Materials and Their Failure Mechanisms in Total Disc Replacement
Abstract
:1. Introduction
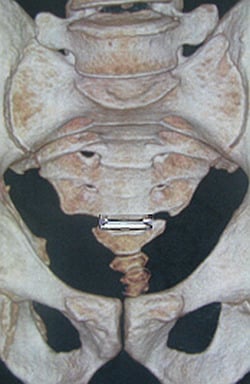
2. Lumbar Total Disc Replacements
2.1. Types of Lumbar TDR
| Device | Classification | Biomaterials | Bearing Design | References | Examples of Manufacturer |
|---|---|---|---|---|---|
| CHARITE | MoP | CoCr-UHMWPE | Mobile | [10,15,16,17,18] | DePuy Spine |
| Prodisc-L | MoP | CoCr-UHMWPE | Fixed | [15,19] | DePuy Synthes |
| Activ-L | MoP | CoCr-UHMWPE | Mobile | [20] | Aesculap |
| Mobidisc | MoP | CoCr-UHMWPE | Mobile | [10,21] | LDR Medical |
| Baguera | MoP | DLC coated Ti-UHMWPE | Fixed | [15] | Spineart |
| NuBac | PoP | PEEK-PEEK | Fixed | [22] | Pioneer |
| Maverick | MoM | CoCr-CoCr | Fixed | [15] | Medtronic |
| Kineflex | MoM | CoCr-CoCr | Mobile | [10,15] | SpinalMotion |
| Flexicore | MoM | CoCr-CoCr | Constrained | Stryker | |
| XL-TDR | MoM | CoCr-CoCr | Fixed | [10,23] | NuVasive |
| CAdisc-L | One piece (1P) | PU-PC graduated modulus | 1P | [10,15,24] | Rainier Technology |
| Freedom | 1P | Ti plates; silicone PU-PC core | 1P | [10,15] | Axiomed |
| eDisc | 1P | Ti plates; elastomer core | 1P | [10,15] | Theken |
| Physio-L | 1P | Ti plates; elastomer core | 1P | [10,15,25,26] | NexGen Spine |
| M6-L | 1P | Ti plates; PU-PC core with UHMWPE fiber encapsulation | 1P | [15] | Spinal Kinetics |
| LP-ESP (elastic spine pad) | 1P | Ti endplates; PUPC coated silicone gel with microvoids | 1P | [3] | FH Orthopedics |
2.1.1. Ball-and-Socket
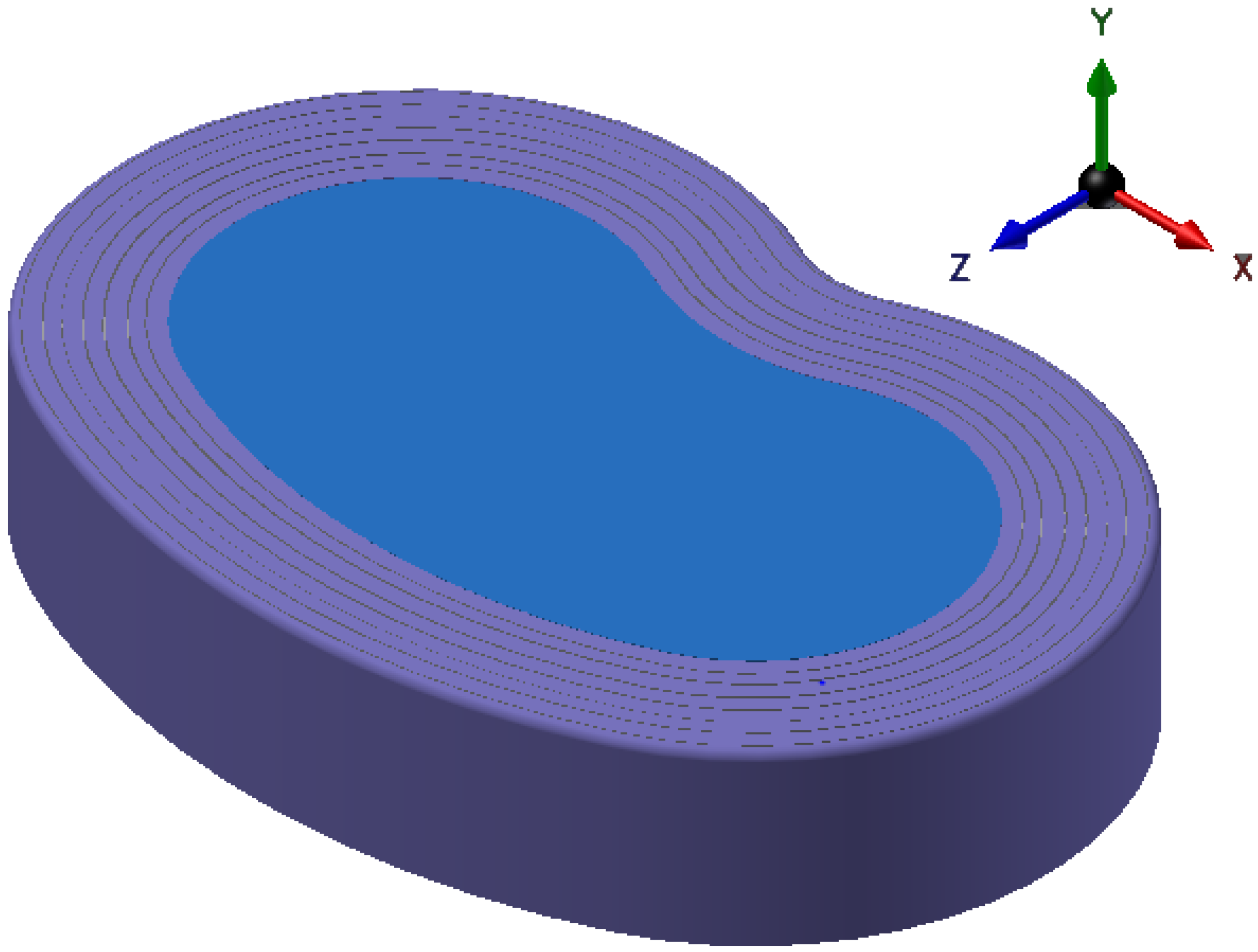
2.1.2. One Piece
2.2. Implant Materials
3. Causes for Failure
3.1. Device Degradation
3.1.1. Metal-on-Polymer
3.1.2. Metal-on-Metal
3.1.3. Polymer-on-Polymer
3.1.4. Diamond-Like Carbon
3.1.5. One Piece
3.2. The Body’s Response
3.2.1. Biological Response
3.2.2. Mechanical Response
(a) Ball-and-Socket Bearing
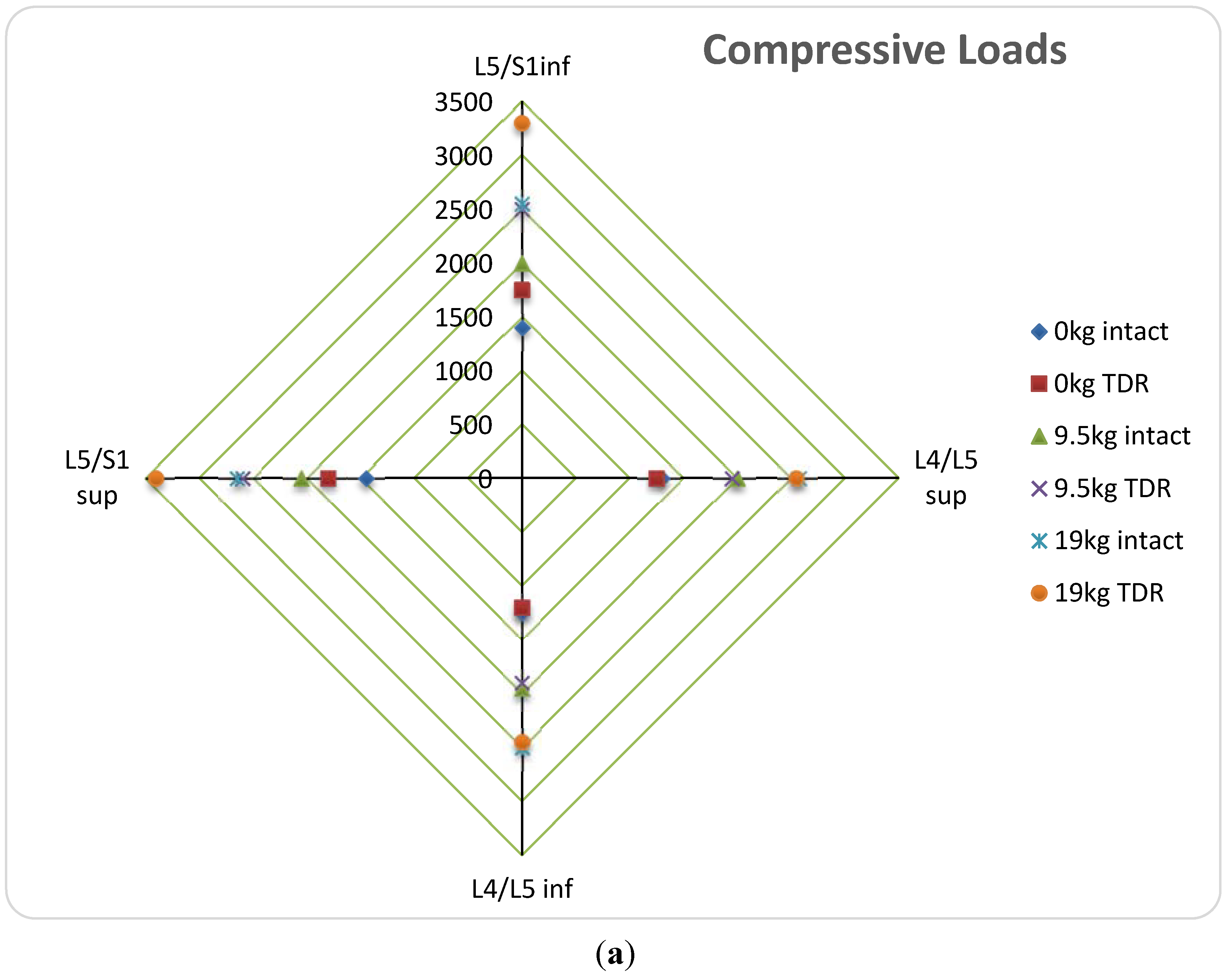
(b) Vertebral Body Stresses
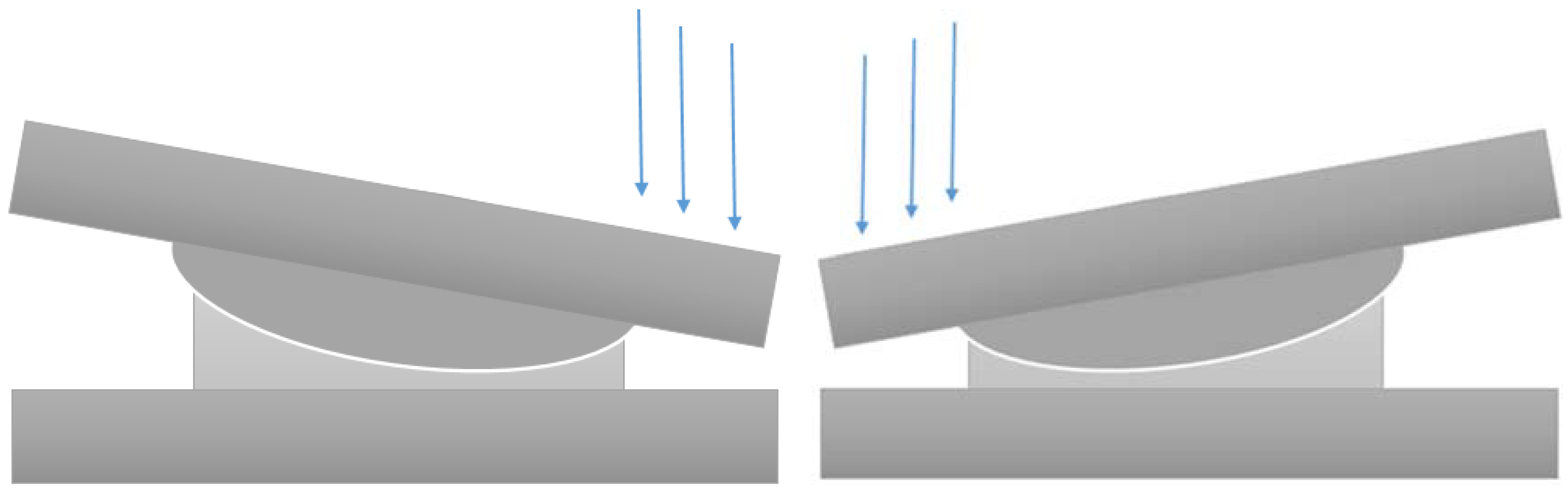
(c) ROM; Facet and Ligament Stresses
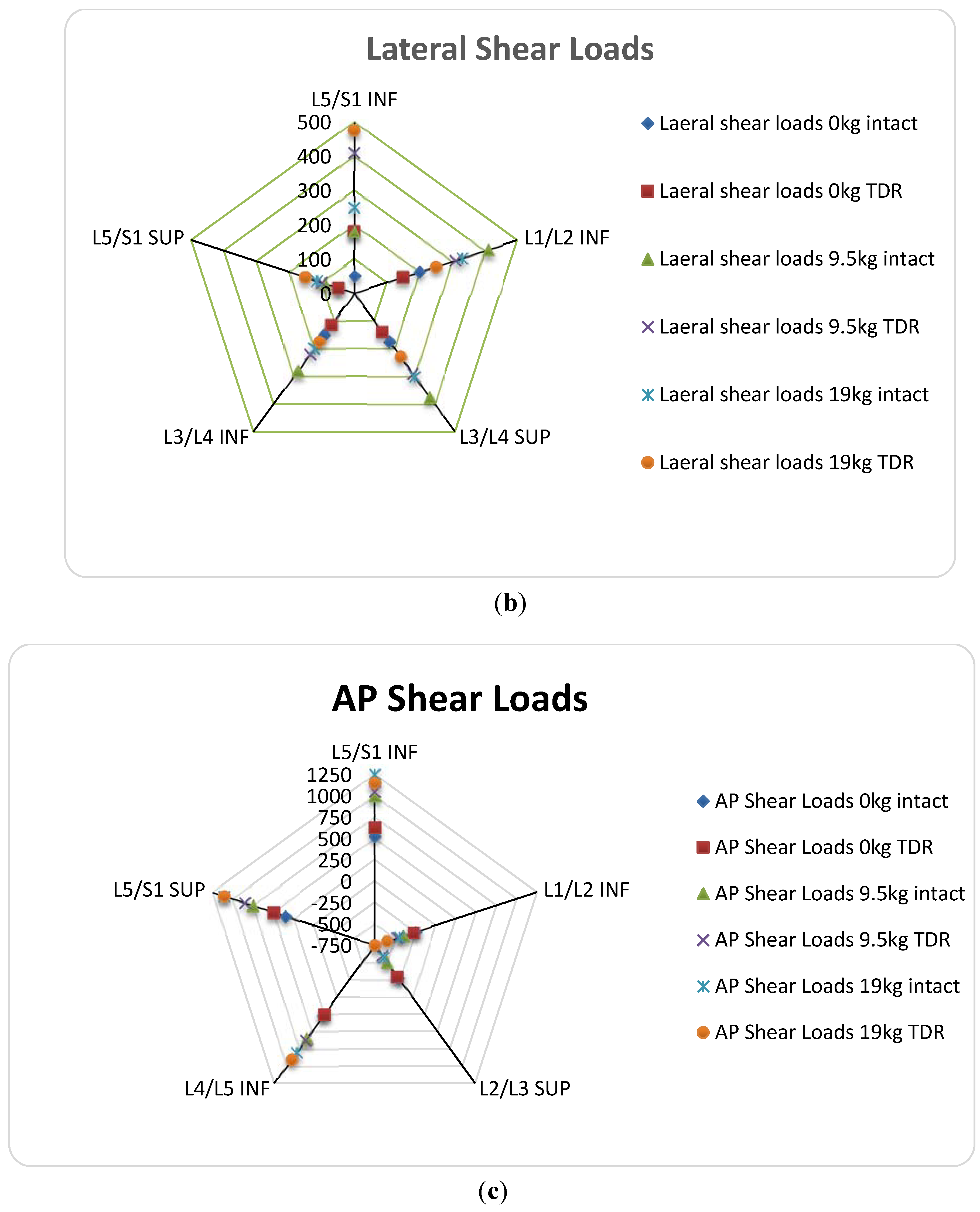
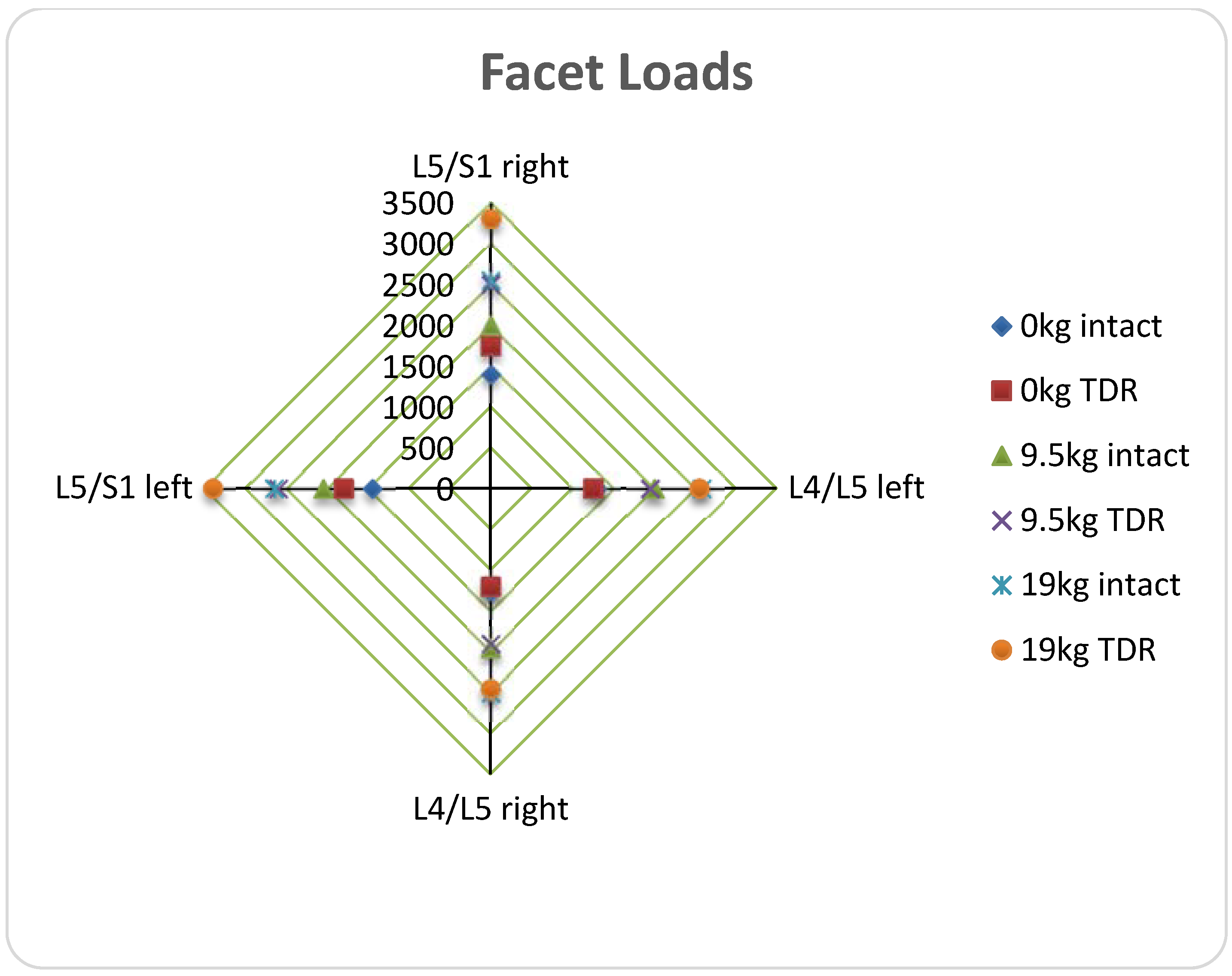
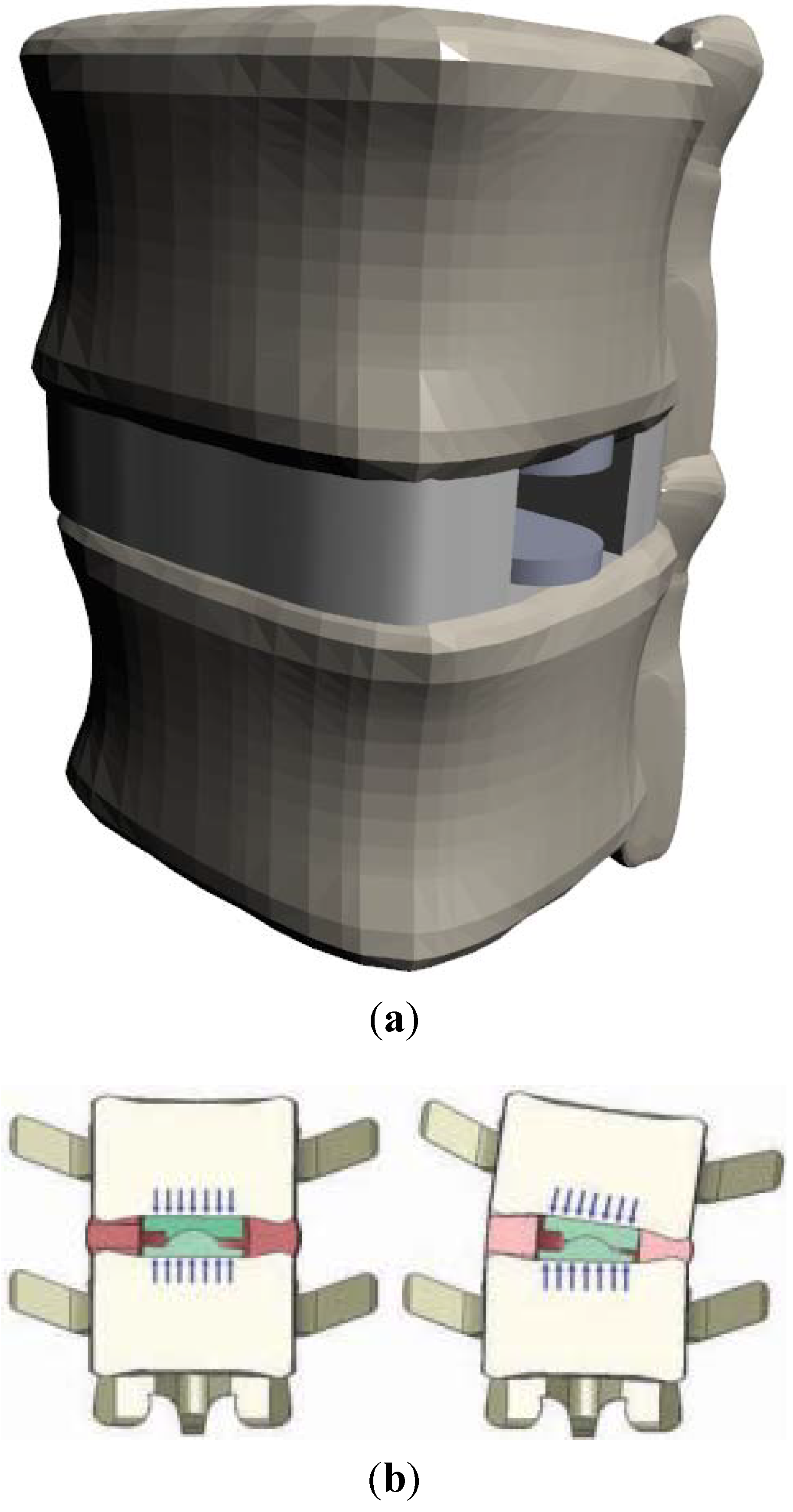
4. One Piece
| Bearing Type | Material | Problems | Effects | Reference |
|---|---|---|---|---|
| Ball and socket | CoCr | Reactive wear ions Fibrous particules | Metal sensitivity reactions, Inflammation, Osteolysis | [19,31,68,91,92] |
| Metallosis | [15,44,61] | |||
| No shock absorption | Compressive stresses on vertebral bodies | [9] | ||
| UHMWPE | Wear debris large wear volume | Bone resorption, Osteolysis | [31,92,93] | |
| [18,31] | ||||
| Plastic deformation | [18] | |||
| Increased ROM | Facet and ligament loading | [16] | ||
| No shock absorption | Compressive stresses on vertebral bodies | [9] | ||
| PEEK | Prosthesis migration | Biomechanical incompatibility, stress on remaining annulus, total ejection of device | [61] | |
