Grateau, S.; Lirand, T.; Aupinel, P.; Richard, F.J. Honeybee queen exposure to a widely used fungicide disrupts reproduction and colony dynamic. Environ. Pollut. 2023, 322, 121131. [Google Scholar] [CrossRef]
- Tosi, S.; Nieh, J.C.; Sgolastra, F.; Cabbri, R.; Medrzycki, P. Neonicotinoid pesticides and nutritional stress synergistically reduce survival in honey bees. Proc. R. Soc. B Biol. Sci. 2017, 284, 20171711. [Google Scholar] [CrossRef] [PubMed]
- Barascou, L.; Requier, F.; Sene, D.; Crauser, D.; Le Conte, Y.; Alaux, C. Delayed effects of a single dose of a neurotoxic pesticide (sulfoxaflor) on honeybee foraging activity. Sci. Total Environ. 2022, 805, 150351. [Google Scholar] [CrossRef] [PubMed]
- Belzunces, L.P.; Tchamitchian, S.; Brunet, J.L. Neural effects of insecticides in the honey bee. Apidologie 2012, 43, 348–370. [Google Scholar] [CrossRef]
- Cabirol, A.; Haase, A. The Neurophysiological bases of the impact of neonicotinoid pesticides on the behaviour of honeybees. Insects 2019, 10, 344. [Google Scholar] [CrossRef]
- Mengoni Gonalons, C.; Farina, W.M. Impaired associative learning after chronic exposure to pesticides in young adult honey bees. J. Exp. Biol. 2018, 221, jeb176644. [Google Scholar] [CrossRef]
- Collison, E.; Hird, H.; Cresswell, J.; Tyler, C. Interactive effects of pesticide exposure and pathogen infection on bee health-a critical analysis. Biol. Rev. 2016, 91, 1006–1019. [Google Scholar] [CrossRef]
- Grassl, J.; Holt, S.; Cremen, N.; Peso, M.; Hahne, D.; Baer, B. Synergistic effects of pathogen and pesticide exposure on honey bee (Apis mellifera) survival and immunity. J. Invertebr. Pathol. 2018, 159, 78–86. [Google Scholar] [CrossRef]
- Pettis, J.S.; vanEngelsdorp, D.; Johnson, J.; Dively, G. Pesticide exposure in honey bees results in increased levels of the gut pathogen Nosema. Naturwissenschaften 2012, 99, 153–158. [Google Scholar] [CrossRef]
- Balbuena, M.S.; Tison, L.; Hahn, M.L.; Greggers, U.; Menzel, R.; Farina, W.M. Effects of sublethal doses of glyphosate on honeybee navigation. J. Exp. Biol. 2015, 218, 2799–2805. [Google Scholar] [CrossRef]
- Müller, C. Impacts of sublethal insecticide exposure on insects—Facts and knowledge gaps. Basic. Appl. Ecol. 2018, 30, 1–10. [Google Scholar] [CrossRef]
- Aliouane, Y.; El Hassani, A.K.; Gary, V.; Armengaud, C.; Lambin, M.; Gauthier, M. Subchronic exposure of honeybees to sublethal doses of pesticides: Effects on behavior. Environ. Toxicol. Chem. 2009, 28, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Hung, Y.T.; Cheng, Q. A review of sub-lethal neonicotinoid insecticides exposure and effects on pollinators. Curr. Pollut. Rep. 2020, 6, 137–151. [Google Scholar] [CrossRef]
- Almasri, H.; Tavares, D.A.; Tchamitchian, S.; Pelissier, M.; Sene, D.; Cousin, M.; Brunet, J.L.; Belzunces, L.P. Toxicological status changes the susceptibility of the honey bee Apis mellifera to a single fungicidal spray application. Environ. Sci. Pollut. Res. Int. 2021, 28, 42807–42820. [Google Scholar] [CrossRef]
- Johnson, R.M.; Percel, E.G. Effect of a fungicide and spray adjuvant on queen-rearing success in honey bees (Hymenoptera: Apidae). J. Econ. Entomol. 2013, 106, 1952–1957. [Google Scholar] [CrossRef]
