Oral Lubrication, Xerostomia, and Advanced Macromolecular Lubricants for Treatment of Dry Mouth
Abstract
1. Introduction
2. Causes of Xerostomia
2.1. Medication Side Effects
2.2. Cancer
2.3. Other Conditions Leading to Xerostomia
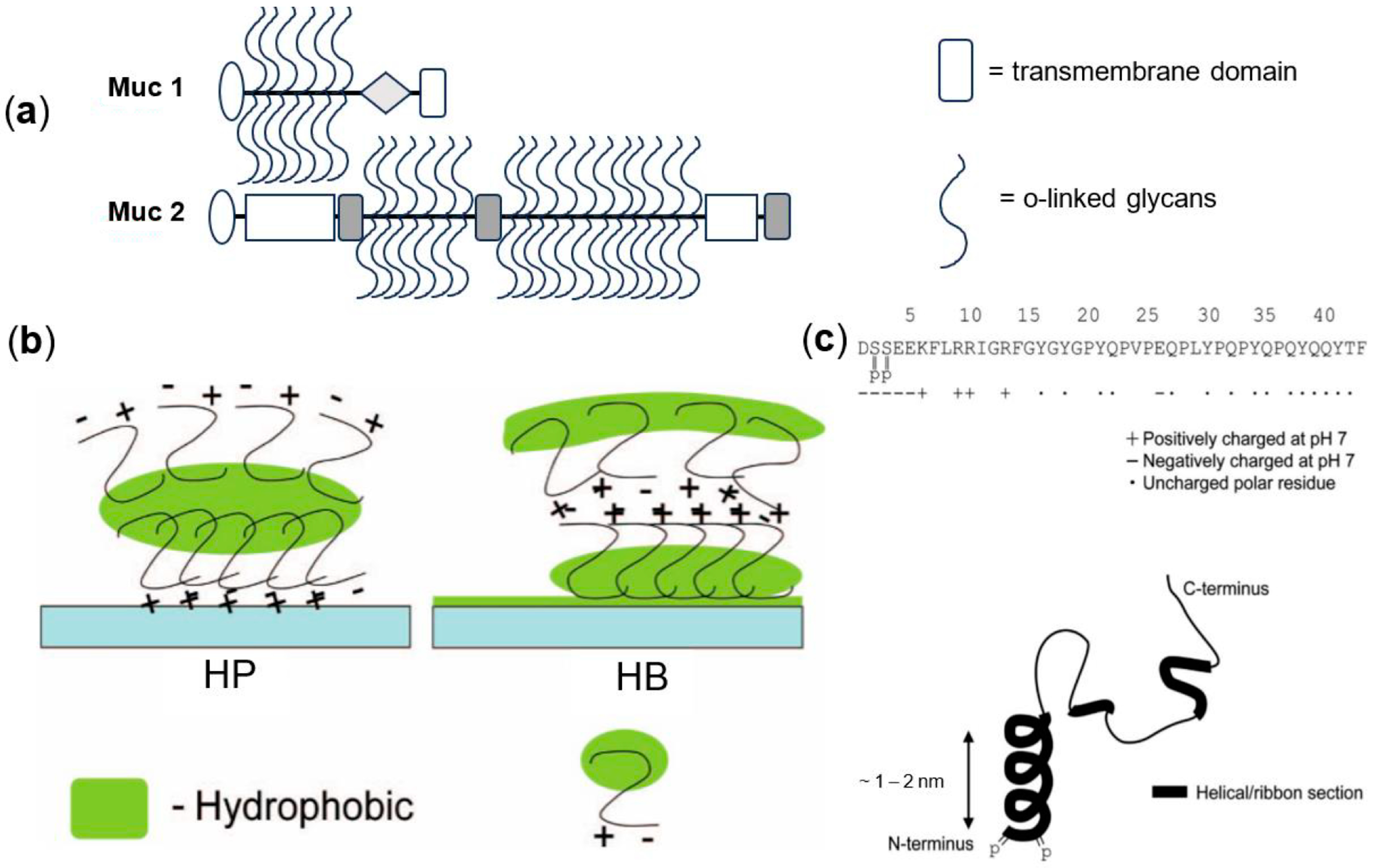
3. Mechanism of Lubrication in the Mouth
| Parotid | Submandibular | Sublingual | Minor Glands | |
|---|---|---|---|---|
| Mucins MG1/MG2 | 0 [61,62,63] | ↑/↑ [61,63] | ↑↑/↑↑ [63] | ↑/0 [63] |
| Statherin (μM) | 12.8 [64] | ND | ND | ND |
| Amylases (U/mL) | 161.8 [63] | 15.9 [63] | 15.9 [63] | 101.4 [63] |
| Water | 99% [50,62,63] | 99% [50,62,63] | 99% [50,62,63] | 99% [50,62,63] |
| Proline-rich Proteins (mg/mL) | 1.7 [63] | 1.3 [63] | 1.8 [63] | 2.1 [63] |
| Cystatin S (μg/mL) | 0.5 [63] | 177 [63] | 28 [63] | 56 [63] |
| Lysozymes | ~0 [63] | ↑ [63] | ↑↑ [63] | ~0 [63] |
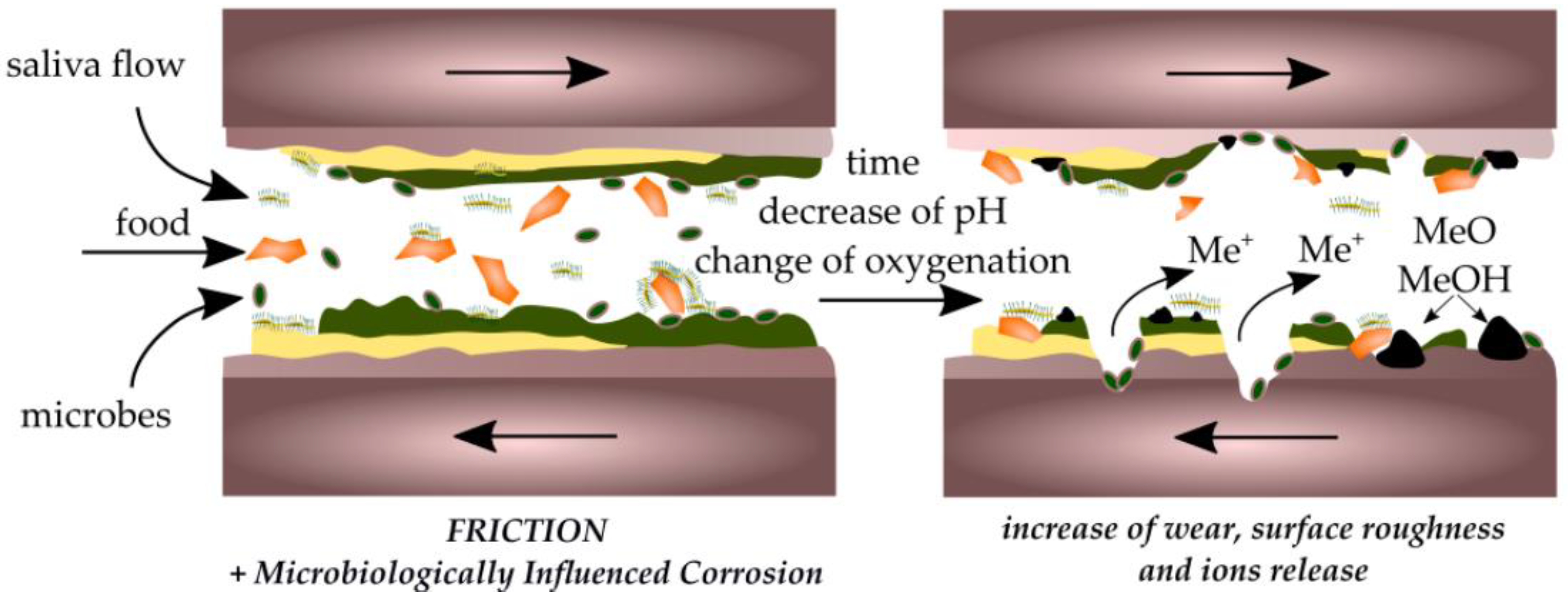
4. Effect of Biofilm and Implants on Oral Friction and Wear
5. Methods of Studying Oral Friction
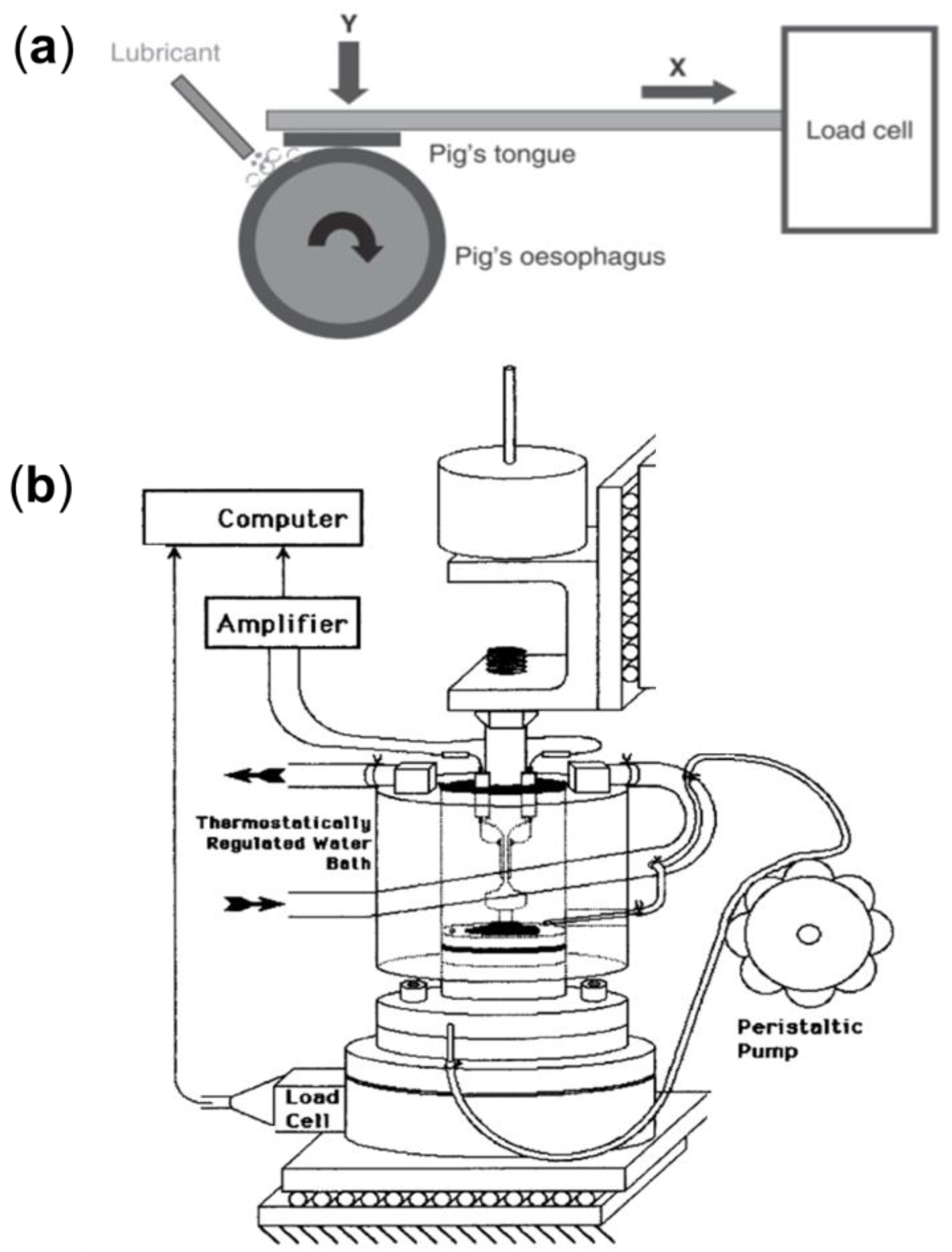
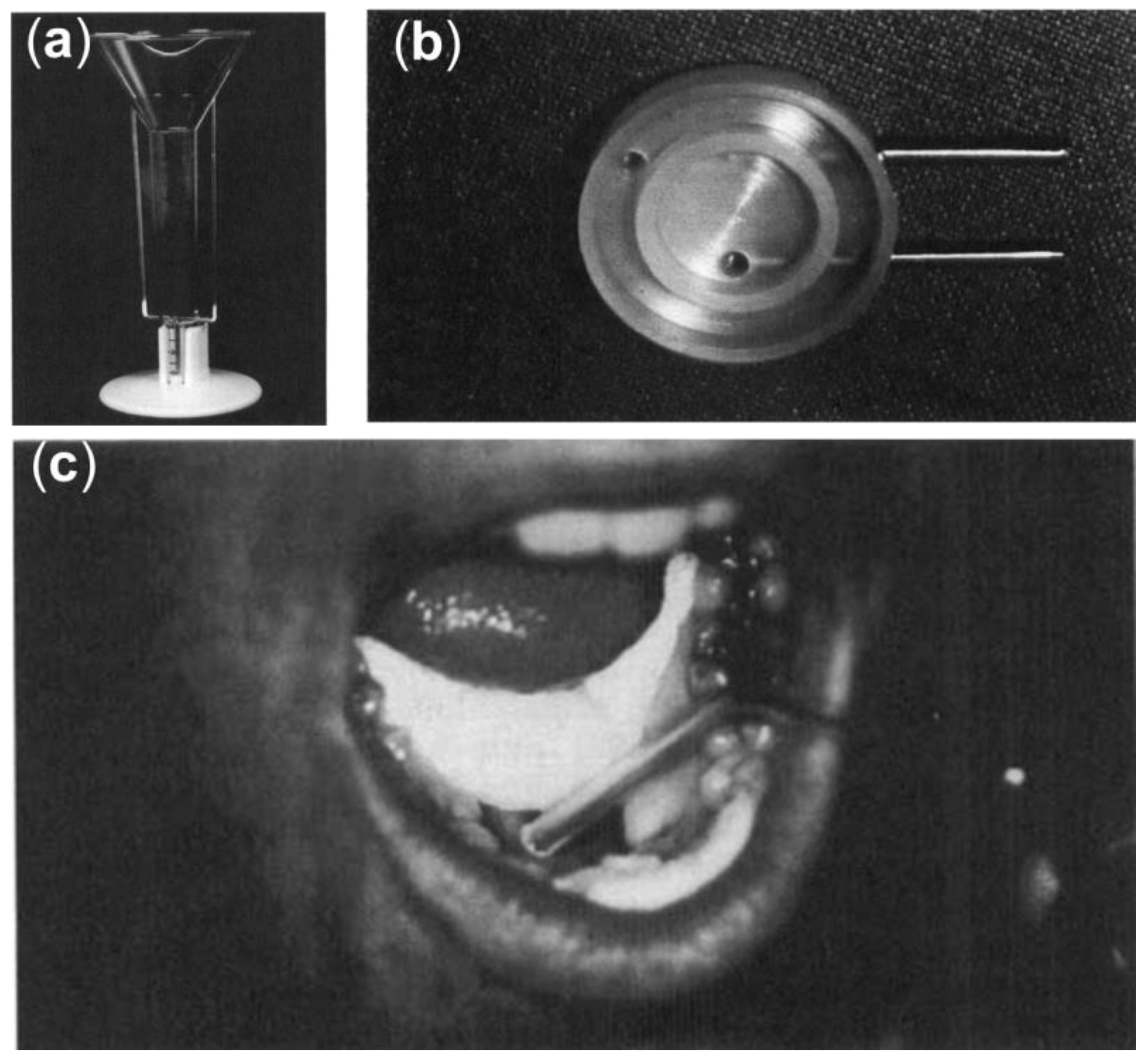
5.1. Ex Vivo Models for Studying Oral Friction
5.2. Clinical Approaches for Studying Dry Mouth
6. Lubricating Saliva Substitutes
6.1. Natural Macromolecular Lubricants for the Treatment of Xerostomia
6.1.1. Lubricating Proteins and Glycoproteins
6.1.2. Lubricating Lipids
6.1.3. Lubricating Polysaccharides
6.1.4. Complex Mixtures
6.2. Synthetic Macromolecular Mouth Lubricants
6.2.1. Chemically Modified Biomacromolecules
6.2.2. Synthetic Polymers
6.2.3. Synthetic Polypeptides
7. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, Y.J. Xerostomia and Its Cellular Targets. Int. J. Mol. Sci. 2023, 24, 5358. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Andablo-Reyes, E.; Mighell, A.; Pavitt, S.; Sarkar, A. Dry Mouth Diagnosis and Saliva Substitutes—A Review from a Textural Perspective. J. Texture Stud. 2021, 52, 141–156. [Google Scholar] [CrossRef]
- Bhayani, M.K.; Lai, S.Y. Xerostomia. In Gland-Preserving Salivary Surgery; Springer: Berlin/Heidelberg, Germany, 2017; pp. 175–183. [Google Scholar] [CrossRef]
- Tanasiewicz, M.; Hildebrandt, T.; Obersztyn, I. Xerostomia of Various Etiologies: A Review of the Literature. Adv. Clin. Exp. Med. 2016, 25, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Porter, S.R.; Scully, C.; Hegarty, A.M. An Update of the Etiology and Management of Xerostomia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 97, 28–46. [Google Scholar]
- Sarkar, A.; Xu, F.; Lee, S. Human Saliva and Model Saliva at Bulk to Adsorbed Phases—Similarities and Differences. Adv. Colloid Interface Sci. 2019, 273, 102034. [Google Scholar]
- Ozdemir, T.; Fowler, E.W.; Hao, Y.; Ravikrishnan, A.; Harrington, D.A.; Witt, R.L.; Farach-Carson, M.C.; Pradhan-Bhatt, S.; Jia, X. Biomaterials-Based Strategies for Salivary Gland Tissue Regeneration. Biomater. Sci. 2016, 4, 592–604. [Google Scholar] [CrossRef]
- Gittings, S.; Turnbull, N.; Henry, B.; Roberts, C.J.; Gershkovich, P. Characterisation of Human Saliva as a Platform for Oral Dissolution Medium Development. Eur. J. Pharm. Biopharm. 2015, 91, 16–24. [Google Scholar] [CrossRef]
- Carpenter, G. Artificial Salivas. Clin. Dent. Rev. 2018, 2, 24. [Google Scholar] [CrossRef]
- Guggenheimer, J.; Moore, P.A. Xerostomia: Etiology, Recognition, and Treatment. J. Am. Dent. Assoc. 2003, 134, 61–69. [Google Scholar] [CrossRef]
- Iorgulescu, G. Saliva between Normal and Pathological. Important Factors in Determining Systemic and Oral Health. J. Med. Life 2009, 2, 303–307. [Google Scholar]
- Malicka, B.; A-F, U.K.; Skośkiewicz-Malinowska, K. Prevalence of Xerostomia and the Salivary Flow Rate in Diabetic Patients. Adv. Clin. Exp. Med. 2014, 23, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Edgar, W.M. Saliva: Its Secretion, Composition and Functions. Br. Dent. J. 1992, 172, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Adibnia, V.; Mirbagheri, M.; Faivre, J.; Robert, J.; Lee, J.; Matyjaszewski, K.; Lee, D.W.; Banquy, X. Bioinspired Polymers for Lubrication and Wear Resistance. Prog. Polym. Sci. 2020, 110, 101298. [Google Scholar] [CrossRef]
- Ranc, H.; Elkhyat, A.; Servais, C.; Mac-Mary, S.; Launay, B.; Humbert, P. Friction Coefficient and Wettability of Oral Mucosal Tissue: Changes Induced by a Salivary Layer. Colloids Surfaces A Physicochem. Eng. Asp. 2006, 276, 155–161. [Google Scholar] [CrossRef]
- Kagami, H.; Wang, S.; Hai, B. Restoring the Function of Salivary Glands. Oral Dis. 2008, 14, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Veeregowda, D.H.; Kolbe, A.; Van Der Mei, H.C.; Busscher, H.J.; Herrmann, A.; Sharma, P.K. Recombinant Supercharged Polypeptides Restore and Improve Biolubrication. Adv. Mater. 2013, 25, 3426–3431. [Google Scholar] [CrossRef] [PubMed]
- Furness, S.; Worthington, H.V.; Bryan, G.; Birchenough, S.; McMillan, R. Interventions for the Management of Dry Mouth: Topical Therapies. Cochrane Database Syst. Rev. 2011, CD008934. [Google Scholar] [CrossRef] [PubMed]
- Satoh-Kuriwada, S.; Iikubo, M.; Shoji, N.; Sakamoto, M.; Sasano, T. Diagnostic Performance of Labial Minor Salivary Gland Flow Measurement for Assessment of Xerostomia. Arch. Oral Biol. 2012, 57, 1121. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Borgnakke, W.S.; Yoshihara, A.; Ito, K.; Ogawa, H.; Nohno, K.; Sato, M.; Minagawa, K.; Ansai, T. Hyposalivation and 10-Year All-Cause Mortality in an Elderly Japanese Population. Gerodontology 2018, 35, 87–94. [Google Scholar] [CrossRef]
- Åstrøm, A.N.; Lie, S.A.; Ekback, G.; Gülcan, F.; Ordell, S. Self-Reported Dry Mouth among Ageing People: A Longitudinal, Cross-National Study. Eur. J. Oral Sci. 2019, 127, 130–138. [Google Scholar] [CrossRef]