| Endplate reaction | Severe biological rejection | [61] | ||
| 1P | PUPC | More studies necessary | [25] |
5. Future Prospects
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pleis, J.; Schiller, J.; Benson, V. Summary health statistics for U.S. adults: National Health Interview Survey, 2010. Vital Health Stat. 2012, 10, 1–207. [Google Scholar]
- Karajan, N. Multiphasic intervertebral disc. mechanics: Theory and application. Arch. Comput. Methods Eng. 2012, 19, 261–339. [Google Scholar] [CrossRef]
- Lazennec, J.Y.; Even, J.; Skalli, W.; Rakover, J.P.; Brusson, A.; Rousseau, M.A. Clinical outcomes, radiologic kinematics, and effects on sagittal balance of the 6 df LP-ESP lumbar disc prosthesis. Spine J. 2014, 14, 1914–1920. [Google Scholar] [CrossRef] [PubMed]
- Neumann, D.A. Kinesiology of the Musculoskeletal System: Foundations for Rehabilitation; Elsevier Health Sciences: St. Louis, MO, USA, 2013. [Google Scholar]
- Bogduk, N. Clinical Anatomy of the Lumbar Spine and Sacrum; Churchill Livingstone: London, UK, 2005. [Google Scholar]
- Markolf, K.L.; Morris, J.M. The structural components of the intervertebral disc. J. Bone Joint Surg. 1974, 56, 675–687. [Google Scholar] [PubMed]
- Rothman, R.H.; Simeone, F.A. The spine. J. Pediatr. Orthop. 1992, 12, 549. [Google Scholar] [CrossRef]
- Gloria, A.; De Santis, R.; Ambrosio, L.; Causa, F.; Tanner, K.E. A multi-component fiber-reinforced PHEMA-based hydrogel/HAPEX device for customized intervertebral disc prosthesis. J. Biomater. Appl. 2011, 25, 795–810. [Google Scholar] [CrossRef] [PubMed]
- Knapik, G. Use of a personalized hybrid biomechanical model to assess change in lumbar spine function with a TDR compared to an intact spine. Eur. Spine J. 2012, 21, 641–652. [Google Scholar] [CrossRef]
- Serhan, H.; Mhatre, D.; Defossez, H.; Bono, C.M. Motion-preserving technologies for degenerative lumbar spine: The past, present, and future horizons. SAS J. 2011, 5, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Gornet, M.F.; Burkus, J.K.; Dryer, R.F.; Peloza, J.H. Lumbar disc arthroplasty with Maverick disc versus stand-alone interbody fusion: A prospective, randomized, controlled, multicenter investigational device exemption trial. Spine 2011, 36, E1600–E1611. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M. Correlation between sagittal plane changes and adjacent segment degeneration following lumbar spine fusion. Eur. Spine J. 2001, 10, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M. Long-term follow-up of functional outcomes and radiographic changes at adjacent levels following lumbar spine fusion for degenerative disc disease. Eur. Spine J. 2001, 10, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Cakir, B.; Richter, M.; Schmoelz, W.; Schmidt, R.; Reichel, H.; Wilke, H.J. Resect or not to resect: The role of posterior longitudinal ligament in lumbar total disc replacement. Eur. Spine J. 2012, 21, 592–598. [Google Scholar] [CrossRef]