- DeGrandi-Hoffman, G.; Corby-Harris, V.; DeJong, E.W.; Chambers, M.; Hidalgo, G. Honey bee gut microbial communities are robust to the fungicide Pristine® consumed in pollen. Apidologie 2017, 48, 340–352. [Google Scholar] [CrossRef]
- Fisher, A., II; DeGrandi-Hoffman, G.; Smith, B.H.; Johnson, M.; Kaftanoglu, O.; Cogley, T.; Fewell, J.H.; Harrison, J.F. Colony field test reveals dramatically higher toxicity of a widely-used mito-toxic fungicide on honey bees (Apis mellifera). Environ. Pollut. 2021, 269, 115964. [Google Scholar] [CrossRef]
- DesJardins, N.S.; Fisher, A., II; Ozturk, C.; Fewell, J.H.; DeGrandi-Hoffman, G.; Harrison, J.F.; Smith, B.H. A common fungicide, Pristine(R), impairs olfactory associative learning performance in honey bees (Apis mellifera). Environ. Pollut. 2021, 288, 117720. [Google Scholar] [CrossRef]
- Duan, X.; Wang, L.; Wang, R.; Xiong, M.; Qin, G.; Huang, S.; Li, J. Variation in the physiological response of adult worker bees of different ages (Apis mellifera L.) to pyraclostrobin stress. Ecotoxicol. Environ. Saf. 2024, 269, 115754. [Google Scholar] [CrossRef]
- Xiong, M.; Qin, G.; Wang, L.; Wang, R.; Zhou, R.; Luo, X.; Lou, Q.; Huang, S.; Li, J.; Duan, X. Field recommended concentrations of pyraclostrobin exposure disturb the development and immune response of worker bees (Apis mellifera L.) larvae and pupae. Front. Physiol. 2023, 14, 1137264. [Google Scholar] [CrossRef]
- Liao, L.H.; Wu, W.Y.; Dad, A.; Berenbaum, M.R. Fungicide suppression of flight performance in the honeybee (Apis mellifera) and its amelioration by quercetin. Proc. R. Soc. B Biol. Sci. 2019, 286, 20192041. [Google Scholar] [CrossRef] [PubMed]
- Desclos le Peley, V.; Moreau-Vauzelle, C.; Raboteau, D.; Laverre, T.; Grateau, S.; Aupinel, P.; Richard, F.-J. Impact of exposure of larvae to boscalid at field concentrations on gene expression in honey bees. Apidologie 2025, 56, 12. [Google Scholar] [CrossRef]
- Dewhurst, I.; Dellarco, V. Propiconazole. Pesticide Residues in Food–2004 Evaluations; WHO: Geneva, Switzerland, 2006; pp. 281–323. [Google Scholar]
- European Food Safety Authority, E.; Arena, M.; Auteri, D.; Barmaz, S.; Bellisai, G.; Brancato, A.; Brocca, D.; Bura, L.; Byers, H.; Chiusolo, A.; et al. Peer review of the pesticide risk assessment of the active substance propiconazole. EFSA J. 2017, 15, e04887. [Google Scholar] [CrossRef]
- Singh, S.; Singh, N.; Kumar, V.; Datta, S.; Wani, A.B.; Singh, D.; Singh, K.; Singh, J. Toxicity, monitoring and biodegradation of the fungicide carbendazim. Environ. Chem. Lett. 2016, 14, 317–329. [Google Scholar] [CrossRef]
- Carlson, E.A.; Melathopoulos, A.; Sagili, R. The Value of hazard quotients in honey Bee (Apis mellifera) ecotoxicology: A review. Front. Ecol. Evol. 2022, 10, 824992. [Google Scholar] [CrossRef]
- EPPO(European andMediterranean Plant Protection Organization). Guidelines for the efficacy evaluation of plant protection products. PP1/170(4): Side effects on honeybees. IOBC/WPRS Bull. 2000, 23, 51–55. [Google Scholar]
- FAO. Revised Guidelines on Environmental Criteria for the Registration of Pesticides: Part 2: Guidelines for Appropriate Test Procedures; FAO: Rome, Italy, 1989. [Google Scholar]
- OECD. OECD Guidance for Industry Data Submission on Plant Protecton Products and Their Active Substances (Revision 2), Appendix 8, Part 3: Ecotoxicological Studies and Risk Assessment; OECD: Paris, France, 2005. [Google Scholar]