- Marcott, S.; Dewan, K.; Kwan, M.; Baik, F.; Lee, Y.; Sirjani, D. Where Dysphagia Begins: Polypharmacy and Xerostomia. Fed. Pract. 2020, 37, 234–241. [Google Scholar] [PubMed]
- Arany, S.; Kopycka-Kedzierawski, D.T.; Caprio, T.V.; Watson, G.E. Anticholinergic Medication: Related Dry Mouth and Effects on the Salivary Glands. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 132, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Gilani, A.H. Antispasmodic and Bronchodilator Activities of Artemisia Vulgaris Are Mediated through Dual Blockade of Muscarinic Receptors and Calcium Influx. J. Ethnopharmacol. 2009, 126, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Tardy, M.; Dold, M.; Engel, R.; Leucht, S. Flupenthixol versus Low-Potency First-Generation Antipsychotic Drugs for Schizophrenia. Cochrane Database Syst. Rev. 2014, CD009227. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.N.; Panchaksharappa, M.G.; Annigeri, R.G. Modified Schirmer Test-a Screening Tool for Xerostomia among Subjects on Antidepressants. Arch. Oral Biol. 2014, 59, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Habbab, K.M.; Moles, D.R.; Porter, S.R. Potential Oral Manifestations of Cardiovascular Drugs. Oral Dis. 2010, 16, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Mohandoss, A.A.; Thavarajah, R. Salivary Flow Alteration in Patients Undergoing Treatment for Schizophrenia: Disease-Drug-Target Gene/Protein Association Study for Side-Effects. J. Oral Biol. Craniofacial Res. 2019, 9, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Murdoch-Kinch, C.A.; Kim, H.M.; Vineberg, K.A.; Ship, J.A.; Eisbruch, A. Dose-Effect Relationships for the Submandibular Salivary Glands and Implications for Their Sparing by Intensity Modulated Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 373–382. [Google Scholar] [CrossRef]
- Stroup, T.S.; Gray, N. Management of Common Adverse Effects of Antipsychotic Medications. World Psychiatry 2018, 17, 341–356. [Google Scholar] [CrossRef]
- Fortuna, G.; Whitmire, S.; Sullivan, K.; Alajbeg, I.; Andabak-Rogulj, A.; Pedersen, A.M.; Vissink, A.; di Fede, O.; Aria, M.; Jager, D.J.; et al. Impact of Medications on Salivary Flow Rate in Patients with Xerostomia: A Retrospective Study by the Xeromeds Consortium. Clin. Oral Investig. 2023, 27, 235–248. [Google Scholar] [CrossRef]
- Hunter, K.D.; Wilson, W.S. The Effects of Antidepressant Drugs on Salivary Flow and Content of Sodium and Potassium Ions in Human Parotid Saliva. Arch. Oral Biol. 1995, 40, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Nederfors, T.; Dahlöf, C. Effects on Salivary Flow Rate and Composition of Withdrawal of and Re-Exposure to the Β1 -Selective Antagonist Metoprolol in a Hypertensive Patient Population. Eur. J. Oral Sci. 1996, 104, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Wolff, A.; Narayana, N.; Aframian, D.; Vissink, A.; Ekström, J.; Proctor, G.; McGowan, R.; Narayana, N.; Aliko, A. World Workshop on Oral Medicine VI: A Systematic Review of Medication-Induced Salivary Gland Dysfunction: Prevalence, Diagnosis, and Treatment. Clin. Oral Investig. 2016, 19, 365–382. [Google Scholar] [CrossRef] [PubMed]
- Wilberg, P.; Hjermstad, M.J.; Ottesen, S.; Herlofson, B.B. Chemotherapy-Associated Oral Sequelae in Patients With Cancers Outside the Head and Neck Region. J. Pain Symptom Manag. 2014, 48, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Main, B.E.; Calman, K.C.; Ferguson, M.M.; Kaye, S.B.; MacFarlane, T.W.; Mairs, R.J.; Samaranayake, L.P.; Willos, J.; Welsh, J. The Effect of Cytotoxic Therapy on Saliva and Oral Flora. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 1984, 58, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.C.; Qazali, A.; Zaveri, J.; Chambers, M.S.; Gunn, G.B.; Fuller, C.D.; Lai, S.Y.; Mott, F.E.; Hutcheson, K.A. Self-Reported Oral Morbidities in Long-Term Oropharyngeal Cancer Survivors: A Cross-Sectional Survey of 906 Survivors. Oral Oncolology 2018, 84, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.B.; Vissink, A.; Limesand, K.H.; Reyland, M.E. Salivary Gland Hypofunction and Xerostomia in Head and Neck Radiation Patients. J. Natl. Cancer Inst. Monogr. 2019, 2019, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Jasmer, K.J.; Gilman, K.E.; Muñoz Forti, K.; Weisman, G.A.; Limesand, K.H. Radiation-Induced Salivary Gland Dysfunction: Mechanisms, Therapeutics and Future Directions. J. Clin. Med. 2020, 9, 4095. [Google Scholar] [CrossRef] [PubMed]
- Vissink, A.; Mitchell, J.B.; Baum, B.J.; Limesand, K.H.; Jensen, S.B.; Fox, P.C.; Elting, L.S.; Langendijk, J.A.; Coppes, R.P.; Reyland, M.E. Clinical Management of Salivary Gland Hypofunction and Xerostomia in Head and Neck Cancer Patients: Successes and Barriers. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 983–991. [Google Scholar] [CrossRef]
- Beetz, I.; Schilstra, C.; Visink, A.; van der Schaaf, A.; Bijl, H.P.; van der Laan, B.F.; Steenbakkers, R.J.H.M.; Langendijk, J.A. Role of Minor Salivary Glands in Developing Patient-Rated Xerostomia and Sticky Saliva during Day and Night. Radiother. Oncol. 2013, 1092, 311–316. [Google Scholar] [CrossRef]
- Winter, S.C.; Cassell, O.; Corbridge, R.J.; Goodacre, T.; Cox, G.J. Quality of Life Following Resection, Free Flap Reconstruction and Postoperative External Beam Radiotherapy for Squamous Cell Carcinoma of the Base of Tongue. Clin. Otolaryngol. Allied Sci. 2004, 29, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017, 69, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, A.; Knaś, M.; Waszkiewicz, N.; Waszkiel, D.; Sierakowski, S.; Zwierz, K. Rheumatoid Arthritis Patients with Xerostomia Have Reduced Production of Key Salivary Constituents. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Aitken-Saavedra, J.; Rojas-Alcayaga, G.; Maturana-Ramírez, A.; Escobar-Álvarez, A.; Cortes-Coloma, A.; Reyes-Rojas, M.; Viera-Sapiain, V.; Villablanca-Martínez, C.; Morales-Bozo, I. Salivary Gland Dysfunction Markers in Type 2 Diabetes Mellitus Patients. J. Clin. Exp. Dent. 2015, 7, 501–505. [Google Scholar] [CrossRef] [PubMed][Green Version]