- Veruva, S.Y.; Steinbeck, M.J.; Toth, J.; Alexander, D.D.; Kurtz, S.M. Which design and biomaterial factors affect clinical wear performance of total disc replacements? A systematic review. Clin. Orthop. Related Res. 2014, 472, 3759–3769. [Google Scholar] [CrossRef]
- Takigawa, T.; Espinoza Orías, A.A.; An, H.S.; Gohgi, S.; Udayakumar, R.K.; Sugisaki, K.; Natarajan, R.N.; Wimmer, M.A.; Inoue, N. Spinal kinematics and facet load transmission after total disc replacement. Spine 2010, 35, E1160–E1166. [Google Scholar] [CrossRef] [PubMed]
- Kettler, A.; Bushelow, M.; Wilke, H.J. Influence of the loading frequency on the wear rate of a polyethylene-on-metal lumbar intervertebral disc replacement. Eur. Spine J. 2012, 21, S709–S716. [Google Scholar] [CrossRef] [PubMed]
- Shkolnikov, Y.P.; Bowden, A.; MacDonald, D.; Kurtz, S.M. Wear pattern observations from TDR retrievals using autoregistration of voxel data. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94, 312–317. [Google Scholar] [PubMed]
- Chen, W.M.; Park, C.; Lee, K.; Lee, S. In situ contact analysis of the prosthesis componentsof Prodisc-L in lumbar spine following total disc replacement. Spine 2009, 34, E716–E723. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.C.; Girardi, F.P.; Cammisa, F.P.; Wright, T.M. The implications of constraint in lumbar total disc replacement. J. Spinal Disord. Tech. 2003, 16, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Austen, S.; Punt, I.M.; Cleutjens, J.P.; Willems, P.C.; Kurtz, S.M.; MacDonald, D.W.; van Rhijn, L.W.; van Ooij, A. Clinical, radiological, histological and retrieval findings of Activ-L and Mobidisc total disc replacements: A study of two patients. Eur. Spine J. 2012, 21, S513–S520. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.B.; Songer, M.; Pimenta, L.; Werner, D.; Reyes-Sanchez, A.; Balsano, M.; Agrillo, U.; Coric, D.; Davenport, K.; Yuan, H. Nubac disc arthroplasty: Preclinical studies and preliminary safety and efficacy evaluations. SAS J. 2007, 1, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Marchi, L.; Oliveira, L.; Coutinho, E.; Pimenta, L. The importance of the anterior longitudinal ligament in lumbar disc arthroplasty: 36-Month follow-up experience in extreme lateral total disc replacement. Int. J. Spine Surg. 2012, 6, 18–23. [Google Scholar] [CrossRef] [PubMed]
- McNally, D.; Naylor, J.; Johnson, S. An in vitro biomechanical comparison of Cadisc™-L with natural lumbar discs in axial compression and sagittal flexion. Eur. Spine J. 2012, 21, 612–617. [Google Scholar] [CrossRef]