- Matsumoto, Y.; Menzel, R.; Sandoz, J.C.; Giurfa, M. Revisiting olfactory classical conditioning of the proboscis extension response in honey bees: A step toward standardized procedures. J. Neurosci. Methods 2012, 211, 159–167. [Google Scholar] [CrossRef]
- Scheiner, R.; Abramson, C.I.; Brodschneider, R.; Crailsheim, K.; Farina, W.M.; Fuchs, S.; Grünewald, B.; Hahshold, S.; Karrer, M.; Koeniger, G.; et al. Standard methods for behavioural studies of Apis mellifera. J. Apicult Res. 2013, 52, 1–58. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, D.; Ma, W.; Guo, Y.; Wang, A.; Wang, Q.; Lee, D.J. Denitrifying sulfide removal process on high-salinity wastewaters in the presence of Halomonas sp. Appl. Microbiol. Biot. 2016, 100, 1421–1426. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microb. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Siviter, H.; Muth, F. Do novel insecticides pose a threat to beneficial insects? Proc. R. Soc. B Biol. Sci. 2020, 287, 20201265. [Google Scholar] [CrossRef] [PubMed]
- Walton, A.; Herman, J.J.; Rueppell, O. Social life results in social stress protection: A novel concept to explain individual life-history patterns in social insects. Biol. Rev. 2024, 99, 1444–1457. [Google Scholar] [CrossRef]
- Stanley, J.; Sah, K.; Jain, S.K.; Bhatt, J.C.; Sushil, S.N. Evaluation of pesticide toxicity at their field recommended doses to honeybees, Apis cerana and A. mellifera through laboratory, semi-field and field studies. Chemosphere 2015, 119, 668–674. [Google Scholar] [CrossRef]
- Doublet, V.; Labarussias, M.; de Miranda, J.R.; Moritz, R.F.A.; Paxton, R.J. Bees under stress: Sublethal doses of a neonicotinoid pesticide and pathogens interact to elevate honey bee mortality across the life cycle. Environ. Microbiol. 2015, 17, 969–983. [Google Scholar] [CrossRef]
- Tosi, S.; Burgio, G.; Nieh, J.C. A common neonicotinoid pesticide, thiamethoxam, impairs honey bee flight ability. Sci. Rep. 2017, 7, 1201. [Google Scholar] [CrossRef]
- Cabirol, A.; Gómez-Moracho, T.; Monchanin, C.; Pasquaretta, C.; Lihoreau, M. Considering variation in bee responses to stressors can reveal potential for resilience. J. Appl. Ecol. 2023, 60, 1435–1445. [Google Scholar] [CrossRef]
- Poquet, Y.; Vidau, C.; Alaux, C. Modulation of pesticide response in honeybees. Apidologie 2016, 47, 412–426. [Google Scholar] [CrossRef]
- Zhi, J.; Tian, T.; Wen, J.; Liu, Y. Effects of kidney bean damaged by Frankliniella occidentalis (Thysanoptera: Thripidae) or Tetranychus urticae (Acari: Tetranychidae) on the activities of protective and detoxification enzymes in the other subsequent herbivore of both. Acta Entomol. Sin. 2016, 59, 707–715. [Google Scholar]
- Badiou-Bénéteau, A.; Carvalho, S.M.; Brunet, J.L.; Carvalho, G.A.; Buleté, A.; Giroud, B.; Belzunces, L.P. Development of biomarkers of exposure to xenobiotics in the honey bee Apis mellifera: Application to the systemic insecticide thiamethoxam. Ecotoxicol. Environ. Saf. 2012, 82, 22–31. [Google Scholar] [CrossRef]
- Berenbaum, M.R.; Johnson, R.M. Xenobiotic detoxification pathways in honey bees. Curr. Opin. Insect Sci. 2015, 10, 51–58. [Google Scholar] [CrossRef]