- López-Verdín, S.; Andrade-Villanueva, J.; Zamora-Perez, A.L.; Bologna-Molina, R.; Cervantes-Cabrera, J.J.; Molina-Frechero, N. Differences in Salivary Flow Level, Xerostomia, and Flavor Alteration in Mexican HIV Patients Who Did or Did Not Receive Antiretroviral Therapy. AIDS Res. Treat. 2013, 2013, 613278. [Google Scholar] [CrossRef] [PubMed]
- Wey, S.J.; Chen, Y.M.; Lai, P.J.; Chen, D.Y. Primary Sjögren Syndrome Manifested as Localized Cutaneous Nodular Amyloidosis. J. Clin. Rheumatol. 2011, 17, 368–370. [Google Scholar] [CrossRef] [PubMed]
- López-Pintor, R.M.; Casañas, E.; González-Serrano, J.; Serrano, J.; Ramírez, L.; De Arriba, L.; Hernández, G. Xerostomia, Hyposalivation, and Salivary Flow in Diabetes Patients. J. Diabetes Res. 2016, 2016, 4372852. [Google Scholar] [CrossRef]
- Saleh, J.; Figueiredo, M.A.Z.; Cherubini, K.; Salum, F.G. Salivary Hypofunction: An Update on Aetiology, Diagnosis and Therapeutics. Arch. Oral Biol. 2015, 60, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, G.H. The Secretion, Components, and Properties of Saliva. Annu. Rev. Food Sci. Technol. 2013, 4, 267–276. [Google Scholar] [CrossRef]
- Yakubov, G.E. Lubrication. Monogr. Oral Sci. 2014, 24, 71–87. [Google Scholar]
- Käsdorf, B.T.; Weber, F.; Petrou, G.; Srivastava, V.; Crouzier, T.; Lieleg, O. Mucin-Inspired Lubrication on Hydrophobic Surfaces. Biomacromolecules 2017, 18, 2454–2462. [Google Scholar] [CrossRef] [PubMed]
- Adibnia, V.; Mirbagheri, M.; Salimi, S.; De Crescenzo, G.; Banquy, X. Nonspecific Interactions in Biomedical Applications. Curr. Opin. Colloid Interface Sci. 2020, 47, 70–83. [Google Scholar] [CrossRef]
- Elkayar, A.; Elshazly, Y.; Assaad, M. Properties of Hydroxyapatite from Bovine Teeth. Bone Tissue Regen. Insights 2009, 2, 31–36. [Google Scholar] [CrossRef]
- Lindberg, K.; Rheinwald’, J.G. Three Distinct Keratinocyte Subtypes Identified in Human Oral Epithelium by Their Patterns of Keratin Expression in Culture and in Xenografts. Differentiation 1990, 45, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Kono, T.; Sakae, T.; Nakada, H.; Kaneda, T.; Okada, H. Confusion between Carbonate Apatite and Biological Apatite (Carbonated Hydroxyapatite) in Bone and Teeth. Minerals 2022, 12, 170. [Google Scholar] [CrossRef]
- Kay, D.M.I.; Young, P.R.A.; Posner, D.A.S. Crystal Structure of Hydroxyapatite. Nature 1964, 204, 1050–1052. [Google Scholar] [CrossRef] [PubMed]
- Eckhart, L.; Lippens, S.; Tschachler, E.; Declercq, W. Cell Death by Cornification. Biochim. Biophys. Acta—Mol. Cell Res. 2013, 1833, 3471–3480. [Google Scholar] [CrossRef] [PubMed]
- Tabak, L.A.; Levine, M.J.; Mandel, I.D.; Ellison, S.A. Role of Salivary Mucins in the Protection of the Oral Cavity. J. Oral Pathol. Med. 1982, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Moran, A.P.; Gupta, A.; Joshi, L. Sweet-Talk: Role of Host Glycosylation in Bacterial Pathogenesis of the Gastrointestinal Tract. Gut 2011, 60, 1412–1425. [Google Scholar] [CrossRef]
- Prinz, J.F.; de Wijk, R.A.; Huntjens, L. Load Dependency of the Coefficient of Friction of Oral Mucosa. Food Hydrocoll. 2007, 21, 402–408. [Google Scholar] [CrossRef]
- Humphrey, S.P.; Williamson, R.T. A Review of Saliva: Normal Composition, Flow and Function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Veerman, E.C.I.; Van Den Keybus, P.A.M.; Vissink, A.; Amerongen, A.V.N. Human Glandular Salivas: Their Separate Collection and Analysis. Eur. J. Oral Sci. 1996, 104, 346–352. [Google Scholar] [CrossRef]
- Hay, D.I.; Smith, D.J.; Schluckebier, S.K.; Moreno, E.C. Basic Biological Sciences Relationship between Concentration of Human Salivary Statherin and Inhibition of Calcium Phosphate Precipitation in Stimulated Human Parotid Saliva. J. Dent. Res. 1984, 63, 857–863. [Google Scholar] [CrossRef]
- Harvey, N.M.; Carpenter, G.H.; Proctor, G.B.; Klein, J. Normal and Frictional Interactions of Purified Human Statherin Adsorbed on Molecularly-Smooth Solid Substrata. Biofouling 2011, 27, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Boze, H.; Marlin, T.; Durand, D.; Pérez, J.; Vemhet, A.; Canon, F.; Sami-Manchado, P.; Cheynier, V.; Cabane, B. Proline-Rich Salivary Proteins Have Extended Conformations. Biophys. J. 2010, 99, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Gorr, S.U. Antimicrobial Peptides of the Oral Cavity. Periodontology 2000 2009, 51, 152–180. [Google Scholar] [CrossRef]
- Dickinson, P.D. Salivary (SD-Type) Cystatins: Over One Billion Yearsin the Making—But to What Purpose? Crit. Rev. Oral Biol. Med. 2002, 13, 485–508. [Google Scholar] [CrossRef]
- Choi, S.; Baik, J.E.; Jeon, J.H.; Cho, K.; Seo, D.G.; Kum, K.Y.; Yun, C.H.; Han, S.H. Identification of Porphyromonas Gingivalis Lipopolysaccharide-Binding Proteins in Human Saliva. Mol. Immunol. 2011, 48, 2207–2213. [Google Scholar] [CrossRef]
- Ferraboschi, P.; Ciceri, S.; Grisenti, P. Applications of Lysozyme, an Innate Immune Defense Factor, as an Alternative Antibiotic. Antibiotics 2021, 10, 1534. [Google Scholar] [CrossRef]
- Douglas, W.H.; Reeh, E.S.; Ramasubbu, N.; Raj, P.A.; Bhandary, K.K.; Levine, M.J. Statherin: A Major Boundary Lubricant of Human Saliva. Biochem. Biophys. Res. Commun. 1991, 180, 91–97. [Google Scholar] [CrossRef]