- Pimenta, L.; Springmuller, R.; Lee, C.K.; Oliveira, L.; Roth, S.E.; Ogilvie, W.F. Clinical performance of an elastomeric lumbar disc replacement: Minimum 12 months follow-up. SAS J. 2010, 4, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Van den, B. Design of next generation total disk replacements. J. Biomech. 2012, 45, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Hallab, N. A review of the biologic effects of spine implant debris: Fact. from fiction. SAS J. 2009, 3, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Frelinghuysen, P.; Huang, R.C.; Girardi, F.P.; Cammisa, F.P. Lumbar total disc replacement part I: Rationale, biomechanics, and implant types. Orthop. Clin. North Am. 2005, 36, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Vicars, R.; Hyde, P.J.; Brown, T.D.; Tipper, J.L.; Ingham, E.; Fisher, J.; Hall, R.M. The effect of anterior-posterior shear load on the wear of ProDisc-L TDR. Eur. Spine J. 2010, 19, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Vicars, R.; Prokopovich, P.; Brown, T.D.; Tipper, J.L.; Ingham, E.; Fisher, J.; Hall, R.M. The effect of anterior-posterior shear on the wear of CHARITE total disc replacement. Spine 2012, 37, E528–E534. [Google Scholar] [CrossRef] [PubMed]
- Taki, N.; Tatro, J.M.; Nalepka, J.L.; Togawa, D.; Goldberg, V.M.; Rimnac, C.M.; Greenfield, E.M. Polyethylene and titanium particles induce osteolysis by similar, lymphocyte-independent, mechanisms. J. Orthop. Res. 2005, 23, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Sieving, A. Morphological characteristics of total joint arthroplasty-derived ultra-high molecular weight polyethylene (UHMWPE) wear debris that provoke inflammation in a murine model of inflammation. J. Biomed. Mater. Res. 2003, 64, 457–464. [Google Scholar] [CrossRef]
- Agarwal, S. Osteolysis—basic science incidence and diagnosis. Curr. Orthop. 2004, 18, 220–231. [Google Scholar] [CrossRef]
- Kurtz, S.M.; van Ooij, A.; Ross, R.; de Waal Malefijt, J.; Peloza, J.; Ciccarelli, L.; Villarraga, M.L. Polyethylene wear and rim fracture in total disc arthroplasty. Spine J. 2007, 7, 12–21. [Google Scholar] [CrossRef] [PubMed]
- McKellop, H.A.; Campbell, P.; Park, S.H.; Schmalzried, T.P.; Grigoris, P.; Amstutz, H.C.; Sarmiento, A. The origin of submicron polyethylene wear debris in total hip arthroplasty. Clin. Orthop. Related Res. 1995, 311, 3–20. [Google Scholar]
- Lebl, D. In vivo functional performance of failed Prodisc-L devices: Retrieval analysis of lumbar total disc replacements. Spine 2012, 37, E1209–E1217. [Google Scholar] [CrossRef] [PubMed]
- Choma, T. Retrieval analysis of a ProDisc-L total disc replacement. J. Spinal Disord. Tech. 2009, 22, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Rundell, S.A.; Day, J.S.; Isaza, J.; Guillory, S.; Kurtz, S.M. Lumbar total disc replacement impingement sensitivity to disc height distraction, spinal sagittal orientation, implant position, and implant lordosis. Spine 2012, 37, E590–E598. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Essner, A.; Polineni, V.K.; Stark, C.; Dumbleton, J.H. Lubrication and wear of ultra-high molecular weight polyethylene in total joint replacements. Tribol. Int. 1998, 31, 17–33. [Google Scholar] [CrossRef]