- Prezenská, M.; Sobeková, A.; Sabová, L. Antioxidant enzymes of honeybee larvae exposed to oxamyl. Folia Vet. 2019, 63, 9–14. [Google Scholar] [CrossRef]
- Wang, K.; Pang, Q.; Zhang, W.W.; Ji, T. Effects of sublethal doses of carbendazim on the growth and detoxifying enzyme activities of honeybee (Apis mellifera ligustica) larvae. Acta Entomol. Sin. 2017, 60, 642–649. [Google Scholar]
- Li, Z.H.; Zlabek, V.; Grabic, R.; Li, P.; Randak, T. Modulation of glutathione-related antioxidant defense system of fish chronically treated by the fungicide propiconazole. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 152, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Maheshwari, N.; Khan, F.H.; Mahmood, R. Carbendazim toxicity in different cell lines and mammalian tissues. J. Biochem. Mol. Toxic. 2022, 36, e23194. [Google Scholar] [CrossRef]
- Duan, X.L.; Xiong, M.Q.; Liu, W.B.; Zhao, B.A.; Huang, S.K.; Li, J.H. Effects of three fungicides on the activities of protective enzymes and detoxifying enzymes in Apis mellifera. Acta Prataculturae Sin. 2020, 29, 74. [Google Scholar] [CrossRef]
- Claudianos, C.; Ranson, H.; Johnson, R.M.; Biswas, S.; Schuler, M.A.; Berenbaum, M.R.; Feyereisen, R.; Oakeshott, J.G. A deficit of detoxification enzymes: Pesticide sensitivity and environmental response in the honeybee. Insect Mol. Biol. 2006, 15, 615–636. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.M. Honey bee toxicology. Annu. Rev. Entomol. 2015, 60, 415–434. [Google Scholar] [CrossRef] [PubMed]
- Khoury, S.E.; Giovenazzo, P.; Derome, N. Endogenous honeybee gut microbiota metabolize the pesticide clothianidin. Microorganisms 2022, 10, 493. [Google Scholar] [CrossRef]
- Jaffar, S.; Ahmad, S.; Lu, Y.Y. Contribution of insect gut microbiota and their associated enzymes in insect physiology and biodegradation of pesticides. Front. Microbiol. 2022, 13, 979383. [Google Scholar] [CrossRef]
- Cuesta-Maté, A.; Renelies-Hamilton, J.; Kryger, P.; Jensen, A.B.; Sinotte, V.M.; Poulsen, M. Resistance and vulnerability of honeybee (Apis mellifera) gut bacteria to commonly used pesticides. Front. Microbiol. 2021, 12, 717990. [Google Scholar] [CrossRef]
- Hotchkiss, M.Z.; Poulain, A.J.; Forrest, J.R.K. Pesticide-induced disturbances of bee gut microbiotas. Fems Microbiol. Rev. 2022, 46, fuab056. [Google Scholar] [CrossRef] [PubMed]
- Kwong, W.K.; Engel, P.; Koch, H.; Moran, N.A. Genomics and host specialization of honey bee and bumble bee gut symbionts. Proc. Natl. Acad. Sci. USA 2014, 111, 11509–11514. [Google Scholar] [CrossRef]
- Kwong, W.K.; Moran, N.A. Gut microbial communities of social bees. Nat. Rev. Microbiol. 2016, 14, 374–384. [Google Scholar] [CrossRef]
- Kakumanu, M.L.; Reeves, A.M.; Anderson, T.D.; Rodrigues, R.R.; Williams, M.A. Honey bee gut microbiome is altered by in-hive pesticide exposures. Front. Microbiol. 2016, 7, 1255. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Mu, X.H.; Cao, Q.; Shi, Y.; Hu, X.; Zheng, H. Honeybee gut Lactobacillus modulates host learning and memory behaviors via regulating tryptophan metabolism. Nat. Commun. 2022, 13, 2037. [Google Scholar] [CrossRef]
- Klein, S.; Cabirol, A.; Devaud, J.M.; Barron, A.B.; Lihoreau, M. Why bees are so vulnerable to environmental stressors. Trends Ecol. Evol. 2017, 32, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Chen, J.T.; Lang, H.Y.; Zheng, H. Bartonella choladocola sp. nov. and Bartonella apihabitans sp. nov., two novel species isolated from honey bee gut. Syst. Appl. Microbiol. 2022, 45, 126372. [Google Scholar] [CrossRef]