- Makrodimitris, K.; Masica, D.L.; Kim, E.T.; Gray, J.J. Structure Prediction of Protein-Solid Surface Interactions Reveals a Molecular Recognition Motif of Statherin for Hydroxyapatite. J. Am. Chem. Soc. 2007, 129, 13713–13722. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, I.E.; Arnebrant, T.; Lindh, L. Human Palatal Saliva: Adsorption Behaviour and the Role of Low-Molecular Weight Proteins. Biofouling 2004, 20, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Proctor, G.B.; Hamdan, S.; Carpenter, G.H.; Wilde, P. A Statherin and Calcium Enriched Layer at the Air Interface of Human Parotid Saliva. Biochemistry 2005, 389, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Hay, D.I.; Schluckebier, K.; Moreno, E.C. Calcified Tissue International Saturation of Human Salivary Secretions with Respect to Calcite and Inhibition of Calcium Carbonate Precipitation by Salivary Constituents. Calcif. Tissue Int. 1986, 39, 151–160. [Google Scholar] [CrossRef]
- Chawhuaveang, D.D.; Yu, O.Y.; Yin, I.X.; Lam, W.Y.H.; Mei, M.L.; Chu, C.H. Acquired Salivary Pellicle and Oral Diseases: A Literature Review. J. Dent. Sci. 2021, 16, 523–529. [Google Scholar] [CrossRef]
- White, D.J. Dental Calculus: Recent Insights into Occurrence, Formation, Prevention, Removal and Oral Health Effects of Supragingival and Subgingival Deposits. Eur. J. Oral Sci. 1997, 105, 508–522. [Google Scholar] [CrossRef]
- White, D.J. Processes Contributing to the Formation of Dental Calculus. Biofouling 1991, 4, 209–218. [Google Scholar] [CrossRef]
- Berg, I.C.H.; Rutland, M.W.; Arnebrant, T. Lubricating Properties of the Initial Salivary Pellicle—An AFM Study. Biofouling 2003, 19, 365–369. [Google Scholar] [CrossRef]
- Bongaerts, J.H.H.; Rossetti, D.; Stokes, J.R. The Lubricating Properties of Human Whole Saliva. Tribol. Lett. 2007, 27, 277–287. [Google Scholar] [CrossRef]
- Mystkowska, J.; Niemirowicz-Laskowska, K.; Łysik, D.; Tokajuk, G.; Dąbrowski, J.R.; Bucki, R. The Role of Oral Cavity Biofilm on Metallic Biomaterial Surface Destruction–Corrosion and Friction Aspects. Int. J. Mol. Sci. 2018, 19, 743. [Google Scholar] [CrossRef]
- Lin, N.J. Biofilm over Teeth and Restorations: What Do We Need to Know? Dent. Mater. 2017, 33, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Kozmos, M.; Virant, P.; Rojko, F.; Abram, A.; Rudolf, R.; Raspor, P.; Zore, A.; Bohinc, K. Bacterial Adhesion of Streptococcus Mutans to Dental Material Surfaces. Molecules 2021, 26, 1152. [Google Scholar] [CrossRef] [PubMed]
- Maddi, A.; Scannapieco, F.A. Oral Biofilms, Oral and Periodontal Infections, and Systemic Disease. Am. J. Dent. 2013, 26, 249–254. [Google Scholar] [PubMed]
- Mathew, M.T.; Barão, V.A.; Yuan, J.C.C.; Assunção, W.G.; Sukotjo, C.; Wimmer, M.A. What Is the Role of Lipopolysaccharide on the Tribocorrosive Behavior of Titanium? J. Mech. Behav. Biomed. Mater. 2012, 8, 71–85. [Google Scholar] [CrossRef]
- Messer, R.L.W.; Tackas, G.; Mickalonis, J.; Brown, Y.; Lewis, J.B.; Wataha, J.C. Corrosion of Machined Titanium Dental Implants under Inflammatory Conditions. J. Biomed. Mater. Res. 2009, 88, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Vissink, A.; Sharma, P.K. Enhancement in Xerostomia Patient Salivary Lubrication Using a Mucoadhesive. J. Dent. Res. 2020, 99, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Vinke, J.; Kaper, H.J.; Vissink, A.; Sharma, P.K. An Ex Vivo Salivary Lubrication System to Mimic Xerostomic Conditions and to Predict the Lubricating Properties of Xerostomia Relieving Agents. Sci. Rep. 2018, 8, 9087. [Google Scholar] [CrossRef] [PubMed]
- Pailler-Mattei, C.; Vargiolu, R.; Tupin, S.; Zahouani, H. Ex Vivo Approach to Studying Bio-Adhesive and Tribological Properties of Artificial Salivas for Oral Dryness (Xerostomia). Wear 2015, 332–333, 710–714. [Google Scholar] [CrossRef]
- Dresselhuis, D.M.; de Hoog, E.H.A.; Stuart, M.A.C.; van Aken, G.A. Application of Oral Tissue in Tribological Measurements in an Emulsion Perception Context. Food Hydrocoll. 2008, 22, 323–335. [Google Scholar] [CrossRef]
- Stokes, J.R.; Boehm, M.W.; Baier, S.K. Oral Processing, Texture and Mouthfeel: From Rheology to Tribology and Beyond. Curr. Opin. Colloid Interface Sci. 2013, 18, 349–359. [Google Scholar] [CrossRef]
- Reeh, E.S.; Douglas, W.H.; Levine, M.J. Pergamon Lubrication of Human and Bovine Enamel Compared in an Artificial Mouth. Arch. Oral Biol. 1995, 40, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Thomson, W.M.; Spencer, A.J.; Williams, S. The Xerostomia Inventory: A Multi-Item Approach to Measuring Dry Mouth Prevalence of Periodontal Diseases in Oman View Project The Xerostomia Inventorv: A Multi-Item Approach to Measuring Dry Mouth. Community Dent. Health 1999, 16, 12–17. [Google Scholar] [PubMed]
- Challacombe, S.J.; Osailan, S.M.; Proctor, G.B.; Carpenter, G. Dry Mouth A Clinical Guide on Causes, Effects and Treatments; Carpenter, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Villa, A.; Connell, C.L.; Abati, S. Diagnosis and Management of Xerostomia and Hyposalivation. Ther. Clin. Risk Manag. 2014, 11, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Navazesh, M. Methods for Collecting Saliva. Ann. N. Y. Acad. Sci. 1993, 694, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Chung, J.W.; Kim, Y.K.; Chung, S.C.; Kho, H.S. Viscosity and Wettability of Animal Mucin Solutions and Human Saliva. Oral Dis. 2007, 13, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Park, W.K.; Chung, J.W.; Kim, Y.K.; Chung, S.C.; Kho, H.S. Influences of Animal Mucins on Lysozyme Activity in Solution and on Hydroxyapatite Surfaces. Arch. Oral Biol. 2006, 51, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Vissink, A.; ’s-Gravenmade, E.J.; Panders, A.K.; Vermey, A.; Petersen, J.K.; Visch, L.L.; Schaub, R.M.H. A Clinical Comparison between Commercially Available Mucin- and CMC-Containing Saliva Substitutes. Int. J. Oral Surg. 1983, 12, 232–238. [Google Scholar] [CrossRef]
- Larsson, B.; Olivecrona, G.; Ericson, T. Lipids in Human Saliva. Arch. Oral Biol. 1996, 41, 105–110. [Google Scholar] [CrossRef]