- Charnley, J.; Halley, D.K. Rate of wear in total hip replacement. Clin. Orthop. Related Res. 1975, 112, 170–179. [Google Scholar]
- Wright, T.; Bartel, D. The problem of surface damage in polyethylene total knee components. Clin. Orthop. Related Res. 1986, 205, 67–74. [Google Scholar]
- Gornet, M. Prospective study on serum metal levels in patients with metal-on-metal lumbar disc arthroplasty. Eur. Spine J. 2013, 22, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S. The latest lessons learned from retrieval analyses of ultra-high molecular weight polyethylene, metal-on-metal, and alternative bearing total disc replacements. Semin. Spine Surg. 2012, 24, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Guyer, R.D.; Shellock, J.; MacLennan, B.; Hanscom, D.; Knight, R.Q.; McCombe, P.; Jacobs, J.J.; Urban, R.M.; Bradford, D.; Ohnmeiss, D.D. Early failure of metal-on-metal artificial disc prostheses associated with lymphocytic reaction: Diagnosis and treatment experience in four cases. Spine 2011, 36, E492–E497. [Google Scholar] [CrossRef] [PubMed]
- Behl, B.; Papageorgiou, I.; Brown, C.; Hall, R.; Tipper, J.L.; Fisher, J.; Ingham, E. Biological effects of cobalt-chromium nanoparticles and ions on dural fibroblasts and dural epithelial cells. Biomaterials 2013, 34, 3547–3558. [Google Scholar] [CrossRef] [PubMed]
- Golish, S.R.; Anderson, P.A. Bearing surfaces for total disc arthroplasty: Metal-on-metal versus metal-on-polyethylene and other biomaterials. Spine J. 2012, 12, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Gotman, I. Characteristics of metals used in implants. J. Endourol. 1997, 11, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Asphahani, A.I.; Kumar, P.; Hickl, A.J.; Lawley, A. Properties and characteristics of cast, wrought, and powder metallurgy (P/M) processed cobalt-chromium-molybdenum implant materials. In Corrosion and Degradation of Implant Materials: Second Symposium; Fraker, A.C., Griffin, C.D., Eds.; ASTM International: West Conshohocken, PA, USA, 1985. [Google Scholar]
- Perkins, L. Evaluation of Bone Fixation Implants. Texas A&M University, College Station, TX, USA, December 2012. [Google Scholar]
- Gurappa, I. Characterization of different materials for corrosion resistance under simulated body fluid conditions. Mater. Charact. 2002, 49, 73–79. [Google Scholar] [CrossRef]
- Gurrappa, I. Corrosion and its importance in selection of materials for biomedical applications. Corros. Prev. Control 2001, 48, 23–37. [Google Scholar]
- Taksali, S. Material considerations for intervertebral disc replacement implants. Spine J. 2004, 4, 231S. [Google Scholar] [CrossRef] [PubMed]