- Castelli, L.; Branchiccela, B.; Garrido, M.; Invernizzi, C.; Porrini, M.; Romero, H.; Santos, E.; Zunino, P.; Antunez, K. Impact of nutritional stress on honeybee gut microbiota, immunity, and Nosema ceranae infection. Int. Microbiol. 2020, 80, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Kesnerova, L.; Emery, O.; Troilo, M.; Liberti, J.; Erkosar, B.; Engel, P. Gut microbiota structure differs between honeybees in winter and summer. ISME J. 2020, 14, 801–814. [Google Scholar] [CrossRef]
- Naggar, Y.A.; Singavarapu, B.; Paxton, R.J.; Wubet, T. Bees under interactive stressors: The novel insecticides flupyradifurone and sulfoxaflor along with the fungicide azoxystrobin disrupt the gut microbiota of honey bees and increase opportunistic bacterial pathogens. Sci. Total Environ. 2022, 849, 157941. [Google Scholar] [CrossRef]
- Cornet, L.; Cleenwerck, I.; Praet, J.; Leonard, R.R.; Vereecken, N.J.; Michez, D.; Smagghe, G.; Baurain, D.; Vandamme, P. Phylogenomic analyses of isolates from honeybees and bumblebees reveal taxonomic and functional diversity. mSystems 2022, 7, e01500-21. [Google Scholar] [CrossRef]
- Zheng, H.; Powell, J.E.; Steele, M.I.; Dietrich, C.; Moran, N.A. Honeybee gut microbiota promotes host weight gain via bacterial metabolism and hormonal signaling. Proc. Natl. Acad. Sci. USA 2017, 114, 4775–4780. [Google Scholar] [CrossRef] [PubMed]
- Steele, M.I.; Motta, E.V.S.; Gattu, T.; Martinez, D.; Moran, N.A. The gut microbiota protects bees from invasion by a bacterial pathogen. Microbiol. Spectr. 2021, 9, e00394-21. [Google Scholar] [CrossRef]
- Bonilla-Rosso, G.; Engel, P. Functional roles and metabolic niches in the honey bee gut microbiota. Curr. Opin. Microbiol. 2018, 43, 69–76. [Google Scholar] [CrossRef]
- Li, W.L.; Huang, Q.; Li, J.L.; Wu, P.; Wei, B.; Li, X.J.; Tang, Q.H.; Dong, Z.X.; Xiong, J.; Tang, H.; et al. Gut microbiota-driven regulation of queen bee ovarian metabolism. Microbiol. Spectr. 2023, 11, e0214523. [Google Scholar] [CrossRef]
- Wong, J.T.; Tran, K.; Pierce, G.N.; Chan, A.C.; Karmin, O.; Choy, P.C. Lysophosphatidylcholine stimulates the release of arachidonic acid in human endothelial cells. J. Biol. Chem. 1998, 273, 6830–6836. [Google Scholar] [CrossRef] [PubMed]
- Kabarowski, J.H. G2A and LPC: Regulatory functions in immunity. Prostaglandins Other Lipid Mediat. 2009, 89, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, I.; Edsfeldt, A.; Ko, N.Y.; Grufman, H.; Berg, K.; Björkbacka, H.; Nitulescu, M.; Persson, A.; Nilsson, M.; Prehn, C.; et al. Evidence supporting a key role of Lp-PLA2-generated lysophosphatidylcholine in human atherosclerotic plaque inflammation. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1505–1512. [Google Scholar] [CrossRef]
- Nesnow, S. Integration of toxicological approaches with “omic” and related technologies to elucidate mechanisms of carcinogenic action: Propiconazole, an example. Cancer Lett. 2013, 334, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.F.; Wang, L.; Ma, L.; Geng, X.Q. P450-mediated detoxification of botanicals in insects. Phytoparasitica 2016, 44, 585–599. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Mu, X.H.; Shi, Y.; Zheng, H. Distinct roles of honeybee gut bacteria on host metabolism and neurological processes. Microbiol. Spectr. 2022, 10, e02438-21. [Google Scholar] [CrossRef]