- Matczuk, J.; Zendzian-Piotrowska, M.; Maciejczyk, M.; Kurek, K. Salivary Lipids: A Review. Adv. Clin. Exp. Med. 2017, 26, 1023–1031. [Google Scholar] [CrossRef]
- Piazza, G.; Foglia, T. Rapeseed Oil for Oleochemical Usage. Eur. J. Lipid Sci. Technol. 2001, 103, 450–454. [Google Scholar] [CrossRef]
- Shahidi, F. Bailey’s Industrial Oil and Fat Products, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Momm, F.; Volegova-Neher, N.J.; Schulte-Mönting, J.; Guttenberger, R. Different Saliva Substitutes for Treatment of Xerostomia Following Radiotherapy a Prospective Crossover Study. Strahlenther. Onkol. 2005, 181, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Marina, A.M.; Man, Y.B.C.; Nazimah, S.A.H.; Amin, I. Chemical Properties of Virgin Coconut Oil. J. Am. Oil Chem. Soc. 2009, 86, 301–307. [Google Scholar] [CrossRef]
- Rahamat, S.F.; Hayati, W.N.; Manan, W.A.; Jalaludin, A.A.; Abllah, Z. Enamel Subsurface Remineralization Potential of Virgin Coconut Oil, Coconut Milk and Coconut Water. Mater. Today Proc. 2019, 16, 2238–2244. [Google Scholar] [CrossRef]
- Jayadas, N.H.; Nair, K.P.; Ajithkumar, G. Tribological Evaluation of Coconut Oil as an Environment-Friendly Lubricant. Tribol. Int. 2007, 40, 350–354. [Google Scholar] [CrossRef]
- Quimby, A.E.; Hogan, D.; Khalil, D.; Hearn, M.; Nault, C.; Johnson-Obaseki, S. Coconut Oil as a Novel Approach to Managing Radiation-Induced Xerostomia: A Primary Feasibility Study. Int. J. Otolaryngol. 2020, 2020, 8537643. [Google Scholar] [CrossRef] [PubMed]
- Boskou, D.; Blekas, G.; Tsimidou, M. Fatty Acids, Triacylglycerols, and Partial Glycerides. In Olive Oil—Chemistry and Technology; AOCS Press: Champaign, IL, USA, 2006; pp. 41–42. [Google Scholar]
- Ship, J.A.; McCutcheon, J.A.; Spivakovsky, S.; Kerr, A.R. Safety and Effectiveness of Topical Dry Mouth Products Containing Olive Oil, Betaine, and Xylitol in Reducing Xerostomia for Polypharmacy-Induced Dry Mouth. J. Oral Rehabil. 2007, 34, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Dost, F.; Farah, C.S. Stimulating the Discussion on Saliva Substitutes: A Clinical Perspective. Aust. Dent. J. 2013, 58, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Adibnia, V.; Ma, Y.; Halimi, I.; Walker, G.C.; Banquy, X.; Kumacheva, E. Phytoglycogen Nanoparticles: Nature-Derived Superlubricants. ACS Nano 2021, 15, 8953–8964. [Google Scholar] [CrossRef] [PubMed]
- Petri, D.F.S. Xanthan Gum: A Versatile Biopolymer for Biomedical and Technological Applications. J. Appl. Polym. Sci. 2015, 132, 42035. [Google Scholar] [CrossRef]
- Bhat, I.M.; Wani, S.M.; Mir, S.A.; Masoodi, F.A. Advances in Xanthan Gum Production, Modifications and Its Applications. Biocatal. Agric. Biotechnol. 2022, 42, 102328. [Google Scholar] [CrossRef]
- Jansson, P.-E.; Kenne, L.; Lindberg, B. Structure of the Extracellular Polysaccharide from Xanthomonas Campestris. Carbohydr. Res. 1975, 45, 275–282. [Google Scholar] [CrossRef]
- Rosalam, S.; England, R. Review of Xanthan Gum Production from Unmodified Starches by Xanthomonas comprestris sp. Enzyme Microb. Technol. 2006, 39, 197–207. [Google Scholar] [CrossRef]
- Jellema, A.P.; Langendijk, H.; Bergenhenegouwen, L.; Reijden, W.V.D.; Leemans, R.; Smeele, L.; Slotman, B.J. The Efficacy of Xialine in Patients with Xerostomia Resulting from Radiotherapy for Head and Neck Cancer: A Pilot-Study. Radiother. Oncol. 2001, 59, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shao, X.; Ling, P.; Liu, F.; Han, G.; Wang, F. Recent Advances in Polysaccharides for Osteoarthritis Therapy. Eur. J. Med. Chem. 2017, 139, 926–935. [Google Scholar] [CrossRef]
- Takemura, A.; Hashimoto, K.; Ho, A.; Bessinger, M.; Law, S.; Schifferle, R.E.; Ciancio, S.G. Efficacy of New Oral Rinse Containing Sodium Hyaluronate in Xerostomia: A Randomized Crossover Study. Oral Dis. 2022, 29, 2747–2755. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front. Vet. Sci. 2019, 6, 192–216. [Google Scholar] [CrossRef] [PubMed]
- Pogrel, M.A.; Lowe, M.A.; Stern, R. Hyaluronan (Hyaluronic Acid) in Human Saliva. Arch. Oral Biol. 1996, 41, 667–671. [Google Scholar] [CrossRef]
- Park, M.S.; Chang, J.Y.; Kang, J.H.; Park, K.P.; Kho, H.S. Rheological Properties of Hyaluronic Acid and Its Effects on Salivary Enzymes and Candida. Oral Dis. 2010, 16, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chang, J.Y.; Kim, Y.Y.; Kim, M.J.; Kho, H.S. Effects of Molecular Weight of Hyaluronic Acid on Its Viscosity and Enzymatic Activities of Lysozyme and Peroxidase. Arch. Oral Biol. 2018, 89, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-marquez, C.D.; Arteaga-marin, S.; Rivas-sánchez, A.; Autrique-hernández, R.; Castro-muñoz, R. A Review on Current Strategies for Extraction and Purification of Hyaluronic Acid. Int. J. Mol. Sci. 2022, 23, 6038. [Google Scholar] [CrossRef]
- Kho, H.S.; Park, M.S.; Chang, J.Y.; Kim, Y.Y. Yam Tuber Mucilage as a Candidate Substance for Saliva Substitute: In Vitro Study of Its Viscosity and Influences on Lysozyme and Peroxidase Activities. Gerodontology 2014, 31, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Misaki, A.; Ito, T.; Harada, T. Constitutional Studies on the Mucilage of “Yamanoimo,” Dioscorea Batatas Decne, Forma Tsukune. Agric. Biol. Chem. 1972, 36, 761–771. [Google Scholar] [CrossRef]
- Johansson, G.; Andersson, G.; Attström, R.; Glantz, P.O.; Larsson, K. The Effect of Salinum on the Symptoms of Dry Mouth: A Pilot Study. Gerodontology 1994, 11, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Herod, E.L. The Use of Milk as a Saliva Substitute. J. Public Health Dent. 1994, 54, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Horne, D.S. Casein Structure, Self-Assembly and Gelation. Curr. Opin. Colloid Interface Sci. 2002, 7, 456–461. [Google Scholar] [CrossRef]