- Pourbaix, M. Electrochemical corrosion of metallic biomaterials. Biomaterials 1984, 5, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, J. Corrosion on spinal implants. J. Spinal Disord. Tech. 2005, 18, 247–251. [Google Scholar] [PubMed]
- Michel, R. Trace element burdening of human tissues due to the corrosion of hip-joint prostheses made of cobalt-chromium alloys. Arch. Orthop. Trauma. Surg. 1984, 103, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Pound, B.G. Passive films on metallic biomaterials under simulated physiological conditions. J. Biomed. Mater. Res. Part A 2014, 102, 1595–1604. [Google Scholar] [CrossRef]
- Jacobs, J.J. Corrosion of metal orthopaedic implants. JBJS J. Bone Joint Surg. 1998, 80, 268–282. [Google Scholar]
- Jacobs, J.J.; Shanbhag, A.; Glant, T.T.; Black, J.; Galante, J.O. Wear debris in total joint replacements. J. Am. Acad. Orthop. Surg. 1994, 2, 212–220. [Google Scholar] [PubMed]
- Waterhouse, R.B. Fretting wear. Wear 1984, 100, 107–118. [Google Scholar] [CrossRef]
- Zeh, A.; Becker, C.; Planert, M.; Lattke, P.; Wohlrab, D. Time-dependent release of cobalt and chromium ions into the serum following implantation of the metal-on-metal Maverick type artificial lumbar disc (Medtronic Sofamor Danek). Arch. Orthop. Trauma Surg. 2009, 129, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, L. Lessons learned after 9 yearsʼ clinical experience with 3 different nucleus replacement devices. Semin. Spine Surg. 2012, 24, 43–47. [Google Scholar] [CrossRef]
- Hauert, R.; Thorwarth, K.; Thorwarth, G. An overview on diamond-like carbon coatings in medical applications. Surf. Coat. Technol. 2013, 233, 119–130. [Google Scholar] [CrossRef]
- Chandra, L. The effect of biological fluids on the adhesion of diamond-like carbon films to metallic substrates. Diamond Related Mater. 1995, 4, 852–856. [Google Scholar] [CrossRef]
- Falub, C.V. In vitro studies of the adhesion of diamond-like carbon thin films on CoCrMo biomedical implant alloy. Acta Mater. 2011, 59, 4678–4689. [Google Scholar] [CrossRef]
- Enker, P. Artificial disc replacement. Preliminary report with a 3-year minimum follow-up. Spine 1993, 18, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Serhan, H.; Ross, R.; Lowery, G.; Fraser, R. Biomechanical characterization of a new lumbar disc prosthesis. J. Bone Joint Surg. Br. 2002, 84, 215. [Google Scholar]
- Moore, R. The biologic response to particles from a lumbar disc prosthesis. Spine 2002, 27, 2088–2094. [Google Scholar] [CrossRef] [PubMed]
- Fraser, R. AcroFlex design and results. Spine J. 2004, 4, S245–S251. [Google Scholar] [CrossRef]
- Credo Reference (Firm). Merriam-Websterʼs Medical Desk Dictionary; Thomson Delmar Learning: Clifton Park, NY, USA, 2006; p. 1. [Google Scholar]