- Zhao, M.L.; Ren, Z.X.; Zhao, A.H.; Tang, Y.J.; Kuang, J.L.; Li, M.C.; Chen, T.L.; Wang, S.L.; Wang, J.Y.; Zhang, H.H.; et al. Gut bacteria-driven homovanillic acid alleviates depression by modulating synaptic integrity. Cell Metab. 2024, 36, 1000–1012.e6. [Google Scholar] [CrossRef]



| Reagents | Manufacturer | Cat.# Number | Quantity |
|---|---|---|---|
| Chunmanchun®: 35% propiconazole·carbendazim suspoemulsion (7% propiconazole, 28% carbendazim) suspension–emulsion | Zhaoyuan Sanlian Chemical Factory Co., Ltd., Zhaoyuan, China | PD20111232 | 200 g |
| 98.7% Dimethoate | Tanmo Quality Inspection Technology Co., Ltd., Shanghai, China | 70529 | 100 mg |
| 99.5% Acetone, analytically pure | Yonghua Chemical Technology (Jiangsu) Co., Ltd., Changshu, China | XK13-201-00115 | 500 mL |
| 99.7% Absolute ethanol, analytically pure | Tianjin Yongda Chemical Reagent Co., Ltd., Tianjin, China | XK13-011-00011 | 500 mL |
| Citral | Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China | A2303116 | 25 mL |
| PBS solution | Bio-Channeln Biotechnology Co., Ltd., Shenzhen, China | BC20220606 | 500 mL |
| Superoxide dismutase (SOD) kit | Beijing Solarbio Science & Technology Co., Ltd., Beijing, China | BC0170 | 50T/24S |
| Peroxidase (POD) kit | Beijing Solarbio Science & Technology Co., Ltd., Beijing, China | BC0090 | 50T/48S |
| Catalase (CAT) kit | Beijing Solarbio Science & Technology Co., Ltd., Beijing, China | BC0200 | 50T/48S |
| Carboxylesterase (CarE) kit | Beijing Solarbio Science & Technology Co., Ltd., Beijing, China | BC0840 | 50T/48S |
| Glutathione-S transferase (GSTs) kit | Beijing Solarbio Science & Technology Co., Ltd., Beijing, China | BC0350 | 50T/48S |
| Insect cytochrome P450 (CYP450) enzyme-linked immunoassay kit | Shanghai Enzyme Biotechnology Co., Ltd., Shanghai, China | MI037418 | 48T/96T |
| Ultrapure water | Shanghai Kanglei Analytical Instrument Co., Ltd., Shanghai, China | 1503106SN15VF | SMART-N |
| Sucrose | Jiangsu Baimei Sugar Industry Co., Ltd., Huaian, China | - | 50 kg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Cao, Q.; Wang, F.; Du, Y.; Zhao, W.; Guo, Y.; Rueppell, O. Diverse Sublethal Effects of a Common Fungicide Impact the Behavior and Physiology of Honey Bees. Insects 2025, 16, 603. https://doi.org/10.3390/insects16060603
Zhang X, Cao Q, Wang F, Du Y, Zhao W, Guo Y, Rueppell O. Diverse Sublethal Effects of a Common Fungicide Impact the Behavior and Physiology of Honey Bees. Insects. 2025; 16(6):603. https://doi.org/10.3390/insects16060603
Chicago/Turabian StyleZhang, Xufeng, Qian Cao, Feng Wang, Yinyin Du, Wen Zhao, Yuan Guo, and Olav Rueppell. 2025. "Diverse Sublethal Effects of a Common Fungicide Impact the Behavior and Physiology of Honey Bees" Insects 16, no. 6: 603. https://doi.org/10.3390/insects16060603
APA StyleZhang, X., Cao, Q., Wang, F., Du, Y., Zhao, W., Guo, Y., & Rueppell, O. (2025). Diverse Sublethal Effects of a Common Fungicide Impact the Behavior and Physiology of Honey Bees. Insects, 16(6), 603. https://doi.org/10.3390/insects16060603