- Huppertz, T. Chemistry of the Caseins. Adv. Dairy Chem. 2013, 1A, 135–160. [Google Scholar]
- Park, M.S.; Chang, J.Y.; Kim, Y.Y.; Kang, J.H.; Kho, H.S. Physical and Biological Properties of Yam as a Saliva Substitute. Arch. Oral Biol. 2010, 55, 177–183. [Google Scholar] [CrossRef]
- Andersson, G.; Johansson, G.; Attström, R.; Edwardsson, S.; Glantz, P.; Larsson, K. Comparison of the Effect of the Linseed Extract Salinum® and a Methyl Cellulose Preparation on the Symptoms of Dry Mouth. Gerodontology 1995, 12, 12–17. [Google Scholar] [CrossRef]
- Hirst, L.S. Fundamentals of Soft Matter Science, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Jensen, R.G.; Ferris, A.M.; Lammi-Keefe, C.J. The Composition of Milk Fat. J. Dairy Sci. 1991, 74, 3228–3243. [Google Scholar] [CrossRef]
- Barbour, M.E.; Shellis, R.P.; Parker, D.M.; Allen, G.C.; Addy, M. Inhibition of Hydroxyapatite Dissolution by Whole Casein: The Effects of PH, Protein Concentration, Calcium, and Ionic Strength. Eur. J. Oral Sci. 2008, 116, 473–478. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, Y.; Ji, Z.; Chen, J. Lubrication and Sensory Properties of Emulsion Systems and Effects of Droplet Size Distribution. Foods 2021, 10, 3024. [Google Scholar] [CrossRef]
- Quispe, C.; Villalobos, M.; Bórquez, J.; Simirgiotis, M. Chemical Composition and Antioxidant Activity of Aloe vera from the Pica Oasis (Tarapacá, Chile) by UHPLC-Q/Orbitrap/MS/MS. J. Chem. 2018, 2018, 6123850. [Google Scholar] [CrossRef]
- Femenia, A.; Sánchez, E.S.; Simal, S.; Rosselló, C. Compositional Features of Polysaccharides from Aloe vera (Aloe barbadensis Miller) Plant Tissues. Carbohydr. Polym. 1999, 39, 109–117. [Google Scholar] [CrossRef]
- McAnalley, B.H. Process for Preparation of Aloe Products. Patent ZA889733B, 29 August 1990. [Google Scholar]
- Xu, J.; Luo, J.B.; Liu, S.H.; Xie, G.X.; Ma, L. Tribological Characteristics of Aloe Mucilage. Tribol.—Mater. Surf. Interfaces 2008, 2, 72–76. [Google Scholar] [CrossRef]
- Javanbakht, S.; Shaabani, A. Carboxymethyl Cellulose-Based Oral Delivery Systems. Int. J. Biol. Macromol. 2019, 133, 21–29. [Google Scholar] [CrossRef]
- Sarideechaigul, W.; Priprem, A.; Limsitthichaikoon, S.; Phothipakdee, P.; Chaijit, R.; Jorns, T.P.; Lungruammit, N.; Chaiya, K. Efficacy and Safety of Two Artificial Saliva-Based Polymers Containing 0.1% Pilocarpine for Treatment of Xerostomia: A Randomized Clinical Pilot Trial. J. Clin. Exp. Dent. 2021, 13, 994–1000. [Google Scholar] [CrossRef]
- Adibnia, V.; Mirbagheri, M.; Latreille, P.L.; Faivre, J.; Cécyre, B.; Robert, J.; Bouchard, J.F.; Martinez, V.A.; Delair, T.; David, L.; et al. Chitosan Hydrogel Micro-Bio-Devices with Complex Capillary Patterns via Reactive-Diffusive Self-Assembly. Acta Biomater. 2019, 99, 211–219. [Google Scholar] [CrossRef]
- Neto, A.I.; Cibrão, A.C.; Correia, C.R.; Carvalho, R.R.; Luz, G.M.; Ferrer, G.G.; Botelho, G.; Picart, C.; Alves, N.M.; Mano, J.F. Nanostructured Polymeric Coatings Based on Chitosan and Dopamine-Modified Hyaluronic Acid for Biomedical Applications. Small 2014, 10, 2459–2469. [Google Scholar] [CrossRef]
- Boddupalli, B.M.; Mohammed, Z.N.K.; Nath, R.A.; Banji, D. Mucoadhesive Drug Delivery System: An Overview. J. Adv. Pharm. Technol. Res. 2010, 1, 381–387. [Google Scholar] [CrossRef]
- Adamczak, M.I.; Martinsen, Ø.G.; Smistad, G.; Hiorth, M. Polymer Coated Mucoadhesive Liposomes Intended for the Management of Xerostomia. Int. J. Pharm. 2017, 527, 72–78. [Google Scholar] [CrossRef]
- Hiorth, M.; Mihailovic, L.; Adamczak, M.; Goycoolea, F.M.; Sarkar, A. Lubricating Performance of Polymer-Coated Liposomes. Biotribology 2023, 35–36, 100239. [Google Scholar] [CrossRef]
- Vance, D.E.; Vance, J.E. Phospholipid Biosynthesis in Eukaryotes. Biochem. Lipids Lipoproteins Membr. 2008, 2008, 213–244. [Google Scholar]
- Ridgway, N.D. Phospholipid Synthesis in Mammalian Cells. In Biochemistry of Lipids, Lipoproteins and Membranes; Elsevier: Amsterdam, The Netherlands, 2021; pp. 227–258. [Google Scholar]
- Abdelkafi, S.; Abousalham, A. The Substrate Specificities of Sunflower and Soybean Phospholipases D Using Transphosphatidylation Reaction. Lipids Health Dis. 2011, 10, 196. [Google Scholar] [CrossRef]
- Kanno, K.; Wu, M.K.; Scapa, E.F.; Roderick, S.L.; Cohen, D.E. Structure and Function of Phosphatidylcholine Transfer Protein (PC-TP)/StarD2. Biochim. Biophys. Acta—Mol. Cell Res. 2007, 1771, 654–662. [Google Scholar] [CrossRef]
- Israelachvili, J.N.; Marcelja, S.; Horn, R.G.; Israelachvili, J.N. Physical Principles of Membrane Organization. Q. Rev. Biophys. 1980, 13, 121–200. [Google Scholar] [CrossRef]
- Adibnia, V.; Olszewski, M.; De Crescenzo, G.; Matyjaszewski, K.; Banquy, X. Superlubricity of Zwitterionic Bottlebrush Polymers in the Presence of Multivalent Ions. J. Am. Chem. Soc. 2020, 142, 14843–14847. [Google Scholar] [CrossRef]
- Faivre, J.; Shrestha, B.; Xie, G.; Olszewski, M.; Adibnia, V.; Moldovan, F.; Montembault, A.; Sudre, G.; Delair, T.; David, L.; et al. Intermolecular Interactions between Bottlebrush Polymers Boost the Protection of Surfaces against Frictional Wear. Chem. Mater. 2018, 30, 4140–4149. [Google Scholar] [CrossRef]
- Goldberg, R.; Schroeder, A.; Silbert, G.; Turjeman, K.; Barenholz, Y.; Klein, J. Boundary Lubricants with Exceptionally Low Friction Coefficients Based on 2D Close-Packed Phosphatidylcholine Liposomes. Adv. Mater. 2011, 23, 3517–3521. [Google Scholar] [CrossRef]
- Trunfio-Sfarghiu, A.M.; Berthier, Y.; Meurisse, M.H.; Rieu, J.P. Role of Nanomechanical Properties in the Tribological Performance of Phospholipid Biomimetic Surfaces. Langmuir 2008, 24, 8765–8771. [Google Scholar] [CrossRef]