- Per Aspenberg, P.H. Periprosthetic bone resorption. Particles versus movement. J. Bone Joint Surg. Br. 1996, 78, 641–646. [Google Scholar] [PubMed]
- Kang, J. Chronic failure of a lumbar total disc. Replacement with osteolysis. report of a case with nineteen-year follow-up. JBJS J. Bone Joint Surg. 2008, 90, 2230. [Google Scholar]
- Bisseling, P.; Zeilstra, D.J.; Hol, A.M.; van Susante, J.L.C. Metal ion levels in patients with a lumbar metal-on-metal total disc replacement SHOULD WE BE CONCERNED? J. Bone Joint Surg. Br. 2011, 93, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Black, J. In Vivo Corrosion of a Cobalt-Base Alloy and Its Biological Consequences, in Biocompatibility of Co-Cr-Ni Alloys; Springer: New York, NY, USA, 1988; pp. 83–100. [Google Scholar]
- Cavanaugh, D. Delayed hyper-reactivity to metal ions after cervical disc arthroplasty: A case report and literature review. Spine 2009, 34, E262–E265. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Wang, L.; Kou, D.; Jia, X.; Yang, X.; Zhang, M.; Tang, Y.; Wang, P.; Wang, S.; Xu, Y.; Wang, H. Metal. hypersensitivity in patient with posterior lumbar spine fusion: A case report and its literature review. BMC Musculoskelet. Disord. 2014, 15, 314. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.J.; Hallab, N.J.; Urban, R.M.; Wimmer, M.A. Wear particles. J. Bone Joint Surg. 2006, 88, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Laquerriere, P. Importance of hydroxyapatite particles characteristics on cytokines production by human monocytes in vitro. Biomaterials 2003, 24, 2739–2747. [Google Scholar] [CrossRef] [PubMed]
- Bruch, J. Response of cell cultures to asbestos fibers. Environ. Health Perspect. 1974, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Dostert, C. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 2008, 320, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Botolin, S. Facet joint biomechanics at the treated and adjacent levels after total disc replacement. Spine 2011, 36, E27–E32. [Google Scholar] [PubMed]
- Chung, S. Biomechanical effect of constraint in lumbar total disc replacement: A study with finite element analysis. Spine 2009, 34, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, P.; Nicolakis, M.; Lorenz, M.A.; Voronov, L.I.; Zindrick, M.R.; Ghanayem, A.; Havey, R.M.; Carandang, G.; Sartori, M.; Gaitanis, I.; et al. Response of CHARITE total disc replacement under physiologic loads: Prosthesis component motion patterns. Spine J. 2005, 5, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Heuer, F.; Schmidt, H.; Klezl, Z.; Claes, L.; Wilke, H.J. Stepwise reduction of functional spinal structures increase range of motion and change lordosis angle. J. Biomech. 2007, 40, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Botolin, S.; Puttlitz, C.; Baldini, T.; Petrella, A.; Burger, E.; Abjornson, C.; Patel, V. Facet joint biomechanics at the treated and adjacent levels after total disc replacement. J. Bone Joint Surg. Br. 2012, 94, 143–143. [Google Scholar]