- Takeuchi, H.; Yamamoto, H.; Niwa, T.; Hino, T.; Kawashima, Y. Mucoadhesion of Polymer-Coated Liposomes to Rat Intestine in Vitro. Chem. Pharm. Bull. 1994, 42, 1954–1956. [Google Scholar] [CrossRef]
- Hanning, S.M.; Yu, T.; Jones, D.S.; Andrews, G.P.; Kieser, J.A.; Medlicott, N.J. Lecithin-Based Emulsions for Potential Use as Saliva Substitutes in Patients with Xerostomia—Viscoelastic Properties. Int. J. Pharm. 2013, 456, 560–568. [Google Scholar] [CrossRef]
- Hu, J. Aqueous Lubricants for Dry Mouth Applications; University of Leeds: Leeds, UK, 2020. [Google Scholar]
- Blakeley, M.; Sharma, P.K.; Kaper, H.J.; Bostanci, N.; Crouzier, T. Lectin-Functionalized Polyethylene Glycol for Relief of Mucosal Dryness. Adv. Healthc. Mater. 2022, 11, 2101719. [Google Scholar] [CrossRef]
- He, X.; Smart, P.; Taufiqurrakhman, M.; Wang, C.; Bryant, M. Stable Oral Lubrication Enhancer Obtained from Thiolated Polyethylene Glycol and Mucin. Friction 2023, 11, 617–634. [Google Scholar] [CrossRef]
- Kaur, G.; Grewal, J.; Jyoti, K.; Jain, U.K.; Chandra, R.; Madan, J. Oral Controlled and Sustained Drug Delivery Systems: Concepts, Advances, Preclinical, and Clinical Status. Drug Target. Stimuli Sensitive Drug Deliv. Syst. 2018, 2018, 567–626. [Google Scholar]
- Baliga, S.; Muglikar, S.; Kale, R. Salivary PH: A Diagnostic Biomarker. J. Indian Soc. Periodontol. 2013, 17, 461–465. [Google Scholar] [CrossRef]
- Suhail, M.; Wu, P.C.; Minhas, M.U. Using Carbomer-Based Hydrogels for Control the Release Rate of Diclofenac Sodium: Preparation and in Vitro Evaluation. Pharmaceuticals 2020, 13, 399. [Google Scholar] [CrossRef]
- Mastropietro, D.; Park, K.; Omidian, H. 4.23 Polymers in Oral Drug Delivery. Compr. Biomater. II 2017, 4, 430–444. [Google Scholar]
- Maslii, Y.; Ruban, O.; Kasparaviciene, G.; Kalveniene, Z.; Materiienko, A.; Ivanauskas, L.; Mazurkeviciute, A.; Kopustinskiene, D.M.; Bernatoniene, J. The Influence of PH Values on the Rheological, Textural and Release Properties of Carbomer Polacril® 40P-Based Dental Gel Formulation with Plant-Derived and Synthetic Active Components. Molecules 2020, 25, 5018. [Google Scholar] [CrossRef]
- Subramanian, D.A.; Langer, R.; Traverso, G. Mucus Interaction to Improve Gastrointestinal Retention and Pharmacokinetics of Orally Administered Nano-Drug Delivery Systems. J. Nanobiotechnol. 2022, 20, 362. [Google Scholar] [CrossRef]
- Mehravaran, N.; Moghimi, H.; Mortazavi, S.A. The Influence of Various Mucoadhesive Polymers on In Vitro Performance of the Resulting Artificial Saliva Pump Spray Formulations. Iran. J. Pharm. Res. 2009, 8, 3–13. [Google Scholar]
- Vinke, J.; Kaper, H.J.; Vissink, A.; Sharma, P.K.; Nl, K.S. Dry Mouth: Saliva Substitutes Which Adsorb and Modify Existing Salivary Condition Films Improve Oral Lubrication Salivary Conditioning Film SCF AT Salivary Conditioning Film after Treatment SN Saliva Natura. Clin. Oral Investig. 2020, 24, 4019–4030. [Google Scholar] [CrossRef]
- Gookizadeh, A.; Emami, H.; Najafizadeh, N.; Roayaei, M. Clinical Evaluation of BIOXTRA in Relieving Signs and Symptoms of Dry Mouth after Head and Neck Radiotherapy of Cancer Patients at Seyed-Al-Shohada Hospital, Isfahan, Iran. Adv. Biomed. Res. 2012, 1, 72. [Google Scholar]
- Wan, H.; Ma, C.; Vinke, J.; Vissink, A.; Herrmann, A.; Sharma, P.K. Next Generation Salivary Lubrication Enhancer Derived from Recombinant Supercharged Polypeptides for Xerostomia. ACS Appl. Mater. Interfaces 2020, 12, 34524–34535. [Google Scholar] [CrossRef]
- Banquy, X.; Burdyńska, J.; Lee, D.W.; Matyjaszewski, K.; Israelachvili, J. Bioinspired Bottle-Brush Polymer Exhibits Low Friction and Amontons-like Behavior. J. Am. Chem. Soc. 2014, 136, 6199–6202. [Google Scholar] [CrossRef]
- Kluzek, M.; Oppenheimer-Shaanan, Y.; Dadosh, T.; Morandi, M.I.; Avinoam, O.; Raanan, C.; Goldsmith, M.; Goldberg, R.; Klein, J. Designer Liposomic Nanocarriers Are Effective Biofilm Eradicators. ACS Nano 2022, 16, 15792–15804. [Google Scholar] [CrossRef]

| Substance | Components that Provide Lubrication |
|---|---|
| Yam tuber | Mucilages (mannan glycoproteins) [124,125,126] |
| Linseed extract | polysaccharides, glycoproteins, and proteins that mimic mucins [127] |
| Milk | Fats and protein [128,129,130] |
| Aloe vera gel | Acemannan polysaccharides [106] |
| Saliva Substitute | Advantages | Disadvantages |
|---|---|---|
| Natural | ||
| Porcine/Bovine Mucins | Similar in structure and function to mucins in human saliva [98], loses effectiveness within 30 min of applying [99] | No noted disadvantages |
| Rapeseed Oil | Low erucic acid and glucosinolate indicates it is healthier than other oils [103]; is on par with mucins, carboxymethyl cellulose, and Aloe vera with regards to effectivity [104] | Poor taste [104] |
| Coconut Oil | Easily digested and preserves well without becoming rancid [105], 41% of study participants chose to continue using coconut oil after the study [108] | Calorie dense [105], may demineralize tooth enamel [106] |
| Olive Oil | Artificial saliva containing olive oil increased unstimulated whole saliva flow rate and improved patient’s xerostomia [110], antimicrobial and anti-inflammatory properties [111] | No clear correlation between olive oil itself and improved xerostomia |
| Xanthan Gum | Mimics native saliva’s mouthfeel during eating and speech [117] | No distinct advantages of xanthan gum over placebos (Xialine® without Xanthan Gum) aside from speech production [117] |
| Sodium Hyaluronate | High water content providing lubrication [122]; biocompatible, non-immunogenic, and degraded by the body’s hyaluronidase [119]; similar rheometric and non-Newtonian qualities as saliva under the same shearing forces [122]; increases unstimulated salivary flow rates compared to placebos [122] | Inferior wettability and film-forming ability to whole human saliva [122]; high molecular weight HA (up to 20,000 kDa) reduces lysozyme and peroxidase, leading to infection [123], so low molecular weight HA from bacteria and yeast will have to be used [123,124] |