- Rundell, S. Total disc replacement positioning affects facet contact forces and vertebral body strains. Spine 2008, 33, 2510–2517. [Google Scholar] [CrossRef] [PubMed]
- Phillips, F.; Diaz, R.; Pimenta, L. The fate of the facet joints after lumbar total disc replacement: A clinical and MRI study. Spine J. 2005, 5, S75. [Google Scholar] [CrossRef]
- Shim, C.S. CHARITE versus ProDisc—A comparative study of a minimum 3-year follow-up. Spine 2007, 32, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Mathew, P. Bilateral pedicle fractures following anterior dislocation of the polyethylene inlay of a ProDisc artificial disc replacement: A case report of an unusual complication. Spine 2005, 30, E311–E314. [Google Scholar] [CrossRef] [PubMed]
- Schulte, T. Acquired spondylolysis after implantation of a lumbar ProDisc II prosthesis: Case report and review of the literature. Spine 2007, 32, E645–E648. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, L.H.; Marchi, L.; Oliveira, L. Lumbar total disc replacement with a ball and socket metal on metal device: Up to 60-months follow-up. Spine J. 2012, 12, S104. [Google Scholar] [CrossRef]
- Moghadas, P. Wear in metal-on-metal total disc arthroplasty. Proc. Inst. Mech. Eng. 2013, 227, 356–361. [Google Scholar] [CrossRef]
- Dong-wook, K.; Lee, K.-Y.; Jun, Y.; Lee, S.J.; Park, C.K. Friction and wear characteristics of UHMWPE against Co-Cr alloy under the wide range of contact pressures in lumbar total disc. replacement. Int. J. Precis. Eng. Manuf. 2011, 12, 1111–1118. [Google Scholar] [CrossRef]
- van Ooij, A.; Kurtz, S.M.; Stessels, F.; Noten, H.; van Rhijn, L. Polyethylene wear debris and long-term clinical failure of the CHARITE disc prosthesis: A study of 4 patients. Spine 2007, 32, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Lee, C. Development of a prosthetic intervertebral disc. Spine 1991, 16, S253–S255. [Google Scholar] [CrossRef] [PubMed]
- Huang, R. Biomechanics of nonfusion implants. Orthop. Clin. N. Am. 2005, 36, 271–280. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reeks, J.; Liang, H. Materials and Their Failure Mechanisms in Total Disc Replacement. Lubricants 2015, 3, 346-364. https://doi.org/10.3390/lubricants3020346
Reeks J, Liang H. Materials and Their Failure Mechanisms in Total Disc Replacement. Lubricants. 2015; 3(2):346-364. https://doi.org/10.3390/lubricants3020346
Chicago/Turabian StyleReeks, John, and Hong Liang. 2015. "Materials and Their Failure Mechanisms in Total Disc Replacement" Lubricants 3, no. 2: 346-364. https://doi.org/10.3390/lubricants3020346
APA StyleReeks, J., & Liang, H. (2015). Materials and Their Failure Mechanisms in Total Disc Replacement. Lubricants, 3(2), 346-364. https://doi.org/10.3390/lubricants3020346