| Yam tuber extract | Can achieve a similar viscosity to saliva by mixing with simulated salivary buffer solutions [131], greater wettability on resins compared to whole human saliva [131] | No noted disadvantages |
| Linseed extract | Similar properties to native saliva [127], lasts longer (60 min compared to 30 min) than commercial carboxymethyl cellulose saliva substitutes [132], reduces gingival bleeding and plaque index [132], improved subjective taste, speech, chewing, swallowing, and overall relief compared to commercial carboxymethyl cellulose saliva substitutes [132] | No noted disadvantages |
| Whole bovine milk | Protect enamel by buffering acids, decreasing enamel’s solubility, and helping remineralize enamel [128]; contains casein which can inhibit hydroxyapatite dissolution [135]; combining whole bovine milk with xanthan gum decreases the coefficient of friction on PDMS [136] | High sugar content leading to dental caries [128], no studies on whole bovine milk as a saliva substitute to date |
| Aloe vera | Lowest frequency of use compared to carboxymethyl cellulose, animal mucins, and rapeseed oil [104]; statistically significant improvement in xerostomia symptoms [104]; improved sleep quality more than carboxymethyl cellulose, rapeseed oil, and animal mucins [104] | No noted disadvantages |
| Synthetic | ||
| Carboxymethyl cellulose | Statistically significant improvement in xerostomia symptoms [104], mucoadhesive and high water retention properties [141], improves stimulated and unstimulated whole saliva flow rates [142] | Stickiness, caking, loses effectiveness within 10 min of applying [99] |
| Chitosan | Mucoadhesive properties [145], can be modified with catechol to become softer and adsorb more salivary proteins [145], chitosan-coated liposomes have the highest water sorption properties compared to other polymer-coated liposomes [146] | No noted disadvantages |
| Alginate-coated liposomes | Outperform chitosan, methoxylated pectin, and hydrophobically modified ethyl hydroxyethyl cellulose coated liposomes for water retention [146] | No noted disadvantages |
| Phosphatidylcholine-modified macromolecules | Phosphatidylcholine (PC) is abundant in all organisms and easy to obtain [150]; imbues macromolecules with excellent hydration, hydration lubrication, and allows them to rapidly relax when under shearing forces [153,154,155] | No noted disadvantages |
| DPPC | Densely packed phospholipids indicate a low coefficient of friction and resistance to deformation in the presence of shear forces [155], highly mechanically stable [155], very low coefficient of friction of 0.002 ± 0.0008 [155] | Needs extensive chemical modification to become mucoadhesive [157] |
| DOPC | Very low coefficient of friction of 0.01 ± 0.005 [155] | Less resistant to deformation than DPPC due to its liquid bilayer state and the lower density of its phospholipids [155], needs extensive modification to become mucoadhesive [157] |
| Polyethylene glycol (PEG) | Sustainable lubrication while providing lasting hydration [160], thiolated PEG is more efficient at lubricating than mucin [161] | PEG must be coated with wheat-germ agglutinin to become bioadhesive to avoid being removed from the mouth during swallowing [160] |
| Carbomers | Excellent water uptake and swelling due to the high concentration of carboxylic acid groups that become negatively charged in the mouth [164], more mucoadhesive than natural human saliva due to the high number of carboxyl groups [167], highly resistant to being dislodged from the oral cavity [168], exhibit similar changes in viscosity in response to force as natural human saliva [168] | Must be combined with other hydrophilic materials to become effective in artificial saliva formulations [169], artificial saliva using carbomers and polyacrylic acid do not improve swallowing despite improving other xerostomia symptoms and speech [170] |
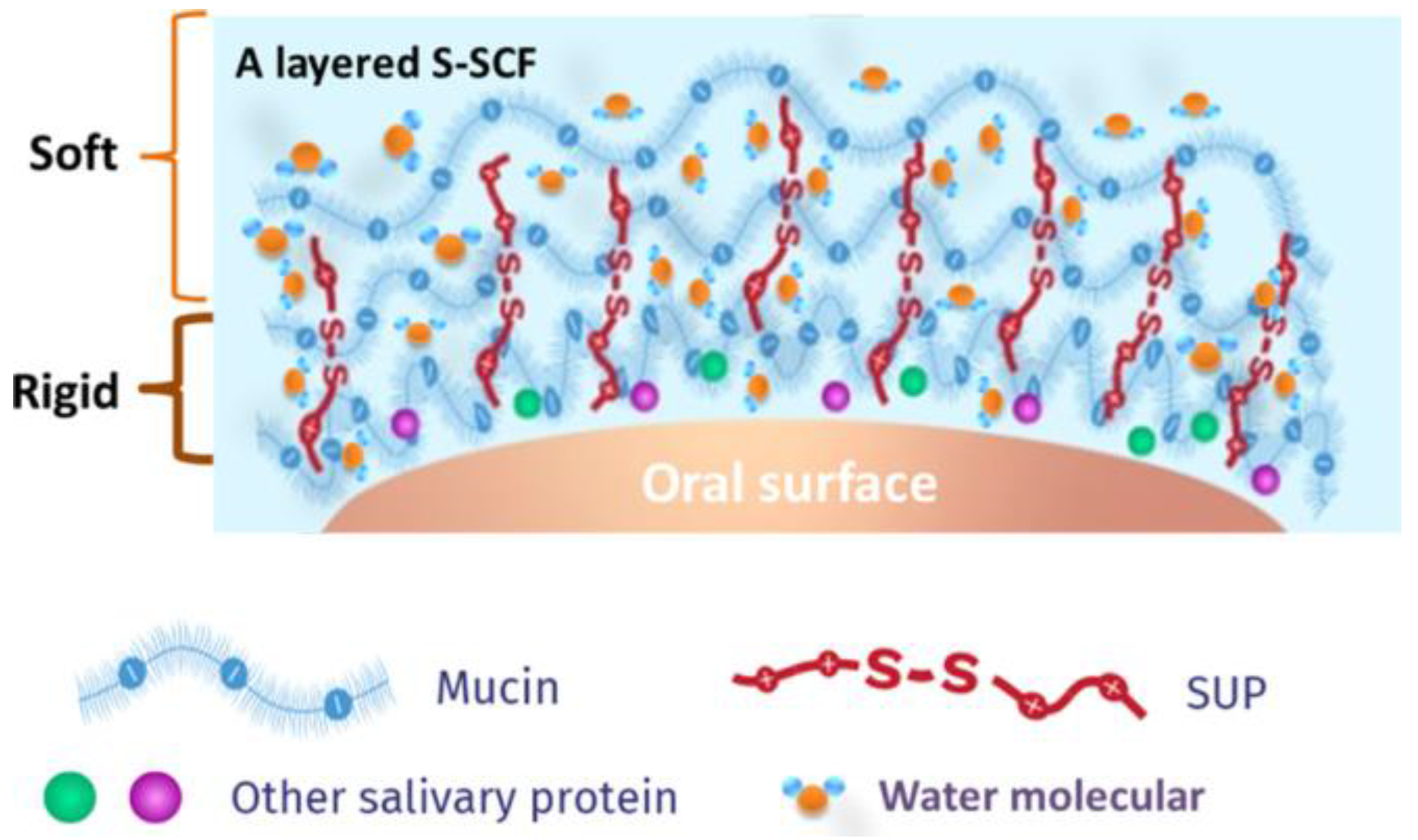
| SUPs | Improve oral lubrication and maintains its structural integrity during high contact pressures [17], SUPs with a sufficient number of positive charges can adsorb onto the SCF and retrieve mucins from the saliva [17], K108cys modified SUPs resulted in higher salivary glycoprotein adsorption and softness while also doubling the period of effectiveness of natural human saliva [17] | No noted disadvantages |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Austin, W.; Hdeib, M.; Fraser, P.; Goldchtaub, M.; Shams, E.; Han, T.; Michaud, P.-L.; Adibnia, V. Oral Lubrication, Xerostomia, and Advanced Macromolecular Lubricants for Treatment of Dry Mouth. Lubricants 2024, 12, 126. https://doi.org/10.3390/lubricants12040126
Austin W, Hdeib M, Fraser P, Goldchtaub M, Shams E, Han T, Michaud P-L, Adibnia V. Oral Lubrication, Xerostomia, and Advanced Macromolecular Lubricants for Treatment of Dry Mouth. Lubricants. 2024; 12(4):126. https://doi.org/10.3390/lubricants12040126
Chicago/Turabian StyleAustin, William, Maryam Hdeib, Paige Fraser, Maya Goldchtaub, Elika Shams, Tianyi Han, Pierre-Luc Michaud, and Vahid Adibnia. 2024. "Oral Lubrication, Xerostomia, and Advanced Macromolecular Lubricants for Treatment of Dry Mouth" Lubricants 12, no. 4: 126. https://doi.org/10.3390/lubricants12040126
APA StyleAustin, W., Hdeib, M., Fraser, P., Goldchtaub, M., Shams, E., Han, T., Michaud, P.-L., & Adibnia, V. (2024). Oral Lubrication, Xerostomia, and Advanced Macromolecular Lubricants for Treatment of Dry Mouth. Lubricants, 12(4), 126. https://doi.org/10.3390/lubricants12040126








